Abstract
Viruses are quasi-inert macromolecular assemblies. Their metastable conformation changes during entry into cells, when chemical and mechanical host cues expose viral membrane-interacting proteins. This leads to membrane rupture or fusion and genome uncoating. Importantly, virions tune their physical properties and enhance penetration and uncoating. For example, influenza virus softens at low pH to uncoat. The stiffness and pressure of adenovirus control uncoating and membrane penetration. Virus and host mechanics thus present new opportunities for antiviral therapy.
INTRODUCTION
Viruses contain a nucleic acid genome in a shell of proteins and sometimes a lipid envelope and sugars. They enter cells by interactions with host attachment factors and receptors (20). Enveloped viruses deliver their genomes into the cytoplasm or the nucleus by fusion of their lipid membranes with a host membrane, in most cases an endosomal membrane (1). Nonenveloped viruses either directly penetrate a host membrane or deliver a subviral particle containing the genome into the cytosol (2). Both enveloped and nonenveloped viruses mask their membrane-penetrating peptide, typically in a glycoprotein of the virion membrane, within the coat, or in the virion lumen. Membrane-penetrating peptides are unmasked by cellular cues that act on the virus during entry (20). Examples of cellular cues controlling virus entry are receptors, enzymes, and chemicals such as proteases, metal ions, and reducing agents (2). More recently, mechanical processes mediated by motor proteins or virus maturation have been observed to control virus entry.
Mechanics is considered to be a branch of physics that deals with the action of forces on materials. In biology, cells can sense, generate, and bear mechanical forces and also convert them into particular responses, in processes of mechano-transduction, for example (3). Unlike cells or organelles, which respond to external forces and adjust their stiffness to resist tension, viruses are not commonly known to adjust their mechanical properties under biological force, but rather to break or rupture. Such “inertia” offers opportunities for the virus to use mechanical events to break the capsid open and release the viral genome for transcriptional activation during entry into cells. This Gem highlights how cellular cues modulate the physical properties of viruses and how this affects viral entry into cells and infectivity.
VIRUS MECHANICS ARE KEY FOR INFECTION
Virus particles (virions) protect the viral nucleic acid and release it upon instruction by host cues. They can break inertia, since they are metastable. Metastability allows a stable virion to change its conformation when disturbed. Disturbance can lead to loss of infectivity by heating, pressurization, radiation, or action of chemicals, for example, or by antiviral immunity. Virus particles can also be disturbed by cues from the host and thereby gain function in cell entry (20).
In addition, viruses use internal genome pressure to destabilize their capsids. Bacteriophages, such as phage lambda, show an inverse correlation between the size of the packaged genome and the temperature for genome uncoating in vitro (4). Likewise, atomic force microscopy (AFM) measurements with adenovirus, a nonenveloped eukaryotic DNA virus and vector widely used in clinics, provided evidence for internal capsid pressure of about 30 atm (5). The nature of this internal pressure from hydrated DNA is mainly entropic and in part electrostatic. It is similar in extent to the ejection pressure of herpesvirus capsids (6).
Internal capsid pressure not only helps to pop off the penton base proteins at the verteces and eventually release the genome from the capsid, but also strengthens the capsid against external deformation forces, as shown by AFM experiments with phage lambda (reference 4 and references therein). Interestingly, the stiffness of icosahedral viral capsids is reinforced anisotropically: for example, with the least enforcement at the 5-fold and the strongest enforcement at the 2-fold symmetry axis. Accordingly, the elastic properties of the penton region around the 5-fold symmetry axis of adenovirus are modulated by host factors, such as integrins and defensins, which play important roles in virus disassembly in cells (7, 8). This evidence highlights the importance of capsid mechanics and how host interactions tune viral mechanics.
