Abstract
LD78α and LD78β are 2 highly related nonallelic genes that code for different isoforms of the human CC chemokine macrophage inflammatory protein-1α (MIP-1α). Two molecular forms of natural LD78β (7.778 and 7.793 kDa) were identified from conditioned media of stimulated peripheral blood mononuclear cells. Although LD78α and LD78β only differ in 3 amino acids, both LD78β variants were 100-fold more potent chemoattractants for mouse lymphocytes than was LD78α. On the contrary, LD78β was only 2-fold more efficient than LD78α in chemoattracting human lymphocytes and monocytes. Using CC chemokine receptor–transfected cells, both molecular forms of LD78β proved to be much more potent than LD78α in inducing an intracellular calcium rise through CCR5. Compared with LD78α and RANTES, this preferential binding of LD78β to CCR5 resulted in a 10- to 50-fold higher potency in inhibiting infection of peripheral blood mononuclear cells by CCR5-using (R5) HIV-1 strains. To date, LD78β is the most potent chemokine for inhibiting HIV-1 infection, and can be considered as a potentially important drug candidate for the treatment of infection with R5 HIV-1 strains.
J. Clin. Invest. 104: R1-R5 (1999)
Introduction
Chemokines are a family of chemotactic cytokines that interfere with normal (leukocyte homing, hematopoiesis, angiogenesis) and pathological (inflammation, HIV-1 infection, atherosclerosis) processes (1–5). Depending on the positioning of the cysteine residues, chemokines are classified as C, CC, CXC and CX3C chemokines. Each of the numerous chemokines stimulates migration of a unique set of leukocytic cell types. This selectivity is due to the restricted expression pattern of chemokine receptors on a particular cell type. Most chemokines recognize several receptors; e.g., human macrophage inflammatory protein-1α (MIP-1α) signals through the CC chemokine receptors CCR1 and CCR5. On the other hand, a single receptor can bind more than 1 chemokine; e.g., CCR5 is a receptor for RANTES, MIP-1α, MIP-1β, and monocyte chemotactic protein-2 (MCP-2) and is expressed on monocytes and activated lymphocytes. Some of these 7 transmembrane–spanning, G-protein–coupled receptors are also necessary for HIV entry (1–5). Macrophage-tropic (M-tropic) (R5) HIV-1 strains predominantly use CCR5 as a coreceptor for infection, whereas T cell–tropic (T-tropic) (X4) HIV-1 strains enter via CXCR4, the unique but widely expressed receptor for stromal cell–derived factor-1 (SDF-1) (3, 5).
MIP-1α has originally been isolated from murine macrophages as an inflammatory mediator and inhibitor of hematopoietic stem cell proliferation (6, 7). Human MIP-1α, the first human CC chemokine for which the cDNA sequence has been reported, is encoded by 2 highly related nonallelic genes (8–11), designated LD78α and LD78β, that are expressed in lymphocytes and monocytes. So far, only recombinant LD78α has been investigated in detail; it was found to chemoattract monocytes, lymphocytes, natural killer cells, dendritic cells, eosinophils, and basophils (3–5). The 2 MIP-1α isoforms differ only in 3 amino acids: the penultimate NH2-terminal residue and amino acids 39 and 47. Many studies have indicated the importance of the NH2-terminal regions of both CXC and CC chemokines for their biological activity (2, 12–15). Because modification or deletion of a single residue may result in altered chemokine receptor usage, and hence target cell specificity, we have investigated both isoforms of MIP-1α in more detail. We believe that this study describes for the first time the isolation of natural LD78β. We show that this MIP-1α isoform is a potent lymphocyte and monocyte chemoattractant that preferentially signals through CCR5 and is the most potent chemokine in inhibiting infection of peripheral blood mononuclear cells (PBMCs) by R5 HIV-1.
Methods
Cell cultures and chemokines.
