Abstract
Few studies have reported on the long-term prognosis of anti-neutrophil cytoplasmic antibody (ANCA)-negative renal vasculitis. Between April 2003 and December 2013, 48 patients were diagnosed with renal vasculitis. Their ANCA status was tested using indirect immunofluorescence and enzyme-linked immunosorbent assays. During a median (interquartile range) follow-up duration of 933.5 (257.5–2,079.0) days, 41.7% of patients progressed to end stage renal disease (ESRD) and 43.8% died from any cause. Of 48 patients, 6 and 42 were ANCA-negative and positive, respectively. The rate of ESRD within 3 months was higher in ANCA-negative patients than in ANCA-positive patients (P = 0.038). In Kaplan-Meier survival analysis, ANCA-negative patients showed shorter renal survival than did ANCA-positive patients (log-rank P = 0.033). In univariate Cox-proportional hazard regression analysis, ANCA-negative patients showed increased risk of ESRD, with a hazard ratio 3.190 (95% confidence interval, 1.028–9.895, P = 0.045). However, the effect of ANCA status on renal survival was not statistically significant in multivariate analysis. Finally, ANCA status did not significantly affect patient survival. In conclusion, long-term patient and renal survival of ANCA-negative renal vasculitis patients did not differ from those of ANCA-positive renal vasculitis patients. Therefore, different treatment strategy depending on ANCA status might be unnecessary.
Keywords: Anti-Neutrophil Cytoplasmic Antibody (ANCA); Kidney Failure, Chronic; Vasculitis; Mortality; Sex; Proteinuria; Koreans; Prognosis; Survival; Cohort Studies
Graphical Abstract
INTRODUCTION
Anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis is a life-threatening disease characterized by inflammation and necrosis of small to medium-sized vessels (1). Renal vasculitis, also known as pauci-immune necrotizing crescentic glomerulonephritis is a severe form of ANCA associated vasculitis (2,3,4). ANCA has recently been proposed to be pathognomonic in ANCA associated vasculitis due to neutrophil, monocyte and endothelial cell activation, which leads to endothelial damage (1). However, patients with vasculitis are not always positive for ANCA. Approximately, 10% of ANCA associated vasculitis patients are ANCA-negative (5,6,7,8,9,10,11,12). To our knowledges, no study has focused specifically on the treatment of ANCA-negative patients (5). Moreover, few studies have directly compared long-term patient and renal outcomes between ANCA-negative and positive renal vasculitis patients (6,7,9). Hence, the long-term prognosis of ANCA-negative renal vasculitis remains unknown (6,7,9). Despite the paucity of evidence, current guidelines recommend that ANCA-negative receive treatments similar to those of ANCA-positive patients (5).
Therefore, the present study compared the long-term prognoses of ANCA-negative and positive renal vasculitis.
MATERIALS AND METHODS
Patients
This retrospective cohort study included 48 patients diagnosed with renal vasculitis at Seoul National University Bundang Hospital, a tertiary care hospital, between April 2003 and December 2013. The requirement for informed consent was waived because the study did not infringe the patients' privacy or health status. Demographic, physiologic, therapeutic and laboratory data were gathered from the electronic medical records database. Manual data verification was performed after patient datasets were merged. The long-term outcomes included end stage renal disease (ESRD) and all-cause mortality. ESRD development and all-cause mortality were determined from the ESRD registry database of the Korean Society of Nephrology and the Korea Statistics Service database, respectively.
Measurement and definitions
All 48 patients were tested for ANCA by indirect immunofluorescence (IIF) assay, but only 66.7% (32/48) were tested by enzyme-linked immunosorbent assay (ELISA) for myeloperoxidase (MPO) and/or proteinase 3 (PR3). ANCA testing was performed following the manufacturer’s instructions by the immunology department of Green Cross Laboratories (Yongin-si, Korea). During the study period, the IIF test kit was changed from INOVA Lite ANCA (INOVA Diagnostics, San Diego, California, USA) to Kallestad ANCA IFA (Bio-Rad, Hercules, California, USA) in April 2010, and the results of the two kits were found to be comparable. Quanta Lite (INOVA Diagnostics) was used for the ELISA.
