ABSTRACT
Attrition within the CD4+ T cell compartment, high viremia, and a cytokine storm characterize the early days after HIV infection. When the first emerging HIV-specific CD8+ T cell responses gain control over viral replication it is incomplete, and clearance of HIV infection is not achieved even in the rare cases of individuals who spontaneously control viral replication to nearly immeasurably low levels. Thus, despite their partial ability to control viremia, HIV-specific CD8+ T cell responses are insufficient to clear HIV infection. Studying individuals in the first few days of acute HIV infection, we detected the emergence of a unique population of CD38+ CD27− CD8+ T cells characterized by the low expression of the CD8 receptor (CD8dim). Interestingly, while high frequencies of HIV-specific CD8+ T cell responses occur within the CD38+ CD27− CD8dim T cell population, the minority populations of CD8bright T cells are significantly more effective in inhibiting HIV replication. Furthermore, the frequency of CD8dim T cells directly correlates with viral load and clinical predictors of more rapid disease progression. We found that a canonical burst of proliferative cytokines coincides with the emergence of CD8dim T cells, and the size of this population inversely correlates with the acute loss of CD4+ T cells. These data indicate, for the first time, that early CD4+ T cell loss coincides with the expansion of a functionally impaired HIV-specific CD8dim T cell population less efficient in controlling HIV viremia.
IMPORTANCE A distinct population of activated CD8+ T cells appears during acute HIV infection with diminished capacity to inhibit HIV replication and is predictive of viral set point, offering the first immunologic evidence of CD8+ T cell dysfunction during acute infection.
INTRODUCTION
Immense levels of human immunodeficiency virus (HIV) replication during the first days of infection are accompanied by dramatic changes in the immune system that may determine the quality of the subsequent immune response and ability to control HIV replication (1). The acute destruction of over half of the body's memory CD4+ T cells (2) is accompanied by changes in the immune system, including a drop in the B cell compartment and a major innate cytokine storm (3). Subsequent development of the adaptive HIV-specific CD8+ T cell response exerts selection pressure on the virus, forcing it to evolve to evade immune recognition and resulting in a lower level and semistable viral set point (4, 5). The level of the early viral set point is highly predictive for long-term disease outcome (6, 7), supporting the notion that the earliest events shaping the T cell responses are setting the stage for disease progression. Indeed, some individual HIV-specific CD8+ T cell responses during acute HIV infection have been identified to dictate long-term disease outcome (8, 9).
However, while the first emerging HIV-specific CD8+ T cell responses are able to gain initial control over viral replication, CD8+ T cell-mediated control is incomplete, immunological clearance of HIV infection is never observed, and viral replication persists (10). This is partially due to HIV's ability to escape from CD8+ T cell targeted epitopes (11, 12), resulting in either the lack of recognition or generation of de novo CD8+ T cell responses against the variant epitope (13).
In the best (but rare) cases, HIV-specific CD8+ T cell responses are able to effectively control viral replication to nearly immeasurably low levels. However, even then HIV cannot be cleared, and the ongoing fight between virus and T cells leads to a deterioration and exhaustion of the CD8+ T cell responses (14–16). This exhaustion is characterized by a hierarchical loss of functions and significant changes in the surface receptors, including the upregulation of inhibitory receptors such as programmed death 1 (PD1). Thus, besides the generation of the large breadth and magnitude of CD8+ T cell responses, the adaptive immune response appears to suffer from insufficient effector function after acute HIV infection that can be explained neither by exhaustion nor CD8+ T cell escape. Here, we assessed HIV-infected individuals at the earliest phase of acute infection to determine whether the failure to mount effective HIV-specific CD8+ T cell responses can be traced to early immunological changes and describe a population of CD8+ T cells that is associated with a lack of subsequent control.
MATERIALS AND METHODS
Study participants.
Twenty-four HIV-1 acutely infected participants identified from the RV217 early-capture HIV cohort were selected based on preinfection sample availability and at least two time points sampled after infection and prior to peak viremia. RV217 is a multicenter, nonrandomized clinical observational study designed to describe the biological characteristics of acute HIV-1 infection in high-risk volunteers from Africa and Southeast Asia. Acute HIV-1 infection was determined from twice-weekly blood draws of at-risk populations using a nucleic acid test, the Aptima HIV-1 RNA qualitative assay (Hologic Gen-Probe Inc., San Diego, CA, USA), and confirmed by enzyme linked immunoassays and Western blotting after the advent of detectable antibodies. HIV-1 viral load was determined at every study visit with longitudinal samples using the Abbott real-time HIV-1 assay with a detection limit of 40 HIV RNA copies/ml (Abbott Laboratories, Abbott Park, IL, USA). The HIV-1 viral set point was defined as the average of all viral load measurements between day 80 and day 365 in the absence of treatment, and it required at least two measurements. Two study participants were considered to have a missing set point viral load due to one individual initiating antiretroviral therapy (ART) at day 35 and the other having only one measurement available after day 80 prior to ART initiation; therefore, they were excluded from analysis with disease progression. Lymphocyte immunophenotyping was performed on fresh whole blood using the single-platform BD Multiset TruCount method to determine absolute counts and percentages (BD Biosciences, San Jose, CA, USA). Table 1 summarizes patient demographics. CD8+ T cell evaluation was completed through a median of 54 days for all participants with an average of 7 time points analyzed for the 13 East Africans and 11 Southeast Asians. One individual initiated antiretroviral therapy prior to the establishment of set point HIV-1 viral load.
TABLE 1.
