ABSTRACT
Inflammasomes are cytosolic multimolecular protein complexes that stimulate the activation of caspase-1 and the release of mature forms of interleukin-1β (IL-1β) and IL-18. We previously demonstrated that the influenza A virus M2 protein stimulates IL-1β secretion following activation of the nucleotide-binding oligomerization domain (NOD)-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome. The nonstructural protein 1 (NS1) of influenza virus inhibits caspase-1 activation and IL-1β secretion. However, the precise mechanism by which NS1 inhibits IL-1β secretion remains unknown. Here, we showed that J774A.1 macrophages stably expressing the NS1 protein inhibited IL-1β secretion after infection with recombinant influenza virus lacking the NS1 gene. Coimmunoprecipitation assay revealed that the NS1 protein interacts with NLRP3. Importantly, the NS1 protein inhibited the NLRP3/ASC-induced single-speck formation required for full activation of inflammasomes. The NS1 protein of other influenza virus strains, including a recent pandemic strain, also inhibited inflammasome-mediated IL-1β secretion. The NS1 RNA-binding domain (basic residues 38 and 41) and TRIM25-binding domain (acidic residues 96 and 97) were required for suppression of NLRP3 inflammasome-mediated IL-1β secretion. These results shed light on a mechanism by which the NS1 protein of influenza virus suppresses NLRP3 inflammasome-mediated IL-1β secretion.
IMPORTANCE Innate immune sensing of influenza virus via pattern recognition receptors not only plays a key role in generating type I interferons but also triggers inflammatory responses. We previously demonstrated that the influenza A virus M2 protein activates the NLRP3 inflammasome, leading to the secretion of interleukin-1β (IL-1β) and IL-18 following the activation of caspase-1. Although the nonstructural protein 1 (NS1) of influenza virus inhibits IL-1β secretion, the precise mechanism by which it achieves this remains to be defined. Here, we demonstrate that the NS1 protein interacts with NLRP3 to suppress NLRP3 inflammasome activation. J774A.1 macrophages stably expressing the NS1 protein suppressed NLRP3-mediated IL-1β secretion. The NS1 RNA-binding domain (basic residues 38 and 41) and TRIM25-binding domain (acidic residues 96 and 97) are important for suppression of NLRP3 inflammasome-mediated IL-1β secretion. These results will facilitate the development of new anti-inflammatory drugs.
INTRODUCTION
Influenza A virus, a member of the family Orthomyxoviridae, is an enveloped virus with an eight-segmented single-stranded negative-sense RNA genome. The virus causes a highly contagious disease of the human upper respiratory tract. The recognition of viruses (e.g., influenza virus) plays a key role not only in limiting virus replication and inflammatory responses at early stages of infection but also in initiating and orchestrating virus-specific adaptive immune responses (1–6). Infection by influenza virus is recognized by at least three classes of host pattern recognition receptors, including Toll-like receptor 7 (TLR7), retinoic acid-inducible gene-I (RIG-I), and NLRP3 (nucleotide-binding oligomerization domain [NOD]-like receptor family pyrin domain-containing 3) (7, 8). First, influenza virus genomic RNA is recognized by TLR7 within endosomal compartments (9, 10). Second, the cytosolic sensor RIG-I directly interacts with the panhandle structure of the viral nucleocapsid and detects the uncapped 5′-triphosphate RNA of the viral genome (11–14). Third, the influenza virus M2 protein, a proton-selective ion channel, stimulates ion flux from the trans-Golgi network and activates the NLRP3 inflammasome (15). Upon activation, NLRP3 is recruited to the mitochondria via mitochondrial antiviral signaling (MAVS) or mitofusin 2 (Mfn2) and forms the multimolecular protein complex termed the NLRP3 inflammasome (16, 17). This event activates the downstream molecule caspase-1, which cleaves the precursor forms of proinflammatory cytokines, such as interleukin-1β (IL-1β) and IL-18, and stimulates their secretion across the plasma membrane (18). These inflammasome-dependent cytokines play a key role in the induction of influenza virus-specific adaptive immune responses and the initiation of tissue repair following infectious damage (1, 3, 5, 6).
Recent reports indicate that several distinct inflammasomes, including the NLRP3 inflammasome, the NLRP1 inflammasome, the RIG-I inflammasome, the absent-in-melanoma 2 (AIM2) inflammasome, and the interferon gamma (IFN-γ)-inducible protein 16 (IFI16) inflammasome, are involved in viral recognition (7). NLRP3 senses cellular damage or distress induced by the viroporins of RNA viruses such as influenza virus (15), encephalomyocarditis virus (EMCV) (19), rhinovirus (20), or severe acute respiratory syndrome (SARS) coronavirus (21). In contrast, infection with vesicular stomatitis virus (VSV) or transfection with 5′-triphosphate RNA may activate the RIG-I inflammasome (22). Furthermore, the AIM2 inflammasome is activated by intracellular double-stranded DNA derived from DNA viruses (23–26). The IFI16 inflammasome in the nucleus is activated by the DNA genome of Kaposi's sarcoma-associated herpesvirus (KSHV) (27). Therefore, each of these viruses has developed strategies to evade host innate immune recognition systems.
The influenza virus PB1-F2 protein dissipates the mitochondrial membrane potential [ΔΨ(m)], which is required for full activation of the NLRP3 inflammasome and for MAVS-mediated antiviral signaling (16, 28, 29). In addition, the NS1 protein of influenza virus inhibits host interferon (IFN) responses either by sequestering viral RNA or by binding to RIG-I and other proteins required for RIG-I and IFN signaling pathways (30–34). Although the NS1 protein of influenza virus inhibits virus-induced IL-1β secretion following activation of caspase-1 (35), the precise mechanism by which it achieves this is unclear. Here, we examined the role of the NS1 protein in activating the NLRP3 inflammasome.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from The Jackson Laboratory. All animal experiments were approved by the Animal Committees of the Institute of Medical Science (The University of Tokyo).
Cells and viruses.
Bone marrow-derived plasmacytoid dendritic cells (pDCs) were prepared as described previously (36). In brief, bone marrow was extracted from the tibia and femur by flushing with RPMI 1640 medium (Nacalai Tesque). Bone marrow cells were then cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and recombinant human Flt3 ligand (Flt3L; 100 μg/ml [PeproTech]) at 37°C for 7 days. J774A.1 and HeLa cells and the human embryonic kidney cell line, 293T (HEK293T), were maintained in Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque) supplemented with 10% FBS. Madin-Darby canine kidney (MDCK) cells were grown in Eagle's minimal essential medium (E-MEM; Nacalai Tesque) supplemented with 10% FBS.
