ABSTRACT
Rapid responses to changes in incident light are critical to the guidance of behavior and development in most species. Phytochrome light receptors in particular play key roles in bacterial physiology and plant development, but their functions and regulation are less well understood in fungi. Nevertheless, genome-wide expression measurements provide key information that can guide experiments that reveal how genes respond to environmental signals and clarify their role in development. We performed functional genomic and phenotypic analyses of the two phytochromes in Neurospora crassa, a fungal model adapted to a postfire environment that experiences dramatically variable light conditions. Expression of phy-1 and phy-2 was low in early sexual development and in the case of phy-2 increased in late sexual development. Under light stimulation, strains with the phytochromes deleted exhibited increased expression of sexual development-related genes. Moreover, under red light, the phy-2 knockout strain commenced sexual development early. In the evolution of phytochromes within ascomycetes, at least two duplications have occurred, and the faster-evolving phy-2 gene has frequently been lost. Additionally, the three key cysteine sites that are critical for bacterial and plant phytochrome function are not conserved within fungal phy-2 homologs. Through the action of phytochromes, transitions between asexual and sexual reproduction are modulated by light level and light quality, presumably as an adaptation for fast asexual growth and initiation of sexual reproduction of N. crassa in exposed postfire ecosystems.
IMPORTANCE
Environmental signals, including light, play critical roles in regulating fungal growth and pathogenicity, and balance of asexual and sexual reproduction is critical in fungal pathogens’ incidence, virulence, and distribution. Red light sensing by phytochromes is well known to play critical roles in bacterial physiology and plant development. Homologs of phytochromes were first discovered in the fungal model Neurospora crassa and then subsequently in diverse other fungi, including many plant pathogens. Our study investigated the evolution of red light sensors in ascomycetes and confirmed—using the model fungus Neurospora crassa—their roles in modulating the asexual-sexual reproduction balance in fungi. Our findings also provide a key insight into one of the most poorly understood aspects of fungal biology, suggesting that further study of the function of phytochromes in fungi is critical to reveal the genetic basis of the asexual-sexual switch responsible for fungal growth and distribution, including diverse and destructive plant pathogens.
INTRODUCTION
Beyond its role supplying the energy for photosynthesis, and thus its direct or indirect energetic support of most forms of life on earth, light also serves as a nearly ubiquitous source of temporal and spatial information concerning environmental changes. Most fungi, like many microbes and most plants, cannot rapidly navigate their environment to move toward desirable conditions. Unlike many microbes, a fungus can comprise a large biomass extending across two or three spatial dimensions as well as time, thereby experiencing diverse environmental conditions. Intense light—a key indicator of environmental changes—is detrimental to most fungi and other microbes over large periods of their life history (1–4). Nevertheless, it serves as one of the direct environmental signals by which they can regulate life cycle decisions (5) and is a facet of fungal biology that has been targeted for the prevention of plant pathogenesis (6). Accordingly, fungal gene expression responds rapidly and dynamically to light stimulation (7–11), presumably and in some cases demonstrably through the action of sensory molecules. Although effects of light on the pathogenicity, reproduction, and life cycle of ascomycetous fungi have been studied for a long time, little genetic analysis of light-responsive pathways has been conducted in these fungi. There is a lack of knowledge about fungal responses to the light spectrum in non-model species, which include many plant-associated fungi. Moreover, even in model species, such as Neurospora crassa and Aspergillus nidulans, the systems biology behind fungal photobiology has been found to be highly complicated (5, 8, 12, 13).
Four kinds of sensory molecules, which bind chromophores that respond to blue, green, and red light, respectively, have been discovered in fungal genomes. The blue light sensors, which are proteins with a flavin chromophore, have been most intensively investigated in plants, animals, microbes, including fungi (5, 8, 14, 15). In Neurospora crassa, blue light regulates circadian rhythms and fungal response to stress agents (16–19). Blue light also plays a role in carotenoid production, conidiation, protoperithecium formation, and phototropism of perithecial beaks (20–23). For example, under nutrient starvation, exposure of N. crassa to brief pulses of blue light can induce formation of protoperithecia or conidiation (24, 25), and blue light affects the polarity of the perithecial beak (21, 22, 26). Rhodopsins (opsins) sense blue and green light and have been found in various fungal genomes (27). Homologs of red-light-sensing proteins, known as phytochromes (PHY) in plants, have also been identified in Neurospora, Aspergillus, Sclerotinia, and some other fungal species (6). Plant and fungal phytochromes share an origin with bacterial phytochromes that bind their chromophore through a thioether bond to conserved cysteine residues, but they followed two distinct evolutionary trajectories subsequent to their divergence (5, 28, 29). In plants, the phytochromes exist as two distinct but photoreversible forms: the red-light-absorbing form (Pr) and the far-red-light-absorbing form (Pfr). The Pfr form is generally considered the active form, interacting with various transcription factors to regulate plant development (30, 31).
Fungal phytochromes, in contrast, are light-regulated “hybrid” histidine kinases that carry a histidine kinase (HK) domain in addition to the N-terminal response regulator (RR) domain in the output module (32–34). Within the photosensory module at the N terminus, there are three domains—PAS, GAF, and PHY (phytochrome). Light-driven HK activity has been reported in phytochrome FphA of Aspergillus species, in which full asexual reproduction (conidiation) requires stimulation by both blue light and red light to effectively inhibit sexual development, and sexual reproduction of these fungi usually requires culture under complete darkness (35–44). After binding to the biliverdin chromophore, both FphA and its Neurospora homolog, PHY-2, exhibit spectral properties similar to phytochrome in plants (33, 34, 37). FphA also interacts with A. nidulans proteins Light response A (LreA) and LreB, which together form a complex homologous to the Neurospora White Collar Complex (WCC) (40). In A. nidulans exposed to white light, the LreA/LreB complex represses asexual development and promotes sexual development, whereas FphA represses sexual development and promotes asexual development (2, 38–40, 45); in contrast, asexual development in A. fumigatus is neither influenced by blue light nor red light nor by mutation of lreA or fphA orthologs (46). Light-dependent gene activation in A. nidulans was reported as strictly dependent on function of the phytochrome: FphA regulates expression of clock-controlled gene A (ccgA), probably via White Collar-regulated histone H3 acetylation (13). Upregulation of ccgA in response to red light in A. nidulans was almost entirely abrogated by deletion of fphA. In contrast, the phytochrome in the fungal entomopathogen Beauveria bassiana—which lacks a sexual cycle—was reported to control conidiation in response to red/far-red light and daylight length (47). In N. crassa, there are two phytochromes, PHY-1 and PHY-2 (48). Genetic and molecular analysis of N. crassa phytochromes revealed a chromophore binding capability of PHY-2 in vitro, and phosphorylated and unphosphorylated forms of PHY-1 exclusively in the cytoplasm (37). Transcript levels of phy-1 and phy-2 were not demonstrably regulated by light, and no photoresponse phenotypes have been identified in common photobiological assays, including vegetative growth, phototropism, conidiation assays, and assays of perithecial beak development (37). In asexual development under darkness, expression of phy-1 was not detectable in N. crassa and Neurospora tetrasperma, and phy-2 was expressed significantly higher in N. tetrasperma than in N. crassa. Under the same dark culture conditions, the two genes were similarly upregulated across protoperithecial development within both of the Neurospora species, as well as between the orthologs (49). This correlation implies that expression of phy-1 and phy-2 is not induced by light but rather is constrained by the initiation of sexual development in both Neurospora species. Recently, a complex model of the regulation of expression of conidiation genes con-10 and con-6 by photoreceptors was proposed for N. crassa, in which the activity of WCC was suggested to be negatively regulated by several photoreceptors, including cryptochrome (cry-1), opsin (nop-1), and PHY-2 proteins (23).
Sexual and asexual reproduction in ascomycetous fungi can be viewed as two modes of reproduction competing for resources, with one process inhibiting the other and vice versa. The balance between the two modes is generally regulated by combined environmental and developmental signals (43). The fungus N. crassa presents a potential model for investigating this balance, especially for non-strict plant pathogens and endophytic fungi (50–54). Its asexual ecology is accessible and well known, it is often found in postfire environments with both endophytic and saprotrophic lifestyles, colonizing exposed dead plant substrates during fast growth and distribution (55). In these environments, N. crassa produces a large amount of small conidiospores via repeated asexual reproduction (56). In contrast, sexual reproductive structures of Neurospora are not commonly observed in natural settings, but initial colonization after fire very likely occurs via thermally cued germination of robustly persistent ascospores in the soil (57, 58). N. crassa produces impact-, desiccation-, and heat-resistant ascospores under environmental conditions that are unfavorable to fast growth. Initiation of sexual reproduction starts with the accumulation of a few thin-walled cells. Then, in 12 to 18 h, these cell masses darken and expand to form mature protoperithecia (59). There is little known regarding how light affects sexual development in N. crassa. Inhibition of sexual development by a phytochrome responding to red light has been reported in Aspergillus nidulans, which preferentially undergoes sexual reproduction in the dark (5, 38). In contrast, Neurospora is known for fast growth and colonization via asexual reproduction in the postfire environment, and sexual reproduction in N. crassa is often triggered by a low nutrient level and requires specific light signals during late perithecial development: i.e., precise regulation of sexual reproduction negatively mediated by light is critical, especially during the initiation stage. Both asexual development and sexual development of its congener N. intermedia have been observed to occur above ground, perithecia have been found under the surface of plant epidermal tissue, and well-developed beaks have been found protruding through cracked, burned plant tissue (58), indicating a developmental program in which light likely plays a key and important role.
Genome-wide expression measurements provide detailed information about gene activity during the processes and facilitate the design of focused experiments to reveal how genes respond to environmental signals. In return, new understanding of the roles that genes play across development expands our ability to interpret transcriptomic observations. Here, based on published transcriptomic data previously unexamined for these questions, we reveal expression patterns of phytochromes during sexual development in N. crassa, N. tetrasperma, and N. discreta as well as two species of Fusarium. We designed and executed assays to directly compare developmental behaviors between wild-type strains and phytochrome knockout (KO) mutants under different light conditions at developmental stages where phytochromes were especially abundantly or meagerly expressed. To investigate genome-wide impacts on gene expression of phytochromes, we used a Bayesian approach to reanalyze genome-wide expression data collected at multiple time points for wild-type and KO strains of phytochromes under different light stimulation conditions. To assess the potential ecological roles of these light-dependent genes, we reconstructed the gene phylogenies of phytochromes from diverse annotated genomes in ascomycetous fungi.
