ABSTRACT
Persistent pathogens, such as herpes simplex virus 1 (HSV-1), have evolved a variety of immune evasion strategies to avoid being detected and destroyed by the host's immune system. A dynamic cross talk appears to occur between the HSV-1 latency-associated transcript (LAT), the only viral gene that is abundantly transcribed during latency, and the CD8+ T cells that reside in HSV-1 latently infected human and rabbit trigeminal ganglia (TG). The reactivation phenotype of TG that are latently infected with wild-type HSV-1 or with LAT-rescued mutant (i.e., LAT+ TG) is significantly higher than TG latently infected with LAT-null mutant (i.e., LAT− TG). Whether LAT promotes virus reactivation by selectively shaping a unique repertoire of HSV-specific CD8+ T cells in LAT+ TG is unknown. In the present study, we assessed the frequency, function, and exhaustion status of TG-resident CD8+ T cells specific to 40 epitopes derived from HSV-1 gB, gD, VP11/12, and VP13/14 proteins, in human leukocyte antigen (HLA-A*0201) transgenic rabbits infected ocularly with LAT+ versus LAT– virus. Compared to CD8+ T cells from LAT– TG, CD8+ T cells from LAT+ TG (i) recognized a broader selection of nonoverlapping HSV-1 epitopes, (ii) expressed higher levels of PD-1, TIM-3, and CTLA-4 markers of exhaustion, and (iii) produced less tumor necrosis factor alpha, gamma interferon, and granzyme B. These results suggest a novel immune evasion mechanism by which the HSV-1 LAT may contribute to the shaping of a broader repertoire of exhausted HSV-specific CD8+ T cells in latently infected TG, thus allowing for increased viral reactivation.
IMPORTANCE A significantly larger repertoire of dysfunctional (exhausted) HSV-specific CD8+ T cells were found in the TG of HLA transgenic rabbits latently infected with wild-type HSV-1 or with LAT-rescued mutant (i.e., LAT+ TG) than in a more restricted repertoire of functional HSV-specific CD8+ T cells in the TG of HLA transgenic rabbits latently infected with LAT-null mutant (i.e., LAT– TG). These findings suggest that the HSV-1 LAT locus interferes with the host cellular immune response by shaping a broader repertoire of exhausted HSV-specific CD8+ T cells within the latency/reactivation TG site.
INTRODUCTION
Following a primary corneal infection, herpes simplex virus 1 (HSV-1) enters the local nerve termini and travels up the axons by retrograde transport to the body of sensory neurons of the trigeminal ganglia (TG), where it establishes lifelong latency (1–4). Recurrent corneal disease results from spontaneous sporadic reactivation of the virus from latently infected sensory neurons of the TG, the anterograde transportation of virus back to nerve termini, and the reinfection of the cornea (5, 6). Virus-specific CD8+ T cells that express an activated effector memory T-cell phenotype are selectively retained in latently infected TG of humans, rabbits, and mice (4, 7–12). These TG-resident CD8+ T cells may control the establishment of HSV-1 latency and prevent virus reactivation from TG (6, 13). Our recent preclinical vaccine studies that used the human leukocyte antigen (HLA-A*0201) transgenic rabbit model of ocular herpes (HLA Tg rabbit) suggest that HSV-1 human epitope-specific CD8+ T cells play a crucial role in reducing virus reactivation from latently infected TG (1, 4, 14). Thus, in latently infected HLA Tg rabbits, TG-resident human epitope-specific CD8+ T cells appear to help control spontaneous HSV-1 reactivation and thus subsequent virus shedding in tears (6, 9, 11, 15).
Dynamic cross talk between the virus, the neurons, and the HSV-specific CD8+ T cells occur in latently infected TG (5, 6, 13, 14). Although many studies have focused on elucidating the mechanisms by which HSV-specific CD8+ T cells control virus reactivation from latently infected neurons (5, 6, 13, 14), few studies have assessed the reverse. Namely, which immune evasion mechanism does HSV-1 use to interfere with the immunosurveillance by the host's TG-resident CD8+ T cells? The latency-associated transcript (LAT) is the only viral gene that is consistently and abundantly transcribed in latently infected TG (16–18). Both mice and rabbits latently infected with LAT+ viruses have significantly higher reactivation phenotypes than mice and rabbits latently infected with LAT– viruses, suggesting that LAT plays an important role in the HSV-1 reactivation phenotype (16–18). LAT appears to regulate the latency/reactivation cycle, at least in part, by blocking apoptosis (18), and through its immune evasion functions, which includes interfering with the function of HSV-specific CD8+ T cells in the TG (5, 15, 19, 20).
CD8+ T cells surround a small number of latently infected neurons in mice, rabbits, and humans. It has been proposed that these CD8+ T cells act to decrease HSV-1 reactivation or at least abort reaction once it is initiated (4, 14, 21). Rabbit TGs infected with wild-type HSV-1 McKrae (LAT+ TG) have a significantly higher spontaneous reactivation phenotype compared to rabbit TGs infected with dLAT2903 (LAT– TG) (22–26). Recently, we found that local CD8+ T cells specific to HSV-1 human epitopes in TG of “humanized” HLA Tg rabbits latently infected with LAT+ virus are exhausted, and this correlated with more virus reactivation (4). The LAT immune evasion mechanisms contributing to HSV-1 reactivation remain to be fully determined (6, 14, 27). Based on these observations, we hypothesized that LAT may contribute to selecting a distinct repertoire of exhausted HSV-specific CD8+ T cells that are selectively retained in latently infected TG, allowing for enhanced viral reactivation. To test this hypothesis, we chose the HLA Tg rabbit model of ocular herpes because, similar to humans, latently infected HLA Tg rabbits (i) support in vivo spontaneous reactivation from latently infected TG that can be monitored by virus shedding in tears (28, 29) and (ii) elicit HLA-restricted CD8+ T cell responses specific to human epitopes (4, 14).
In the present study, HLA Tg rabbits were ocularly infected with either LAT+ or LAT– HSV-1. The frequency, function, and exhaustion status of local CD8+ T cells, specific to 40 different HSV-1 human epitopes selected from HSV-1 glycoproteins B and D (gB and gD) and tegument virion phosphoproteins 11/12 and 13/14 (VP11/12 and VP13/14) (3, 4, 14, 30–32), were compared in LAT+ TG and LAT– TG. Compared to CD8+ T cells from LAT– TG, CD8+ T cells from LAT+ TG (i) recognized a different and broader profile of HLA-restricted HSV-1 epitopes and (ii) were significantly more exhausted, expressing more PD-1, TIM-3, and CTLA-4, but less tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and granzyme B (GzmB). Thus, HSV-1-specific CD8+ T cells were more frequent in LAT+ TG but had reduced functionality. These results suggest a novel mechanism of immune evasion where HSV-1 LAT contributes to the shaping of a broader repertoire of exhausted HSV-specific CD8+ T cells that are selectively retained in latently infected TG, thus allowing for increased viral reactivation.
MATERIALS AND METHODS
HLA-A*02:01 transgenic rabbits.
A colony of human leukocyte antigens (HLA) transgenic (Tg) rabbits maintained at UC Irvine, were used for all experiments. HLA Tg rabbits were derived from New Zealand White rabbits (33). The HLA Tg rabbits retain their endogenous rabbit major histocompatibility complex (MHC) locus and express human HLA-A*02:01 under the control of its normal promoter (33). Prior to this study, the expression of HLA-A*02:01 molecules on the PBMC of each HLA-Tg rabbit was confirmed by fluorescence-activated cell sorting (FACS). Briefly, rabbits' peripheral blood mononuclear cells (PBMCs) were stained with 2 μl of anti-HLA-A2 monoclonal antibody (MAb; clone BB7.2; BD Pharmingen, USA) at 4°C for 30 min. The cells were washed and analyzed by flow cytometer using a LSRII (Becton Dickinson, Mountain View, CA). The acquired data were analyzed with FlowJo software (TreeStar, Ashland, OR). Only rabbits with high HLA expression in >90% of PBMC were used in these studies. Thus, all of the HLA rabbits used had similar high level expression of HLA-A*02:01. This avoided potential bias due to variability of HLA-A*0201 molecule levels in different animals. High expression of HLA-A*02:01 molecules is expected to (i) force rabbit CD8+ T cells to use human HLA-A*02:01 molecules at both thymic educational and peripheral effector levels (14) and (ii) minimize the competition between rabbits MHC class I molecules and human HLA-A*02:01-restricted responses (14). New Zealand White rabbits (non-Tg control rabbits; Western Oregon Rabbit Co.) were used as controls.
Viruses.
Throughout the study, wild-type HSV-1 strain McKrae and LAT-rescued dLAT2903R mutant (25), expressing LAT, are designated LAT+. LAT-null virus dLAT2903 mutant is designated LAT–. Plaque-purified HSV-1 strain McKrae (wild type) and its derived mutants: LAT– dLAT2903 and LAT+ dLAT2903R were grown in rabbit skin (RS) cell monolayers in minimal essential medium that contained 5% fetal calf serum, as described previously (25, 34, 35).
Ocular infection.
Without corneal scarification, female HLA Tg rabbits (8 to 10 weeks old) were ocularly infected by eye drops (both eyes) of 5 μl of tissue culture media containing 2 × 105 PFU of LAT+ or LAT– HSV-1, as we previously described (36). At the dose used (2 × 105 PFU/eye), all surviving rabbits harbor the latent virus in both TG, with high spontaneous reactivation and shedding in the tears of both eyes (28, 37–40). Acute ocular infection was confirmed by HSV-1-positive tear film cultures collected on days 3 to 4 p.i, as we described (40).
PBMC isolation.
Portions (20 ml) of blood were collected from each rabbit into yellow-top Vacutainer tubes (Becton Dickinson). The serum were isolated and centrifuged for 10 min at 800 × g. Rabbit PBMCs were isolated by gradient centrifugation using leukocyte separation medium (Cellgro, USA). Cells were washed in phosphate-buffered saline (PBS) and resuspended in complete culture medium consisting of RPMI 1640 containing 10% fetal bovine serum (FBS; Bio-Products, Woodland, CA) supplemented with l-glutamine and penicillin-streptomycin, sodium pyruvate, nonessential amino acids, and 50 μM 2-mercaptoethanol (Life Technologies, Rockville, MD). Aliquots of freshly isolated PBMCs were also cryopreserved in 90% fetal calf serum and 10% dimethyl sulfoxide in liquid nitrogen for future testing.
Flow cytometry analysis.
