Abstract
Few orthotopic heart transplantations have been performed in patients infected with the human immunodeficiency virus since the first such case was reported in 2001. Since that time, advances in highly active antiretroviral therapy have resulted in potent and durable suppression of the causative human immunodeficiency virus—accompanied by robust immune reconstitution, reversal of previous immunodeficiency, a marked decrease in opportunistic and other infections, and near-normal long-term survival. Although human immunodeficiency virus infection is not an absolute contraindication, few centers in the United States and Canada have performed heart transplantations in this patient population; these patients have been de facto excluded from this procedure in North America.
Re-evaluation of the reasons for excluding these patients from cardiac transplantation is warranted in light of such significant advances in antiretroviral therapy. This case report documents successful orthotopic heart transplantation in 2 patients infected with human immunodeficiency virus, and we describe their antiretroviral therapy and immunosuppressive management challenges. Both patients were doing well without sequelae 43 and 38 months after transplantation.
Keywords: Antiretroviral therapy, highly active/interactions; HAART; heart transplantation, orthotopic; HIV seropositivity/drug therapy; HIV-1; human immunodeficiency virus; immunosuppressive regimens; solid organ transplantation
In 1984, the human immunodeficiency virus-1 (HIV) was first identified as the causative agent of acquired immune deficiency syndrome (AIDS). The first orthotopic heart transplantation (OHT) was performed in an HIV-seropositive(+) patient in 2001.1 Since that time, significant breakthroughs in the understanding of the pathophysiology of HIV have produced treatment regimens now known as highly active antiretroviral therapy (HAART). Current HAART regimens effectively suppress HIV replication for prolonged periods of time, thereby leading to near-normal life expectancy with ever-decreasing side effects and ever-increasing therapeutic simplicity.2
As a result of HAART, patients infected with HIV are living longer lives and are now presenting at the operating room for a wide variety of surgical procedures, including cardiothoracic surgery.3,4 Patients infected with HIV may now appear as candidates for kidney, liver, and heart transplantation. Although HIV infection is no longer considered an absolute contraindication to cardiac transplantation, the procedure is still rarely performed in this patient population. Kidney and liver transplantation5–11 have been extensively studied and more frequently performed in HIV+ patients, yet the literature reports only 9 cases of heart transplantation (since 2003) in patients who were HIV+ at the time of transplant.1,12–16
This report describes the cases of 2 additional HIV-seropositive patients who successfully underwent cardiac transplantation, together with the highly unusual challenges of HAART and of concomitant immunosuppressive therapy. Our Institutional Review Board approved these case reports, and the 2 patients gave their written permission to publish.
Case Reports
Patient 1. A 65-year-old white man presented with a 10-year history of ischemic cardiomyopathy and coexisting morbidities notable for hyperlipidemia, hypertension, diabetes mellitus, myocardial infarction (11 years earlier), ventricular tachycardia, and HIV infection (23 years in duration). The patient had previously undergone percutaneous coronary interventions, followed by coronary artery bypass grafting. He had no history of cerebrovascular accident or hepatitis A, B, or C. His HAART medications are listed in Table I; additional medications included aldactone, aspirin, atorvastatin, sotalol, carvedilol, famotidine, fish oil, furosemide, lisinopril, mexilitine, and warfarin.
TABLE I.
