Abstract
Epicardial adipose tissue thickness is associated with the severity and extent of atherosclerotic coronary artery disease. We prospectively investigated whether epicardial adipose tissue thickness is related to coronary artery disease extent and complexity as denoted by Gensini and Syntax scores, and whether the thickness predicts critical disease.
After performing coronary angiography in 183 patients who had angina or acute myocardial infarction, we divided them into 3 groups: normal coronary arteries, noncritical disease (≥1 coronary lesion with <70% stenosis), and critical disease (≥1 coronary lesion with <70% stenosis). We used transthoracic echocardiography to measure epicardial adipose tissue thickness, then calculated Gensini and Syntax scores by reviewing the angiograms.
Mean thicknesses were 4.3 ± 0.9, 5.2 ± 1.5, and 7.5 ± 1.9 mm in patients with normal coronary arteries, noncritical disease, and critical disease, respectively (P <0.001). At progressive thicknesses (<5, 5–7, and >7 mm), mean Gensini scores were 4.1 ± 5.5, 19.8 ± 15.6, and 64.9 ± 32.4, and mean Syntax scores were 4.7 ± 5.9, 16.6 ± 8.5, and 31.7 ± 8.7, respectively (both P <0.001). Thickness had strong and positive correlations with both scores (Gensini, r =0.82, P <0.001; and Syntax, r =0.825, P <0.001). The cutoff thickness value to predict critical disease was 5.75 mm (area under the curve, 0.875; 95% confidence interval, 0.825–0.926; P <0.001).
Epicardial adipose tissue thickness is independently related to coronary artery disease extent and complexity as denoted by Gensini and Syntax scores, and it predicts critical coronary artery disease.
Keywords: Adipose tissue/pathology/physiology/ultrasonography, atherosclerosis/physiopathology, coronary artery disease/diagnosis, echocardiography, forecasting, patient selection, predictive value of tests, prospective studies, sensitivity and specificity
Epicardial adipose tissue (EAT), found between the visceral pericardium and myocardium, is a specialized visceral adipose tissue in which the coronary arteries are embedded.1 The lack of an anatomic barrier between EAT and the myocardium enables them to share microcirculation. Epicardial adipose tissue produces numerous proinflammatory and proatherogenic mediators that might promote the initiation and progression of coronary atherosclerosis,1,2 including subclinical atherosclerosis.3 High EAT volume has been shown to increase the prognostic value of coronary artery calcium score in predicting future cardiac events.4 The thickness and volume of EAT are related to the severity and extent of atherosclerotic coronary artery disease (CAD)5–9; however, some investigators have found no significant association between EAT volume10 or thickness11 and CAD presence or severity. Although the relationship between EAT and the severity and extent of CAD has been extensively investigated,5–11 comparatively few studies pertain to the association of EAT with CAD complexity.12,13 The complexity of CAD, an important factor in clinical decision-making, is typically evaluated by means of the Syntax score.14,15 Another important factor in choosing treatment approaches is the presence of critical lesions,16 which potentially qualifies the patient for interventional treatment. In addition, we used the Gensini score to evaluate the extent and severity of CAD.
In this study, we tested the hypotheses that greater EAT thickness identifies patients with more-complex CAD, and that greater EAT thickness predicts the presence of critical lesions that potentially warrant interventional treatment.
Patients and Methods
We screened 221 consecutive patients who had no histories of myocardial revascularization and who had undergone coronary angiography from June through October 2012 because of clinical diagnoses of CAD. The prospective study was performed in a single tertiary center. We excluded 38 patients for the following reasons: forms of CAD other than angina pectoris or acute myocardial infarction (MI), including coronary vasospasm, coronary ectasia, or turbulent or slow flow (n=17); pericardial effusion (n=4); severe valvular pathologic conditions (n=5); poor echocardiographic images (n=3); or refusal to give written informed consent (n=9). The clinical presentations of the remaining 183 patients were as follows: 116 patients had stable angina pectoris, 34 patients had unstable angina pectoris, and 33 patients had acute MI. Of these last, 16 presented with ST-segment-elevation MI and 17 with non-ST-segment-elevation MI.
This study was conducted in accordance with the recommendations of the Declaration of Helsinki on Biomedical Research involving human subjects. The study protocol was approved by our institutional ethics committee. Written informed consent was obtained from each participant.
