ABSTRACT
Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is in the family Baculoviridae, genus Alphabaculovirus. AcMNPV me53 is a highly conserved immediate early gene in all lepidopteran baculoviruses that have been sequenced and is transcribed up to late times postinfection. Although me53 is not essential for viral DNA synthesis, infectious budded virus (BV) production is greatly attenuated when it is deleted. ME53 associates with the nucleocapsid on both budded virus and occlusion-derived virus, but not with the virus envelope. ME53 colocalizes in plasma membrane foci with the envelope glycoprotein GP64 in a GP64-dependent manner. ME53 localizes in the cytoplasm early postinfection, and despite the lack of a reported nuclear localization signal (NLS), ME53 translocates to the nucleus at late times postinfection. To map determinants of ME53 that facilitate its nuclear translocation, recombinant AcMNPV bacmids containing a series of ME53 truncations, internal deletions, and peptides fused with hemagglutinin (HA) or green fluorescent protein (GFP) tags were constructed. Intracellular-localization studies identified residues within amino acids 109 to 137 at the N terminus of ME53 that acted as the nuclear translocation sequence (NTS), facilitating its nuclear transport at late times postinfection. The first 100 N-terminal amino acids and the last 50 C-terminal amino acids of ME53 are dispensable for high levels of budded virus production. The region within amino acids 101 to 398, which also contains the NTS, is critical for optimal levels of budded virus production.
IMPORTANCE Baculovirus me53 is a conserved immediate early gene found in all sequenced lepidopteran alpha- and betabaculoviruses. We first identified residues within amino acids 109 to 137 at the N terminus that act as the ME53 nuclear translocation sequence (NTS) to facilitate its nuclear translocation and defined an internal region within amino acids 101 to 398, which includes the NTS, as being necessary for optimal budded virus production. Altogether, these results indicate a previously unidentified nuclear role that ME53 plays in virus replication.
INTRODUCTION
The Baculoviridae are a family of insect DNA viruses that infect insects, mostly from the order Lepidoptera but also from the orders Diptera and Hymenoptera. Baculoviruses are characterized by a circular double-stranded DNA genome ranging from 80 to 180 kb in size, packaged within a rod-shaped capsid and enclosed by a lipid envelope (1).
The viral DNA genome is uncoated into the nucleus, followed by virus gene transcription, DNA replication, and eventually nucleocapsid assembly in the nucleus prior to budding from the cells or occlusion into polyhedra (2, 3). Baculovirus gene expression and regulation follow a temporal cascade. Immediate early genes are transcribed first within 30 min postinfection, followed by transactivation of viral early genes (4). The early gene products then allow viral DNA replication and late/very late gene transcription (5, 6). Late and very late gene transcription is dependent on viral early gene products and viral DNA replication (6).
Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is in the species Autographa californica nucleopolyhedrovirus, the type species of the genus Alphabaculovirus. AcMNPV me53 (ACNVgp140) is a conserved immediate early gene found in all sequenced lepidopteran alpha- and betabaculoviruses. me53 is transcribed both early and late during infection from a dual early/late promoter (7, 8, 9). The putative 449-amino-acid (aa) ME53 protein contains a proline-rich region at the N terminus and a C-terminal C4 zinc finger domain whose function is not yet clear. Although ME53 is not essential for viral DNA synthesis, infectious budded virus (BV) production is greatly attenuated if me53 is knocked out (10). Western blot analysis of purified virions revealed that ME53 is associated with both BV and occlusion-derived virus (ODV), suggesting that ME53 may act as a packaging protein or as a structural component associated with intranuclear baculovirus virion assembly (10). Fractionation of budded virions further demonstrated that ME53 associates exclusively with the nucleocapsid and not with the envelope (10). Moreover, besides colocalizing at foci in the cell membrane along with the viral major envelope protein GP64 in an infection- and GP64-dependent manner, ME53 also translocates into the nucleus in the late phase (11). Since ME53 translocates to the nucleus, we were interested in identifying the amino acid sequence responsible for this.
Protein nuclear import typically requires a nuclear localization signal (NLS), which normally consists of a short cluster of positively charged arginine and/or lysine residues or a short sequence of basic amino acids flanked by prolines. The NLSs are recognized by nuclear transport receptors, such as importins, in the cytoplasm, which assist transport through the nuclear pore complex into the nucleus. Typical NLSs can be further classified as bipartite or monopartite (12). A monopartite NLS is composed of only one element, whereas a bipartite NLS is composed of two separate elements. For example, the AcMNPV nuclear protein LEF-3 residues 26 to 32, PKKIREN, were identified as the LEF-3 core NLS, with the adjacent residues, 18K and 19R, augmenting nuclear transport as part of the bipartite element (13). Similarly, AcMNPV DNApol, which is essential for viral DNA replication, also utilizes a bipartite NLS within residues 804 to 827 and a monopartite NLS within residues 939 to 948, both essential for its nuclear localization (14). Unlike the typical NLS, the nontypical NLSs do not have much in common. Some of the motifs are within one short region, while some are far apart. Most of them depend on specific protein structure formation and/or protein-protein interaction to facilitate their nuclear localization function (15, 16, 17). For instance, residues 534 to 538, KVNRR, in AcMNPV IE-1 form a positively charged domain that contributes to a novel nuclear localizer to initiate IE-1 nuclear transport upon homodimerization (18). AcMNPV P143 (helicase) itself, like ME53, does not have an NLS, but it is recruited and cotransported to the nucleus when bound to LEF-3, which has a bipartite NLS (19). Similarly, while ME53 localizes primarily to the cytoplasm early postinfection, it adopts a nuclear localization at late times. Moreover, ME53 nuclear translocation is infection dependent, as plasmid-expressed ME53 remains only cytoplasmic (11). This suggests that ME53, which lacks an identifiable NLS, is more likely to rely on a late nuclear protein to escort ME53 to the nucleus. However, the region in ME53 responsible for its nuclear translocation and the mechanism ME53 uses to translocate to the nucleus are still unknown. We hypothesized that ME53 has a nuclear translocation sequence (NTS) responsible for its nuclear translocation. In this study, we constructed a series of ME53 mutations by truncations, internal deletions, internal peptides, and site-directed mutagenesis focusing on the N terminus. These analyses have identified residues critical for ME53 nuclear translocation that would constitute an NTS. We also identified a minimal region of ME53 required for optimal BV production in vitro.
MATERIALS AND METHODS
Viruses and cell lines.
Bacmid bMON14272, containing an AcMNPV genome and propagated in Escherichia coli strain DH10B, was purchased from Invitrogen Life Technology. AcMNPV me53 was deleted by replacing it with the chloramphenicol acetyltransferase gene (10). This me53 knockout (KO) bacmid was used as the backbone for all the recombinant bacmid constructs developed here. The Sf21 insect cell line, derived from the fall armyworm (Spodoptera frugiperda), was cultured at 27°C in Grace's medium (Invitrogen Life Technologies) supplemented with 10% fetal bovine serum, penicillin (100 μg/ml), and streptomycin (30 μg/ml). In all experiments, the virus inoculum was allowed to adsorb for 1 h upon infection.
Bioinformatic analysis.
To obtain an overview of ME53 evolutionary relationships, conservation among baculoviruses, identification of conserved regions, and secondary-structure prediction, lepidoptera nucleopolyhedroviruses (NPV) from group I alphabaculoviruses (with GP64 as the major envelope protein) and group II alphabaculoviruses (with F protein as the major envelope protein) and from betabaculoviruses (granuloviruses [GVs]) were selected for ME53 bioinformatic analysis by pairwise comparison and phylogeny. The viruses selected were the group I alphabaculoviruses Autographa californica MNPV (AcMNPV), Anticarsia gemmatalis MNPV (AgMNPV), Antheraea pernyi NPV (AnpeNPV), Bombyx mori NPV (BmNPV), Choristoneura fumiferana DEF MNPV (CfDEFNPV), Choristoneura fumiferana MNPV (CfMNPV), Epiphyas postvittana NPV (EppoNPV), Hyphantria cunea NPV (HycuNPV), Maruca vitrata NPV (MaviNPV), Orgyia pseudotsugata MNPV (OpMNPV), Plutella xylostella multiple NPV (PlxyNPV), Rachiplusia ou MNPV (RoMNPV), and Thysanoplusia orichalcea NPV (ThorNPV); the group II alphabaculoviruses Adoxophyes honmai NPV (AdhoNPV), Agrotis segetum NPV (AgseNPV), Ecotropis obliqua NPV (EcobNPV), Helicoverpa armigera NPV (HearNPV), Lymantria dispar MNPV (LdMNPV), Leucania separata MNPV (LsMNPV), Mamestra configurata NPV (MacoNPV), Orgyia leucostigma NPV (OrleNPV), Spodoptera exigua MNPV (SeMNPV), Spodoptera frugiperda MNPV (SfMNPV), Spodoptera litura NPV (SpltNPV), and Trichoplusia ni single nucleopolyhedrovirus (TnSNPV); and the betabaculoviruses Adoxophyes honmai GV (AdhoGV), Agrotis segetum GV (AgseGV), Choristoneura occidentalis GV (ChocGV), Cydia pomonella GV (CpGV), Cryptophlebia leucotreta GV (CrleGV), Epinotia aporema GV (EpapGV), Helicoverpa armigera GV (HearGV), Phthorimaea operculella GV (PhopGV), Plutella xylostella GV (PlxyGV), Spodoptera frugiperda GV (SfGV), and Spodoptera litura GV (SpliGV). To infer the evolutionary relationships of ME53, the amino acid sequences of ME53 protein homologues were aligned with ClustalX, and MEGA 5.0.2 was used to generate a phylogenetic tree based on the differences and similarities of ME53s (20). SIAS (http://imed.med.ucm.es/Tools/sias.html) was applied to calculate the pairwise similarities and identities of ME53s from each group based on their amino acid sequences. Furthermore, T-COFFEE (21; http://tcoffee.crg.cat/apps/tcoffee/index.html) was applied to evaluate the amino acid conservation of ME53s from group I alphabaculoviruses. In order to investigate the related secondary structure within the conserved region based on its amino acid sequence, Ali2D (22; http://toolkit.tuebingen.mpg.de/sections/secstruct) was used to predict all the possible alpha helix and beta strand secondary structures formed in ME53s from group I alphabaculoviruses.