RV217 patient demographics
| Patient ID | Gender | Age (yr) | Country | CD4 T cell nadir (cells/μl) | Time to peak VLa (days) | Peak VL (log10 copies/ml) | Set point VLb (log10 copies/ml) |
|---|---|---|---|---|---|---|---|
| 10220 | F | 33 | Uganda | 796 | 18 | 5.49 | 3.69 |
| 10428 | F | 27 | Uganda | 421 | 10 | 6.92 | 4.08 |
| 10435 | F | 18 | Uganda | 392 | 14 | 6.74 | 3.52 |
| 20225 | F | 24 | Kenya | 819 | 9 | 7.99 | 4.28 |
| 20263 | F | 20 | Kenya | 462 | 11 | 8.20 | 2.52 |
| 20337 | F | 24 | Kenya | 142 | 14 | 7.64 | 5.35 |
| 20355 | F | 24 | Kenya | 430 | 11 | 6.02 | 3.01 |
| 20368 | F | 18 | Kenya | 676 | 13 | 6.12 | * |
| 20509 | F | 24 | Kenya | 673 | 14 | 6.93 | 3.04 |
| 20511 | F | 24 | Kenya | 285 | 14 | 6.73 | 4.54 |
| 30112 | F | 25 | Tanzania | 884 | 18 | 7.50 | 4.38 |
| 30124 | F | 24 | Tanzania | 464 | 14 | 6.76 | 4.83 |
| 30190 | F | 25 | Tanzania | 511 | 11 | 7.34 | 5.96 |
| 40007 | M | 25 | Thailand | 406 | 15 | 7.31 | 5.35 |
| 40061 | F | 48 | Thailand | 312 | 14 | 5.79 | * |
| 40094 | M | 19 | Thailand | 866 | 16 | 6.40 | 4.64 |
| 40100 | M | 18 | Thailand | 467 | 10 | 8.46 | 5.22 |
| 40123 | M | 23 | Thailand | 616 | 14 | 6.24 | 4.47 |
| 40168 | MtFc | 25 | Thailand | 444 | 11 | 6.66 | 5.28 |
| 40250 | M | 35 | Thailand | 522 | 19 | 6.67 | 4.83 |
| 40257 | MtF | 18 | Thailand | 370 | 10 | 7.35 | 5.39 |
| 40265 | M | 23 | Thailand | 476 | 12 | 6.49 | 4.83 |
| 40353 | M | 21 | Thailand | 516 | 17 | 5.81 | 3.60 |
| 40363 | MtF | 29 | Thailand | 576 | 14 | 6.86 | 4.77 |
| Average | 25 | 522 | 13 | 6.85 | 4.44 |
Peak indicates the true peak; VL indicates HIV-1 load (copies/ml).
Set point VL is the average of all measured viral loads between day 80 and day 365 in the absence of treatment (requiring at least two measurements). An asterisk indicates that a set point cannot be defined because of treatment.
MtF, male-to-female transgender.
Ethics statement.
All individuals participating in this study provided written informed consent. Ethical approval was obtained from institutional review boards in each country as well as the Human Subjects Protection Branch at the Walter Reed Army Institute of Research, which approved the overall protocol.
Phenotypic analysis.
One hundred sixty-seven cryopreserved peripheral blood mononuclear cell (PBMC) samples of 24 individuals were thawed and used for this study. Cells were washed in serum-free medium and stained with Live/Dead fixable aqua (Life Technologies, Grand Island, NY, USA) to exclude nonviable cells. PBMC then were washed with staining buffer containing 0.5% bovine serum albumin (BSA)–0.01% azide and stained with 2 panels of monoclonal antibodies for polychromatic flow-cytometric analysis. Antibodies used included CD4 Qdot605 (clone S3.5), CD8a phycoerythrin (PE)-TR (clone 3B5), CD14 Tri-Color (clone Tük4), and CD19 Tri-Color (clone SJ25-C1), all from Life Technologies. CD3 allophycocyanin (APC)-eF780 (clone UCHT1), CD45R0 eFluor 650NC (clone UCHL1), and HLA-DR eFluor 450 (clone L243) were from eBioscience, Inc., San Diego, CA, USA. CD27 peridinin chlorophyll protein (PerCP)-Cy5.5 and Alexa Fluor 700 (clone O323), CD28 biotinylated (clone CD28.2), CD45RA PerCP-Cy5.5 (clone HI100), CD57 fluorescein isothiocyanate (FITC) (clone HCD57), CD62L Alexa Fluor 700 (clone DREG-56), CD95 Alexa Fluor 647 (clone DX2), and CD127 BV421 (clone A019D5) were all from BioLegend (San Diego, CA, USA). CD38 APC (clone HB7), CD56 PE-Cy5 (clone B159), CD95 PE-Cy7 (clone DX2), CCR5 (CD195) FITC (clone 2D7/CCR5), CCR7 (CD197) PE-Cy7 (clone 3D12), CXCR3 (CD183) PE (clone 1C6/CXCR3), and PD-1 (CD279) PE (clone EH12.1) all were procured from BD Biosciences. α4β7 (ACT-1 clone) (NIH AIDS Research and Reference Reagent Program) was conjugated in-house with an eFluor 650 nanocrystal conjugation kit–amine-reactive kit (eBioscience). After incubation, cells were washed with staining buffer and secondary Qdot 800 Streptavidin conjugate (Life Technologies) was applied for CD28. Cells were washed, fixed with 2% formaldehyde for 20 min, washed again, and resuspended in staining buffer. Samples then were acquired on an LSRII with a 4-laser configuration (BD Biosciences) and analyzed using Flow Jo, version 9.7.5 (TreeStar, Inc., Ashland, OR, USA).
Assessment of autologous HIV-specific CD8+ T cell responses.
Founder viruses of three study participants were deduced from plasma from the first time point preceding peak viremia with greater than 10,000 copies/ml following full-length single-genome amplification sequencing using Sanger technology as described elsewhere (17). Autologous overlapping peptides (18mer overlapping by 10 amino acids) were synthesized on the basis of the viral sequence for each individual. Five peptide pools then were generated for Gag, Env, Nef, Pol, and accessory proteins. PBMC were thawed, counted, and plated as described above. Cells were stimulated in the presence of 3 μg/ml HIV peptide pools, costimulatory antibodies CD28/CD49d and CD107a− FITC (clone H4A3) (BD Biosciences), and incubated at 5% CO2 at 37°C for 6 h. Brefeldin A and monensin were added 2 h after stimulation. Cells were processed as described above. Antibodies include CD14 Tri-Color (clone Tük4) and CD19 Tri-Color (clone SJ25-C1), both from Life Technologies, CD38 APC (clone HB7), CD56 PE-Cy5 (clone B159), CD197 PE-Cy7 (clone 3D12), and HLA-DR APC-H7 (clone L243), all from BD Biosciences, CD45RO eFluor 650NC (clone UCHL1) from eBioscience, and CD27 AL700 (clone O323) from BioLegend. Cells then were washed, fixed in 2% formaldehyde, and permeabilized using Perm/Wash buffer (BD Biosciences). Cells then were stained intracellularly with CD3 PE-TR (clone S4.1) and CD4 Qdot605 (clone S3.5) from Life Technologies, CD8 PerCP-Cy5.5 (clone SK1) and interleukin-2 (IL-2) FITC (clone 5344.111) from BD Bioscience, and gamma interferon (IFN-γ) eFluor 450NC (clone 4S.B3) from eBioscience. Cells were washed, resuspended in staining buffer, and acquired on an LSRII flow cytometer (BD Biosciences).