Renal vasculitis was defined as cases which fulfilled the following two criteria: 1) evidence of glomerular crescent and/or necrosis but no other explainable pathologic diagnosis, such as IgA nephropathy or lupus nephritis and 2) no evidence of immune-complex deposition in immunofluorescence staining and electron microscopic examination. Hypertension was defined as systolic blood pressure (BP) ≥ 140 mmHg or diastolic BP ≥ 90 mmHg, use of antihypertensive drugs, or a physician’s diagnosis of hypertension. Diabetes was defined as random glucose ≥ 200 mg/dL, use of insulin and/or oral hypoglycemic agents, or a physician’s diagnosis of diabetes. The disease extent index was scored as described previously (13). The physiologic and laboratory data used for analysis were the worst values observed at index admission. Severe proteinuria and hematuria were defined as ≥ 3 positive on the dipstick protein test and ≥ 100 red blood cells per high power field in urine sediment microscopic examination, respectively. The estimated glomerular filtration rate (eGFR) was calculated using the equation from the chronic kidney disease epidemiology collaboration (14).
For renal pathology, each glomerulus was evaluated for the presence of global sclerosis, segmental sclerosis, crescent, and necrosis. Glomerular lesions were reported as a proportion of the total number of glomeruli in each specimen. Glomerular ischemia was defined as the presence of ischemic wrinkling and/or collapse. Glomerular cellularity and size and tubule-interstitial lesions were assessed semi-quantitatively as normal to severe (15). Vascular abnormality was defined as the presence of arteriolar hyalinosis and arteriosclerosis.
Statistical analysis
Values were expressed as median (interquartile range, IQR) for continuous variables and No./total (%) for categorical variables. Difference was analyzed by Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. The Kaplan-Meier method was used to estimate survival, and statistical significance was determined using the log-rank test. Univariate and multivariate Cox-proportional hazard regression analyses were performed for the factors related to renal and patient survival. Variables associated with clinical outcomes or ANCA status were entered in the multivariate analysis, along with age and sex. P < 0.05 was considered statistically significant. All analyses were performed using IBM SPSS for Windows, version 22 (IBM Corp., Armonk, NY, USA).
Ethics statement
This study was approved by the Seoul National University Bundang Hospital institutional review board (IRB number: B-1410/272-119). The requirement for informed consent was waived because the study did not infringe the patients’ privacy or health status.
RESULTS
Of 48 patients, the median (IQR) follow-up duration was 933.5 (257.5-2079.0) days. The median (IQR) age was 71.0 (61.5-78.8) years, and nearly half of the patients were male (26/48, 54.2%). During the follow-up period, 21 patients died from any cause (all-cause mortality 43.8%), and 20 progressed to ESRD (ESRD rate 41.7%). Among 48 patients with renal vasculitis, 6 (12.5%) were ANCA-negative and 42 (87.5%) were ANCA-positive.
We compared baseline characteristics according to ANCA status (Table 1). Unlike patients with ANCA, those without ANCA were predominantly male. Furthermore, ANCA-negative patients had a lower body temperatures and white blood cell counts than ANCA-positive patients. Although the serum creatinine level and eGFR were similar between ANCA-negative and positive patients, the rate of severe proteinuria was higher in ANCA-negative patients. We also compared pathologic findings depending on ANCA status, but there were no statistically significant differences between groups (Table 2). The treatment strategy did not differ between ANCA-negative and positive patients (Table 3).
