Abstract
The Korea Chronic Obstructive Pulmonary Disorders Subgroup Study Team (Korea COPD Subgroup Study team, KOCOSS) is a multicenter observational study that includes 956 patients (mean age 69.9 ± 7.8 years) who were enrolled from 45 tertiary and university-affiliated hospitals from December 2011 to October 2014. The initial evaluation for all patients included pulmonary function tests (PFT), 6-minute walk distance (6MWD), COPD Assessment Test (CAT), modified Medical Research Council (mMRC) dyspnea scale, and the COPD-specific version of St. George’s Respiratory Questionnaire (SGRQ-C). Here, we report the comparison of baseline characteristics between patients with early- (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage I and II/groups A and B) and late-stage COPD (GOLD stage III and IV/groups C and D). Among all patients, the mean post-bronchodilator FEV1 was 55.8% ± 16.7% of the predicted value, and most of the patients were in GOLD stage II (520, 56.9%) and group B (399, 42.0%). The number of exacerbations during one year prior to the first visit was significantly lower in patients with early COPD (0.4 vs. 0.9/0.1 vs. 1.2), as were the CAT score (13.9 vs. 18.3/13.5 vs. 18.1), mMRC (1.4 vs. 2.0/1.3 vs.1.9), and SGRQ-C total score (30.4 vs. 42.9/29.1 vs. 42.6) compared to late-stage COPD (all P < 0.001). Common comorbidities among all patients were hypertension (323, 37.7%), diabetes mellitus (139, 14.8%), and depression (207, 23.6%). The data from patients with early COPD will provide important information towards early detection, proper initial management, and design of future studies.
Keywords: Respiratory Function Tests; Questionnaires; Pulmonary Disease, Chronic Obstructive
Graphical Abstract
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases (1). The most important risk factor for COPD is cigarette smoking. Adults who have a history of more than 40 pack years of smoking show an approximately twelve-fold higher positive likelihood ratio for airflow obstruction (2). Other risk factors may include environmental exposures other than smoking, atopy, and antioxidant deficiency. The estimated worldwide prevalence of COPD is 7.5% to 10% (3).
According to the 2008 Korean National Health and Nutrition Examination Survey-IV, 13.4% of the population aged over 40 years in Korea had spirometrically-detected airflow obstruction consistent with COPD (4) and approximately 90% of them were classified as patients with early COPD. Even as a higher prevalence of COPD has been reported, the disease remains under-diagnosed, and it is believed that most patients with COPD here are under treated (4). One study reported that approximately 62% of moderate to severe COPD patients have variability in symptoms, which could precede potentially fatal delays (5) in diagnosis and treatment. The prognosis for patients with COPD can improve greatly if it is diagnosed in its early stages and promptly addressed with medications and lifestyle changes (including smoking cessation and regular exercise) aimed at preventing disease progression (6). However, in Korea, studies regarding the contribution of early detection and treatment to prevention of COPD progression have been scarce. Thus, the KOrea COpd Subgroup Study team (KOCOSS) cohort was developed to address the above issues through the serial observation of disease progression and outcomes, to identify risk factors that will form a foundation for early detection of COPD patients who may be at higher risk of progression, and to provide guidance for further studies. We present herein a cross-sectional analysis of the data collected at the time of enrollment in KOCOSS to describe the baseline characteristics of the KOCOSS cohort.
MATERIALS AND METHODS
Data collection
Recruitment and measurement occurred between December 2011 and October 2014. There are 45 study centers throughout Korea (Seoul, Pusan, Daegu, Incheon, Chungcheong-do, Gyeonggi-do, Gangwon-do, Gyeongsang-do, and Jeju-do) that are enrolling patients. Inclusion criteria are diagnosis of COPD by a pulmonologist, age ≥ 40 years, symptoms including cough, sputum, dyspnea, and post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) of 70% less than normal predicted value. The medical history at the first visit included frequency and severity of exacerbations in the previous 12 months, smoking status, patient-reported education level, medications, including those already prescribed for COPD, and comorbidities. For diagnosis of depression, we used Beck Depression Inventory (BDI), which confirmed of its validity and reliability (7). The medical research council (mMRC) dyspnea score was recorded, as were results of the COPD assessment test (CAT) and the COPD-specific version of St. George’s Respiratory Questionnaire (SGRQ-C). A 6-minute walk distance (6MWD) test was also performed. All of the data were reported using case-report forms (CRFs) completed by physicians or trained nurses, and patients were to be evaluated at regular 6-month intervals after the initial examination. The initial data sets were analyzed to identify the baseline patient characteristics that are reported in this study. Major exclusion criteria were asthma, other obstructive lung diseases including bronchiectasis, tuberculosis destroyed lung, inability to complete pulmonary function test, myocardial infarction or cerebrovascular event within the previous 3 months, pregnancy, rheumatoid disease, malignancy, irritable bowel disease, and steroid use for conditions other than COPD exacerbation within 8 weeks before enrollment. Exacerbations were defined as worsening of any respiratory symptom, such as increased sputum volume, purulence, or increased dyspnea, which required treatment with systemic corticosteroids, antibiotics, or both.