RESULTS
Expression of phytochromes was upregulated during sexual development in Neurospora crassa and related fungi.
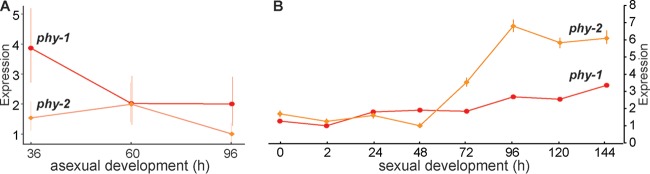
The genome-wide gene expression was profiled during the course of asexual development (60) and over sexual development in N. crassa (61, 62), N. tetrasperma and N. discreta (63), and two Fusarium species (64) (Table 1). Across asexual development in N. crassa, expression of phy-1 and phy-2 exhibited sequential—but not statistically significant—downregulation. Across sexual development, expression of phy-1 and phy-2 exhibited sequential upregulation; expression of phy-2 increased over 6-fold (P < 0.01) starting at 48 h after crossing (Fig. 1). Phytochromes in N. tetrasperma, N. discreta, and two Fusarium spp. also exhibited increased expression during sexual development (see Fig. S1 in the supplemental material), measured as reads per kilobase of exon model per million mapped reads (RPKM) (65). Five genes, including wc-1, wc-2, vvd, cry, and velvet, are known as blue-light-responsive genes. They exhibited a peak expression at 48 h after crossing, a stage characterized by meiosis-specific expression and ascus/ascospore development. Unlike upregulated phytochromes, these genes were generally downregulated during late perithecial development, except cry, in N. crassa (see Fig. S2 in the supplemental material).
TABLE 1 .
Transcriptomic data analyzed for this study
| Expt | Species | Conditions | Accession no. | Reference |
|---|---|---|---|---|
| Asexual development | Neurospora crassa | Bird medium, constant light, wild type | GSE26209 | 60 |
| Sexual development | N. crassa | Carrot medium, constant light, wild type | GSE41484 | 62 |
| N. tetrasperma | Carrot medium, constant light, wild type | GSE60255 | 63 | |
| N. discreta | Carrot medium, constant light, wild type | GSE60266 | 63 | |
| Fusarium graminearum | Carrot medium, constant light, wild type | GSE61865 | 64 | |
| F. verticillioides | Carrot medium, constant light, wild type | GSE61865 | 64 | |
| Light response | N. crassa | Bird medium, dark and light conditions, wild-type and KO strains | GSE8932 | 7 |
FIG 1 .
Expression of N. crassa phytochrome genes phy-1 and phy-2. (A) Expression level of phytochromes across asexual development, assessed using gene expression microarrays (60). (B) Expression of phytochromes during sexual development using transcriptome sequencing (62). Expression levels are presented in fold change relative to the lowest time point, which was normalized to 1. Error bars represent 95% confidence intervals.
Knockouts of phytochromes affected light-induced expression of conidiation-related genes and early sexual developmental genes.
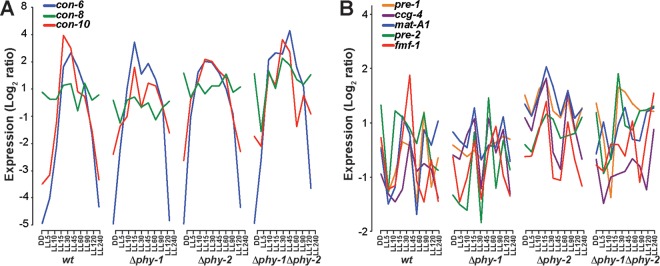
A hierarchical regulatory program governing responses to white light has been described in N. crassa based on analysis of transcriptional profiles of cultures held in white light up to 6 h (7). These extensive data were reanalyzed with a new focus on phy-1 and phy-2 knockout strains and a phy-1 phy-2 double-knockout strain (see Tables S1 and S2 in the supplemental material). The Δphy-1, Δphy-2, and Δphy-1 Δphy-2 gene deletion strains all exhibited genome-wide impacts on gene expression patterns and levels (Fig. 2; see Fig. S3 in the supplemental material). Among eight light-responsive transcription factors investigated, WC-1, VVD (VIVID, a PAS protein), and SUB-1 (submerged protoperithecia-1) strongly influenced early or late light-responsive genes (affecting 3% of the N. crassa genome). A weaker but similar influence was observed for CSP-1 (conidial separation-1), SAH-1 (short aerial hyphae-1), VAD-3 (vegetative asexual development-3), NOP-1 (new eukaryotic opsin-1), and a hypothetical protein, NCU03643 (4). Overall one-third or more of the genome showed significantly higher expression (P < 0.05) in Δphy-2, and Δphy-1 Δphy-2 strains compared with wild-type and Δphy-1 strains, especially under light stimulation longer than 60 min (see Fig. S3 in the supplemental material). Similar results were obtained imposing an exclusive effect-size threshold of 2-fold for biological significance. Cell-type differentiation genes were significantly enriched for upregulation in single- and double-knockout mutants shortly after exposure to light (see Table S2A in the supplemental material). Some differentially expressed cell-type differentiation genes had roles in conidiation, including con-8 (NCU10997), sporulation protein gene sps19 (NCU07958), high conidial production gene hcp-1 (NCU07221), G-protein gene gna-1 (NCU06493), and conidiophore development gene hym-1 (NCU03576 [51, 66]). We observed a typical photoadaptation response (acute light induction followed by a decrease in expression for carotenoid biosynthesis genes [7, 8, 67]) for the three conidiation genes con-6, con-10, and con-8 (Fig. 2A). The Δphy-2 knockout strain exhibited highly correlated upregulation for genes related to sexual development right after exposure to light (Fig. 2B), including mating type locus mat-A1 (NCU01958), pheromone precursor coding gene ccg-4 (NCU02500), and pheromone receptor coding genes pre-1 (NCU00138) and pre-2 (NCU05758).
FIG 2 .
Wild-type and phytochrome knockout strains all respond dynamically to light, but respond differently, especially for genes involved in sexual reproduction. Disruption of phytochromes affected expression of early sexual development genes in response to light but did not markedly affect expression of conidiation genes. (A) The conidiation genes con-6, con-8, and con-10, showed similar expression profiles among wild-type and mutant strains. (B) Highly correlated upregulation of expression of sexual development-related genes occurred immediately following light stimulation in the Δphy-2 KO strain. The log2 ratio of expression level was estimated based on analysis of microarray data (7). Expression is depicted under conditions of constant darkness (D) and following light exposure (5 to 240 min [LL5 to LL240]).
Knockouts of phytochromes exhibit phenotypes of altered initiation of sexual development in Neurospora crassa.
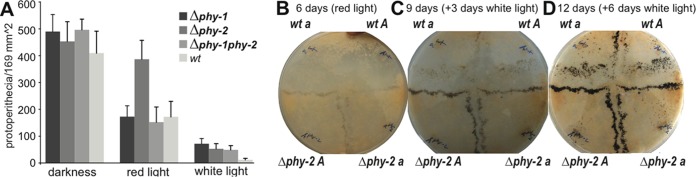
Light influenced the production of protoperithecia in both wild-type and knockout strains (Fig. 3; see Fig. S4 and S5 in the supplemental material). The greatest abundance of perithecia formed in cultures grown in darkness and, in the case of the Δphy-2 strain, also under red light (Fig. 3; see Fig. S5). We examined the role of the phy genes in sexual development on synthetic complete medium (SCM) and carrot media under darkness and exposed to consistent red, far-red, blue, and white light conditions, by culturing crosses between wild-type strains and phy-1 and phy-2 knockout strains (see Fig. S4 and S5A and B in the supplemental material). Under diverse light conditions, sexual development manifests itself similarly in N. crassa cultured on SCM and carrot medium. However, perithecial morphology is markedly easier to assess in SCM cultures: orange-colored conidia, profuse along the crossing zone, seriously obscure the orange protoperithecia and young perithecia, making the discernment of sexual phenotypes extremely challenging to perform against the similarly orange background of the carrot medium. Cultured under constant blue light, both Δphy-1 and Δphy-2 strains produced protoperithecia and then perithecia slightly earlier than the wild-type strains; however, this phenotype was irregular, exhibiting levels of variation among replicates and differences between mating types that rendered any strong conclusions in this culture condition unwarranted in our judgment (see Fig. S5C). Perhaps extensive experimentation could enlighten the reasons for this variability and provide a means to yield a consistent phenotype under blue light. Exposed to constant red light, mating pairs of Δphy-2 strains produced protoperithecia much earlier than did mating pairs of wild-type and Δphy-1 strains (Fig. 3B to D; see Fig. S4). Pairs of Δphy-1 strains exhibited almost the same phenotypes as mating pairs of wild-type strains under red-light conditions. A double-knockout mat a Δphy-1 Δphy-2 strain was generated, and under all light conditions, phenotypes of this mutant exhibited the same phenotypes as the Δphy-1 strain. Under weak white light, Δphy-2 strains produced protoperithecia slightly earlier than did mating pairs of the wild type. Under intense white light, the earlier development of perithecia in mutants was too slight to be readily apparent, implying the possibility of light intensity or other light-dependent regulations. In cultures that were tightly sealed, in cultures grown on conidiation medium under any light conditions, in all cultures grown in the dark, and in late perithecial development stages, no developmental difference observed between knockout and wild-type strains. Cosegregation experiments were performed to confirm that the intended deletion is responsible for the mutant phenotype. Cosegregation of the hygromycin resistance marker and earlier protoperithecia phenotypes was observed for Δphy-1 and Δphy-2 knockout mutants. All 30 individual ascospore progenies that were resistant to hygromycin from a cross of wild-type and Δphy-2 strains exhibited dramatically earlier production of protoperithecia cultured under red light (see Fig. S5D for three representative images). Phenotypes under the blue light condition in the cosegregation experiment were ambiguous. For the Δphy-1 strain, only 12 out of 30 ascospore progeny from a cross with the wild type exhibited both hygromycin resistance and weaker protoperithecial production.
FIG 3 .
Effects of light on sexual development in N. crassa. (A) Abundance of protoperithecia in constant darkness or exposed to red light, and white light for the Δphy-1, Δphy-2, Δphy-1 Δphy-2, and wild-type strains after 6 days of incubation. Error bars represent standard deviations. (B to D) The Δphy-2 strain was cultured for 6 days under constant red light and exhibited early sexual development of protoperithecia and perithecia. (B) Protoperithecia and early perithecia formed on the Δphy-2 strain side. (C) The asymmetry was even more evident after the cultures were placed under 3 days of constant white light for an additional 3 days. (D) The wild-type side of the crossing plate begins to mature at 6 days after shifting to continuous white light.