Influenza virus A/Puerto Rico/8/34 (H1N1) (PR8) was grown in the allantoic cavities of 10-day-old fertile chicken eggs at 35°C for 2 days (3). Influenza A virus lacking the NS1 gene (37) was grown in MDCK cells stably expressing the influenza virus NS1 protein at 37°C for 2 days. Viruses were stored at −80°C, and the viral titer was quantified in a standard plaque assay using MDCK cells.
Plasmids.
cDNAs encoding the influenza virus NS1 proteins of PR8 (H1N1) and A/Narita/1/2009 (H1N1) (Narita/2009) were obtained by reverse transcription and PCR of total RNA extracted from influenza virus-infected MDCK cells, followed by PCR using specific primers. Oligonucleotides corresponding to both strands of the full-length sequence of NS1 proteins from influenza virus strains A/Brevig Mission/1/18 (H1N1) (BM/1918), A/Hong Kong/483/1997 (H5N1) (HK/1997), and A/Anhui/1-BALF_RG1/2013 (H7N9) (Anhui/2013) and containing EcoRI and NotI sites at the 5′ and 3′ ends were synthesized (Eurofins Genomics) and cloned into the eukaryotic expression vectors pCA7-Flag (38) (a derivative of pCAGGS [39]) to produce Flag-tagged proteins. The R38A/K41A and E96A/E97A NS1 mutants were constructed by standard PCR-based methods. The integrity of the inserts was verified by sequencing. Plasmids encoding Flag-, myc-, or enhanced green fluorescent protein (EGFP)-tagged or untagged human NLRP3, RIG-I, ASC (apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain), procaspase-1, pro-IL-1β, uncoupling protein-2 (UCP-2), or influenza virus PB1-F2 protein were described previously (16, 19, 28, 29, 40).
Lentiviral vectors.
To generate lentiviruses expressing the influenza virus NS1 protein, the full-length cDNA encoding the NS1 protein was cloned into the pLenti6.3/V5-TOPO vector (Invitrogen). 293FT cells cultured in a collagen-coated 10-cm dish were transfected with 3 μg of NS1 protein-expressing pLenti6.3/V5-TOPO vector together with ViraPower Packaging Mix (Invitrogen) using Lipofectamine 2000 (Invitrogen). The culture medium was replaced with fresh medium 24 h later. At 72 to 96 h posttransfection, the lentivirus-containing supernatants were harvested. A lentivirus encoding an irrelevant protein (EGFP) served as a control. The stock virus, containing Polybrene (10 μg/ml), was then inoculated into J774A.1 or MDCK cells. The culture medium was replaced with fresh medium 24 h later. Finally, the cells were cultured for 2 to 3 weeks in complete medium containing blasticidin (10 μg/ml) to kill nontransduced cells.
Infection.
J774A.1 cells were infected with influenza virus at a multiplicity of infection (MOI) of 5 for 1 h at 37°C, washed with phosphate-buffered saline (PBS), and then cultured in complete DMEM for 18 to 24 h. Unless otherwise stated, all experiments were performed in lipopolysaccharide (LPS)-primed J774A.1 cells. Bone marrow-derived pDCs (5 × 105 cells in 96-well round-bottom plates) were stimulated with CpG-A (10 μg/ml; Invivogen) or influenza virus at an MOI of 0.5 for 24 h at 37°C.
ELISA.
Cell-free supernatants were collected at 18 to 24 h postinfection or at 6 h after stimulation with LPS plus ATP. The supernatants were analyzed for the presence of IL-1β or IFN-α using an enzyme-linked immunosorbent assay (ELISA) utilizing paired antibodies (eBiosciences) (15, 41). To measure intracellular pro-IL-1β levels, cells were lysed by repeated cycles of freezing and thawing in PBS containing 2% FBS. The lysates were then analyzed by ELISA (16, 22, 40).
Coimmunoprecipitation and Western blot analysis.
Subconfluent monolayers of HEK293T cells in 24-well cluster plates were cotransfected with 0.5 μg each of pCA7-EGFP, pCA7-Flag-NLRP3, pCA7-Flag-ASC, or pCA7-Flag-RIG-I together with 0.5 μg of pcDNA3.1-myc-NS1. At 24 h posttransfection, the cells were washed with PBS and lysed in 500 μl of 1× TNT buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10% glycerol) containing protease inhibitors (Sigma). Lysates were centrifuged at 20,630 × g for 10 min at 4°C. A small amount (50 μl) of each supernatant was mixed with sodium dodecyl sulfate (SDS) loading buffer (50 mM Tris [pH 6.8], 100 mM dithiothreitol [DTT], 2% SDS, 0.1% bromophenol blue, 10% glycerol) and boiled for 5 min. The rest of the supernatant was incubated for 60 min at 4°C with protein G-Sepharose (GE Healthcare AB), which had been pretreated with an anti-Flag (M2; Sigma) or normal mouse IgG1 (sc-3877; Santa Cruz) antibody overnight at 4°C. Complexes were obtained by centrifugation and washed three times with coimmunoprecipitation buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1 mM EDTA). The polypeptides within the precipitated complexes were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) (10 to 15% gels) and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore). The membranes were incubated with rabbit anti-mouse IL-1β (Chemicon), mouse anti-influenza A virus M2 (14C2; Abcam), mouse anti-influenza A virus NS1 (NS1-23-1; Santa Cruz), mouse anti-tubulin (DM1A; Santa Cruz), mouse anti-NLRP3 (Cryo-2; AdipoGen), mouse anti-myc (9E10), or mouse anti-Flag (M2; Sigma) antibody, followed by incubation with horseradish peroxidase-conjugated anti-mouse IgG (Jackson Immuno Research Laboratories) or anti-rabbit IgG (Invitrogen). The PVDF membranes were then treated with Chemi-Lumi One Super (Nacalai Tesque) to elicit chemiluminescent signals, which were detected and visualized using an LAS-4000 Mini apparatus (GE Healthcare).
Flow cytometry analysis.
The mitochondrial membrane potential [ΔΨ(m)] was measured by staining the cells with a cationic fluorescent dye, tetramethylrhodamine methyl ester (TMRM) (Molecular Probes/Invitrogen), according to the manufacturer's instructions. In brief, cells (∼1 × 106 cells per ml) were treated with carbonyl cyanide m-chlorophenylhydrazone (CCCP; Sigma-Aldrich) or transfected with plasmids, washed once with PBS, and harvested into a centrifuge tube. The cells were then resuspended in 1 ml of PBS containing 2 μM TMRM and incubated at 37°C for 30 min. After three washes with PBS, flow cytometry analysis was performed in a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Inc.).
