Abstract
Purpose
Despite several advantages of proton therapy over megavoltage x-ray therapy its lack of proximal-tissue sparing is a concern. The method presented here adds proximal-tissue sparing to protons and light ions by turning their uniform incident beams into arrays of parallel, small or thin (0.3 mm) pencil or planar minibeams, which are known to spare tissues. As these minibeams penetrate the tissues they gradually broaden and merge with each other to produce a solid beam.
Methods and Materials
Broadening of 0.3-mm-diameter, 109-MeV proton pencil minibeams was measured using a stack of radiochromic films with plastic spacers. Monte Carlo simulations were used to evaluate the broadening in water of minibeams of protons and several light ions and the dose from neutron generated by collimator.
Results
A central parameter was the tissue depth where the beam full-width-at-half-maximum (FWHM) reached 0.7 mm, beyond which tissue sparing decrease. This depth was measured to be 22 mm for 109-MeV protons in a film-stack. It was also found by simulations a) for protons to be 23.5 mm for 109-MeV pencil minibeams and 26 mm for 116-MeV planar minibeams, and b) for light ions, all with 10-cm range in water, to increase with particle size; specifically it was 51 mm for Li-7 ions. The ~2.7% photon equivalent neutron skin dose from the collimator was reduced 7-fold by introducing a gap in between the collimator and the skin.
Conclusions
Proton minibeams can be implemented at existing particle therapy centers. Because they spare the shallow tissues they could augment the efficacy of proton therapy and light particle therapy particularly in treating tumors that benefit from sparing of proximal tissues such as pediatric brain tumors. They should also allow hypofractionated treatment of all tumors by allowing the use of higher incident doses with less concern about proximal tissue damage.
INTRODUCTION
Brain tumors are the second most common type of pediatric cancer in their incidence in the United States. As survival among children treated for brain tumors improves, more attention is being focused on the late effects of treatment (1–4), which include neurocognitive and neurological deficits. Similar effects, although at a lower intensity, have also been detected in adults undergoing radiotherapy of brain tumors (5). Radiation therapy (RT) is an integral component of the treatment of brain tumors. Theoretical advantages of proton therapy include better dose confinement to the target due to its Bragg peak feature, and its slightly higher relative biological effectiveness (RBE) at the target compared to photons (6). Despite these advantages, except for treating pediatric brain tumors (7), studies on the advantages of proton therapy over x-rays to reduce cognitive effects are not conclusive.
The method presented here spares proximal tissues by turning the uniform beams of protons and light ions into arrays of parallel pencil-shaped or planar small beams, with ~0.3 mm incident size, called minibeams. These beams gradually broaden because of multiple Coulomb scattering and merge deeper in the body to produce a solid beam. The method’s impact can be large for RT of the brain because the cerebral cortex is known to be a major organ contributing to the neurological and cognitive side effects of radiation. Specifically, the radiation damage to the brain cortex’s white matter in children has been blamed for decline in the intelligence quotient (1) and for delayed visual-motor performance (3). Also, the earliest change in the canine brain irradiated with with He and Ne ions were the decreased metabolic activity in the cortex, mostly in its white matter (8). Finally, the radiation-induced pediatric cognitive effects have also been attributed, to a certain extent, to the disturbance of the cortex’s angiogenesis, a process producing neural progenitor cells; these cells later differentiate to produce new oligodendrocytes (4).
The general tissue-sparing effect of minibeams and their smaller counterpart microbeams [<0.3 mm FWHM] has been established with deuterons (9), synchrotron x-rays (10–18), carbon ions (18), and microchannel protons (19). First, it was found that the threshold dose for damage in the mouse cerebellum from deuteron pencil beams of 0.025, 0.075, 0.25, and 1.0 mm diameter are 4,000, 500, 360, and 140 Gy, respectively (9). Second, the rat cerebellum tolerated arrays of parallel, 37-μm planar synchrotron x-rays spaced 75 μm on-center at 250 Gy in-beam dose (10). The study triggered a line of research called microbeam radiation therapy in the U.S. (11,14,15,17) and in France (12,13,16,18). Among others, the studies showed that planar synchrotron x-ray beams as thick as 0.68 mm still retain much of their tissue-sparing effect (14). Third, arrays of 0.3-mm planar carbon minibeams, aimed at a rabbit brain from orthogonal directions, interlaced and ablated a 6.5 mm target at 40 Gy target physical absorbed dose, which would be ~120 photon Gy equivalent (GyE), without damaging the surrounding brain (19). Finally, exposing a 3-dimensional human skin model to 2-Gy, 20 MeV arrays of 10- and 50-μm microchannel protons spaced 500 μm showed smaller damage than that from homogeneous protons by 5.3-fold and 3.0-fold, respectively (20).