In addition, the concept of pressure-dependent biological function applies to adenovirus in several ways (Fig. 1A). The stiffness of the mature adenovirus is about 20% greater than that of immature virus, which suggests that the internal pressure of mature virus is greater than that of immature virus (5, 9). This was confirmed by virus disruption experiments using AFM, where the mature core (which is the viral DNA-protein complex) was spread out to a larger area and was more accessible to a fluorescent DNA-binding dye than the immature core upon disruption (10). Thus, pressurization during proteolytic virus maturation destabilizes the virion and brings it into a metastable state, where the low activation energy barrier to disruption facilitates the release of penton base, exposes the lytic protein VI, and eventually leads to genome release. This scenario likely explains why the immature virus TS1 is uncoating defective when it enters cells in a coxsackievirus adenovirus receptor (CAR) and integrin-dependent pathway like wild-type virus, yet unlike the wild type does not shed the fibers, does not expose the membrane lytic protein VI, and cannot penetrate through the endosomal membrane (7). All these data show that virus pressurization is an evolutionarily conserved mechanism used in bacteriophages and today's human viruses. In particular, physical properties of virions facilitate infectious uncoating triggered by host cues and offer new possibilities for therapeutic interventions.
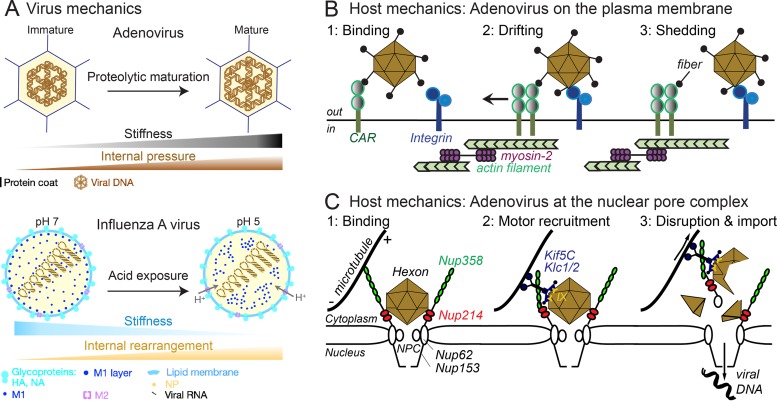
FIG 1.
Schematic depiction of virus and host mechanics with impact on virus entry into cells. (A) Proteolytic maturation during adenovirus progeny assembly increases internal pressure and, concomitantly, stiffness of the capsid coat (upper panel). (Lower panel) Acid exposure of influenza A virus during entry, e.g., leads to proton influx through the matrix protein 2 (M2) channel into the virion and reduces viral stiffness by enhancing rearrangement of internal proteins, including M1 and viral ribonucleoprotein particles (vRNPs) composed of viral RNA and nucleoprotein (NP). (B) Acto-myosin-mediated drifting motions of adenovirus bound to the coxsackievirus adenovirus receptor (CAR) and virus confinement by integrins on the cell surface lead to shedding of viral fibers, the first uncoating step in the viral disassembly program. (C) Incoming (fiberless) adenovirus binds to the nuclear pore complex (NPC) protein Nup214 by the major capsid protein hexon. The microtubule-dependent motor kinesin-1 (Kif5C heavy chain) attaches to the capsid by binding of its light chain Klc1/2 to protein IX (IX). Activation of the motor occurs upon heavy chain binding to Nup358 and initiates capsid disruption by displacement on microtubules, which may be tethered to the distal domain of Nup358. Concomitantly, capsid disruption removes Nup62 from the central channel structure of the NPC (but not Nup153 of the nuclear basket), and viral DNA is imported into the nucleus.
MECHANICAL PROPERTIES OF INFLUENZA VIRUS CHANGE UPON HOST CUES
Influenza virus passes through acidic endosomes, an essential step for uncoating and infection (1, 20). While residing under low-pH conditions, protons pass through the viral ion channel M2 into the virus lumen and there separate the viral ribonucleoprotein cores (vRNPs) from the M1 protein by inducing a conformational change in M1 (Fig. 1A). This is consistent with the observation that the M1 protein has multiple functions in the virion and occurs in a ribbon-like form or a coil structure. Influx of protons into the virion lumen then softens the viral envelope, most likely by dissociating vRNPs from the inner side of the envelope (11, 12). Evidence for this notion was obtained by atomic force microscopy measurements with both native virions and “bald” particles lacking viral glycoproteins. The latter result implies that low-pH-induced changes in the viral glycoproteins do not significantly contribute to softening of the envelope under low-pH conditions.