Generation of AcMNPV bacmids with HA-tagged ME53 peptides and internal deletions.
The me53 promoter was amplified from the AcMNPV bacmid using the primers me53pro-F (GAGCTCAGCGTGTGCGCCGGAGCACA; the SacI site is in italics) and me53pro-R (TCTAGATGTAACTGTTAGTTAGCACT; the XbaI site is in italics) and cloned into pBluescript using SacI and XbaI, generating pBlue-pro. The simian virus 40 (SV40) poly(A) signal was amplified from the plasmid pFACT with primers sv40-F (GATATCGATCATAATCAGCCATACCA; the EcoRV site is in italics) and sv40-R (CTCGAGGATCCAGACATGATAAGATA; the XhoI site is in italics) and cloned into pBlue-pro using EcoRV and XhoI, generating pBlue-pro-sv40. Each me53 fragment for making bacmids expressing ME53 peptides ME5333–82, ME5383–152, and ME53153–225 and internal deletions ME53Δ33–82, ME53Δ83–152, and ME53Δ153–225 was amplified from AcMNPV bacmid DNA and fused with a double-hemagglutinin (HA)-epitope tag at the C terminus by using primers listed in Table 1. The fragments were then cloned into pBlue-pro-sv40 using XbaI and EcoRV. The fragment containing the me53 promoter, C-terminally HA-tagged ME53 truncation, and SV40 poly(A) signal was then subcloned into pFACT-GFP using SacI and XhoI, generating the donor plasmid for transposition. The Tn7 cassette from the donor plasmid was transposed to the atti-Tn7 transposition site in the me53 knockout bacmid as described in the Bac-to-Bac expression manual (Invitrogen) to generate recombinant bacmids. All constructs containing ME53 mutations were confirmed by sequencing.
TABLE 1.
Primers for HA-tagged ME53 peptides or internal deletions
| Promoter, peptide, or deletion | Primer name | Sequencea |
|---|---|---|
| me53 promoter | ME53PRO-F | GAGCTCAGCGTGTGCGCCGGAGCACA |
| ME53PRO-R | TCTAGATGTAACTGTTAGTTAGCACT | |
| SV40 poly(A) | SV40-F | GATATCGATCATAATCAGCCATACCA |
| SV40-R | CTCGAGGATCCAGACATGATAAGATA | |
| ME5333–822HA | ME53(33-82)-F | TCTAGAATGCCGCCGTCGCCTGTTCGT |
| ME53(33-82)2HA-R | GATATCTTAAGCGTAATCTGGAACATCGTATG | |
| GGTAAGCGTAATCTGGAACATCGTATGGGTAATCTTTTCTGTTGACGACT | ||
| ME5383–1522HA | ME53(83-152)-F | TCTAGAATGGGATATTTTGTGCCGCCCGAGTT |
| ME53(83-152)2HA-R | GATATCTTAAGCGTAATCTGGAACATCGTATG | |
| GGTAAGCGTAATCTGGAACATCGTATGGGTATGCAAATTTGCCCGTCATGCGCAT | ||
| ME53153–2252HA | ME53(153-225)-F | TCTAGAATGAGCAGGCCTGTGAAATACAA |
| ME53(153-225)2HA-R | GATATCTTAAGCGTAATCTGGAACATCGTATG | |
| GGTAAGCGTAATCTGGAACATCGTATGGGTAAGAAGGATATATTTCGTAC | ||
| ME531–32 | ME53(1-449)-F | TCTAGAATGAACCGTTTTTTTCGAGA |
| ME53(1-32)-R | GGATCCCGAGTTGGCGGCAGGCGCTGGCAA | |
| ME5383–4492HA | ME53(83-449)-F | GGATCCGGATATTTTGTGCCGCCCGAGTT |
| ME53 (1-449)2HA-R | GATATCTTAAGCGTAATCTGGAACATCGTATG | |
| GGTAAGCGTAATCTGGAACATCGTATGGGTAGACATTGTTATTTACAAT | ||
| ME531–82 | ME53(1-82)-R | GGATCCATCTTTTCTGTTGACGACT |
| ME53153–4492HA | ME53(153-449)-F | GGATCCAGCAGGCCTGTGAAATACAA |
| ME531–152 | ME53(1-152)-R | GGATCCTGCAAATTTGCCCGTCATGCGCAT |
| ME53226–4492HA | ME53(226-449)-F | GGATCCATCAATTTGGTCGACCTCAGCTA |
Restriction sites are in italics and underlined.
Generation of GFP-fused ME53-truncated AcMNPV bacmids.
The green fluorescent protein gene (gfp) was amplified from plasmid pFACT-GFP by using primers gfp-F (CTGCAGGTGAGCAAGGGCGAGGAGCTG; thePstI site is in italics) and gfp-R (GATATCTTACTTGTACAGCTCGTCCATGC; the EcoRV site is in italics) and cloned into pBlue-pro-sv40 using PstI and EcoRV, generating pblue-pro-gfp-sv40. Each me53 fragment used for making the ME53 truncations ME53NTS, ME53Δ2–106, ME53Δ2–108, ME53Δ2–112, ME53Δ2–113, ME53Δ2–121, ME53Δ2–150, and ME53Δ250–449 was amplified from AcMNPV bacmid by using primers listed in Table 2. The fragments were then cloned into pBlue-pro-gfp-sv40 using XbaI and PstI. The insert containing the me53 promoter, C-terminal GFP-fused me53 truncation, and SV40 poly(A) signal was then subcloned into pFACT using SacI and XhoI, generating the donor plasmid for transposition, and used to generate recombinant bacmids in the me53 knockout bacmid as described above. All constructs containing ME53 truncations were confirmed by sequencing.
TABLE 2.
Primers for GFP-fused ME53 truncations
| Truncation | Primer name | Sequencea |
|---|---|---|
| GFP tag | GFP-F | CTGCAGGTGAGCAAGGGCGAGGAGCTG |
| GFP-R | GATATCTTACTTGTACAGCTCGTCCATGC | |
| ME53Δ2–106 | ME53(107-449)GFP-F | TCTAGAATGAAACAAGAGCGCGATCTACG |
| ME53(1-449)GFP-R | CTGCAGGACATTGTTATTTACAATA | |
| ME53Δ2–108 | ME53(109-449)GFP-F | TCTAGAGAGCGCGATCTACGTATGCAT |
| ME53Δ2–112 | ME53(113-449)GFP-F | TCTAGAATGCGTATGCATTTCATGAGCGATT |
| ME53Δ2–113 | ME53(114-449)GFP-F | TCTAGAATGCATTTCATGAGCGATTTAGAAC |
| ME53Δ2–121 | ME53(122-449)GFP-F | TCTAGAATGCGCGACATCATGAAAGCCACG |
| ME53Δ2–150 | ME53(151-449)GFP-F | TCTAGAAAATTTGCAAGCAGGCCTGTGA |
| ME53Δ250–449 | ME53(1-249)GFP-F | CTGCAGTGACAGCAGATGTCTATGCGGTC |
| ME53NTS | ME53NTS:GFP-F | TCTAGAATGGAGCGCGATCTACGTATGCA |
| ME53NTS:GFP-R | CTGCAGCATAATGTAATTGGTGGA |
Restriction sites are in italics and underlined.
GFP-tagged ME53 site-directed mutagenesis and internal deletions.
