ABSTRACT
Impairment of Nef function, including reduced CD4 downregulation, was described in a subset of HIV-1-infected individuals that control viral replication without antiretroviral treatment (elite controllers [EC]). Elimination of HIV-1-infected cells by antibody-dependent cellular cytotoxicity (ADCC) requires the presence of envelope glycoproteins (Env) in the CD4-bound conformation, raising the possibility that accumulating CD4 at the surface of virus-infected cells in EC could interact with Env and thereby sensitize these cells to ADCC. We observed a significant increase in the exposure of Env epitopes targeted by ADCC-mediating antibodies at the surface of cells expressing Nef isolates from EC; this correlated with enhanced susceptibility to ADCC. Altogether, our results suggest that enhanced susceptibility of HIV-1-infected cells to ADCC may contribute to the EC phenotype.
IMPORTANCE Nef clones derived from elite controllers (EC) have been shown to be attenuated for CD4 downregulation; how this contributes to the nonprogressor phenotype of these infected individuals remains uncertain. Increasing evidence supports a role for HIV-specific antibody-dependent cellular cytotoxicity (ADCC) in controlling viral infection and replication. Here, we show that residual CD4 left at the surface of cells expressing Nef proteins isolated from ECs are sufficient to allow Env-CD4 interaction, leading to increased exposure of Env CD4-induced epitopes and increased susceptibility of infected cells to ADCC. Our results suggest that ADCC might be an active immune mechanism in EC that helps to maintain durable suppression of viral replication and low plasma viremia level in this rare subset of infected individuals. Therefore, targeting Nef's ability to downregulate CD4 could render HIV-1-infected cells susceptible to ADCC and thus have therapeutic utility.
INTRODUCTION
HIV-1 Nef is a small (27 to 35 kDa) accessory protein critical for viral replication and progression to AIDS (1). Infection with nef deletion or nef-defective strains of HIV or SIV was shown to lead to a slow or nonprogressive disease phenotype (2–4). Nef possesses several activities that are important for viral replication and pathogenesis, including downregulation of CD4 (5, 6) and HLA class I (7) molecules and enhancement of viral infectivity and replication (8, 9). Impairment of these Nef activities was demonstrated in HIV-1 elite controllers (EC), rare infected individuals who spontaneously suppress plasma viremia to <50 RNA copies/ml without antiretroviral therapy (10). In particular, Nef clones derived from EC displayed a significantly lower ability to downregulate CD4 compared to clones isolated from individuals during chronic progressive infections (CP) (10, 11). However, how impaired Nef function contributes to the EC phenotype remains unclear.
Increasing evidence supports a role for HIV-specific antibody (Ab)-dependent cellular cytotoxicity (ADCC) in controlling viral infection and replication (12–18). Analysis of the correlates of protection in the RV144 vaccine trial suggested that increased ADCC activity was linked with decreased HIV-1 acquisition (19), and Abs with potent ADCC activity were isolated from some RV144 vaccinees (20). We reported that the CD4-bound conformation of HIV-1 envelope glycoproteins (Env) was preferentially targeted by ADCC-mediating Abs or sera from HIV-1-infected individuals (21–24). Of note, ADCC-mediating (non-neutralizing) Abs represent a significant proportion of anti-Env Abs elicited during natural HIV infection (24, 25), and particularly high levels of ADCC-mediating Abs have been observed in EC subjects (26, 27). However, in order to limit the exposure of CD4-bound Env at the surfaces of infected cells, HIV-1 has developed sophisticated mechanisms to efficiently internalize Env (28), to counteract host restriction factor BST-2 by Vpu (23, 29, 30), and to downregulate CD4 by Nef and Vpu (23, 24). It was also reported that Env-CD4 interaction plays a role in CD4 downregulation (31). Here, we evaluated whether the inability of EC Nef clones to fully downregulate CD4 results in the adoption of a CD4-bound Env conformation on HIV-1-infected cells and enhanced susceptibility of these cells to ADCC.
MATERIALS AND METHODS
Cell lines and isolation of primary cells.
293T human embryonic kidney and HOS cell lines were obtained from the American Type Culture Collection (ATCC) and the National Institutes of Health (NIH) AIDS and Research and Reference Reagent Program, respectively. Peripheral blood mononuclear cells (PBMCs) from healthy donors were obtained under research regulations approved by CRCHUM; written informed consent was obtained from each individual. Cells were grown as previously described (23, 32). CD4 T lymphocytes were purified from rested PBMCs by negative selection and activated as previously described (24).
Study participants and nef cloning.
