Abstract
BACKGROUND
Bariatric surgery is increasingly considered for the treatment of adolescents with severe obesity, but few prospective adolescent-specific studies examining the efficacy and safety of weight-loss surgery are available to support clinical decision making.
METHODS
We prospectively enrolled 242 adolescents undergoing weight-loss surgery at five U.S. centers. Patients undergoing Roux-en-Y gastric bypass (161 participants) or sleeve gastrectomy (67) were included in the analysis. Changes in body weight, coexisting conditions, cardiometabolic risk factors, and weight-related quality of life and postoperative complications were evaluated through 3 years after the procedure.
RESULTS
The mean (±SD) baseline age of the participants was 17±1.6 years, and the mean body-mass index (the weight in kilograms divided by the square of the height in meters) was 53; 75% of the participants were female, and 72% were white. At 3 years after the procedure, the mean weight had decreased by 27% (95% confidence interval [CI], 25 to 29) in the total cohort, by 28% (95% CI, 25 to 30) among participants who underwent gastric bypass, and by 26% (95% CI, 22 to 30) among those who underwent sleeve gastrectomy. By 3 years after the procedure, remission of type 2 diabetes occurred in 95% (95% CI, 85 to 100) of participants who had had the condition at baseline, remission of abnormal kidney function occurred in 86% (95% CI, 72 to 100), remission of prediabetes in 76% (95% CI, 56 to 97), remission of elevated blood pressure in 74% (95% CI, 64 to 84), and remission of dyslipidemia in 66% (95% CI, 57 to 74). Weight-related quality of life also improved significantly. However, at 3 years after the bariatric procedure, hypoferritinemia was found in 57% (95% CI, 50 to 65) of the participants, and 13% (95% CI, 9 to 18) of the participants had undergone one or more additional intraabdominal procedures.
CONCLUSIONS
In this multicenter, prospective study of bariatric surgery in adolescents, we found significant improvements in weight, cardiometabolic health, and weight-related quality of life at 3 years after the procedure. Risks associated with surgery included specific micro-nutrient deficiencies and the need for additional abdominal procedures. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; Teen-LABS ClinicalTrials.gov number, NCT00474318.)
Severe obesity affects 4.4 million children and adolescents in the United States,1 and few effective treatments are available.2 Particular concern has centered on health problems among severely obese adolescents and possible treatment with bariatric surgery.3 Indeed, adolescent bariatric surgical case volumes doubled from approximately 800 cases in 20034 to 1600 procedures in 2009.5 Few prospective studies have examined changes in body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) and outcomes of the currently used surgical procedures, and little is known about clinical events after bariatric surgery in adolescents.6,7
To address important questions regarding the efficacy and safety of bariatric surgery in adolescents, the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study collects longitudinal, prospective clinical and laboratory data on teenagers undergoing bariatric surgery at five centers in the United States. The current report presents data on weight loss, coexisting conditions, weight-related quality of life, micronutrient levels, and additional abdominal procedures during the 3 years after the bariatric procedure.
METHODS
STUDY DESIGN AND PARTICIPANTS
In this prospective, multicenter, observational study, we enrolled consecutive adolescents (≤19 years of age) who were undergoing any bariatric surgical procedure from March 2007 through February 2012 at participating centers. The steering committee, which is made up of the principal investigator at each site, in collaboration with the data coordinating center and the project scientist from the National Institute of Diabetes and Digestive and Kidney Diseases, designed and implemented the study. The protocol and statistical analysis plan are available with the full text of this article at NEJM.org. The first author wrote the first draft of the manuscript, and all the authors participated in critical reviews and editing. The protocol and data and safety monitoring plans were approved by the institutional review board at each institution and by a data and safety monitoring board for the study as a whole. All participants provided written informed consent.
DATA COLLECTION
The standardized methods we used for data collection have been described previously.8,9 Follow-up data were collected at the 6-month, 1-year, 2-year, and 3-year postoperative research visits. Most visits occurred at the clinical centers or at the participant's home; in 22 instances, assessments were conducted through self-report (Fig. S1 in the Supplementary Appendix, available at NEJM.org). Research coordinators, nurse practitioners, and physicians were trained in protocol procedures for the collection of data. For home visits, a field examiner who was trained in protocol procedures conducted a visit at the participant's residence. Data collected during study visits were maintained in a central database by the data coordinating center. Missed visits did not necessarily indicate withdrawal from the study, because participants commonly returned for later visits even after missing a visit. Weight-related quality of life was assessed with the use of the total score from the Impact of Weight on Quality of Life–Kids10 instrument (scores range from 0 to 100, with higher scores indicating a better quality of life).
DEFINITIONS
Standard conventions were followed for the assessment of prevalence, remission, and incidence of coexisting conditions, and micronutrients were measured as described in the Supplementary Appendix. Information on additional surgical and endoscopic procedures that were performed between 31 days after bariatric surgery and the 3-year study visit was collected with the use of a scripted interview at each visit.