Table 1. Baseline characteristics according to anti-neutrophil cytoplasmic antibody status.
| Parameters | ANCA status | P value | |
|---|---|---|---|
| Negative (n = 6) | Positive (n = 42) | ||
| Age (yr) | 68.5 (56.7-76.4) | 71.0 (62.1-78.9) | 0.554 |
| Male sex | 6/6 (100.0) | 20/42 (47.6) | 0.025 |
| Hypertension | 6/6 (100.0) | 37/42 (88.1) | 1.000 |
| Diabetes | 2/6 (33.3) | 14/42 (33.3) | 1.000 |
| Interval between symptom onset and diagnosis | 135.0 (53.8-782.5) | 90.5 (60.0-255.8) | 0.473 |
| Disease extent index | 5.0 (3.5-5.5) | 5.0 (3.0-6.3) | 0.962 |
| Systolic BP (mmHg) | 175.5 (160.5-187.8) | 172.0 (160.0-187.8) | 0.755 |
| Diastolic BP (mmHg) | 91.0 (84.8-105.0) | 88.5 (81.8-94.5) | 0.341 |
| Body temperature (℃) | 37.5 (37.0-37.9) | 38.3 (37.5-38.9) | 0.041 |
| White blood cells (× 103/µL) | 13.5 (10.3-16.1) | 19.0 (14.1-25.3) | 0.031 |
| Hemoglobin (g/dL) | 7.5 (6.1-8.7) | 7.4 (6.9-8.1) | 0.803 |
| Platelet (× 103/µL) | 183.0 (101.5-289.8) | 173.5 (105.5-229.8) | 0.708 |
| Albumin (g/dL) | 2.6 (2.1-2.8) | 2.6 (2.2-2.9) | 0.876 |
| Bilirubin (mg/dL) | 0.7 (0.4-1.6) | 0.7 (0.5-1.0) | 0.742 |
| Cholesterol (mg/dL) | 109.0 (85.5-133.0) | 109.0 (84.0-132.3) | 0.972 |
| Creatinine (mg/dL) | 4.3 (1.5-8.4) | 1.9 (0.9-5.9) | 0.374 |
| eGFR (mL/min/1.73 m2) | 21.2 (6.4-51.9) | 35.7 (8.1-81.2) | 0.575 |
| Peak Creatinine (mg/dL) | 6.5 (5.1-10.4) | 5.1 (3.4-8.4) | 0.185 |
| Lowest eGFR (mL/min/1.73 m2) | 8.0 (4.9-10.2) | 9.2 (5.0-18.9) | 0.493 |
| Severe proteinuria | 4/6 (66.7) | 8/41 (19.5) | 0.030 |
| Severe hematuria | 3/6 (50.0) | 25/41 (61.0) | 0.674 |
| C-reactive protein (mg/L) | 7.2 (2.9-10.9) | 10.1 (2.3-16.4) | 0.366 |
Values are expressed as median (interquartile range) for continuous variables and No./total (%) for categorical variables. Difference was analyzed by Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Severe proteinuria and hematuria were defined as dipstick urine protein ≥ 3+ and urine red cells ≥ 100/high power field, respectively. ANCA, anti-neutrophil cytoplasmic antibody; BP, blood pressure; AKI, acute kidney injury; eGFR, estimated glomerular filtration rate.
Table 2. Pathologic finding according to anti-neutrophil cytoplasmic antibody status.