Reconstitution of the NLRP3 inflammasome in HEK293T cells.
HEK293T cells, grown to ∼90% confluence in 24-well cluster plates, were transfected with pCA7-NLRP3 (30 ng), pCA7-ASC (5 ng), pCA7-procaspase-1 (5 ng), and pCA7-pro-IL-1β (150 ng) and 500 ng of either pCA7-EGFP, pCA7-hUCP-2 (where hUCP-2 is human UCP-2), or pCA7-NS1 using polyethylenimine (PEI) Max. Cell-free supernatants were collected 24 h after transfection, and IL-1β levels were measured in an ELISA.
To analyze the activation of caspase-1, HEK293T cells in 24-well cluster plates were transfected with pCA7-NLRP3 (30 ng), pCA7-ASC (5 ng), pCA7-procaspase-1 (500 ng), and pCA7-pro-IL-1β (150 ng) and 500 ng of either pCA7-EGFP, pCA7-Flag-NS1, pCA7-Flag-R38A/K41A, or pCA7-Flag-E96A/E97A using PEI Max. Cell extracts were collected at 24 h posttransfection, and samples were analyzed by immunoblotting with rabbit monoclonal antibody against human caspase-1 (D7F10; Cell Signaling).
Confocal microscopy.
HeLa cells were seeded on coverslips in 24-well cluster plates and transfected with 0.5 μg each of pCA7-myc-NS1, pCA7-Flag-NS1, pCA7-PB1-F2, or control plasmid (empty vector) together with 30 ng of pCA7-EGFP-NLRP3 and 5 ng of pCA7-Flag-ASC or pCA7-HA-ASC (where HA is hemagglutinin). At 24 h posttransfection, cells were fixed and permeabilized with PBS containing 4% formaldehyde and 1% Triton X-100. The cells were then washed with PBS and incubated with rabbit anti-Flag (F7425; Sigma), mouse anti-HA (F-7; Santa Cruz), or mouse anti-myc (9E10) antibody, followed by incubation with Alexa Fluor 647-conjugated goat anti-mouse IgG(H+L) and Alexa Fluor 568-conjugated goat anti-rabbit IgG(H+L) antibodies (Life Technologies).
To analyze the subcellular localization of influenza virus NS1 and PB1-F2 proteins, HeLa cells were seeded on coverslips in 24-well plates and transfected with 0.5 μg each of pCA7-myc-NS1 or pCA7-PB1-F2. At 24 h posttransfection, cells were fixed and permeabilized with PBS containing 4% formaldehyde and 1% Triton X-100. The cells were then washed with PBS and incubated with a rabbit anti-Tom20 (FL-145; Santa Cruz) antibody together with mouse anti-myc (9E10) or mouse anti-PB1-F2 antibody (29), followed by incubation with Alexa Fluor 647-conjugated goat anti-mouse IgG(H+L) and Alexa Fluor 568-conjugated goat anti-rabbit IgG(H+L) (Life Technologies). Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI). The stained cells were then observed under a confocal microscope (A1R+; Nikon).
Statistical analysis.
Statistical significance was tested using a two-tailed Student's t test. P values of <0.05 were considered statistically significant.
RESULTS
The influenza virus NS1 protein inhibits NLRP3 inflammasome-mediated IL-1β secretion.
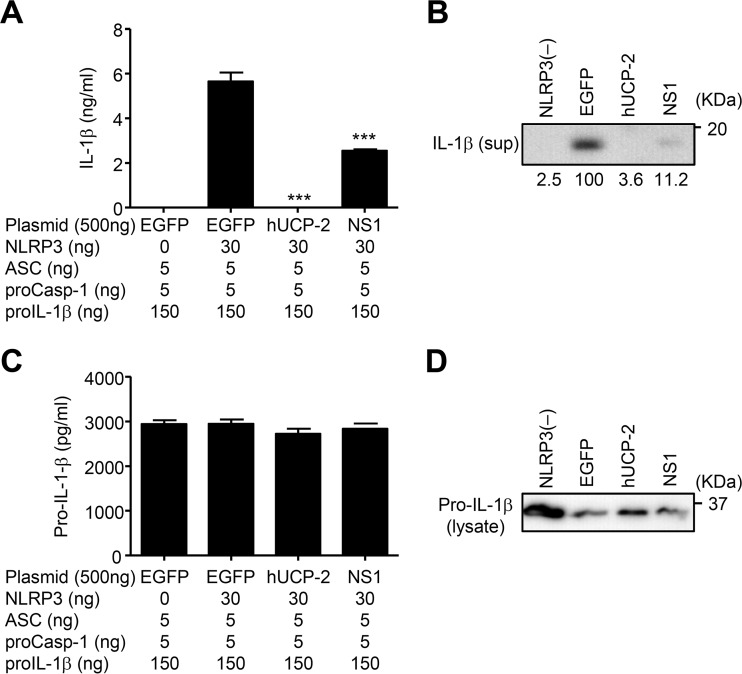
We used an NLRP3 reconstitution assay in HEK293T cells to test whether the NS1 protein of influenza virus inhibits NLRP3 inflammasome-mediated IL-1β secretion because HEK293T cells are deficient in endogenous NLRP3 inflammasomes (42); however, they can produce mature IL-1β upon transfection with plasmids encoding NLRP3, ASC, procaspase-1, and pro-IL-1β (40, 43). Indeed, reconstitution of the NLRP3 inflammasome resulted in IL-1β secretion by HEK293T cells, for which NLRP3 was absolutely required (Fig. 1A and B). Consistent with previous reports (16, 29), transfection of HEK293T cells with human uncoupling protein-2 (hUCP-2), which reduces ΔΨ(m) via proton leakage (44, 45), suppressed IL-1β secretion (Fig. 1A and B). Similarly, NS1 protein significantly inhibited IL-1β secretion (Fig. 1A and B) without affecting the expression of pro-IL-1β in the cytosol (Fig. 1C and D).
FIG 1.
NS1 inhibits NLRP3 inflammasome-mediated IL-1β secretion. (A) HEK293T cells were transfected with expression plasmids encoding either NLRP3, ASC, procaspase-1, pro-IL-1β, and human UCP-2 (hUCP-2) or NS1. pCA7-EGFP was used as a control. (A and B) Cell-free supernatants were collected at 24 h posttransfection, and the mature (p17) form of IL-1β was analyzed by ELISA (A) or immunoblot analysis (B). sup, supernatant. (C and D) Cell extracts were collected at 24 h posttransfection, and the immature form of IL-1β (pro-IL-1β) was analyzed by ELISA (C) or immunoblot analysis (D). Data in panels A and C are representative of at least three independent experiments and are expressed as the means ± standard deviations. ***, P < 0.001.