Pulmonary function, disease severity, and exercise assessment
Spirometry and 6MWD were performed according to standard techniques (8,9). COPD severity was categorized by spirometry alone, in accordance with the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. Stage I COPD was present at FEV1 ≥ 80% predicted, stage II at FEV1 ≤ 50% to 80% predicted, stage III at FEV1 ≤ 30% to 50% predicted, and stage IV at FEV1 < 30% predicted. In addition, a risk/symptom-classification system (A to D) consistent with the 2011 GOLD guidelines was used.
Quality of life and dyspnea scores
The SGRQ-C was administered to assess the health status from the patient’s perspective (10). The SGRQ-C is a 14-item questionnaire that can be summarized as a total score, as well as by three component scores for symptoms, activities, and impacts. Total and component scores were calculated according to algorithms provided in the SGRQ-C instruction manual (11). Dyspnea was evaluated using the mMRC dyspnea scale, which is five-point scale with higher scores indicating more severe dyspnea. The CAT score was also used for evaluation of dyspnea. It consists of 8 items, each scored from 0 to 5, with higher scores indicating a more severe symptom (12).
Statistical analyses
In case of continuous variables, descriptive statistics are reported as means with standard deviations, and in the case of categorical variables as the number of patients per category and frequency of responses. Continuous variables with different severity classifications were analyzed using two-sample t-test and Chi-squared tests or Fisher’s exact test for comparisons of categorical variables. The correlation between GOLD group A to D and symptom scores (mMRC, CAT score), SGRQ-C total score, and 6MWD test result was checked using the Spearman rank correlation coefficient (rho), because GOLD group A, B, C, D is an ordinal categorical variable. Differences were considered statistically significant at P < 0.05.
Ethics statement
The protocol was approved by the institutional review board at each center (Konkuk University Chungju Hospital, IRB No. 2012-001). All of the patients provided written informed consent for participation in the study.
RESULTS
Baseline patient characteristics
Table 1 shows the baseline characteristics of the COPD patients who were included in this study. Their average age was 69.9 ± 7.8 years, and the most of them were male (n = 872, 91.2%). Almost all patients (91.5%) were former or current smokers. Nonsmoker-COPD counted as 80 cases (8.5%). The post-bronchodilator FEV1 was 55.8% ± 16.7% predicted, the FEV1/FVC ratio was 49.0 ± 12.0, and the mean 6MWD was 365.6 ± 117.3 meter. Mean CAT and mMRC scores were 15.5 ± 7.7 and 1.6 ± 1.0. The CAT score was ≥ 10 in 76.7% of the patients and the mMRC score was ≥ 2 in 45.8% of the patients. The total SGRQ-C score was 34.8 ± 19.6. The mean number of acute exacerbations during one year before enrollment was 0.6 ± 1.6. Of the total study population, the percentage in GOLD stage I, II, III, and IV was 5.6 (n = 51), 56.9 (n = 520), 31.8 (n = 291), and 5.7 (n = 52) and the percentage in GOLD group A, B, C, and D was 12.8 (n = 122), 42.0 (n = 399), 5.1 (n = 48), and 36.9 (n = 351).
Table 1. Baseline characteristics of the KOCOSS subjects.
| Variables | No. of subjects | No. (%) or observation |
|---|---|---|
| Age (yr, mean ± SD) | 956 | 69.9 ± 7.8 |
| Male, No. (%) | 956 | 872 (91.2) |
| BMI (kg/m2, mean ± SD) | 926 | 22.7 ± 3.4 |
| Smoking, pack/yr, (mean ± SD) | 812 | 43.9 ± 25.1 |
| Current smoker (%) | 938 | 262 (27.9) |
| Former smoker (%) | 596 (63.5) | |
| Non – smoker (%) | 80 (8.5) | |
| Number of acute exacerbation during one year before enroll | 942 | |
| Mean number (mean ± SD) | 0.58 ± 1.6 | |
| 0 (%) | 692 (73.5) | |
| 1 (%) | 145 (15.4) | |
| ≥ 2 (%) | 105 (11.2) | |
| Number of comorbidities (mean ± SD) | 958 | 1.7 ± 1.4 |
| 0 | 208 (21.7) | |
| 1 | 256 (26.7) | |
| 2 | 258 (26.9) | |
| 3 | 145 (15.1) | |
| 4 | 51 (55.3) | |
| 5 | 28 (2.92) | |
| 6 | 7 (0.7) | |
| 7 | 5 (0.5) | |
| Education level | 945 | |
| Middle school and below | 540 (57.1) | |
| High school | 280 (29.6) | |
| College and above | 125 (13.2) | |
| Lung function | ||
| FEV1/FVC (%, mean ± SD) | 916 | 49.0 ± 12.0 |
| Post bronchodilator FEV1 (%, mean ± SD) | 914 | 55.8 ± 16.7 |
| Post bronchodilator FEV1 (L, mean ± SD) | 923 | 1.5 ± 0.58 |
| FVC (L, mean ± SD) | 923 | 3.06 ± 0.79 |
| TLC (L, mean ± SD) | 596 | 5.6 ± 0.8 |
| 6MWD (meters, mean ± SD) | 737 | 365.6 ± 117.3 |
| Subjects requiring oxygen during/after 6MWD | 958 | 2 (0.3) |
| Symptom scores | 958 | |
| CAT (mean ± SD) | 913 | 15.5 ± 7.7 |
| CAT score | 213 (23.3) | |
| CAT score ≥ 10 | 700 (76.7) | |
| mMRC score (mean ± SD) | 951 | 1.6 ± 1.0 |
| mMRC score | 515 (54.2) | |
| mMRC score ≥ 2 | 436 (45.8) | |
| Questionnaire (SGRQ-C) | ||
| Symptom | 947 | 44.2 ± 20.9 |
| Activity | 946 | 46.0± 27.3 |
| Impact | 947 | 25.7 ± 23.3 |
| Total | 944 | 34.8 ± 19.6 |
| GOLD stage (I to IV) | 914 | |
| I | 51 (5.6) | |
| II | 520 (56.9) | |
| III | 291 (31.8) | |
| IV | 52 (5.7) | |
| GOLD (A to D) | 920 | |
| A | 122 (12.8) | |
| B | 399 (42.0) | |
| C | 48 (5.1) | |
| D | 351 (36.9) |
SD, standard deviation; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity; 6MWD, six minutes walk distance test; CAT, COPD assessment test; mMRC, modified Medical Research Council dyspnea scale; SGRQ-C, St. George’s respiratory questionnaire; GOLD, global initiative for chronic obstructive lung disease.