| Findings | ANCA status | P value | |
|---|---|---|---|
| Negative (n = 6) | Positive (n = 42) | ||
| Number of glomeruli/biopsy | 32.0 (19.0-47.0) | 28.0 (14.5-49.3) | 0.827 |
| Global sclerosis (%) | 9.8 (4.2-17.4) | 13.9 (7.1-45.4) | 0.242 |
| Segmental sclerosis (%) | 0.0 (0.0-13.8) | 0.0 (0.0-0.4) | 0.512 |
| Crescent (%) | 52.6 (23.2-88.4) | 27.9 (15.0-60.1) | 0.156 |
| Necrosis (%) | 0.0 (0.0-12.5) | 0.0 (0.0-15.1) | 0.271 |
| Other glomerular finding | |||
| Increased cellularity | 3/6 (50.0) | 12/42 (28.6) | 0.360 |
| Increased size | 2/6 (33.3) | 9/42 (21.4) | 0.609 |
| Ischemia | 0/6 (0.0) | 4/48 (9.5) | 1.000 |
| Tubular atrophy | 0.671 | ||
| Normal | 0/6 (0.0) | 5/42 (14.0) | 1.000 |
| Mild | 1/6 (16.7) | 9/42 (21.4) | 1.000 |
| Moderate | 2/6 (33.3) | 7/42 (16.7) | 0.312 |
| Severe | 3/6 (50.0) | 21/42 (50.0) | 1.000 |
| Interstitial inflammation | 0.812 | ||
| Normal | 0/5 (0.0) | 0/42 (0.0) | |
| Mild | 1/5 (20.0) | 5/42 (11.9) | 0.511 |
| Moderate | 1/5 (20.0) | 13/42 (31.0) | 1.000 |
| Severe | 3/5 (60.0) | 24/42 (57.1) | 1.000 |
| Interstitial fibrosis | 0.663 | ||
| Normal | 0/5 (0.0) | 10/42 (23.8) | 0.569 |
| Mild | 1/5 (20.0) | 8/42 (19.0) | 1.000 |
| Moderate | 1/5 (20.0) | 6/42 (14.3) | 0.571 |
| Severe | 3/5 (60.0) | 18/42 (42.9) | 0.644 |
| Vascular abnormality | 3/6 (50.0) | 34/42 (81.0) | 0.124 |
Values are median (interquartile range) for continuous variables and No./total (%) for categorical variables. Difference was analyzed by Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. ANCA, anti-neutrophil cytoplasmic antibody.
Table 3. Therapeutic and clinical courses according to anti-neutrophil cytoplasmic antibody status.
| Status | ANCA status | P value | |
|---|---|---|---|
| Negative (n = 6) | Positive (n = 42) | ||
| Plasmapheresis | 0/6 (0.0) | 8/42 (19.0) | 0.571 |
| Steroid use | 6/6 (100.0) | 39/42 (92.9) | 1.000 |
| Steroid dose (g) | 6.7 (3.4-13.8) | 7.7 (3.1-10.9) | 0.803 |
| Steroid duration (days) | 119.0 (50.3-866.8) | 185.5 (56.0-572.3) | 0.803 |
| Cyclophosphamide use | 6/6 (100.0) | 32/42 (76.2) | 0.320 |
| Oral cyclophosphamide use | 4/6 (66.7) | 29/42 (69.0) | 1.000 |
| Cyclophosphamide dose (g) | 1.1 (0.9-3.3) | 1.2 (0.1-6.6) | 0.851 |
| Cyclophosphamide duration (days) | 23.5 (7.8-56.5) | 30.0 (0.8-146.0) | 0.802 |
| ESRD | 4/6 (66.7) | 16/42 (38.1) | 0.218 |
| ESRD within 3 mon | 4/6 (66.7) | 9/42 (21.4) | 0.038 |
| All-cause mortality | 2/4 (33.3) | 19/42 (45.2) | 0.683 |
Values were median (interquartile range) for continuous variables and No./total (%) for categorical variables. The difference was analyzed by Mann-Whitney U test in continuous variables and Fisher’s exact test in categorical variables. ANCA, anti-neutrophil cytoplasmic antibody; ESRD, end stage renal disease.