Viral inhibition assay.
PBMC from six patients identified in early acute infection were sorted using a FACSAria SORP (BD Biosciences) into CD4, CD8bright, and CD8dim T cells. Sorted cells were rested overnight at 37°C in RPMI containing 20% fetal bovine serum (FBS), IL-2 (50 IU/ml), and IL-7 (5 ng/ml) in preparation for a modified viral inhibition assay as previously described (18). The target cells (autologous CD4+ T cells) were infected with a nevirapine-resistant virus at a multiplicity of infection (MOI) of 0.01 for 2 h. Excess virus was washed off and 100,000 cells were added to a 96-well plate in RPMI–10% fetal calf serum (FCS). Effector cells (CD8bright and CD8dim T cells) were added to the targets at a ratio of 1:1. Nevirapine (NIH AIDS Reagent Program) was added to cultures on day 0 at a concentration of 10 μg/ml from a stock solution of 0.1 mg/ml. Supernatant was removed 3, 5, and 7 days postinfection and frozen at −80 before quantitative PCR (qPCR).
Soluble factor analysis.
The cytokine levels of IL-2, IL-15, and IL-21 were assessed using a standard Luminex multiplexed bead system (Milliplex) according to the manufacturer's instructions and as previously described (19). Results obtained from the Bio-Plex system were analyzed automatically by the Bio-Plex Manager software program (Bio-Rad Laboratories Inc., Hercules, CA, USA) using a (5PL-fit) standard curve derived from recombinant cytokine and chemokine standards. IL-7 was quantified from longitudinal citrate plasma samples by electrochemiluminescent detection enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (Meso Scale Discovery, Rockville, MD). Briefly, samples were diluted 1:2 in assay buffer and loaded onto the MSD plate in duplicate along with an 8-point, 4-fold standard curve, also in duplicate. After 2 h, plates were washed, and detection antibody bound to an electrochemiluminescent label was added for 2 h. Plates were washed again and read buffer added before the image was captured using a Meso QuickPlex SQ120 imager and analyzed with Discovery Workbench 4.0 software.
Statistical analysis.
Statistical analysis was performed using Graph Pad Prism software version 6.0a for Mac OS X (GraphPad Software, La Jolla, CA, USA), JMP version 10.0.0, and SAS v9.3 (SAS, Cary, NC, USA). The standard score (Z score) for each analyte at each time point was calculated as sample measurement minus mean at baseline divided by standard deviations at baseline. Analysis of immunologic variables before and after infection was completed using the Friedman test for matched nonparametric data. Comparisons between groups were performed using the nonparametric Mann-Whitney U test or Wilcoxon method for continuous data. Correlations between continuous outcomes were determined by Spearman's rank correlation. For predictive associations of set point HIV viral load, linear regression models were generated. Assessing model fit for maximum CD8dim CD38+ CD27− T cell frequency revealed two observations with a high Cook's distance, and models were fit with and without these observations. Statistical significance was assessed at the 0.05 level.
RESULTS
Acute emergence of HIV-specific CD8+ T cells with a unique phenotype.
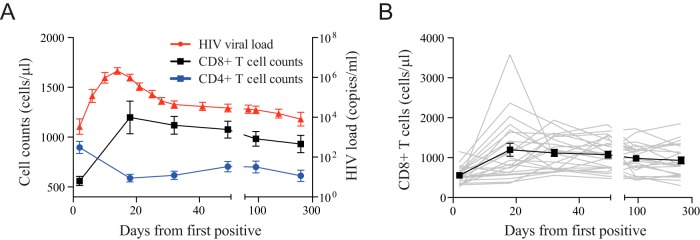
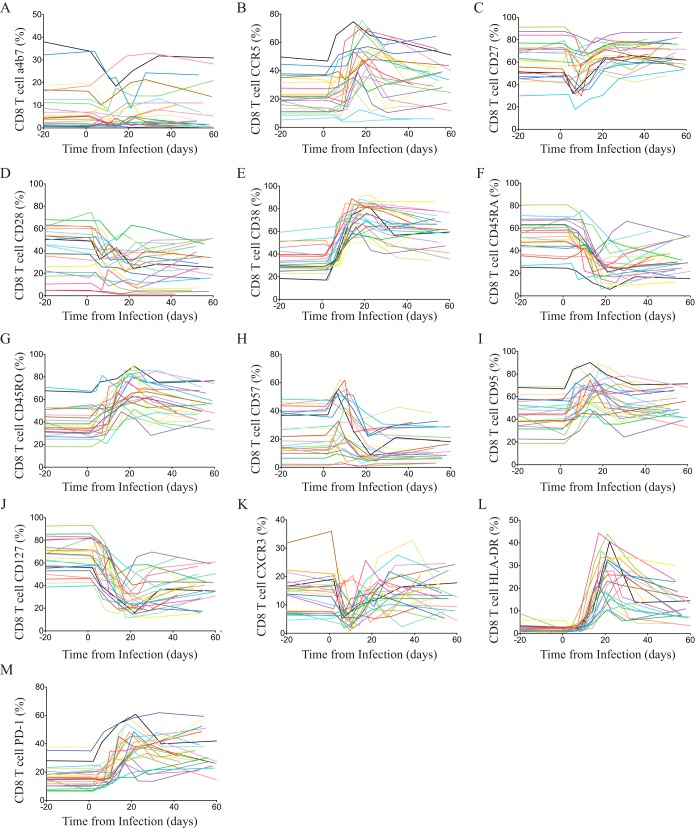
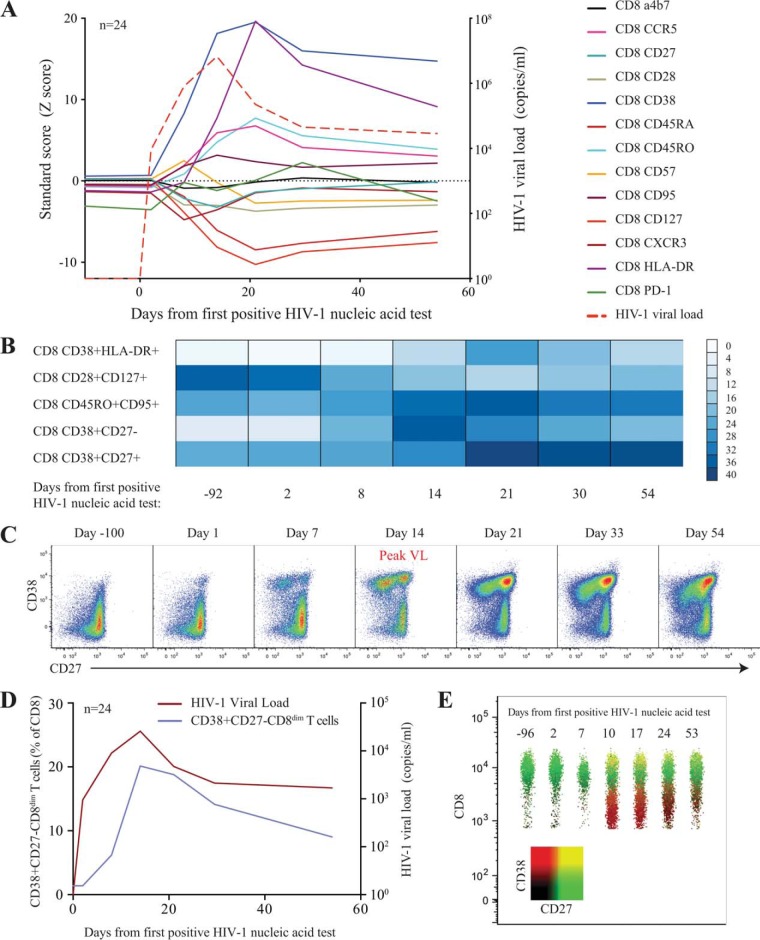