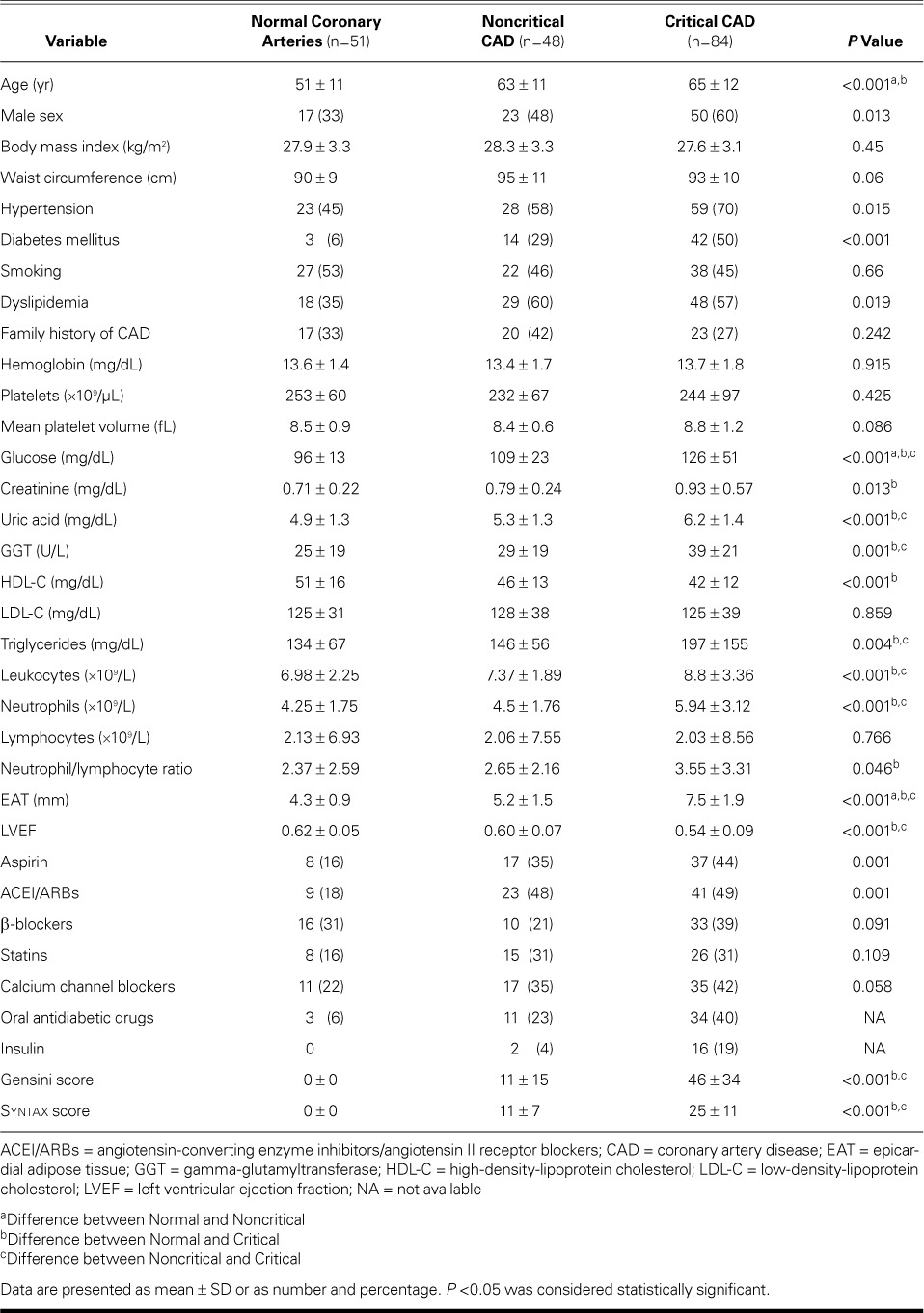
Baseline characteristics, including medical history, anthropometric measurements, risk factors for atherosclerosis, and medications, were recorded for each patient (Table I). Before performing coronary angiography, we obtained blood samples for complete blood counts with use of a Cell-Dyn® 3700 (Abbott Laboratories; Abbott Park, Ill), and for biochemistry and lipid values with use of a UniCel® DxC 800 Synchron® Clinical System (Beckman Coulter, Inc.; Brea, Calif).
TABLE I.
Baseline Characteristics in the Study Population (N=183)
Echocardiography
All echocardiographic measurements were performed by the same cardiologist, who was blinded to the patients' clinical information. A Vivid 7® cardiac ultrasound system (GE Medical Systems; Horten, Norway) with a 2.5- to 3.5-MHz transducer was used before coronary angiography was performed. Parasternal and apical views were obtained in accordance with the recommendations of the American Society of Echocardiography.17 The EAT thickness was measured from the standard parasternal long-axis view on the free wall of the right ventricle, perpendicular to the aortic annulus at end-systole.18 The EAT was identified as the echo-free space between the outermost border of the myocardium and the visceral layer of the pericardium. The thickest point of the EAT was measured in each of 3 cycles, and the average value was calculated.