Human monocytic THP-1 cells were cultured in RPMI-1640 (BioWhittaker Europe, Verviers, Belgium) enriched with 10% FCS (FCS; GIBCO BRL, Paisley, Scotland, United Kingdom). Murine lymphocytic ESb/MP cells (16), kindly provided by J.M. Wang (National Cancer Institute/National Institutes of Health, Frederick, Maryland, USA), were grown in DMEM (BioWhittaker Europe) supplemented with β-mercaptoethanol and 10% FCS. Human osteosarcoma (HOS) cells transfected with CD4 and either CCR1 or CCR5 (17) were cultured in DMEM supplemented with 10% FCS and puromycin (1 μg/mL; Sigma Chemical Co., St. Louis, Missouri, USA). PBMCs were isolated by hydroxyethyl starch sedimentation and Ficoll-sodium metrizoate centrifugation (13). Lymphocytes and monocytes were further separated on magnetic beads, coated with anti-CD3 (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
RANTES and the human recombinant MIP-1α isoform LD78α were purchased from PeproTech Inc. (Rocky Hill, New Jersey, USA) and BioSource Europe (Fleurus, Belgium). The 7.793-kDa LD78β was synthesized by FMOC solid-phase peptide synthesis (18). 125I-RANTES was purchased from Amersham Pharmacia Biotech (Roosendaal, the Netherlands).
Production, purification, and identification of natural MIP-1α isoforms.
PBMCs (54 × 109 cells) from 132 blood donations were incubated for 48 hours in EMEM (GIBCO BRL) plus 2% FCS supplemented with 10 μg/mL Con A (Calbiochem-Novabiochem Corp., San Diego, California, USA) and 2 μg/mL LPS (from Escherichia coli 0111:B4; Difco Laboratories, Detroit, Michigan, USA). Natural MIP-1α isoforms were isolated through a 4-step concentration and purification procedure (13) comprising adsorption to silicic acid, heparin-Sepharose chromatography, cation exchange chromatography, and reverse-phase HPLC (RP-HPLC). Fractions derived from RP-HPLC were checked for purity by SDS-PAGE under reducing conditions (13). MIP-1α proteins were viewed by silver staining, and the following molecular weight markers were used: carbonic anhydrase (Mr = 31 kDa), soybean trypsin inhibitor (Mr = 21 kDa), lysozyme (Mr = 14 kDa), and aprotinin (Mr = 6.5 kDa). NH2-terminal amino acid sequences of pure MIP-1α forms were obtained by Edman degradation, using a pulsed liquid-phase 477A/120A protein sequencer (Perkin-Elmer Applied Biosystems, Foster City, California, USA). To distinguish LD78β forms, purified proteins were subjected to electrospray mass spectrometry (LCQ-Iontrap; Finnigan, San Jose, California, USA).
Chemokine assays.
MIP-1α immunoreactivity was measured by a specific sandwich ELISA (BioSource Europe), kindly supplied by J.-M. Jaspar. The cellular expression of chemokine receptors was examined by flow cytometric analysis, using mAb’s against CCR1 (R&D Systems Inc., Minneapolis, Minnesota, USA) and CCR5 (PharMingen, San Diego, California, USA). Competition for 125I-RANTES binding was analyzed on CCR1- and CCR5-transfected HOS cells as previously described (19). MIP-1α isoforms were tested for their chemotactic potency on freshly isolated monocytes (2 × 106 cells/mL), CD3+ lymphocytes (10 × 106 cells/mL), monocytic THP-1 cells (0.5 × 106 cells/mL), and lymphocytic ESb/MP cells (2 × 106 cells/mL), in the Boyden microchamber (Neuro Probe Inc., Cabin John, Maryland, USA) (13, 16). The chemotactic index (number of chemoattracted cells divided by the number of randomly migrated cells) was used as a parameter for potency. Intracellular calcium concentrations ([Ca2+]i) were measured by fluorescence spectrophotometry and calculated according to the Grynkiewicz equation (13).
Inhibition of HIV-1 infection.