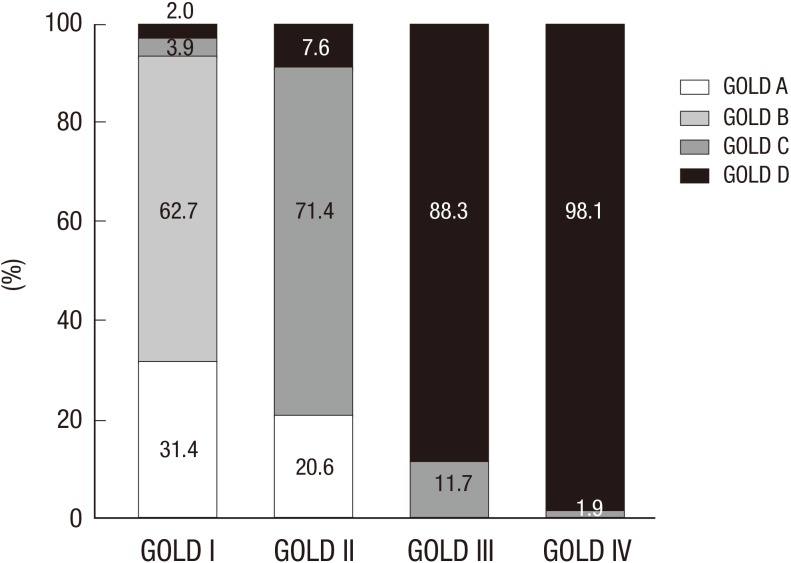
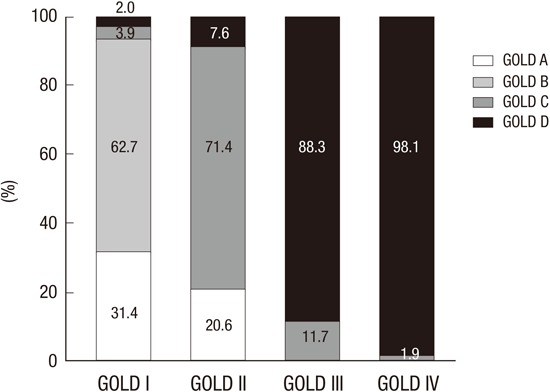
Fig. 1 shows the cumulative distribution of each GOLD (2011) group within each GOLD (2007) stage. GOLD group B patients comprised a large proportion (62.7%) compared to group A (31.4%) in the GOLD stage II patients. Similarly, GOLD stages III and IV included more patients from group D than group C (88.3 vs. 11.7 in stage III and 98.1% vs. 1.9% in stage IV). As specified in the GOLD guideline, stages III and IV do not include patients from group A or group B.
Fig. 1.
Distribution of subjects according to the GOLD classification.
Correlation of baseline characteristics and disease severity (global initiative for chronic obstructive lung disease criteria)
When patients were classified according to the 2007 GOLD classification, those with early stage (I and II) COPD had fewer exacerbations (mean number of exacerbations, 0.4 vs. 0.9, P < 0.001), significantly lower CAT, mMRC, and SGRQ-C total scores (13.9 vs. 18.3; 1.4 vs. 2.0; 30.4 vs. 42.9, respectively, all P < 0.001), and higher BMI (23.4 vs. 21.6), post-bronchodilator FEV1% (66.0 vs. 38.9), 6MWD (375.9 vs. 344.2), and education levels compared to patients with advanced (stage III and IV) COPD (all P < 0.001). According to the GOLD revision (2011), patients in group A and B had fewer exacerbations (mean number of exacerbations 0.1 vs. 1.2, P < 0.001), significantly lower CAT (13.5 vs. 18.1), mMRC (1.3 vs. 1.9), and SGRQ-C total score (29.1 vs. 42.6), and higher BMI (23.4 vs. 21.8), post bronchodilator FEV1% (66.2 vs. 41.6), 6MWD (376.4 vs. 350.0 meter), and education level compared patients in groups C and D (all P < 0.001) (Table 2).