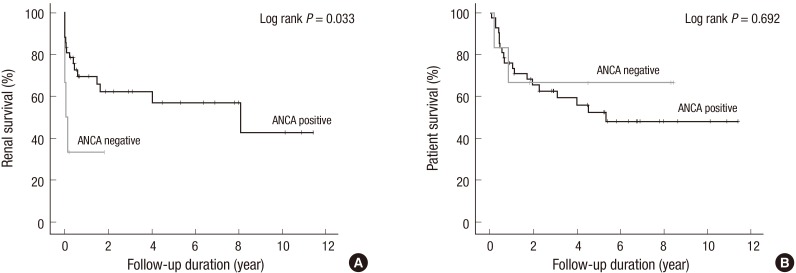
ANCA-negative patients had a higher rate of ESRD within 3 months than did ANCA-positive patients (Table 3). In Kaplan-Meier survival curves, the renal survival of ANCA-negative patients was significantly shorter than that of ANCA-positive patients: median (95% confidence interval [CI]) 15.0 (0.0-63.0) days vs. 2,941.0 (90.9-5,791.1) days (log-rank P = 0.033, Fig. 1A). In univariate Cox proportional hazard regression analysis, ANCA-negative patients showed significantly higher risk of ESRD than did ANCA-positive patients with a hazard ratio of 3.190 (95% CI, 1.028-9.895, P = 0.045). We performed multivariate analysis to adjust for confounding effects among the variables. Adjusting only for age did not affect the significance of ANCA status on renal survival. However, after adjusting for sex and severe proteinuria, the association between ANCA status and renal survival was not statistically significant (Table 4). Patient survival did not differ between groups (Table 3, Fig. 1B).
Fig. 1.
Kaplan-Meier survival curves according to anti-neutrophil cytoplasmic antibody anti-neutrophil cytoplasmic antibody (ANCA) status. Renal and patient survival are shown in (A) and (B), respectively. The gray and black lines represent ANCA-negative and positive groups, respectively.
Table 4. Factors associated with renal survival in Cox-proportional hazard model.
| Factors | Univariate | Multivariate* | Multivariate† | Multivariate‡ | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| ANCA (- vs. + ) | 3.190 (1.028-9.895) | 0.045 | 3.200 (1.030-9.938) | 0.044 | 1.923 (0.492-7.522) | 0.347 | 1.568 (0.347-7.081) | 0.559 |
| Age (yr)§ | 1.004 (0.966-1.044) | 0.826 | 1.005 (0.966-1.046) | 0.805 | 1.018 (0.976-1.063) | 0.403 | 1.057 (0.995-1.122) | 0.070 |
| Sex (M vs. F) | 1.317 (0.536-3.235) | 0.549 | - | - | 1.121 (0.406-3.098) | 0.825 | 0.809 (0.228-2.872) | 0.743 |
| Severe PU (+ vs. -) | 2.859 (1.145-7.138) | 0.024 | - | - | 2.833 (0.989-8.118) | 0.053 | 3.314 (1.109-9.903) | 0.032 |
| Cr (mg/dL)§ | 1.213 (1.090-1.349) | < 0.001 | - | - | - | - | 1.328 (1.133-1.556) | < 0.001 |
| WBC (103/µL)§ | 0.978 (0.922-1.037) | 0.460 | - | - | - | - | 1.015 (0.932-1.105) | 0.732 |
| BT (℃)§ | 0.894 (0.507-1.577) | 0.700 | - | - | - | - | 0.524 (0.165-1.669) | 0.274 |
*Adjusted for age; †Adjusted for age, sex and severe PU; ‡Variables associated with either renal survival or ANCA status were included for the full adjustment; §Per 1 unit increase. BT, body temperature; Cr, creatinine; PU, proteinuria; WBC, white blood cells; ANCA, anti-neutrophil cytoplasmic antibody; HR, hazard ratio; CI, confidence interval.