VIRUS AND HOST MECHANICS CONTROL MEMBRANE RUPTURE AND GENOME UNCOATING
Besides mechanics of the virus, mechanics of the host have been implicated in virus entry based on cell biological infection studies with adenoviruses and influenza virus. A combination of studies, including single-virus tracking at high spatial and temporal resolution, fluorescence recovery after photobleaching, immunological analyses, drug interference, electron microscopy, and virus retargeting, has shown that adenovirus is exposed to mechanical cues on the cell surface (7). Within the first seconds of virus interactions with cells, the virus is bound to the receptor CAR and moves in random motions (Fig. 1B). It then engages in acto-myosin-mediated slow drifting motions (<0.1 μm/s), which persist over several micrometers without apparent interruption. Slow drifts of virus are interrupted by stalling or short periods of random motions. Mechanical cues on the virus arise from the slow drifts of CAR and the stalling motions of the second virus receptor, integrins. These cues are thought to be directly transmitted to the virus particle and lead to mechanical stress, which initiates the stepwise virus-uncoating program at the cell surface. For example, pharmacological interference with the slow drifts blocks the release of the virus fibers and the exposure of the membrane lytic protein VI from the inside of the virus, notably before the virus is engulfed into endosomes.
The exposure of protein VI on the plasma membrane can have several effects. One is that protein VI binds to phospholipids, and the second is that it disrupts the membrane. A recent study has found evidence that the exposure of protein VI on the plasma membrane from incoming adenovirus leads to small membrane lesions (13). Piercing of the plasma membrane was demonstrated with the cell-impermeable dye propidium iodide (PI), and it was dependent on protein VI, as indicated by the use of a mutant adenovirus. PI becomes fluorescent when it binds to nucleic acids, such as DNA in the nucleus or RNA in the cytoplasm. Both population measurements by flow cytometry and single-cell/single-lesion analyses by confocal microscopy showed that the exposure of protein VI leads to increased uptake of PI into the cytosol. This occurs within a few minutes of virus addition to cells, and individual lesions last for typically 10 to 20 s. Interestingly, a single cell can have multiple lesions of the plasma membrane, and these lesions occur repetitively, as long as there is virus present on the cell surface. Coincident with the influx of PI, transient increases of cytosolic free calcium ion concentrations, which required extracellular calcium, were also shown by these experiments. Together, these observations indicate that incoming adenovirus induces transient lesions of the plasma membrane.
Compromised integrity of the plasma membrane is a severe threat to cell survival. Eukaryotic cells have evolved multiple repair mechanisms to restore membrane integrity. For example, muscle cells under heavy mechanical load repair lesions in the plasma membrane by secreting a special set of lysosomes, so-called secretory lysosomes (reviewed in reference 14). These acidic organelles are frequently located in the cell periphery. They fuse with the injured plasma membrane, depending on cytosolic calcium ions.
A series of biochemical and genetic experiments has shown that cells inoculated with adenovirus trigger the secretion of acidic lysosomes, and within minutes release the lipid hydrolase acid sphingomyelinase (ASMase) (13). ASMase catalyzes the breakdown of sphingomyelin to ceramide and phosphoryl-choline, and its absence from cells is associated with lysosomal storage disorders such as Niemann-Pick disease. It has optimal activity at pH 5, but it is also considerably active at pH 7. High-resolution lipidomic profiling of infected cells showed that incoming adenovirus triggers rapid and selective increase of ceramide lipid species dependent on ASMase activity. Ceramide is a cone-shaped signaling lipid lacking a large hydrophilic head group and possibly enhances membrane repair. However, ceramide also enhances the interaction of protein VI with synthetic or cell-derived liposomes and boosts liposome disruption. Further in vivo experiments then showed that ceramide accelerates adenovirus endocytosis and penetration from endosomes to the cytosol.
Collectively, these data provide strong evidence for a two-step membrane penetration process in adenovirus infection. The first step is controlled by the mechanical properties of the virus and the cell. High internal pressure stiffens and primes the mature adenovirus for uncoating. It makes the virus receptive to mechanical cues on the cell surface, which derive from motor-dependent movements and confinement of virus receptors. The virus exposes its membrane lytic protein and triggers a membrane repair process involving lysosomal secretion of ASMase. The second step then enhances the levels of ceramide in the plasma membrane and progressively increases membrane lesions coincident with virus endocytosis. It is thought that this occurs by an enrichment of ceramide in virus-containing endosomes. Regardless, ceramide signaling facilitates virus penetration from early endosomes to the cytosol. It is of interest now to explore if the broken endosomes represent a new class of danger signal for the cell. Are these endosomes repaired, secreted from the infected cell, or neutralized by autophagosomes, for example? It will also be interesting to see if other nonenveloped viruses use membrane repair processes for infection and if proviral or antiviral signals emerge from the broken membranes.