Plasma from 47 untreated EC (viral load [VL] < 50 RNA copies/ml plasma) and 48 untreated CP (median VL = 80,500; interquartile range = 25,121 to 221,250) was used to amplify Nef sequences (10, 11, 33–36; see also below). All EC and CP were HIV-1 subtype B-infected from the Boston area, and comparable with respect to ethnicity and diagnosis date of HIV (EC [1985-2006] versus CP [1981-2003]). The study was approved by the institutional review board of the Massachusetts General Hospital, Boston, MA; all participants provided written informed consent.
HIV RNA was extracted from plasma of EC and CP subjects and amplified using nested reverse transcription-PCR, as described previously (36, 37). Nef amplicons were cloned into pIRES2-EGFP expression vector (Clontech). At least three Nef clones were sequenced per patient, and a single clone with an intact Nef reading frame that closely resembled the sequence of the original bulk plasma RNA was chosen, as described previously (10). Nef clones were transferred into a pNL4.3 lacking Nef (N−) plasmid and confirmed by DNA sequencing, as described previously (38). Recombinant viruses harboring nef from the HIV SF2 strain (wt Nef SF2) and lacking nef (N−) were used as positive and negative controls, respectively.
Viral production and infections.
Vesicular stomatitis virus G-pseudotyped pNL4.3-encoding Nef SF2, deleted nef, and nef clones from EC (15 clones) or CP (15 clones) viruses were produced in 293T cells and titrated as previously described (23). A random number generator (GraphPad QuickCalcs) was used to randomly select EC and CP nef proviruses for this study. Viruses were then used to infect approximately 20 to 30% of primary CD4 T cells from healthy donors by spin infection at 800 × g for 1 h in 96-well plates at 25°C.
Antibodies and sera.
The gp120 outer-domain recognizing antibody 2G12 was obtained from the NIH AIDS and Research and Reference Reagent Program. The broadly neutralizing CD4-binding site VRC01 antibody was obtained from Peter Kwong (Vaccine Research Center, National Institute of Allergy and Infectious Disease). The anti-gp120 cluster A (A32, L9-i1, L9-i2, and N26-i1) and anti-gp41 antibodies (7B2 and M785-U1) were previously reported (23, 39–41). The monoclonal antibody (MAb) anti-CD4 OKT4 (BioLegend) binds to the D3 domain of CD4 and was used to measure cell surface levels of CD4, as described previously (23). The secondary goat anti-mouse and anti-human antibodies coupled to Alexa Fluor 647 (Invitrogen) were used in flow cytometry experiments.
HIV+ sera was obtained from the Montreal Primary HIV Infection Cohort (42, 43) and the Canadian Cohort of HIV-Infected Slow Progressors (33, 44, 45). Research adhered to the ethical guidelines of CRCHUM and informed consent was obtained from each volunteer. Sera was collected during Ficoll isolation of PBMCs and conserved at −80°C. Serum aliquots were heat inactivated for 30 min at 56°C and stored at 4°C until ready and used in subsequent experiments as shown (23, 24). A random number generator (GraphPad QuickCalcs) was used to randomly select a number of sera from each cohort for testing.
Cell-based ELISA.
Detection of trimeric Env at the surface of HOS cells was performed by cell-based enzyme-linked immunosorbent assay (ELISA), as previously described (23, 46, 47). Briefly, HOS cells were seeded in 96-white well plates (2 × 104 cells per well) and transfected the next day with a cytoplasmic-tail-deleted HIV-1 EnvYU2 variant, together with a human CD4 expressor and nef-encoding plasmid from EC or CP using standard polyethylenimine (Polyscience, Inc., Warrington, PA) transfection method. At 2 days posttransfection, cells were blocked (10 mg/ml nonfat dry milk, 1.8 mM CaCl2, 1 mM MgCl2, 25 mM Tris [pH 7.5], and 140 mM NaCl) and then incubated with 1:1,000 dilutions of HIV+ sera or 1 μg of relevant MAbs/ml. Env/CD4-specific IgGs were detected using an horseradish peroxidase-conjugated anti-human/mouse IgG-specific secondary Ab (Pierce) with a TriStar LB 941 luminometer (Berthold Technologies).
Flow cytometry: cell surface staining and ADCC responses.
Cell surface staining was performed as previously described (23, 24). Binding of HIV-1-infected cells by sera (1:1,000 dilution) or relevant MAbs (1 μg/ml) were performed 48 after infection. After surface staining, infected cells were permeabilized using the Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences, Mississauga, Ontario, Canada) to detect infected cells (p24+ cells) with the fluorescent anti-p24 MAb (PE-anti-p24, clone KC57; Beckman Coulter/Immunotech, Hialeah, FL) at a 1:100 final concentration, as previously described (23, 48). The percentage of infected cells was determined by gating on the living cell population based on the viability AquaVivid dye (Invitrogen). Samples were analyzed on an LSRII cytometer (BD Biosciences), and data analysis was performed using FlowJo vX.0.7 (Tree Star, Ashland, OR).