Site-directed mutagenesis in me53 was carried out using primers listed in Table 3, which were designed for specific point mutations and internal deletions at the ME53 N terminus. Codons for residue 121 (E), 122 (R), or 126 (K) in ME53 were each changed by point mutation to alanine (A) through PCRs using pFACT-ME53:GFP as the template. All the internal deletions ME53Δ107–121, ME53Δ121–130, ME53Δ126–140, ME53Δ138–145, and ME53Δ159–168 were carried out through PCRs using pFACT-ME53:GFP as the template, as well. PCR mixtures (25 μl) were set up as follows: 1 μl template DNA (200 ng/μl), 2 μl forward primer (200 nM), 2 μl reverse primer (200 nM), 0.5 μl deoxynucleoside triphosphate (dNTP) (200 μM), 2.5 μl PFU buffer (1×) [10× reaction buffer = 200 mM Tris-HCl (pH 8.8 at 25°C), 100 mM KCl, 100 mM (NH4)2SO4, 20 mM MgSO4, 1.0% Triton X-100, and 1 mg/ml nuclease-free bovine serum albumin (BSA); Agilent] , 0.5 μl PFU polymerase (1.25 U), 17 μl water (in a total volume adjusted to 25 μl). The PCR program was as follows: 95°C for 5 min, followed by 17 cycles of 95°C for 30 s, 56°C for 1 min, and 68°C for 12 min. Final incubation was at 4°C for 12 h. The PCR products with point mutations or internal deletions in me53 were then transformed into DH5α competent cells. Colonies with positive donor plasmids were selected, and the donor plasmid was transposed into the atti-Tn7 transposition site in the me53 knockout bacmid for ME53 mutant bacmid construction as described above. All constructs containing me53 point mutations or internal deletions were confirmed by sequencing.
TABLE 3.
Primers for GFP-fused ME53 site-directed mutagenesis (point mutations or internal deletions)
| Mutation or deletion | Primer name | Sequence |
|---|---|---|
| ME53 (121 E to A) | 121 E to A-F | CATTTCATGAGCGATTTAGCCCGCGACATCATGAAAGCC |
| 121 E to A-R | GGCTTTCATGATGTCGCGGGCTAAATCGCTCATGAAATG | |
| ME53 (122 R to A) | 122 R to A-F | CATTTCATGAGCGATTTAGAAGCCGACATCATGAAAGCCACGC |
| 122 R to A-R | GCGTGGCTTTCATGATGTCGGCTTCTAAATCGCTCATGAAATG | |
| ME53 (126 K to A) | 126 K to A-F | GATTTAGAACGCGACATCATGGCCGCCACGCTAAAATTTTCCAC |
| 126 K to A-R | GTGGAAAATTTTAGCGTGGCGGCCATGATGTCGCGTTCTAAATC | |
| ME53Δ107–121 | ME53(Δ107-121)GFP-F | GCGACAAACTGGATTTCGAACGCGACATCATGAAAGCCACG |
| ME53(Δ107-121)GFP-R | ATGATGTCGCGTTCGAAATCCAGTTTGTCGCTGTACGCGGG | |
| ME53Δ121–130 | ME53(Δ121-130)GFP-F | GCATTTCATGAGCGATTTAAATTACATTATGGGCTACATAAACAGCAAAG |
| ME53(Δ121-130)GFP-R | GTAGCCCATAATGTAATTTAAATCGCTCATGAAATGCATACGTAGATCG | |
| ME53Δ126–140 | ME53(Δ126-140)GFP-F | GAACGCGACATCATGAACAGCAAAGATATGCGCATGACGG |
| ME53(Δ126-140)GFP-R | GCGCATATCTTTGCTGTTCATGATGTCGCGTTCTAAATCGCTCATG | |
| ME53Δ138–145 | ME53(Δ138-145)GFP-F | CCACCAATTACATTATGCGCATGACGGGCAAATTTGCAAGC |
| ME53(Δ138-145)GFP-R | CAAATTTGCCCGTCATGCGCATAATGTAATTGGTGGAAAATTTTAGCG | |
| ME53Δ159–168 | ME53(Δ159-168)GFP-F | CAGGCCTGTGAAATACCGATGCACCACTTGCAATTATAGATTC |
| ME53(Δ159-168)GFP-R | GCAAGTGGTGCATCGGTATTTCACAGGCCTGCTTGCAAATTTGC |
Transfection.
Recombinant bacmid DNA was purified using a midi-plasmid extraction kit from Qiagen, and the NanoDrop ND-1000 (Thermo Scientific) was then used to determine the bacmid DNA concentration. For transfection, Sf21 cells were seeded at 1 × 106 cells/35-mm plate overnight. The cells were then transfected with 5 μg of the bacmid DNA by using 8 μl Cellfectin II (Invitrogen) according to the manufacturer's protocol. After incubation for 5 h, the DNA and Cellfectin mixture was removed and replaced with fresh Grace's medium.
Fluorescence and immunofluorescence confocal microscopy.
Sf21 cells were seeded on coverslips at 1 × 106 cells/plate (35-mm plate) overnight and then transfected with each of the recombinant bacmid DNAs. An early time point of 18 h posttransfection (hpt) and a late time point of 48 hpt were selected to observe ME53 localization. At 18 and 48 hpt, cells were first fixed with 4% paraformaldehyde in PBS for 15 min, washed 3 times for 5 min each time with 1 ml PBS, and then blocked for 30 min with 3% bovine serum albumin (BSA) in PBS. After blocking, the cells were incubated with mouse anti-HA monoclonal antibodies (Sigma), which were diluted 1:20 in 3% BSA in PBS for 2 h, and rinsed 3 times in 1 ml PBS for 5 min each time. The cells were then incubated with Alexa Fluor 594 goat anti-mouse secondary antibodies (Invitrogen) diluted 1:100 in 3% BSA in PBS for 1 h and rinsed 3 times for 5 min each time in 1 ml PBS. For a truncated or mutated ME53:GFP fusion, virus-transfected cells were fixed with 4% paraformaldehyde in PBS for 15 min and washed twice with 1 ml PBS (5 min each time). In both cases, the cells were then stained with Hoechst for 30 min in the dark and examined with a Leica SP5 confocal laser scanning microscope (CLSM) using a 63× dipping lens. For ME53:GFP, excitation was at 488 nm and acquisition was between 500 and 523 nm for GFP. For ME53:HA, excitation was at 594 nm and acquisition was between 607 and 671 nm for Alexa Fluor 594. To determine the percentage of successfully transfected cells showing nuclear localization of ME53, 40 cells with evidence of HA- or GFP-mediated fluorescence were chosen at random and scored. A lower magnification of cell monolayers is shown to demonstrate that fluorescence in either the cytoplasm or nucleus, or both, was observed in more than one cell.
Virus titration.
Sf21 cells were seeded at 1 × 106 cells/plate (35-mm plates) overnight. The cells were then transfected with 5 μg of recombinant bacmid DNA by using 8 μl Cellfectin II (Invitrogen) according to the manufacturer's protocol. To determine the level of BV production, a 200-μl sample of the medium was collected at 7 days posttransfection and centrifuged at 1,000 × g for 5 min to pellet the cells. The supernatant was then used for endpoint dilution (100 to 10−9) to determine the virus titer (23). The plates were scored according to the presence of occlusion bodies. For each of the ME53-mutated bacmids, two replicates of virus titrations were performed.
RESULTS
Bioinformatic analysis predicted three highly conserved regions in ME53.
While ME53 homologues are found in alpha- and betabaculoviruses, none could be found in delta- or gammabaculoviruses. To obtain an overview of ME53 evolutionary relationships, ME53s from both alpha- and betabaculoviruses were selected for phylogeny studies and analysis of amino acid sequence conservation. The phylogenetic tree showed evidence for three ME53 clades. All the group I alphabaculovirus ME53s studied belonged to clade 1, but it also contained ME53 from only one group II alphabaculovirus, SfMNPV. Clade 2 contained ME53s from mostly betabaculoviruses, though ME53s from four group II alphabaculoviruses were also part of the clade. Clade 3 contained ME53s from mostly group II alphabaculoviruses but also included ME53s from three betabaculoviruses. Among the group I ME53s, PlxyNPV ME53 is the most highly conserved with AcMNPV ME53. Overall, ME53s from the group II alphabaculoviruses and betabaculoviruses are more distant from group I ME53s (Fig. 1A). While in general the ME53s segregate according to genus and group, this is not absolute. Interestingly, ME53s from the group II alphabaculoviruses segregate into the same two clades as the betabaculovirus ME53s while ME53s from group I alphabaculoviruses are restricted to the first clade. The amino acid similarities and identities of AcMNPV and viruses from each group are shown, by pairwise comparison, in Tables 4 to 6. The conservation of ME53 is in general consistent with the baculovirus classification in the two genera. The fact that there is at least some conservation in ME53s among different genera suggests ME53 may also be conserved in its evolutionary function.
FIG 1.
Bioinformatic analysis of ME53s from group I and II alphabaculoviruses and betabaculoviruses. (A) Neighbor-joining phylogenetic analysis of ME53 protein sequences. The bootstrap scores of the nodes are shown. Clades 1, 2, and 3 are indicated by brackets. (B) Schematics of ME53 conservation relative to AcMNPV ME53 within each group of viruses showing three highly conserved amino acid regions, 111 to 138, 225 to 300, and 379 to 400.
TABLE 4.