Microbeams’ tissue-sparing effect is based on the “dose-volume effect” (21) and the “prompt microscopic biological repair effect” (12,13,15). The former is the basis for Grid Therapy (22) and stereotactic radiosurgery, while the latter is specific for microbeams and minibeams, and is related to the fast repair of capillary blood vessels via the regeneration of angiogenic cells surviving between the microbeams. The effect was documented in the chicken chorio-allantoic membrane (12), and in the mouse brain; the latter, using 25-μm beams at 250-Gy, showed no leakage of intravenously injected FITC-dextran any time after 12 hours (13). This effect makes the present method categorically different from Grid Therapy that uses beams of up to 2 cm and is solely based on the dose-volume effect.
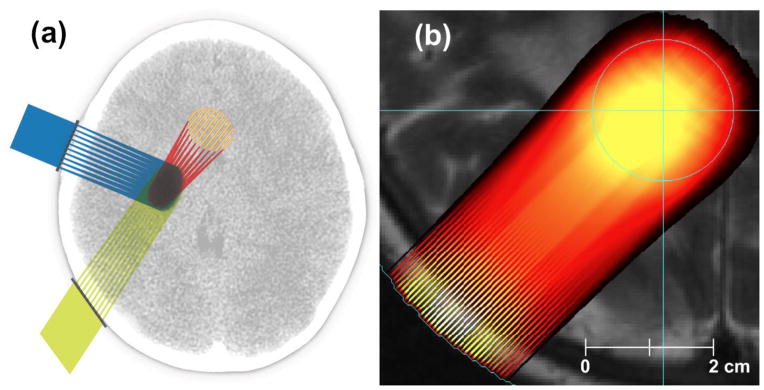
Fig. 1 shows geometries in which proton minibeams merge either before or at the proximal edge of the target.
Fig. 1.
a) Schematic view of treating a brain tumor from three ports, b) proton dose produced by our prototype treatment planning system (TPS) on an MRI image. The method produces physical dose distributions using interpolated Monte-Carlo beam data in conjunction with 3D proton scanning, i.e., active pencil-beam scanning with multiple energy layers in a realistic patient target. The target is the light blue circle.
METHODS AND MATERIALS
a. Measurements
The studies used pristine proton beams from a synchrotron. First, 100 and 109 MeV protons corresponding to range of ~8–10 cm in depth were produced with a 0.3-mm pinhole collimator made of 1-cm thick tungsten-copper alloy. Next, a stack of radiochromic films interspersed with 2-mm plastic sheets was positioned downstream of the collimator and irradiated to 10 Gy peak dose to measure the minibeam’s broadening. In a second experiment, a 5-cm-thick, tungsten multi-slit collimator of 0.3 mm beam-width and 1-mm on-center beam spacing was used to produce an array of planar proton minibeams for exposing a chromographic film.
b. Monte Carlo simulations
Simulations were performed using MCNPX (version 2.7d, Los Alamos National Laboratory) to predict beam broadening for 0.3-mm minibeams of protons and H-2, H-3, He-3, He-4, Li-6, and Li-7 individually in water. Energies were determined for beams such that all had an equal range of 10 cm; these were 116, 157, 188, 410, 462, 873, and 931 MeV, respectively. Ten million particle histories were tracked for each ion species.