Measurement of serum or A32-mediated ADCC responses was performed using a fluorescence-activated cell sorting (FACS)-based ADCC assay (21, 23, 24, 48). Briefly, infected primary CD4+ T cells were stained with viability (AquaVivid; Invitrogen) and cellular (cell proliferation dye eFluor670; eBioscience) markers and used as Target cells (T). Autologous PBMC effector cells (used as effector [E] cells), stained with another cellular marker (cell proliferation dye eFluor450; eBiosciences), were then mixed at an effector/target (E/T) ratio of 10:1 in 96-well V-bottom plates (Corning). A 1:1,000 final concentration of serum or 5 μg/ml of the A32 MAb was added to appropriate wells. Cocultures were centrifuged for 1 min at 300 × g and incubated at 37°C for 5 to 6 h before being fixed in a 2% PBS-formaldehyde solution; infected cells were identified by intracellular p24 staining, as described above. Samples were analyzed on an LSRII cytometer (BD Biosciences). The percent cytotoxicity was calculated with the following formula: (% p24+ cells in targets plus effectors) − [(% p24+ cells in targets plus effectors plus serum or A32)/(% p24+ cells in targets)].
Statistical analyses.
Statistics were analyzed using Prism, version 6.01 (GraphPad, San Diego, CA). P values of <0.05 were considered significant; significance values are indicated by asterisks in the figures (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
RESULTS
Attenuated CD4 downregulation by Nef alleles from elite controllers enhances exposure of ADCC-mediating epitopes on HIV-1 Env.
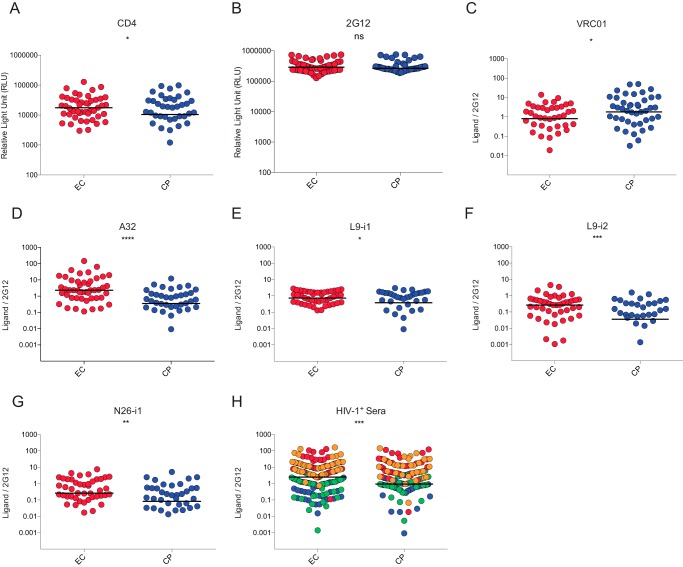
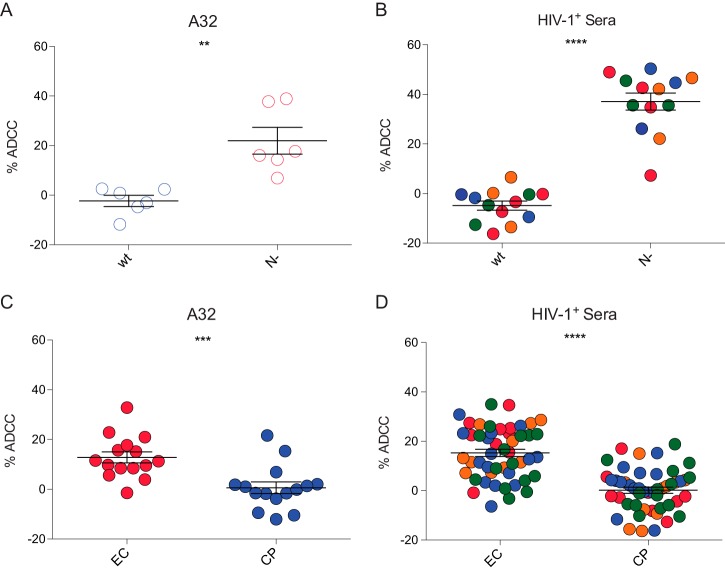
To evaluate the effect of CD4 downregulation mediated by Nef clones isolated from previously characterized EC (47 clones) or chronic progressors (48 clones) (10, 11, 49, 50), on the exposure of Env ADCC-mediating epitopes, we used a previously described cell-based ELISA which allows measurement of Env conformation at the cell surface (22). Briefly, HOS cells were transfected with plasmids expressing Env, CD4, and Nef clones. Two days later, transfected cells were washed and incubated with anti-CD4 and anti-Env Abs or sera from HIV-1-infected individuals. As previously reported (10, 11), Nef clones isolated from EC were less efficient for CD4 downregulation compared to Nef clones from CP (Fig. 1A), but no difference was observed in the overall levels of Env, as measured by the outer-domain recognizing 2G12 Ab (Fig. 1B) or anti-gp41 Abs (Fig. 2). CD4 remaining at the surface of cells expressing EC Nef clones competed for ligands that recognize the CD4-binding site, such as VRC01 Ab (Fig. 1C), suggesting that CD4 recognized its binding site on these cells in a manner similar to that of Env in the context of viral particles, as previously reported (23). Importantly, in the presence of EC Nef clones, Env exposed CD4-induced (CD4i) ADCC-mediating epitopes, such as A32 (Fig. 1D) and other anti-cluster A Abs (Fig. 1E to G), significantly better. Moreover, in the presence of EC Nef clones, we observed that Env was recognized better by sera from HIV-1-infected individuals (Fig. 1H), suggesting that inefficient CD4 downregulation by EC Nef clones might affect Env conformation and sensitize infected cells to ADCC.