Pairwise comparisons of amino acid similarities and identities for ME53s from group I alphabaculoviruses
| Virus | ME53 similarity⃥identity (%)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcMNPV | PlxyNPV | RoMNPV | BmNPV | ThorNPV | MaviNPV | CfDEFNPV | AgMNPV | CfMNPV | OpMNPV | EppoNPV | AnpeNPV | HycuNPV | |
| AcMNPV | 98.71 | 96.03 | 91.87 | 78.44 | 72.21 | 45.55 | 44.61 | 42.53 | 43.66 | 43.10 | 42.91 | 39.31 | |
| PlxyNPV | 99.43 | 96.21 | 91.68 | 78.82 | 72.02 | 45.17 | 44.23 | 42.15 | 43.28 | 42.72 | 42.53 | 39.31 | |
| RoMNPV | 97.16 | 97.35 | 89.79 | 78.07 | 72.77 | 45.17 | 44.42 | 42.72 | 43.47 | 42.91 | 42.53 | 39.50 | |
| BmNPV | 92.81 | 92.62 | 90.73 | 76.18 | 69.18 | 44.61 | 44.42 | 41.39 | 43.28 | 43.10 | 42.15 | 38.18 | |
| ThorNPV | 83.93 | 83.93 | 83.55 | 81.09 | 67.10 | 44.61 | 44.80 | 44.04 | 44.04 | 43.10 | 43.28 | 39.69 | |
| MaviNPV | 76.74 | 76.37 | 76.93 | 74.66 | 74.41 | 40.26 | 40.83 | 38.56 | 39.13 | 39.88 | 39.5 | 36.29 | |
| CfDEFNPV | 56.52 | 56.52 | 56.14 | 55.19 | 55.76 | 51.79 | 94.32 | 62.38 | 62.38 | 67.29 | 60.11 | 56.14 | |
| AgMNPV | 55.00 | 55.00 | 54.63 | 54.44 | 55.00 | 51.22 | 95.08 | 62.75 | 63.32 | 66.91 | 60.49 | 55.95 | |
| CfMNPV | 53.11 | 53.11 | 53.11 | 52.17 | 53.68 | 49.52 | 72.40 | 73.34 | 80.34 | 61.05 | 62.57 | 61.81 | |
| OpMNPV | 53.11 | 53.11 | 52.74 | 52.17 | 52.93 | 49.52 | 72.96 | 74.48 | 86.76 | 59.73 | 64.83 | 63.70 | |
| EppoNPV | 52.55 | 52.55 | 52.36 | 52.36 | 52.55 | 50.28 | 75.04 | 74.66 | 71.07 | 69.75 | 58.03 | 53.49 | |
| AnpeNPV | 52.36 | 52.55 | 51.98 | 50.28 | 52.74 | 49.14 | 69.56 | 70.13 | 74.1 | 73.15 | 67.48 | 51.98 | |
| HycuNPV | 49.33 | 49.33 | 49.33 | 48.39 | 49.71 | 46.12 | 65.02 | 64.65 | 68.99 | 70.13 | 63.32 | 61.43 | |
Identity, top right; similarity, bottom left.
TABLE 6.
Pairwise comparisons of amino acid similarities and identities for ME53s from betabaculoviruses and AcMNPV
| Virus | ME53 similarity⃥identity (%)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcMNPV | PlxyGV | AgseGV | SpliGV | CrleGV | HearGV | CpGV | SfGV | PhopGV | ChocGV | AdhoGV | EpapGV | |
| AcMNPV | 18.53 | 16.70 | 18.94 | 17.31 | 15.88 | 18.12 | 16.08 | 17.10 | 16.49 | 16.90 | 11.40 | |
| PlxyGV | 28.10 | 67.41 | 61.30 | 58.85 | 56.61 | 58.24 | 59.47 | 58.04 | 57.84 | 58.04 | 40.93 | |
| AgseGV | 26.88 | 76.17 | 60.28 | 59.06 | 54.98 | 59.87 | 59.47 | 59.26 | 59.87 | 59.06 | 42.36 | |
| SpliGV | 26.88 | 69.65 | 68.43 | 58.65 | 56.41 | 57.23 | 62.32 | 57.23 | 58.04 | 57.23 | 39.71 | |
| CrleGV | 26.27 | 67.41 | 69.04 | 65.78 | 50.50 | 80.24 | 54.98 | 69.04 | 69.45 | 67.00 | 41.14 | |
| HearGV | 25.66 | 63.95 | 63.74 | 64.56 | 58.45 | 50.71 | 73.52 | 49.69 | 49.08 | 50.10 | 38.69 | |
| CpGV | 25.45 | 66.59 | 68.63 | 64.56 | 85.94 | 58.85 | 55.80 | 71.07 | 71.07 | 66.59 | 42.36 | |
| SfGV | 25.45 | 67.00 | 68.83 | 69.85 | 62.11 | 80.24 | 62.11 | 56.00 | 53.56 | 54.37 | 37.88 | |
| PhopGV | 24.43 | 66.80 | 68.63 | 65.78 | 76.37 | 57.43 | 78.00 | 61.91 | 66.59 | 66.59 | 41.54 | |
| ChocGV | 23.42 | 65.78 | 69.24 | 64.35 | 77.39 | 58.04 | 77.80 | 61.30 | 75.35 | 66.59 | 42.76 | |
| AdhoGV | 23.21 | 67.00 | 68.22 | 64.56 | 74.33 | 58.45 | 74.33 | 62.72 | 72.50 | 74.54 | 41.54 | |
| EpapGV | 19.34 | 47.86 | 50.91 | 47.65 | 50.71 | 48.06 | 50.50 | 47.25 | 48.47 | 50.91 | 49.28 | |
Identity, top right; similarity, bottom left.
TABLE 5.
Pairwise comparisons of amino acid similarities and identities for ME53s from group II alphabaculoviruses and AcMNPV
| Virus | ME53 similarity\identity (%)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcMNPV | AgseNPV | TnSNPV | SfMNPV | MacoNPV | SeMNPV | LsMNPV | LdMNPV | OrleNPV | SpltNPV | AdhoNPV | HearNPV | EcobNPV | |
| AcMNPV | 29.20 | 25.33 | 26.69 | 25.14 | 24.37 | 24.17 | 24.56 | 22.82 | 22.82 | 23.98 | 21.85 | 19.72 | |
| AgseNPV | 36.94 | 58.60 | 68.47 | 64.21 | 64.60 | 46.42 | 48.93 | 44.68 | 45.45 | 50.09 | 47.38 | 29.78 | |
| TnSNPV | 34.42 | 68.27 | 55.31 | 52.80 | 50.29 | 40.61 | 43.90 | 41.58 | 39.45 | 45.84 | 43.13 | 30.36 | |
| SfMNPV | 33.65 | 76.2 | 62.66 | 59.38 | 68.85 | 43.32 | 44.87 | 42.94 | 42.16 | 48.16 | 43.71 | 29.98 | |
| MacoNPV | 33.26 | 71.56 | 60.34 | 66.73 | 54.15 | 44.87 | 46.61 | 43.13 | 44.68 | 47.38 | 47.19 | 29.78 | |
| SeMNPV | 32.68 | 70.01 | 56.86 | 74.46 | 61.50 | 38.87 | 41.58 | 38.68 | 38.49 | 41.77 | 41.00 | 27.85 | |
| LsMNPV | 32.10 | 54.15 | 48.74 | 50.29 | 52.41 | 47.00 | 45.84 | 38.49 | 73.11 | 46.42 | 50.67 | 27.27 | |
| LdMNPV | 31.72 | 55.89 | 52.41 | 52.41 | 52.99 | 48.35 | 52.41 | 51.06 | 44.29 | 47.00 | 43.13 | 35.78 | |
| OrleNPV | 31.14 | 52.80 | 49.32 | 50.09 | 50.09 | 46.22 | 45.26 | 60.54 | 37.13 | 40.81 | 39.07 | 44.10 | |
| SpltNPV | 30.94 | 52.41 | 47.19 | 49.70 | 51.25 | 45.26 | 79.49 | 50.29 | 45.06 | 43.90 | 48.93 | 24.95 | |
| AdhoNPV | 30.75 | 57.44 | 52.41 | 54.73 | 55.12 | 48.54 | 53.19 | 52.80 | 47.77 | 51.64 | 45.06 | 25.91 | |
| HearNPV | 29.98 | 55.51 | 51.25 | 51.64 | 56.47 | 48.35 | 58.41 | 52.22 | 47.77 | 56.47 | 51.45 | 24.56 | |
| EcobNPV | 28.62 | 38.29 | 39.45 | 36.75 | 36.55 | 37.13 | 34.04 | 46.42 | 54.35 | 32.88 | 34.04 | 34.81 | |
Identity, top right; similarity, bottom left.
ME53s from group I alphabaculoviruses (AcMNPV, AgMNPV, AnpeNPV, BmNPV, CfDEFNPV, CfMNPV, EppoNPV, HycuNPV, MaviNPV, OpMNPV, PlxyNPV, RoMNPV, and ThorNPV) have polypeptides of similar sizes (433 aa to 483 aa) and share high amino acid similarity and identity. However, ME53s from group II alphabaculoviruses (AdhoNPV, AgseNPV, EcobNPV, HearNPV, LdMNPV, LsMNPV, MacoNPV, OrleNPV, SeMNPV, SfMNPV, SpltNPV, and TnSNPV) and betabaculoviruses (AdhoGV, AgseGV, ChocGV, CpGV, CrleGV, EpapGV, HearGV, PhopGV, PlxyGV, SfGV, and SpliGV) lack the first 80 to 110 amino acids at the N terminus that are present in group I viruses and consequently are much smaller (289 aa to 450 aa). Nevertheless, there are three highly conserved regions that exist in ME53s from both alpha- and betabaculoviruses. The first conserved region, relative to AcMNPV ME53, is at the amino terminus within aa 107 to 190 of group I, especially from aa 111 to 138 (both alphabaculoviruses and betabaculoviruses), including three 100% conserved residues, D111, R113, and G138. However, the function of this region was not known prior to our work. The second highly conserved region lies within aa 225 to 300 and the third within aa 379 to 400 (both alphabaculoviruses and betabaculoviruses), which includes the previously identified putative zinc finger domain (Fig. 1B).