HOST MECHANICS CONTROL GENOME UNCOATING OF ADENOVIRUS AND INFLUENZA VIRUS
Many viruses, including adenovirus, infect postmitotic cells and replicate in the nucleus. Adenovirus deposits its linear DNA genome, but not the viral capsid, in the nucleus, as recently demonstrated by click chemistry-tagged incoming virus genomes at single-molecule resolution (15). The virus uses the molecular motor kinesin-1 to separate the genome from the capsid (16). This occurs at the cytoplasmic face of the nuclear pore complex (NPC), where the major virus capsid protein docks to the nucleoporin Nup214. The kinesin-associated light chain 1,2 binds to another virus capsid protein (protein IX), and the motor domain in the heavy chain gets activated by binding to Nup358 of the cytoplasmic pore complex filaments. Microtubules are positioned proximal to nuclear pore complexes because they have a binding site in the distal domain of Nup358. This quinary complex of virus, two Nups and two motor components, then executes the disruption of the virus and, coincidentially, also in part the nuclear pore complex. The viral DNA (presumably in a complex with the viral protein VII) is then imported into the nucleus with the assistance of cellular transport factors, such as importins and transportin. The nuclear import process of viral DNA is, however, not perfectly accurate, and a variable fraction of the incoming viral DNA is misdelivered to the cytoplasm (17). This raises questions about innate immune recognition of the cytosolic viral DNA and possible viral antagonism.
Besides that of adenovirus, the uncoating of the influenza virus genome is dependent on cellular motor proteins, in this case dynein and myosin (18). Under the low pH conditions in the endosome, influenza virus alters the conformation of the hemagglutinin glycoprotein, which leads to exposure of the hydrophobic fusion peptide and fusion of the viral membrane with the limiting endosomal membrane. The cytosolic RNPs are dissociated from the site of membrane fusion by a process mimicking aggresome formation and clearance. This involves the unanchored ubiquitin-binding domain (ZnF-UBP) and dynein-binding domains of histone deacetylase 6 (HDAC6), as well as microtubule and actin-based motors (18). Given that the ionic milieu in the endosomal compartments is subject to regulation—late but not early endosomes contain high concentrations of potassium ions and low concentrations of sodium ions, for example—it can be anticipated that additional cues act on the endosomal virus. In fact, high concentrations of potassium ions together with low pH in late endosomes prime influenza A virus for separating vRNPs long before fusion (19).
CONCLUSIONS AND OUTLOOK
This essay has attempted to integrate physical properties of single virus particles with mechanical or chemical cues from host cells. Physical and structural virology will continue to elucidate novel and exciting features of single virus particles and show that they are important for infection, virus transmission, or vaccination against viral disease. The full power and importance of these features, however, only comes to bear if physical and structural features are integrated into the context of cellular or immunological mechanisms. This approach considers the environment under which viruses have been selected in the course of evolution. In addition, it is important to keep in mind that viruses act as a swarm of particles and genetic elements, and a single virus particle rarely succeeds in infecting a single cell. Rather, more frequently, virus particles cooperate or compete during infection. This notion gives rise to the emerging field of pathogen coinfection: for example, virus-virus and virus-bacteria coinfections.
ACKNOWLEDGMENTS
I thank Maarit Suomalainen and Yohei Yamauchi for comments on the manuscript and apologize to those authors whose work could not be cited due to space restrictions.
Funding Statement
Medical Research and Development Project VirX (SystemsX.ch, evaluated by the Swiss National Science Foundation) provided funding to Urs F. Greber.