The R5 HIV-1 strain BaL was obtained through the Medical Research Council AIDS Reagent Project (National Institute for Biological Standards and Control, Herts, United Kingdom). Purified PBMCs, stimulated with PHA (1 μg/mL for 3 days), were infected with virus in the presence of 25 U/mL of IL-2 and various concentrations of LD78α, LD78β, or RANTES (12). Cell supernatant was collected at day 10, and HIV-1 core antigen in the supernatant was analyzed by a p24 antigen ELISA kit (NEN Life Science Products Inc., Boston, Massachusetts, USA).
Results
Identification of natural LD78β isoforms from stimulated mononuclear cells.
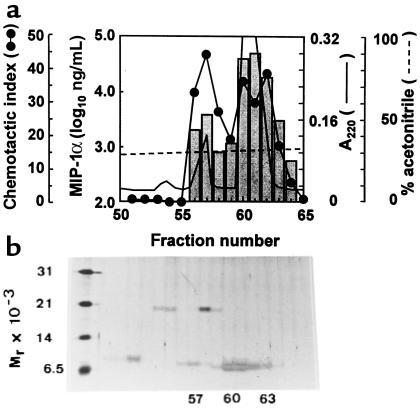
Conditioned medium from PBMCs stimulated with mitogen and endotoxin was analyzed for its MIP-1α content using a specific ELISA and the sensitive lymphocyte (ESb/MP cells) chemotactic assay. With an established procedure for chemokine isolation (13), MIP-1α isoforms were fractionated and finally purified to homogeneity by RP-HPLC (Figure 1a). MIP-1α immunoreactivity and chemotactic activity corresponded to a minor peak containing a single protein of 8 kDa and to a major peak of 7–8 kDa MIP-1α (Figure 1b). NH2-terminal amino acid sequence analysis revealed that the minor peak (fraction no. 56–57) corresponded to a pure, intact LD78β isoform of MIP-1α, whereas the major peak (fraction no. 60) contained a mixture of intact LD78β and truncated LD78α or β, each missing 4 NH2-terminal residues. Because the sequences of LD78α and LD78β differ only in 3 amino acids, including the penultimate and 2 internal but interchanged residues, NH2-terminal sequence analysis and mass spectrometry allowed us to discriminate between the intact α and β forms but not between the truncated LD78 isoforms. Nevertheless, extended Edman degradation (up to 45 amino acids identified) showed the presence of both truncated LD78α and LD78β in the major MIP-1α peak. Intact LD78α could not be recovered from any of the natural MIP-1α preparations analyzed. When both MIP-1α peaks were subjected to mass spectrometry, a different molecular mass was obtained for NH2-terminally intact LD78β in RP-HPLC fraction no. 56 (7.7776 ± 0.0012 kDa) and fraction no. 60 (7.7939 ± 0.6 kDa). Because the theoretical monoisotopic molecular mass of LD78β is 7.7927 kDa, it was concluded that only fraction no. 60 contains authentic LD78β. The loss of 16 kDa, observed in the LD78β form present in fraction no. 56, may correspond to an amino acid substitution (polymorphism) or the reduction (loss of oxygen) or deamination of a single residue. To biologically compare these 2 forms of LD78β, we have synthesized the authentic 7.793-kDa molecule, because the latter was not obtained in its pure natural form.
Figure 1.
Purification of PBMC-derived MIP-1α to homogeneity by RP-HPLC. (a) Prepurified MIP-1α immunoreactivity from conditioned medium of PBMCs was loaded on a C8 RP-HPLC column. Proteins were eluted with an acetonitrile gradient (dashed line) and detected by measuring UV absorption at 220 nm (solid line). Fractions were tested for the presence of MIP-1α immunoreactivity (histogram) and ESb/MP cell chemotactic activity (solid line broken by circles). (b) Proteins eluted from the HPLC column were analyzed for purity by SDS-PAGE under reducing conditions and were stained with silver. Markers used are indicated in Methods.
Diverging chemotactic potencies of LD78α and LD78β isoforms on monocytic and lymphocytic cells.