RESULTS
a. Broadening of proton pencil minibeam at 109 MeV
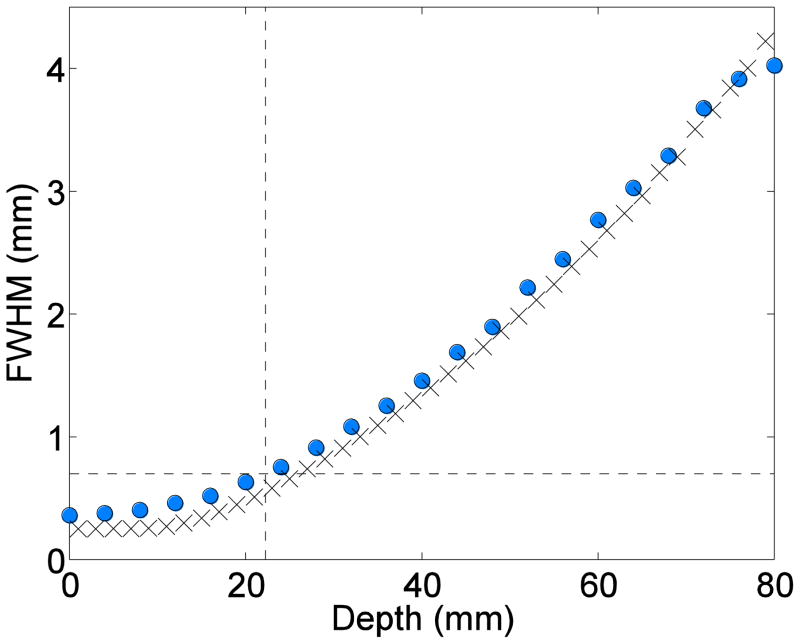
Fig. 2 shows the analyses of the beam FWHM as a function of depth for the measurements in film (circles) and Monte Carlo simulations in water (x marks). The target depths at which the minibeams’ FWHM reached 0.7 mm were 22 and 23.5 mm, respectively.
Fig. 2.
Proton pencil minibeams’ broadening for 109 MeV beam energy (circles: film measurements; x marks: the simulations in water).
b. Array profile of proton planar minibeams
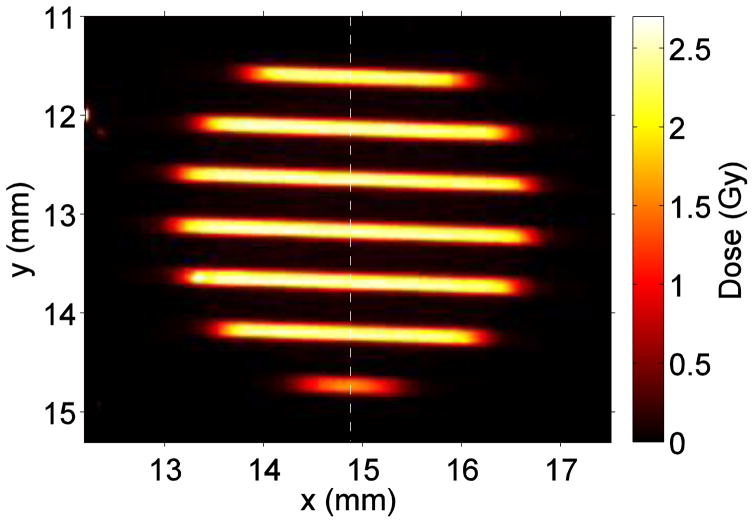
The results are shown in Fig. 3.
Fig. 3.
An array of 100-MeV planar proton minibeams of 0.3 mm thickness and 1.0 mm spacing on-center.