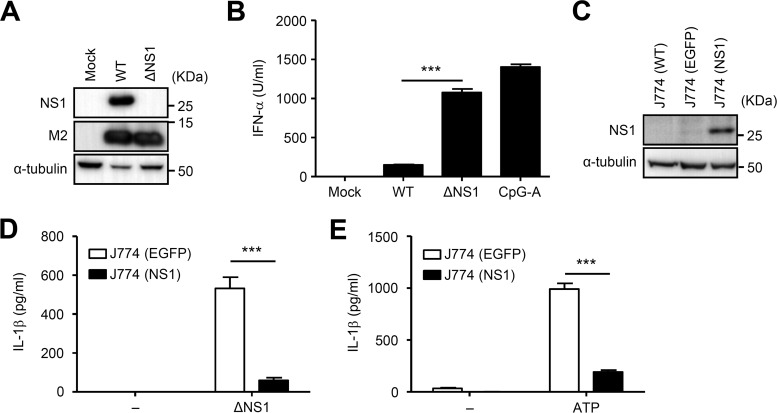
We next examined whether the suppression of NLRP3 inflammasome-mediated IL-1β secretion by NS1 protein in HEK293T cells can be reproduced by recombinant influenza virus lacking the NS1 gene (ΔNS1 virus). We first confirmed the deletion of the NS1 protein in HEK293T cells infected with ΔNS1 virus by immunoblotting (Fig. 2A). Consistent with a previous report (12), bone marrow-derived pDCs produced more IFN-α in response to mutant ΔNS1 virus than in response to the wild-type (WT) influenza virus (Fig. 2B). Since the mutant ΔNS1 virus has a lower replicative capacity than wild-type influenza virus in vitro (37), we decided to compare IL-1β secretion by EGFP-expressing and NS1 protein-expressing J774A.1 macrophages infected with ΔNS1 virus. To this end, we established J774A.1 macrophages stably expressing EGFP or the NS1 protein (Fig. 2C). J774A.1 macrophages stably expressing the NS1 protein secreted significantly less IL-1β in response to ΔNS1 virus or LPS plus ATP (NLRP3 agonist) than control cells (Fig. 2D and E), indicating that the NS1 protein inhibits NLRP3 inflammasome-mediated IL-1β secretion. Thus, the inhibitory activity of the NS1 protein appears to be specific for the NLRP3 inflammasome.
FIG 2.
Influenza virus lacking the NS1 gene failed to stimulate IL-1β secretion from J774A.1 cells stably expressing the NS1 protein. (A) HEK293T cells were infected with wild-type (WT) or recombinant influenza virus lacking the NS1 gene (ΔNS1). Cell extracts were collected at 24 h postinfection, and NS1 and M2 protein expression was analyzed by immunoblotting. (B) Flt3L-cultured bone marrow pDCs were stimulated with CpG-A DNA (10 μg/ml) or WT or ΔNS1 influenza virus (MOI of 0.5) for 24 h. Cell-free supernatants were collected at 24 h poststimulation and analyzed for IFN-α by ELISA. (C) Samples from J774A.1 cells stably expressing EGFP or NS1 were analyzed by immunoblot analysis. (D and E) LPS-primed J774A.1 cells stably expressing EGFP or NS1 were stimulated with ΔNS1 or ATP. Cell-free supernatants were collected at 24 h poststimulation and analyzed for IL-1β by ELISA. Data are expressed as the means ± standard deviations. Similar results were obtained in three separate experiments. ***, P < 0.001.
The mitochondrial membrane potential is not affected by the NS1 protein.
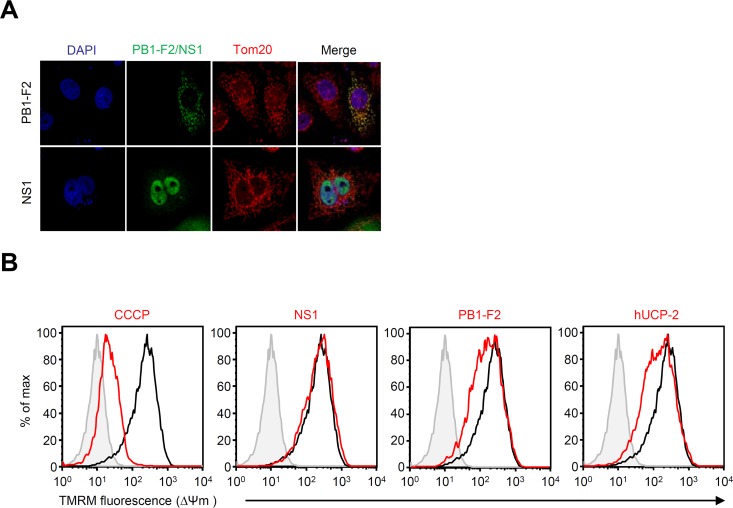
We previously demonstrated that the PB1-F2 protein of influenza virus dissipated ΔΨ(m) and suppressed NLRP3 inflammasome-mediated IL-1β secretion (29). To gain insight into the mechanism by which the NS1 protein inhibits NLRP3 inflammasome activation, we first measured ΔΨ(m) (which is required for full activation of the NLRP3 inflammasome) (16). Consistent with previous reports (16, 29), the PB1-F2 protein localized to mitochondria and dissipated ΔΨ(m) (Fig. 3A and B). Similarly, ΔΨ(m) was dissipated in cells treated with the protonophore carbonyl cyanide m-chlorophenylhydrazine (CCCP) and in cells transfected with hUCP-2 (Fig. 3B). In contrast, the NS1 protein of influenza virus did not localize to mitochondria and alter ΔΨ(m) (Fig. 3A and B), indicating that the mechanism by which the NS1 protein inhibits NLRP3 inflammasome-mediated IL-1β secretion is different from that of the PB1-F2 protein.
FIG 3.
NS1 does not reduce mitochondrial membrane potential. (A) HeLa cells were transfected with an expression plasmid encoding influenza virus PB1-F2 or myc-tagged NS1. Twenty-four hours later, cells were stained with anti-myc, anti-PB1-F2, and anti-Tom20 (mitochondrion marker) antibodies and analyzed by confocal microscopy. Nuclei were visualized by staining with DAPI. (B) HEK293T cells were treated with CCCP (40 μM) or transfected with an expression plasmid encoding influenza virus PB1-F2 or NS1. Twenty-four hours later, the cells were stained with TMRM and analyzed by flow cytometry. Similar results were obtained in three separate experiments. max, maximum.