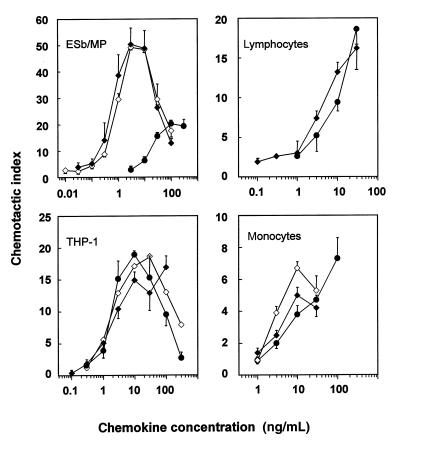
Because natural LD78β protein has not yet been biologically evaluated, pure NH2-terminally intact LD78β forms (natural = 7.778 kDa; synthetic = 7.793 kDa) were compared with intact LD78α for their capacity to stimulate monocytic (freshly isolated blood monocytes and THP-1 cells) and lymphocytic (freshly isolated CD3+ blood lymphocytes and ESb/MP cells) cell migration in vitro. In the microchamber assay, both LD78β forms induced lymphocytic ESb/MP cell chemotaxis from 0.3 ng/mL onward, whereas for LD78α, 30 ng/mL was necessary (Figure 2). Moreover, at optimal concentrations, 2.5 times more ESb/MP cells migrated in response to LD78β compared with LD78α. In contrast, LD78α and LD78β were equally potent as chemoattractants for monocytic THP-1 cells, with a minimal effective concentration of 1 ng/mL. Similarly, LD78α was equipotent to LD78β in inducing calcium mobilization in THP-1 cells (data not shown). Additional experiments on freshly isolated monocytes and CD3+ lymphocytes indicated that LD78β was only 2-fold more potent than LD78α. Taken together, these findings indicated that, despite minor structural differences between the MIP-1α isoforms, LD78α and LD78β show diverging chemotactic potencies. Because monocyte and lymphocyte populations were found to express both CCR1 and CCR5, as determined by flow cytometric analysis (data not shown), the effect of the ligands LD78α and LD78β on these chemokine receptors was further investigated.
Figure 2.
Comparison of the chemotactic potencies of LD78α and the LD78β isoforms. The chemotactic activities of LD78α (filled circles), 7.778-kDa LD78β (filled diamonds), and 7.793-kDa LD78β (open diamonds) were determined in the Boyden microchamber assay, using ESb/MP lymphocytic cells, THP-1 monocytic cells, and PBMC CD3+ lymphocytes and monocytes. Results represent the mean chemotactic index ± SEM of 3 or more independent experiments (each performed in triplicate).
Inverse chemokine receptor recognition by LD78α and LD78β.
First, we studied whether the 7.793-kDa LD78β differed from LD78α in order to compete with 125I-labeled RANTES for receptor binding on CCR1- and CCR5-transfected HOS cells. LD78α and LD78β displaced 125I-RANTES from CCR1 equally well, whereas on CCR5 transfectants, LD78β was superior to LD78α (data not shown).
LD78α strongly induced calcium mobilization in CCR1 transfectants (minimal effective concentration: 3 ng/mL), whereas LD78β (7.778 and 7.793 kDa) yielded a significant effect only from 10 ng/mL onward (Figure 3). In contrast, in CCR5 transfectants, both LD78β forms induced calcium mobilization more efficiently than RANTES and LD78α (minimal effective concentration: 3 vs. 30 and 100 ng/mL, respectively), indicating an inverse receptor preference of these MIP-1α isoforms. A small difference between both LD78β forms, in favor of the 7.778-kDa variant, was observed on CCR5 transfectants. The maximal calcium response of LD78β on CCR5 was 3-fold higher than that of LD78α and RANTES. The 30-fold higher potency of LD78β, compared with LD78α, to signal through CCR5 suggested that LD78α and LD78β might differ in their ability to block CCR5 binding by M-tropic HIV-1 strains.
Figure 3.