Coronary Angiography and Evaluation of Coronary Artery Disease
Selective coronary angiography was performed via the femoral approach by means of the Judkins technique and with use of an Innova® angiographic system (GE Healthcare; Wausheka, Wisc). Multiple views were obtained in all patients, including the left anterior descending coronary artery (LAD) and left circumflex coronary artery (LCx) in at least 4 projections, and the right coronary artery in at least 2 projections. Coronary angiograms were recorded in Digital Imaging and Communications in Medicine (DICOM) format.
We used the Syntax score to evaluate the complexity of CAD. The Syntax score, developed to better predict the risks of percutaneous coronary intervention (PCI) and coronary artery bypass grafting,14 is also a surrogate of disease complexity.15 The location of the lesions and their impact on blood flow, the degree of vessel stenosis, lesion classifications, and diameter and calcification of the vessel are important factors that affect the technical feasibility of performing PCI. The Syntax score takes into account the functional impact of the coronary circulation and all anatomic aspects, including bifurcation, trifurcation lesions, calcification, tortuosity, thrombus, and occlusions.19 Each coronary lesion resulting in a luminal stenosis >50% in vessels ≥1.5 mm in diameter is separately scored and summed to obtain the overall Syntax score. We calculated the Syntax score, which is also related to cardiovascular outcomes, by using the Web-based calculator at the http://www.syntaxscore.com/ internet address.
We evaluated the extent and severity of CAD by using the Gensini score.20 In this system, a severity score is derived for each coronary stenosis on the basis of the degree of luminal narrowing and its topographic importance. Reductions in luminal diameter of 1% to 25%, 26% to 50%, 51% to 75%, 76% to 90%, 91% to 99%, and 100% (occlusion) are scored as 1, 2, 4, 8, 16, and 32, respectively. Each principal vascular segment is assigned a multiplier that represents its functional importance in maintaining myocardial blood supply. Multipliers are 5 for the left main coronary artery; 2.5 for the proximal segments of the LAD and LCx; 1.5 for the mid segment of the LAD; 1 each for the right coronary artery, distal segment of the LAD, posterolateral artery, and obtuse marginal branch; and 0.5 for other segments.
All angiographic scoring was performed by 2 interventional cardiologists who were blinded to the echocardiographic measurements and clinical data. The Gensini and Syntax scores for each angiogram were obtained by averaging the scores assigned by these 2 observers. In case of discrepancy between the observers, the angiogram was rescored.
Critical CAD has been defined as ≥70% stenosis in a coronary artery.16 Accordingly, we classified the study population into 3 angiographic groups: individuals with normal coronary arteries (NCA), patients with noncritical CAD (<70% stenosis in ≥1 coronary artery), and patients with critical CAD (≥70% stenosis in ≥1 coronary artery).
Statistical Analysis
Continuous variables were expressed as mean ± SD; categorical variables were defined as numbers and percentages. Data were tested for normal distribution with use of the Shapiro-Wilk test. For continuous variables, mean values were compared by analysis of variance among different groups. Homogeneity of variance underwent the Levene test, and post hoc analysis was performed with either the Tukey test or Dunnett's T3 test, whichever was appropriate. The χ2 test was used to compare categorical variables among the groups. All correlation analyses were performed with use of the Pearson or Spearman correlation test, whichever was appropriate. To determine the independent predictors of critical CAD, univariate linear regression analysis was performed first; then, multivariate linear regression analysis was performed with the independent variables that were found to be significant correlates in the univariate analysis. An optimal cutoff value of EAT thickness for the detection of critical CAD was determined by means of receiver operating characteristics (ROC) analysis, and area under the curve (AUC) was calculated. All tests of significance were 2-tailed. Statistical significance was defined as P <0.05. We used SPSS statistical software version 20.0 for Windows (IBM Corporation; Armonk, NY) for all statistical calculations.
Results
Patients with critical and noncritical CAD were older and were more likely male than were individuals with NCA. There were no significant differences between the groups with respect to anthropometric measurements, including body mass index (BMI) and waist circumference (WC). There was no significant association between BMI and EAT after adjustment for Gensini and Syntax scores (adjusted r =0.13; P =0.082); however, we found a weak yet significant correlation between WC and EAT after adjustment for Gensini and Syntax scores (adjusted r =0.196; P=0.006).
Mean EAT thickness was 4.3 ± 0.9 mm in the NCA group, 5.2 ± 1.5 mm in the noncritical-CAD group, and 7.5 ± 1.9 mm in the critical-CAD group (P <0.001). Upon post hoc analysis, differences were also significant for each pairwise comparison (Table I).