The rabbits were euthanized, the draining lymph node (DLN), PBMCs, and trigeminal ganglia (TG) were individually harvested, and single-cell suspensions were prepared and were analyzed using flow cytometry as we described previously (4, 14, 41). The following cross-reactive MAbs were used: mouse anti-rabbit CD8 (clone MCA1576F; AbD Serotec), hamster anti-mouse PD-1 (clone J4; BD Pharmingen), anti-human-CTLA-4 (L3D10), anti-human TIM-3 (clone F38-2E2; BioLegend), anti-human/mouse GzmB (clone GB1; BioLegend), rat anti-mouse IFN-γ (clone XMG1.2; BD Pharmingen), and anti-human TNF-α (BD Pharmingen). For surface staining, MAbs against various cell markers were added to a total of 106 cells in PBS containing 1% FBS and 0.1% sodium azide (FACS buffer) and left for 45 min at 4°C. After a washing step with FACS buffer, the cells were permeabilized for 20 min on ice using a Cytofix/Cytoperm kit (BD Biosciences) and then washed twice with Perm/Wash buffer (BD Bioscience). For intracellular staining, GzmB MAb was added to the cells, followed by incubation for 45 min on ice in the dark. The cells were washed again with Perm/Wash and FACS buffer and fixed in PBS containing 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). For the measurement of IFN-γ, the cells were in vitro stimulated with HSV-1 peptides. Briefly, 106 cells were transferred into 96-well flat bottom plates and stimulated with HSV-1 peptides (10 μg/ml in 200 μl of complete culture medium) in the presence of BD Golgi stop (10 μg/ml) for 6 h at 37°C. Phytohemagglutinin (5 μg/ml; Sigma) and no peptides were used as positive and negative controls, respectively. At the end of the incubation period, the cells were transferred into a 96-well round-bottom plate and washed once with FACS buffer. Surface and intracellular staining were performed as described above. A total of 50,000 events were acquired by LSRII (Becton Dickinson). Data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Tetramer assay.
Single-cell suspensions from DLN, PBMCs, and TG were analyzed for the frequency of CD8+ T cells specific to each of the HSV epitopes using corresponding HLA-A2 peptide/tetramers, as previously described (30, 42, 43). Human β2-microglobulin was incorporated into the tetramers, since no rabbit β2-microglobulins are currently available. Briefly, cells were first incubated with 1 μg/ml phycoerythrin (PE)-labeled HLA-A2-peptide/tetramer at 37°C for 30 to 45 min. The cells were then washed twice and stained with 1 μg/ml fluorescein isothiocyanate (FITC)-conjugated mouse anti-rabbit CD8 MAb (clone 215B; Serotec). After two additional washings, the cells were fixed with 1% formaldehyde in PBS. A total of 50,000 events were acquired using the LSRII FACS (Becton Dickinson). Data were analyzed using the FlowJo software (TreeStar, Ashland, OR). The absolute number of gD peptide-specific CD8+ T cells was calculated using the following formula: (number of events in CD8+/tetramer+ cells) × (number of events in gated lymphocytes)/(number of total events acquired).
Immunohistochemistry.
Immunostaining of latently infected TG was performed on day 35, as previously described (14, 15, 44–46). Rabbits were euthanized and the TG were harvested, embedded in Tissue-Tek (OCT compound; VWR International, West Chester, PA), and snap-frozen in nitrogen. Cryosections of approximately 10-μm thickness were cut, fixed in acetone (10 min, room temperature), air dried, and stored at −80°C. For immunostaining, TG sections were rehydrated in PBS (10 min, room temperature), Fc blocked, and then stained with anti-PD-L1. After three successive washings in PBS (three times for 5 min each time), sections were stained with 14.3 mM DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes, Eugene, OR) for 2 min at room temperature to stain cell nuclei. Excess DAPI was removed by washing with PBS (three times for 5 min each time), and slides were mounted in 50% glycerol-PBS and then analyzed by fluorescence microscopy.
Quantification of infectious virus.
Tear swabs were collected daily from both eyes using Dacron swabs (type 1; Spectrum Laboratories, Los Angeles, CA). Individual swabs were transferred to a 2-ml sterile cryogenic vial containing 500 μl of culture medium and stored at −80°C until use. HSV-1 titers in tear samples were determined by standard plaque assays on RS cells as previously described (36).
Statistical analyses.
Data for each assay were compared by using analysis of variance (ANOVA) and a Student t test with Prism (version 5; GraphPad, La Jolla, CA). Differences between the groups were identified by ANOVA and multiple comparison procedures, as we previously described (43). Data are expressed as the means ± the standard deviations (SD). Results were considered statistically significant when the P value was <0.05.
RESULTS
Increased expression of HLA-A*02:01 molecules in TG of HLA Tg rabbits latently infected with LAT+ compared to LAT– virus.
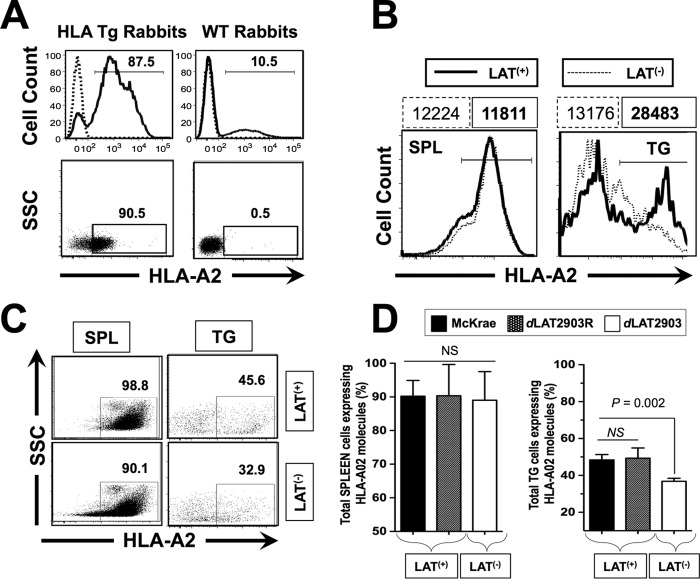
We first selected HLA Tg rabbits with the highest expression of HLA-A*02:01 molecules, since expression of the rabbits' own MHC class I molecules might interfere with the human HLA-A*02:01-restricted responses (14). Both the levels of HLA-A*0201 molecules and the percentages of cells expressing HLA-A*02:01 were assessed on PBMCs isolated from fresh blood samples. High levels of HLA-A*02:01 expression were detected by FACS in PBMCs from the HLA Tg rabbits (Fig. 1A, left panels). The specificity of anti-human HLA-A*02:01 antibody was confirmed by using an isotype IgG control. As expected, HLA-A*02:01 expression was not detected in PBMCs from wild-type (nontransgenic) rabbits (negative controls; Fig. 1A, right panels). Although up to 90.5% of PBMCs from HLA Tg rabbits expressed HLA-A*02:01 molecules, their expression levels appeared to vary among animals (data not shown). In order to avoid any biases that might be introduced by variable HLA expression levels, HLA Tg rabbits with the highest expression of HLA-A*02:01 molecules were selected. Increased expression of HLA-A*02:01 molecules in selected HLA Tg rabbits should cause rabbit CD8+ T cells to make use of the human HLA-A*02:01 molecules both at the thymic selection level and at the peripheral effector level (14).
FIG 1.
HLA Tg rabbits infected with LAT+ virus have increased spontaneous virus shedding in tears and express high levels of HLA-A2 molecules in TG compared to HLA Tg rabbits infected with LAT– virus. (A) Peripheral blood mononuclear cells (PBMCs) from either HLA transgenic rabbits (HLA Tg rabbits) or from wild-type nontransgenic rabbit controls (WT rabbits) were stained with PE-conjugated anti-HLA-A2 MAb (clone BB7.2) and analyzed by flow cytometry for the relative expression of HLA-A2 molecules (top two panels) and for the percentage of cells expressing HLA-A2 molecules (bottom two panels). Rabbits with the highest levels of HLA-A2 molecules and with the highest percentages of cells expressing HLA-A2 molecules were selected for the remainder of the study. (B) LAT+ TG have increased levels of HLA-A2 molecules compared to LAT– TG. TG and spleens (control) from HLA Tg rabbits infected with either LAT+ or LAT– virus were removed on day 35 postocular infection. (C) Representative data of the percentages of total TG-derived cells expressing HLA-A2 molecules detected by FACS were compared in LAT+ TG versus LAT– TG. (D) Each bar represents the means ± the SD of the fluorescence intensity from two independent experiments from the spleen (control) and from TG harvested from five HLA Tg rabbits at 35 days postinfection. *, P < 0.05 (ANOVA).
We next determined whether the expression levels of HLA-A*02:01 molecules would be affected in latently infected TG by LAT. Groups of 10 HLA Tg rabbits were ocularly infected with 2 × 105 PFU/eye of wild-type HSV-1 strain McKrae (LAT+) or dLAT2903, a McKrae-derived LAT– mutant. TGs and spleen (control) were harvested at 35 days postinfection (i.e., after latency was well established). The levels of HLA-A*02:01 molecules (Fig. 1B) and the percentages (Fig. 1C) of cells expressing HLA-A*0201 were assessed by FACS on total TG cells and splenocytes. The average percentage of cells pooled from 10 TG and 10 spleens ± the SD from two independent experiments is shown in Fig. 1D.
Significantly more HLA-A*02:01 molecules were detected in the TG of LAT+ latently infected rabbits in the TG of LAT– rabbits (Fig. 1B). Approximately 45% of cells in LAT+ TG expressed detectable levels of HLA-A*02:01 molecules. However, less than 33% of cells in LAT– TG had detectable levels of HLA-A*02:01 molecules (Fig. 1C and D). In contrast, splenocytes had similar HLA-A*02:01 in LAT+ and LAT– infected rabbits. These results indicate that TG from LAT+ latently infected HLA rabbits contained more HLA-A*02:01+ cells than the corresponding LAT– TG (P = 0.002). These results suggest that, compared to LAT– virus, latent infection with LAT+ virus results in increased numbers of HLA-A*02:01+ cells in the TG. This might imply increased amount of antigen presented to T cells in LAT+ TG compared to LAT– TG.
Increased number of CD8+ T cells are present in the TG of HLA Tg rabbits infected with LAT+ versus LAT– virus.
In a pilot experiment, we compared the percentage and the total number of T cells in the TGs of rabbits that were latently infected with wild-type HSV-1 (strain McKrae) that had been either perfused or not perfused prior to euthanasia. The percentages and the numbers of HSV-1 specific CD8+ T cells were similar in perfused and nonperfused TG (4, 41; data not shown). This was expected, since during HSV-1 latency there are few, if any, HSV-1-specific CD8+ T cells in the circulation. Subsequent experiments were carried out with nonperfused rabbits.