Evolutionary history of phytochromes is revealed with available fungal genomes.
Phytochrome genes experienced several duplication and loss events during the evolution of higher fungi (subkingdom Dikarya) that are likely related to their function in divergent taxa. Accordingly, across ascomycetes, homologs of N. crassa phy-2 are fast evolving. ModelTest (68) suggested HKY85 (Hasegawa-Kishino-Yano 85) as an optimal model for assessing phytochrome evolutionary history. The relative ratio test in HyPhy (69) exhibited elevated numbers of nucleotide substitutions in phy-2 compared to phy-1 (P < 0.01). Furthermore, phy-2 exhibited a higher nonsynonymous mutation rate than phy-1 (0.63 versus 0.33, P < 0.01, calculated with CODEML of PAML [70, 71]). The index of substitution saturation (Iss, 0.71) and the critical index of substitution saturation (Iss.c, 0.76), are not significantly different (72), indicating that synonymous mutations are substantially saturated for phytochromes over the time scale of the evolution of ascomycetes (minimum most recent common ancestor [MRCA] of 400 million years ago [mya] [73]).
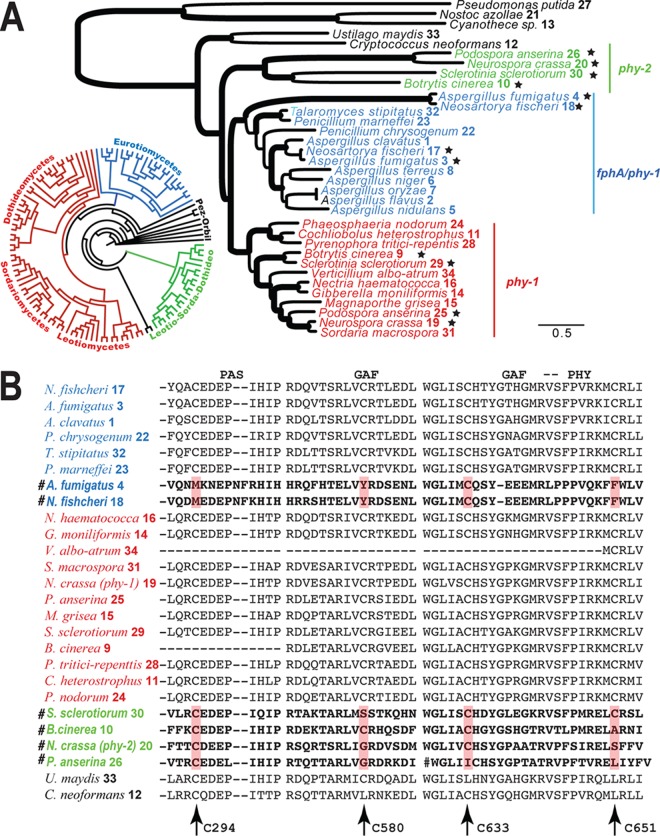
The molecular phylogeny of fungal phytochromes and their functional domains suggested a single origin of ascomycete phytochromes (Fig. 4; see Fig. S6 and Table S2B and C in the supplemental material); no significant conflicts in topology were observed among phylogenies obtained using likelihood, Bayesian parsimony, or distance criteria. The polytomy of the phylogeny based only on the sensory module, including the P2, GAF, and PHY domains, implies quick diversification and rapid settling into conserved function (Fig. 4A; see Fig. S7 in the supplemental material). N. crassa phy-2 and its homologs were part of a strongly supported clade present in 96% of bootstrap replicates (bootstrap proportions [BP]) and 1.0 Bayesian posterior probability (BPP), referred to here as the phy-2 clade. N. crassa phy-1 and its homologs were part of a second strongly supported clade (BP = 99%, BPP = 1.0) referred to here as the phy-1 clade. Phytochromes in Aspergillus and related species formed the fphA/phy-1 clade, which included two phytochrome genes in two closely related genomes, A. fumigatus and A. fischeri. The phylogeny indicated that phy-2 homologs and other phytochromes in ascomycetes were the results of gene duplications. There were subsequent losses of phy-2 in many ascomycetes, especially in the Dothideomycetes and Sordariomycetes, and many losses of phy-1 in the Leotiomycetes (see Fig. S6 and S7 in the supplemental material). With the sole exception of Saitoella, no phytochrome was identified in annotated yeast genomes in Saccharomycotina and Taphrinomycotina, the deepest-diverging lineages in Ascomycota.
FIG 4 .
Evolution of phytochromes in fungi. Homologs were color coded for phy-1 (red), phy-2 (green), and fphA (blue), and sequences were numbered as in Table S2B in the supplemental material. (A) Maximum likelihood phylogeny of fungal phytochromes. Branches with strong support (BP of >85% or BPP of >0.98) are in boldface. Species with multiple copies of phytochromes are marked with stars. A circular tree shows a distribution of phytochromes in major ascomycetous groups. (Phylogenetic details are provided in Fig. S6 in the supplemental material.) (B) Excerpts of amino acid alignments of the PAS, GAF, and PHY domains. The cysteine sites putatively functioning in chromophore binding are indicated by arrows. PHY-1 of N. crassa was used as a reference for positions of the cysteine sites. Fast-evolving phytochromes are marked with the symbol #.
Amino acid sequences of PHY-1 associated with functional domains were highly conserved in fungi (Fig. 4B). Three to four cysteines located in the PAS and GAF domains serve as chromophore-binding sites: two of these cysteine residues are conserved in bacteria (the 294th and 633rd), and two (the 580th and 651st) are conserved in plants (74). N. crassa PHY-1 has cysteines in all four of these positions (Fig. 4B). All ascomycete phytochromes examined featured at least one of the cysteines conserved in plants, except in the fast-evolving phytochrome lineages and in the two basidiomycete outgroups.
DISCUSSION
We have demonstrated that in the model filamentous fungus Neurospora crassa, light affects the initiation of sexual development via phytochromes, mainly the fast-evolving phy-2 gene. Expression of these two genes was at its nadir at early sexual development. Consistent with previous studies (7, 8, 37), our data demonstrate that expression of phy-1 and phy-2 is not regulated by light, yet show for the first time that their functions are light dependent. Deletions of phy-2 and/or phy-1 positively impacted genome-wide expression levels and patterns, especially for genes regulating mating and early sexual development, implying a negative regulatory role for phy-2 in sexual reproduction initiation in the response to light. Strains with knockouts of phytochromes also exhibited phenotypes of altered initiation of sexual development in N. crassa; protoperithecial development commenced earlier in the Δphy-2 strain than in the wild type when cultured under red light, suggesting that phytochromes are regulating the balance between asexual and sexual reproduction in N. crassa as in A. nidulans (40). The repressive regulation imposed mainly by the phytochrome phy-2 gene impacts both the timing of the transition to sexual development and the abundance with which sexual development is initiated in N. crassa. Our observations provide evidence that phytochromes may play a role regulating the asexual-sexual balance in numerous fungi. Considering its unique postfire ecology, the use of light signals and nutrient sensing to balance asexual and sexual development could ensure that N. crassa can maximize its spatial expansion and biomass via quick asexual growth on rich, easily acquired nutrients, before turning to sexual development for the production of genetically diversified, resistant sexual structures and spores that can persistently await the next fire event.
Despite the individual function required to retain them both within single lineages, there may be a degree of functional redundancy between the phytochrome paralogs. In N. crassa, the Δphy-1 and Δphy-2 strains exhibited no malfunctions in growth and development in a previous study (37). Furthermore, our analysis of amino acid conservation demonstrates that all of the recognized domains of the sensory and output modules of PHY-1 and PHY-2 are conserved between paralogs. Generally, strong conservation is equated to essentiality and importance to wild-type fitness (75). However, tests of this assumption using genome-wide data have led to contradictory conclusions (76). The functions of genes that duplicate and then are retained are often split between paralogs (de novo functions are—arguably—rarely gained via gene duplication [77, 78]). The results from our sexual development assay under the blue light condition were indicative but noisy, implying that additional pathways, including some role for phy-1, might be actively regulating sexual development in a light-dependent manner.
Inference of the detailed role of phy-1 in sexual development is made challenging by the complex regulatory effects of light on both asexual and sexual development. In a previous study (37), expression of phy-1 was not observed to be affected by light conditions, yet was under the control of the circadian clock, which is regulated mainly by blue light. Full cosegregation was not observed here for the Δphy-1 strain, which suggested that observed variable phenotypes of earlier protoperithecial production in the Δphy-1 strain could be evidence of a less robust regulatory apparatus, greater sensitivity to microenvironmental variation, or possibly other segregating mutations. The complexity of interactions among light reception genes is clear in A. nidulans, where function of fphA is likely realized through histone H3 acetylation by blue light sensors LreA and velvet protein VeA on clock-controlled gene ccg-4 and where both asexual reproduction and sexual reproduction are regulated by light responses of many genes (13). Upon light exposure, in N. crassa the White Collar Complex (WCC) binds to hundreds of genomic regions as a transcription factor—including the promoter region of Phy-1 (19). Knowledge of the multiple effects of blue light on N. crassa fruiting body maturation would assist efforts to develop an assay that yielded consistent results. The level of detail of knowledge of the circadian response to light during asexual development is striking in comparison to how little is understood regarding how light is perceived and used to modulate sexual reproduction—a challenging but potentially highly informative component of the photobiology of Neurospora and of fungi in general.