STATISTICAL ANALYSIS
A complete description of the statistical methods is provided in the Supplementary Appendix. Weight loss, quality of life, coexisting conditions, and micronutrient outcomes were evaluated with the use of linear mixed and generalized mixed models, with separate models according to surgical procedure. Each model included only the study visit as the independent predictor term. Estimates of least-squares means and 95% confidence intervals were generated. These models addressed missing data values by means of the maximum-likelihood method, under the data-missing-at-random assumption. Sensitivity analyses were performed to evaluate this assumption. Using linear interpolation, we generated body-weight values from the values at previous and subsequent visits. For weights that were missing at the 3-year follow-up visit, we applied a conservative 10% increase from the latest visit. On the basis of these analyses, the missing-at-random assumption was considered to be reasonable (see the Supplementary Appendix).
Event rates for subsequent abdominal procedures were calculated as the number of events that occurred from 31 days after the procedure through the 3-year study visit (visit window, 2.5 to 3.5 years), divided by person-years of observation. Poisson regression with the logarithm of person-years as an offset parameter was used to calculate unadjusted rates and 95% confidence intervals (expressed per 300 person-years).
RESULTS
PARTICIPANTS
We enrolled 242 participants in the study; 161 (67%) underwent Roux-en-Y gastric bypass, 67 (28%) underwent sleeve gastrectomy, and 14 (6%) underwent adjustable gastric banding. Because of the small size of the gastric-band cohort, these results were not included in the main analyses (see the Supplementary Appendix). At baseline, 29% of the participants were in the early teenage age groups (13 to 15 years of age), 41% were in the middle age groups (16 to 17 years of age), and 30% were in the late age groups (18 to 19 years of age) (Table 1). The mean BMI was 53 (range, 34 to 88); 98% of the participants had a BMI higher than 40. The majority of participants were from families with household incomes of less than $50,000 per year. The majority of caregivers had completed high school, and 40% had obtained some college education.
Table 1.
Demographic, Anthropometric, and Procedural Characteristics of the Participants.*
| Characteristic | All Participants (N = 228) | Gastric Bypass (N = 161) | Sleeve Gastrectomy (N = 67) |
|---|---|---|---|
| Age — yr | 17±1.6 | 17±1.5 | 17±1.7 |
| Age group — no. (%) | |||
| 13–15 yr | 66 (29) | 42 (26) | 24 (36) |
| 16–17 yr | 94 (41) | 71 (44) | 23 (34) |
| 18–19 yr | 68 (30) | 48 (30) | 20 (30) |
| Sex — no. (%) | |||
| Female | 171 (75) | 126 (78) | 45 (67) |
| Male | 57 (25) | 35 (22) | 22 (33) |
| Race or ethnic background — no. (%)† | |||
| White | 164 (72) | 119 (74) | 45 (67) |
| Black | 50 (22) | 35 (22) | 15 (22) |
| Asian | 1 (<1) | 1 (1) | 0 |
| American Indian or Alaskan native | 1 (<1) | 0 | 1 (1) |
| More than one race or ethnic background | 12 (5) | 6 (4) | 6 (9) |
| Hispanic ethnic background — no. (%)† | 16 (7) | 15 (9) | 1 (1) |
| Household income — no./total no. (%) | |||
| <$25,000 | 83/218 (38) | 51/156 (33) | 32/62 (52) |
| $25,000–$49,999 | 44/218 (20) | 31/156 (20) | 13/62 (21) |
| $50,000–$74,999 | 38/218 (17) | 28/156 (18) | 10/62 (16) |
| ≥$75,000 | 53/218 (24) | 46/156 (29) | 7/62 (11) |
| Caregiver level of education — no./total no. (%) | |||
| Less than high school | 23/221 (10) | 11/157 (7) | 12/64 (19) |
| High-school graduate | 68/221 (31) | 47/157 (30) | 21/64 (33) |
| Some college | 89/221 (40) | 67/157 (43) | 22/64 (34) |
| College graduate | 41/221 (19) | 32/157 (20) | 9/64 (14) |
| Mean weight (95% CI) | |||
| Baseline — kg | 149 (145 to 153) | 151 (146 to 156) | 144 (136 to 152) |
| 3 Yr‡ — kg | 108 (103 to 113) | 109 (104 to 115) | 105 (96 to 113) |
| Absolute change‡ — kg | –41 (–45 to –37) | –42 (–47 to –38) | –38 (–44 to –31) |
| Percent change‡ | –27 (–29 to –25) | –28 (–30 to –25) | –26 (–30 to –22) |
| Model-estimated percent change | –28 (–26 to –30) | –29 (–26 to –31) | –27 (–23 to –31) |
| Mean height (95% CI) | |||
| Baseline — cm | 167.9 (166.7 to 169.1) | 167.5 (166.2 to 168.9) | 168.7 (166.1 to 171.2) |
| 3 Yr§ — cm | 168.3 (166.9 to 169.7) | 168.3 (166.7 to 169.8) | 168.5 (165.1 to 171.9) |
| Absolute change§ — cm | 0.51 (0.23 to 0.80) | 0.54 (0.20 to 0.88) | 0.44 (–0.12 to 1.00) |
| Percent change§ | 0.31 (0.14 to 0.48) | 0.32 (0.12 to 0.53) | 0.25 (–0.07 to 0.57) |
| Model-estimated percent change | 0.29 (0.09 to 0.49) | 0.31 (0.12 to 0.51) | 0.27 (–0.07 to 0.60) |
| Mean BMI (95% CI) | |||
| Baseline | 53 (51 to 54) | 54 (52 to 55) | 50 (48 to 52) |
| 3 Yr¶ | 38 (37 to 40) | 39 (37 to 41) | 37 (34 to 39) |
| Absolute change¶ | –15 (–16 to –13) | –15 (–17 to –14) | –13 (–15 to –11) |
| Percent change¶ | –28 (–30 to –25) | –28 (–31 to –25) | –26 (–30 to –22) |
| Model-estimated percent change | –29 (–27 to –31) | –29 (–27 to –32) | –27 (–23 to –31) |
Plus-minus values are means ±SD. CI denotes confidence interval.