Diverging signaling capacities of LD78α, LD78β, and RANTES through CCR1 and CCR5. Intracellular calcium mobilization experiments were performed on CCR1- and CCR5-transfected HOS cells. Results represent the mean [Ca2+]i rise ± SEM, obtained in 2 or more independent experiments. The limit for significant increases in [Ca2+]i (20 nM) is indicated by the dashed line.
Higher potency of LD78β, compared with LD78α and RANTES, in inhibiting HIV-1 infection.
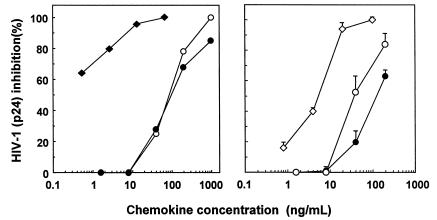
LD78α and LD78β were compared for their capacity to prevent HIV-1 infection of PBMCs by the M-tropic BaL strain, which uses CCR5 as a coreceptor to enter the cells. As could be predicted by the receptor recognition patterns, LD78β (7.778 and 7.793 kDa) was found to be a more potent inhibitor of HIV-1 infection than LD78α and RANTES, both known to block M-tropic strains via CCR5 (Figure 4). The 7.778-kDa LD78β form seemed to be more effective compared with the 7.793-kDa LD78β form. On average, the IC50 values were about 1, 5, 60 and 100 ng/mL for 7.778-kDa LD78β, 7.793-kDa LD78β, RANTES, and LD78α, respectively. This more than 20-fold difference in antiviral potency between LD78α and LD78β reflects their differences in CCR5 signaling capacity.
Figure 4.
Inhibition of R5 HIV-1 infection by LD78β. PBMCs were protected from infection with the R5 BaL strain by the CC chemokines LD78α (filled circles), 7.778-Da LD78β (filled diamonds), 7.793-kDa LD78β (open diamonds), or RANTES (open circles). HIV-1 inhibition was evaluated by measuring viral p24 core antigen. Results at left show 1 representative experiment out of 2, whereas the results at right represent the mean ± SEM of 4 independent experiments.
Discussion
The human macrophage inflammatory proteins MIP-1α and MIP-1β, mainly produced by mononuclear leukocytes (2), display different chemotactic activities despite their high structural relationship (70% identical amino acids). The diverging effects of MIP-1α and MIP-1β on leukocytes can be explained by the fact that MIP-1α signals through CCR1 and CCR5, whereas MIP-1β does not bind CCR1 (1–5). The anti–HIV-1 activities of MIP-1α and MIP-1β are both mediated through CCR5 binding (17, 20–23). For MIP-1α, 2 isoforms (LD78α and LD78β) have been reported, but so far only the biological activities and receptor binding properties of LD78α have been investigated in detail (8–11, 20–23). The primary structure of LD78α and LD78β differs in 3 amino acids, including 1 residue in the NH2-terminal region, which may be important for receptor binding, as shown for other chemokines (2, 12–15).