DISCUSSION
Although ANCA is thought to play a pathogenic role in vasculitis (1), it is not always positive. According to Kidney Disease: Improving Global Outcome guideline, 10% of patients with signs and symptoms of vasculitis are persistently ANCA-negative; these patients are treated similarly to ANCA-positive patients (5). To our knowledge, only three studies have directly compared the long-term prognosis of ANCA-negative and positive renal vasculitis (6,7,9); however, their findings were not consistent. Weidner et al. (9) analyzed 80 patients with vasculitis, and showed excellent long-term outcome in ANCA negative patients: patients for an average of 46.7 months; in this population, no death or ESRD developed in 6 ANCA-negative patients, but 17 deaths and 18 cases of ESRD were identified in 74 ANCA-positive patients. In contrast, Chen et al. (6) reported poor renal, but similar patient survival in 28 ANCA-negative vasculitis patients, compared to 57 ANCA-positive vasculitis patients. Meanwhile, Hung et al. (7) reported similar renal and patient survival between 15 ANCA-negative and 25 ANCA-positive patients. In our study, we found similar patient survival between ANCA-negative and positive renal vasculitis patients after 933.5 days’ follow-up, concordant with previous studies (6,7). However, the seemingly poor renal survival in ANCA-negative renal vasculitis patients could be a result confounded by various factors.
According to our study results, ANCA-negative renal vasculitis patients showed shorter renal survival than ANCA-positive renal vasculitis patients. The rate of ESRD within 3 months was significantly higher in ANCA-negative renal vasculitis patients than in ANCA-positive renal vasculitis patients. The hazard of ESRD in ANCA-negative patients was 3.190 times higher than that in ANCA-positive patients. These findings are concordant with those of Chen et al. (6). However, the poor renal outcome in ANCA-negative renal vasculitis patients might result from the confounding effects of sex and proteinuria. In our study, the ANCA-negative patients were predominantly male and had higher rates of severe proteinuria, both of which are known risk factors for the progression of kidney disease (16,17). The fact that the significance of ANCA status on renal survival was not statistically significant after adjusting for sex and proteinuria supports our assumption. The similar clinical presentation and pathologic results between ANCA-negative and positive renal vasculitis patients in our study, which are different from previous studies (6,7), also strengthened the suspicion that the seemingly poor renal outcome in ANCA-negative patients is not real.
This study has several limitations. First, because the incidence of vasculitis is low (18,19), the number of study patients was small. Because of the small sample size, the differences in clinical presentation and pathologic results between ANCA-negative and positive patients could be under-represented for statistical significance. However, despite this weakness, renal survival between ANCA-negative and positive patients was significantly different. Moreover, the similarity of clinical and pathologic findings between ANCA-negative and positive patients was paralleled by similar patient and renal survival. Therefore, the problem of small sample size could be acceptable in this respect. Second, because of the retrospective study design, data such as crescent characteristics or the relapse pattern were unavailable. Third, the ELISA findings to confirm ANCA and classify whether the ANCA is for MPO or PR3 were not always available. In addition, complete ANCA data during the follow-up period was not available. Finally, the study patients were from a single center and single nation, which limits the generalizability of the findings.
In conclusion, long-term patient and renal survival of ANCA-negative renal vasculitis patients was not good or bad, compared to that of ANCA positive renal vasculitis patients. The results of our study support current treatment recommendations for ANCA-negative vasculitis patients. However, future large studies are necessary to confirm our results.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Na KY, Chae DW, Chin HJ. Data production and collection: Lee SW, Na KY, Chin HJ. Data analysis and interpretation: Lee SW, Yu MY, Chae DW, Baek SH, Ahn SY, Kim S, Chin HJ. Writing: Lee SW. Review and revision of manuscript: Chin HJ. Approval of final manuscript: all authors.