Among the highly conserved alphabaculoviruses, group I ME53s share high amino acid similarity, with aa 111 to 145 relative to AcMNPV ME53 at its N terminus being highly conserved (Fig. 2A). In particular, ME53 residues D111, L112, R113, H115, F116, S118, E121, R122, M125, L129, F131, T133, N134, Y135, G138, and Y139 are 100% conserved (Fig. 2A, asterisks). Moreover, three residues, D111, R113, and G138, are conserved, not just in group I alphabaculoviruses, but also in group II alphabaculoviruses and betabaculoviruses. Among all the conserved sites, two are acidic residues (D111 and E121) and three are basic residues (R113, H115, and R122). Such charged amino acids may contribute to the conformation of ME53 and form domains that are capable of binding to chaperone proteins to facilitate ME53 nuclear localization. The secondary structure predicted in the conserved N-terminal aa 111-to-145 region is an alpha helix from aa 113 to 136 (Fig. 2A), which is also the longest alpha helix predicted in ME53. Another highly conserved region in ME53 is a zinc finger domain from aa 379 to 400 that is conserved in all alpha- and betabaculoviruses (Fig. 1B and 2B). In particular, AcMNPV ME53 residues C379, C382, K383, K386, N391, P392, C396, C399, G400, F401, T402, F407, and Y411 are 100% conserved among all group I alphabaculoviruses analyzed (Fig. 2B). The conserved cysteines C379, C382, C396, and C399 have the appropriate spacing for a C4 zinc finger (Fig. 2B).
FIG 2.
Analysis of amino acid sequence conservation of ME53s from group I alphabaculoviruses. Shown are amino acid sequence alignments of the ME53 N terminus (A) and C terminus (B). The asterisks indicate 100% identical residues, the colons indicate conserved substitutions, and the periods indicate semiconserved substitutions. The sequences within the boxes represent the predicted alpha helix of aa 113 to 136 (A) and putative zinc finger domain of aa 379 to 399 (B). The numbered amino acids are the 100% conserved residues mentioned in the text.
Preliminary mapping of ME53 fragments required for nuclear translocation.
By sequence analysis, ME53 does not contain any recognized mono- or bipartite NLSs. Moreover, ME53 translocates to the nucleus only in the late phase during virus infection, which further suggests that ME53 does not have an intrinsic NLS. Thus, it may utilize viral and/or host chaperone proteins to facilitate its nuclear translocation. To identify which region is needed for the nuclear translocation of AcMNPV ME53 in virus-transfected cells, a series of ME53 peptides and corresponding internal deletions were constructed (Fig. 3A). Since the 200 amino acids in the C terminus of ME53 are not essential for its nuclear translocation (24), the focus of this study was on the 250 amino acids in the N terminus. Based on the amino acid compositions of different regions, the ME53 N terminus was further divided into 3 contiguous smaller regions, ME5333–82, ME5383–152, and ME53153–225. Each region contains a cluster of positively charged arginine or lysine residues that are often part of a typical NLS. A double-HA epitope tag was fused to the C termini of the ME53 mutants (ME53:2HA) to follow their intracellular localization by immunofluorescence microscopy. Since most of the ME53 peptides and internal deletions reduced the normal levels of BV production, there was insufficient virus available for infection studies. Consequently, we used transfection with bacmid DNAs throughout the study. ME53 localization was analyzed at 18 and 48 hpt. Transfected cells identified as showing ME53 nuclear translocation were those in which fluorescence could be seen largely in the nucleus. By 18 hpt, which is equivalent to approximately 6 h postinfection (hpi), the full-length ME53, the ME53 peptides, and internal deletions from each construct all localized mainly in the cytoplasm, with only negligible levels showing in the nucleus (Fig. 4, ME53:2HA). By 48 hpt, which is equivalent to approximately 36 hpi, the full-length ME53 was localized equally in the cytoplasm and nucleus. Subsequently, only the 48-hpt time point was used for analysis of nuclear translocation. As shown in Fig. 4, by 48 hpt, the full-length ME53 localized primarily in the nucleus, and 85% of the successfully transfected cells showed obvious nuclear translocation. However, peptide ME5333–82 showed only cytoplasmic localization, while the corresponding internal deletion, ME53Δ33–82, did not inhibit its nuclear transport. Similarly, the peptide ME53153–225 localized exclusively to the cytoplasm, while the corresponding internal deletion, ME53Δ153–225, still translocated primarily to the nucleus. These data suggested that neither the peptide ME5333–82 nor the peptide ME53153–225 was important for ME53 nuclear localization. In contrast, by 48 hpt, the peptide ME5383–152 translocated to the nucleus in 65% of the successfully transfected cells, mimicking the full-length ME53 localization, while the corresponding internal deletion, ME53Δ83–152, failed to localize to the nucleus (Fig. 4). Thus, the peptide ME5383–152, which includes the N-terminal conserved region of aa 111 to 145 shown in the bioinformatic analysis, was found to be sufficient for its ME53 nuclear translocation.
FIG 3.
Schematics of ME53 bacmid constructions. (A) Cloning strategy for HA-tagged ME53 peptides and internal deletions using the AcMNPV me53 knockout bacmid as the backbone. An HA epitope tag (open squares) was fused to the C terminus of ME53 (solid rectangles) to allow intracellular localization of ME53 by immunofluorescence microscopy. (B) Strategy for GFP-fused ME53 truncations and internal deletions using the AcMNPV me53 knockout bacmid as the backbone. A GFP tag (gray rectangles) was fused to the C terminus of ME53 (black rectangles) to follow ME53 localization by fluorescence microscopy. The white rectangles represent internal deletions.
FIG 4.
Intracellular localization of HA-tagged ME53 peptides and internal deletions following transfection with bacmid DNA at 18 and 48 hpt. Cells were fixed and stained with mouse anti-HA primary antibody (1:20) and Alexa Fluor 594 goat anti-mouse secondary antibody (1:100). Forty transfected cells showing red fluorescence were counted, and the percentages of cells showing nuclear localization are indicated. Peptide aa 83 to 152 localized largely to the nucleus, while ME53 lacking aa 83 to 152 remained cytoplasmic. The monolayer column is at a lower magnification to show several cells in the same view. Scale bar is 2 µm.
Fine mapping the ME53 NTS sequence.
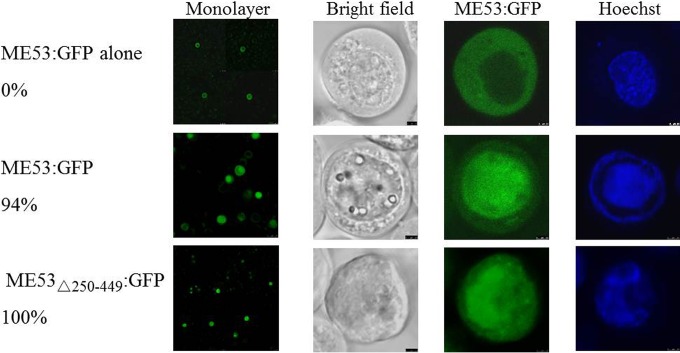
To confirm the preliminary results from immunofluorescence microscopy mapping the NTS to aa 83 to 152 and to more finely map the minimal residues in the peptide essential for ME53 nuclear transport, N- and C-terminal truncations of ME53 were constructed. In addition, each of the constructs was fused with a GFP tag at its carboxyl end (Fig. 3B). Each of the AcMNPV bacmids with ME53:GFP truncations was transfected into Sf21 cells and monitored for nuclear localization. As an infection negative control, only the expression plasmid pME53:GFP was transfected. ME53:GFP in the absence of bacmid cotransfection was localized to the cytoplasm only, while that expressed from a bacmid did localize to the nucleus (Fig. 5). Previous data have shown that the C terminus of ME53 is not required for its nuclear translocation (24). To confirm this result, all 200 amino acids in the C terminus were deleted (Δ250–449) from ME53. As expected, ME53Δ250–449:GFP accumulated in the nucleus at late times posttransfection, confirming that the C terminus of ME53 is not required for its nuclear transport (Fig. 5).
FIG 5.
Intracellular localization of plasmid-only expressed GFP fused to ME53 (ME53:GFP alone), bacmid DNA-expressed full-length ME53 fused to GFP (ME53:GFP), and ME53 lacking aa 250 to 449 at the C terminus fused to GFP (ME53Δ250–449:GFP) at 48 hpt. Cells were fixed and examined under a Leica SP5 CLSM using a 63× dipping lens. Forty transfected cells with green fluorescence were counted, and the percentages of cells showing nuclear localization are indicated. Transient expression of ME53:GFP in the absence of virus infection showed only cytoplasmic localization, while truncation of the C terminus of ME53 did not abolish its nuclear translocation.