c. Simulation results for the broadening of proton and light-ion minibeams
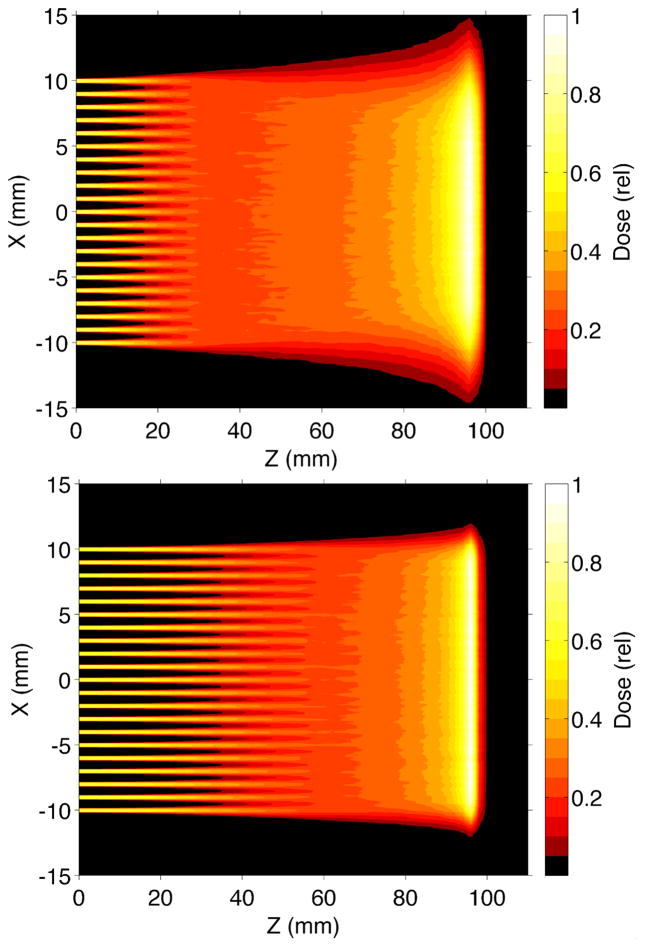
The results, shown in Fig. 4, indicate that the depths at which the 0.7-mm FWHM was reached were 27 mm for proton pencil beams and 25, 33, 37, 39, 41, 47, and 49 mm for planar minibeams of proton, H-2, H-3, He-3, He-4, Li-6, and Li-7, respectively; this means that proton planar beams broaden slightly faster than proton pencil beams. Fig. 5 shows the simulations of such protons and Li-7 minibeams merging in 10-beam arrays.
Fig. 4.
MCNPX simulations of broadening in water of planar minibeams of protons and several light ions, and that for pencil minibeams of protons, all at energies producing 100 mm range in water.
Fig. 5.
MCNPX simulations of arrays of planar minibeams of 116-MeV protons (top) and 931-MeV Li-7 ions (bottom) of Fig. 4.
d. Choice of the beam spacing
The minibeams’ ideal on-center spacing to allow merging of 0.3-mm incident minibeams when they are 0.7 mm in size is 0.7 mm. However, for a deeper proximal side of the target, either larger beam spacing (at the cost of reduced collimator transmission) or a heavier ion species can be used.
e. Neutron dose
MCNPX also calculated the skin dose from neutrons produced in the collimator. For planar proton minibeams and for collimator positioned on the skin, the neutron’s physical dose was 0.3% of the in-beam proton physical dose. Assuming an RBE of 10 for neutrons and 1.1 for protons, the biological neutron dose will be 2.7%. This dose was reduced by ~7 fold when a 5-cm gap was introduced between the collimator and the skin. The frame used for his purpose can push on the skin instead of the collimator for patient immobilization.
f. Dosimetry
Designing the optimal configuration of individual ports for covering a tumor and maximally sparing normal tissues takes into account the ion species and the minibeams’ spacing. Table 1 presents the MCNPX results for three different target geometries using 0.3 mm planar beams. The ion species was chosen on the basis of the depth of the target’s proximal edge, although the larger RBE of heavier ions could be also a consideration for treating radioresistant tumors. Table 2 shows the minibeam merging depth in water for planar minibeams of 10-cm range; for pencil minibeams the depths are 1–2 mm longer.
Table 1.
Dosimetric considerations
| Target’s proximal depth (cm) | Target’s distal depth (cm) | Recommended ion | Beam energy (MeV) | Beam’s FWHM at target’s proximal edge (mm) | Recommended beam spacing (mm) | Collimator’s transmission |
|---|---|---|---|---|---|---|
|
| ||||||
| 4 | 8 | H | 102 | 1.6 | 0.7 | 43% |
|
| ||||||
| 4 | 12 | H | 129 | 1.2 | 0.7 | 43% |
| He-4 | 512 | 0.6 | 0.6 | 50% | ||
| Li-7 | 1,032 | 0.5 | 0.5 | 60% | ||
|
| ||||||
| 8 | 16 | Li-7 | 1,214 | 1.1 | 0.7 | 43% |
Table 2.