Considering the overall conservation of these phytochrome genes, the functional divergence that we do observe between PHY-2 and PHY-1 is slightly puzzling. Except for the highly divergent copy of phytochrome in Aspergillus fumigatus and Neosartorya fischeri, all examined fungal phytochromes are conserved at the chromophore-binding cysteine site (Cys-294 in N. crassa PHY-1) in the PAS domain. However, additional cysteine sites in the GAF and PHY domains are conserved among phy-1 and phy-1/fphA homologs, whereas the PHY-2 amino acid sequence features a glycine or serine instead of cysteine at two of these sites (Cys-580 and Cys-651). The conserved cysteine sites at key locations within phytochromes are critical for chromophore binding and function (28, 29). Cyanobacterial photosensors that have evolved insertions of additional cysteines within the GAF domain all share a ground-state absorbance of near-UV to blue light and a common mechanism of light perception (79). Absorption spectra for phy-1 and phy-2 are needed to test if additional cysteines in the GAF domain might also restrict the photosensory range of phy-1 to shorter wavelengths of visible light in N. crassa. Since the blue light sensor WCC is known to play multiple roles in Neurospora circadian biology, asexual development and sexual development (8, 16, 22), including binding to the phy-1 promoter region (19), an additional response mediated by phy-1 and by blue light would be complex to characterize. Accordingly, we observed inconsistent precociousness of sexual development among Δphy-1 knockout strains during development under blue light. The phenotype of the Δphy-2 strain was more perceptible under red light than under blue or white light; both complexities of phenotype imply the existence of diverse regulatory interactions affecting the asexual-sexual switch.
Repression of sexual development in N. crassa in response to reception of light by phytochromes is probably a secondary or fine-tuning regulatory mechanism: production of perithecia is not fully suppressed in wild-type strains even under intense white light under laboratory conditions. Accordingly, while phytochromes are functioning to sense changes in light and to ensure that protoperithecial development begins under appropriate conditions, other environmental factors and interactions can significantly influence sexual development. Intriguingly, phy-1 and phy-2 knockout mutants exhibited a wild-type phenotype when cultured under intense white light, implying that under natural conditions, initiation of sexual development is regulated by an interaction of light sensors that are sensitive to different components of the spectrum. As evidence of even further complexity, the phy-1 phy-2 double knockout exhibited a wild-type phenotype cultured under blue, red, and white light, so that the consonant effects of Δphy-1 and Δphy-2 are antagonistic rather than synergistic with regard to the asexual-sexual life history decision. Since both the asexual development and sexual development of N. crassa are stimulated by different light conditions, further investigation of the absorbance spectrum and gene interactions of phy-1 and phy-2 would facilitate investigation of the regulatory interactions mediated by different light sensors. Detailed sensing of environmental factors likely helps fungal species to precisely program developmental regulation of life history decisions.
The examination of the function and evolution of phytochromes in ascomycetes provides additional insight into how fungi adapt and respond to environmental signals. In Aspergillus nidulans, fphA mutants exhibited an increase in the number of sexual fruiting bodies compared to the wild type under white light but not in the dark (38). Unlike N. crassa, which requires light during late sexual development, Aspergillus reproduces sexually underground in darkness. The function of fphA in repressing sexual reproduction is considered an adaptation to Aspergillus ecology (5). Catlett et al. (32) reported no phytochromes in early lineages in Ascomycota. We did not find phytochromes in any available yeast genomes in the Saccharomycotina, but we identified a phytochrome in Taphrina and in the yeast Saitoella, deeply diverging ascomycetous lineages in Taphrinomycotina. A very short phytochrome was found in the largest sequenced ascomycete genome, that of Tuber melanosporum (80), an underground mycorrhizal species in Pezizomycetes. Interestingly, aquatic fungus Loramyces juncicola, which grows on submerged substrates, has eight phytochrome-like proteins—the largest number observed in genomes examined here.
Plant pathogens Sclerotinia sclerotiorum and Botrytis cinerea are very closely related, yet show divergent regulation of life history regarding sporulation type: while S. sclerotiorum produces only ascospores and not conidia, B. cinerea is dispersed mainly via conidia (81). Both pathogens can survive during winter in the form of sclerotia, from which sexual apothecia or conidiophores arise, extend beyond covering duff or soil, and then release sexual or asexual spores to attack budding and blooming plants. In contrast to Aspergillus and Neurospora, asexual sporulation in B. cinerea is inhibited by blue light but can be promoted by far-red light, and sexual development of S. sclerotiorum is regulated by light in a narrow range of UV to blue light (36, 82). Furthermore, the stipe bearing the apothecia of S. sclerotiorum exhibits phototropism in red light that is not essential for apothecial development (82), and stipe morphology appears to be regulated in part by a CRY-DASH type photolyase/cryptochrome (83). Two to three phytochromes are found in B. cinerea and S. sclerotiorum, which exhibit evidence of domain losses and substitutions at key functional sites, including a histidine-to-alanine change at the histidine kinase-related domain in the S. sclerotiorum homolog of PHY-2. Disruption of Bcphy3 in B. cinerea (one of the two orthologs of N. crassa phy-2) led to a significant reduction in growth, production of sclerotia, and pathogenicity (84). Although only a single phytochrome (a Phy-1 homolog) is identified in pathogenic Fusarium, expression of this phytochrome gene in two Fusarium species exhibited upregulation during the late sexual reproduction, as seen in phy-2 in N. crassa. There has been no report of the effects of red light on Fusarium development and pathogenicity. Measurement of the absorption spectrum and better identification of the roles of conserved phy-1 in N. crassa would be helpful for investigation of the function of the single phy-1 homolog in Fusarium, and further study of the function and evolution of phytochromes in fungi such as Aspergillus, Sclerotinia, Botrytis, and Neurospora has great potential to reveal the genetic basis of sexual development in response to light perception by rapidly and slowly evolving copies of red light sensors in ascomycetous fungi, including diverse and destructive plant pathogens.
MATERIALS AND METHODS
Strains and growth conditions.
To examine the strains and growth conditions for genome-wide profiles of gene expression during asexual and sexual development, the N. crassa strains FGSC4200 (mat a) and FGSC2489 (mat A) were provided by the Fungal Genetic Stock Center. The conditions for growth were as described in detail elsewhere (60, 61). For assays of gene expression across asexual development, the strains were grown on Bird medium (85). Assays of gene expression across perithecial development were conducted on both synthetic crossing medium (SCM) and carrot agar (86).
Expression of phytochromes in asexual and sexual development.
Expression of phytochromes in asexual and sexual development and genome-wide gene expression in Neurospora and Fusarium species were assessed using microarrays and RNA sequencing (Table 1). LOX v1.6 (87) and BAGEL (88, 89) were used to estimate gene expression levels across the time courses of this study.
Assessment of phenotypes of the knockout mutants.
To assess the phenotypes of the knockout mutants, N. crassa gene knockouts were acquired from the Fungal Genetic Stock Center, including knockout strains FGSC11235 (mat a) and FGSC11236 (mat A) for Δphy-1 and FGSC11240 (mat a) and FGSC11241 (mat A) for Δphy-2. A double-knockout mat a strain, 560-8 (phy-1::hph; phy-2::hph), was generated for a previous study (7) and was investigated in this study as well. All KO strains used in this study were produced and verified by Southern blotting (90). Verification of the genotype of the Δphy-1 and Δphy-2 gene deletion mutants was performed by PCR-based genotyping (see Fig. S8 in the supplemental material), following a highly accurate methodology (91, 92).
Gene expression phenotypes of the KO strains.
To determine the gene expression phenotypes of the KO strains, data from 40 microarrays originally reported by Chen et al. (7) and available from the GEO database (GSE8932) were reanalyzed. These data included measurements for the wild-type, Δphy-1, Δphy-2, and Δphy-1 Δphy-2 double-knockout strains at all sample points. The signal intensities of MRAT (the median of the set of background-corrected single-pixel ratios for all pixels with the spot) were compared between the reference and samples, and the ratios were used as input for a Bayesian analysis of gene expression levels (BAGEL) (88, 89). Data from serial data points were combined and treated as replicates for four different light conditions, including constant dark (“DD” [as in reference 7]), short light exposure (1,200 lx provided with cool white fluorescent bulbs) exposure up to 30 min (LL5, -10, -15, and 30), medium light exposure from 45 to 90 min (LL45, -60, and -90), and long light exposure from 120 to 240 min (LL120 and -240). BAGEL output was then further analyzed to identify genes with significant changes in expression among different samples, as well as to identify gene enrichment patterns for different treatments on different genotypes, using the Functional Catalogue annotation scheme (93).
Developmental phenotypes of the KO strains.
Using developmental phenotypes of the KO strains to screen for developmental phenotypes of phytochromes, crosses were prepared between opposite mating types on synthetic complete medium (SCM [94]). All permutations of crosses were performed in triplicate, and fungi were cultured at room temperature (25°C) under five light conditions, including complete darkness, constant white light provided by Ecolux bulbs with two intensity settings of 14 µmol/m2/s and about 700 µmol/m2/s, respectively, and constant red light (670 nm), far-red light (735 nm), and constant blue light (470 nm), which was provided by an E-30LED incubator (Percival Scientific) as the sole light source with a high intensity of 450 µmol/m2/s. Abundance of protoperithecia was assayed using a 100 by 100 by 15-mm gridded petri dish (Fisher Scientific). Plates with 40 ml SCM seeded with 3,000 conidia (hemocytometer counts) were incubated for 6 days. Numbers of protoperithecia were recorded for four blocks in the center of each plate, each 13 by 13 mm2, with two biological replicates. All comparisons were based on mat A strains. Under the blue light condition for all tested strains, protoperithecia were produced in unevenly distributed patches and were concealed under dense aggregations of conidia: thus, we elected not to attempt to count protoperithecia under this condition. Development of protoperithecia and perithecia was examined to reveal the impact of light on sexual development between wild-type and KO strains. Opposite mating types were inoculated on opposite sides of small plates (9 cm in diameter) or in alternate quadrants of larger plates (14 cm in diameter).
Complementation of the knockout mutants.
For complementation of the knockout mutants, cosegregation experiments were performed to ensure that the intended deletion is responsible for the mutant phenotype (95, 96). A hygromycin resistance cassette at the location of the deletion mutation provides a selectable marker (97). To assess cosegregation, the mat A Δphy-1 and Δphy-2 KO strains were crossed with a wild-type strain (FGSC4200 mat a). Thirty individual ascospore progenies were isolated and tested for resistance to hygromycin and genotyped by PCR to confirm the existence of phy-1 and phy-2 in hygromycin-resistant strains. Their phenotypes were then examined when cultured on SCM under red and blue light. Cosegregation of hygromycin resistance and the observed phenotype constitutes evidence that the observed phenotype was a result of the deletion of the specified gene.
Molecular evolution analyses.