NS1 protein interacts with NLRP3.
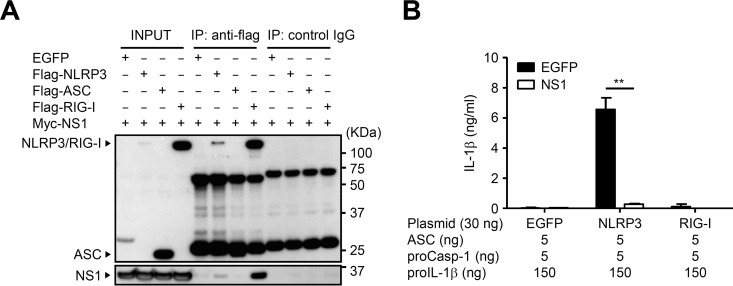
We next examined the association between the NS1 protein and components of the NLRP3 inflammasome by performing coimmunoprecipitation experiments using HEK293T cells. In agreement with a previous report (12), the NS1 protein coimmunoprecipitated with RIG-I in HEK293T cells. The NS1 protein coimmunoprecipitated with NLRP3 to a lesser extent than in cells cotransfected with RIG-I (Fig. 4A). Recent reports indicate that both the NLRP3 and RIG-I inflammasomes are involved in recognition of influenza virus or cytosolic viral RNA (1, 3, 6, 15, 22). So, we next examined whether the NS1 protein of influenza virus inhibits NLRP3 and RIG-I inflammasome-mediated IL-1β secretion. Reconstitution of the NLRP3, but not RIG-I, inflammasome resulted in IL-1β secretion by HEK293T cells (Fig. 4B). Inclusion of the NS1 protein significantly inhibited NLRP3 inflammasome-mediated IL-1β secretion (Fig. 4B). These results suggest that the NS1 protein interacts with NLRP3 and inhibits the NLRP3 inflammasome-mediated IL-1β secretion.
FIG 4.
NS1 protein interacts with NLRP3. (A) HEK293T cells were cotransfected with expression plasmids encoding Flag-tagged NLRP3, ASC, or RIG-I together with myc-tagged NS1. pCA7-EGFP was used as a control. At 24 h posttransfection, proteins were immunoprecipitated with an anti-Flag antibody, followed by immunoblotting of total lysate (Input) and immunoprecipitates (IP) with anti-Flag or anti-myc antibodies. (B) HEK293T cells were transfected with expression plasmids as indicated. pCA7-EGFP was used as a control. Cell-free supernatants were collected at 24 h posttransfection and analyzed for IL-1β by ELISA. Data in panel B are representative of at least three independent experiments and are expressed as the means ± standard deviations. **, P < 0.01.
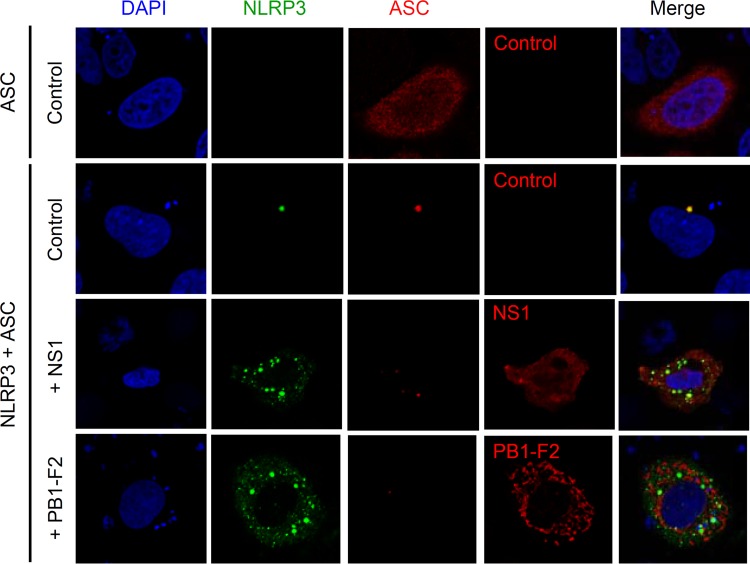
NS1 protein inhibits a single-speck formation of NLRP3.
Upon activation of the inflammasomes, ASC forms speck-like structures, termed pyroptosomes (25, 46). It has been reported that ASC forms specks typically with one speck per cell when HEK293T cells are transfected with plasmids encoding ASC. The lack of a single-speck formation correlates with a loss of IL-1β processing in response to NLRP3 agonists in RAW264.7 cell (47). Thus, we next examined whether the NS1 protein of influenza virus inhibits a single-speck formation. To this end, we cotransfected HeLa cells with EGFP-tagged NLRP3 and Flag-tagged ASC to visualize speck formation. When HeLa cells were transfected with Flag-tagged ASC alone, most of the ASC protein localized to the nucleus and other cytoplasmic structures (Fig. 5). Transfection of HeLa cells with EGFP-tagged NLRP3 and Flag-tagged ASC led to a single-speck formation, which colocalized with NLRP3 and ASC (Fig. 5). In contrast, NLRP3 and ASC formed discrete specks when HeLa cells were cotransfected with EGFP-tagged NLRP3, Flag-tagged ASC, and myc-tagged NS1 or PB1-F2 (Fig. 5). Taken together, these results suggest that the NS1 protein of influenza virus inhibits a single-speck formation of NLRP3 and ASC.
FIG 5.
Inhibition of a single-speck formation by influenza virus NS1 protein. HeLa cells were cotransfected with expression plasmids encoding Flag-tagged ASC, EGFP-NLRP3, and myc-tagged NS1. PB1-F2 was used as a positive control. At 24 h after transfection, cells were stained with anti-Flag, anti-myc, and anti-PB1-F2 antibodies and analyzed by confocal microscopy. Nuclei were visualized by staining with DAPI. Data are representative of at least three independent experiments.