FIG 1.
Attenuated CD4 downregulation by Nef alleles from elite controllers enhance exposure of ADCC-mediating epitopes. HOS cells coexpressing HIV-1YU2 Env and CD4 in the presence of Nef isolates from EC (47 clones) or CP (48 clones) were evaluated at 48 h posttransfection by cell-based ELISA (22). Nef clones from EC present attenuated CD4-downregulation compared to CP (A) but do not affect levels of Env present at the cell surface, as measured by 2G12 (B). Inefficient CD4 downregulation by Nef from EC resulted in competition for the CD4-binding site VRC01 antibody (C) but enhanced recognition by anti-cluster A ADCC-mediating antibodies A32 (D), L9i1 (E), L9i2 (F), N26-i1 (G), and sera from four HIV-1-infected individuals (H). The results for each serum are depicted in a different color. Data shown are representative of those of at least two independent experiments acquired in triplicate. In panels A and B, the relative light units (RLU) are shown; in panels C to H, the signals were normalized to Env levels, as evaluated by 2G12 binding. Shown is the median of EC (in red) versus CP (in blue) clones. Statistical significance was tested using unpaired t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant).
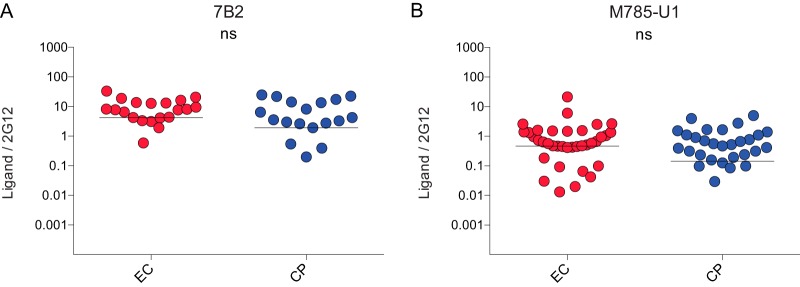
FIG 2.
Gp41 detection is not affected in the presence of Nef proteins from ECs. Env at the surfaces of HOS cells coexpressing HIV-1YU2 Env and CD4 in the presence of Nef isolates from EC or CP is detected to similar levels by 7B2 (A) and M785-U1 (B) anti-gp41 Abs. The data shown are representative of those of at least two independent experiments acquired in triplicate. Signals were normalized to Env levels, as evaluated by 2G12 binding. Shown is the median of ECs (in red) versus CPs (in blue) clones. (ns, not significant.)
Cells infected with viruses coding for Nef from elite controllers expose Env ADCC-mediating epitopes.