For molecular evolution analyses, multiple BLAST searches were performed against the NCBI and JGI databases (98) for homologs of N. crassa PHY-1 (NCU04834) and PHY-2 (NCU05790) in other fungal genomes. Bacterial phytochromes were downloaded to root the phylogeny (5). A set of 116 unique amino acid sequences from major groups of Ascomycetes was assembled from 302 phytochrome-like proteins acquired from protein BLAST searches (minimum length of 700 amino acids and sequence similarity above 40%). To confirm our finding of no phytochrome-like proteins in the Saccharomycotina yeasts, a more relaxed search (minimum length of 300 amino acids and sequence similarity above 20%) was performed against all available yeast genomes in Saccharomycotina. Amino acid sequences were aligned using SATé-II (99) with MAFFT as the aligner, MUSCLE as the merger, and RAxML as the tree estimator under the WAG model. The robustness of branching topologies was estimated with 1,000 maximum likelihood searches of bootstrapped sequence data using PhyML (100) under the WAG model. Robustness of the topology was further confirmed by comparison to phylogenetic analyses applying parsimony and distance criteria, performed with PAUP* 4.0 (101), as well as Bayesian analyses using MrBayes 3.2 (102). Branches with bootstrap proportions (BP) higher than 85% or posterior probability (PP) higher than 0.98 were considered significantly supported. A smaller data set that included homologs of Phy-1 and Phy-2 in species closely related to Neurospora and homologs of FphA in species closely related to Aspergillus was prepared for further analyses. ModelTest (68) was used to select an appropriate model of nucleotide substitution for the fungal phytochromes, and the relative ratio test in HyPhy (69) was applied to evaluate differences in substitution rates between lineages of interest. Synonymous and nonsynonymous mutation rates of fungal phytochromes were calculated with CODEML of PAML (70, 71), and substitution saturation was estimated with index of substitution saturation analysis (72). Evolutionary conservation in the sequence and structure of each domain in phytochromes was calculated by Bayesian inference, specifying a WAG substitution model with ConSeq at the ConSurf (103) online server (104, 105).
SUPPLEMENTAL MATERIAL
Expression level of phytochrome genes phy-1 and phy-2, measured by transcriptomic sequencing in Neurospora and Fusarium species during sexual development from protoperithecium (0 h) to fully matured perithecia (144 h after crossing) on carrot agar (63, 64). Expression levels are presented as fold change relative to the lowest time point, which was normalized to 1. Error bars represent 95% confidence intervals. (A) N. tetrasperma. (B) N. discreta. (C) Only phy-1 is present in Fusarium graminearum (fg) and F. vercillioides (fv). Download
Expression level of genes’ responses to blue light, measured by transcriptomic sequencing in N. crassa during sexual development from protoperithecium (0 h) to fully matured perithecia (144 h after crossing) on carrot agar (62). Expression levels are presented as fold change relative to the lowest time point, which was normalized to 1. Genes are color coded, and error bars of 95% confidence intervals are too small to exhibit. Download
Knockout mutants of phytochromes affected expression levels genome-wide. Expression levels were estimated from published microarray results (7) using BAGEL (see Table S1 in the supplemental material). Inferences of expression were made for constant darkness (D), short light exposure (S [incorporating time points from 10, 15, and 30 min]), medium light exposure (M [incorporating time points from 45 and 60 to 90 min]), and long light exposure (L [incorporating time points from 120 to 240 min]) for genotypes of the wild-type, Δphy-1, Δphy-2, and Δphy-1 Δphy-2 strains. (A) Number of genes (y axis) showing a significant difference in expression level (P < 0.05) between genotypes under each light condition. (B) Number of genes showing a significant difference in expression (P < 0.05), including both higher and lower expression, between genotypes under different light conditions. Download
Crosses between Δphy-1 and Δphy-2 strains of N. crassa and sexual development under red light. Protoperithecia and perithecia were apparently more abundant on the phy-2 sides along the crossing zones. Cultures were photographed 6 days after inoculation. (A) View from above the culture plate. (B) View through the bottom of the plate. Download
Impacts of light on different N. crassa cultures. (A) Crosses and sexual development in the N. crassa wild type on SCM and carrot medium under five different light conditions. While both media showed similar impacts on sexual development under different light conditions, morphological development is observed much easier on SCM plates. Incidentally, the finding that the phenotype of a sexual development-related gene does not change between carrot agar and SCM is consistent with the finding that developmental gene expression does not change much between these media (61). (B) Crosses were performed between wild-type mat A and mat a strains. From left to right, every triplet of crossing was under complete dark, constant white light, and red light conditions separately. Cultures were photographed 6 days after inoculation. (C) Noisy phenotypes of phytochrome knockouts under the blue light condition. Triplet crosses between phy-1 and phy-2 knockout strains and between knockout and wild-type strains of N. crassa and sexual development under blue light. The culture was photographed 6 days after inoculation. Protoperithecia and perithecia were slightly more abundant on the phy-1 sides along the crossing zones. (D) Three representative images (of the 30 hygromycin-resistant progeny from a WT × Δphy-2 strain cross on hygromycin SCM plates cultured under red light) demonstrate cosegregation of the removal of inhibition to initiation of sexual development and deletion of phy-2. Download
Phylogeny of phytochromes in ascomycetes. Amino acid sequences were achieved from JGI fungal genome databases from eight major classes of ascomycetes (see Table S2C in the supplemental material), and homologs were color coded for phy-1 (red), phy-2 (green), and fphA (blue). The alignment has 116 sequences and 2,738 aligned positions. (A) Fifty percent majority consensus bootstrap tree based on 1,000 replicates of heuristic search for maximum parsimony trees using PAUP 4.0*. There were no significant conflicts in topology and branch support among maximum parsimony and neighbor joining analyses using PAUP 4.0* and maximum likelihood analyses using RAxML 8 (substitution matrix WAG with fixed base frequencies). (B) Consensus tree based on the last 8,000 trees sampled per 1,000 generations in Bayesian analyses using MrBayes 3.2 (2 runs, 10,000,000 generations, 4 chains, and mixed state frequencies with substitution rates). Branches with a Bayesian posterior probability higher than 98% are marked with *. Download
Maximum likelihood phylogeny of sensory and output modules of ascomycetous phytochromes, rooted with basidiomycetous phytochromes. Strongly supported branches (BP of >85%) are represented by thick lines. Weakly supported branches (BP of <70%) are collapsed. Species with multiple copies of phytochromes are indicated by stars. Segments of several extremely long branches were deleted and replaced with “—//—” to optimize figure legibility. (A) Sensory module, including the PAS, GAF, and PHY domains. (B) Output module, including the HK and RR domains. Download
The presence of the hph cassette in the target locus of the Δphy-1 and Δphy-2 strains was verified by PCR genotyping. The existence of targeting genes was verified by PCR products of primer pairs covering internal region of the gene (phy-1 or phy-2) and covering the 3′ and 5′ ends of the gene and its fringe regions. Correct replacements of targeting genes by the hph cassette are verified by PCR products of primers covering the 3′ and 5′ ends of the hph cassette and fringe regions of targeting genes. The crossing background of mus-51 and/or mus-52 was checked for knockout strains, and the gene coding for actin was used as a standard reference. Download
Expression level was estimated from microarray results in the 2009 article by Chen et al. (7) using BAGEL. Inferences of expression were made for constant darkness (D), short light exposure (S [incorporating time points from 10, 15, and 30 min]), medium light exposure (M [incorporating time points from 45, 60, and 90 min]), and long light exposure (L [incorporating time points from 120 and 240 min]) for genotypes of wild-type, Δphy-1, Δphy-2, and Δphy-1 Δphy-2 strains.
Supplemental information for gene expression and gene identification used in this study. (A) Functional enrichment analysis for genes significantly differentially expressed between genotypes under various light conditions, based on results in Table S1 in the supplemental material. (B) GenBank accession numbers for sequences analyzed and presented in Fig. 4. (C) JGI protein identifications for proteins analyzed and presented in Fig. S6 in the supplemental material.
ACKNOWLEDGMENTS
We are grateful to the Neurospora community and FGSC for making KO strains available for this study. We thank, I. Grigoriev, F. Martin, P. Dyer, P. Crittenden, D. Archer, M. Binder, F. Lutzoni, D. Armaleo, and J. K. Magnuson for providing access to unpublished genome data produced by the U.S. Department of Energy Joint Genome Institute, a DOE Office of Science User Facility, supported under contract no. DE-AC02-05CH11231. We also thank D. O. Natvig, D. J. Jacobson, N. L. Glass, and J. W. Taylor, who wrote the community-based fungal genome sequencing proposal (JGI CSP 777450) and pioneered sequencing and sharing of fungal genome data within the community.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Wang Z, Li N, Li J, Dunlap JC, Trail F, Townsend JP. 2016. The fast-evolving phy-2 gene modulates sexual development in response to light in the model fungus Neurospora crassa. mBio 7(2):e02148-15. doi:10.1128/mBio.02148-15.