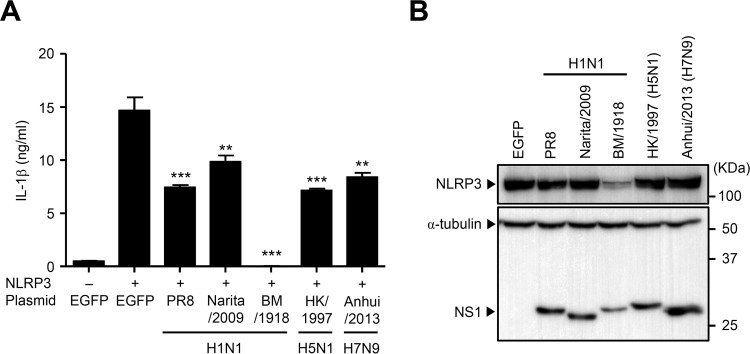
The NS1 proteins from other influenza virus strains inhibit NLRP3 inflammasome-mediated IL-1β secretion.
So far, we used the NS1 gene derived from the mouse-adapted A/Puerto Rico/8/34 (H1N1) (denoted PR8) strain. Next, we compared the inhibitory effect of NS1 proteins from other influenza viruses, including those from recent pandemic strains A/Narita/1/2009 (H1N1) (denoted Narita/2009), A/Brevig Mission/1/1918 (H1N1) (denoted BM/1918), A/Hong Kong/483/1997 (H5N1) (denoted HK/1997), and A/Anhui/1-BALF-RG1/2013 (H7N9) (denoted Anhui/2013), on NLRP3 inflammasome activation to determine whether viral pathogenesis could be explained (at least in part) by the ability of the respective NS1 proteins to inhibit host inflammatory responses. The NS1 protein from all five viral strains significantly inhibited NLRP3-mediated IL-1β secretion compared with the level in EGFP-transfected cells (Fig. 6A). Strikingly, IL-1β secretion was completely abrogated by transfecting cells with a plasmid encoding the BM/1918 NS1 protein. It has been reported that the NS1 protein of BM/1918 has an intact CPSF30-binding site that blocks host mRNA maturation (48, 49). Indeed, this inhibitory effect of IL-1β secretion was due to suppression of NLRP3 expression in BM/1918 NS1-transfected cells (Fig. 6B). Taken together, these data indicate that the NS1 protein from not only a mouse-adapted laboratory strain but also other human isolates inhibits NLRP3 inflammasome-mediated IL-1β secretion.
FIG 6.
The NS1 protein from different strains suppresses NLRP3 inflammasome-mediated IL-1β secretion. (A) HEK293T cells were transfected with expression plasmids encoding NLRP3, ASC, procaspase-1, pro-IL-1β, and NS1 from different virus strains as indicated. pCA7-EGFP was used as a control. Cell-free supernatants were collected at 24 h posttransfection, and IL-1β was analyzed by ELISA. (B) HEK293T cells were transfected as described for panel A. Cell extracts were collected at 24 h posttransfection, and NLRP3 expression was analyzed by immunoblotting. Data in panel A are representative of at least three independent experiments and are expressed as the means ± standard deviations. **, P < 0.01; ***, P < 0.001.
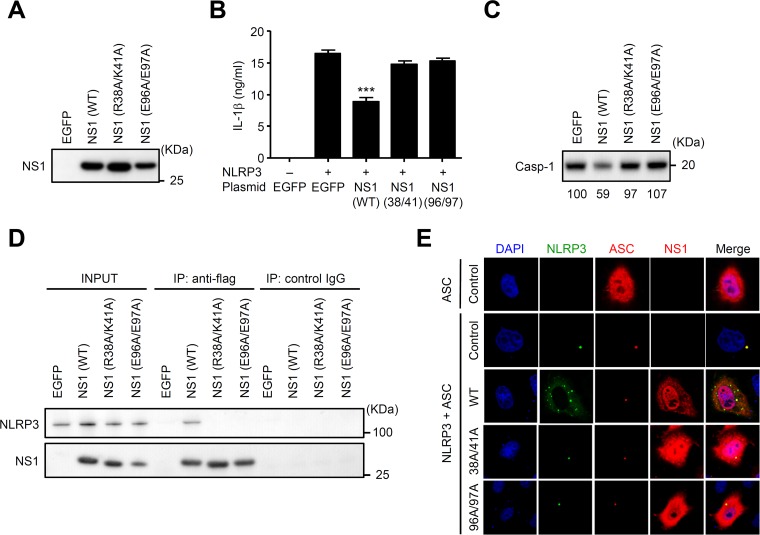
R38A/K41A and E96A/E97A NS1 mutants are defective in blocking NLRP3 inflammasome-mediated IL-1β secretion.
Finally, we examined the role of functional regions of the NS1 protein, including RNA-binding and TRIM25-binding domains, for the inhibition of NLRP3 inflammasome-mediated IL-1β secretion. To this end, we first generated plasmids encoding Flag-tagged NS1 protein in which amino acids R38/K41 or E96/E97 were mutated to A residues and confirmed their expression in HEK293T cells by immunoblot analysis (Fig. 7A). To assess the role of the RNA-binding domain (R38A/K41A) and TRIM25-binding domain (E96A/E97A) of NS1 protein in NLRP3 inflammasome activation, we first transfected HEK293T cells with plasmids encoding NLRP3, ASC, procaspase-1, pro-IL-1β, and either EGFP, Flag-tagged WT, R38A/K41A, or E96A/E97A NS1. Reconstitution of the NLRP3 inflammasome resulted in IL-1β secretion by HEK293T cells, in which NLRP3 was absolutely required (Fig. 7B). Inclusion of Flag-tagged WT NS1 but not the R38A/K41A and E96A/E97A NS1 mutants inhibited NLRP3 inflammasome-mediated IL-1β secretion (Fig. 7B) and caspase-1 activation (Fig. 7C). These NS1 mutants failed to associate with NLRP3 (Fig. 7D). Furthermore, the WT but not mutant NS1 proteins inhibited a single-speck formation of NLRP3 and ASC (Fig. 7E). Taken together, these results suggest that the RNA- and TRIM25-binding domains of NS1 protein are important for NLRP3 inflammasome-mediated IL-1β secretion.
FIG 7.