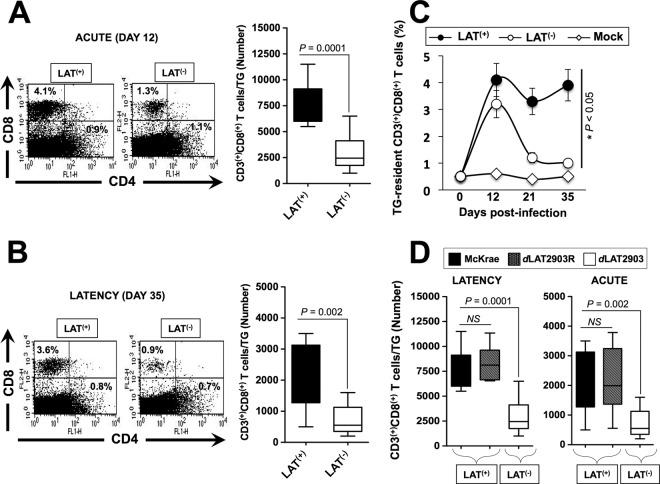
Groups of 20 HLA Tg rabbits were ocularly infected with the wild-type McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–) or the marker-rescued virus dLAT2902R counterpart (LAT+). TGs were harvested before infection (five rabbits), on day 12 (acute infection, five rabbits), and on day 35 (latency, five rabbits) postinfection. Representative experiments of percentages of TG-resident total CD3+ CD4+ and CD3+ CD8+ T cells are shown in the left panels of Fig. 2A and B. The average numbers ± the SD of TG-resident CD3+ CD8+ T cells pooled from 10 TG in two independent experiments are shown in the right panels of Fig. 2A and B. Both the percentages and the average numbers of CD8+ T cells were significantly higher in LAT+ TG compared to LAT– TG during both acute (P = 0.0001) and latent infection (P = 0.002). Fewer TG-resident CD4+ T cells were present during both acute and latent infection and were similar with LAT+ and LAT– viruses (Fig. 2A and B, left panels). At 21 and 35 days postinfection, significantly higher percentages of total CD3+ CD8+ T cells were observed in LAT+ TG compared to LAT– TG (P < 0.05) (Fig. 2C). As expected, on both day 12 (Fig. 2D, left panel) and day 35 (Fig. 2D, right panel), the average numbers of total CD3+ CD8+ T cells/TG were similarly high in HLA Tg rabbits infected with the marker-rescued virus dLAT2903R and the wild-type McKrae compared to LAT-null virus mutant dLAT2903 (P < 0.005). These results indicate that the higher numbers of CD8+ T cells seen in TG infected with LAT+ virus was associated with LAT. The differences in the total CD8+ T cells seen in LAT+ versus LAT– infected HLA Tg rabbits appeared to be specific to TG-resident T cells since no significant differences in the percentages and numbers of circulating CD8+ T cells (PBMCs) were observed in LAT+ versus LAT– infected HLA Tg rabbits during acute and latent infections (data not shown).
FIG 2.
More total and HSV-specific CD8+ T cells are detected in LAT+ TG than in LAT– TG during latent herpes virus infection. Three groups of HLA Tg rabbits (n = 20) were ocularly infected with 2 × 105 PFU/eye of the wild-type McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–), or the marker-rescued virus dLAT2903R counterpart (LAT+). Both TG were harvested from each rabbit on days 0 (5 rabbits), 12 (5 rabbits), 21 (5 rabbits), or 35 (5 rabbits) postinfection. The 10 TG were pooled and treated with collagenase I, and the numbers and percentages of total CD3+ CD4+ T cells and CD3+ CD8+ T cells were determined in TG cell suspensions by FACS. (A) Representative dot plot showing the percentages (left panel) and the average numbers ± the SD/TG (right panel) of CD3+ CD4+ and CD3+ CD8+ T cells per TG on day 12 postinfection (i.e., during acute infection). The bar graphs indicate the means and the SD of the two independent experiments with rabbits at each time point. (B) Dot plot showing the percentages (left panel) and the average numbers ± the SD/TG (right panel) of CD3+ CD4+ and CD3+ CD8+ T cells per TG on day 35 postinfection (i.e., during latency). The bar graphs show the means and the SD of the two independent experiments with rabbits at each time point. (C) Kinetics of the percentages of total CD3+ CD8+ T cells detected in LAT+ TG, LAT– TG, and mock TG. (D) Average numbers of total CD3+ CD8+ T cells per TG from HLA Tg rabbits infected with marker-rescued virus dLAT2903R, wild-type McKrae, and LAT-null virus mutant dLAT2903 on day 12 (left panel) and day 35 (right panel).
LAT gene expression is associated with a broader repertoire of HSV-1 epitope-specific CD8+ T cells in latently infected TG.
We next sought to determine whether LAT would modulate the size of the repertoire of HSV-1 epitope-specific CD8+ T cells that are selectively retained in humanized rabbit latently infected TG. HLA Tg rabbits were infected with McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–), or the marker-rescued virus dLAT2903R counterpart (LAT+), as described above. The frequencies of CD8+ T cells specific to 40 different nonoverlapping HSV-1 human epitopes, selected from glycoproteins B and D (gB and gD) and tegument phosphoproteins (VP11/12 and VP13/14) (Tables 1 and 2), were compared in latently infected LAT+ versus LAT– TG (Fig. 3).
TABLE 1.
HLA-A*02:01-restricted epitopes selected from HSV glycoproteinsa
| Antigen and peptide | Sequence | MW | No. (aa) | HLA-A*0201 (IC50 [nM]) |
|---|---|---|---|---|
| gD | ||||
| gD-18 to –10 | RLGAVILFV | 987 | 9 | 62 |
| gD-13 to –5 | ILFVVIVGL | 972 | 9 | >50,000 |
| gD-11 to –2 | AVVIVGLHGV | 963 | 10 | >50,000 |
| gD53-61 | SLPITVYYA | 1,026 | 9 | 20 |
| gD70-78 | VLLNAPSEA | 913 | 9 | 19 |
| gD95-103 | NLTIAWPRM | 1,101 | 9 | >50,000 |
| gD153-161 | LMHAPAFET | 1,016 | 9 | 2,002 |
| gD224-232 | FIPENQRTV | 1,053 | 9 | 4,218 |
| gD253-261 | LLPPELSET | 998 | 9 | 4,891 |
| gD278-286 | ALLEDPVGT | 916 | 9 | 28 |
| gB | ||||
| gB17-25 | ALLGLTLGV | 856 | 9 | 1.3 |
| gB183-191 | GIFEDRAPV | 1,003.1 | 9 | |
| gB286-294 | FVLATGDFV | 968.1 | 9 | |
| gB342-350 | NLLTTPKFT | 1,034.2 | 9 | |
| gB343-351 | LLTTPKFTV | 1,019.2 | 9 | |
| gB441-449 | YLANGGFLI | 967.1 | 9 | 0.56 |
| gB447-455 | FLIAYQPLL | 1,077.3 | 9 | 0.31 |
| gB561-569 | RMLGDVMAV | 991.2 | 9 | 1.7 |
| gB675-683 | TMLEDHEFV | 1,120.2 | 9 | 118 |
The sequence ofHSV-1 gD and gB was subjected to screening of potential HLA-A*02:01 epitopesusing several computer algorithms. Ten peptides were selected on the basis ofthe HLA-A*0201 binding motif sequence from HSV-1 strain 17. Immunodominant epitopes are indicated in boldface. MW, molecular weight; aa, amino acids.
TABLE 2.
HLA-A*02:01-restricted epitopes selected from HSV tegument proteinsa
| Antigen and peptide | Sequence | MW | No. (aa) | HLA-A*0201 (IC50 [nM]) |
|---|---|---|---|---|
| VP11/12 | ||||
| VP11/1239–47 | CLLPTPEGL | 942.1 | 9 | |
| VP11/1266–74 | FLTCTDRSV | 1,041.1 | 9 | 83 |
| VP11/12127–135 | ILTQYWKYL | 1,227.4 | 9 | |
| VP11/12197–205 | RIQQYMFFM | 1,263.5 | 9 | 81 |
| VP11/12220–228 | RLNELLAYV | 1,090.2 | 9 | 709 |
| VP11/12230–238 | VLYRWASWM | 1,211.4 | 9 | |
| VP11/12238–246 | MLWTTDKHV | 1,130.3 | 9 | 1,293 |
| VP11/12346–354 | TLTGYGVWA | 967.0 | 9 | |
| VP11/12622–630 | RVYEEIPWM | 1,222.4 | 9 | |
| VP11/12702–710 | ALSALLTKL | 929.1 | 9 | 313 |
| VP13/14 | ||||
| VP13/14286–294 | FLADAVVRL | 1,003.2 | 9 | 52 |
| VP13/14374–382 | ALLDRDCRV | 1,060.2 | 9 | 5.6 |
| VP13/14410–418 | VLTREAAFL | 1,019.2 | 9 | |
| VP13/14417–425 | FLGRVLDVL | 1,131.2 | 9 | |
| VP13/14464–472 | ALPLGSPAV | 823.9 | 9 | |
| VP13/14497–505 | VLGAAVYAL | 876.0 | 9 | 100 |
| VP13/14504–512 | ALHTALATV | 896.0 | 9 | 16 |
| VP13/14544–552 | RLLGFADTV | 991.1 | 9 | 27 |
| VP13/14545–553 | LLGFADTVV | 934.1 | 9 | |
| VP13/14657–665 | IMSQFRKLL | 1,135.4 | 9 |
The sequence of HSV-1 VP11/12, and VP13/14 protein was subjected to screening of potential HLA-A*02:01 epitopes using several computer algorithms. Ten peptides were selected on the basis of the HLA-A*0201 binding motif sequence from HSV-1 strain 17. Immunodominant epitopes are indicated in boldface. MW, molecular weight; aa, amino acids.
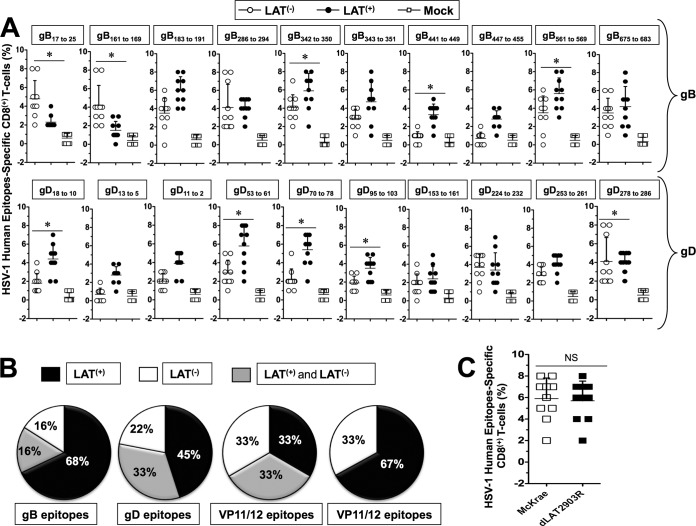
FIG 3.
Profiles of HSV-1 gB- and gD-derived human epitope-specific CD8+ T cells are detected in infected LAT+ TG versus LAT– TG. Three groups of HLA Tg rabbits Tg rabbits (n = 10) were ocularly infected with 2 × 105 PFU/eye of the wild-type McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–), or the marker-rescued virus dLAT2903R counterpart (LAT+), as in Fig. 2. Both TG were harvested from each rabbit on day 35 (10 rabbits) postinfection. The 20 TG were pooled and treated with collagenase I, and the percentages of CD8+ T cells specific to HSV-1 human epitopes selected from glycoproteins B and D were determined in TG cell suspensions by FACS. (A) Percentages of HSV-1 human gD, gB, VP11/12, and VP13/14 epitope-specific CD8+ T cells detected during latency (day 35) in 10 LAT+ TG versus 10 LAT– TG infected HLA Tg rabbits. Each black and white circle represents individual HLA Tg rabbits. (B) Pie charts represent the frequencies of CD8+ T cells specific to HSV-1 human gD, gB, VP11/12, and VP13/14 epitopes detected in LAT+ TG compared LAT– TG. *, P < 0.05 (for the percentage of epitope-specific CD8+ T cells in LAT+ TG versus LAT– TG). (C) Percentages of HSV-1 gB342–350 epitope-specific CD8+ T cells detected in TG of rabbits infected with the marker-rescued virus dLAT2903R versus wild-type McKrae.