Table 2. Baseline characteristics of the subjects according to the GOLD stage (I + II vs. III + IV) and GOLD group (A + B vs. C + D).
| Parameters | Stage I + II | Stage III + IV | P value | Grade A + B | Grade C + D | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | value | No. | value | No. | value | No. | value | |||
| Age (yr, mean ± SD) | 569 | 69.8 ± 7.8 | 343 | 70.0 ± 7.9 | 0.828 | 520 | 69.7 ± 7.9 | 398 | 70.1 ± 7.8 | 0.550 |
| Male, No. (%) | 570 | 516 (90.5) | 342 | 315 (92.1) | 0.417 | 520 | 474 (91.2) | 398 | 362 (91.0) | 0.917 |
| BMI (kg/m2, mean ± SD) | 562 | 23.4 ± 3.3 | 340 | 21.6 ± 3.3 | < 0.001 | 513 | 23.4 ± 3.3 | 391 | 21.8 ± 3.4 | < 0.001 |
| Current smoker | 567 | 169 (29.8) | 338 | 82 (24.3) | 0.076 | 519 | 155 (29.9) | 391 | 98 (25.1) | 0.253 |
| Smoking, pack/yr, (mean ± SD) | 487 | 43.8 ± 24.3 | 297 | 44.0 ± 26.0 | 0.905 | 447 | 43.5 ± 23.9 | 341 | 44.3 ± 27.0 | 0.649 |
| Number of acute exacerbation during one year before enroll | 565 | 340 | 521 | 396 | ||||||
| Mean ± SD | 0.4 ± 1.6 | 0.9 ± 1.6 | < 0.001 | 0.1 ± 0.3 | 1.2 ± 2.3 | < 0.001 | ||||
| 0 | 457 (80.9) | 204 (60.0) | < 0.001 | 457 (87.7) | 212 (53.5) | < 0.001 | ||||
| 1 | 64 (11.3) | 78 (22.9) | 64 (12.3) | 79 (20.0) | ||||||
| ≥ 2 | 44 (7.8) | 58 (17.1) | 0 (0.0) | 105 (26.5) | ||||||
| Number of comorbidities | 571 | 1.7 ± 1.3 | 343 | 1.8 ± 1.5 | 0.162 | 521 | 1.6 ± 1.3 | 399 | 1.8 ± 1.5 | 0.082 |
| Education level | 569 | 343 | 0.049 | 519 | 398 | 0.043 | ||||
| Middle school and below | 312 (54.8) | 214 (62.4) | 279 (53.8) | 246 (61.8) | ||||||
| High school | 176 (30.9) | 95 (27.7) | 164 (31.6) | 108 (27.1) | ||||||
| College and above | 81 (14.2) | 34 (9.9) | 76(14.6) | 44 (11.1) | ||||||
| Lung function | ||||||||||
| FEV1/FVC (%, mean ± SD) | 570 | 54.5 ± 9.6 | 343 | 39.9 ± 10.0 | < 0.001 | 520 | 54.7 ± 9.7 | 388 | 41.3 ± 10.7 | < 0.001 |
| Post BD FEV1 (%, mean ± SD) | 571 | 66.0 ± 11.6 | 343 | 38.9 ± 7.6 | < 0.001 | 521 | 66.2 ± 11.5 | 387 | 41.6 ± 11.4 | < 0.001 |
| FEV1 (L, mean ± SD) | 571 | 1.8 ± 0.5 | 343 | 1.0 ± 0.3 | < 0.001 | 521 | 1.8 ± 0.5 | 389 | 1.1 ± 0.5 | < 0.001 |
| FVC (L, mean ± SD) | 571 | 3.3 ± 0.8 | 343 | 2.7 ± 0.7 | < 0.001 | 521 | 3.3 ± 0.8 | 389 | 2.7 ± 0.8 | < 0.001 |
| TLC (L, mean ± SD) | 393 | 5.6 ± 0.8 | 193 | 5.6 ± 0.8 | 0.824 | 364 | 5.6 ± 0.8 | 226 | 5.6 ± 0.8 | 0.550 |
| 6MWD (meters, mean ± SD) | 474 | 375.9 ± 112.9 | 250 | 344.2 ± 120.1 | < 0.001 | 431 | 376.4 ± 110.9 | 294 | 349.8 ± 124.4 | < 0.001 |
| Subjects requiring oxygen during/after 6MWD | 466 | 2 (0.4) | 242 | 0 (0.0) | 0.549 | 422 | 2 (0.5) | 286 | 0 (0.00) | 0.518 |
| Symptom scores | ||||||||||
| CAT score (mean ± SD) | 541 | 13.9 ± 6.9 | 335 | 18.3 ± 8.1 | < 0.001 | 493 | 13.5 ± 6.7 | 389 | 18.1 ± 8.3 | < 0.001 |
| CAT score | 151 (27.9) | 51 (15.2) | < 0.001 | 146 (29.6) | 65 (16.7) | < 0.001 | ||||
| CAT score ≥ 10 | 390 (72.1) | 284 (84.8) | < 0.001 | 347 (70.4) | 324 (83.3) | < 0.001 | ||||
| mMRC score (mean ± SD) | 571 | 1.4 ± 0.9 | 343 | 2.0 ± 1.0 | < 0.001 | 521 | 1.3 ± 0.8 | 399 | 1.9 ± 1.1 | < 0.001 |
| mMRC score < 2 | 367 (64.3) | 122 (35.6) | < 0.001 | 343 (65.8) | 153 (38.4) | < 0.001 | ||||
| mMRC score ≥ 2 | 204 (35.7) | 221 (64.4) | 178 (34.2) | 246 (61.7) | ||||||
| SGRQ-C | ||||||||||
| Symptom | 571 | 40.4 ±19.1 | 343 | 50.9 ± 21.8 | < 0.001 | 521 | 39.2 ± 18.7 | 398 | 50.7 ± 21.6 | < 0.001 |
| Activity | 570 | 40.3 ±23.8 | 343 | 56.7 ± 29.2 | < 0.001 | 520 | 38.9 ± 23.4 | 398 | 55.8 ± 29.3 | < 0.001 |
| Impact | 571 | 21.0 ±19.8 | 343 | 34.3 ± 26.2 | < 0.001 | 521 | 19.5 ± 18.7 | 398 | 34.3 ± 26.1 | < 0.001 |
| Total | 570 | 30.4 ± 16.5 | 342 | 42.9 ± 21.6 | < 0.001 | 520 | 29.1 ± 15.6 | 397 | 42.6 ± 21.6 | < 0.001 |
GOLD, global initiative for chronic obstructive lung disease; SD, standard deviation; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity; 6MWD, six minutes walk distance test; CAT, COPD assessment test; mMRC, modified Medical Research Council dyspnea scale; SGRQ-C, St. George’s respiratory questionnaire.