The RNA- and TRIM25-binding domains of NS1 protein are essential for inhibition of NLRP3 inflammasome activation. (A) HEK293T cells were transfected with plasmids encoding Flag-tagged WT or mutant NS1. Cell extracts were collected at 24 h posttransfection, and NS1 protein expression was analyzed by immunoblotting. (B) HEK293T cells were transfected with expression plasmids encoding NLRP3, ASC, procaspase-1, and pro-IL-1β together with WT or mutant NS1. pCA7-EGFP was used as a control. Cell-free supernatants were collected at 24 h posttransfection, and IL-1β was analyzed by ELISA. (C) HEK293T cells were transfected as described for panel B. Cell extracts were collected at 24 h posttransfection, and active caspase-1 (p20) was analyzed by immunoblotting. (D) HEK293T cells were cotransfected with expression plasmids encoding NLRP3 together with Flag-tagged wild-type or mutant NS1. pCA7-EGFP was used as a control. At 24 h posttransfection, proteins were immunoprecipitated with an anti-Flag antibody, followed by immunoblotting of total lysate (Input) and immunoprecipitates (IP) with anti-Flag or anti-NLRP3 antibodies. (E) HeLa cells were cotransfected with expression plasmids encoding HA-tagged ASC, EGFP-NLRP3, and Flag-tagged NS1. Twenty-four hours after transfection, cells were stained with anti-Flag and anti-HA antibodies and analyzed by confocal microscopy. Nuclei were visualized by staining with DAPI. Data in panel B are representative of at least three independent experiments and are expressed as the means ± standard deviations. ***, P < 0.001.
DISCUSSION
Here, we showed that the NS1 protein of influenza virus interacts with NLRP3 and inhibits the NLRP3/ASC-induced single-speck formation required for full activation of inflammasome, thereby inhibiting the NLRP3 inflammasome-mediated secretion of IL-1β.
Both DNA and RNA viruses induce the secretion of IL-1β from infected cells. Recent reports indicate that the viroporins of RNA viruses, such as influenza virus M2 protein (15), EMCV 2B protein (19), rhinovirus 2B protein (20), or SARS coronavirus E protein (21), induce IL-1β secretion. In contrast, other studies suggested that viral RNA is involved in NLRP3- or RIG-I-mediated inflammasome activation (1, 22, 50, 51). In addition, Chakrabarti et al. showed that RNA cleavage products generated by RNase L bind to the DEXD/H-box helicase, DHX33, and activate the NLRP3 inflammasome (52). These results indicate that while influenza virus M2 protein triggers NLRP3 inflammasome activation in macrophages and dendritic cells (15), other cell types, such as lung epithelial cells, employ partially redundant recognition mechanisms and that virus-induced type I interferon through the RIG-I/TRIM25-dependent pathway stimulates RIG-I and NLRP3 expression and their assembly to form the RIG-I and NLRP3 inflammasome (51). Given that viral RNA triggers RIG-I and NLRP3 inflammasome activation, the NS1 protein may inhibit RIG-I and NLRP3 inflammasome-mediated IL-1β secretion by sequestering viral RNA or by binding to RIG-I in influenza virus-infected cells. Recently, Cheong et al. demonstrated that NS1 proteins derived from both highly pathogenic (A/Hong Kong/483/1997 [H5N1]) and low-pathogenic (A/WSN/1933 [H1N1]) strains suppressed IL-1β secretion from THP-1 cells after stimulation with LPS plus ATP (NLRP3 agonist) (53). In the present study, we demonstrated that NS1 protein derived from different virus strains suppressed NLRP3 inflammasome-mediated IL-1β secretion by different mechanisms. The BM/1918 NS1 protein, which has an intact CPSF30-binding site that blocks host mRNA maturation, suppressed expression of NLRP3, thereby abrogating NLRP3 inflammasome-mediated IL-1β secretion (Fig. 6). In contrast, other NS1 proteins did not change the expression levels of NLRP3 or ΔΨ(m). Instead, the RNA-binding domain (basic residues 38 and 41) and TRIM25-binding domain (acidic residues 96 and 97) of NS1 protein are required for suppression of NLRP3 inflammasome-mediated IL-1β secretion. Since type I IFN signals enhance IL-1β secretion in response to influenza virus infection (3, 51), the NS1 protein appears to play a key role in inhibition of RIG-I and NLRP3 inflammasome-mediated IL-1β secretion from nonimmune cells such as lung epithelia.
Viruses utilize many strategies to inhibit inflammasome activation. We previously demonstrated that the PB1-F2 protein of influenza virus translocates to the mitochondrial inner membrane space and dissipates ΔΨ(m), thereby suppressing NLRP3 inflammasome-mediated IL-1β secretion (29). In contrast, the NS1 protein did not dissipate ΔΨ(m). Instead, the NS1 protein interacted with NLRP3 and inhibited a single-speck formation, which is required for full activation of inflammasomes (47). Similarly, the nonstructural V protein of measles virus interacted with NLRP3 to specifically inhibit NLRP3 inflammasome activity (40). KHSV Orf63, a viral homolog of human NLRP1, inhibits inflammasome activation by interacting with NLRP1 or NLRP3. In addition, the pyrin domain (PYD)-containing M13L protein of myxoma virus, a rabbit-specific poxvirus, interacts with ASC and inhibits inflammasome activation. The Basic Local Alignment Search Tool for proteins, blastp, and ClustalW2 alignment confirmed that the NS1 protein of influenza virus does not contain any regions homologous with NLRP3, indicating that the interaction is not dependent on homologous conserved sequences.
It was reported that IL-1 receptor (IL-1R) signaling enhanced the ability of DCs to stimulate T cells (5, 54). In addition, inflammasome-dependent cytokine production plays a key role in the induction of virus-specific adaptive immunity and in the initiation of host repair responses to lung tissue damage after influenza virus infection (1, 3, 55). These observations suggest that inhibition of inflammasome activation by the NS1 protein may be associated with influenza virus pathogenesis.
In summary, the findings of the present study reveal a mechanism by which NS1 protein inhibits inflammasome activation. These results will aid the development of new anti-inflammatory drugs. Also, inhibiting NS1 activity might be a potential novel therapeutic approach.
ACKNOWLEDGMENTS
We thank A. García-Sastre (Icahn School of Medicine) for providing the recombinant influenza virus lacking the NS1 gene.
This work was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (25713018 and 15H01254), the Japanese Ministry of Health, Labor, and Welfare, the Takeda Science Foundation, the Astellas Foundation for Research on Metabolic Disorders, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Tokyo Biochemical Research Foundation, the Uehara Memorial Foundation, the SENSHIN Medical Research Foundation, the Kao Foundation for Arts and Sciences, the Kanae Foundation for the Promotion of Medical Science, and the Daiichi Sankyo Foundation of Life Science.