Higher and broader frequencies of CD8+ T cells specific to nine epitopes (gB342–350, gB441–449, gB561–569, gD-18–10, gD53–61, gD70–78, gD95–103, VP11/1266–74, and VP13/14544–552) were detected in LAT+ TG (Fig. 3A). In contrast, higher frequencies of CD8+ T cells specific to only four HSV-1 human epitopes (gB17–25, gB161–169, gD278–286, and VP11/12220–228) were detected in latently infected LAT– TG (Fig. 3A). Significant frequencies of CD8+ T cells specific to eight epitopes (gB183–191, gB286–294, gB342–350, gB675–683, gD224–232, VP11/12702–710, VP13/14286–294, and VP13/14504–512) were detected in both LAT+ TG and LAT– TG. Overall, higher and broader frequencies of CD8+ T cells specific to HSV-1 human epitopes were detected in LAT+ TG compared to LAT– TG (i.e., 42% in LAT+ TG versus 19% in LAT– TG of all responding epitopes).
The differences in the frequency of CD8+ T cells between LAT+ and LAT– TG were particularly broader for epitopes derived from gB and VP13/14 epitopes (i.e., 68% versus 16% and 67% versus 33% of all responding epitopes, respectively; P < 0.001). In contrast, the differences in the frequency of CD8+ T cells between LAT+ and LAT– TG were narrower for epitopes derived from gB epitopes (i.e., 45% versus 22% responding epitopes, P < 0.005). Similar frequencies of CD8+ T cells specific to VP11/12 epitopes were detected in LAT+ and LAT– TG (Fig. 3B). As expected, similar percentages of HSV-1 epitope-specific CD8+ T cells were detected in the TG of rabbits infected with the marker-rescued virus dLAT2903R and wild-type McKrae (Fig. 3C).
Increased frequency of HSV-1 human epitope-specific PD-1+ CD8+, TIM-3+ CD8+, and CTLA-4+ CD8+ T cells in TG of HLA Tg rabbits infected with LAT+ virus versus LAT– virus.
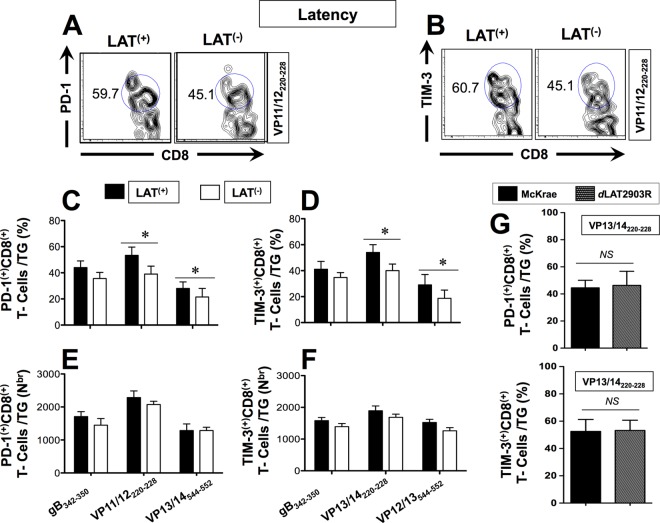
We next determined whether HSV-specific CD8+ T cells in the TG would be functionally affected by LAT. HLA Tg rabbits were infected with McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–), or the marker-rescued virus dLAT2903R counterpart (LAT+), as described above. The percentages and numbers of CD8+ T cells specific to the VP11/12220–228 epitope (one of the most immunodominant HSV-1 epitopes) expressing PD-1, TIM-3, and CTLA-4, three markers of phenotypic exhaustion, were determined in the TG during both acute (day 11) (Fig. 4) and latent (day 35) (Fig. 5) phases of infection.
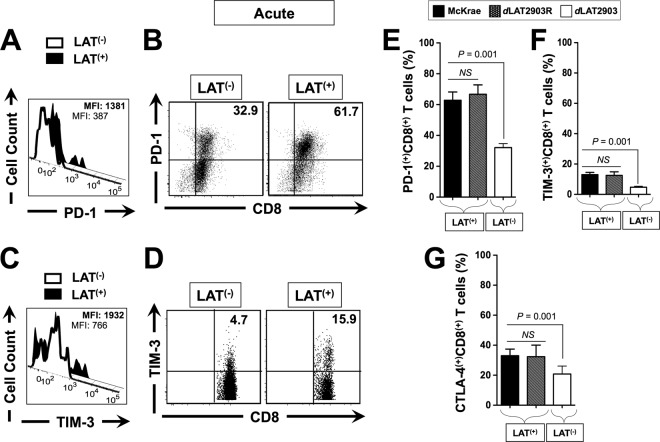
FIG 4.
More exhausted HSV-specific CD8+ T cells were detected in acutely HSV-1 LAT+ infected TG than in HSV-1 LAT– infected TG. Three groups of five HLA Tg rabbits were ocularly infected as described above with the wild-type McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–), or the marker-rescued virus dLAT2903R counterpart (LAT+), as in Fig. 2. Ten TG/group were harvested at 12 days postinfection, pooled, and processed, as described above. The cells were triple stained with FITC-labeled anti-PD-1, PerCP-anti-CD8 T-cell, and PE-labeled HSV-1 VP11/12220–228/HLA Tg rabbit-A*0201 tetramer. The levels of PD-1 and TIM-3 expression and the percentages of HSV-1 VP11/12220–228 epitope-specific CD8+ T cells expressing PD-1, TIM-3, and CTLA-4 were determined by FACS. (A) Representative histogram of levels of PD-1 on gated VP11/12220–228 epitope-specific CD8+ T cells in LAT+ TG versus LAT– TG. (B) Representative dot plots of the percentages of VP11/12220–228 epitope-specific PD-1+ CD8+ T cells in LAT+ TG versus LAT– TG. (C) Representative histograms of the levels of TIM-3 gated on VP11/12220–228 epitope-specific CD8+ T cells in LAT+ TG versus LAT– TG. (D) Representative dot plot of the percentages of VP11/12220–228 epitope-specific TIM-3 CD8+ T cells in LAT+ TG versus LAT– TG. (E) Percentage of VP11/12220–228 epitope-specific CD8+ T cells expressing PD-1. (F) Percentage of VP11/12220–228 epitope-specific CD8+ T cells expressing TIM-3. (G) Percentage of VP11/12220–228 epitope-specific CD8+ T cells expressing CTLA-4. Each bar in panels C and F represents the mean and SD from two independent experiments. *, P < 0.05.
FIG 5.
More exhausted HSV-specific CD8+ T cells were detected in latently HSV-1 LAT+ infected TG than in HSV-1 LAT– infected TG. Groups of five HLA Tg rabbits were ocularly infected as described above with wild-type McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–), or the marker-rescued virus dLAT2903R counterpart (LAT+). Pools of 10 LAT+ TG versus 10 LAT– TG were harvested during latent infection and processed as described above. The cells were triple stained with FITC-labeled anti-PD-1, PerCP-anti-CD8 T-cell, and PE-labeled HSV-1 gB342–350, VP11/12220–228, or VP13/14544–552/HLA Tg rabbit-A*0201 tetramers. The percentages and absolute numbers of HSV-1 gB342–350, VP11/12220–228, or VP13/14504–512 epitope-specific CD8+ T cells expressing PD-1 and TIM-3 markers of exhaustion were determined by FACS. (A) Representative dot plots of VP11/12220–228 epitope-specific PD-1+ CD8+ T cell percentages in LAT+ TG versus LAT– TG. (B) Representative dot plots of the percentages of VP11/12220–228 epitope-specific TIM-3+ CD8+ T cells in LAT+ TG versus LAT– TG. (C and D) Average percentages of HSV-1 gB342–350, VP11/12220–228, or VP13/14544–552 epitope-specific CD8+ T cells expressing PD-1 and TIM-3, respectively. (E and F) Average numbers of HSV-1 gB342–350, VP11/12220–228, or VP13/14544–552 epitope-specific CD8+ T cells expressing PD-1 and TIM-3, respectively. (G) Percentages of exhausted PD-1+ CD8+ T cells/TG and TIM-3+ CD8+ T-cells/TG were detected in rabbits infected the marker-rescued virus dLAT2903R and wild-type McKrae. Each bar in panels C to F represents the mean and SD of two independent experiments. *, P < 0.05.
The results of a representative experiment of the level of PD-1 (Fig. 4A), as well as the percentages of PD-1+ CD8+ T cells (Fig. 4B), during acute infection are shown. The results of a representative experiment of the level of TIM-3 (Fig. 4C), as well as the percentages of TIM-3+ CD8+ T cells (Fig. 4D), during latent infection are also shown. The average percentages ± the SD of PD-1+ CD8+ T cells, TIM-3+ CD8+ T cells, and CTLA-4+ CD8+ T cells detected from 10 LAT+ TG versus 10 LAT– TG are shown in Fig. 4E, F, and G, respectively. Significantly higher expression levels of PD-1 (Fig. 4A) and TIM-3 (Fig. 4C) were detected on VP11/12220–228 epitope-specific CD8+ T cells in LAT+ TG than in LAT– TG (P < 0.05). The mean fluorescence intensity (MFI) of PD-1 on CD8+ T cells from LAT+ TG was higher than the MFI of PD-1 on CD8+ cells from LAT– TG (1,381 ± 5 versus 387 ± 5, respectively; P < 0.05, Fig. 4A). Similarly, the MFI of TIM-3 on CD8+ T cells from LAT+ TG was higher than the MFI of TIM-3 on CD8+ cells from LAT– TG (1,932 ± 5 versus 766 ± 5, respectively; P < 0.05, Fig. 4C). Additionally, compared to LAT– TG, LAT+ TG had significantly higher percentages of PD-1+ CD8+ T cells (Fig. 4B and E), TIM-3+ CD8+ T cells (Fig. 4D and F), and CTLA-4+ CD8+ T cells (Fig. 4G, P < 0.001). These results indicate that during acute infection, not only do a greater proportion of HSV-specific CD8+ T cells in LAT+ TG express PD-1, TIM-3, and CTLA-4, but also the expression levels of these exhaustion markers were significantly higher in LAT+ TG than in LAT– TG. As expected, similar percentages of exhausted CD8+ T cells were detected in the TG of rabbits infected with the marker-rescued virus dLAT2903R and wild-type McKrae (Fig. 4E, F, and G). These results suggests that the exhaustion of HSV-specific CD8+ T cells seen during acute infection in rabbit TG infected with LAT+ virus was associated with LAT.