To investigate the limited ability of HIV-specific CD8+ T cells to completely control viremia during acute HIV infection, we studied a prospective cohort of high-risk HIV-uninfected individuals from East Africa and Thailand (RV 217/ECHO) undergoing twice-weekly nucleic acid testing (Aptima HIV-1 RNA qualitative assay) to identify individuals in the earliest period after HIV infection (20). Twenty-four participants with extensive peripheral blood sampling before infection and also prior to peak HIV viral load were included in this study. Patient characteristics and demographics are shown in Table 1. Viral load peaked on average 13 days after the first reactive HIV RNA test and ranged from 5.49 log10 to 8.46 log10 HIV RNA copies/ml, with a median of 6.7 log10 HIV RNA copies/ml. Viral set point occurred an average of 41 days after peak and ranged from 2.52 log10 to 5.96 log10 HIV RNA copies/ml. The initial CD4 count dropped from 855 to 472 cells/μl (range, 142 to 884 cells/μl) within 20 days from the first reactive HIV RNA test. In contrast, the CD8+ T cell count increased from 500 cells/μl (range, 209 to 1,150 cells/μl) to 1,413 cells/μl (range, 558 to 3,581 cells/μl; P < 0.001) (Fig. 1). The flow-cytometric assessment of CD8+ T cell phenotype before and during acute HIV infection demonstrated dramatic and synchronized changes to T cell surface receptor expression in parallel to HIV viral load kinetics (Fig. 2 and 3A). In particular, CD8+ T cell activation indices coincided with HIV expansion and were associated with the loss of IL-7 receptor (CD127) expression. However, we found most phenotypic markers did not occur singularly on CD8+ T cells; rather, we noted a shift or emergence of specific clusters of CD8+ T cell populations (Fig. 3B). In particular, we noted a profound contraction of the CD28+ CD127+ CD8+ T cell population and a corresponding increase of activated CD8+ T cells with the CD38+ HLADR+ phenotype. The most dramatic changes were observed with the emergence of a CD8+ T cell population characterized by the CD38+ CD27− phenotype (Fig. 3B and C), which appeared a median of 8 days after the detection of HIV-1 RNA in the periphery, closely followed viral load trajectory, but it continued to be significantly elevated for the remainder of the study (P < 0.001) (Fig. 3D). This population, clearly distinguishable by the lower expression of the CD8 receptor (CD8dim) (Fig. 3E), was absent prior to HIV infection. Taken together, we found dramatic harmonized changes within the CD8+ T cell compartment characterized by the emergence of a unique CD38+ CD27− CD8dim T cell subset.
FIG 1.
Longitudinal changes in HIV-1 load and CD4 and CD8 T cell absolute counts. (A) Average absolute CD4 T cell count (blue box), absolute CD8 T cell count (black box), and viral load (red circle) are presented for 24 individuals identified during acute HIV infection. (B) Aggregate CD8 T cell absolute counts are displayed for all 24 individuals during acute HIV infection.
FIG 2.
Bulk CD8 T cell receptor changes through acute HIV-1 infection. Changes of individual markers on CD8 T cells for all patients during acute HIV infection. The relative frequency of CD8 T cells expressing α4β7 (A), CCR5 (B), CD27 (C), CD28 (D), CD38 (E), CD45RA (F), CD45RO (G), CD57 (H), CD95 (I), CD127 (J), CXCR3 (K), HLA-DR (L), and PD-1 (M) is shown. Color-coding across panels indicates each subject, and day 0 indicates the day of the first reactive HIV-1 Aptima nucleic acid test.
FIG 3.
Longitudinal changes of surface maker expression on CD8+ T cells during acute HIV infection. (A) Relative changes (Z-score) of surface markers on CD8+ T cells during acute HIV infection. Average viral loads are depicted as red-segmented lines from the first RNA-positive HIV nucleic acid test. (B) Color intensity map of the most dramatic shifts within the CD8+ T cell populations during acute HIV infection. Populations are shown as a median frequency of CD8+ T cells based on specific combinations of receptors from green (no expression) to red (∼40% expression). (C) Successive pseudocolor plots from CD8+ T cells, demonstrating CD38 against CD27 longitudinally, through acute HIV-1 infection and the emergence of a CD38+ CD27− population in a representative donor. (D) Expansion of cells in association with viral loads. (E) Concatenated polychromatic plot of CD8 over time depicts the emergence of the CD38+ CD27− population as a CD8dim subset.