This study describes the isolation and biological characterization of natural LD78β from stimulated leukocytes. Two variants of natural NH2-terminally intact LD78β, characterized by different molecular masses, were recovered. The 7.794-kDa LD78β variant, with a molecular mass corresponding to the theoretical mass (7.793 kDa), was impure and was synthesized to be compared with the 7.778-kDa variant, lacking 0.016 kDa. Compared with LD78α, both LD78β variants were found to be 100-fold more potent in chemoattracting ESb/MP lymphocytic cells, whereas no difference was observed on THP-1 monocytic cells. On freshly isolated blood monocytes and CD3+ lymphocytes, both LD78β variants seemed to be only 2-fold better chemoattractants than LD78α. By means of the calcium mobilization assay, using CCR transfectants, it was further shown that LD78α signals predominantly via CCR1. In contrast, LD78β activates CCR5 more efficiently than LD78α or RANTES. This probably explains the differences in chemotactic potencies observed between LD78α and LD78β. Finally, the preference of both LD78β isoforms for CCR5 was biologically confirmed on PBMCs by their 10- to 50-fold higher antiviral activity against M-tropic HIV-1 strains compared with RANTES and LD78α. The reported rank order amongst CC chemokines for antiviral potency is RANTES>MIP-1β>MIP-1α/LD78α (24). Our findings that RANTES and LD78α have an IC50 of about 60 and 100 ng/mL, respectively, are in agreement with these published data (24). The fact that the IC50 for SDF-1, when evaluated against different T-tropic HIV isolates, was as high as 200–3,500 ng/mL demonstrates that LD78β (IC50: 1–5 ng/mL) is the most potent chemokine reported to date for inhibiting HIV-1 infection. This superior anti–HIV-1 activity of LD78β needs to be reconsidered in the light of the isolation of MIP-1α, MIP-1β, and RANTES from cultured T cells as suppressors of HIV-1 infection (20). Although not investigated in the latter study (20), it can be deduced from our data that the antiviral effect corresponding to MIP-1α was likely due to LD78β. A reported overproduction of the CC chemokines MIP-1α, MIP-1β, and RANTES correlated with the protection against HIV-1 infection (25–26). Moreover, MIP-1α appeared faster and reached higher concentrations than MIP-1β or RANTES (25–26). These clinical data demonstrating natural resistance to HIV-1 infection, which was not linked to a mutation deletion in the CCR5 gene, are in agreement with the higher antiviral potency of the LD78β isoforms of MIP-1α observed in our study.
The fact that we could only recover intact LD78β from stimulated mononuclear cells points to a higher resistance of LD78β to posttranslational processing by proteolytic cleavage. Nevertheless, both truncated LD78α and LD78β, missing 4 NH2-terminal residues, were purified from leukocyte-conditioned media. In a previous study, using the HTLV-1–infected MT4 cells as a cellular source, only NH2-terminally processed MIP-1α was recovered (27). NH2-terminal truncation of 2 amino acids, including a penultimate proline, by dipeptidyl peptidase IV/CD26 differentially affected the chemotactic and anti–HIV-1 activities of both CXC (SDF-1) and CC (RANTES, macrophage-derived chemokine [MDC]) chemokines (12, 14–15). Although posttranslational processing of MDC and RANTES seemed to improve their antiviral capacity, both truncated MDC and RANTES remained far inferior for blocking HIV-1 replication in PBMCs when compared with LD78β (12, 15). In view of the importance of the NH2-terminal region of chemokines for receptor recognition, the substitution of the penultimate serine residue in LD78α by a proline in LD78β is probably responsible for the receptor switch from CCR1 to CCR5.
In addition to HIV-1 infection, the role of MIP-1α has been studied in other human pathologies, including airway inflammation and autoimmune disease (1). Using immunotests recognizing both LD78α and LD78β, elevated MIP-1α levels have been detected in bronchoalveolar lavage fluids of asthma patients. Similarly, MIP-1α immunoreactivity is significantly present in the synovial fluid during rheumatoid arthritis. The involvement of MIP-1α in eosinophilic and mononuclear leukocyte infiltration has been confirmed in animal models by blocking antibodies (1). Because no MIP-1α isoforms have been described in the mouse, and human LD78α and LD78β are immunologically indistinguishable, the individual roles of LD78α and LD78β in these human diseases remain, at present, unknown.
Acknowledgments
The work has been supported by the Fund for Scientific Research of Flanders (FWO-Vlaanderen), the Concerted Research Actions (GOA) of the Regional Government of Flanders, the Interuniversity Attraction Pole (IUAP) initiative of the Belgian Federal Government, and the Biotech program of the European Union. P. Menten, P. Proost, and S. Struyf hold fellowships of the FWO. The authors thank C. Govaerts (Lab Pharmaceutical Chemistry, Leuven, Belgium) and A. De Jong and H. Meiring (Rijksinstituut voor Volksgezondheid en Milieu, Bilthoven, the Netherlands) for mass determinations. We appreciate the editorial help of D. Brabants and the technical assistance of S. Claes, R. Conings, E. Fonteyn, J.-P. Lenaerts, and W. Put. The chemokine receptor–transfected HOS cells were obtained from Nathaniel Landau through the AIDS Research and Reference Program (Division of AIDS, National Institute of Allergy and Infectious Diseases/National Institutes of Health, Bethesda, Maryland, USA).