Next, we determined the percentages and numbers of PD-1+ CD8+ T cells and TIM-3+ CD8+ T cells from pools of 10 LAT+ TG and 10 LAT– TG harvested during latent infection (Fig. 5). Instead of relying on just one epitope (i.e., VP11/12220–228) in these experiments, we expanded our range and included three additional epitopes from three different HSV-1 proteins for which CD8+ T cells has previously been detected in both LAT+ TG and LAT– TG (i.e., gB342–350, VP11/12220–228, and VP13/14544–552; Fig. 3 and Tables and 2). Compared to latently infected LAT– TG, latently infected LAT+ TG displayed a significantly higher percentage of PD-1+ CD8+ T cells and of TIM3+ CD8+ T cells specific to VP11/12220–228 and VP13/14544–552 epitopes from tegument proteins (Fig. 5A to D). As expected, similar percentages of exhausted PD-1+ CD8+ T cells/TG and TIM-3+ CD8+ T cells/TG was detected in rabbits infected with the marker-rescued virus dLAT2903R and wild-type McKrae (Fig. 5G).
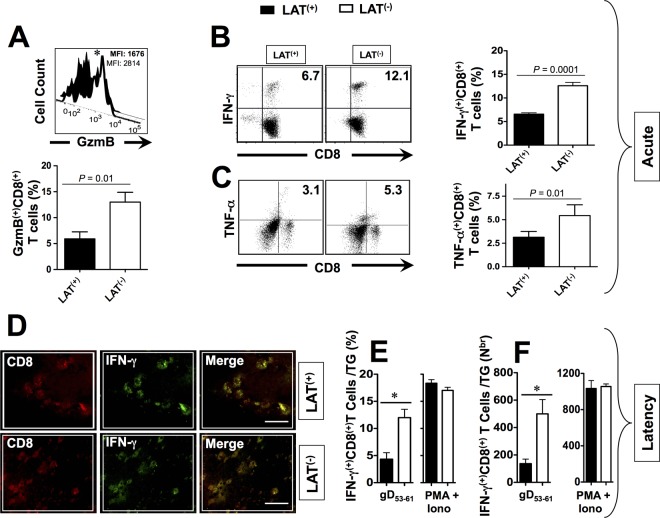
Dramatically more functionally exhausted HSV-1 human epitope-specific CD8+ T cells are retained in LAT+ TG than in LAT– TG.
Since phenotypic expression of markers of exhaustion of T cells may not necessarily translate into functional exhaustion, we next compared the ability of LAT+ versus LAT– TG-resident CD8+ T cells, specific to HSV-1 human epitopes, to produce cytokines and display cytotoxic activity. In Fig. 6, we examined the function of both VP11/12220–228-specific CD8+ T cells (which are highly frequent in LAT– TG) and of gB53–61-specific CD8+ T cells (which are highly frequent in LAT+ TG) in both LAT+ TG and LAT– TG, during the acute (top panels) and latent (lower panels) phases of infection. In vitro stimulation was performed using VP11/12220–228 or gB53–61 peptides, as we previously described (4). The nonspecific stimulant, PMA+IONO, which should stimulate both VP11/12220–228 and gB53–61 peptide-specific and nonspecific CD8 T cells, was used as a positive control. Intracellular staining for GzmB, IFN-γ, and TNF-α cytokines was performed on tetramer gated CD8+ T cells using FACS. The expression levels, frequencies, and numbers of HSV-specific CD8+ T cells expressing IFN-γ, TNF-α, and GzmB were compared in LAT+ and LAT– TG. As shown in the representative FACS histogram in the top panel of Fig. 6A, a higher level of GzmB was detected in VP11/12220–228-specific CD8+ T cells that were retained in LAT– TG compared to LAT+ TG. The lower panel of Fig. 6A shows higher levels of GzmB expressed by VP11/12220–228-specific CD8+ T cells from LAT– TG than from LAT– TG. A similar trend was observed when we compared the levels of GzmB on gB53–61-specific CD8+ T cells retained in LAT– TG to those in LAT+ TG (data not shown). The left two panels in Fig. 6B and C show representative dot plots of higher percentages of VP11/12220–228-specific CD8+ T cells producing IFN-γ and TNF-α in LAT– TG compared to LAT+ TG. The right two panels in Fig. 6B and C show significantly higher percentages of VP11/12220–228-specific CD8+ T cells producing IFN-γ and TNF-α detected from 10 LAT– TG versus 10 LAT+ TG (P = 0.0001). A similar trend was observed when we compared the levels of GzmB on gB53–61-specific CD8+ T cells retained in LAT– TG to those in LAT+ TG (data not shown). More exhausted HSV-specific CD8+ T cells were also seen in LAT+ TG versus LAT– TG during latent infection (Fig. 6D, E, and F).
FIG 6.
HSV-specific CD8+ T cells that are retained in HSV-1 LAT+ infected TG are functionally exhausted compared to CD8+ T cells from HSV-1 LAT– infected TG. Three groups of HLA Tg rabbits (n = 10) were ocularly infected with the wild-type McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–), or the marker-rescued virus dLAT2903R counterpart (LAT+). At 12 (Acute) and 35 (Latency) days postocular infection, 10 TG from five rabbits/group were harvested and pooled, and single-cell suspensions were prepared as described above. For GzmB, cell suspensions from LAT+ TG versus LAT– TG were stimulated with HSV-1 VP11/12220–228 peptide for 6 h at 37°C in the presence of 0.7 μl/ml BD Golgi-Stop and 10 μl of FITC-labeled anti-human GzmB MAb, washed with FACS buffer, and stained with PerCP-conjugated anti-rabbit CD8 MAb at 4°C. Tetramer staining and FACS assays were used to determine the levels of expression of GzmB on HSV-1 VP11/12220–228 epitope-specific CD8+ T cells that are retained in LAT+ TG versus LAT– TG. For intracellular cytokine staining, cell suspensions from LAT+ TG versus LAT– TG were stimulated with VP11/12220–228 peptide (10 μg/ml) for 6 h at 37°C in the presence of Golgi Plug (BD Biosciences). The cells were then stained with PerCP-conjugated anti-rabbit CD8 for 30 min, permeabilized, and stained with PE-labeled anti-human IFN-γ or TNF-α antibodies. (A) Representative histogram of level of GzmB on gated VP11/12220–228 epitope-specific CD8+ T cells in LAT+ TG versus LAT– TG (upper panel). Average percentages of VP11/12220–228 epitope-specific CD8+ T cells expressing significant levels of GzmB are given in the lower panel. (B) Representative dot plots of the percentages of VP11/12220–228 epitope-specific IFN-γ+ CD8+ T cells in LAT+ TG versus LAT– TG (left panel). Average percentages/TG of VP11/12220–228 epitope-specific CD8+ T cells producing IFN-γ in LAT+ TG versus LAT– TG are shown in the right panel. (C) Representative dot plots of percentage VP11/12220–228 epitope-specific TNF-α+ CD8+ T cells in LAT+ TG versus LAT– TG (left panel). Average percentages/TG of VP11/12220–228 epitope-specific CD8+ T cells producing TNF-α in LAT+ TG versus LAT– TG are shown in the right panel. (D) TG from HLA Tg rabbits latently infected with either LAT+ or LAT– virus were removed at 35 days postocular infection, immunostained with MAbs specific to rabbit CD8 and IFN-γ, and then analyzed by confocal microscopy. The results shown are representative of four independent experiments. (E) Average percentages/TG of gD53–61 epitope-specific CD8+ T cells producing IFN-γ detected during latency in LAT+ TG versus LAT– TG, following restimulation with either gD53–61 peptide or PMA+IONO (positive control). (F) Average numbers/TG of gD53–61 epitope-specific CD8+ T cells producing IFN-γ detected during latency in LAT+ TG versus LAT– TG, following restimulation with either gD53–61 peptide or PMA+IONO (positive control). Each bar represents the mean and the SD of two independent experiments each using 10 TG. *, P < 0.05 (LAT+ TG versus LAT– TG [ANOVA]).
Altogether, these results indicate that during both acute and latent infection, a significantly higher percentage of HSV-specific CD8+ T cells that were retained in LAT+ TG were exhausted than in LAT– TG. The results confirmed that LAT interferes with the function of TG-resident HSV-specific CD8+ T cells and alters, by a yet-to-be-determined mechanism, their ability to produce IFN-γ and TNF-α and to express GzmB.
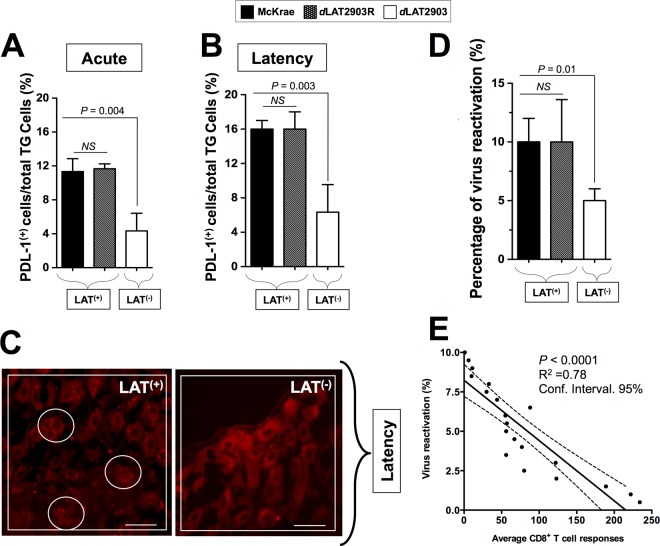
PD-L1, the ligand of PD-1, is highly expressed in LAT+ TG compared to LAT– TG.
Since larger amounts of exhausted CD8+ T cells expressing PD-1 receptor were observed in HSV-1 LAT+ infected TG compared to HSV-1 LAT– infected TG, it was of interest to determine whether the two ligands of this receptor (i.e., PD-L1 and PD-L2) are also upregulated in LAT+ TG. Compared to LAT– TG, LAT+ TG had significantly higher levels of PD-L1 during both acute (Fig. 7A; P = 0.004) and latent (Fig. 7B; P = 0.003) phases of infection. During the acute phase (day 12 postinfection), 10 to 12% of cells in LAT+ TG expressed significantly enhanced the levels of PD-L1, as detected by FACS, compared to only 0.9 to 3.9% of cells from LAT– TG. Likewise, during latency (day 35 postinfection), 15 to 16% of cells in LAT+ TG expressing a significant level of PD-L1, were detected by FACS compared to only 4 to 8% of cells from LAT– TG. In contrast, only low, and similar, levels of PD-L2 were detected in LAT+ TG and LAT– TG (data not shown). Similar high levels of PDL-1 were detected in the TG of rabbits infected with McKrae compared to dLAT2903R during both acute (Fig. 7A) and latent (Fig. 7B) phases of infection. Moreover, during latency, significantly more PD-L1 was observed by immunostaining of sections of LAT+ TG than by immunostaining of sections of LAT– TG (Fig. 7C). In contrast, PD-L2, the other ligand of PD-1, was not detected in either LAT+ TG or LAT– TG (not shown). Thus, LAT may stimulate the PD-1/PD-L1 pathway, but not the PD-1/PD-L2 pathway, in latently infected TG. Thus, the PD-L1/PD-1 negative T cell costimulatory pathway appears to be involved in exhaustion of TG-resident HSV-specific CD8+ T cells in LAT[supb]+ TG.
FIG 7.