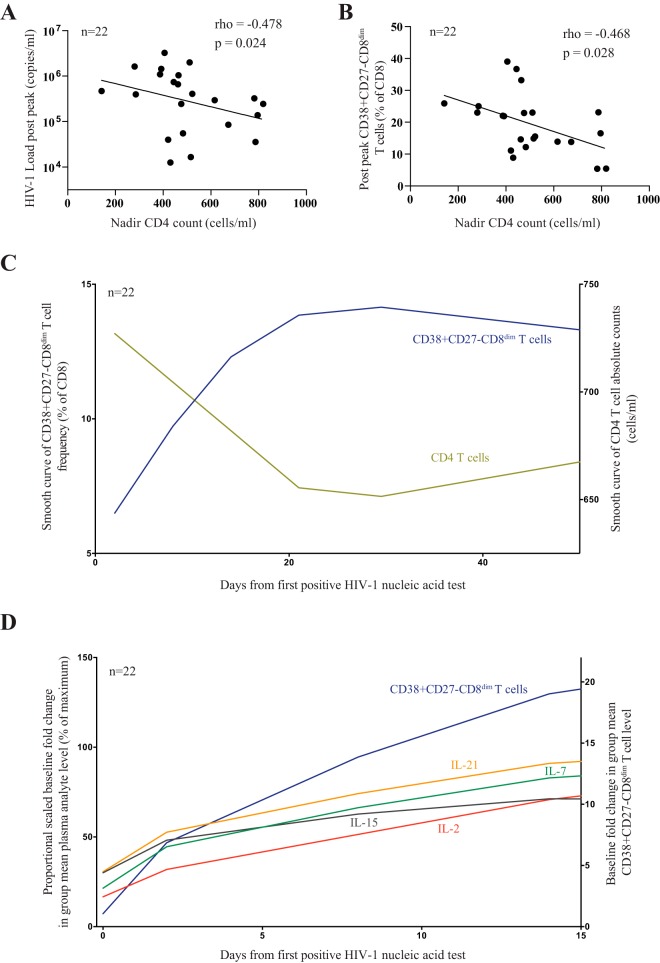
Early changes in the CD8+ T cell compartment is associated with loss of CD4+ T helper cells.
Given the dramatic loss of peripheral CD4+ T cells during acute HIV infection, we wondered whether the emergence of this aberrant CD8+ T cell population is associated with the early changes in the CD4+ T cell compartment. While CD4+ and CD8+ T cell homeostasis are believed to be rather independent (21), we found that the CD4 nadir, defined as the minimum CD4+ T cell absolute count in acute HIV infection prior to day 80, was inversely correlated with HIV viral load (rho = −0.478, P = 0.024) (Fig. 4A) and HIV levels of CD38+ CD27− CD8dim T cells (rho = −0.495, P = 0.014) (Fig. 4B) at a time when viral decline is most profound, suggesting that at least in part the damage to the CD4+ T cell compartment is associated with the emergence of this unique CD8+ T cell phenotype. Indeed, we observed inverse trajectories of CD4 absolute counts and CD38+ CD27− CD8dim T cell frequency longitudinally during acute HIV-1 infection (Fig. 4C). To further understand the link between CD4 loss and CD8+ T cell proliferation, we next determined plasma levels of proliferative cytokines, including IL-2, IL-7, IL-15, and IL-21 (22). While we found no direct link between a single cytokine and the emergence of CD8dim T cells, we nevertheless observed that a canonical burst of the cytokines preceded the expansion of the CD38+ CD27− CD8dim T cell subset (Fig. 4D). Thus, our data suggest that the extent of damage to the CD4+ T cell compartment during acute HIV infection may in part contribute to the development of unique phenotypes of activated CD8+ T cells.
FIG 4.
Temporal dynamics of CD4+ T cell count, HIV-1 viral load, CD38+ CD27− CD8dim T cell population, and plasma cytokine levels during acute HIV infection (n = 22). Two study participants were considered to have missing set point viral loads and were excluded. (A and B) Correlation of acute CD4 count nadir and HIV-1 viral load (A) and frequency of CD38+ CD27− CD8dim T cells an average 7 days after peak viral load (B). (C) Smoothed curve of means showing trends in 22 HIV-1-infected individuals of CD4 absolute counts (green) and CD38+ CD27− CD8dim T cell frequency (blue). (D) Scaled proportional group means of IL-2 (red), IL-7 (green), IL-15 (gray), and IL-21 (orange) relative to plasma baseline levels for each analyte. Mean fold difference from the background is presented for CD38+ CD27− CD8dim T cell frequency.
High frequency of HIV-specific CD8+ T cells are CD38+ CD27− CD8dim T cells.
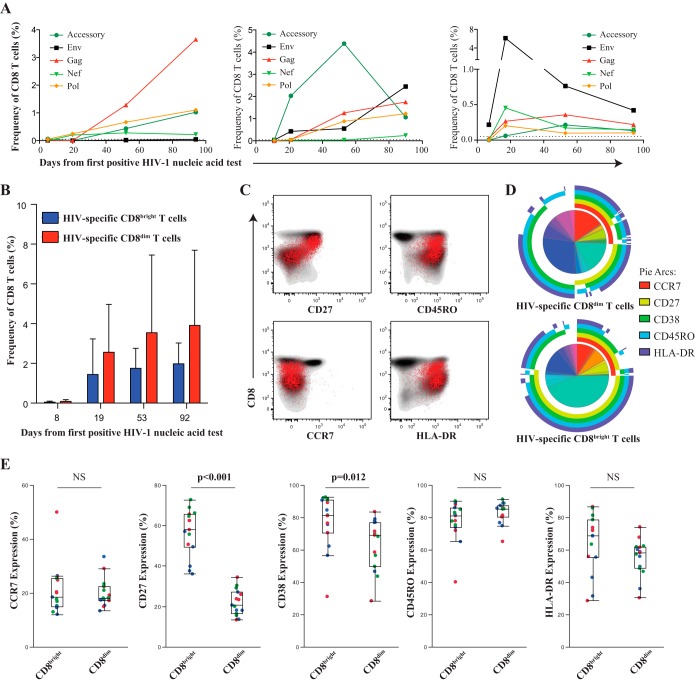
We next investigated whether the expansion of the CD8dim population is virus specific or a nonspecific alteration within the CD8+ T cell compartment. Given the vast amount of HIV sequence diversity and recombinant forms in particular in the East-African population, we sequenced the founder virus from three study participants and generated autologous peptide sets spanning the entire HIV proteome to accurately determine the contribution of virus-specific CD8+ T cells to the increase of CD38+ CD27− CD8dim T cells (23, 24). In all three longitudinally studied individuals, we observed the emergence of HIV-specific CD8+ T cells, determined by either degranulation (CD107a) or cytokine production (IFN-γ or IL-2), early in acute HIV infection with various kinetics and specificities (Fig. 5A). While the majority of the responses increased over time, there were some individual responses targeting more variable segments of the viral proteome that showed an early expansion but then declined over the initial period of infection, as previously described (23, 24). Interestingly, both CD8bright and CD8dim T cells contained HIV-specific responses, yet a higher frequency of HIV-specific CD8+ T cell responses are within the CD8dim population (Fig. 5B). It is important to note that no specific trends or patterns emerged with regard to the epitope targeting between the CD8bright and CD8dim T cells. We further characterized the HIV-specific CD8+ T cell population and found that they demonstrated lower expression of CD27, with an effector memory phenotype (CCR7− CD45RO+), and were consistently HLADR+ (Fig. 5C). Coordinated expression patterns of CCR7, CD27, CD38, CD45RO, and HLA-DR were significantly different between HIV-specific CD8bright and CD8dim T cells (P < 0.001) (Fig. 5D). Interestingly, the only distinct subset we observed within this homogenous virus-specific CD8+ T cell population was the CD38+ CD27− CD8dim T cell population (Fig. 5E).