REFERENCES
- 1.Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu Rev Biochem 79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 2.Suomalainen M, Greber UF. 2013. Uncoating of non-enveloped viruses. Curr Opin Virol 3:27–33. doi: 10.1016/j.coviro.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Bao G, Suresh S. 2003. Cell and molecular mechanics of biological materials. Nat Mater 2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- 4.Bauer DW, Evilevitch A. 2015. Influence of internal DNA pressure on stability and infectivity of phage λ. J Mol Biol 427:3189–3200. doi: 10.1016/j.jmb.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortega-Esteban A, Condezo GN, Pérez-Berná AJ, Chillón M, Flint SJ, Reguera D, San Martín C, de Pablo PJ. 2015. Mechanics of viral chromatin reveals the pressurization of human adenovirus. ACS Nano 9:10826–10833. doi: 10.1021/acsnano.5b03417. [DOI] [PubMed] [Google Scholar]
- 6.Bauer DW, Huffman JB, Homa FL, Evilevitch A. 2013. Herpes virus genome, the pressure is on. J Am Chem Soc 135:11216–11221. doi: 10.1021/ja404008r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burckhardt CJ, Suomalainen M, Schoenenberger P, Boucke K, Hemmi S, Greber UF. 2011. Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe 10:105–117. doi: 10.1016/j.chom.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Snijder J, Reddy VS, May ER, Roos WH, Nemerow GR, Wuite GJ. 2013. Integrin and defensin modulate the mechanical properties of adenovirus. J Virol 87:2756–2766. doi: 10.1128/JVI.02516-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Berná AJ, Ortega-Esteban A, Menéndez-Conejero R, Winkler DC, Menéndez M, Steven AC, Flint SJ, de Pablo PJ, San Martín C. 2012. The role of capsid maturation on adenovirus priming for sequential uncoating. J Biol Chem 287:31582–31595. doi: 10.1074/jbc.M112.389957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortega-Esteban A, Bodensiek K, San Martin C, Suomalainen M, Greber UF, de Pablo PJ, Schaap IA. 2015. Fluorescence tracking of genome release during mechanical unpacking of single viruses. ACS Nano 9:10571–10579. doi: 10.1021/acsnano.5b03020. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Sieben C, Ludwig K, Hofer CT, Chiantia S, Herrmann A, Eghiaian F, Schaap IA. 2014. pH-Controlled two-step uncoating of influenza virus. Biophys J 106:1447–1456. doi: 10.1016/j.bpj.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greber UF. 2014. How cells tune viral mechanics–insights from biophysical measurements of influenza virus. Biophys J 106:2317–2321. doi: 10.1016/j.bpj.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luisoni S, Suomalainen M, Boucke K, Tanner LB, Wenk MR, Guan XL, Grzybek M, Coskun U, Greber UF. 2015. Co-option of membrane wounding enables virus penetration into cells. Cell Host Microbe 18:75–85. doi: 10.1016/j.chom.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Andrews NW, Almeida PE, Corrotte M. 2014. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol 24:734–742. doi: 10.1016/j.tcb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang IH, Suomalainen M, Andriasyan V, Kilcher S, Mercer J, Neef A, Luedtke NW, Greber UF. 2013. Tracking viral genomes in host cells at single-molecule resolution. Cell Host Microbe 14:468–480. doi: 10.1016/j.chom.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Strunze S, Engelke MF, Wang IH, Puntener D, Boucke K, Schleich S, Way M, Schoenenberger P, Burckhardt CJ, Greber UF. 2011. Kinesin-1-mediated capsid disassembly and disruption of the nuclear pore complex promote virus infection. Cell Host Microbe 10:210–223. doi: 10.1016/j.chom.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Flatt JW, Greber UF. 2015. Misdelivery at the nuclear pore complex—stopping a virus dead in its tracks. Cells 4:277–296. doi: 10.3390/cells4030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee I, Miyake Y, Nobs SP, Schneider C, Horvath P, Kopf M, Matthias P, Helenius A, Yamauchi Y. 2014. Influenza A virus uses the aggresome processing machinery for host cell entry. Science 346:473–477. doi: 10.1126/science.1257037. [DOI] [PubMed] [Google Scholar]
- 19.Stauffer S, Feng Y, Nebioglu F, Heilig R, Picotti P, Helenius A. 2014. Stepwise priming by acidic pH and a high K+ concentration is required for efficient uncoating of influenza A virus cores after penetration. J Virol 88:13029–13046. doi: 10.1128/JVI.01430-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamauchi Y, Greber UF. 15 February 2016, posting date Principles of virus uncoating: cues and the snooker ball. Traffic doi: 10.1111/tra.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]