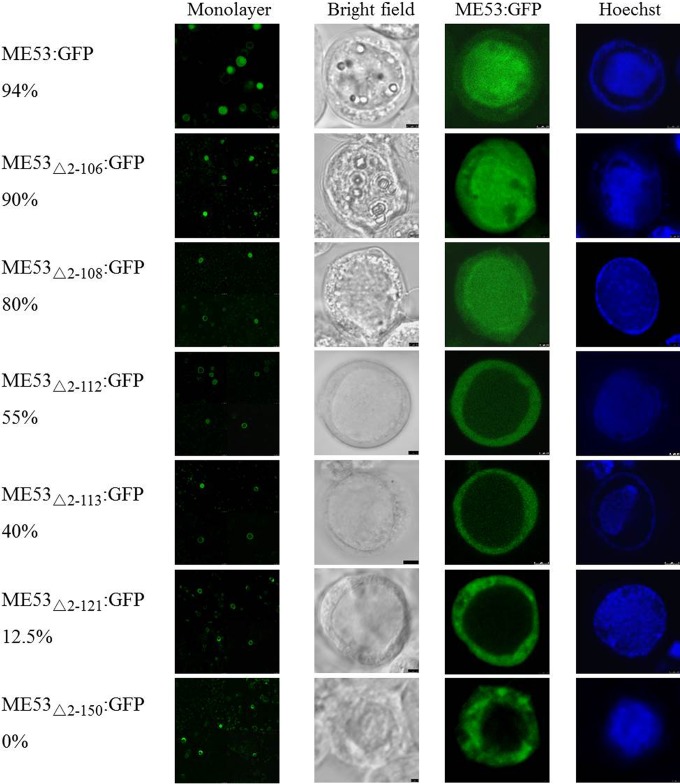
To more finely map the NTS, AcMNPV bacmids with different N-terminal truncations of ME53:GFP were transfected into Sf21 cells (Fig. 6). ME53Δ2–106:GFP and ME53Δ2–108:GFP showed no or minimal impact on nuclear translocation. A major reduction in cell numbers showing nuclear transport was observed, starting with ME53Δ2–112:GFP, where only 55% of the cells showed nuclear translocation. Even lower levels of nuclear localization were observed for deletions beyond aa 112, including ME53Δ2–113:GFP with 40%, ME53Δ2–121:GFP with 12.5%, and finally ME53Δ2–150:GFP with 0% nuclear localization (Fig. 6). This showed that the ME53 N-terminal aa 2 to 108 are not required for nuclear translocation. Therefore, the ME53 NTS required for optimal nuclear translocation mapped to residues within aa 109 to 249. Combined with the immunofluorescence data of the HA-tagged constructs (Fig. 4), the N-terminal-truncation studies tentatively mapped the NTS to residues within aa 109 to 152.
FIG 6.
Intracellular localization of GFP-fused ME53 N-terminal truncations with bacmid DNA at 48 hpt. Cells were fixed and examined under a Leica SP5 CLSM using a 63× dipping lens. Forty transfected cells with green fluorescence were counted, and the percentages of cells showing any nuclear localization are indicated. Nuclear localization started to be reduced for truncations of ME53 downstream of amino acid 108.
To more accurately define the C terminus of the NTS within aa 109 to 152, an additional series of internal deletions at the C terminus of the region in ME53:GFP was generated (Fig. 1B). The internal deletions were Δ107–121, Δ121–130, Δ126–140, Δ138–145, and Δ159–168. Of these five internal deletions, only ME53Δ138–145:GFP and ME53Δ159–168:GFP had minimal or no impact on nuclear translocation (Fig. 7). ME53Δ107–121:GFP, ME53Δ121–130:GFP, and ME53Δ126–140:GFP all greatly compromised the nuclear translocation at 48 hpt (Fig. 7). Based on these data, the C terminus of the ME53 NTS does not go beyond amino acid 137. These results, therefore, mapped the ME53 NTS to residues within aa 109 to 137, a highly conserved region that includes a predicted alpha helix of aa 113 to 136.
FIG 7.
Intracellular localization of GFP-fused ME53 with internal deletions at the C terminus of the putative NTS at 48 hpt. Cells were fixed and examined under a Leica SP5 CLSM using a 63× dipping lens. Forty transfected cells with green fluorescence were counted, and the percentages of cells showing any nuclear localization are indicated. ME53 nuclear translocation was observed for deletions of residues downstream of amino acid 137.
To confirm the function of the NTS as a nuclear translocation sequence, ME53 bacmids containing an NTS (aa 109 to 137) fused to GFP (NTS:GFP) and a fusion between GFP and ME53 lacking an NTS (ΔNTS:GFP) were constructed. Compared to GFP alone, which localized equally between the cytoplasm and nucleus, the NTS bearing GFP concentrated mostly in the nucleus in the successfully transfected cells by 48 hpt. In contrast, the internal deletion of the NTS (aa 113 to 139) in ME53 of an ME53:GFP fusion (ΔNTS:GFP), which is also predicted to disrupt the alpha helical structure in this area, totally abolished the nuclear translocation (Fig. 8). Therefore, the ME53 NTS was found to be essential and sufficient for its nuclear translocation.
FIG 8.
Intracellular localization of transiently expressed GFP (GFP alone), bacmid-expressed ME53 fused to GFP (ME53:GFP), GFP only tagged with the NTS (aa 109 to 137) (NTS:GFP), and GFP fused to ME53 with the NTS (aa 113 to 139) deleted (ME53ΔNTS:GFP) at 48 hpt. Cells were fixed and examined under a Leica SP5 CLSM using a 63× dipping lens. Forty transfected cells with green fluorescence were counted, and the percentages of cells showing any nuclear localization are indicated. NTS:GFP localized mostly to the nucleus, while deletion of the NTS from ME53 abolished its nuclear localization.
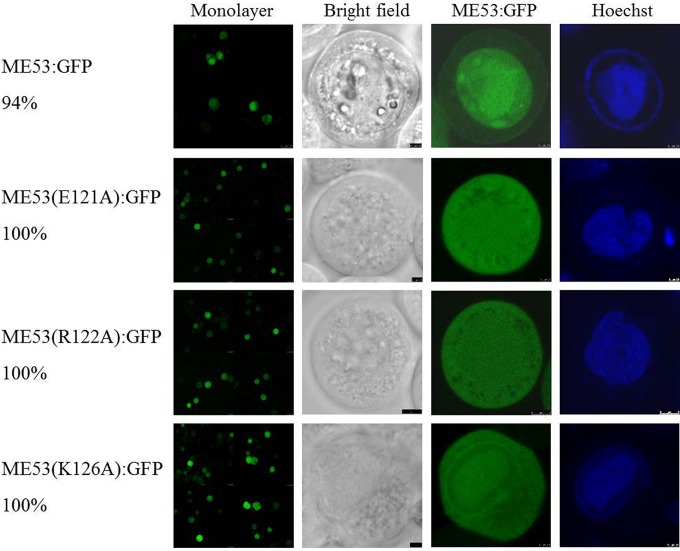
Alanine mutagenesis within the ME53 NTS.
Site-directed mutagenesis was introduced to disrupt the predicted alpha helix component within the ME53 NTS to determine if the highly conserved residues E121, R122, and K126 were critical for ME53:GFP nuclear translocation. Residues E121, R122, and K126 were mutated to alanine to determine the effect on nuclear localization. These mutations were predicted by COILS (http://www.ch.embnet.org/software/COILS_form.html) to disrupt the alpha helix secondary structure in this region. However, ME53 translocated to the nucleus for all of the single mutations E121A, R122A, and K126A (Fig. 9). Even a mutation of both E121 and R122 (E121A R122A) failed to reduce its nuclear translocation (data not shown). The fact that the ME53s expressed by these mutants localize to the nucleus suggested that these specific amino acids are not critical for nuclear translocation.
FIG 9.
Intracellular localization of GFP-fused ME53 site-directed mutations with bacmid DNA at 48 hpt. Cells were fixed and examined under a Leica SP5 CLSM using a 63× dipping lens. Forty transfected cells with green fluorescence were counted, and the percentages of cells showing nuclear localization are indicated. The highly conserved residues E121, R122, and K126 were mutated to alanine (A). None of the single-site mutations altered its nuclear translocation.
The ME53 NTS and other residues within amino acids 101 to 398 are necessary for optimal budded virus production.
In addition to nuclear translocation, ME53 also forms foci at the cell membrane and is required for high-level BV production. A series of bacmids containing ME53 mutations were used to determine the minimal region necessary for efficient BV production. Virus titrations for each of the ME53 mutants were performed at 7 days posttransfection, and the percent virus yields shown in Fig. 10 were based on the average titers of two replicates.
FIG 10.
Virus titration of ME53 truncations and internal deletions at 7 days posttransfection. The horizontal dotted lines represent internally deleted regions. Supernatant with BVs was collected and used in endpoint dilution (100 to 10−9) to determine the virus titer. The values are relative to 100% virus yield for the wild-type ME53 and are the averages of two determinations.