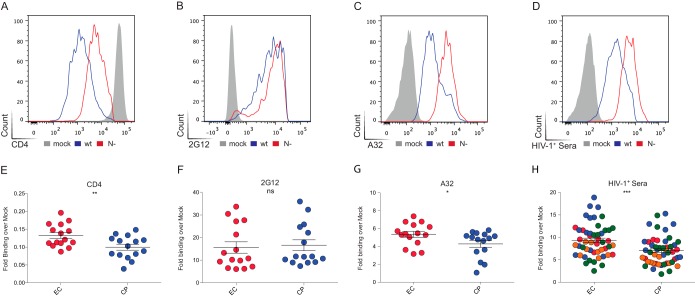
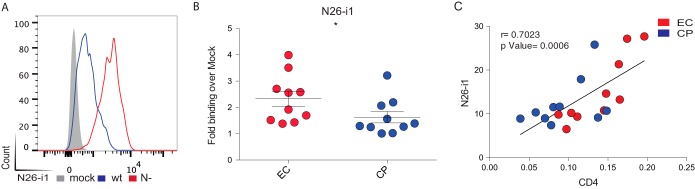
To address whether ADCC-mediating epitopes were better exposed at the surface of infected cells, 15 randomly selected Nef clones from EC and 15 from CP were cloned into replication-competent pNL4.3 proviruses. Infectious viral particles were generated by transfection into 293T cells and used to infect primary CD4 T cells isolated from healthy donors. As positive and negative controls for CD4 downregulation, viruses coding for wild-type NefSF2 or with Nef deleted (Nef−) were also generated. At 2 days postinfection, the cells were surface stained with anti-CD4, anti-Env or HIV+ sera, followed by permeabilization and intracellular p24 staining. As expected, cells infected with wild-type virus displayed greater CD4 downregulation than those infected with Nef− virus (Fig. 3A). No difference was observed in the amount of Env at the cell surface between the two viruses, based on recognition by 2G12 Ab (Fig. 3B). Remarkably, relatively modest difference in CD4 levels at the surfaces of wild-type versus Nef− virus-infected cells was sufficient to induce Env conformational changes and expose CD4i epitopes, such as those recognized by A32 (Fig. 3C), N26-i1 (Fig. 4A), or HIV+ (Fig. 3D) sera. As expected, sera from healthy uninfected individuals failed to recognize HIV-1-infected cells (Fig. 5). Of note, recognition of infected cells by N26-i1 correlated with the amount of CD4 remaining at the cell surface (Fig. 4C).
FIG 3.
Env conformation at the surface of cells infected with viruses coding for Nef proteins from ECs versus CPs. Primary CD4+ T cells from healthy donors were infected with a panel of pNL4.3-based viruses: wild-type virus (wt, coding for NefSF2), virus lacking Nef (N−), or virus encoding Nef from 15 randomly selected clones from ECs or CPs. Infected primary CD4 T cells were stained at 48 h postinfection with an anti-CD4 (OKT4; A and E) or anti-Env 2G12 (B and F) or A32 (C and G) antibodies or with HIV+ sera from four different donors (shown in different colors) (D and H). In the upper panels, results obtained with wild-type versus Nef(−)-infected cells are shown. In the lower panels, results from cells infected with viruses coding for Nef from ECs (in red) or CPs (blue) are shown. Quantification of data represented as the fold binding over mock representative of at least three independent experiments is shown. Error bars indicate means ± the standard errors of the mean (SEM). Statistical significance was evaluated using an unpaired t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant).
FIG 4.
Env conformation at the surfaces of cells infected with viruses coding for Nef proteins from ECs versus CPs. Primary CD4+ T cells from healthy donors were infected with a panel of pNL4.3-based viruses: wild-type virus (wt, coding for NefSF2), virus lacking Nef (N−), or virus encoding Nef from 10 randomly selected clones from ECs or CPs. (A and B) Infected primary CD4 T cells were stained at 48 h postinfection with the anti-cluster A ADCC-mediating antibody N26-i1. In panel A, the results obtained with wild-type versus Nef(−)-infected cells are shown. In panel B, the results from cells infected with viruses coding for Nef from ECs (red) or CPs (blue) are shown. Quantification of data represented as the fold binding over mock representatives of at least three independent experiments is shown. (C) A positive correlation between cell-surface levels of CD4 and N26-i1 recognition was observed with a Spearman rank correlation. Error bars indicate means ± the SEM. Statistical significance was evaluated using an unpaired t test (*, P < 0.05).
FIG 5.
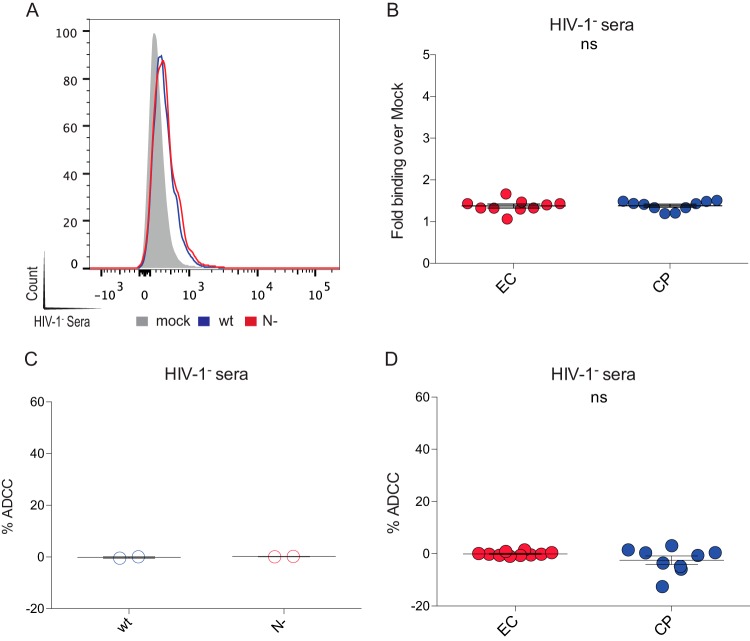
HIV-negative sera does not recognize or mediate the ADCC of cells infected with viruses coding for Nef from either ECs or CPs. Primary CD4+ T cells from healthy donors were infected with a panel of pNL4.3-based viruses: wild-type virus (wt, coding for NefSF2), virus lacking Nef (N−), or virus encoding Nef from 10 randomly selected clones from ECs or CPs. (A and B) Infected primary CD4 T cells were stained at 48 h postinfection with sera from healthy HIV-negative donors. (C and D) Cells were also used to evaluate the ability of HIV-negative sera to mediate ADCC. In the left panels, results obtained with wild-type versus Nef-infected cells are shown. In the right panels, results from cells infected with viruses coding for Nef from ECs (red) or CPs (blue) are shown. In panel B, the data are presented as the quantification of the fold binding over mock in at least three independent experiments. (ns, not significant.)