Depth of merging in water for planar beams of 10-cm range.
| Beam spacing on-center (mm) | Incident beam thickness (mm) | Merging depth for H-1, He-4, and Li-7, respectively (mm) |
|---|---|---|
| 0.5 | 0.3 | 21, 35, 41 |
| 0.7 | 0.3 | 25, 41, 49 |
| 1.2 | 0.3 | 37, 60, 70 |
| 1.2 | 0.5 | 37, 59, 69 |
DISCUSSION
The results confirm the physical feasibility of using the particle minibeam method to spare proximal tissues by demonstrating that the rates at which proton and light ion minibeams broaden with depth are adequate for such tissue sparing in a wide range of clinical applications. They also indicate that although the planar and pencil-shaped minibeams should have similar tissue-sparing effects the former’s ~3-fold larger production yield, i.e., 43% versus 14.4%, and ease of collimator custom fabrication may make planar beams preferable for clinical translation. Assuming proton dose delivery of 2 Gy/minute to one liter tumor, i.e., ~5 Gy/minute to a typical brain tumor, leading to 2.2 Gy/minute through our planar collimator, delivering each of four 12-Gy dose fractions takes ~6 minutes, which is manageable with head immobilization. Bragg-peak spreading is completely compatible with the method as long as all its steps cover the entire target with a solid beam.
Although the minibeams’ decline of tissue sparing with increasing beam size is gradual, nevertheless any treatment planning will require defining an upper size limit for the minibeams’ usage. Our choice of 0.7 mm is based on the results from ref. 9 and ref. 14; while the former shows that 140 Gy given with 1.0-mm deuteron beams is adequate to damage the mouse cerebellum, the latter indicates that arrays of 0.68 mm x-ray minibeams are tolerated by the rat spinal cord at 400 Gy and by the entire rat brain at 170 Gy.
An estimate of the tolerance advantage of minibeams vis-à-vis solid beams can be obtained by comparing the above work of ref. 14 with that of ref. 23, both using x-rays on the rat brain. While in the former the entire brain tolerated 0.68 mm planar minibeams spaced 1.36 mm at 170 Gy both behaviorally and histologically for the 7-month observation period, the latter’s local 22.5-Gy solid beam irradiation led to “histological evidence for the development of necrosis in the white matter after a latent period of > 26 weeks” (23). This puts the minibeam at a >7.5-fold advantage. This factor is not in contradiction with that reported in ref. 9 indicating a ~3-fold advantage in tissue tolerance between 0.25 mm deuterons (360 Gy damage limit) and 1.0 mm (140 Gy) because a) that work does not use a uniform definition of the threshold dose for different beam sizes, and b) it is less relevant than refs. 14 and 23 for defining dose tolerance limits because refs. 14 and 23 irradiate significant portions of the brain.
The method’s potential clinical applications include brain tumors, particularly those of children. In this regard the method’s tissue sparing, depth, ~25 mm for protons and much larger for light ions, is sufficiently large to cover the skull and much of the cortex. Therefore, the method can improve the already favorable performance of proton therapy in treating pediatric brain tumors by sparing the cortex. The method’s other applications could include head and neck tumors where the parotid glands are to be spared, tumors near the orbit, and pediatric spinal cord tumors. A second application category would be hypofractionation, particularly of lung and liver tumors where the chest- and the abdomen-wall could be exposed to much higher doses. The method is compatible with intensity modulated proton therapy (IMPT). Its implementation is simple. It can use both passively scattered beams and scanned pencil beams because the natural beam divergence in both are adequately small to have a significant yield through the minibeam collimator.
Dose fractionation of proton minibeams given from the same direction administered every other day should not be a problem as tissues recover quickly from low-LET radiation (24). In this regard, synchrotron microbeam results show that a) much of the prompt repair occurs within a day (15,17), and b) unpublished results show that rat brain tolerates 27 μm beams spaced 200 μm at 200 Gy given every 12 hours and at 400 and 600 Gy given daily. However, longer inter-fraction periods might be required for light ions.
The smaller broadening for heavier ions rates shown in Figs. 4 and 5 is caused by their inverse relationship to the particle momenta (25) which, under the equal-range condition, are larger for heavier ions. Therefore, ions heavier than protons offer a larger tissue-sparing depth and higher RBE, while their disadvantage is that they require a greater tissue depth before they merge at the target’s proximal edge.