Correlation of medication history and disease severity (global initiative for chronic obstructive lung disease criteria, 2011)
The most commonly used medications before and after initial enrollment in the study, regardless of GOLD group, were long acting muscarinic antagonists (LAMA; Group A to D, n = 68 [48.2%], n = 204 [40.6%], n = 29 [41.4%], and n = 227 [34.2]; P < 0.001), followed by inhaled corticosteroid (ICS) plus long acting beta-2 agonist (LABA) (Group A to D, n = 28 [19.9%], n = 122 [24.3%], n = 21 [30.0%], and n = 189 [28.5%]; P < 0.001) (Table 3).
Table 3. Medications of the COPD subjects according to the GOLD group A to D at inclusion.
| Medication* | No. (%) of patients | P value | |||
|---|---|---|---|---|---|
| GOLD A | GOLD B | GOLD C | GOLD D | ||
| LAMA | 68 (48.2) | 204 (40.6) | 29 (41.4) | 227 (34.2) | < 0.001 |
| LABA | 18 (12.8) | 47 (9.4) | 6 (8.6) | 33 (4.8) | < 0.001 |
| ICA + LABA | 28 (19.9) | 122 (24.3) | 21 (30.0) | 189 (28.5) | < 0.001 |
| ICA + LABA + LAMA | 18 (12.8) | 74 (14.7) | 18 (25.7) | 153 (23.0) | < 0.001 |
| PDE4 Inhibitor | 2 (1.4) | 5 (1.0) | 2 (2.9) | 35 (5.3) | < 0.001 |
| Xanthine oxidase inhibitor (Theophylline) | 22 (15.6) | 107 (21.3) | 10 (14.3) | 141 (21.3) | < 0.001 |
| Oral beta 2 agonist | 3 (2.1) | 17 (3.4) | 2 (2.9) | 38 (5.7) | < 0.001 |
*Multiple responses for medication. COPD, chronic obstructive pulmonary disease; GOLD, global initiative for chronic obstructive lung disease; LAMA, long acting muscarinic antagonist; LABA, long acting beta-2 agonist; ICA, inhaled corticosteroid; PDE4 inhibitor, phosphodiesterase 4 inhibitor.
Comorbidities
The most common comorbidities among the study patients were counted hypertension (n = 323, 37.7%), depression (207, 23.6%), diabetes mellitus (139, 14.8%), GERD (83, 9.5%), coronary heart disease (43, 4.9%), heart failure (32, 3.7%), and peripheral vascular disease (15, 1.7%) (Table 4).
Table 4. Comorbidities of the total COPD subjects at inclusion.
| Co-morbidity | No. | (%) |
|---|---|---|
| Hypertension | 323 | (37.7) |
| Diabetes mellitus | 139 | (14.8) |
| Coronary heart disease | 43 | (4.9) |
| Heart failure | 32 | (3.7) |
| Gastro Esophageal Reflux Disease | 83 | (9.5) |
| Osteoporosis | 28 | (3.2) |
| Peripheral vascular disease | 15 | (1.7) |
| Depression | 207 | (23.6) |
COPD, chronic obstructive pulmonary disease.
Correlation between disease severity (global initiative for chronic obstructive lung disease 2011 criteria) and symptom scores
Table 5 shows the Spearman’s rank correlation coefficients (R2) for GOLD group and symptom scores (mMRC, CAT, SGRQ-C total, and 6MWD). GOLD groups (A to D) were significantly correlated with mMRC (R2 0.42, P < 0.001), CAT (R2 0.49, P < 0.001), and SGRQ-C (R2 0.45, P < 0.001). The relationship between GOLD group 6MWD was significant but weak and showed a negative correlation (R2 −0.16, P < 0.001).