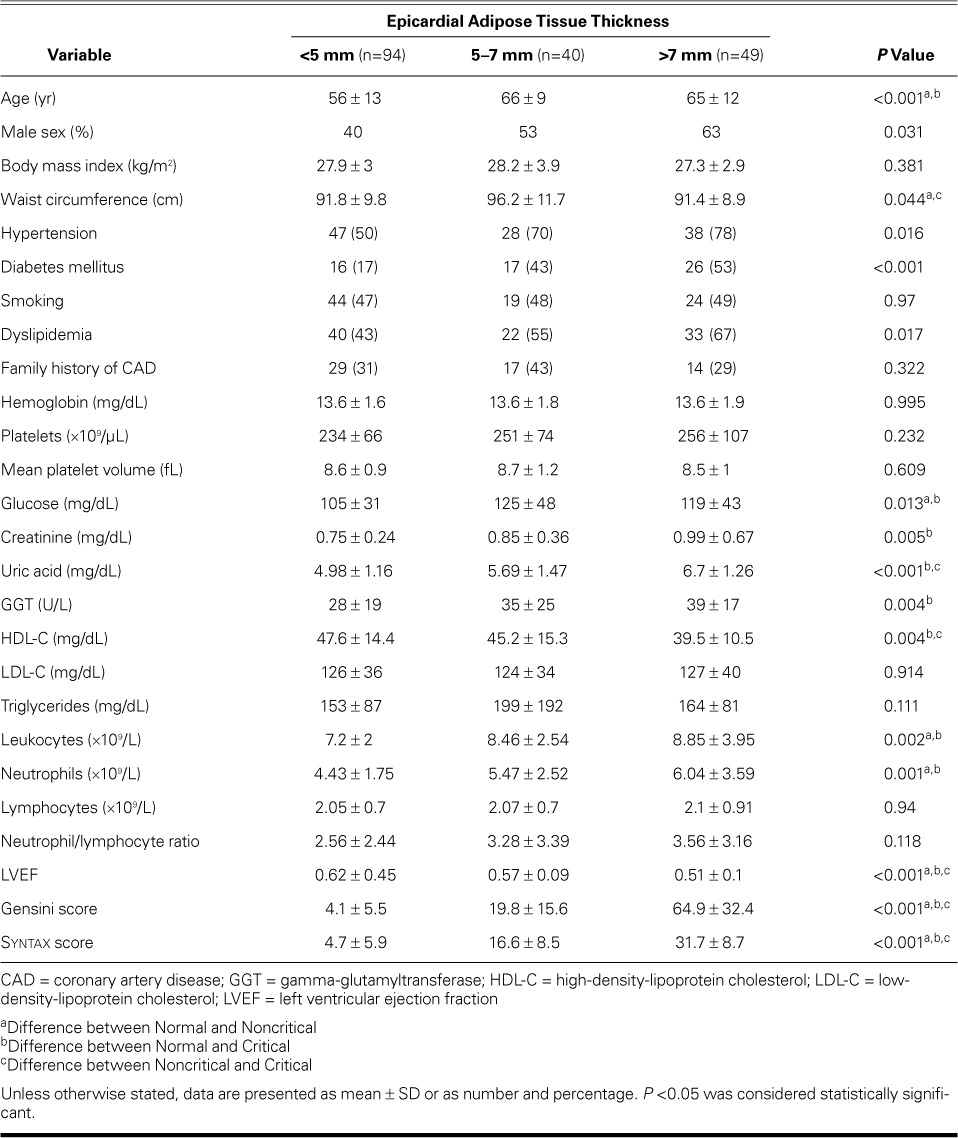
Table II shows changes in values at different EAT thickness strata. As EAT thickness increased, so did Syntax score and Gensini score: for EAT thicknesses <5 mm, 5–7 mm, and >7 mm, the mean Syntax scores were 4.7 ± 5.9, 16.6 ± 8.5, and 31.7 ± 8.7, respectively; and the mean Gensini scores were 4.1 ± 5.5, 19.8 ± 15.6, and 64.9 ± 32.4, respectively (both P <0.001). Upon post hoc analysis, all differences with respect to Gensini and Syntax scores among subgroups were also significant (Table II).
TABLE II.
Comparison of Clinical and Laboratory Values by Epicardial Adipose Tissue Thickness Measurement
We found a significant and positive correlation between EAT thickness and the extent of CAD, as evidenced by the Gensini score (r =0.82; P <0.001) (Fig. 1). A similarly strong correlation existed between EAT thickness and CAD complexity, as evidenced by the Syntax score (r =0.825; P <0.001) (Fig. 2).
Fig. 1.
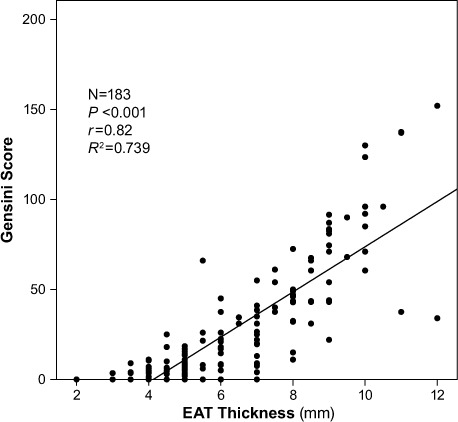
Scatter plot shows a significant and positive correlation between epicardial adipose tissue (EAT) thickness and the extent of coronary artery disease as evidenced by Gensini score. P <0.05 was considered statistically significant.