References
- 1.Strieter RM, et al. The good, the bad and the ugly: the role of chemokines in models of human disease. J Immunol. 1996;156:3583–3586. [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines: CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 4.Taub DD. Chemokine-leukocyte Interactions. Cytokine & Growth Factor Rev. 1996;7:355–376. doi: 10.1016/s1359-6101(97)89237-4. [DOI] [PubMed] [Google Scholar]
- 5.Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 6.Wolpe SD, et al. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;176:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham GJ, et al. Identification and characterization of an inhibitor of haematopoietic stem cell proliferation. Nature. 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 8.Obaru K, Fukuda M, Maeda S, Shimada K. A cDNA clone used to study mRNA inducible in human tonsillar lymphocytes by a tumor promoter. J Biochem. 1986;99:885–894. doi: 10.1093/oxfordjournals.jbchem.a135549. [DOI] [PubMed] [Google Scholar]
- 9.Blum S, Forsdyke RE, Forsdyke DR. Three human homologs of a murine gene encoding an inhibitor of stem cell proliferation. DNA Cell Biol. 1990;9:589–602. doi: 10.1089/dna.1990.9.589. [DOI] [PubMed] [Google Scholar]
- 10.Irving SG, et al. Two inflammatory mediator cytokine genes are closely linked and variably amplified on chromosome 17q. Nucleic Acids Res. 1990;18:3261–3270. doi: 10.1093/nar/18.11.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakao M, Nomiyama H, Shimada K. Structures of human genes coding for cytokine LD78 and their expression. Mol Cell Biol. 1990;10:3646–3658. doi: 10.1128/mcb.10.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proost P, et al. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1 infection. J Biol Chem. 1998;273:7222–7227. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- 13.Wuyts A, et al. Isolation of the CXC chemokines ENA-78, GROa and GROg from tumor cells and leukocytes reveals NH2-terminal heterogeneity. Functional comparison of different natural isoforms. Eur J Biochem. 1999;260:421–429. doi: 10.1046/j.1432-1327.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 14.Proost P, et al. Processing by CD26/dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell-derived factor-1α. FEBS Lett. 1998;432:73–76. doi: 10.1016/s0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- 15.Struyf S, et al. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor. J Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- 16.Wang JM, et al. Purification and identification of chemokines potentially involved in kidney specific metastasis by a murine lymphoma variant: induction of migration and NFκB activation. Int J Cancer. 1998;75:900–907. doi: 10.1002/(sici)1097-0215(19980316)75:6<900::aid-ijc13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Deng HK, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 18.Proost P, et al. Chemical synthesis, purification and folding of the human monocyte chemotactic proteins MCP-2 and MCP-3 into biologically active chemokines. Cytokine. 1995;7:97–104. doi: 10.1006/cyto.1995.1013. [DOI] [PubMed] [Google Scholar]
- 19.Schols D, et al. CD26-processed RANTES(3-68), but not intact RANTES, has potent anti-HIV-1 activity. Antiviral Res. 1998;39:175–187. doi: 10.1016/s0166-3542(98)00039-4. [DOI] [PubMed] [Google Scholar]
- 20.Cocchi F, et al. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 21.Dragic T, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 22.Choe H, et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 23.Doranz BJ, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 24.Trkola A, et al. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zagury D, et al. CC chemokines, pivotal in protection against HIV type 1 infection. Proc Natl Acad Sci USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxton WA, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 27.Bertini R, et al. Identification of MIP-1α/LD78 as a monocyte chemoattractant released by the HTLV-I-transformed cell line MT4. AIDS Res Hum Retroviruses. 1995;11:155–160. doi: 10.1089/aid.1995.11.155. [DOI] [PubMed] [Google Scholar]