Table 5. Spearman’s rank correlations coefficients between GOLD group and symptom score parameters including mMRC, CAT, SGRQ-C total and 6 minutes walk distance test.
| GOLD Group | mMRC (n = 920) | CAT (n = 882) | SGRQ-C (n = 917) | 6MWD (n = 728) | ||||
|---|---|---|---|---|---|---|---|---|
| Spearman's rho | P value | Spearman's rho | P value | Spearman's rho | P value | Spearman's rho | P value | |
| A to D | 0.42 | < 0.001 | 0.49 | < 0.001 | 0.45 | < 0.001 | -0.17 | < 0.001 |
GOLD, global initiative for chronic obstructive lung disease; mMRC, modified Medical Research Council dyspnea scale; CAT, COPD assessment test; SGRQ-C, St. George’s respiratory questionnaire; 6MWD, six minutes walk distance test.
DISCUSSION
The most prominent characteristic of the KOCOSS cohort is that it is composed of a relatively greater number of patients with early COPD (mean post bronchodilator FEV1 55.8%) compared to other large cohorts, such as the genetic epidemiology of COPD cohort (COPDGene, mean FEV1 48.3%) and the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort (ECLIPSE, mean FEV1 48.9%) (14,15). In line with this, the mean number of exacerbations during the year prior to enrolment was lower in our cohort than in the ECLIPSE cohort (0.6 vs. 0.9) (14,15). The patients included in another large cohort study, the subpopulations and intermediate outcome in COPD study (SPIROMICS) had higher FEV1 values at enrollment, which may be a reflection of the different inclusion criteria for SPIROMICS (FEV1/FVC < 0.7 and > 20 pack years smoking history regardless FEV1) (13). It is well known that FEV1, alone cannot capture the complexity of COPD to an adequate degree for prediction of exacerbation or disease progression, but it can be useful for interpreting discrepancies between the studies. The differences may also be partly due to differences among populations in terms of willingness to participate in clinical research. Another large cohort study in Korea, named Korean Obstructive Lung Disease (KOLD) showed relatively higher FEV1 (52.0% ± 19.4%) compared to other studies but still lower than our study even though they included asthma, and other obstructive lung disease for better understanding of COPD heterogeneity (16,17). As mentioned above, we wanted to know the natural courses of COPD, excluding other causes of obstructive lung disease, in priority for early COPD. In this study, only the preliminary data analysis was done. But, future work, we could verify differences with other large cohort studies.
The relatively poor correlation between GOLD group and the 6MWD test results and the better correlation between the other symptom scores (mMRC and CAT) and the SGRQ-C and GOLD group in our result are supported by findings for the ECLIPSE cohort (15,18). The multinational ECLIPSE cohort is a well-characterized group of patients with clinically stable moderate to severe COPD (15,18). The determinants of 6MWD are multifactorial and include both physical (pulmonary and non-pulmonary) and psychological factors (18). Favorable exercise capacity among patients with early-stage COPD in our cohort might also have affected the 6MWD result. We expect to examine these hypotheses in greater detail during the ten-year longitudinal follow-up of the KOCOSS cohort.
The Canadian cohort obstructive lung disease (CanCOLD) study is another large cohort study that aims to develop and validate a practical risk index for COPD that predicts the clinical course in a primary care setting (19). Even in large-scale, well designed studies such as CanCOLD, evaluations focusing on each GOLD group, has the potential for ambiguous results because of using different classification compared with GOLD, a limited (3-year) follow-up period, and the primary care setting. As mentioned, the KOCOSS cohort is to be followed for 10 years, and we anticipate that this will help to further elucidate the clinical course of COPD, particularly after diagnosis at an early stage, which should provide a better chance of reducing damage to the lungs.
The inclusion of a larger number of patients with early COPD in KOCOSS as compared to the other large cohort studies (ECLIPSE, SPIROMICS, CanCOLD, and COPDGene) may provide more information about the characteristics of patients with early COPD within the classic definition of COPD. Our preliminary data will allow follow-up for improved quality of life and symptom scores after treatment started according to the GOLD guideline, especially for patients with mild to moderate COPD (data not shown). Furthermore, considering heterogeneity of COPD, results derived from a large, 45-center, well-characterized population of individuals with COPD diagnosed by pulmonary specialists and covering all areas in Korea should allow generalization of our results. The next study of the KOCOSS cohort is focused upon longitudinal changes and natural course of the COPD, including COPD in an at-risk population in an outpatient setting. We expect that it will be an important step toward identifying important variables for tailoring individualized treatment plans before and after the diagnosis of COPD.
Regardless of COPD stage, co-morbid conditions are common and are of importance in COPD since they are frequent and they affect prognosis and the costs (20). However, quantifying their burden is difficult (21). One study that evaluated data from COPD patients in the Korean Health Insurance Review and Assessment Service (KHIRA) database reported that hypertension, diabetes, ischemic heart disease, and osteoporosis as the leading comorbidities in COPD patients (22). This might be because an operational definition was used to extracting data on COPD patients from the KHIRA database. It is also possible that the somewhat higher rate of comorbidities in our study is associated with cases of hospital-diagnosed COPD and worse prognosis in the end. However, there are no tools for evaluation of these associations, although one study has shown that data from two large multicenter cohort studies (COPDGene and SPIROMICS), it is possible to formulate a simplified score to quantify comorbidity, which provided a more thorough understanding of the risk in terms of patient-centered outcomes (23). These results were derived from self-reported comorbidities without objective evaluation of these comorbidities, and severity was not reported. This situation is similar to the situation in our present study. We will need to perform additional studies to better understand the associations between the comorbidities and prognosis of COPD at each level of COPD severity. More thorough evaluation and management will also be needed for these purposes.