Fig. 2.
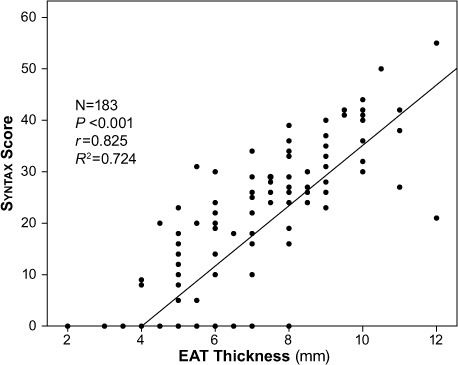
Scatter plot shows a significant and positive correlation between epicardial adipose tissue (EAT) thickness and the complexity of coronary artery disease as evidenced by Syntax score. P <0.05 was considered statistically significant.
Multivariate analyses (Tables III and IV) and ROC analysis revealed that EAT thickness can be used as an independent predictor of critical CAD. The optimal cutoff EAT thickness value to predict critical CAD was 5.75 mm (AUC=0.875; 95% confidence interval [CI], 0.825–0.926; P <0.001) (Fig. 3). We found a sensitivity of 77%, a specificity of 83%, a positive predictive value of 48%, a negative predictive value of 95.8%, and 82.4% diagnostic accuracy of the cutoff value of 5.75 mm to predict critical CAD.
TABLE III.
Regression Analysis with Gensini Score as the Dependent Variable
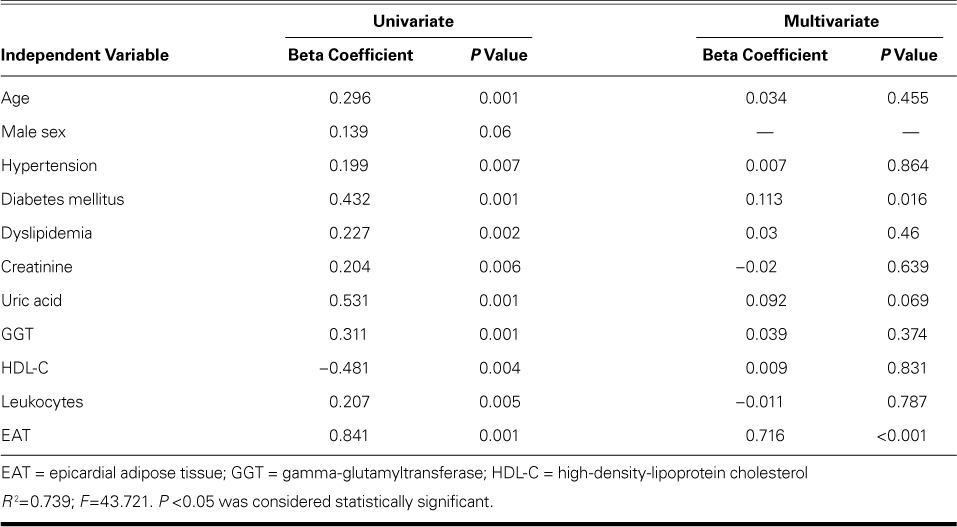
TABLE IV.
Regression Analysis with Syntax Score as the Dependent Variable
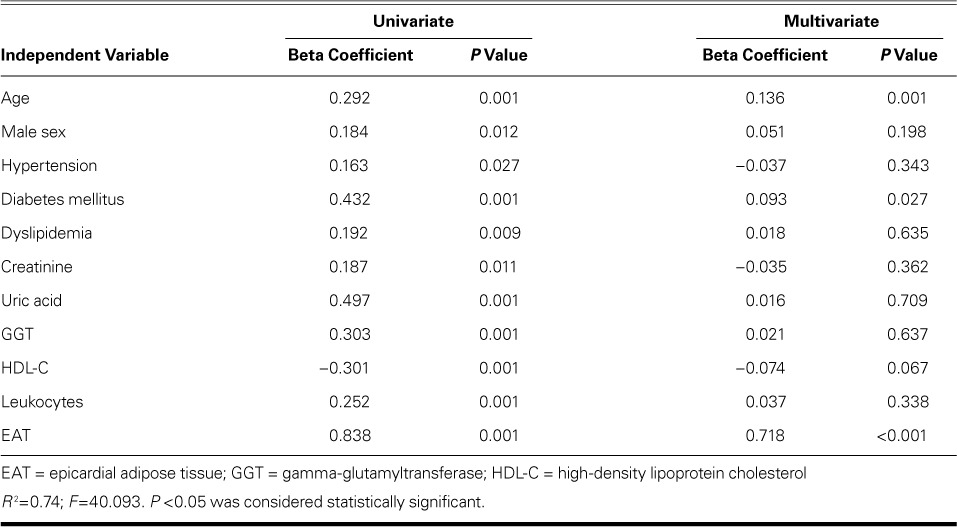
Fig. 3.