Acknowledgments
The authors thanks Tatiana Wolfe and Istvan Dioszegi for their assistance with measurements and simulations, respectively, Jann Stavro for comments on the manuscript, Kelly Tharp for machining expertise and assistance to fabricate minibeam collimator prototypes, Katherine Gebhart for graphic arts assistance, Uwe Titt and Dragan Mirkovic for helpful conversations regarding MCNPX simulation, and the John E. and Dorothy J. Harris Endowed Professorship to SK. The studies were carried out with partial support from the Radiation Oncology Departments of both Stony Brook Medical Center and M.D. Anderson Cancer Center.
Footnotes
Conflicts of interest: Dilmanian, Eley, and Krishnan have a pending patent application on the technology presented here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004 Jul;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 2.Khong PL, Leung LH, Fung AS, Fong DY, Qiu D, Kwong DL, Ooi GC, McAlonan G, Cao G, Chan GC. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006 Feb 20;24(6):884–90. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 3.Dockstader C, Gaetz W, Bouffet E, Tabori U, Wang F, Bostan SR, Laughlin S, Mabbott DJ. Neural correlates of delayed visual-motor performance in children treated for brain tumours. Cortex. 2013 Sep;49(8):2140–50. doi: 10.1016/j.cortex.2012.09.004. [DOI] [PubMed] [Google Scholar]; Cortex. 2013 Sep;49(8):2140–50. doi: 10.1016/j.cortex.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Rodgers SP, Trevino M, Zawaski JA, Gaber MW, Leasure JL. Neurogenesis, exercise, and cognitive late effects of pediatric radiotherapy. Neural Plast. 2013;2013:698528. doi: 10.1155/2013/698528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene-Schloesser D1, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: A review. Front Oncol. 2012 Jul 19;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giantsoudi D, Grassberger C, Craft D, Niemierko A, Trofimov A, Paganetti H. Linear energy transfer-guided optimization in intensity modulated proton therapy: feasibility study and clinical potential. Int J Radiat Oncol Biol Phys. 2013 Sep 1;87(1):216–22. doi: 10.1016/j.ijrobp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant TE, Farr JB. Proton beam therapy: a fad or a new standard of care. Curr Opin Pediatr. 2014 Feb;26(1):3–8. doi: 10.1097/MOP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 8.Brennan KM, Roos MS, Budinger TF, Higgins RJ, Wong ST, Bristol KS. A study of radiation necrosis and edema in the canine brain using positron emission tomography and magnetic resonance imaging. Radiat Res. 1993 Apr;134(1):43–53. [PubMed] [Google Scholar]
- 9.Zeman W, Curtis HJ, Baker CP. Histopathologic effect of high-energy-particle microbeams on the visual cortex of the mouse brain. Radiat Res. 1961;15:496–514. [PubMed] [Google Scholar]
- 10.Slatkin DN, Spanne PO, Dilmanian FA, Gebbers J-O, Laissue JA. Subacute neuropathological effects on rats of micro-planar beams of x-rays from a synchrotron wiggler. Proc Nat’l Acad Sci USA. 1995;92:8783–7. doi: 10.1073/pnas.92.19.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dilmanian FA, Morris GM, Le Duc G, Huang X, Ren B, Bacarian T, Allen JC, Kalef-Ezra J, Orion I, Rosen EM, Sandhu T, Sathé P, Wu XY, Zhong Z, Shivaprasad HL. Response of avian embryonic brain to spatially segmented x-ray microbeams. Cell Molec Biol. 2001;47:485–94. [PubMed] [Google Scholar]
- 12.Blattmann H, Burkard W, Di Michiel M, Brauer E, Stepanek J, Bravin A, et al. Scientific Report 2001/Vol II, Life Sciences. Paul Scherrer Institute; 2002. Microbeam irradiation of the corrio-allantoic membrane CAM) of the chicken embryo. [Google Scholar]