Clinical Summary for Patients 1 and 2 at the Time of Orthotopic Heart Transplantation
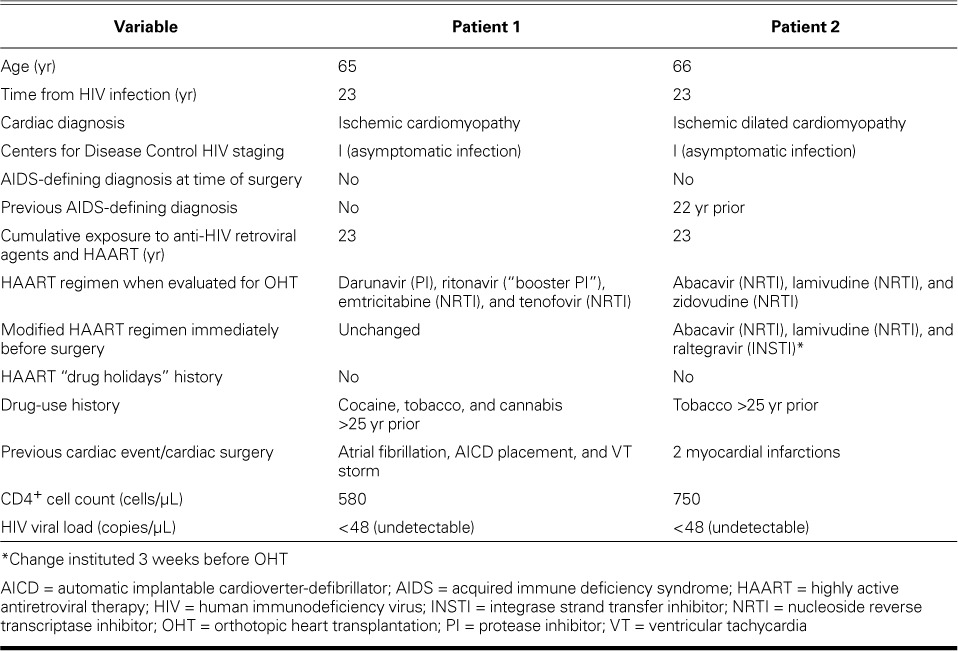
Patient 2. A 66-year-old white man had a history of idiopathic dilated cardiomyopathy with New York Heart Association functional class IV symptoms. His medical history included hypertension, hyperlipidemia, 2 myocardial infarctions (17 and 7 years earlier), chronic kidney disease, and HIV infection (23 years in duration, consistent with Patient 1). The patient had no history of diabetes mellitus, peripheral vascular disease, or hepatitis A, B, or C, and he had quit smoking 25 years before surgery. His medications included HAART (Table I), as well as calcitriol, clonazepam, digoxin, erythropoietin, fish oil, furosemide, gabapentin, gemfibrozil, loratadine, magnesium oxide, omega-3 fatty acids, and pravastatin.
Patient 2 had chronic kidney disease accompanied by anemia and thrombocytopenia. Therefore, HAART was initiated (in the following manner) during the preoperative period. He had been taking a single-pill, fixed-dose, triple combination of abacavir, lamivudine, and zidovudine. Zidovudine was discontinued from his regimen to minimize bone marrow suppression and was replaced by raltegravir, a potent anti-HIV integrase inhibitor with minimal drug–drug interactions. With these modifications, his new HAART regimen—consisting of raltegravir (400 mg 2×/d), abacavir (300 mg 2×/d) and lamivudine (150 mg 2×/d)—was initiated, and it proved to be effective and well-tolerated during the 6 weeks before transplantation. In order to minimize the pill burden, both abacavir and lamivudine were dispensed in a combination tablet (abacavir 600 mg/lamivudine 300 mg) by mouth, 1 tablet daily. The patient had been confirmed not to have the HLA-B*5701 allele, that excludes the risk of hypersensitivity reactions to abacavir. Abacavir is primarily metabolized by hepatic glucuronyl transferase (36%) and alcohol dehydrogenase (30%), resulting in inactive metabolites which, along with the unchanged drug, are eliminated in the urine. Raltegravir is primarily metabolized by the UGT1A1 glucuronidation, and lamivudine is primarily eliminated by renal excretion. There were no significant drug–drug interactions with this modified antiretroviral therapy, and the management of immunosuppressive therapy was facilitated with these changes.