Race and ethnic background were self-reported.
Data are for 183 participants in total (131 participants who underwent gastric bypass and 52 participants who underwent sleeve gastrectomy), with values from 7 patients (6 participants who underwent gastric bypass and 1 participant who underwent sleeve gastrectomy) excluded because of pregnancy.
Data are for 179 participants in total (131 participants who underwent gastric bypass and 48 participants who underwent sleeve gastrectomy).
Data are for 173 participants in total (125 participants who underwent gastric bypass and 48 participants who underwent sleeve gastrectomy), with values from 7 patients (6 participants who underwent gastric bypass and 1 participant who underwent sleeve gastrectomy) excluded because of pregnancy.
Through the 3-year study end point, 99% of the cohort (225 of 228 participants) participated actively and completed 88% of all postoperative visits (805 of 912 visits) (Fig. S1 in the Supplementary Appendix). The rates of visit completion according to follow-up time point were 89% at 6 months (203 of 228 participants), 90% at 1 year (205 of 228), 89% at 2 years (203 of 228), and 85% at 3 years (194 of 228). A total of 89% of the postoperative visits (715 of 805) were completed at the clinical center and 8% (68 of 805) were conducted at the participant's home; 3% (22 of 805) were self-reported assessments conducted through telephone contact or electronic correspondence.
ANTHROPOMETRIC CHANGES
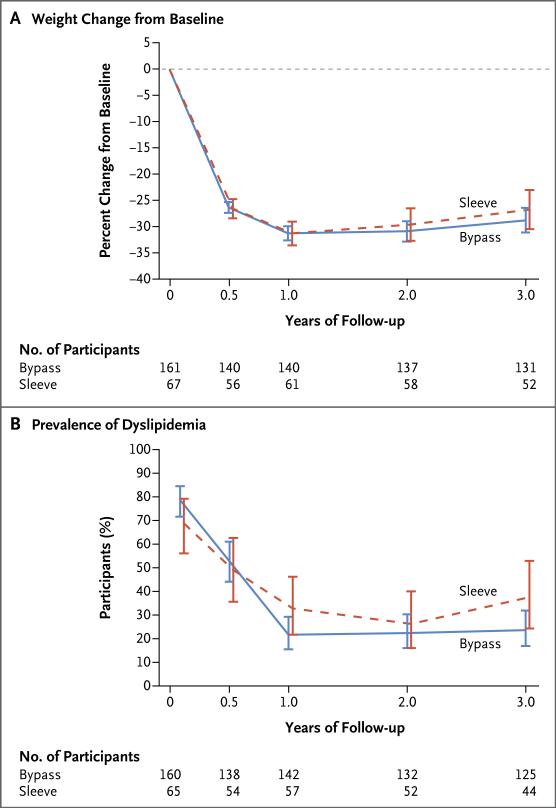
At 3 years, the mean weight reduction among all participants was 41 kg, with little increase in height (Table 1). The mean percent weight loss was 27% (95% confidence interval [CI], 25 to 29) in the overall cohort: 28% (95% CI, 25 to 30; P<0.001) in the group that underwent gastric bypass and 26% (95% CI, 22 to 30; P<0.001) in the group that underwent sleeve gastrectomy (Fig. 1A). The magnitude of BMI reduction was nearly identical to that of weight reduction (Table 1, and Fig. S3 in the Supplementary Appendix). Sensitivity analyses indicated that missing values had a negligible effect on the results for weight loss (Fig. S2 in the Supplementary Appendix). At baseline, all the participants were obese (BMI >30), whereas at 3 years, 26% of the participants were no longer obese (Fig. S3A and S3B in the Supplementary Appendix). The proportion of participants who had a 10% or greater reduction in BMI was 89% among participants who underwent gastric bypass and 85% among participants who underwent sleeve gastrectomy. At 3 years, 2% of the participants who underwent gastric bypass and 4% of those who underwent sleeve gastrectomy exceeded their baseline weight.
Figure 1. Weight Changes and Prevalence of Dyslipidemia during the 3-Year Period after Bariatric Surgery.
Panel A shows the modeled least-squares mean percent changes in weight from baseline at each study visit during the 3 years after Roux-en-Y gastric bypass surgery (bypass) or vertical sleeve gastrectomy (sleeve). Panel B shows the modeled least-squares mean prevalences of dyslipidemia at each study visit during the 3 years after Roux-en-Y gastric bypass surgery (bypass) or vertical sleeve gastrectomy (sleeve). I bars in both panels represent 95% confidence intervals.