HLA Tg rabbits infected with LAT+ virus have increased spontaneous virus shedding in tears and express high levels of PD-L1 molecules in TG compared to HLA Tg rabbits infected with LAT– virus. (A) HLA Tg rabbits infected with the wild-type McKrae (LAT+), its LAT-null virus mutant dLAT2903 (LAT–), or the marker-rescued virus dLAT2903R counterpart (LAT+). TG were removed 12 (Acute) or 35 (Latency) days after ocular infection. Half of the TG were treated with collagenase, and the other half were fixed, embedded in OCT, immunostained as indicated, and examined for expression of PD-L1 by confocal microscopy as described in Materials and Methods. Cell suspensions from LAT+ TG and LAT– TG harvested on five rabbits on day 12 (acute infection) or day 35 (latency) were stained with FITC-anti-human PD-L1 MAb (clone M1/42). The percentages of total TG-derived cells expressing PD-L1 detected by FACS in 10 LAT+ TG versus 10 LAT– TG during acute (A) and latent (B) phases are shown. (C) TG cells expressing significant detectable levels of PD-L1 were visualized by immunostaining in LAT+ TG versus LAT– TG during latency. (D) HLA Tg rabbits latently infected with LAT+ virus show increased spontaneous virus shedding in tears. HSV-1 shedding detected in tears was monitored for 30 days postinfection, as described in Materials and Methods. (E) Correlation between the percentage of positive tears and the average T cell responses during a 30-day monitoring period. *, P < 0.05 (ANOVA).
As expected, a larger amount of spontaneous reactivation was detected in HLA Tg rabbits that were latently infected with the LAT+ virus than those infected with the LAT– virus (Fig. 7D). A statistically significant correlation was observed between the high levels of TG-resident CD8+ T-cell responses and low rates of virus reactivation, as shown in Fig. 7E (P < 0.0001). The average T cell responses pooled from 10 TG ± the SD from two independent experiments are shown.
DISCUSSION
Persistent infectious viral pathogens, including HSV-1, have evolved a variety of immunoevasion tactics to avoid being detected by the host's immune system. The present report revealed a novel immune evasion mechanism whereby HSV-1 LAT, the only gene that is abundantly transcribed during latency, directly or indirectly impairs host cellular immunity by shaping a repertoire of dysfunctional HSV-specific CD8+ T cells that are selectively retained in latently infected TG. Such a strategy would allow for increased viral reactivations (4–14). Thus, we found that the frequency and size of the HSV-1 human epitope-specific CD8+ T cell population that is selectively retained in LAT+ TG were quantitatively and qualitatively different from the frequency and size of HSV-1 human epitope-specific CD8+ T cell population that is retained in LAT– TG. Moreover, compared to HSV-specific CD8+ T cells that were retained in LAT– TG, HSV-specific CD8+ T cells that were retained in latently infected LAT+ TG were phenotypically and functionally exhausted. To the best of our knowledge, this is the first report to suggest that the LAT gene not only affects the function/dysfunction of TG-resident HSV-specific CD8+ T cells but also contributes to shaping a unique repertoire of HSV-specific CD8+ T cells that are selectively retained in latently infected TG. The different HSV-specific CD8+ T cell responses in LAT+ TG compared to LAT– TG may explain, at least in part, why LAT+ viruses have an increased spontaneous reactivation phenotype compared to LAT– viruses.
After primary corneal infection, HSV-1 establishes latency in sensory neurons of the TG (4, 20, 47–49). HSV-1 spontaneously reactivates from latently infected TG, and the viral particles are sporadically shed in tears. During HSV-1 latency, the HSV-1 LAT is the only viral gene that is abundantly transcribed in TG. LAT+ viruses (i.e., wild-type McKrae and the marker-rescued virus dLAT2903R) have a significantly higher reactivation phenotype (explant TG induced reactivation in mice and spontaneous reactivation in rabbits) than their LAT– mutant counterparts (i.e., the LAT-null virus mutant dLAT2903) (22–26), and this appears to be due in part to LAT's antiapoptosis activity (25, 34, 50–55). We found that CD8+ T cells from rabbit LAT+ TG recognized a broader selection of nonoverlapping human HSV-1 epitopes, expressed higher levels of exhaustion markers, and exhibited reduced levels of TNF-α, IFN-γ, and GzmB. These results suggest that HSV-1 LAT contributes, by yet-to-be-determined mechanisms, to the homing of a large repertoire of exhausted HSV-specific CD8+ T cells within latently infected LAT+ TG, thereby promoting more virus reactivation. CD8+ T cells are found surrounding some neurons in latently infected human TG (6, 8, 11, 56–58). Since CD8+ T cells are presumably attracted to these neurons by viral antigens, it is assumed that the neurons surrounded by CD8+ T cells are those in which the virus has initiated the early stages of reactivation from latency. The more frequent reactivation seen in LAT+ TG is expected to produce more viral antigens to which local TG-resident CD8+ T cells are constantly exposed. This likely produced more exhausted PD-1+ TIM-3+ CTLA+ CD8+ T cells detected in LAT+ TG (Fig. 4 and 5). Exhausted CD8+ T cells are likely unable to functionally prevent virus reactivation seen in LAT+ TG. The current largely accepted paradigm is that the exhaustion of T cells requires persistent exposure to antigen. Thus, it is likely that LAT gene, which does not make a protein, induces exhaustion of CD8+ T cells in TG through mechanisms other than antigen persistence, as we previously discussed (20). The amount of viral antigen produced in TG is quite low, less than 1 positive neuron/TG with LAT+ (59–62). Thus, additional factors likely contributed to CD8+ T cell exhaustion detected in LAT+ TG. LAT encodes eight miRNAs (63, 64) and two additional sRNAs (65), which might contribute to CD8+ T cell exhaustion (66–69). However, elucidating the mechanisms by which these miRNAs and sRNAs might be involved in CD8+ T cell exhaustion is beyond the scope of the present study.
HSV-specific CD8+ T cells that are retained in latently infected TG appear to play a role in decreasing spontaneous reactivation from latency (5, 8, 10, 12, 20, 70–73). An intriguing and unexpected finding in this report was that quantitatively and qualitatively different repertoires of HSV-1 human epitope-specific CD8+ T cells were selectively retained in LAT+ TG compared to LAT– TG. The absolute numbers and percentages of CD8+ T cells specific to nine different HSV-1 human epitopes were significantly higher in LAT+ TG than in LAT– TG. In contrast, the absolute numbers and percentages of CD8+ T cells specific to four HSV-1 human epitopes were significantly higher in LAT– TG than in LAT+ TG. The mechanisms by which a different hierarchy of CD8+ T cell dominance occurs in latently infected LAT+ TG versus LAT– TG remain to be elucidated. What is clear, however, is that the hierarchy of dominance of CD8+ T cells specific to human epitopes, detected in the TG of the HLA Tg rabbit, does not reflect the unusual H2-restricted CD8+ T cell immunodominance in TG of B6 mice. In B6 mice ∼50% of HSV-specific CD8+ T cells are directed against a single gB498–505 mouse epitope, with the remaining HSV-specific CD8+ T cells being directed against 18 other subdominant mouse epitopes (74). The larger repertoire of CD8+ T cells in LAT+ TG may suggest (i) a relatively uniform proliferation of human epitope-specific CD8+ T cells which might expand at a significantly higher rate, hence creating a uniform exposure to the viral proteins that express these epitopes compared to CD8+ T cells from LAT– TG, and/or (ii) that LAT+ TG are more immunologically open to the homing of circulating HSV-specific T cells than are LAT– TG. Whether these TG-resident HSV-specific CD8+ T cells are TCM, TEM, or TRM cells is currently being determined and will be the subject of future reports. Regardless of the mechanisms by which more T cell clones are maintained in LAT+ TG, this is, to the best of our knowledge, the first report of a new immune evasion mechanism in which the HSV-1 LAT might contribute, by a yet-to-be-determined mechanism, to the shaping of a well-built repertoire of HSV-specific CD8+ T cell clones selectively retained in latently infected TG. Since spontaneous HSV-1 reactivation is significantly higher in LAT+ TG compared to LAT– TG (24, 25, 55) (Fig. 7D), the new finding may suggest that HSV-1 LAT favors the retention in the latently infected TG of particular epitope-specific CD8+ T cell clones that are less efficient at preventing and/or aborting reactivation, while preventing the hosting of CD8+ T cell clones specific to nonoverlapping epitopes that are more efficient at blocking/aborting reactivation. Such a LAT-mediated immune evasion strategy should lead to a higher spontaneous reactivation rate in LAT+ latently infected TG than in LAT– latently infected TG. It is also possible that the higher reactivation frequency in LAT+ compared to LAT– latently infected rabbits, and hence the increased exposure to HSV-1 antigens, may contribute to shaping a specific repertoire of T cells. We speculate that whatever mechanisms are involved in shaping the TG-resident T cell repertoire in our LAT+ infected HLA Tg rabbits are also involved in shaping the TG-resident T cell repertoire in humans (21, 75), since all HSV-1 isolates express LAT.
The finding that LAT+ TG-resident CD8+ T cells recognized multiple HSV-1 human gB, gD, VP11/12, and VP13/14 epitopes, express high levels of PD-1, TIM-3, and CTLA-4, and produce low levels of IFN-γ, TNF-α, and GzmB differs from a recent suggestion of fully functional T cells that react only to one or to a few epitopes in humans TG (7). It should be noted that a high level of PD-1 expression alone does not necessarily prove functional exhaustion. Both activated memory T cells and exhausted T cells express PD-1, with exhausted CD8+ T cells expressing higher and more persistent levels of PD-1 than do activated memory CD8+ T cells (61, 76, 77). With lymphocytic choriomeningitis virus, PD-1 is markedly upregulated on exhausted CD8+ T cells but only transiently expressed on effector memory CD8+ T cells during acute infections and is not present on functionally competent memory CD8+ T cells (77–79). Instead of relying only on the level of expression of PD-1, in the present study, we also analyzed TIM-3 and CTLA-4 expression levels (additional cell surface markers of exhaustion) on HSV-specific CD8+ T cells that are retained in latently infected LAT+ TG versus LAT– TG. PD-1, TIM-3, and CTLA-4 were all more highly expressed on HSV-specific CD8+ T cells from LAT+ TG than in cells from LAT– TG. The high levels of expression of PD-1, TIM-3, and CTLA-4 helped to discriminate between exhaustion and activation. The percentage and number of PD-1+ CD8+ T cells, TIM-3+ CD8+ T cells, and CTLA-4+ CD8+ T cells were significantly increased in TG of HLA Tg rabbits latently infected with LAT+ compared to LAT– virus (P < 0.001). In addition, in this study, exhaustion (dysfunction) of LAT+ TG-resident CD8+ T cells was confirmed at the function level by a decrease in the levels of GzmB (i.e., cytotoxicity activity), IFN-γ, and TNF-α. To the best of our knowledge, this is the first report showing LAT-related functional exhaustion of HSV-specific CD8+ T cells in the TG in an animal model with spontaneous HSV-1 reactivation (i.e., not induced). These results are consistent with our recent finding of significantly more exhaustion of HSV-1 human gD epitope-specific CD8+ T cells from the TG of HLA Tg rabbits with higher virus reactivation compared to the TG of HLA Tg rabbits with less virus reactivation (1, 4, 14). In that study, the majority (67%) of HSV-1 human gD epitope-specific CD8+ T cells in TG of HLA Tg rabbits with higher virus reactivation coexpressed PD-1, TIM-3, and CTLA-4 (4). In contrast, only 22% of HSV-1 human gD epitope-specific CD8+ T cells in TG of HLA Tg rabbits with less virus reactivation coexpressed PD-1, TIM-3, and CTLA-4 (4). In addition, CD8+ T cells from HLA Tg rabbits with less virus reactivation, but not from HLA Tg rabbits with more virus reactivation, significantly decreased induced HSV-1 reactivation in vitro in explanted autologous TG; thus, directly confirming the role of functional TG-resident CD8+ T cells in controlling virus reactivation from latency (4). Results from the present study (Fig. 7E), together with results from our recent report (4), suggest that the high frequency of HSV-specific exhausted CD8+ T cells positively correlated with high rates of virus reactivated from latently infected TG (4). Regardless of the mechanism by which a specific repertoire of exhausted PD-1+ TIM-3+ CTLA-4+ CD8+ T cells is selectively retained in the LAT+ TG of latently infected HLA Tg rabbits, the present report supports our original hypothesis that LAT might enhance virus reactivations by reducing the size of the HSV-specific functional CD8+ T-cell population that resides in TG. The results presented here also indicate that our previous finding using CD8+ T cells in a mouse model of HSV-1 infection (20) can be extended to several HSV-1 human epitopes and to HLA Tg rabbits, an animal model that displays spontaneous virus reactivation and shedding of virus similar to humans.