In accordance with protocol at our institution, pre-transplantation evaluation included the extent of the heart disease, functional status, and indications for OHT; the medical history of both patients was reviewed, with specific attention to their history of HIV infection. Our institution's exclusion criteria for HIV+ candidates for OHT include the following: 1) active opportunistic or other infections, 2) current history of AIDS-defining diagnoses (opportunistic infections or cancers), and 3) lack of stable HAART regimens in place. Inclusion criteria include the following: 1) HIV+ serostatus; 2) over the past 6 months to 1 year, CD4+ T-cell counts that are stable and within our clinical laboratory's normal range (450–2,500 cells/μL); 3) stable HAART regimen for over 1 year; and 4) undetectable HIV ribonucleic acid (RNA) by polymerase chain reaction (PCR) (<50 copies/μL).
Patients 1 and 2 fulfilled all inclusion criteria: they had been on stable HAART regimens for multiple years, the result of which was long-time undetectable HIV RNA, and they showed evidence of robust immune reconstitution (stable and normal CD4+ T-cell counts) (Table I).
At the time of transplantation, Patient 1 was listed as Status II, and Patient 2 was listed as Status Ia, in accordance with the United Network for Organ Sharing (UNOS) classification method. The UNOS classification is based upon severity of heart disease and urgency for heart transplantation. Patient 1 was hemodynamically stable with no pressor support and was admitted from outpatient status, whereas Patient 2 was hemodynamically unstable and needed urgent transplantation or ventricular assist device placement. Transplantation occurred when suitable organs became available.
Postoperative HAART and Immunosuppressive Management
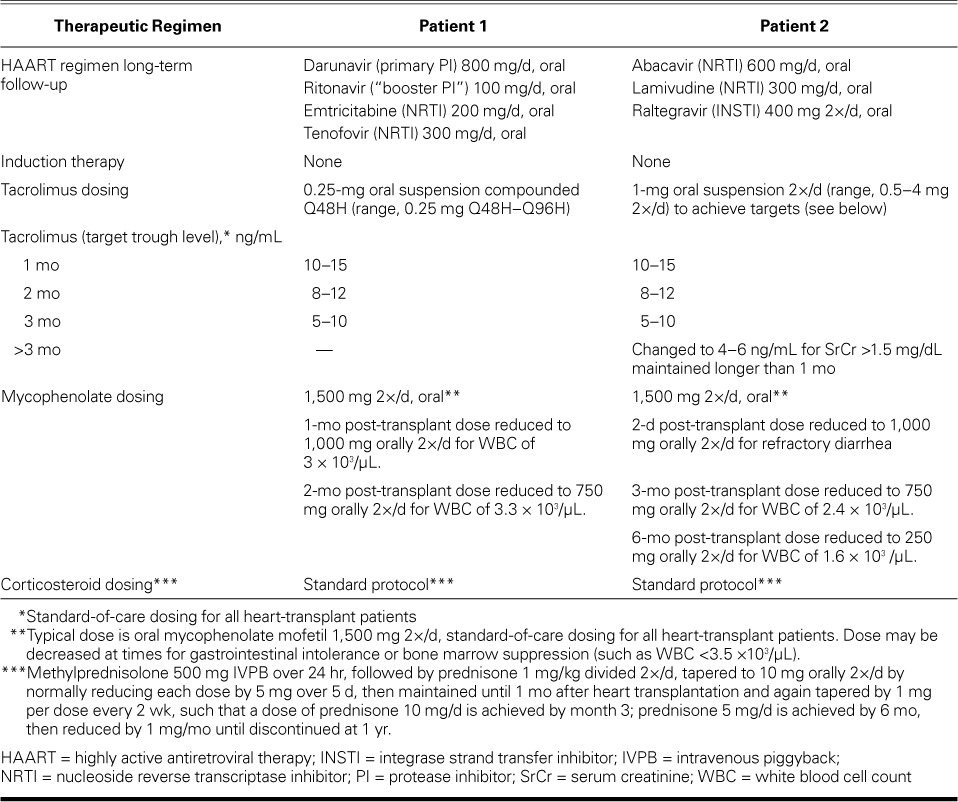
After orthotopic heart transplantation, Patient 1's preoperative HAART regimen was modified in both schedule and dosage. His protease inhibitor (PI) darunavir, 600 mg 2×/d, was reduced to 800 mg/d, and the required concomitant pharmacoenhancer ritonavir (PI booster) was reduced from 100 mg 2×/d to 100 mg/d. These were given with emtricitabine/tenofovir (200/300 mg). HAART was restarted on postoperative day 2, and Patient 1 was not given induction immunosuppressive therapy. However, the patient's transplant immunosuppressive regimen, specifically tacrolimus dosing, was tailored to account for the pharmacokinetic interactions expected to occur between tacrolimus and darunavir (PI) boosted with ritonavir (PI). We refrained from our standard dosing of tacrolimus (0.5–1 mg, orally 2×/d), and tacrolimus dosing was determined on the basis of trough levels. Despite this change, supratherapeutic levels resulted, and we subsequently considered daily trough levels in the dosing. By the end of postoperative week 3, Patient 1's tacrolimus levels remained stable. He was discharged from the hospital with instructions to take oral tacrolimus (0.25 mg) every 48 hours; levels were effectively maintained between 11.5 and 14.7 ng/mL on this regimen. Patient 1 was discharged 21 days after OHT.