- 13.Serduc R, Vérant P, Vial JC, Farion R, Rocas L, Rémy C, Fadlallah T, Brauer E, Bravin A, Laissue J, Blattmann H, van der Sanden B. In vivo two-photon microscopy study of short-term effects of microbeam irradiation on normal mouse brain microvasculature. Int J Radiat Oncol Biol Phys. 2006;64:1519–27. doi: 10.1016/j.ijrobp.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 14.Dilmanian FA, Zhong Z, Bacarian T, Benveniste H, Romanelli P, Wang R, Welwart J, Yuasa T, Rosen EM, Anschel DJ. Interlaced X-ray Microplanar Beams: A Radiosurgery Approach with Clinical Potential. Proc Nat’l Acad Sci USA. 2006;103(25):9709–14. doi: 10.1073/pnas.0603567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilmanian FA, Qu Y, Feinendegen LE, Peña LA, Bacarian T, Henn FA, Kalef-Ezra J, Liu S, Zhong Z, McDonald JW. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: Is a bystander effect involved? Exp Hematol. 2007;35(4 Suppl 1):69–77. doi: 10.1016/j.exphem.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Laissue JA, Blattmann H, Wagner HP, Grotzer MA, Slatkin DN. Prospects for microbeam radiation therapy of brain tumours in children to reduce neurological sequelae. Dev Med Child Neurol. 2007;49(8):577–81. doi: 10.1111/j.1469-8749.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 17.Dilmanian FA, Jenkins AL, Olschowka J, Zhong Z, Park JY, Desnoyers NR, Sobotka S, Fois GR, Messina CR, Morales M, Hurley SD, Trojanczyk LA, Ahmad S, Shahrabi N, Coyle PK, Meek AG, O’Banion MK. X-ray microbeam irradiation of the contusion-injured rat spinal cord temporarily improves hind-limb function. Radiat Res. 2013;179:76–88. doi: 10.1667/RR2921.1. [DOI] [PubMed] [Google Scholar]
- 18.Laissue JA, Bartzsch S, Blattmann H, Bräuer-Krisch E, Bravin A, Dalléry D, Djonov V, Hanson AL, Hopewell JW, Kaser-Hotz B, Keyriläinen J, Laissue PP, Miura M, Serduc R, Siegbahn AE, Slatkin DN. Response of the rat spinal cord to X-ray microbeams. Radiother Oncol. 2013;106(1):106–11. doi: 10.1016/j.radonc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Dilmanian FA, Rusek A, Fois GR, Olschowka J, Desnoyers NR, Park JY, Dioszegi I, Dane B, Wang R, Tomasi D, Lee H, Hurley SD, Coyle PK, Meek AG, O’Banion MK. Interleaved carbon minibeams: An experimental radiosurgery method with clinical potential. Int J Radiat Oncol Biol Phys. 2012;84(2):514–9. doi: 10.1016/j.ijrobp.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Zlobinskaya O, Girst S, Greubel C, Hable V, Siebenwirth C, Walsh DW, Multhoff G, Wilkens JJ, Schmid TE, Dollinger G. Reduced side effects by proton microchannel radiotherapy: study in a human skin model. Radiat Environ Biophys. 2013 Mar;52(1):123–33. doi: 10.1007/s00411-012-0450-9. [DOI] [PubMed] [Google Scholar]
- 21.Withers HR, Taylor JMG, Maciejewski B. Treatment volume and tissue tolerance. Int J Rad Oncol Biol Phys. 1998;14:751–759. doi: 10.1016/0360-3016(88)90098-3. [DOI] [PubMed] [Google Scholar]
- 22.Harris W. Recent clinical experience with the grid in the x-ray treatment of advanced cancer; preliminary report. Radiology. 1952 Mar;58(3):343–50. doi: 10.1148/58.3.343. [DOI] [PubMed] [Google Scholar]
- 23.Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Brit J Radiol. 1998;61:1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- 24.Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Ciammella P, Franco P, Mantovani C, Borasio P, Scagliotti GV, Ragona R. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010 Apr;68(1):72–7. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Schimmerling W, Rapkin M, Wong M, Howard J. The propagation of relativistic heavy ions in multielement beam lines. Med Phys. 1986 Mar-Apr;13(2):217–28. doi: 10.1118/1.595900. [DOI] [PubMed] [Google Scholar]