Receiver operating characteristic (ROC) curve depicts the optimal cutoff value of epicardial adipose tissue (EAT) thickness to predict critical coronary artery disease: 5.75 mm (area under the curve=0.875; 95% confidence interval, 0.825–0.926; P <0.001). The sensitivity of the cutoff value was 77%; the specificity was 83%. P <0.05 was considered statistically significant.
Discussion
We found that EAT thickness measured by means of echocardiography is independently associated with the complexity of CAD that is evidenced by the Syntax score. Although previous study results linked EAT to CAD,5–9 our findings highlight the association between echocardiographic EAT thickness and the Syntax score, which incorporates lesion characteristics and functional impact and thus denotes CAD complexity. We propose that EAT thickness is of value in the early identification of patients who have complex CAD. This knowledge might prompt clinicians to choose more aggressive prevention and treatment strategies or to refer these patients for earlier diagnostic cardiac catheterization.
In addition, we found significant differences among the 3 EAT thickness strata in terms of traditional cardiovascular risk factors, including older age, diabetes mellitus, hypertension, and serum HDL cholesterol levels. These observations support earlier findings: age is a strong independent correlate of EAT,21 and EAT is significantly thicker in diabetic subjects.22 (Moreover, Iacobellis and colleagues23 identified a significant correlation of EAT thickness with fasting plasma glucose in nondiabetic individuals.) Hypertension (especially diastolic blood pressure) and serum HDL cholesterol levels have been well correlated to EAT thickness.24
Furthermore, we observed a significant difference in terms of WC, but not BMI, across the EAT thickness strata. The weak yet significant correlation between WC and EAT agrees with previous data concerning EAT thickness that significantly correlated with WC but not with BMI.24 This finding highlights the importance of visceral adiposity but not total adiposity with respect to cardiovascular risk. Thickness in EAT is also associated with metabolic syndrome,25 insulin resistance,26 endothelial dysfunction,27 and overt atherosclerotic CAD.28 The influence of conventional cardiovascular risk factors on EAT thickness is well established; regardless, EAT has additional predictive value over these risk factors in regard to the presence of critical and complex CAD, according to our multivariate regression analysis.
Clinical observations suggest that proximal portions of the coronary arteries are more deeply embedded in epicardial fat than are distal portions and are thus more susceptible to atherosclerosis. This has been attributed to the relative paucity of periadventitial adipose tissue, which is in continuity with the epicardial adipose tissue.29 Vela and associates30 pointed out the importance of periadventitial adipose tissue in the development of atherosclerosis, highlighting the aggregation of macrophages in this tissue.
Epicardial adipose tissue produces miscellaneous proinflammatory and proatherogenic mediators, including interleukin-6, interleukin-1, tumor necrosis factor-α, monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, angiotensinogen, leptin, resistin, and visfatin.31–33 On the other hand, adiponectin, which exerts an antiatherogenic effect via the improvement of endothelial function and the mitigation of inflammation, has been shown to be underexpressed in the EAT of patients who have CAD.33
In 2014, Yanez-Rivera and colleagues11 found no significant relationship between echocardiographic EAT thickness and the angiographic severity of CAD. This discrepancy might have resulted from the method used to evaluate CAD severity. Whereas those investigators11 used the number of stenotic major coronary arteries as the surrogate of CAD severity, we used the Gensini score—a more quantitative method. Furthermore, we evaluated CAD complexity by using Syntax scores and correlating those values with EAT thickness.
Gökdeniz and associates12 studied the relationship of EAT thickness to the complexity of CAD in nondiabetic subjects. They found EAT thickness to be significantly correlated to Syntax score (r =0.629; P <0.001). They also determined a cutoff value of 5-mm EAT thickness for the prediction of an intermediate-to-high Syntax score (AUC=0.851; 95% CI, 0.775–0.91) at a specificity of 92.2% and a sensitivity of 77.4%. This cutoff value is close to ours (5.75 mm) for critical-lesion prediction. However, those investigators12 studied nondiabetic patients only, whereas we identified the relationship of EAT thickness with CAD complexity in a diabetic and nondiabetic population.
In 2014, Wang and co-authors34 reported an association of echocardiographically determined EAT thickness with Syntax score in patients who had acute MI. Mean EAT thickness was greater in the investigators' high-score group (Syntax score, ≥33) than in their lower-score group (Syntax score, <33): 5.6 ± 1.1 versus 4.1 ± 1 mm (P <0.01). As did we, they found that EAT thickness was positively and significantly correlated with Syntax score; however, their study involved patients who had acute MI only. We identified a similar association of EAT thickness with CAD complexity in a study population that presented with the entire clinical spectrum of CAD, including stable angina pectoris, unstable angina pectoris, and acute MI.