The virus with wild-type (WT) ME53 showed a high titer at 2.05 × 108 50% tissue culture infective doses (TCID50)/ml, while virus without ME53 (ME53 KO) had a decreased yield of 1.48 × 104 TCID50/ml, only less than 0.01% that of the WT. Viral titers from cell monolayers transfected with the N-terminal truncations demonstrated that the BV production from a bacmid lacking the first 50 amino acids (Δ3–50) in the N terminus of ME53 was equivalent to that of the WT, while BV production from a bacmid lacking the first 100 amino acids (Δ3–100) in the N terminus was reduced to 11% compared to the WT. However, further deletion from the N terminus (Δ3–121) severely impaired virus yield, reducing it to less than 0.01% that of the WT, similar to that of the ME53 KO bacmid (Fig. 10). This suggests that a region at the N terminus beginning between aa 50 and 100 is required for optimal BV production, though not for nuclear translocation, while the N-terminal region beginning between aa 100 and 121 is essential for BV production at a level above that for the ME53 KO bacmid. As the latter truncation would disrupt the putative NTS, this suggested that the ME53 nuclear localization is also required for optimal BV production. In addition, viral titers from cell monolayers transfected with C-terminal truncations of different lengths demonstrated that the C-terminal 50 amino acids were not essential for normal levels of BV production. With the C-terminal 50 amino acids deleted (Δ399–449), the virus yield was 48% that of the WT. However, further deletion up to the C-terminal 100 amino acids (Δ349–449) resulted in a much lower viral titer, 0.078% that of the WT, demonstrating that ME53 ending between aa 349 and 399 is essential for an optimal virus yield. Therefore, a region within aa 101 to 398 in AcMNPV ME53 is necessary for optimal BV production (Fig. 10).
BV production was greatly impaired for the internal deletion bacmid Δ113–139, which deletes the ME53 NTS (ΔNTS), and the internal deletion bacmid Δ169–191. The BV yields of Δ113–139 and Δ169–191 were only 0.015% and 0.013% that of the WT, respectively. The C-terminal internal deletion Δ278–302 also reduced the virus titer to 0.058% that of the WT (Fig. 10). This suggested that the NTS region aa 109 to 137 in ME53 itself is not just important for nuclear translocation but is also required for optimal BV production. Interestingly, the virus yield of the zinc finger deletion (ΔZnF) in ME53 was 53% that of the WT, essentially indistinguishable from that of WT ME53 at 7 days posttransfection (Fig. 10). The virus titers from the ΔZnF bacmid were also measured and compared to those of the wild-type bacmid at 24, 48, 60, and 72 hpt, and the virus yields at those time points were similar to those of the WT ME53 (data not shown). This suggests that there is no delay in virus production when the zinc finger is deleted and that the C4 zinc finger domain itself in ME53 is not necessary for optimal BV production.
DISCUSSION
The AcMNPV me53 gene is continuously expressed from immediate early to late phases during infection (9). me53 is essential for wild-type levels of BV production, and deletion of me53 results in a 10,000-fold reduction in virus yield (10). Our bioinformatic analysis showed that ME53 is conserved in both alpha- and betabaculoviruses but is not found in gamma- or deltabaculoviruses. Compared to group I alphabaculoviruses, ME53s from group II alphabaculoviruses and betabaculoviruses lack about 100 aa of the N terminus. This amino-terminal region of the AcMNPV ME53 protein is the same one we found to be dispensable for NTS activity and optimal virus production. However, there are three highly conserved regions in ME53 in both the alpha- and betabaculoviruses. The first is at the N terminus within aa 107 to 190, which includes the ME53 NTS at aa 109 to 137 identified in this study. The second lies at the C terminus within the range aa 225 to 300. The third conserved sequence includes the previously identified putative zinc finger domain at aa 379 to 400.
Intracellular localization of ME53 revealed a switch from an early cytoplasmic localization to a nuclear localization later during infection (11). The fact that ME53 localized only to the cytoplasm early in infection suggested the lack of an NLS in ME53. Moreover, as previously reported (11) and confirmed in this study, when ME53:GFP alone was transiently expressed in a plasmid, ME53 remained in the cytoplasm. Since ME53 remained cytoplasmic in the absence of virus infection, this suggested the involvement of viral chaperone proteins available late postinfection, enabling ME53 nuclear translocation. For nuclear proteins with a typical NLS to initiate nuclear transport, the NLS interacts with two major cellular proteins, importin α and importin β, to complete the protein translocation through the nuclear pore complex (12, 25, 26). However, for nuclear proteins that do not have a typical NLS, such as ME53, little is known about the nuclear translocation mechanism. A series of ME53 truncations and internal deletions were constructed to more accurately map the NTS to residues within aa 109 to 137, which were shown to be essential for the nuclear translocation. Within the alphabaculovirus ME53 NTS region, aa 113 to 136 consists of a cluster of highly conserved amino acids that form a predicted alpha helix that may contribute to forming a domain capable of binding to chaperone proteins to facilitate ME53 nuclear localization. The fact that deletion of the NTS prevents nuclear localization of an ME53:GFP fusion while addition of the NTS to GFP enhances GFP localization to the nucleus further confirms the nuclear translocation function of the AcMNPV ME53 NTS. If the region including the alpha helix is deleted, the ME53 nuclear translocation efficiency is much lower than that of the wild-type ME53. This suggests that the alpha helix region in the NTS is required for the interaction between ME53 and a putative chaperone protein. The fact that changing some of the conserved charged amino acids in the predicted alpha helix did not alter the NTS activity suggests that the NTS is not dependent on the alpha helical nature of these specific charged amino acids and that other amino acids within the NTS might complement their function. ME53 transiently expressed in uninfected cells is distributed predominantly in the cytoplasm, suggesting that virus infection is required to escort ME53 to the nucleus. Since the deletion of ME53 does not compromise viral DNA replication, this suggests that the nuclear-localized ME53 is required for a purpose other than DNA replication. For example, the fact that virus assembly occurs in the nucleus and ME53 is detected on both BV and ODV nucleocapsids suggests that ME53 might act as a structural or scaffolding protein involved in virus assembly. A similar example of baculovirus proteins chaperoning other viral proteins to the nucleus is baculovirus P78/83 and BV/ODV-C42, which are both required for virus assembly. The highly conserved baculovirus protein BV/ODV-C42 contains a putative NLS at its C terminus and is capable of binding to the viral nucleocapsid protein P78/83. Although P78/83 itself does not have an NLS, it is capable of translocating to the nucleus, but only when BV/ODV-C42 binds to P78/83, resulting in its recruitment to the nucleus for actin polymerization and nucleocapsid assembly (27, 28).
The results of this study showed that the NTS and other residues within aa 101 to 398 in ME53 are critical for optimal levels of BV production. The fact that the ME53s in group II alphabaculoviruses and betabaculoviruses lack the N-terminal 100 amino acids of the group I alphabaculoviruses further demonstrates that they are not essential for ME53 function and BV production. Surprisingly, the viral titer from the zinc finger knockout bacmid was similar to that of WT ME53 throughout the replication cycle, indicating that the putative zinc finger domain is dispensable for normal levels of BV production. It is significant that the deletion of only the ME53 NTS greatly compromised BV production, with a 10,000-fold decrease in the virus titer, similar to the me53 knockout virus. Thus, BV production levels correlate with the nuclear localization of ME53. Although ME53 nuclear translocation is important for normal BV production, it seems that virus production also requires the regions surrounding the NTS from aa 101 to 398. When aa 169 to 191 or aa 278 to 302 downstream of the nuclear translocation region were deleted, ME53 was able to enter the nucleus, but the virus production level was still compromised. Though we lack direct evidence, one possible nuclear function of ME53 is as a matrix or even a structural protein directly affecting intranuclear viral nucleocapsid assembly. The ME53 NTS within aa 109 to 137 may thus facilitate ME53 nuclear transport, and the nuclear-localized ME53 may be required for efficient viral nucleocapsid assembly and eventual egress. Nevertheless, since deletion of the full-length ME53 still yields infectious BV production, albeit at extremely low levels, other viral or host nuclear proteins may be able to substitute for the function of ME53 in virus production, although at much reduced efficiency.
Moreover, since ME53 localizes to both the nucleus and cell membrane foci, it is likely that ME53 influences virus production during both the assembly and budding steps. Preliminary data from immunoprecipitation assays revealed that ME53 potentially interacts with both viral envelope GP64 and capsid protein VP39 (unpublished data). Since VP39 is a nuclear viral protein and participates in virus assembly, VP39 might act as a chaperone protein to facilitate ME53 nuclear translocation. When VP39 was deleted, ME53 still translocated to the nucleus, albeit at a much lower level (11). While this suggests that VP39 might be one ME53 chaperone protein, other viral or host proteins might chaperone ME53 nuclear translocation, as well. In addition, the association of ME53 with the viral envelope protein GP64 is also consistent with their colocalization and focus formation at the plasma membrane. Previous studies (24) do not support a direct interaction between GP64 and ME53, because GP64 and ME53 do not form foci when only the two proteins are expressed in uninfected cells. Nevertheless, a third viral protein might bind both ME53 and GP64 for focus formation and potential virus budding.