References
- 1.Wilde B, van Paassen P, Witzke O, Tervaert JW. New pathophysiological insights and treatment of ANCA-associated vasculitis. Kidney Int. 2011;79:599–612. doi: 10.1038/ki.2010.472. [DOI] [PubMed] [Google Scholar]
- 2.Tarzi RM, Cook HT, Pusey CD. Crescentic glomerulonephritis: new aspects of pathogenesis. Semin Nephrol. 2011;31:361–368. doi: 10.1016/j.semnephrol.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Rutgers A, Sanders JS, Stegeman CA, Kallenberg CG. Pauci-immune necrotizing glomerulonephritis. Rheum Dis Clin North Am. 2010;36:559–572. doi: 10.1016/j.rdc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Han JY, Yoon SA, Woo JY, Park IS, Kim SY, Chang YS, Bang BK. ‘Pauci-immune’ rapidly progressive glomerulonephritis associated with systemic vasculitis. J Korean Med Sci. 1992;7:264–270. doi: 10.3346/jkms.1992.7.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapter 13: pauci-immune focal and segmental necrotizing glomerulonephritis. Kidney Int Suppl. 2011;2012:233–239. doi: 10.1038/kisup.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Yu F, Wang SX, Zou WZ, Zhao MH, Wang HY. Antineutrophil cytoplasmic autoantibody-negative Pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol. 2007;18:599–605. doi: 10.1681/ASN.2006091021. [DOI] [PubMed] [Google Scholar]
- 7.Hung PH, Chiu YL, Lin WC, Chiang WC, Chen YM, Lin SL, Wu KD, Tsai TJ. Poor renal outcome of antineutrophil cytoplasmic antibody negative Pauci-immune glomerulonephritis in Taiwanese. J Formos Med Assoc. 2006;105:804–812. doi: 10.1016/S0929-6646(09)60267-9. [DOI] [PubMed] [Google Scholar]
- 8.Bajema IM, Hagen EC, Hermans J, Noël LH, Waldherr R, Ferrario F, Van Der Woude FJ, Bruijn JA. Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int. 1999;56:1751–1758. doi: 10.1046/j.1523-1755.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- 9.Weidner S, Geuss S, Hafezi-Rachti S, Wonka A, Rupprecht HD. ANCA-associated vasculitis with renal involvement: an outcome analysis. Nephrol Dial Transplant. 2004;19:1403–1411. doi: 10.1093/ndt/gfh161. [DOI] [PubMed] [Google Scholar]
- 10.Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 2002;61:80–89. doi: 10.1046/j.1523-1755.2002.00089.x. [DOI] [PubMed] [Google Scholar]
- 11.Little MA, Nazar L, Farrington K. Outcome in glomerulonephritis due to systemic small vessel vasculitis: effect of functional status and non-vasculitic co-morbidity. Nephrol Dial Transplant. 2004;19:356–364. doi: 10.1093/ndt/gfg551. [DOI] [PubMed] [Google Scholar]
- 12.Hedger N, Stevens J, Drey N, Walker S, Roderick P. Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: a 10-year retrospective study. Nephrol Dial Transplant. 2000;15:1593–1599. doi: 10.1093/ndt/15.10.1593. [DOI] [PubMed] [Google Scholar]
- 13.de Groot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener’s granulomatosis. Clin Nephrol. 2001;55:31–38. [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh SW, Kim S, Na KY, Chae DW, Kim S, Jin DC, Chin HJ. Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes Res Clin Pract. 2012;97:418–424. doi: 10.1016/j.diabres.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Möllsten A, Svensson M, Waernbaum I, Berhan Y, Schön S, Nyström L, Arnqvist HJ, Dahlquist G, Swedish Childhood Diabetes Study Group Cumulative risk, age at onset, and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes: a nationwide population-based cohort study. Diabetes. 2010;59:1803–1808. doi: 10.2337/db09-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gatenby PA. Anti-neutrophil cytoplasmic antibody-associated systemic vasculitis: nature or nurture? Intern Med J. 2012;42:1066–1067. doi: 10.1111/j.1445-5994.2012.02891.x. [DOI] [PubMed] [Google Scholar]
- 19.Sinico RA, Di Toma L, Radice A. Renal involvement in anti-neutrophil cytoplasmic autoantibody associated vasculitis. Autoimmun Rev. 2013;12:477–482. doi: 10.1016/j.autrev.2012.08.006. [DOI] [PubMed] [Google Scholar]