After OHT, Patient 2 was not given induction therapy and was immediately started on triple-drug immunosuppressive therapy: 1) methylprednisolone (500 mg) intravenously over 24 hr, followed by a prednisone taper to 10 mg orally 2×/d, 2) mycophenolate (1,500 mg) intravenously every 12 hr, and 3) tacrolimus suspension (0.5 mg) by nasogastric tube 2×/d. HAART was restarted on postoperative day 2. The patient's mycophenolate mofetil dose was accordingly decreased from 1.5 to 1 g orally 2×/d. Patient 2 was discharged from the hospital 19 days after OHT.
It is of note that neither patient was given induction therapy. Induction therapy, typically with antithymocyte globulin, is given to patients who are sensitized before transplantation (that is, patients with preformed, potentially cytotoxic donor-specific antibodies against the new heart) to potentially prevent delayed hyper-acute rejection. Because neither patient was sensitized, induction therapy was not required. This was fortunate, because induction therapy can increase the risk of infections in the short term after transplantation.
Because of persistent interactions with the HAART-protease inhibitors, Patient 1 was given the lowest dose possible of tacrolimus (0.25 mg orally every 48 hr). His HIV RNA levels and CD4+ T-cell counts were measured at 1 month postoperatively, then every 2 months thereafter for the first year. His HIV RNA levels measured by means of PCR remained stable, at <50 copies/μL.
Patient 1's postoperative white blood counts (WBC) were noted as follows: at 2 weeks, 6 ×103/μL; at 1 month, 8.7 ×103/μL; at 2 months, 3.3 ×103/μL; and at 3 months, 3 ×103/μL. In both patients, left ventricular ejection fraction remained unchanged from the time of transplantation throughout the postoperative period, until the time of this report. The T-cell immune function at 1 and 2 months postoperatively for Patients 1 and 2 was quantitated at 192 and 203, respectively.
Patient 1 was weaned completely from prednisone by 1 year after OHT. He underwent renal artery stent placement 9 months after OHT for refractory hypertension, which subsided postprocedurally. Five months postoperatively, Patient 2's post-discharge course was complicated by one bout of neutropenia, which resolved after a 2-week hold and subsequent de-escalation of the valganciclovir dose (from 450 mg orally 2×/d to 450 mg/d) and escalation of the sulfamethoxazole/trimethoprim dose (from 1 tablet triweekly to 1 tablet biweekly). Patient 2's T-cell immune function was 138, so his mycophenolate mofetil dosage was reduced to 250 mg orally 2×/d. Three months later, after his T-cell immune function had risen above 200 ng ATG/mL, trimethoprim-sulfamethoxazole and acyclovir were resumed.