ME53 might have multiple roles after being transported to the nucleus. Since ME53 has a C4 zinc finger domain that is usually involved in DNA binding and protein interactions and the zinc finger domain is highly conserved across both the alpha- and betabaculoviruses, ME53 might also be involved in viral gene transcription regulation in virus production. ME53 might transactivate the expression of other viral genes encoding structural proteins to increase the pool of the structural proteins for nucleocapsid assembly. Transcription factors are normally related to DNA binding and transcriptional activation and can function only when they enter the nucleus. For instance, herpes simplex virus 1 tegument protein VP16 initiates viral transcriptional activation in the nucleus by recruiting the host protein Oct-1 and host cell factor (29, 30, 31, 32) to form a complex on the regulatory sites of TAATGARAT motifs in each of the immediate early gene promoters to activate its expression (33, 34). ME53 might play a similar role at later times postinfection by transactivating the expression of viral late structural protein genes for BV assembly or ODV formation. This would be consistent with the fact that ME53 is distributed predominantly in the cytoplasm at early times postinfection, while nuclear translocation is observed mostly late in infection.
In summary, this study is the first to report that AcMNPV ME53 utilizes an NTS within aa 109 to 137 to translocate to the nucleus and that a region within aa 101 to 398 is necessary for optimal BV production. The study has for the first time demonstrated the functional significance of ME53 in baculovirus replication and indicates that it has multiple roles, including in the nucleus and later at the plasma membrane for virus egress. Furthermore, identification of an NTS now allows us to better assess the possible nuclear roles, such as in transcriptional regulation and nucleocapsid assembly, for example, by using an ME53 ΔNTS bacmid.
ACKNOWLEDGMENTS
This work was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) discovery (RGPIN-2009-8395 and RGPIN-2014-05472) and strategic (STPGP 365213-2008) grants to P.J.K.
We acknowledge Michaela Strüder-Kypke and David Leishman for technical assistance.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Funk J, Braunagel SC, Rohrmann GF. 1997. Baculovirus structure, p 7–32. Miller LK, () The baculoviruses. Plenum Press, New York, NY. [Google Scholar]
- 2.Federici BA. 1997. Baculovirus pathogenesis, p 33–60. In Miller LK. (ed), The baculoviruses. Plenum Press, New York, NY. [Google Scholar]
- 3.Lauzon HA, Lucarotti CJ, Krell PJ, Feng Q, Retnakaran A, Arif BM. 2004. Sequence and organization of the Neodiprion lecontei nucleopolyhedrovirus genome. J Virol 78:7023–7035. doi: 10.1128/JVI.78.13.7023-7035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisholm GE, Henner DJ. 1988. Multiple early transcripts and splicing of the Autographa californica nuclear polyhedrosis virus IE-1 gene. J Virol 62:3193–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu A, Miller LK. 1995. The roles of eighteen baculovirus late expression factor genes in transcription and DNA replication. J Virol 69:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodems SM, Pullen SS, Friesen PD. 1997. DNA-dependent transregulation by IE1 of Autographa californica nuclear polyhedrosis virus: IE1 domains required for transactivation and DNA binding. J Virol 71:9270–9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YR, Zhong S, Fei Z, Hashimoto Y, Xiang JZ, Zhang S, Blissard GW. 2013. The transcriptome of the baculovirus Autographa californica multiple nucleopolyhedrovirus in Trichoplusia ni cells. J Virol 87:6391–6405. doi: 10.1128/JVI.00194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knebel-Morsdorf D, Flipsen JT, Roncarati R, Jahnel F, Kleefsman AW, Vlak JM. 1996. Baculovirus infection of Spodoptera exigua larvae: lacZ expression driven by promoters of early genes pe38 and me53 in larval tissue. J Gen Virol 77:815–824. doi: 10.1099/0022-1317-77-5-815. [DOI] [PubMed] [Google Scholar]
- 9.Knebel-Morsdorf D, Kremer A, Jahnel F. 1993. Baculovirus gene me53, which contains a putative zinc finger motif, is one of the major early-transcribed genes. J Virol 67:753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong J, Arif BM, Theilmann DA, Krell PJ. 2009. Autographa californica multiple nucleopolyhedrovirus me53 (ac140) is a nonessential gene required for efficient budded-virus production. J Virol 83:7440–7448. doi: 10.1128/JVI.02390-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong J, Theilmann DA, Arif BM, Krell PJ. 2011. Immediate-early protein ME53 forms foci and colocalizes with GP64 and the major capsid protein VP39 at the cell membranes of Autographa californica multiple nucleopolyhedrovirus-infected cells. J Virol 85:9696–9707. doi: 10.1128/JVI.00833-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook A, Bono F, Jinek M, Conti E. 2007. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem 76:647–671. doi: 10.1146/annurev.biochem.76.052705.161529. [DOI] [PubMed] [Google Scholar]
- 13.Au V, Yu M, Carstens EB. 2009. Characterization of a baculovirus nuclear localization signal domain in the late expression factor 3 protein. Virology 385:209–217. doi: 10.1016/j.virol.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Feng G, Krell PJ. 2014. Autographa californica multiple nucleopolyhedrovirus DNA polymerase C terminus is required for nuclear localization and viral DNA replication. J Virol 88:10918–10933. doi: 10.1128/JVI.01167-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bange G, Murat G, Sinning I, Hurt E, Kressler D. 2013. New twist to nuclear import: when two travel together. Commun Integr Biol 6:e24792. doi: 10.4161/cib.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CW, Counago RM, Williams SJ, Boden M, Kobe B. 2013. Distinctive conformation of minor site-specific nuclear localization signals bound to importin-alpha. Traffic 14:1144–1154. doi: 10.1111/tra.12098. [DOI] [PubMed] [Google Scholar]
- 17.Kahms M, Huve J, Wesselmann R, Farr JC, Baumgartel V, Peters R. 2011. Lighting up the nuclear pore complex. Eur J Cell Biol 90:751–758. doi: 10.1016/j.ejcb.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Olson VA, Wetter JA, Friesen PD. 2002. Baculovirus transregulator IE1 requires a dimeric nuclear localization element for nuclear import and promoter activation. J Virol 76:9505–9515. doi: 10.1128/JVI.76.18.9505-9515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Carstens EB. 1998. A baculovirus single-stranded DNA binding protein, LEF-3, mediates the nuclear localization of the putative helicase P143. Virology 247:32–40. doi: 10.1006/viro.1998.9235. [DOI] [PubMed] [Google Scholar]
- 20.Clem SA, Wu W, Passarelli AL. 2014. The Trichoplusia ni single nucleopolyhedrovirus tn79 gene encodes a functional sulfhydryl oxidase enzyme that is able to support the replication of Autographa californica multiple nucleopolyhedrovirus lacking the sulfhydryl oxidase ac92 gene. Virology 460–461:207–216. doi: 10.1016/j.virol.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang JM, Taly JF, Notredame C. 2011. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res 39:W13–W17. doi: 10.1093/nar/gkr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira PE, Holmgren G, Veiga MI, Uhlen P, Kaneko A, Gil JP. 2011. PfMDR1: mechanisms of transport modulation by functional polymorphisms. PLoS One 6:e23875. doi: 10.1371/journal.pone.0023875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Reilly DR, Miller LK, Luckow VA. 1994. Baculovirus expression vectors: a laboratory manual. W. H. Freeman and Company, New York, NY. [Google Scholar]
- 24.de Jong J. 2010. Analysis of the immediate early/late protein me53 from the baculovirus Autographa californica Nucleopolyhedrovirus. Ph.D thesis University of Guelph, Guelph, ON, Canada. [Google Scholar]
- 25.Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. 2004. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol 14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. 2007. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J Biol Chem 282:5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braunagel SC, Guidry PA, Rosas-Acosta G, Engelking L, Summers MD. 2001. Identification of BV/ODV-C42, an Autographa californica nucleopolyhedrovirus orf101-encoded structural protein detected in infected-cell complexes with ODV-EC27 and p78/83. J Virol 75:12331–12338. doi: 10.1128/JVI.75.24.12331-12338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Wang Q, Liang C, Song J, Li N, Shi H, Chen X. 2008. Autographa californica multiple nucleopolyhedrovirus nucleocapsid protein BV/ODV-C42 mediates the nuclear entry of P78/83. J Virol 82:4554–4561. doi: 10.1128/JVI.02510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Hare P, Goding CR. 1988. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell 52:435–445. doi: 10.1016/S0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- 30.Preston CM, Frame MC, Campbell ME. 1988. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell 52:425–434. doi: 10.1016/S0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 31.Stern S, Tanaka M, Herr W. 1989. The Oct-1 homeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature 341:624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- 32.Xiao P, Capone JP. 1990. A cellular factor binds to the herpes simplex virus type 1 transactivator Vmw65 and is required for Vmw65-dependent protein-DNA complex assembly with Oct-1. Mol Cell Biol 10:4974–4977. doi: 10.1128/MCB.10.9.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babb R, Huang CC, Aufiero DJ, Herr W. 2001. DNA recognition by the herpes simplex virus transactivator VP16: a novel DNA-binding structure. Mol Cell Biol 21:4700–4712. doi: 10.1128/MCB.21.14.4700-4712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson AC, Cleary MA, Lai JS, LaMarco K, Peterson MG, Herr W. 1993. Combinatorial control of transcription: the herpes simplex virus VP16-induced complex. Cold Spring Harbor Symp Quant Biol 58:167–178. doi: 10.1101/SQB.1993.058.01.021. [DOI] [PubMed] [Google Scholar]