COEXISTING CONDITIONS AND WEIGHT-RELATED QUALITY OF LIFE
Elevated blood pressure was present in 96 participants at baseline, and at 3 years after bariatric surgery, blood pressure had normalized in 74% of the participants (95% CI, 64 to 84) who had had the condition at baseline and for whom data were available (Table 2, and Fig. S3C in the Supplementary Appendix). Four incident cases of elevated blood pressure were observed among the 98 participants with available data who had not had the condition at baseline (4%; 95% CI, 0 to 8]). Dyslipidemia was present in 171 participants at baseline; at 3 years, lipid levels had normalized (without lipid-lowering therapy) in 66% of the participants (95% CI, 57 to 74) who had had the condition at baseline and for whom data were available (P<0.001) (Table 2 and Fig. 1B, and Fig. S4 in the Supplementary Appendix). Among the 39 participants with available data who had not had dyslipidemia at baseline, 3 incident cases had developed by 3 years (8%; 95% CI, 0 to 16). At 3 years, resolution of abnormal kidney function (defined by low glomerular filtration rate or proteinuria) was observed in 86% (95% CI, 72 to 100) of the participants with available data who had had this condition at baseline, and 12 incident cases of abnormal kidney function had developed among the 124 participants with data who had not had the condition at baseline (10%; 95% CI, 5 to 15).
Table 2.
Prevalence and Remission of Coexisting Conditions.*
| Condition | Baseline |
3 Years |
||||
|---|---|---|---|---|---|---|
| Observed Prevalence of Condition | Modeled Prevalence of Condition† | Observed Prevalence of Remission‡ | Modeled Prevalence of Remission† | |||
| no. of patients/total no. | % (95%, CI) | % (95%, CI) | no. of patients/total no. | % (95% CI) | % (95% CI) | |
| Type 2 diabetes | ||||||
| Total | 29/225 | 13 (9–17) | 13 (8–20) | 19/20 | 95 (85–100) | 90 (65–98) |
| Gastric bypass | 23/159 | 14 (9–20) | 14 (9–23) | 17/18 | 94 (84–100) | 94 (66–99) |
| Sleeve gastrectomy | 6/66 | 9 (2–16) | 9 (3–22) | 2/2 | 100 (100–100) | 68 (7–99) |
| Prediabetes | ||||||
| Total | 19/194 | 10 (6–14) | 10 (6–17) | 13/17 | 76 (56–97) | 77 (48–92) |
| Gastric bypass | 17/135 | 13 (7–18) | 13 (7–22) | 11/15 | 74 (51–96) | 94 (66–99) |
| Sleeve gastrectomy | 2/59 | 3 (0–8) | — § | 2/2 | 100 (100–100) | — § |
| Dyslipidemia | ||||||
| Total | 171/225 | 76 (70–82) | 76 (69–81) | 84/128 | 66 (57–74) | 66 (56–74) |
| Gastric bypass | 126/160 | 79 (72–85) | 79 (71–85) | 66/95 | 69 (60–79) | 70 (59–79) |
| Sleeve gastrectomy | 45/65 | 69 (58–80) | 69 (55–80) | 18/33 | 55 (38–72) | 55 (36–73) |
| Elevated blood pressure | ||||||
| Total | 96/224 | 43 (36–49) | 43 (35–51) | 56/76 | 74 (64–84) | 73 (60–83) |
| Gastric bypass | 73/159 | 46 (38–54) | 46 (37–55) | 47/60 | 78 (68–89) | 78 (64–88) |
| Sleeve gastrectomy | 23/65 | 35 (24–47) | 35 (23–50) | 9/16 | 56 (32–81) | 53 (27–78) |
| Abnormal kidney function | ||||||
| Total | 36/212 | 17 (12–22) | 17 (12–23) | 19/22 | 86 (72–100) | 86 (63–90) |
| Gastric bypass | 29/153 | 19 (13–25) | 19 (13–27) | 16/19 | 84 (68–100) | 84 (59–95) |
| Sleeve gastrectomy | 7/59 | 12 (4–20) | 12 (6–24) | 3/3 | 100 (100–100) | — § |
The criteria used to define each coexisting condition are provided in the Supplementary Appendix.
Generalized mixed models were used to calculate the modeled results.
The proportion of participants in remission was calculated as the number of participants (among those for whom sufficient data were available at 3 years to determine whether the co-existing condition was present) who did not have the condition at 3 years after the procedure, divided by the number of participants (among those for whom sufficient data were available at 3 years to define whether the coexisting condition was present) who had had the condition at baseline.
The model failed because of the small sample size.
A total of 32 participants had diabetes at baseline; 3 of these participants had type 1 diabetes, and no participants with type 1 diabetes had resolution of the condition after the surgical procedure. At baseline, among the 29 participants (13% of all participants) who had type 2 diabetes, the median glycated hemoglobin level was 6.3%, the median fasting glucose level was 110 mg per deciliter (6.1 mmol per liter), and the median insulin level was 43 IU per milliliter. At 3 years, 19 of 20 participants (95%; 95% CI, 85 to 100) with data that could be evaluated were in remission (Table 2), with a median glycated hemoglobin of 5.3%, a median fasting glucose of 88 mg per deciliter (4.9 mmol per liter), and a median insulin level of 12 IU per milliliter. No incident cases of diabetes were observed. Prediabetes was found in 19 participants (10%; 95% CI, 6 to 14) at baseline; of the participants for whom data were available, 76% (95% CI, 56 to 97) no longer had prediabetes at 3 years. Incident prediabetes had developed in 1 participant by 3 years.