REFERENCES
- 1.Wang Z, Johnston PR, Yang ZL, Townsend JP. 2009. Evolution of reproductive morphology in leaf endophytes. PLoS One 4:e4246. doi: 10.1371/journal.pone.0004246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heintzen C. 2012. Plant and fungal photopigments. Wiley Interdiscip Rev Membr Transp Signal 1:1–22. doi: 10.1002/wmts.36. [DOI] [Google Scholar]
- 3.Purschwitz J, Müller S, Kastner C, Fischer R. 2006. Seeing the rainbow: light sensing in fungi. Curr Opin Microbiol 9:566–571. doi: 10.1016/j.mib.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Fischer R. 2008. Developmental biology. Sex and poison in the dark. Science 320:1430–1431. doi: 10.1126/science.1160123. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Romero J, Hedtke M, Kastner C, Müller S, Fischer R, Gottesman S, Harwood C. 2010. Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol 64:585–610. doi: 10.1146/annurev.micro.112408.134000. [DOI] [PubMed] [Google Scholar]
- 6.Idnurm A, Heitman J. 2005. Photosensing fungi: phytochrome in the spotlight. Curr Biol 15:R829–R832. doi: 10.1016/j.cub.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Chen CH, Ringelberg CS, Gross RH, Dunlap JC, Loros JJ. 2009. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J 28:1029–1042. doi: 10.1038/emboj.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CH, Dunlap JC, Loros JJ. 2010. Neurospora illuminates fungal photoreception. Fungal Genet Biol 47:922–929. doi: 10.1016/j.fgb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrochano LM. 2007. Fungal photoreceptors: sensory molecules for fungal development and behaviour. Photochem Photobiol Sci 6:725–736. doi: 10.1039/b702155k. [DOI] [PubMed] [Google Scholar]
- 10.Lee SS, Choi AR, Kim SY, Kang HW, Jung KH, Lee JH. 2011. Acetabularia rhodopsin I is a light-stimulated proton pump. J Nanosci Nanotechnol 11:4596–4600. [DOI] [PubMed] [Google Scholar]
- 11.Wu C, Yang F, Smith KM, Peterson M, Dekhang R, Zhang Y, Zucker J, Bredeweg EL, Mallappa C, Zhou X, Lyubetskaya A, Townsend JP, Galagan JE, Freitag M, Dunlap JC, Bell-Pedersen D, Sachs MS. 2014. Genome-wide characterization of light-regulated genes in Neurosproa crassa. G3 4:1731–1745. doi: 10.1534/g3.114.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller KK, Loros JJ, Dunlap JC. 2015. Fungal photobiology: visible light as a signal for stress, space and time. Curr Genet 61:275–288. doi: 10.1007/s00294-014-0451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedtke M, Rauscher S, Röhrig J, Rodríguez-Romero J, Yu Z, Fischer R. 2015. Light-dependent gene activation in Aspergillus nidulans is strictly dependent on phytochrome and involves the interplay of phytochrome and White Collar-regulated histone H3 acetylation. Mol Microbiol 97:733–745. doi: 10.1111/mmi.13062. [DOI] [PubMed] [Google Scholar]
- 14.Tisch D, Schmoll M. 2010. Light regulation of metabolic pathways in fungi. Appl Microbiol Biotechnol 85:1259–1277. doi: 10.1007/s00253-009-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X, Liu H, Klejnot J, Lin C. 2010. The cryptochrome blue light receptors. Arabidopsis Book 8:e0135. doi: 10.1199/tab.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlap JC, Loros JJ. 2004. The Neurospora circadian system. J Biol Rhythms 19:414–424. doi: 10.1177/0748730404269116. [DOI] [PubMed] [Google Scholar]
- 17.Heintzen C, Liu Y. 2007. The Neurospora crassa circadian clock. Adv Genet 58:25–66. doi: 10.1016/S0065-2660(06)58002-2. [DOI] [PubMed] [Google Scholar]
- 18.Belozerskaya TA, Gessler NN, Isakova EP, Deryabina YI. 2012. Neurospora crassa light signal transduction is affected by ROS. J Signal Transduct 2012:791963, doi: 10.1155/2012/791963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KM, Sancar G, Dekhang R, Sullivan CM, Li S, Tag AG, Sancar C, Bredeweg EL, Priest HD, McCormick RF, Thomas TL, Carrington JC, Stajich JE, Bell-Pedersen D, Brunner M, Freitag M. 2010. Transcription factors in light and circadian clock signaling networks revealed by genomewide mapping of direct targets for Neurospora White Collar Complex. Eukaryot Cell 9:1549–1556. doi: 10.1128/EC.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding RW, Turner RV. 1981. Photoregulation of the carotenoid biosynthetic pathway in albino and White Collar mutants of Neurospora crassa. Plant Physiol 68:745–749. doi: 10.1104/pp.68.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding RW, Melles S. 1983. Genetic analysis of phototropism of Neurospora crassa perithecial beaks using White Collar and albino mutants. Plant Physiol 72:996–1000. doi: 10.1104/pp.72.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degli-Innocenti F, Russo VE. 1984. Isolation of new white collar mutants of Neurospora crassa and studies on their behavior in the blue light-induced formation of protoperithecia. J Bacteriol 159:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olmedo M, Ruger-Herreros C, Luque EM, Corrochano LM. 2010. A complex photoreceptor system mediates the regulation by light of the conidiation genes con-10 and con-6 in Neurospora crassa. Fungal Genet Biol 47:352–363. doi: 10.1016/j.fgb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Innocenti FD, Pohl U, Russo VEA. 1983. Photoinduction of protoperithecia in Neurospora crassa by blue light. Photochem Photobiol 37:49–51. doi: 10.1111/j.1751-1097.1983.tb04432.x. [DOI] [PubMed] [Google Scholar]
- 25.Lauter F, Yamashiro CT, Yanofsky C. 1997. Light stimulation of conidiation in Neurospora crassa: studies with the wild-type strain and mutants Wc-1, Wc-2 and acon-2. J Photochem Photobiol B 37:203–211. doi: 10.1016/S1011-1344(96)07405-2. [DOI] [Google Scholar]
- 26.Tan Y, Merrow M, Roenneberg T. 2004. Photoperiodism in Neurospora crassa. J Biol Rhythms 19:135–143. doi: 10.1177/0748730404263015. [DOI] [PubMed] [Google Scholar]
- 27.Waschuk SA, Bezerra AG, Shi L, Brown LS. 2005. Leptosphaeria rhodopsin: bacteriorhodopsin-like proton pump from a eukaryote. Proc Natl Acad Sci U S A 102:6879–6883. doi: 10.1073/pnas.0409659102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auldridge ME, Forest KT. 2011. Bacterial phytochromes: more than meets the light. Crit Rev Biochem Mol Biol 46:67–88. doi: 10.3109/10409238.2010.546389. [DOI] [PubMed] [Google Scholar]
- 29.Wagner JR, Zhang J, Brunzelle JS, Vierstra RD, Forest KT. 2007. High resolution structure of Deinococcus bacteriophytochrome yields new insights into phytochrome architecture and evolution. J Biol Chem 282:12298–12309. doi: 10.1074/jbc.M611824200. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Li G, Wang H, Wang Deng X. 2011. Phytochrome signaling mechanisms. Arabidopsis Book 9:e0148. doi: 10.1199/tab.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galvão RM, Li M, Kothadia SM, Haskel JD, Decker PV, Van Buskirk EK, Chen M. 2012. Photoactivated phytochromes interact with HEMERA and promote its accumulation to establish photomorphogenesis in Arabidopsis. Genes Dev 26:1851–1863. doi: 10.1101/gad.193219.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catlett NL, Yoder OC, Turgeon BG. 2003. Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot Cell 2:1151–1161. doi: 10.1128/EC.2.6.1151-1161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purschwitz J, Müller S, Fischer R. 2009. Mapping the interaction sites of Aspergillus nidulans phytochrome FphA with the global regulator VeA and the White Collar protein LreB. Mol Genet Genomics 281:35–42. doi: 10.1007/s00438-008-0390-x. [DOI] [PubMed] [Google Scholar]
- 34.Jones CA, Greer-Phillips SE, Borkovich KA. 2007. The response regulator RRG-1 functions upstream of a mitogen-activated protein kinase pathway impacting asexual development, female fertility, osmotic stress, and fungicide resistance in Neurospora crassa. Mol Biol Cell 18:2123–2136. doi: 10.1091/mbc.E06-03-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan KK. 1975. Recovery from blue-light inhibition of sporulation in Botrytis cinerea. Trans Br Mycol Soc 64:223–228. doi: 10.1016/S0007-1536(75)80106-9. [DOI] [Google Scholar]
- 36.Tan KK. 1975. Interaction of near-ultraviolet, blue, red, and far-red light in sporulation of Botrytis cinerea. Trans Br Mycol Soc 64:215–222. doi: 10.1016/S0007-1536(75)80105-7. [DOI] [Google Scholar]
- 37.Froehlich AC, Noh B, Vierstra RD, Loros J, Dunlap JC. 2005. Genetic and molecular analysis of phytochromes from the filamentous fungus Neurospora crassa. Eukaryot Cell 4:2140–2152. doi: 10.1128/EC.4.12.2140-2152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg-Dinkel N, Fischer R. 2005. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol 15:1833–1838. doi: 10.1016/j.cub.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 39.Brandt S, von Stetten D, Günther M, Hildebrandt P, Frankenberg-Dinkel N. 2008. The fungal phytochrome FphA from Aspergillus nidulans. J Biol Chem 283:34605–34614. doi: 10.1074/jbc.M805506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purschwitz J, Müller S, Kastner C, Schöser M, Haas H, Espeso EA, Atoui A, Calvo AM, Fischer R. 2008. Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Curr Biol 18:255–259. doi: 10.1016/j.cub.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 41.O’Gorman CM, Fuller H, Dyer PS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474. doi: 10.1038/nature07528. [DOI] [PubMed] [Google Scholar]
- 42.Dyer PS, O’Gorman CM. 2011. A fungal sexual revolution: Aspergillus and Penicillium show the way. Curr Opin Microbiol 14:649–654. doi: 10.1016/j.mib.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Böhm J, Hoff B, O’Gorman CM, Wolfers S, Klix V, Binger D, Zadra I, Kürnsteiner H, Pöggeler S, Dyer PS, Kück U. 2013. Sexual reproduction and mating-type-mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. Proc Natl Acad Sci U S A 110:1476–1481. doi: 10.1073/pnas.1217943110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayram O, Braus GH, Fischer R, Rodriguez-Romero J. 2010. Spotlight on Aspergillus nidulans photosensory systems. Fungal Genet Biol 47:900–908. doi: 10.1016/j.fgb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Röhrig J, Kastner C, Fischer R. 2013. Light inhibits spore germination through phytochrome in Aspergillus nidulans. Curr Genet 59:55–62. doi: 10.1007/s00294-013-0387-9. [DOI] [PubMed] [Google Scholar]
- 46.Fuller KK, Ringelberg CS, Loros JJ, Dunlap JC. 2013. The fungal pathogen Aspergillus fumigatus regulates growth, metabolism, and stress resistance in response to light. mBio 4:e00142-13. doi: 10.1128/mBio.00142-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu L, Wang JJ, Chu ZJ, Ying SH, Feng MG. 2014. Phytochrome controls conidiation in response to red/far-red light and daylight length and regulates multistress tolerance in Beauveria bassiana. Environ Microbiol 16:2316–2328. doi: 10.1111/1462-2920.12486. [DOI] [PubMed] [Google Scholar]