Similar differences were seen for cells infected with viruses encoding EC or CP Nef proteins. Indeed, cells infected with EC Nef viruses presented significantly more CD4 at their surfaces compared to CP (Fig. 3E), while the overall levels of Env remained similar (Fig. 3F). However, in the context of EC Nef, Env appeared to sample a conformation closer to the CD4-bound state, since it was better recognized by A32 (Fig. 3G), N26-i1 (Fig. 4B), and HIV+ (Fig. 3H) sera.
Enhanced susceptibility of cells infected with EC Nef viruses to ADCC-mediated killing.
Using a previously described FACS-based ADCC assay (21, 23), we then asked whether the higher levels of A32 and HIV+ sera staining at the surface of HIV-1-infected cells observed for EC Nef viruses enhanced their susceptibility to ADCC mediated by PBMCs from healthy individuals. Primary CD4 T cells infected with wild-type and Nef− viruses were used as positive and negative controls. As previously reported (21, 23, 24), wild-type-infected cells were not sensitive to ADCC mediated by autologous PBMCs (Fig. 6A and B); thus, highlighting the ability of HIV-1 to escape from this adaptative immune response. Confirming Nef's role in protection from ADCC (21, 23, 24), cells infected with Nef− virus were susceptible to ADCC killing mediated by A32 (Fig. 6A) and HIV+ sera from five HIV-1-infected individuals (Fig. 6B). Mirroring Env recognition by A32 and HIV+ sera presented in Fig. 3, ADCC activity was higher in the presence of cells infected with viruses encoding Nef proteins from EC compared to those from CP. Indeed, using the same PBMCs, we observed that A32 (Fig. 6C) and HIV+ sera (Fig. 6D) mediated significantly greater killing of infected cells expressing Nefs from EC than from CP (P < 0.0001). Interestingly, recognition of infected cells by HIV+ sera positively correlated with higher levels of CD4 at the cell surface (Fig. 7A) and with enhanced ADCC responses (Fig. 7B and C). This is true for cells expressing Nefs from EC and CP as well. However, as mentioned above, ADCC responses were higher for EC due to attenuated CD4 downregulation. Thus, highlighting the necessity of HIV-1 to downregulate CD4 from the surfaces of infected cells to escape from ADCC.
FIG 6.
Primary CD4+ T cells infected with viruses coding Nef proteins from elite controllers are more susceptible to ADCC-mediated killing. Primary CD4+ T cells from healthy donors were infected with a panel of pNL4.3-based viruses: wild-type virus (wt, coding for NefSF2), virus lacking Nef (N−), or virus encoding Nef from 15 randomly selected clones from ECs or CPs and used 48 h after infection as target cells in a FACS-based ADCC assay (23) to determine their susceptibility to ADCC, by autologous PBMCs. ADCC mediated by A32 (A and C) or HIV+ sera from two to four different donors (shown in different colors) (B and D) is shown. In the upper panels, results obtained with wild-type versus Nef(−)-infected cells are shown. In the lower panels, the results from cells infected with viruses coding for Nef from ECs (in red) or CPs (blue) are shown. The data shown are representative of at least four independent experiments, with means ± the SEM. Statistical significance was tested using an unpaired t test (**, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
FIG 7.