FIG 5.
HIV-1-specific CD8+ T cells within the CD8bright and CD8dim population. PBMC from three donors were chosen an average 8, 19, 53, and 93 days after the 1st RNA-positive HIV nucleic acid test. (A) Shown are the kinetics of total HIV-specific CD8+ T cell responses (CD107a, IFN-γ, and/or IL-2) in each individual measured by flow cytometry. (B) The majority of HIV-specific CD8+ T cell responses emerge from the CD8dim (red) T population. (C) Representative donor demonstrating the effector phenotype of the HIV-specific CD8+ T cell responses (in red) overlaying the total CD8 compartment (contour plot, in gray). (D) Pie charts showing average frequency of HIV-specific CD8bright and CD8dim T cells with combinatorial expression of 5 phenotypic markers assessed. Arcs show individual expression levels of each phenotypic marker. (E) Box-and-whisker plots show the relative expression of phenotypic markers between HIV-specific CD8bright and CD8dim T cells 39 days after peak viremia. Different donor responses are indicated by different colors for various peptide pools. Nonparametric comparisons were performed using the Wilcoxon method, and nonsignificant relationships are denoted as NS.
CD38+ CD27− CD8dim T cells are inefficient in control of viral replication.
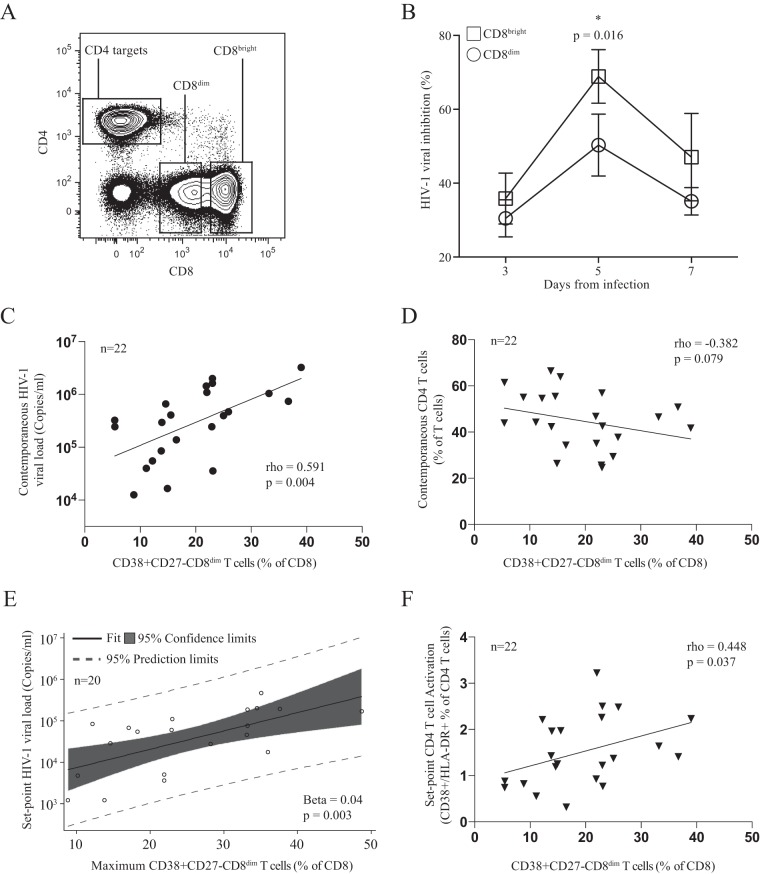
We next asked whether the two phenotypically distinct CD8+ T cell populations with HIV-specific functional activity were equally capable of controlling HIV viremia. Therefore, we purified CD8bright and CD8dim T cells from 6 patients immediately following peak viremia (average of 4 days after peak viremia [14 to 21 days following the first positive HIV determination]), where the most precipitous viral load decline occurs. We simultaneously activated and infected autologous CD4+ T cells as target cells with nevirapine-resistant virus in culture (Fig. 6A). Immunofluorescently stained and sorted CD8bright and CD8dim T cells were added as effector cells at a ratio of 1:1. Strikingly, after 5 days of infection, we found that the CD8bright T cell population was significantly more efficient at viral inhibition than the CD8dim T cell population (65% versus 45%, respectively; P = 0.016) despite the higher frequency of HIV-specific CD8+ T cells observed in the CD8dim T cell population (Fig. 6B). We next assessed whether this population shows any association with levels of HIV viral load. Indeed, we found that 7 days after peak viremia the frequency of CD38+ CD27− CD8dim T cells was positively associated with viral load (rho = 0.591, P = 0.004) (Fig. 6C). Examining absolute CD4+ T cell counts at contemporaneous time points revealed a trend toward an inverse relationship with the frequency of CD38+ CD27− CD8dim T cells (rho = −0.382, P = 0.079) (Fig. 6D). The maximum frequency of CD38+ CD27− CD8dim T cells, which occurred an average of 18 days after the first positive HIV-1 nucleic acid test, was significantly correlated with set point viral load (Spearman rho = 0.498, P = 0.018). Fitting a linear regression model with the maximum frequency of CD38+ CD27− CD8dim T cells as a predictor yielded a positive relationship with a marginally significant P value for set point viral load (beta = 0.03, P = 0.066). Further examination of model fit revealed two data points with a large Cook's distance. Removing these potential outliers and refitting the model yielded a similar result with a significant P value (beta = 0.04, P = 0.003) (Fig. 6E). In addition, the frequency of less functional CD38+ CD27− CD8dim T cells was positively associated with higher set-point CD4+ T cell activation (rho = 0.448, P = 0.037) (Fig. 6F), another marker of rapid HIV disease progression. Taken together, these data suggest that the loss of CD4+ T cells in acute HIV infection drives the emergence of a suboptimal CD8+ T cell population that is less capable of controlling viral replication and may reflect faster disease progression.