Study Limitations
The most important limitation of this study is the relatively small sample size. Serum levels of interleukins, cytokines, and adipokines, which could hint at the underlying mechanisms, were not evaluated. In addition, we measured EAT thickness by means of echocardiography, rather than the comparatively more precise computed tomography. Last, although patients admitted with acute coronary syndromes have reportedly had higher EAT values than do patients with stable angina, we did not categorize patients with respect to clinical presentation. Had we subdivided the population into unstable angina, stable angina, and atypical chest pain, and then further divided those groups into NCA, noncritical-CAD, and critical-CAD categories, the number of subjects in those subgroups would not have been adequate to yield statistically significant results. Rather, the purpose of our study was to investigate whether EAT thickness could predict more critical and complex CAD.
Conclusion
Quantification of EAT thickness by means of echocardiography—a relatively inexpensive, readily available method—might be beneficial in the early identification of patients who have complex or critical CAD. This knowledge could enable earlier referral of these patients for diagnostic coronary angiography and timely interventions. Our findings warrant further research in larger study populations.
Footnotes
From: Department of Cardiology (Drs. Erkan, Tanindi, Tore, and Ugurlu), Ufuk University Faculty of Medicine, 06520 Ankara; and Department of Cardiology (Dr. Kocaman), Ankara Guven Hospital, 06700 Ankara; Turkey
References
- 1.Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 2011;43(12):1651–4. doi: 10.1016/j.biocel.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis. 2012;23(4):227–33. doi: 10.1097/MCA.0b013e328351ab2c. [DOI] [PubMed] [Google Scholar]
- 3.Comert N, Yucel O, Ege MR, Yaylak B, Erdogan G, Yilmaz MB. Echocardiographic epicardial adipose tissue predicts subclinical atherosclerosis: epicardial adipose tissue and atherosclerosis. Angiology. 2012;63(8):586–90. doi: 10.1177/0003319711432452. [DOI] [PubMed] [Google Scholar]
- 4.Kunita E, Yamamoto H, Kitagawa T, Ohashi N, Oka T, Utsunomiya H et al. Prognostic value of coronary artery calcium and epicardial adipose tissue assessed by non-contrast cardiac computed tomography. Atherosclerosis. 2014;233(2):447–53. doi: 10.1016/j.atherosclerosis.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Eroglu S, Sade LE, Yildirir A, Bal U, Ozbicer S, Ozgul AS et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2009;19(3):211–7. doi: 10.1016/j.numecd.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Topuz M, Dogan A, Celik A, Can C, Ozdogru I, Kemal Eryol N. Investigation of the relationship between non-alcoholic fatty liver disease and coronary artery disease. Clin Ter. 2014;165(1):e46–51. doi: 10.7417/CT.2014.1671. [DOI] [PubMed] [Google Scholar]
- 7.Groves EM, Erande AS, Le C, Salcedo J, Hoang KC, Kumar S et al. Comparison of epicardial adipose tissue volume and coronary artery disease severity in asymptomatic adults with versus without diabetes mellitus. Am J Cardiol. 2014;114(5):686–91. doi: 10.1016/j.amjcard.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihl C, Loeffen D, Versteylen MO, Takx RA, Nelemans PJ, Nijssen EC et al. Automated quantification of epicardial adipose tissue (EAT) in coronary CT angiography; comparison with manual assessment and correlation with coronary artery disease. J Cardiovasc Comput Tomogr. 2014;8(3):215–21. doi: 10.1016/j.jcct.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Chung JH, Kwon BJ, Song SW, Choi WS. The associations of epicardial adipose tissue with coronary artery disease and coronary atherosclerosis. Int Heart J. 2014;55(3):197–203. doi: 10.1536/ihj.13-303. [DOI] [PubMed] [Google Scholar]
- 10.Yuce G, Turkvatan A, Yener O. Can aortic atherosclerosis or epicardial adipose tissue volume be used as a marker for predicting coronary artery disease? J Cardiol. 2015;65(2):143–9. doi: 10.1016/j.jjcc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Yanez-Rivera TG, Banos-Gonzalez MA, Ble-Castillo JL, Torres-Hernandez ME, Torres-Lopez JE, Borrayo-Sanchez G. Relationship between epicardial adipose tissue, coronary artery disease and adiponectin in a Mexican population. Cardiovasc Ultrasound. 2014;12:35. doi: 10.1186/1476-7120-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gokdeniz T, Turan T, Aykan AC, Gul I, Boyaci F, Hatem E et al. Relation of epicardial fat thickness and cardio-ankle vascular index to complexity of coronary artery disease in nondiabetic patients. Cardiology. 2013;124(1):41–8. doi: 10.1159/000345298. [DOI] [PubMed] [Google Scholar]