- 48.Borkovich KA, Alex LA, Yarden O, Freitag M, Turner GE, Read ND, Seiler S, Bell-Pedersen D, Paietta J, Plesofsky N, Plamann M, Goodrich-Tanrikulu M, Schulte U, Mannhaupt G, Nargang FE, Radford A, Selitrennikoff C, Galagan JE, Dunlap JC, Loros JJ, Catcheside D, Inoue H, Aramayo R, Polymenis M, Selker E, Sachs M, Marzluf G, Paulsen I, Davis R, Ebbole D, Zelter A, Kalkman E, O’Rourke R, Bowring F, Yeadon J, Ishii C, Suzuki K, Sakai W, Pratt R. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev 68:1–108. doi: 10.1128/MMBR.68.1.1-108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samils N, Gioti A, Karlsson M, Sun Y, Kasuga T, Bastiaans E, Wang Z, Li N, Townsend JP, Johannesson H. 2013. Sex-linked transcriptional divergence in the hermaphrodite fungus Neurospora tetrasperma. Proc Biol Sci 280:20130862. doi: 10.1098/rspb.2013.0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H, Nelson MA. 2005. Molecular and functional analyses of poi-2, a novel gene highly expressed in sexual and perithecial tissues of Neurospora crassa. Eukaryot Cell 4:900–910. doi: 10.1128/EC.4.5.900-910.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H, Wright SJ, Park G, Ouyang S, Krystofova S, Borkovich KA. 2012. Roles for receptors, pheromones, G proteins, and mating type genes during sexual reproduction in Neurospora crassa. Genetics 190:1389–1404. doi: 10.1534/genetics.111.136358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li D, Bobrowicz P, Wilkinson HH, Ebbole DJ. 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170:1091–1104. doi: 10.1534/genetics.104.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gladieux P, Wilson BA, Perraudeau F, Montoya LA, Kowbel D, Hann-Soden C, Fischer M, Sylvain I, Jacobson DJ, Taylor JW. 2015. Genomic sequencing reveals historical, demographic and selective factors associated with the diversification of the fire-associated fungus Neurospora discreta. Mol Ecol 24:5657–5675. doi: 10.1111/mec.13417. [DOI] [PubMed] [Google Scholar]
- 54.Ellison CE, Stajich JE, Jacobson DJ, Natvig DO, Lapidus A, Foster B, Aerts A, Riley R, Lindquist EA, Grigoriev IV, Taylor JW. 2011. Massive changes in genome architecture accompany the transition to self-fertility in the filamentous fungus Neurospora tetrasperma. Genetics 189:55–69. doi: 10.1534/genetics.111.130690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raju NB. 2009. Neurospora as a model fungus for studies in cytogenetics and sexual biology at Stanford. J Biosci 34:139–159. doi: 10.1007/s12038-009-0015-5. [DOI] [PubMed] [Google Scholar]
- 56.Springer ML. 1993. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. Bioessays 15:365–374. doi: 10.1002/bies.950150602. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson DJ, Powell AJ, Dettman JR, Saenz GS, Barton MM, Hiltz MD, Dvorachek WH Jr, Glass NL, Taylor JW, Natvig DO. 2004. Neurospora in temperate forests of western North America. Mycologia 96:66–74. doi: 10.2307/3761989. [DOI] [PubMed] [Google Scholar]
- 58.Pandit A, Maheshwari R. 1996. Life-history of Neurospora intermedia in a sugar cane field. J Biosci 21:57–79. doi: 10.1007/BF02716813. [DOI] [Google Scholar]
- 59.Rothschild H, Suskind SR. 1966. Protoperithecia in Neurospora crassa: technique for studying their development. Science 154:1356–1357. doi: 10.1126/science.154.3754.1356. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Kin K, López-Giráldez F, Johannesson H, Townsend JP. 2012. Sex-specific gene expression during asexual development of Neurospora crassa. Fungal Genet Biol 49:533–543. doi: 10.1016/j.fgb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Lehr N, Trail F, Townsend JP. 2012. Differential impact of nutrition on developmental and metabolic gene expression during fruiting body development in Neurospora crassa. Fungal Genet Biol 49:405–413. doi: 10.1016/j.fgb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Lopez-Giraldez F, Lehr N, Farré M, Common R, Trail F, Townsend JP. 2014. Global gene expression and focused knockout analysis reveals genes associated with fungal fruiting body development in Neurospora crassa. Eukaryot Cell 13:154–169. doi: 10.1128/EC.00248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehr NA, Wang Z, Li N, Hewitt DA, López-Giráldez F, Trail F, Townsend JP. 2014. Gene expression differences among three Neurospora species reveal genes required for sexual reproduction in Neurospora crassa. PLoS One 9:e110398. doi: 10.1371/journal.pone.0110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sikhakolli UR, López-Giráldez F, Li N, Common R, Townsend JP, Trail F. 2012. Transcriptome analyses during fruiting body formation in Fusarium graminearum and Fusarium verticillioides reflect species life history and ecology. Fungal Genet Biol 49:663–673. doi: 10.1016/j.fgb.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 66.Krystofova S, Borkovich KA. 2006. The predicted G-protein-coupled receptor GPR-1 is required for female sexual development in the multicellular fungus Neurospora crassa. Eukaryot Cell 5:1503–1516. doi: 10.1128/EC.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwerdtfeger C, Linden H. 2001. Blue light adaptation and desensitization of light signal transduction in Neurospora crassa. Mol Microbiol 39:1080–1087. doi: 10.1046/j.1365-2958.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 68.Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 69.Pond SL, Frost SD, Muse SV. 2005. HyPhy: hypothesis testing using phylogenies. Bioinformatics 21:676–679. doi: 10.1093/bioinformatics/bti079. [DOI] [PubMed] [Google Scholar]
- 70.Nei M, Gojobori T. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol 3:418–426. [DOI] [PubMed] [Google Scholar]
- 71.Yang Z, Nielsen R. 2000. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol 17:32–43. doi: 10.1093/oxfordjournals.molbev.a026236. [DOI] [PubMed] [Google Scholar]
- 72.Xia X, Xie Z, Salemi M, Chen L, Wang Y. 2003. An index of substitution saturation and its application. Mol Phylogenet Evol 26:1–7. doi: 10.1016/S1055-7903(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 73.Taylor JW, Berbee ML. 2006. Dating divergences in the fungal tree of life: review and new analyses. Mycologia 98:838–849. doi: 10.3852/mycologia.98.6.838. [DOI] [PubMed] [Google Scholar]
- 74.Lamparter T, Michael N, Mittmann F, Esteban B. 2002. Phytochrome from Agrobacterium tumefaciens has unusual spectral properties and reveals an N-terminal chromophore attachment site. Proc Natl Acad Sci U S A 99:11628–11633. doi: 10.1073/pnas.152263999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dolinski K, Botstein D. 2007. Orthology and functional conservation in eukaryotes. Annu Rev Genet 41:465–507. doi: 10.1146/annurev.genet.40.110405.090439. [DOI] [PubMed] [Google Scholar]
- 76.Krylov DM, Wolf YI, Rogozin IB, Koonin EV. 2003. Gene loss, protein sequence divergence, gene dispensability, expression level, and interactivity are correlated in eukaryotic evolution. Genome Res 13:2229–2235. doi: 10.1101/gr.1589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynch M, Conery JS. 2000. The evolutionary fate and consequences of duplicate genes. Science 290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 78.He X, Zhang J. 2005. Rapid subfunctionalization accompanied by prolonged and substantial neofunctionalization in duplicate gene evolution. Genetics 169:1157–1164. doi: 10.1534/genetics.104.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rockwell NC, Martin SS, Feoktistova K, Lagarias JC. 2011. Diverse two-cysteine photocycles in phytochromes and cyanobacteriochromes. Proc Natl Acad Sci U S A 108:11854–11859. doi: 10.1073/pnas.1107844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin F, Kohler A, Murat C, Balestrini R, Coutinho PM, Jaillon O, Montanini B, Morin E, Noel B, Percudani R, Porcel B, Rubini A, Amicucci A, Amselem J, Anthouard V, Arcioni S, Artiguenave F, Aury JM, Ballario P, Bolchi A, Brenna A, Brun A, Buee M, Cantarel B, Chevalier G, Couloux A, Da Silva C, Denoeud F, Duplessis S, Ghignone S, Hilselberger B, Iotti M, Marcais B, Mello A, Miranda M, Pacioni G, Quesneville H, Riccioni C, Ruotolo R, Splivallo R, Stocchi V, Tisserant E, Viscomi AR, Zambonelli A, Zampieri E, Henrissat B, Lebrun MH, Paolocci F, Bonfante P, Ottonello S, Wincker P. 2010. Perigord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 464:1033–1038. doi: 10.1038/nature08867. [DOI] [PubMed] [Google Scholar]
- 81.Amselem J, Cuomo CA, van Kan JA, Viaud M, Benito EP, Couloux A, Coutinho PM, de Vries RP, Dyer PS, Fillinger S, Fournier E, Gout L, Hahn M, Kohn L, Lapalu N, Plummer KM, Pradier JM, Quévillon E, Sharon A, Simon A, ten Have A, Tudzynski B, Tudzynski P, Wincker P, Andrew M, Anthouard V, Beever RE, Beffa R, Benoit I, Bouzid O, Brault B, Chen Z, Choquer M, Collemare J, Cotton P, Danchin EG, Da Silva C, Gautier A, Giraud C, Giraud T, Gonzalez C, Grossetete S, Guldener U, Henrissat B, Howlett BJ, Kodira C, Kretschmer M, Lappartient A, Leroch M, Levis C, Mauceli E, Neuveglise C, Oeser B, Pearson M, Poulain J, Poussereau N, Quesneville H, Rascle C, Schumacher J, Segurens B, Sexton A, Silva E, Sirven C, Soanes DM, Talbot NJ, Templeton M, Yandava C, Yarden O, Zeng Q, Rollins JA, Lebrun MH, Dickman M. 2011. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet 7:e1002230. doi: 10.1371/journal.pgen.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thaning C, Nilsson HE. 2000. A narrow range of wavelengths active in regulating apothecial development in Sclerotinia sclerotiorum. J Phytopathol (Berl) 148:627–631. [Google Scholar]
- 83.Veluchamy S, Rollins JA. 2008. A CRY-DASH-type photolyase/cryptochrome from Sclerotinia sclerotiorum mediates minor UV-A-specific effects on development. Fungal Genet Biol 45:1265–1276. doi: 10.1016/j.fgb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 84.Hu YZ, He JY, Wang YW, Zhu PK, Zhang CH, Lu RS, Xu L. 2014. Disruption of a phytochrome-like histidine kinase gene by homologous recombination leads to a significant reduction in vegetative growth, sclerotia production, and the pathogenicity of Botrytis cinerea. Physiol Mol Plant P85:25–33. [Google Scholar]
- 85.Metzenberg RL. 2004. Bird medium: an alternative to Vogel medium. Fungal Genet Newsl 51:19–20. [Google Scholar]