At our institution, the T-cell immune-function assay is followed routinely in heart-transplant recipients to determine whether there is over-immunosuppression. If so, the target tacrolimus level or mycophenolate mofetil dose is reduced. The Cylex® ImmuKnow® T-cell immune-function assay (Viracor-IBT Laboratories; Lee's Summit, Mo) has been approved by the U.S. Food and Drug Administration for the detection and quantification of cell-mediated immunity in an immunosuppressed population. This assay measures adenosine triphosphate (ATP) release from activated lymphocytes and correlates with the level of immune responsiveness. We have shown that a T-cell immune function <200 ng ATP/mL is associated with an increased risk of infection over the next 30 days.17 At our center, the rate of cellular or antibody-mediated rejection within the first 2 years of transplantation is less than 10%.
Of note, it is our policy to continue cytomegalovirus (CMV) prophylaxis for only 6 months after heart transplantation in CMV recipient-positive–donor-negative patients (as in our 2 patients). Our standard protocol is to use valganciclovir therapy for CMV prophylaxis after transplantation, depending on the CMV status of the heart-transplant donor and recipient. The highest-risk patients for CMV infection are those recipients who have never been exposed to CMV and receive a heart from a donor who has CMV infection (an anti-CMV immunoglobulin [Ig]G-positive donor and an anti-CMV IgG-negative recipient is termed a “CMV mismatch”). These patients receive one year of prophylactic valganciclovir therapy. If the recipient is CMV IgG-positive, he or she is given 6 months of prophylactic valganciclovir therapy regardless of the donor's CMV IgG status. If both the donor and recipient are CMV IgG-negative, the recipient is given acyclovir for 6 months after transplantation (an inferior agent that is acceptable because of the recipient's lower risk of CMV infection and the high cost of valganciclovir). The CMV DNA PCR is checked only in patients in whom we suspect clinical CMV infection (that is, those with some combination of diarrhea, fever, and leukopenia). Tables II and III summarize the immunosuppressive therapy and monitoring of Patients 1 and 2.
TABLE II.
Postoperative Results and Long-Term Follow-Up Values
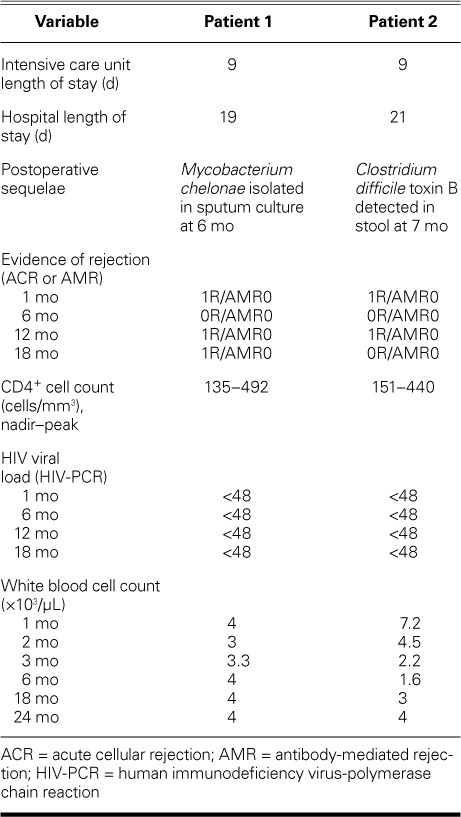
TABLE III.
Therapeutic Regimens after Orthotopic Heart Transplantation
After discharge from the hospital, Patients 1 and 2 were monitored at regular intervals for potential cardiac rejection. In addition, monitoring of the development of premature atherosclerosis was performed via coronary angiography because transplanted hearts are denervated, and patients with transplant coronary artery disease rarely experience angina. Consequently, coronary angiography is the most reliable way to detect cardiac allograft vasculopathy. At our institution, post-OHT patients undergo yearly angiograms. If there is no development of vasculopathy by 6 years after transplant, these patients are at lower risk for vasculopathy in the long term, and it is our practice to evaluate ischemia by alternating angiography (every other year) with myocardial perfusion stress imaging.
Subsequent to heart transplantation, Patients 1 and 2 remained free of any major negative sequelae (atherosclerosis, cardiac rejection, reactivated HIV infection, or increased immunodeficiency) at 43 and 38 months, respectively. Both patients are participating in full activities of daily living.