- 13.Borekci A, Gur M, Ozaltun B, Baykan AO, Harbalioglu H, Seker T et al. Epicardial fat thickness in stable coronary artery disease: its relationship with high-sensitive cardiac troponin T and N-terminal pro-brain natriuretic peptide. Coron Artery Dis. 2014;25(8):685–90. doi: 10.1097/MCA.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 14.Ong AT, Serruys PW, Mohr FW, Morice MC, Kappetein AP, Holmes DR, Jr et al. The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: design, rationale, and run-in phase. Am Heart J. 2006;151(6):1194–204. doi: 10.1016/j.ahj.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Schwalm JD, Wijeysundera HC, Tu JV, Guo H, Kingsbury KJ, Natarajan MK. Influence of coronary anatomy and SYNTAX Score on the variations in revascularization strategies for patients with multivessel disease. Can J Cardiol. 2014;30(10):1155–61. doi: 10.1016/j.cjca.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Paul SD, Eagle KA, Kuntz KM, Young JR, Hertzer NR. Concordance of preoperative clinical risk with angiographic severity of coronary artery disease in patients undergoing vascular surgery [published erratum appears in Circulation 1996; 94(10):2668] Circulation. 1996;94(7):1561–6. doi: 10.1161/01.cir.94.7.1561. [DOI] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22(12):1311–9. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Head SJ, Farooq V, Serruys PW, Kappetein AP. The SYNTAX score and its clinical implications. Heart. 2014;100(2):169–77. doi: 10.1136/heartjnl-2012-302482. [DOI] [PubMed] [Google Scholar]
- 20.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 21.Karadag B, Ozulu B, Ozturk FY, Oztekin E, Sener N, Altuntas Y. Comparison of epicardial adipose tissue (EAT) thickness and anthropometric measurements in metabolic syndrome (MS) cases above and under the age of 65. Arch Gerontol Geriatr. 2011;52(2):e79–84. doi: 10.1016/j.archger.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Iacobellis G, Diaz S, Mendez A, Goldberg R. Increased epicardial fat and plasma leptin in type 1 diabetes independently of obesity. Nutr Metab Cardiovasc Dis. 2014;24(7):725–9. doi: 10.1016/j.numecd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G, Barbaro G, Gerstein HC. Relationship of epicardial fat thickness and fasting glucose. Int J Cardiol. 2008;128(3):424–6. doi: 10.1016/j.ijcard.2007.12.072. [DOI] [PubMed] [Google Scholar]
- 24.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88(11):5163–8. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 25.Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013;111(1):73–8. doi: 10.1016/j.amjcard.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA et al. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) 2009;70(6):876–82. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 27.Kocaman SA, Durakoglugil ME, Cetin M, Erdogan T, Ergul E, Canga A. The independent relationship of epicardial adipose tissue with carotid intima-media thickness and endothelial functions: the association of pulse wave velocity with the active facilitated arterial conduction concept. Blood Press Monit. 2013;18(2):85–93. doi: 10.1097/MBP.0b013e32835ebbb5. [DOI] [PubMed] [Google Scholar]
- 28.Doesch C, Haghi D, Suselbeck T, Schoenberg SO, Borggrefe M, Papavassiliu T. Impact of functional, morphological and clinical parameters on epicardial adipose tissue in patients with coronary artery disease. Circ J. 2012;76(10):2426–34. doi: 10.1253/circj.cj-12-0301. [DOI] [PubMed] [Google Scholar]
- 29.Tellides G. Periadventitial fat. Arch Pathol Lab Med. 2007;131(3):346–7. doi: 10.5858/2007-131-346-PF. [DOI] [PubMed] [Google Scholar]
- 30.Vela D, Buja LM, Madjid M, Burke A, Naghavi M, Willerson JT et al. The role of periadventitial fat in atherosclerosis. Arch Pathol Lab Med. 2007;131(3):481–7. doi: 10.5858/2007-131-481-TROPFI. [DOI] [PubMed] [Google Scholar]
- 31.Sengul C, Ozveren O. Epicardial adipose tissue: a review of physiology, pathophysiology, and clinical applications. Anadolu Kardiyol Derg. 2013;13(3):261–5. doi: 10.5152/akd.2013.075. [DOI] [PubMed] [Google Scholar]
- 32.Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17(2):115–30. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 33.Eiras S, Teijeira-Fernandez E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine. 2008;43(2):174–80. doi: 10.1016/j.cyto.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Wang T, Liu Q, Liu C, Sun L, Li D, Liu A, Jia R. Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease in patients with acute myocardial infarction. Echocardiography. 2014;31(10):1177–81. doi: 10.1111/echo.12545. [DOI] [PubMed] [Google Scholar]