FIG 6.
CD8dim CD38+ CD27− T cell population demonstrates a less efficient ability to inhibit viral replication in vitro and is associated with higher viral loads in vivo. (A) Sorting strategy for CD8bright and CD8dim cells. (B) Differences in viral inhibition between CD8bright and CD8dim T cells. (C) CD8dim CD38+ CD27− T cell frequency correlates positively with contemporaneous viral loads an average of 7 days after peak viral load and (D) negatively correlates with contemporaneous CD4+ T cell count. (E) Maximum CD8dim CD38+ CD27− T cell frequency was predictive of HIV set point using linear regression after the removal of two potential outliers. (F) CD8dim CD38+ CD27− T cell frequency an average of 7 days after peak viral load correlates directly with set point activation of CD4+ T cells (CD38+ HLADR+).
DISCUSSION
The efficacy of CD8+ T cell-mediated control of HIV replication is one of the leading underlying mechanisms that determine the long-term control and disease progression in the absence of antiretroviral therapy. However, in most cases of HIV-infected individuals, CD8+ T cells are incapable of achieving durable control of HIV replication, and there is no documented case of natural clearance or cure from HIV infection. Here, we found that the inefficiency of CD8+ T cells to control viral replication is associated with the emergence of a unique CD8+ T cell phenotype. These CD8+ T cells are characterized by the expression of CD38+ CD27− and low expression of the CD8+ T cell receptor. While these cells harbor a high frequency of HIV-specific CD8+ T cell responses, they are less efficient at suppressing viral replication. Moreover, we found that the frequency of this particular phenotype was associated with viral loads and markers for disease progression, suggesting that the generation of CD8+ T cells with the aberrant phenotype greatly influences long-term disease outcome.
CD8+ T cells with low expression of the CD8+ receptor have been observed previously in persistent viral infections and described to have inefficient cytotoxic activity (25–27). Indeed, the presence of a CD8dim population also has been described in chronic HIV infection and associated with poor disease outcome (28). The physiologic role for CD8dim T cells currently is unknown. It has been speculated that either antigen persistence drives the development of an inefficient cellular T cell population or that these cells emerge as a regulatory population that controls the intensity of ongoing cytolytic and tissue-damaging activity (29). While both scenarios have been described in chronic infection and later stages of viral infections, the emergence of these cells during the acute phase of HIV infection is surprising and has been described previously only in simian immunodeficiency virus (SIV) infection (26). In particular, as a large fraction of HIV-specific CD8+ T cell responses are contained in the CD8dim population, their overall contribution to the control of viral replication appears to be relatively minor. Indeed, the frequency of these CD8+ T cells positively correlates with HIV viral load and portend a worse prognosis for disease progression. A potential explanation for the emergence of a dysfunctional CD8+ T cell population is the lack of CD4 help during the acute phase of HIV infection due to the massive destruction in the CD4+ T cell compartment. While studies have demonstrated that efficient CD8+ T cell responses can be primed in the absence of CD4 help (reviewed in reference 30), CD4-mediated helper signals nevertheless often are required for the generation of long-lived, functional memory CD8+ T cell responses. Indeed, it has been described that the lack of CD4+ T cell help in the acute phase of infections can influence the frequency, phenotype, and function of CD8+ T cell responses (31, 32). Here, we found that the size of the CD8dim T cell population was associated with the early CD4+ T cell nadir, suggesting that the loss of early CD4+ T cells and their helper function is involved in the emergence of the unique CD8+ T cell populations. Previous studies have already suggested that a low acute CD4 nadir is associated with a poor long-term disease outcome (33), but a link to the CD8+ T cell population has not been established. A further indicator that insufficient CD4 help is driving the CD8dim phenotype is the activated CD8+ T cell phenotype (CD38) but lack of CD27 expression. Previous studies suggested that CD8+ T cells primed in the absence of CD4 help lack the expression of CD27, which renders them inefficient to expand in the recall phase of the response (34, 35).
Thus, our data suggest an explanation for differences in prognosis as estimated here by viral load set point and CD4 activation in which CD4 count loss and canonical bursts of proliferative cytokines drive the expansion of a less functional CD8+ T cell population that is not able to fully suppress viremia. Furthermore, our data support the notion that ART treatment during early acute HIV infection leads to an overall improvement of CD8+ T cell function by preventing or limiting the emergence of the CD8dim population and ultimately permit durable control of HIV with future treatment interruptions due to a more dominant and functional CD8bright HIV-specific immune response (36–39).
ACKNOWLEDGMENTS
We thank the women and men who participated in the RV217 study. We also thank the RV217 study team: Jeffrey R. Currier, Peter Dawson, Fatim Jallow, Silvia Ratto-Kim, Eugene Kroon, Cornelia Lueer, Jennifer Malia, Mark Manak, Mark Milazzo, Robert O'Connell, Joseph Oundo, Donald Stablein, Erica Sanga, Somchai Sriplichien, and Rapee Trichavaroj.
Financial support for this study was provided by the U.S. Army under cooperative agreement W81XWH-11-2-0174 and the National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: α4-β7 monoclonal antibody (catalog no. 11718) from A. A. Ansari and nevirapine.
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense.
Funding Statement
This work was supported by a cooperative agreement (W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DoD).