Discussion
During the past decade, patients infected with HIV have been presenting for surgery, including cardiothoracic surgery, at an increasing rate and with successful outcomes.3,4,18 Although patients infected with HIV remain an exceptional subcategory of patients undergoing cardiothoracic surgery, HIV+ patients who undergo cardiac transplantation are even rarer. The debate over whether to perform heart transplantation in HIV+ individuals continues, and it appears that most centers either explicitly or de facto consider HIV infection a relative contraindication for heart transplantation.19
Initial concerns for HIV+ patients undergoing surgery focused on immune compromise. However, HAART regimens have proved effective and durable in maintaining immune competence. Of particular significance and importance is the ability to continue HAART regimens after cardiac surgery and to minimize that time of interruption.
Our 2 cases illustrate the resolvable challenges in HAART and immunosuppressive management when an HIV+ patient's HAART regimen includes protease inhibitors (PIs). Subtle gradations in the preoperative, intraoperative, and postoperative management of the HIV+ patient can further improve outcomes and reduce morbidity and mortality rates. Our 2 cases show that HIV-seropositive patients can successfully undergo heart transplantation with complication-free survival at 43 and 38 months, respectively. However, this patient population most likely should undergo heart transplantation only in centers that have extensive experience with heart transplantation and cardiologic management of HIV+ patients. A multidisciplinary approach in the management of HIV+ transplant recipients should include cardiologists, cardiac surgeons, infectious-disease specialists, anesthesiologists, perfusionists, and pharmacists.
From 2011 through 2015, our center has performed nearly 500 heart transplantations, but only 2 of them have been in HIV+ patients. However, over the past 15 years, our center has performed cardiac surgery or other cardiologic interventions in approximately 400 HIV+ patients. We continue to consider HIV+ patients for heart transplantation if they meet inclusion criteria (stable CD4+ cell count within the normal range, stable HAART regimen for >1 year, and undetectable HIV viral load) and do not possess exclusion criteria (active infection of any type, current AIDS-defining diagnosis, lack of stable HAART regimen, or detectable HIV viral load). We anticipate that the HIV+ patient population will increasingly undergo heart transplantation in the 21st century.
Acknowledgments
The authors thank Drs. Alfredo Trento, Lawrence Czer, Mark Zukowski, and Manxu Zhao for their time and support in the management of the patients in this case report.
Footnotes
From: Division of Cardiothoracic Anesthesiology, Department of Anesthesiology (Dr. Conte), Department of Pharmacy (Dr. Dilibero), and Division of Infectious Diseases, Department of Medicine (Dr. Hardy), Cedars-Sinai Medical Center; and Division of Cardiothoracic Surgery, Department of Surgery (Dr. Esmailian), and Division of Cardiology (Drs. Kittleson and Kobashigawa), Cedars-Sinai Heart Institute; Los Angeles, California 90048
References
- 1.Calabrese LH, Albrecht M, Young J, McCarthy P, Haug M, Jarcho J, Zackin R. Successful cardiac transplantation in an HIV-1-infected patient with advanced disease. N Engl J Med. 2003;348(23):2323–8. doi: 10.1056/NEJMoa022935. [DOI] [PubMed] [Google Scholar]
- 2.van Sighem AI, Gras LA, Reiss P, Brinkman K, deWolf F, ATHENA national observational cohort study Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24(10):1527–35. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- 3.Horberg MA, Hurley LB, Klein DB, Follansbee SE, Quesenberry C, Flamm JA et al. Surgical outcomes in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Arch Surg. 2006;141(12):1238–45. doi: 10.1001/archsurg.141.12.1238. [DOI] [PubMed] [Google Scholar]