Similar higher numbers of exhausted CD8+ T cells were detected in TG of HLA Tg rabbits infected with the parental wild-type virus (McKrae) and the marker-rescued virus dLAT2903R that express a restored LAT gene in an otherwise LAT-null mutant (dLAT2903). These results confirm our published results over the last 10 years which demonstrate that the wild-type McKrae and the dLAT2903R are phenotypically similar at both virological and immunological levels: (i) McKrae and dLAT2903R replicated similarly in rabbit eyes and TG (25); (ii) most of the HSV-1-specific CD8+ T cells that reside in mouse TG infected with McKrae and dLAT2903R were phenotypically and functionally exhausted, as judged by the high level of expression of PD-1 and TIM-3 and the low cytotoxic function and decreased IFN-γ and TNF-α production. This resulted in LAT+ TG having less functional HSV-gB495–505 tetramer+ CD8+ T cells compared to LAT– TG (20); (iii) transfection with a plasmid expressing LAT, in the absence of other HSV-1 gene products, upregulated both PD-L1 and major histocompatibility complex class I (MHC-I) on mouse neuroblastoma cells (Neuro2A) (20); and (iv) protection of neurons latently infected with either McKrae or dLAT2903R against cytotoxic CD8 T-cell killing appeared to be due to LAT's antiapoptotic activity (15). Altogether, these results clearly demonstrate that exhaustion of TG-resident CD8+ T cells is associated with LAT.
Elucidation of the cellular and molecular immune mechanisms that control spontaneous HSV-1 reactivation from latency is critical for the development of future immunotherapies to prevent blinding ocular herpes. Since the memory CD8+ T cells retained in TG do not always prevent reactivation from latency, a successful therapeutic vaccine will have to induce a repertoire of TG-resident HSV-specific CD8+ T cells that are qualitatively different and/or quantitatively stronger than the “natural” repertoire of CD8+ T cells (80, 81). An eventual goal is to develop a therapeutic vaccine strategy that will “fine-tune” the human TG-resident CD8+ T cells to more strongly react to “LAT–” protective epitopes and thereby reduce virus reactivation and recurrent disease. Therapeutic vaccine strategies aiming at boosting the number and/or the function of TG-resident CD8+ T cell repertoire specific to “LAT+” epitopes could also potentially reduce the frequency of virus reactivation and recurrent disease. Because HSV-1 coopts PD-1/PD-L1, CTLA-4/Gal-9, and BTLA/HVEM immune checkpoints as a strategy to evade CD8+ T cell immune surveillance (20, 49), a T cell-based immunotherapy will likely have to be combined with immune checkpoints blockade. This can be accomplished by a single- or a multiple-antibody-mediated blockade of PD-1/PD-L1, CTLA-4/Gal-9, and/or BTLA/HVEM pathways in order to reverse the dysfunction of CD8+ T cells. Antibodies targeting the PD-1/PD-L1 immune checkpoint have recently shown remarkable clinical safety and efficacy in other systems (82–84). In addition, if a recent suggestion that latently infected TG are immunologically “closed” to the homing of circulating HSV-specific CD8+ T cells holds true in humans (85), then an immunotherapeutic vaccine will most likely need to be complemented with a neurotropic strategy that will attract T cells specifically into infected TG (e.g., by specifically producing T-cell-attracting chemokines within infected TG). Altogether, these strategies would help to reduce HSV-1 reactivation and decrease the incidence of recurrent ocular herpetic disease.
In conclusion, there are three principal findings in this report. (i) TG from “humanized” HLA Tg rabbits latently infected with LAT+ virus had more exhausted CD8+ T cells specific to human epitopes than did TG from HLA Tg rabbits latently infected with LAT– virus. (ii) This increased exhaustion correlated with increased reactivation in LAT+ versus LAT– latently infected HLA Tg rabbits. (iii) LAT+ TG appeared to selectively retain a repertoire of HSV-specific CD8+ T cell clones that differed from those retained in LAT– TG. This suggests a new immune evasion mechanism whereby the HSV-1 LAT contributes, by a yet-to-be-determined mechanism, to preventing protective CD8+ T cells to be retained in latently infected TG that would otherwise prevent virus reactivation.
To the best of our knowledge, this report is the first to show that a virus latency-related gene interferes with the host's adaptive immune response by modulating the frequency and the size of the T cell repertoire retained in the infected tissues. Shaping a broader and specific repertoire of exhausted virus-specific CD8+ T cells that are selectively retained in latently infected tissues appears to be a new immune evasion mechanism and may be an important maneuver used by HSV-1 to escape from immune surveillance. This is particularly relevant during the establishment and maintenance of latency, as well as during the initial stages of virus reactivation from latency at times when LAT is the only abundantly transcribed HSV-1 gene.
ACKNOWLEDGMENTS
This study is supported by Public Health Service research grants EY14900, EY019896, EY024618, EY013191, 1R56AI098985, 1R56AI093133, and 1R21AI110902, from the National Institutes of Health (NIH), The Discovery Center for Eye Research, and a Research to Prevent Blindness Challenge grant.
We thank Dale Long from the NIH Tetramer Facility (Emory University, Atlanta, GA) for providing the tetramers used in this study.
REFERENCES
- 1.Srivastava R, Khan AA, Spencer D, Vahed H, Lopes PP, Thai NT, Wang C, Pham TT, Huang J, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes. J Immunol 194:2232–2248. doi: 10.4049/jimmunol.1402606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perng GC, Osorio N, Jiang X, Geertsema R, Hsiang C, Brown D, BenMohamed L, Wechsler SL. 2015. Large amounts of reactivated virus in tears precedes recurrent herpes stromal keratitis in stressed rabbits latently infected with herpes simplex virus. Curr Eye Res 19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AA, Srivastava R, Spencer D, Garg S, Fremgen D, Vahed H, Lopes PP, Pham TT, Hewett C, Kuang J, Ong N, Huang L, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. Phenotypic and functional characterization of herpes simplex virus glycoprotein B epitope-specific effector and memory CD8+ T cells from ocular herpes symptomatic and asymptomatic individuals. J Virol 89:3776–3792. doi: 10.1128/JVI.03419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AA, Srivastava R, Chentoufi AA, Geertsema R, Thai NT, Dasgupta G, Osorio N, Kalantari M, Nesburn AB, Wechsler SL, BenMohamed L. 2015. Therapeutic immunization with a mixture of herpes simplex virus type 1 glycoprotein D-derived “asymptomatic” human CD8+ T-cell epitopes decreases spontaneous ocular shedding in latently infected HLA transgenic rabbits: association with low frequency of local PD-1+ TIM-3+ CD8+ exhausted T cells. J Virol 89:6619–6632. doi: 10.1128/JVI.00788-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen SR, Hamrah P, Gate DM, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H. 2011. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J Virol 85:4184–4197. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Velzen M, Jing L, Osterhaus AD, Sette A, Koelle DM, Verjans GM. 2013. Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog 9:e1003547. doi: 10.1371/journal.ppat.1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A 104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Lint AL, Kleinert L, Clarke SR, Stock A, Heath WR, Carbone FR. 2005. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J Virol 79:14843–14851. doi: 10.1128/JVI.79.23.14843-14851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593–603. doi: 10.1016/S1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol 163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derfuss T, Segerer S, Herberger S, Sinicina I, Hufner K, Ebelt K, Knaus HG, Steiner I, Meinl E, Dornmair K, Arbusow V, Strupp M, Brandt T, Theil D. 2007. Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol 17:389–398. doi: 10.1111/j.1750-3639.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mott KR, Bresee CJ, Allen SJ, BenMohamed L, Wechsler SL, Ghiasi H. 2009. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J Virol 83:2246–2254. doi: 10.1128/JVI.02234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. 2010. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol 184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Chentoufi AA, Hsiang C, Carpenter D, Osorio N, Benmohamed L, Fraser NW, Jones C, Wechsler SL. 2011. The herpes simplex virus type 1 latency associated transcript (LAT) can protect neuron-derived C1300 and Neuro2A cells from granzyme B induced apoptosis and CD8 T-cell killing. J Virol 85:2325–2332. doi: 10.1128/JVI.01791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BenMohamed L, Osorio N, Khan AA, Srivastava R, Huang L, Krochma JJ, Garcia LM, Simpson JL, Wechsler SL. 2015. Prior corneal scarification and injection of immune serum are not required before ocular HSV-1 infection for UV-B induced virus reactivation and recurrent herpetic corneal disease in latently infected mice. Curr Eye Res 23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BenMohamed L, Osorio N, Srivastava R, Khan AA, Simpson JL, Wechsler SL. 2015. Decreased reactivation of a herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) mutant using the in vivo mouse UV-B model of induced reactivation. J Neurovirol 21:508–517. doi: 10.1007/s13365-015-0348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perng GC, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, Slanina SM, Hofman FM, Ghiasi H, Nesburn AB, Wechsler SL. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 19.Allen SJ, Mott KR, Chentoufi AA, BenMohamed L, Wechsler SL, Ballantyne CM, Ghiasi H. 2011. CD11c controls herpes simplex virus 1 responses to limit virus replication during primary infection. J Virol 85:9945–9955. doi: 10.1128/JVI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, Hsiang C, Nesburn AB, Wechsler SL, BenMohamed L. 2011. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J Virol 85:9127–9138. doi: 10.1128/JVI.00587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Held K, Junker A, Dornmair K, Meinl E, Sinicina I, Brandt T, Theil D, Derfuss T. 2011. Expression of herpes simplex virus 1-encoded microRNAs in human trigeminal ganglia and their relation to local T-cell infiltrates. J Virol 85:9680–9685. doi: 10.1128/JVI.00874-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leib DA, Nadeau KC, Rundle SA, Schaffer PA. 1991. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc Natl Acad Sci U S A 88:48–52. doi: 10.1073/pnas.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawtell NM, Thompson RL. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol 66:2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill JM, Sedarati F, Javier RT, Wagner EK, Stevens JG. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- 25.Perng GC, Dunkel EC, Geary PA, Slanina SM, Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol 68:8045–8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perng GC, Chokephaibulkit K, Thompson RL, Sawtell NM, Slanina SM, Ghiasi H, Nesburn AB, Wechsler SL. 1996. The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J Virol 70:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. 2007. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol 81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. 1998. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology 252:200–209. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- 29.Nesburn AB, Elliott JH, Leibowitz HM. 1967. Spontaneous reactivation of experimental herpes simplex keratitis in rabbits. Arch Ophthalmol 78:523–529. doi: 10.1001/archopht.1967.00980030525021. [DOI] [PubMed] [Google Scholar]