- 86.Klittich C, Leslie JF. 1988. Nitrate reduction mutants of Fusarium moniliforme (Gibberella fujikuroi). Genetics 118:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Z, López-Giráldez F, Townsend JP. 2010. LOX: inferring Level of eXpression from diverse methods of census sequencing. Bioinformatics 26:1918–1919. doi: 10.1093/bioinformatics/btq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Townsend JP. 2004. Resolution of large and small differences in gene expression using models for the Bayesian analysis of gene expression levels and spotted DNA microarrays. BMC Bioinformatics 5:54. doi: 10.1186/1471-2105-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Townsend JP, Hartl DL. 2002. Bayesian analysis of gene expression levels: statistical quantification of relative mRNA level across multiple strains or treatments. Genome Biol 3:RESEARCH0071. doi: 10.1186/gb-2002-3-12-research0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dunlap JC, Borkovich KA, Henn MR, Turner GE, Sachs MS, Glass NL, McCluskey K, Plamann M, Galagan JE, Birren BW, Weiss RL, Townsend JP, Loros JJ, Nelson MA, Lambreghts R, Colot HV, Park G, Collopy P, Ringelberg C, Crew C, Litvinkova L, DeCaprio D, Hood HM, Curilla S, Shi M, Crawford M, Koerhsen M, Montgomery P, Larson L, Pearson M, Kasuga T, Tian C, Basturkmen M, Altamirano L, Xu J. 2007. Enabling a community to dissect an organism: overview of the Neurospora functional genomics project. Adv Genet 57:49–96. doi: 10.1016/S0065-2660(06)57002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lichius A, Lord KM, Jeffree CE, Oborny R, Boonyarungsrit P, Read ND. 2012. Importance of MAP kinases during protoperithecial morphogenesis in Neurospora crassa. PLoS One 7:e42565. doi: 10.1371/journal.pone.0042565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Henegariu O, Heerema NA, Dlouhy SR, Vance GH, Vogt PH. 1997. Multiplex PCR: critical parameters and step-by-step protocol. Biotechniques 23:504–511. [DOI] [PubMed] [Google Scholar]
- 93.Ruepp A, Zollner A, Maier D, Albermann K, Hani J, Mokrejs M, Tetko I, Güldener U, Mannhaupt G, Münsterkötter M, Mewes HW. 2004. The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Res 32:5539–5545. doi: 10.1093/nar/gkh894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark TA, Guilmette JM, Renstrom D, Townsend JP. 2008. RNA extraction, probe preparation, and competitive hybridization for transcriptional profiling using Neurospora crassa long-oligomer DNA microarrays. Fungal Genet Rep 55:18–28. [Google Scholar]
- 95.Fu C, Iyer P, Herkal A, Abdullah J, Stout A, Free SJ. 2011. Identification and characterization of genes required for cell-to-cell fusion in Neurospora crassa. Eukaryot Cell 10:1100–1109. doi: 10.1128/EC.05003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chinnici JL, Fu C, Caccamise LM, Arnold JW, Free SJ. 2014. Neurospora crassa female development requires the PACC and other signal transduction pathways, transcription factors, chromatin remodeling, cell-to-cell fusion, and autophagy. PLoS One 9:e110603. doi: 10.1371/journal.pone.0110603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A 103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nordberg H, Cantor M, Dusheyko S, Hua S, Poliakov A, Shabalov I, Smirnova T, Grigoriev IV, Dubchak I. 2014. The genome portal of the Department of Energy Joint Genome Institute: 2014 updates. Nucleic Acids Res 42:D26–D31. doi: 10.1093/nar/gkt1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu K, Warnow TJ, Holder MT, Nelesen SM, Yu J, Stamatakis AP, Linder CR. 2012. SATe-II: very fast and accurate simultaneous estimation of multiple sequence alignments and phylogenetic trees. Syst Biol 61:90–106. doi: 10.1093/sysbio/syr095. [DOI] [PubMed] [Google Scholar]
- 100.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 101.Wilgenbusch JC, Swofford D. 2003. Inferring evolutionary trees with PAUP*. Curr Protoc Bioinform Chapter 6:Unit 6.4. doi: 10.1002/0471250953.bi0604s00. [DOI] [PubMed] [Google Scholar]
- 102.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. 2010. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res 38:W529–W533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glaser F, Pupko T, Paz I, Bell RE, Bechor-Shental D, Martz E, Ben-Tal N. 2003. ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information. BioInformatics 19:163–164. doi: 10.1093/bioinformatics/19.1.163. [DOI] [PubMed] [Google Scholar]
- 105.Mayrose I, Graur D, Ben-Tal N, Pupko T. 2004. Comparison of site-specific rate-inference methods for protein sequences: empirical Bayesian methods are superior. Mol Biol Evol 21:1781–1791. doi: 10.1093/molbev/msh194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression level of phytochrome genes phy-1 and phy-2, measured by transcriptomic sequencing in Neurospora and Fusarium species during sexual development from protoperithecium (0 h) to fully matured perithecia (144 h after crossing) on carrot agar (63, 64). Expression levels are presented as fold change relative to the lowest time point, which was normalized to 1. Error bars represent 95% confidence intervals. (A) N. tetrasperma. (B) N. discreta. (C) Only phy-1 is present in Fusarium graminearum (fg) and F. vercillioides (fv). Download
Expression level of genes’ responses to blue light, measured by transcriptomic sequencing in N. crassa during sexual development from protoperithecium (0 h) to fully matured perithecia (144 h after crossing) on carrot agar (62). Expression levels are presented as fold change relative to the lowest time point, which was normalized to 1. Genes are color coded, and error bars of 95% confidence intervals are too small to exhibit. Download
Knockout mutants of phytochromes affected expression levels genome-wide. Expression levels were estimated from published microarray results (7) using BAGEL (see Table S1 in the supplemental material). Inferences of expression were made for constant darkness (D), short light exposure (S [incorporating time points from 10, 15, and 30 min]), medium light exposure (M [incorporating time points from 45 and 60 to 90 min]), and long light exposure (L [incorporating time points from 120 to 240 min]) for genotypes of the wild-type, Δphy-1, Δphy-2, and Δphy-1 Δphy-2 strains. (A) Number of genes (y axis) showing a significant difference in expression level (P < 0.05) between genotypes under each light condition. (B) Number of genes showing a significant difference in expression (P < 0.05), including both higher and lower expression, between genotypes under different light conditions. Download
Crosses between Δphy-1 and Δphy-2 strains of N. crassa and sexual development under red light. Protoperithecia and perithecia were apparently more abundant on the phy-2 sides along the crossing zones. Cultures were photographed 6 days after inoculation. (A) View from above the culture plate. (B) View through the bottom of the plate. Download
Impacts of light on different N. crassa cultures. (A) Crosses and sexual development in the N. crassa wild type on SCM and carrot medium under five different light conditions. While both media showed similar impacts on sexual development under different light conditions, morphological development is observed much easier on SCM plates. Incidentally, the finding that the phenotype of a sexual development-related gene does not change between carrot agar and SCM is consistent with the finding that developmental gene expression does not change much between these media (61). (B) Crosses were performed between wild-type mat A and mat a strains. From left to right, every triplet of crossing was under complete dark, constant white light, and red light conditions separately. Cultures were photographed 6 days after inoculation. (C) Noisy phenotypes of phytochrome knockouts under the blue light condition. Triplet crosses between phy-1 and phy-2 knockout strains and between knockout and wild-type strains of N. crassa and sexual development under blue light. The culture was photographed 6 days after inoculation. Protoperithecia and perithecia were slightly more abundant on the phy-1 sides along the crossing zones. (D) Three representative images (of the 30 hygromycin-resistant progeny from a WT × Δphy-2 strain cross on hygromycin SCM plates cultured under red light) demonstrate cosegregation of the removal of inhibition to initiation of sexual development and deletion of phy-2. Download
Phylogeny of phytochromes in ascomycetes. Amino acid sequences were achieved from JGI fungal genome databases from eight major classes of ascomycetes (see Table S2C in the supplemental material), and homologs were color coded for phy-1 (red), phy-2 (green), and fphA (blue). The alignment has 116 sequences and 2,738 aligned positions. (A) Fifty percent majority consensus bootstrap tree based on 1,000 replicates of heuristic search for maximum parsimony trees using PAUP 4.0*. There were no significant conflicts in topology and branch support among maximum parsimony and neighbor joining analyses using PAUP 4.0* and maximum likelihood analyses using RAxML 8 (substitution matrix WAG with fixed base frequencies). (B) Consensus tree based on the last 8,000 trees sampled per 1,000 generations in Bayesian analyses using MrBayes 3.2 (2 runs, 10,000,000 generations, 4 chains, and mixed state frequencies with substitution rates). Branches with a Bayesian posterior probability higher than 98% are marked with *. Download
Maximum likelihood phylogeny of sensory and output modules of ascomycetous phytochromes, rooted with basidiomycetous phytochromes. Strongly supported branches (BP of >85%) are represented by thick lines. Weakly supported branches (BP of <70%) are collapsed. Species with multiple copies of phytochromes are indicated by stars. Segments of several extremely long branches were deleted and replaced with “—//—” to optimize figure legibility. (A) Sensory module, including the PAS, GAF, and PHY domains. (B) Output module, including the HK and RR domains. Download
The presence of the hph cassette in the target locus of the Δphy-1 and Δphy-2 strains was verified by PCR genotyping. The existence of targeting genes was verified by PCR products of primer pairs covering internal region of the gene (phy-1 or phy-2) and covering the 3′ and 5′ ends of the gene and its fringe regions. Correct replacements of targeting genes by the hph cassette are verified by PCR products of primers covering the 3′ and 5′ ends of the hph cassette and fringe regions of targeting genes. The crossing background of mus-51 and/or mus-52 was checked for knockout strains, and the gene coding for actin was used as a standard reference. Download
Expression level was estimated from microarray results in the 2009 article by Chen et al. (7) using BAGEL. Inferences of expression were made for constant darkness (D), short light exposure (S [incorporating time points from 10, 15, and 30 min]), medium light exposure (M [incorporating time points from 45, 60, and 90 min]), and long light exposure (L [incorporating time points from 120 and 240 min]) for genotypes of wild-type, Δphy-1, Δphy-2, and Δphy-1 Δphy-2 strains.
Supplemental information for gene expression and gene identification used in this study. (A) Functional enrichment analysis for genes significantly differentially expressed between genotypes under various light conditions, based on results in Table S1 in the supplemental material. (B) GenBank accession numbers for sequences analyzed and presented in Fig. 4. (C) JGI protein identifications for proteins analyzed and presented in Fig. S6 in the supplemental material.