- 4.Conte AH, Esmailian F, LaBounty T, Lubin L, Hardy WD, Yumul R. The patient with the human immunodeficiency virus-1 in the cardiovascular operative setting. J Cardiothorac Vasc Anesth. 2012;27(1):135–55. doi: 10.1053/j.jvca.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Halpern SD, Ubel PA, Caplan AL. Solid-organ transplantation in HIV-infected patients. N Engl J Med. 2002;347(4):284–7. doi: 10.1056/NEJMsb020632. [DOI] [PubMed] [Google Scholar]
- 6.Kuo PC, Stock PG. Transplantation in the HIV+ patient. Am J Transplant. 2001;1(1):13–7. doi: 10.1034/j.1600-6143.2001.010104.x. [DOI] [PubMed] [Google Scholar]
- 7.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J et al. Outcomes of kidney transplantation in HIV-infected recipients [published erratum appears in N Engl J Med 2011; 364(11):1082] N Engl J Med. 2010;363(21):2004–14. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roland ME, Stock PG. Review of solid-organ transplantation in HIV-infected patients. Transplantation. 2003;75(4):425–9. doi: 10.1097/01.TP.0000046943.35335.18. [DOI] [PubMed] [Google Scholar]
- 9.Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336–7. doi: 10.1056/NEJMc0900837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar MS, Sierka DR, Damask AM, Fyfe B, McAlack RF, Heifets M et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int. 2005;67(4):1622–9. doi: 10.1111/j.1523-1755.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 11.Gruber SA, Doshi MD, Cincotta E, Brown KL, Singh A, Morawski K et al. Preliminary experience with renal transplantation in HIV+ recipients: low acute rejection and infection rates. Transplantation. 2008;86(2):269–74. doi: 10.1097/TP.0b013e318177884e. [DOI] [PubMed] [Google Scholar]
- 12.Uriel N, Jorde UP, Cotarlan V, Colombo PC, Farr M, Restaino SW et al. Heart transplantation in human immunodeficiency virus-positive patients. J Heart Lung Transplant. 2009;28(7):667–9. doi: 10.1016/j.healun.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Ragni MV, Bontempo FA, Lewis JH. Organ transplantation in HIV-positive patients with hemophilia. N Engl J Med. 1990;322(26):1886–7. doi: 10.1056/nejm199006283222613. [DOI] [PubMed] [Google Scholar]
- 14.Tzakis AG, Cooper MH, Dummer JS, Ragni M, Ward JW, Starzl TE. Transplantation in HIV+ patients. Transplantation. 1990;49(2):354–8. doi: 10.1097/00007890-199002000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisleri G, Morgan JA, Deng MC, Mancini D, Oz MC. Should HIV-positive recipients undergo heart transplantation? J Thorac Cardiovasc Surg. 2003;126(5):1639–40. doi: 10.1016/s0022-5223(03)01216-9. [DOI] [PubMed] [Google Scholar]
- 16.Castel MA, Perez-Villa F, Roig E, Miro JM. Heart transplantation in an HIV-1-infected patient with ischemic cardiomyopathy and severe pulmonary hypertension [in Spanish] Rev Esp Cardiol. 2011;64(11):1066–7. doi: 10.1016/j.recesp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Kobashigawa JA, Kiyosaki KK, Patel JK, Kittleson MM, Kubak BM, Davis SN et al. Benefit of immune monitoring in heart transplant patients using ATP production in activated lymphocytes. J Heart Lung Transplant. 2010;29(5):504–8. doi: 10.1016/j.healun.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Mestres CA, Chuquiure JE, Claramonte X, Munoz J, Benito N, Castro MA et al. Long-term results after cardiac surgery in patients infected with the human immunodeficiency virus type-1 (HIV-1) Eur J Cardiothoracic Surg. 2003;23(6):1007–16. doi: 10.1016/s1010-7940(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 19.Uriel N, Nahumi N, Colombo PC, Yuzefpolskaya M, Restaino SW, Han J et al. Advanced heart failure in patients infected with human immunodeficiency virus: is there equal access to care? J Heart Lung Transplant. 2014;33(9):924–30. doi: 10.1016/j.healun.2014.04.015. [DOI] [PubMed] [Google Scholar]