Of note, depressive disorders were common (23.6%) in our study compared to the KHIRA data (9.0%) (22). In line with our study, KOLD data also showed higher prevalence of depression among COPD patients (191/803, 23.8%) and depression was well correlated with CAT score (16). Even that higher prevalence of depression, the treatment or research on mental health of COPD has been insufficient. Recently, cognitive behavioral therapy was approved in a large randomized controlled trial for COPD subjects (23). Pharmacological intervention such as benzodiazepine and antidepressant was also commonly used to treat anxiety in COPD (24). We suggest that, depression should be also evaluated at the time of diagnosis of COPD and should be controlled actively with individual aspects. That will be expected to be associated with quality of life improvement and better outcome for COPD subjects by checking-up during its early stage and consistent management (23,24). Further large randomized study will be needed.
Finally, with regard to treatment options, in our cohort, LAMA and ICS plus LABA were the most commonly used drugs. However, data extracted from the KHIRA records showed that methylxanthines and systemic corticosteroids were more commonly prescribed than LAMA or ICS plus LABA (25). There are several possible reasons for this. Physicians at primary and secondary facilities may prefer prescribing oral medications rather than inhalers, and the KHIRA database may include more patients who visited such centers. Furthermore, there may be patients who prefer oral medications. Treatment trends have also changed over time, and KHIRA data were compiled from 2006 to 2008, while our data collection took place from 2011 to 2014. A major challenge of KOCOSS will be maintaining long-term follow-up with the study participants.
Loss to follow-up is problematic in most cohort studies and often leads to bias. Although guidelines suggest acceptable follow-up rates, the authors are unaware of studies that have tested the validity of these recommendations. We plan to make every effort to reduce follow-up loss by close contact with each patient by scheduling periodic visits and phone calls from the clinical research coordinator and by forming a good rapport with hospitalized patients.
In conclusion, the KOCOSS is the first large cohort study that has the primary objective of describing the features of patients with COPD in each of the GOLD subtypes throughout all of Korea. Moving forward, through inclusion in future work of individuals who are at risk for COPD and healthy controls, we anticipate that our cohort study will be able to define predictive or surrogate markers of disease progression, including annual decline of FEV1, and analyze medical care cost, medical resource use, burden and to identify novel strategies for individualized treatment options.
ACKNOWLEDGMENT
This study was made through the help and support from everyone including KOCOSS members who are working at the pulmonary and critical care medicine at each tertiary and university-affiliated hospitals: Sun-Young Kyung, Chung Wung Bark in Gachon University; Min Kwang Byun in Gangnam Severance Hospital, Yonsei University; Yee Hyung Kim in Kyung Hee University Hospital; Seong Yong Lim in Kangbuk Samsung Hospital, Sungkyunkwan University; Yoonki Hong and Woo Jin Kim in Kangwon National University Hospital; Jaehee Lee in Kyungbuk University Hospital; Yi Yeong Jeong and Ho Choel Kim in Gyeongsang University Hospital; Kyung Hoon Min and Jae Jung Shim in Korea University Hospital; Hye Sook Choi in Dongguk University Hospital; Soo-Jung Um in Dong-A University Hospital; Jeong Ha Mok and Ki Uk Kim in Pusan National University Hospital; Yong Hyun Kim in Bucheon St. Mary's Hospital, The Catholic University of Korea College of Medicine; An-Soo Jang, Sung-Woo Park, and Do-Jin Kim in Soonchunhyang University Bucheon Hospital; Ji-Hyun Lee, Pochon CHA University Hospital; Jae Hyung Lee in Eulji General Hospital; Chang-Hoon Lee in Korea University Anam Hospital; Hye Yun Park in Samsung Medical Center, Sungkyunkwan University Hospital; Sei Won Kim and Yeon-Mok Oh in Ulsan University Asan Hospital, Jick Hwan Hah in Catholic University of Korea St. Paul’s hospital, Sung Kyoung Kim in St. Vincent's Hospital, The Catholic University of Korea College of Medicine; Soo-Taek Uh in Soonchunhyang University Hospital; Joo Hun Park in Ajou University School of Medicine Hospital; Ji Ye Jung in Yonsei University Hospital; Kyeong-Cheol Shin in Yeungnam University Hospital; Seung Won Ra in Ulsan University Hospital; Sang Ha Kim, Won Yeon Lee in Yonsei University Wonju Hospital; Jin Woo Kim in Uijeongbu St. Mary's Hospital, Ju Sang Kim in Incheon St. Mary's Hospital, The Catholic University of Korea College of Medicine; Jae Hwa Cho in Inha University Hospital; Sung-Soon Lee in Inje University Hospital; Heung Bum Lee, Seoung-Ju Park in Chonbuk National University; Jaechun Lee in Jeju National University Hospital; Joo Ock Na in Soonchunhyang University Cheonan Hospital; Tae Rim Shin, Yun Su Sim, So Young Park, Cheol Hong Kim, Myung-Goo Lee, Chang Youl Lee, and Ji Yong Moon in Hallym University Hospital.