We found improvements in participant-reported weight-related quality of life from baseline to the 3-year follow-up. The mean quality-of-life total score was 63 (95% CI, 61 to 65) at baseline and had increased to 83 (95% CI, 81 to 86) by 3 years (P<0.001) (Table S2 in the Supplementary Appendix).
NUTRITIONAL MEASURES
Low ferritin levels were found in 5% (95% CI, 2 to 8) of the participants at baseline, but at 3 years, 57% (95% CI, 50 to 65) had abnormally low levels (P<0.001). Vitamin B12 levels declined by 35% (Table S3 in the Supplementary Appendix), and 8% of the participants had a deficiency at 3 years (Table 3). Deficiencies in vitamin A (levels <301 μg per liter) were found at baseline in 6% (95% CI, 2 to 9) of the participants who underwent gastric bypass; at 3 years, vitamin A deficiencies were found in 16% of participants who underwent this procedure (95% CI, 9 to 24; P = 0.008). Levels of 25-hydroxyvitamin D were insufficient (<20.1 ng per milliliter) in 37% of the participants (95% CI, 31 to 44) before the surgical procedure and did not increase significantly over time.
Table 3.
Nutritional and Related Abnormalities.*
| Abnormality | Baseline |
3 Years |
P Value† | ||||
|---|---|---|---|---|---|---|---|
| Observed Prevalence | Modeled Prevalence‡ | Observed Prevalence | Modeled Prevalence‡ | ||||
| no. of patients/total no. | % (95% CI) | % (95% CI) | no. of patients/total no. | % (95% CI) | % (95% CI) | ||
| Low albumin level | |||||||
| Total | 7/225 | 3 (1–5) | — § | 0/171 | 0 | — § | — |
| Gastric bypass | 7/160 | 4 (1–8) | — § | 0/127 | 0 | — § | — |
| Sleeve gastrectomy | 0/65 | 0 | — § | 0/44 | 0 | — § | — |
| Low folate level | |||||||
| Total | 6/173 | 3 (1–6) | 3 (1–7) | 10/132 | 8 (3–12) | 7 (4–14) | 0.13 |
| Gastric bypass | 4/126 | 3 (<1–6) | 3 (1–8) | 6/100 | 6 (1–11) | 6 (3–13) | 0.29 |
| Sleeve gastrectomy | 2/47 | 4 (0–10) | 4 (1–16) | 4/32 | 12 (1–24) | 10 (3–28) | 0.28 |
| Low vitamin B12 level | |||||||
| Total | 1/222 | <1 (0–1) | <1 (<1–3) | 13/160 | 8 (4–12) | 8 (4–14) | 0.005 |
| Gastric bypass | 1/159 | 1 (0–2) | 1 (<1–4) | 10/121 | 8 (3–13) | 8 (4–15) | 0.01 |
| Sleeve gastrectomy | 0/63 | 0 | — § | 3/39 | 8 (0–16) | — § | — |
| Low 25-hydroxyvitamin D level | |||||||
| Total | 83/223 | 37 (31–44) | 37 (30–45) | 74/172 | 43 (36–50) | 42 (34–50) | 0.37 |
| Gastric bypass | 71/159 | 45 (37–52) | 45 (36–54) | 61/128 | 48 (39–56) | 47 (38–57) | 0.64 |
| Sleeve gastrectomy | 12/64 | 19 (9–28) | 19 (10–32) | 13/44 | 30 (16–43) | 27 (14–44) | 0.36 |
| High parathyroid hormone level | |||||||
| Total | 18/223 | 8 (5–12) | 8 (5–13) | 16/172 | 9 (5–14) | 9 (5–15) | 0.77 |
| Gastric bypass | 17/159 | 11 (6–15) | 11 (6–18) | 15/128 | 12 (6–17) | 12 (7–19) | 0.85 |
| Sleeve gastrectomy | 1/64 | 2 (0–5) | — § | 1/44 | 2 (0–7) | — § | — |
| Low ferritin level | |||||||
| Total | 11/225 | 5 (2–8) | 5 (3–9) | 98/171 | 57 (50–65) | 57 (49–65) | <0.001 |
| Gastric bypass | 4/160 | 2 (<1–5) | 3 (1–7) | 83/127 | 65 (57–74) | 66 (56–74) | <0.001 |
| Sleeve gastrectomy | 7/65 | 11 (3–18) | 11 (5–22) | 15/44 | 34 (20–48) | 32 (19–50) | 0.01 |
| High transferrin level | |||||||
| Total | 7/225 | 3 (1–5) | 3 (1–7) | 27/171 | 16 (10–21) | 16 (11–24) | <0.001 |
| Gastric bypass | 5/160 | 3 (<1–6) | 3 (1–8) | 25/127 | 20 (13–27) | 20 (13–29) | <0.001 |
| Sleeve gastrectomy | 2/65 | 3 (0–7) | 3 (1–13) | 2/44 | 5 (0–11) | 5 (1–19) | 0.63 |
| Low vitamin A level | |||||||
| Total | 13/221 | 6 (3–9) | 6 (3–10) | 22/170 | 13 (8–18) | 13 (8–20) | 0.02 |
| Gastric bypass | 9/158 | 6 (2–9) | 6 (3–11) | 20/126 | 16 (9–24) | 15 (10–24) | 0.008 |
| Sleeve gastrectomy | 4/63 | 6 (<1–12) | 6 (2–17) | 2/44 | 5 (0–11) | 6 (2–20) | 0.93 |
| High vitamin B1 erythrocyte transketolase activity | |||||||
| Total | 2/217 | 1 (0–2) | — § | 1/172 | 1 (0–2) | — § | — |
| Gastric bypass | 2/154 | 1 (0–3) | — § | 1/126 | 1 (0–4) | — § | — |
| Sleeve gastrectomy | 0/63 | 0 | — § | 0/46 | 0 | — § | — |
The reference ranges used to determine abnormal values are provided in the Supplementary Appendix.