- 30.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. 2008. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol 180:426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 31.Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, Scarfone VM, McKinney DM, Sidney J, Sette A, Nesburn AB, Wechsler SL, BenMohamed L. 2013. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol 191:5124–5138. doi: 10.4049/jimmunol.1301415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan AA, Srivastava R, Lopes PP, Wang C, Pham TT, Cochrane J, Thai NT, Gutierrez L, Benmohamed L. 2014. Asymptomatic memory CD8+ T cells: from development and regulation to consideration for human vaccines and immunotherapeutics. Hum Vaccin Immunother 10:945–963. doi: 10.4161/hv.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. 2006. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol 177:8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 34.Perng GC, Ghiasi H, Slanina SM, Nesburn AB, Wechsler SL. 1996. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J Virol 70:976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin L, Peng W, Perng GC, Brick DJ, Nesburn AB, Jones C, Wechsler SL. 2003. Identification of herpes simplex virus type 1 latency-associated transcript sequences that both inhibit apoptosis and enhance the spontaneous reactivation phenotype. J Virol 77:6556–6561. doi: 10.1128/JVI.77.11.6556-6561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. 2005. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine 23:873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. 1998. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol 72:7715–7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. 1998. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci 39:1163–1170. [PubMed] [Google Scholar]
- 39.Webre JM, Hill JM, Nolan NM, Clement C, McFerrin HE, Bhattacharjee PS, Hsia V, Neumann DM, Foster TP, Lukiw WJ, Thompson HW. 2012. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J Biomed Biotechnol 7:12–16. doi: 10.1155/2012/612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jester JV, Morishige N, BenMohamed L, Brown DJ, Osorio N, Hsiang C, Perng GC, Jones C, Wechsler SL. 2016. Confocal microscopic analysis of a rabbit eye model of high-incidence recurrent herpes stromal keratitis. Cornea 35:81–88. doi: 10.1097/ICO.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava R, Khan AA, J H, Nesburn AB, Wechsler SL, BenMohamed L. 2015. A herpes simplex virus type 1 human asymptomatic CD8+ T cell epitope-based vaccine protects against ocular herpes in “humanized” HLA transgenic rabbit model. Invest Ophthalmol Vis Sci 56:4013–4028. doi: 10.1167/iovs.15-17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillere B, BenMohamed L. 2008. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol 82:11792–11802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chentoufi AA, BenMohamed L. 2010. Future viral vectors for the delivery of asymptomatic herpes epitope-based immunotherapeutic vaccines. Future Virol 5:525–528. doi: 10.2217/fvl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chentoufi AA, Dasgupta G, Nesburn AB, Bettahi I, Binder NR, Choudhury ZS, Chamberlain WD, Wechsler SL, BenMohamed L. 2010. Nasolacrimal duct closure modulates ocular mucosal and systemic CD4(+) T-cell responses induced following topical ocular or intranasal immunization. Clin Vaccine Immunol 17:342–353. doi: 10.1128/CVI.00347-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng W, Henderson G, Inman M, BenMohamed L, Perng GC, Wechsler SL, Jones C. 2005. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal ganglia of acutely infected mice. J Virol 79:6162–6171. doi: 10.1128/JVI.79.10.6162-6171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan AA, Srivastava R, Lopes PP, Wang C, Pham TT, Cochrane J, Thai NT, Gutierrez L, BenMohamed L. 2014. Asymptomatic memory CD8 T cells: From development and regulation to consideration for human vaccines and immunotherapeutics. Hum Vaccin Immunother 10:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F, DS, Chentoufi AA, Lemonnier FA, BenMohamed L. 2014. Associations of HLA-A, HLA-B and HLA-C alleles frequency with prevalence of herpes simplex virus infections and diseases across global populations: implication for the development of an universal CD8+ T-cell epitope-based vaccine. Hum Immunol 75:715–729. doi: 10.1016/j.humimm.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chentoufi AA, Dervillez X, Dasgupta G, Nguyen C, Kabbara KW, Jiang X, Nesburn AB, Wechsler SL, BenMohamed L. 2012. The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells. Viral Immunol 25:204–215. doi: 10.1089/vim.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Block TM, Spivack JG, Steiner I, Deshmane S, McIntosh MT, Lirette RP, Fraser NW. 1990. A herpes simplex virus type 1 latency-associated transcript mutant reactivates with normal kinetics from latent infection. J Virol 64:3417–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devi-Rao GB, Aguilar JS, Rice MK, Garza HH Jr, Bloom DC, Hill JM, Wagner EK. 1997. Herpes simplex virus genome replication and transcription during induced reactivation in the rabbit eye. J Virol 71:7039–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perng GC, Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL. 1994. An improved method for cloning portions of the repeat regions of herpes simplex virus type 1. J Virol Methods 46:111–116. doi: 10.1016/0166-0934(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed M, Lock M, Miller CG, Fraser NW. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J Virol 76:717–729. doi: 10.1128/JVI.76.2.717-729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perng GC, Maguen B, Jin L, Mott KR, Osorio N, Slanina SM, Yukht A, Ghiasi H, Nesburn AB, Inman M, Henderson G, Jones C, Wechsler SL. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J Virol 76:1224–1235. doi: 10.1128/JVI.76.3.1224-1235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trousdale MD, Steiner I, Spivack JG, Deshmane SL, Brown SM, MacLean AR, Subak-Sharpe JH, Fraser NW. 1991. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J Virol 65:6989–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hufner K, Derfuss T, Herberger S, Sunami K, Russell S, Sinicina I, Arbusow V, Strupp M, Brandt T, Theil D. 2006. Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J Neuropathol Exp Neurol 65:1022–1030. doi: 10.1097/01.jnen.0000235852.92963.bf. [DOI] [PubMed] [Google Scholar]
- 57.Simmons A, Tscharke DC. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med 175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimeld C, Whiteland JL, Nicholls SM, Grinfeld E, Easty DL, Gao H, Hill TJ. 1995. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol 61:7–16. doi: 10.1016/0165-5728(95)00068-D. [DOI] [PubMed] [Google Scholar]
- 59.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 60.Vali B, Jones RB, Sakhdari A, Sheth PM, Clayton K, Yue FY, Gyenes G, Wong D, Klein MB, Saeed S, Benko E, Kovacs C, Kaul R, Ostrowski MA. 2010. HCV-specific T cells in HCV/HIV coinfection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur J Immunol 40:2493–2505. doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 61.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mueller SN, Ahmed R. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Umbach JL, Wang K, Tang S, Krause PR, Mont EK, Cohen JI, Cullen BR. 2010. Identification of viral microRNAs expressed in human sacral ganglia latently infected with herpes simplex virus 2. J Virol 84:1189–1192. doi: 10.1128/JVI.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. 2008. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454:780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng W, Vitvitskaia O, Carpenter D, Wechsler SL, Jones C. 2008. Identification of two small RNAs within the first 1.5-kb of the herpes simplex virus type 1-encoded latency-associated transcript. J Neurovirol 14:41–52. doi: 10.1080/13550280701793957. [DOI] [PubMed] [Google Scholar]
- 66.Cioffi M, Trabulo SM, Sanchez-Ripoll Y, Miranda-Lorenzo I, Lonardo E, Dorado J, Reis Vieira C, Ramirez JC, Hidalgo M, Aicher A, Hahn S, Sainz B Jr, Heeschen C. 2015. The miR-17-92 cluster counteracts quiescence and chemoresistance in a distinct subpopulation of pancreatic cancer stem cells. Gut doi: 10.1136/gutjnl-2014-308470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lechman ER, Gentner B, van Galen P, Giustacchini A, Saini M, Boccalatte FE, Hiramatsu H, Restuccia U, Bachi A, Voisin V, Bader GD, Dick JE, Naldini L. 2012. Attenuation of miR-126 activity expands HSC in vivo without exhaustion. Cell Stem Cell 11:799–811. doi: 10.1016/j.stem.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swaminathan S, Kelleher AD. 2014. MicroRNA modulation of key targets associated with T cell exhaustion in HIV-1 infection. Curr Opin HIV AIDS 9:464–471. doi: 10.1097/COH.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Zhang Z, Ji D, Chen GF, Feng X, Gong LL, Guo J, Li ZW, Chen CF, Zhao BB, Li ZG, Li QJ, Yan HP, Sempowski G, Wang FS, He YW. 2015. Regulation of T cell function by microRNA-720. Sci Rep 5:12159. doi: 10.1038/srep12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. 2006. Latent virus influences the generation and maintenance of CD8+ T cell memory. J Immunol 177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frank GM, Lepisto AJ, Freeman ML, Sheridan BS, Cherpes TL, Hendricks RL. 2010. Early CD4+ T cell help prevents partial CD8+ T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J Immunol 184:277–286. doi: 10.4049/jimmunol.0902373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neumann J, Eis-Hubinger AM, Koch N. 2003. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J Immunol 171:3075–3083. doi: 10.4049/jimmunol.171.6.3075. [DOI] [PubMed] [Google Scholar]
- 73.Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, Brandt T. 2001. Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol 11:408–413. doi: 10.1111/j.1750-3639.2001.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.St Leger AJ, Jeon S, Hendricks RL. 2013. Broadening the repertoire of functional herpes simplex virus type 1-specific CD8+ T cells reduces viral reactivation from latency in sensory ganglia. J Immunol 191:2258–2265. doi: 10.4049/jimmunol.1300585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Held K, Eiglmeier I, Himmelein S, Sinicina I, Brandt T, Theil D, Dornmair K, Derfuss T. 2011. Clonal expansions of CD8+ T cells in latently HSV-1-infected human trigeminal ganglia. J Neurovirol 18:62–68. doi: 10.1007/s13365-011-0067-9. [DOI] [PubMed] [Google Scholar]
- 76.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 78.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishida Y, Agata Y, Shibahara K, Honjo T. 1992. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C, Douglas MW. 2006. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis 194(Suppl 1):S11–S18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 81.Gebhardt BM, Halford WP. 2005. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J 2:67–73. doi: 10.1186/1743-422X-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K-. 2015. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 83.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. 2015. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small-cell lung cancer. Science 348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. 2015. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. 2011. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae 2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]