Footnotes
Present address: Mary A. Marovich, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
REFERENCES
- 1.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 3.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 68:6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyles RH, Munoz A, Yamashita TE, Bazmi H, Detels R, Rinaldo CR, Margolick JB, Phair JP, Mellors JW. 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS cohort study. J Infect Dis 181:872–880. [DOI] [PubMed] [Google Scholar]
- 7.Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 8.Goulder PJ, Phillips RE, Colbert RA, McAdam S, Ogg G, Nowak MA, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael AJ, Rowland-Jones S. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med 3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 9.Streeck H, Lu R, Beckwith N, Milazzo M, Liu M, Routy JP, Little S, Jessen H, Kelleher AD, Hecht F, Sekaly RP, Alter G, Heckerman D, Carrington M, Rosenberg ES, Altfeld M. 2014. Emergence of individual HIV-specific CD8 T cell responses during primary HIV-1 infection can determine long-term disease outcome. J Virol 88:12793–12801. doi: 10.1128/JVI.02016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatano H, Delwart EL, Norris PJ, Lee TH, Dunn-Williams J, Hunt PW, Hoh R, Stramer SL, Linnen JM, McCune JM, Martin JN, Busch MP, Deeks SG. 2009. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol 83:329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P, Rudersdorf RR, Liebl ME, Krebs K, Vasquez J, Dodds E, Loffredo J, Martin S, McDermott AB, Allen TM, Wang C, Doxiadis GG, Montefiori DC, Hughes A, Burton DR, Allison DB, Wolinsky SM, Bontrop R, Picker LJ, Watkins DI. 2003. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J Virol 77:9029–9040. doi: 10.1128/JVI.77.16.9029-9040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen TM, O'Connor DH, Jing P, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, Kunstman KJ, Wang X, Allison DB, Hughes AL, Desrosiers RC, Altman JD, Wolinsky SM, Sette A, Watkins DI. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 13.Allen TM, Yu XG, Kalife ET, Reyor LL, Lichterfeld M, John M, Cheng M, Allgaier RL, Mui S, Frahm N, Alter G, Brown NV, Johnston MN, Rosenberg ES, Mallal SA, Brander C, Walker BD, Altfeld M. 2005. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J Virol 79:12952–12960. doi: 10.1128/JVI.79.20.12952-12960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 17.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saez-Cirion A, Shin SY, Versmisse P, Barre-Sinoussi F, Pancino G. 2010. Ex vivo T cell-based HIV suppression assay to evaluate HIV-specific CD8+ T-cell responses. Nat Protoc 5:1033–1041. doi: 10.1038/nprot.2010.73. [DOI] [PubMed] [Google Scholar]
- 19.Streeck H, Kwon DS, Pyo A, Flanders M, Chevalier MF, Law K, Julg B, Trocha K, Jolin JS, Anahtar MN, Lian J, Toth I, Brumme Z, Chang JJ, Caron T, Rodig SJ, Milner DA Jr, Piechoka-Trocha A, Kaufmann DE, Walker BD, Altfeld M. 2011. Epithelial adhesion molecules can inhibit HIV-1-specific CD8(+) T-cell functions. Blood 117:5112–5122. doi: 10.1182/blood-2010-12-321588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robb ML. 2012. Viral dynamics and immune response in acute infection and their impact on viral set-point. Abstr AIDS Vaccine 2012. http://aidsvac.capitalreach.com/portal.
- 21.Douek DC, Picker LJ, Koup RA. 2003. T cell dynamics in HIV-1 infection. Annu Rev Immunol 21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 22.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M Jr, Lifson JD, Maino VC, Axthelm MK, Villinger F. 2006. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Investig 116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari G, Korber B, Goonetilleke N, Liu MK, Turnbull EL, Salazar-Gonzalez JF, Hawkins N, Self S, Watson S, Betts MR, Gay C, McGhee K, Pellegrino P, Williams I, Tomaras GD, Haynes BF, Gray CM, Borrow P, Roederer M, McMichael AJ, Weinhold KJ. 2011. Relationship between functional profile of HIV-1 specific CD8 T cells and epitope variability with the selection of escape mutants in acute HIV-1 infection. PLoS Pathog 7:e1001273. doi: 10.1371/journal.ppat.1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, Weinhold KJ, Moore S, Letvin N, Haynes BF, Cohen MS, Hraber P, Bhattacharya T, Borrow P, Perelson AS, Hahn BH, Shaw GM, Korber BT, McMichael AJ. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trautmann A, Ruckert B, Schmid-Grendelmeier P, Niederer E, Brocker EB, Blaser K, Akdis CA. 2003. Human CD8 T cells of the peripheral blood contain a low CD8 expressing cytotoxic/effector subpopulation. Immunology 108:305–312. doi: 10.1046/j.1365-2567.2003.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Wang X, Lackner AA, Veazey RS. 2013. CD8 down-regulation and functional impairment of SIV-specific cytotoxic T lymphocytes in lymphoid and mucosal tissues during SIV infection. J Leukoc Biol 93:943–950. doi: 10.1189/jlb.1112580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouyang L, Li X, Liang Z, Yang D, Gong F, Shen G, Weng X, Wu X. 2013. CD8low T-cell subpopulation is increased in patients with chronic hepatitis B virus infection. Mol Immunol 56:698–704. doi: 10.1016/j.molimm.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Favre D, Stoddart CA, Emu B, Hoh R, Martin JN, Hecht FM, Deeks SG, McCune JM. 2011. HIV disease progression correlates with the generation of dysfunctional naive CD8(low) T cells. Blood 117:2189–2199. doi: 10.1182/blood-2010-06-288035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggi E, Giudizi MG, Biagiotti R, Annunziato F, Manetti R, Piccinni MP, Parronchi P, Sampognaro S, Giannarini L, Zuccati G, Romagnani S. 1994. Th2-like CD8+ T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J Exp Med 180:489–495. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiesel M, Oxenius A. 2012. From crucial to negligible: functional CD8(+) T-cell responses and their dependence on CD4(+) T-cell help. Eur J Immunol 42:1080–1088. doi: 10.1002/eji.201142205. [DOI] [PubMed] [Google Scholar]
- 31.Sun JC, Bevan MJ. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. 2005. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 33.Olson AD, Guiguet M, Zangerle R, Gill J, Perez-Hoyos S, Lodi S, Ghosn J, Dorrucci M, Johnson A, Sannes M, Moreno S, Porter K. 2014. Evaluation of rapid progressors in HIV infection as an extreme phenotype. J Acquir Immune Defic Syndr doi: 10.1097/QAI.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochsenbein AF, Riddell SR, Brown M, Corey L, Baerlocher GM, Lansdorp PM, Greenberg PD. 2004. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. J Exp Med 200:1407–1417. doi: 10.1084/jem.20040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matter MS, Claus C, Ochsenbein AF. 2008. CD4+ T cell help improves CD8+ T cell memory by retained CD27 expression. Eur J Immunol 38:1847–1856. doi: 10.1002/eji.200737824. [DOI] [PubMed] [Google Scholar]
- 36.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C. 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLoS Pathog 9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecuroux C, Girault I, Cheret A, Versmisse P, Nembot G, Meyer L, Rouzioux C, Pancino G, Venet A, Saez-Cirion A. 2013. CD8 T-cells from most HIV-infected patients lack ex vivo HIV-suppressive capacity during acute and early infection. PLoS One 8:e59767. doi: 10.1371/journal.pone.0059767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, Epling L, Lee TH, Busch MP, McCune JM, Pilcher CD, Hecht FM, Deeks SG. 2013. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 208:1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oxenius A, Price DA, Easterbrook PJ, O'Callaghan CA, Kelleher AD, Whelan JA, Sontag G, Sewell AK, Phillips RE. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A 97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]