P values are for the comparison between the value at 3 years and the value at the baseline visit.
Generalized mixed models were used to calculate the modeled results.
The model failed because of the small sample size.
COMPLICATIONS AND DEATHS
Within 3 years, 47 intraabdominal procedures were performed in 30 participants (13% [95% CI, 9 to 18]) (Table 4). Three procedures (1 appendicostomy and 2 appendectomies) were unrelated to the previous bariatric procedure, whereas all others were considered to be related to the procedure. A total of 24% of the procedures were performed within the first year after the bariatric procedure, 55% within the second year, and 21% within third year. Upper endoscopic procedures (including stricture dilations) were performed in 29 participants (13%). One participant with known type 1 diabetes died 3.3 years after gastric bypass surgery, from complications of a hypoglycemic event.
Table 4.
Intraabdominal Operations and Other Related Procedures from 31 Days to 3 Years after the Bariatric Procedure.*
| Procedure | All Participants (N = 228) |
Gastric Bypass (N = 161) |
Sleeve Gastrectomy (N = 67) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Events | Rate (95% CI) | Patients | Events | Rate (95% CI) | Patients | Events | Rate (95% CI) | |
| no. (%) | no. | events per 300 person-years | no. (%) | no. | events per 300 person-years | no. (%) | no. | events per 300 person-years | |
| Intraabdominal operations | 30 (13) | 47 | 22.3 (16.8–29.7) | 23 (14) | 38 | 25.0 (18.2–34.4) | 7 (10) | 9 | 15.4 (8.0–29.5) |
| Ventral hernia repair | 1 (<1) | 1 | 0.5 (0.1–3.4) | 0 | 0 | 0 | 1 (1) | 1 | 1.7 (0.2–12.1) |
| Exploratory laparotomy | 3 (1) | 3 | 1.4 (0.5–4.4) | 3 (2) | 3 | 2.0 (0.6–6.1) | 0 | 0 | 0 |
| Wound drainage | 2 (1) | 2 | 1.0 (0.2–3.8) | 1 (1) | 1 | 0.7 (0.1–4.7) | 1 (1) | 1 | 1.7 (0.2–12.1) |
| Lysis of adhesions | 4 (2) | 6 | 2.9 (1.3–6.3) | 4 (2) | 6 | 3.9 (1.8–8.8) | 0 | 0 | 0 |
| Gastrostomy | 4 (2) | 5 | 2.4 (1.0–5.7) | 4 (2) | 5 | 3.3 (1.4–7.9) | 0 | 0 | 0 |
| Sleeve gastrectomy converted to gastric bypass | 1 (1) | 1 | 1.7 (0.2–12.1) | NA | NA | NA | 1 (1) | 1 | 1.7 (0.2–12.1) |
| Appendicostomy for antegrade enemas | 1 (<1) | 1 | 0.5 (0.1–3.4) | 0 | 0 | 0 | 1 (1) | 1 | 1.7 (0.2–12.1) |
| Repair of internal hernia | 3 (1) | 5 | 2.4 (1.0–5.7) | 3 (2) | 5 | 3.3 (1.4–7.9) | 0 | 0 | 0 |
| Bowel resection or diverting stoma | 2 (1) | 2 | 1.0 (0.2–3.8) | 2 (1) | 2 | 1.3 (0.3–5.3) | 0 | 0 | 0 |
| Luminal stent placement for leak | 1 (<1) | 1 | 0.5 (0.1–3.4) | 0 | 0 | 0 | 1 (1) | 1 | 1.7 (0.2–12.1) |
| Cholecystectomy | 18 (8) | 18 | 8.6 (5.4–13.6) | 15 (9) | 15 | 9.9 (6.0–16.4) | 3 (4) | 3 | 5.1 (1.7–15.9) |
| Appendectomy | 2 (1) | 2 | 1.0 (0.2–3.8) | 1 (1) | 1 | 0.7 (0.1–4.7) | 1 (1) | 1 | 1.7 (0.2–12.1) |
| Endoscopic procedures | 29 (13) | 48 | 22.8 (17.2–30.3) | 24 (15) | 41 | 27.0 (19.9–36.6) | 5 (7) | 7 | 12.0 (5.7–25.1) |
| Upper gastrointestinal tract endoscopy | 21 (9) | 37 | 17.6 (12.7–24.3) | 16 (10) | 31 | 20.4 (14.3–29.0) | 5 (7) | 6 | 10.2 (4.6–22.8) |
| Stricture dilation | 9 (4) | 11 | 5.2 (2.9–9.4) | 8 (5) | 10 | 6.6 (3.5–12.2) | 1 (1) | 1 | 1.7 (0.2–12.1) |
Data are for events that occurred through the 3-year follow-up visit (visit window, 2.5 to 3.5 years).