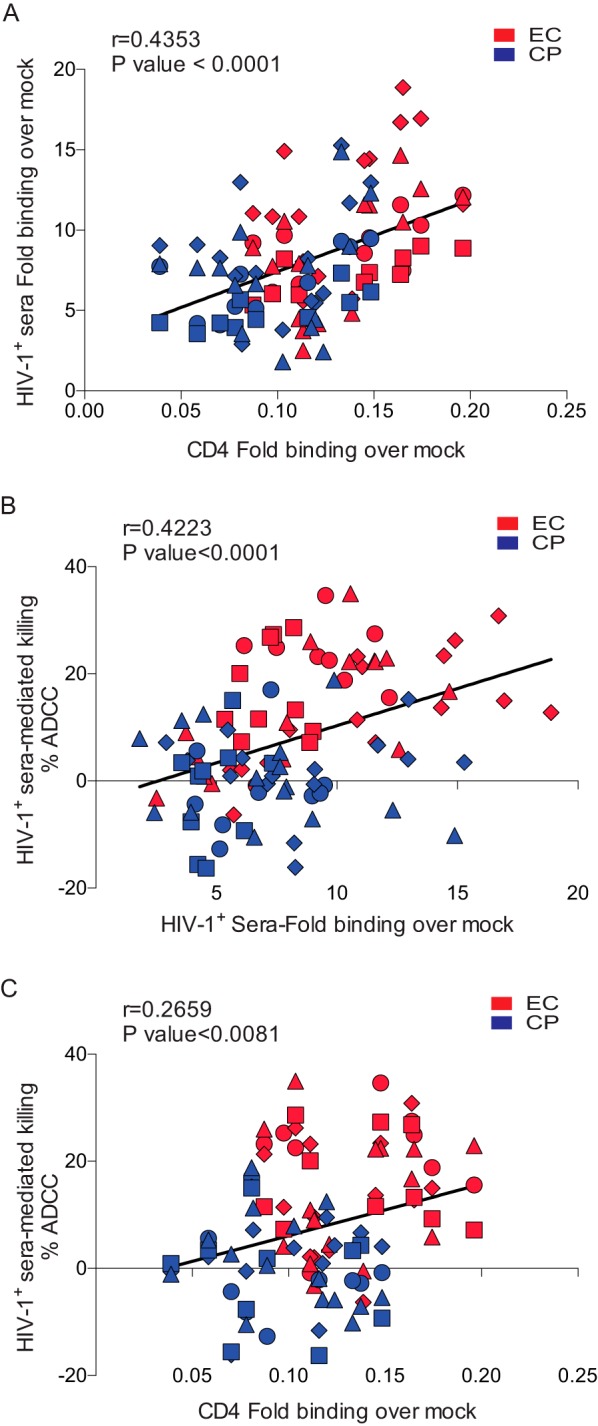
The presence of CD4 at the surfaces of HIV-1-infected cells enhances the susceptibility of infected cells to ADCC mediated by HIV+ sera. (A) CD4 and HIV+ sera staining of primary CD4 T cells infected with viruses coding for Nef from 15 randomly selected clones from ECs (red) or CPs (blue) exhibited a positive correlation. (B) Sera from two to four HIV-1-infected individuals (represented by different shapes) were evaluated for their ability to recognize infected cells and correlated with ADCC killing. (C) The cell surface levels of CD4 correlated with ADCC responses mediated by HIV+ sera. Statistical analysis was tested utilizing a Spearman rank correlation.
DISCUSSION
Previous studies have shown that HIV-1 has evolved several mechanisms to prevent exposing the CD4-bound conformation of Env at the cell surface (23, 24). Accordingly, by forcing Env to sample this conformation using a CD4 mimetic, we found that it was sufficient to sensitize infected cells to ADCC-mediated killing (48). The HIV-1 accessory protein Nef protects HIV-1-infected cells from ADCC by decreasing cell surface levels of CD4 (23, 24), which otherwise engages with Env, induces the CD4-bound conformation and exposes CD4i epitopes. These epitopes are recognized by well-established CD4i ADCC-mediating Abs such as A32 (23) or by sera from HIV-1-infected individuals (24, 25, 51). Importantly, in the present study, Env conformational changes observed at the surfaces of HIV-1-infected cells depended on Nef proteins since EC and CP Nefs were cloned into isogenic proviruses coding for the same Vpu and Env proteins. Interestingly, a role for ADCC in immune control of HIV-1 in EC was previously suggested based on the presence of high levels of ADCC-mediating Abs in these individuals (27). Moreover, it was recently reported that several Nef functions, including CD4 downregulation, are attenuated in this subset of HIV-1-infected individuals (10, 11), but whether this affected ADCC responses was unknown. Here, we evaluated whether the inability of EC Nef clones to fully downregulate CD4 affected the conformation of Env at the cell surface, thereby enhancing susceptibility of HIV-1-infected cells to ADCC.