We sincerely thank to KOCCOS members who provided the support for inclusion and close follow up of the enrolled COPD subjects. The product of this research paper would not be possible without all of them.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept, design, data collection: Lee JY, Chon GR, Rhee CK, Kim DK, Yoon HK, Lee JH, Yoo KH, Lee SH, Lee SY, Kim TE, Kim TH, Park YB, Hwang YI, Kim YS, Jung KS. Writing, revision: Lee JY, Chon GR, Rhee CK, Kim DK, Yoon HK, Lee JH, Yoo KH. Statistic analysis: Kim TE. Review & revision: Yoo KH, Lee SY, Kim TH, Hwang YI, Park YB, Kim YS. Approval of the final version of the manuscript: all authors.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. [accessed on 2 April 2015]. Available at http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Apr2.pdf.
- 2.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 3.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 4.Yoo KH, Kim YS, Sheen SS, Park JH, Hwang YI, Kim SH, Yoon HI, Lim SC, Park JY, Park SJ, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology. 2011;16:659–665. doi: 10.1111/j.1440-1843.2011.01951.x. [DOI] [PubMed] [Google Scholar]
- 5.Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, Ostinelli J. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37:264–272. doi: 10.1183/09031936.00051110. [DOI] [PubMed] [Google Scholar]
- 6.Decramer M, Miravitlles M, Price D, Román-Rodríguez M, Llor C, Welte T, Buhl R, Dusser D, Samara K, Siafakas N. New horizons in early stage COPD--improving knowledge, detection and treatment. Respir Med. 2011;105:1576–1587. doi: 10.1016/j.rmed.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Jo SA, Park MH, Jo I, Ryu SH, Han C. Usefulness of Beck Depression Inventory (BDI) in the Korean elderly population. Int J Geriatr Psychiatry. 2007;22:218–223. doi: 10.1002/gps.1664. [DOI] [PubMed] [Google Scholar]
- 8.Standardization of Spirometry 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 9.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 10.Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George Respiratory Questionnaire. Chest. 2007;132:456–463. doi: 10.1378/chest.06-0702. [DOI] [PubMed] [Google Scholar]
- 11.Jones PW, Forde Y. St George's Respiratory Questionnaire for COPD Patients (SGRQ-C) Manual. London: St George's University of London; 2008. [Google Scholar]
- 12.Tsiligianni IG, van der Molen T, Moraitaki D, Lopez I, Kocks JW, Karagiannis K, Siafakas N, Tzanakis N. Assessing health status in COPD. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ) BMC Pulm Med. 2012;12:20. doi: 10.1186/1471-2466-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, Crapo JD, Silverman EK. COPDGene Investigators. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YS, Park S, Oh YM, Lee SD, Park SW, Kim YS, In KH, Jung BH, Lee KH, Ra SW, et al. Chronic obstructive pulmonary disease assessment test can predict depression: a prospective multi-center study. J Korean Med Sci. 2013;28:1048–1054. doi: 10.3346/jkms.2013.28.7.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park TS, Lee JS, Seo JB, Hong Y, Yoo JW, Kang BJ, Lee SW, Oh YM, Lee SD, KOLD Study Group Study design and outcomes of Korean Obstructive Lung Disease (KOLD) cohort study. Tuberc Respir Dis (Seoul) 2014;76:169–174. doi: 10.4046/trd.2014.76.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spruit MA, Watkins ML, Edwards LD, Vestbo J, Calverley PM, Pinto-Plata V, Celli BR, Tal-Singer R, Wouters EF, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010;104:849–857. doi: 10.1016/j.rmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Bourbeau J, Tan WC, Benedetti A, Aaron SD, Chapman KR, Coxson HO, Cowie R, Fitzgerald M, Goldstein R, Hernandez P, et al. Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD. 2014;11:125–132. doi: 10.3109/15412555.2012.665520. [DOI] [PubMed] [Google Scholar]
- 20.Frei A, Muggensturm P, Putcha N, Siebeling L, Zoller M, Boyd CM, ter Riet G, Puhan MA. Five comorbidities reflected the health status in patients with chronic obstructive pulmonary disease: the newly developed COMCOLD index. J Clin Epidemiol. 2014;67:904–911. doi: 10.1016/j.jclinepi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 21.O’Neal WK, Anderson W, Basta PV, Carretta EE, Doerschuk CM, Barr RG, Bleecker ER, Christenson SA, Curtis JL, Han MK, et al. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J Transl Med. 2014;12:9. doi: 10.1186/1479-5876-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C, Yoo KH, Rhee CK, Yoon HK, Kim YS, Lee SW, Oh YM, Lee SD, Lee JH, Kim KJ, et al. Health care use and economic burden of patients with diagnosed chronic obstructive pulmonary disease in Korea. Int J Tuberc Lung Dis. 2014;18:737–743. doi: 10.5588/ijtld.13.0634. [DOI] [PubMed] [Google Scholar]
- 23.Kunik ME, Veazey C, Cully JA, Souchek J, Graham DP, Hopko D, Carter R, Sharafkhaneh A, Goepfert EJ, Wray N, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med. 2008;38:385–396. doi: 10.1017/S0033291707001687. [DOI] [PubMed] [Google Scholar]
- 24.Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6:46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putcha N, Puhan MA, Drummond MB, Han MK, Regan EA, Hanania NA, Martinez CH, Foreman M, Bhatt SP, Make B, et al. A simplified score to quantify comorbidity in COPD. PLoS One. 2014;9:e114438. doi: 10.1371/journal.pone.0114438. [DOI] [PMC free article] [PubMed] [Google Scholar]