NA denotes not applicable.
DISCUSSION
A majority of participants in our study had marked improvements with respect to weight, obesity-related coexisting conditions, and quality of life. The emergence of specific micronutrient deficiencies and the need for subsequent abdominal procedures indicate that there are also risks associated with bariatric surgery in this age group.
The outcomes of bariatric surgery among adolescents beyond 1 year after the procedure have rarely been described.11,12 The mean decrease in BMI after 1 year among Teen-LABS participants who underwent gastric bypass was similar to that reported in seven previous studies involving 256 adolescents (decreases of 16.5 and 17.2, respectively).11-17 In a study involving 53 younger adolescents (mean age, 14 years), a decrease of 20 in mean BMI and an increase of 5 cm in mean height was observed 3 years after sleeve gastrectomy.18 This decrease in BMI was greater than the decrease of 13.1 that was observed among participants who underwent sleeve gastrectomy in our study, probably in part because of the linear growth in the younger cohort, which was not seen in our cohort. In aggregate, these results suggest that adolescents can lose a clinically significant amount of weight after bariatric surgery, with the majority of patients maintaining meaningful weight loss for at least 3 years.
Among adults who undergo gastric bypass, remission of type 2 diabetes occurs in 50 to 70%, and remission of elevated blood pressure occurs in 40%.19,20 We found remission of diabetes in 95% of participants who had type 2 diabetes at baseline in our study, a finding consistent with our previous findings in adolescents.21 This result, coupled with the findings of normalization of elevated blood pressure in nearly 80% of our participants, leads us to hypothesize that adolescents may have a greater potential than adults for reversal of the cardiometabolic consequences of obesity. We further speculate that these improvements with regard to weight, glycemic control, blood pressure, and dyslipidemia in adolescents may mitigate the progression of adverse anatomical and physiological cardiovascular changes — changes that may be less reversible after the accumulation of more pound-years later in life.22 Additional research may clarify the way in which age, obesity duration, and the timing of surgery could modify the response to surgical treatment.
Gastric bypass and gastric resection may affect the absorption of numerous micronutrients that are necessary for normal metabolism and for good bone, hematologic, and nervous system health; therefore, multivitamin and mineral supplementation is needed, as was prescribed in this cohort. The greatest changes that we observed were in measures related to iron and vitamin B12. Iron-deficiency anemia and vitamin B12 deficiency after gastric bypass are well described.23,24 Vitamin B12 deficiency was not unexpected after sleeve gastrectomy, and it presumably relates to a reduction in intrinsic factor production after the procedure. These results, as well as the decreased vitamin A levels after gastric-bypass surgery, highlight the importance of long-term follow-up to evaluate nutritional measures, as well as the importance of ensuring that appropriate supplementation is provided to minimize the development of clinically significant nutritional deficiencies in adolescents after bariatric surgery.
There are currently few available data regarding the need for subsequent abdominal operations after bariatric surgery in adolescents. Our data, which include information on additional conditions and procedures, may elucidate the types and frequencies of the adverse effects of bariatric surgery in this age group. Previously, we reported that 8% of the participants in our study had major complications within 30 days after the bariatric procedure,9 and here we report that 13% of the participants underwent additional intraabdominal procedures within the subsequent 3 years. The risks of complications may differ according to the type of bariatric procedure; however, the current study was not designed to identify such differences. Further study of larger cohorts and other populations may provide insight regarding this question.
The strengths of the current study include the prospective enrollment of consecutive patients at geographically distinct sites, the standardized methods used to collect data, and strong cohort maintenance over time. The limitations of our study include the small size of certain important subpopulations, such as patients with diabetes. In addition, the observational nature of the study introduces heterogeneity into the data set, including unmeasured covariates and imbalances in race, sex, and socioeconomic status. Without a non–surgically treated control group, it is difficult to place the postoperative changes in weight and health status completely into perspective, since behavioral treatment can result in modest improvements in weight and cardiometabolic health. However, it has been reported that severely obese adolescents who undergo nonsurgical treatment do not have major reductions in weight, and the reductions that they do have are not maintained over 2 years of follow-up.25 Finally, despite a relatively low 3-year missed-visit rate of 15%, missing data — particularly data from laboratory testing, which were missing in 24% of participants — is a limitation. However, statistical techniques that address missing data were applied, and sensitivity analyses indicated that our assumptions with regard to patterns of missing data were reasonable.