REFERENCES
- 1.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. 2007. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol 178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 3.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. 2007. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol 179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 5.Pang IK, Ichinohe T, Iwasaki A. 2013. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. Nat Immunol 14:246–253. doi: 10.1038/ni.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas PG, Dash P, Aldridge JR Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen IY, Ichinohe T. 2015. Response of host inflammasomes to viral infection. Trends Microbiol 23:55–63. doi: 10.1016/j.tim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Ichinohe T. 2010. Respective roles of TLR, RIG-I and NLRP3 in influenza virus infection and immunity: impact on vaccine design. Expert Rev Vaccines 9:1315–1324. doi: 10.1586/erv.10.118. [DOI] [PubMed] [Google Scholar]
- 9.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 10.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A 101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 12.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 13.Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, Reis e Sousa C. 2010. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell 140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Weber M, Gawanbacht A, Habjan M, Rang A, Borner C, Schmidt AM, Veitinger S, Jacob R, Devignot S, Kochs G, Garcia-Sastre A, Weber F. 2013. Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe 13:336–346. doi: 10.1016/j.chom.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichinohe T, Pang IK, Iwasaki A. 2010. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol 11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichinohe T, Yamazaki T, Koshiba T, Yanagi Y. 2013. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci U S A 110:17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. 2013. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller M, Ruegg A, Werner S, Beer HD. 2008. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Yanagi Y, Ichinohe T. 2012. Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome. PLoS Pathog 8:e1002857. doi: 10.1371/journal.ppat.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triantafilou K, Kar S, van Kuppeveld FJ, Triantafilou M. 2013. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am J Respir Cell Mol Biol 49:923–934. doi: 10.1165/rcmb.2013-0032OC. [DOI] [PubMed] [Google Scholar]
- 21.Nieto-Torres JL, DeDiego ML, Verdia-Baguena C, Jimenez-Guardeno JM, Regla-Nava JA, Fernandez-Delgado R, Castano-Rodriguez C, Alcaraz A, Torres J, Aguilella VM, Enjuanes L. 2014. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog 10:e1004077. doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschlager N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, Ruland J. 2010. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol 11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 23.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 27.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. 2011. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. 2011. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal 4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- 29.Yoshizumi T, Ichinohe T, Sasaki O, Otera H, Kawabata S, Mihara K, Koshiba T. 2014. Influenza A virus protein PB1-F2 translocates into mitochondria via Tom40 channels and impairs innate immunity. Nat Commun 5:4713. doi: 10.1038/ncomms5713. [DOI] [PubMed] [Google Scholar]
- 30.Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. 2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hale BG, Randall RE, Ortin J, Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J Gen Virol 89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 32.Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M Jr, Garcia-Sastre A. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol 81:514–524. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min JY, Krug RM. 2006. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2′–5′ oligo(A) synthetase/RNase L pathway. Proc Natl Acad Sci U S A 103:7100–7105. doi: 10.1073/pnas.0602184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajsbaum R, Albrecht RA, Wang MK, Maharaj NP, Versteeg GA, Nistal-Villan E, Garcia-Sastre A, Gack MU. 2012. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. PLoS Pathog 8:e1003059. doi: 10.1371/journal.ppat.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stasakova J, Ferko B, Kittel C, Sereinig S, Romanova J, Katinger H, Egorov A. 2005. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1β and 18. J Gen Virol 86:185–195. doi: 10.1099/vir.0.80422-0. [DOI] [PubMed] [Google Scholar]
- 36.Sasai M, Linehan MM, Iwasaki A. 2010. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science 329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 38.Takeda M, Ohno S, Seki F, Nakatsu Y, Tahara M, Yanagi Y. 2005. Long untranslated regions of the measles virus M and F genes control virus replication and cytopathogenicity. J Virol 79:14346–14354. doi: 10.1128/JVI.79.22.14346-14354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199. doi: 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
- 40.Komune N, Ichinohe T, Ito M, Yanagi Y. 2011. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1β secretion. J Virol 85:13019–13026. doi: 10.1128/JVI.05942-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. 2007. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 42.Chuang YT, Lin YC, Lin KH, Chou TF, Kuo WC, Yang KT, Wu PR, Chen RH, Kimchi A, Lai MZ. 2011. Tumor suppressor death-associated protein kinase is required for full IL-1β production. Blood 117:960–970. doi: 10.1182/blood-2010-08-303115. [DOI] [PubMed] [Google Scholar]
- 43.Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. 2003. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol 171:6154–6163. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- 44.Fink BD, Hong YS, Mathahs MM, Scholz TD, Dillon JS, Sivitz WI. 2002. UCP2-dependent proton leak in isolated mammalian mitochondria. J Biol Chem 277:3918–3925. doi: 10.1074/jbc.M107955200. [DOI] [PubMed] [Google Scholar]
- 45.Krauss S, Zhang CY, Lowell BB. 2002. A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2. Proc Natl Acad Sci U S A 99:118–122. doi: 10.1073/pnas.012410699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. 2009. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ. 2013. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J 449:613–621. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayllon J, Domingues P, Rajsbaum R, Miorin L, Schmolke M, Hale BG, Garcia-Sastre A. 2014. A single amino acid substitution in the novel H7N9 influenza A virus NS1 protein increases CPSF30 binding and virulence. J Virol 88:12146–12151. doi: 10.1128/JVI.01567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hale BG, Steel J, Medina RA, Manicassamy B, Ye J, Hickman D, Hai R, Schmolke M, Lowen AC, Perez DR, Garcia-Sastre A. 2010. Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein. J Virol 84:6909–6922. doi: 10.1128/JVI.00081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W, Xu Y, Li H, Tao W, Xiang Y, Huang B, Niu J, Zhong J, Meng G. 2014. HCV genomic RNA activates the NLRP3 inflammasome in human myeloid cells. PLoS One 9:e84953. doi: 10.1371/journal.pone.0084953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pothlichet J, Meunier I, Davis BK, Ting JP, Skamene E, von Messling V, Vidal SM. 2013. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog 9:e1003256. doi: 10.1371/journal.ppat.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakrabarti A, Banerjee S, Franchi L, Loo YM, Gale M Jr, Nunez G, Silverman RH. 2015. RNase L activates the NLRP3 inflammasome during viral infections. Cell Host Microbe 17:466–477. doi: 10.1016/j.chom.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheong WC, Kang HR, Yoon H, Kang SJ, Ting JP, Song MJ. 2015. Influenza A virus NS1 protein inhibits the NLRP3 inflammasome. PLoS One 10:e0126456. doi: 10.1371/journal.pone.0126456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kruse M, Meinl E, Henning G, Kuhnt C, Berchtold S, Berger T, Schuler G, Steinkasserer A. 2001. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1β. J Immunol 167:1989–1995. doi: 10.4049/jimmunol.167.4.1989. [DOI] [PubMed] [Google Scholar]
- 55.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. 2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]