In conclusion, we documented the durability of clinically meaningful weight loss and improvements in key health conditions and weight-related quality of life among adolescents who underwent gastric bypass surgery or sleeve gastrectomy. These benefits must be viewed in the context of the risks of micronutrient deficiencies and the possibility that future abdominal procedures will be needed in some patients. Studies that assess the longer-term durability of weight loss, potential improvements with respect to coexisting conditions, and the risk of adverse events, as well as the cost, may provide a better understanding of the role of bariatric surgery in the treatment of severe obesity in adolescents.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (U01DK072493 and UM1DK072493 to Dr. Inge, UM1DK095710 to Dr. Buncher, UL1TR000077-04 to Cincinnati Children's Hospital Medical Center, UL1RR025755 to Nationwide Children's Hospital, M01-RR00188 to Texas Children's Hospital/Baylor College of Medicine, UL1RR024153 and UL1TR000005 to the University of Pittsburgh, and UL1TR000165 to the University of Alabama, Birmingham).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatr. 2014;168:561–6. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 2.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689–712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 3.Daniels SR, Kelly AS. Pediatric severe obesity: time to establish serious treatments for a serious disease. Child Obes. 2014;10:283–4. doi: 10.1089/chi.2014.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161:217–21. doi: 10.1001/archpedi.161.3.217. [DOI] [PubMed] [Google Scholar]
- 5.Zwintscher NP, Azarow KS, Horton JD, Newton CR, Martin MJ. The increasing incidence of adolescent bariatric surgery. J Pediatr Surg. 2013;48:2401–7. doi: 10.1016/j.jpedsurg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Treadwell JR, Sun F, Schoelles K. Systematic review and meta-analysis of bariatric surgery for pediatric obesity. Ann Surg. 2008;248:763–76. doi: 10.1097/SLA.0b013e31818702f4. [DOI] [PubMed] [Google Scholar]
- 7.Paulus GF, de Vaan LE, Verdam FJ, Bouvy ND, Ambergen TA, van Heurn LW. Bariatric surgery in morbidly obese adolescents: a systematic review and meta-analysis. Obes Surg. 2015;25:860–78. doi: 10.1007/s11695-015-1581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inge TH, Zeller M, Harmon C, et al. Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS): methodological features of the first prospective multi-center study of adolescent bariatric surgery. J Pediatr Surg. 2007;42:1969–71. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inge TH, Zeller MH, Jenkins TM, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168:47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolotkin RL, Zeller M, Modi AC, et al. Assessing weight-related quality of life in adolescents. Obesity (Silver Spring) 2006;14:448–57. doi: 10.1038/oby.2006.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugerman HJ, Sugerman EL, DeMaria EJ, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102–7. doi: 10.1016/S1091-255X(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 12.Olbers T, Gronowitz E, Werling M, et al. Two-year outcome of laparoscopic Rouxen-Y gastric bypass in adolescents with severe obesity: results from a Swedish Nationwide Study (AMOS). Int J Obes (Lond) 2012;36:1388–95. doi: 10.1038/ijo.2012.160. [DOI] [PubMed] [Google Scholar]
- 13.Strauss RS, Bradley LJ, Brolin RE. Gastric bypass surgery in adolescents with morbid obesity. J Pediatr. 2001;138:499–504. doi: 10.1067/mpd.2001.113043. [DOI] [PubMed] [Google Scholar]
- 14.Loux TJ, Haricharan RN, Clements RH, et al. Health-related quality of life before and after bariatric surgery in adolescents. J Pediatr Surg. 2008;43:1275–9. doi: 10.1016/j.jpedsurg.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 15.Inge TH, Jenkins TM, Zeller M, et al. Baseline BMI is a strong predictor of nadir BMI after adolescent gastric bypass. J Pediatr. 2010;156:103–8. e1. doi: 10.1016/j.jpeds.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Cruz-Muñoz N, Messiah SE, Cabrera JC, et al. Four-year weight outcomes of laparoscopic gastric bypass surgery and adjustable gastric banding among multiethnic adolescents. Surg Obes Relat Dis. 2010;6:542–7. doi: 10.1016/j.soard.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Teeple EA, Teich S, Schuster DP, Michalsky MP. Early metabolic improvement following bariatric surgery in morbidly obese adolescents. Pediatr Blood Cancer. 2012;58:112–6. doi: 10.1002/pbc.23370. [DOI] [PubMed] [Google Scholar]
- 18.Alqahtani AR, Elahmedi MO, Al Qahtani A. Co-morbidity resolution in morbidly obese children and adolescents undergoing sleeve gastrectomy. Surg Obes Relat Dis. 2014;10:842–50. doi: 10.1016/j.soard.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes — 3-year outcomes. N Engl J Med. 2014;370:2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–25. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inge TH, Miyano G, Bean J, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123:214–22. doi: 10.1542/peds.2008-0522. [DOI] [PubMed] [Google Scholar]
- 22.Abdullah A, Wolfe R, Stoelwinder JU, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40:985–96. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]
- 23.Kotkiewicz A, Donaldson K, Dye C, et al. Anemia and the need for intravenous iron infusion after Roux-en-Y gastric bypass. Clin Med Insights Blood Disord. 2015;8:9–17. doi: 10.4137/CMBD.S21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karefylakis C, Näslund I, Edholm D, Sundbom M, Karlsson FA, Rask E. Prevalence of anemia and related deficiencies 10 years after gastric bypass — a retrospective study. Obes Surg. 2015;25:1019–23. doi: 10.1007/s11695-014-1500-y. [DOI] [PubMed] [Google Scholar]
- 25.van der Baan-Slootweg O, Benninga MA, Beelen A, et al. Inpatient treatment of children and adolescents with severe obesity in the Netherlands: a randomized clinical trial. JAMA Pediatr. 2014;168:807–14. doi: 10.1001/jamapediatrics.2014.521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.