We observed that Env levels at the surfaces of cells expressing Nef clones from EC were similar to those of cells expressing CP Nefs. However, in the presence of EC Nef clones, Env sampled the CD4-bound conformation more readily and exposed ADCC-mediating epitopes such as those recognized by the anti-cluster A class of Abs or HIV+ sera from several HIV-1-infected individuals. Enhanced recognition by these ligands was correlated with the amount of CD4 molecules present at the cell surface and resulted in increased ADCC-mediated killing. These results suggest a model (Fig. 8) where a limited amount of CD4 still present at the surface of HIV-1-infected cells in EC is sufficient to induce the CD4-bound conformation of Env, thereby exposing epitopes that are readily recognized by ADCC-mediating Abs, which are also prevalent in sera from these individuals. Upon recognition of Env, these Abs recruit effector cells such as NK cells to eliminate the infected cells through ADCC. These observations may also help to explain the functional and immunological pressure on HIV-1 Nef to maintain its ability to downregulate CD4.
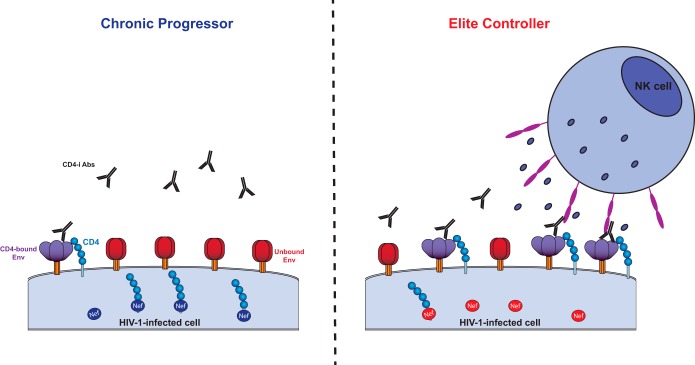
FIG 8.
Nef-mediated CD4 downregulation affects the susceptibility of infected cells to ADCC. Nef proteins from CPs (left panel, shown in dark blue) downregulate CD4 molecules (shown in light blue) more efficiently than Nef proteins from ECs (right panel, shown in light red). Efficient Nef-mediated CD4 downregulation in CPs allows Env to stay in its unbound conformation (shown in red), thus hiding ADCC-mediating epitopes. In ECs, limited amounts of CD4 remain at the cell surface due to attenuated Nef-mediated CD4 downregulation, forcing Env to sample the CD4-bound conformation (shown in purple), thereby exposing epitopes recognized by CD4-induced ADCC-mediating Abs (shown in black), which are highly prevalent in ECs (27). Upon Env recognition, the Fc portion of these antibodies recruits effector cells, such as NK cells, through their Fcγ receptors (shown in pink). Activation of effector cells results in degranulation and secretion of perforin and granzymes, which ultimately leads to cell death.
Revealing mechanisms associated with spontaneous control of HIV might provide important insight with regard to HIV pathogenesis and critical information to develop strategies aimed at eliciting a functional cure in noncontroller subjects. For example, highly effective human lymphocyte antigen (HLA) class I-restricted T cell responses targeting conserved viral peptide epitopes is a major factor modulating the durable control of HIV infection in EC (33). In addition, noncanonical HLA-associated escape mutations in EC have been shown to affect the proper function of several viral proteins, including Nef (10). Indeed, multiple Nef activities, including HLA class I and CD4 downregulation, enhancement of viral infectivity, and replication, were reported to be significantly attenuated in EC Nef clones compared to those from CP (10, 49, 50). An extensive analysis of Nef sequences from ECs and CPs used in the present study was previously conducted and found no common residue signatures involved in decreased CD4 downregulation (10). However, inverse associations between the number of EC-specific B*57-associated polymorphisms and Nef-mediated replication, HLA-I downregulation, and CD74 upregulation were observed (10). CD4 downregulation also displayed a modest, albeit not significant, negative relationship between the burden of B*57-associated escape mutations and function (10). Here, we report that impaired CD4 downregulation by EC Nef clones results in a functional consequence, namely, that infected cells become more susceptible to ADCC.
In conclusion, we observed an incomplete downregulation of CD4 molecules by Nef clones isolated from EC. Residual levels of CD4 at the surface of infected cells were sufficient to allow Env-CD4 interaction, leading to increased exposure of Env CD4i epitopes and increased susceptibility of infected cells to ADCC. Our results suggest that ADCC might be an active immune mechanism in EC that helps to maintain durable suppression of viral replication and low plasma viremia level in this rare subset of infected individuals. Therefore, targeting Nef's ability to downregulate CD4 could render HIV-1-infected cells susceptible to ADCC and thus might have therapeutic utility in strategies aiming to elicit a functional cure in HIV-1-infected individuals.
ACKNOWLEDGMENTS
We thank the CRCHUM Flow Cytometry Platform for technical assistance, as well as Mario Legault for cohort coordination. We thank Maxime Veillette, Mathieu Coutu, and Jean-Philippe Chapleau for helpful discussions; Toshiyuki Miura for isolation of EC virus specimens; and all subjects for their participation.
This study was possible thanks to the International HIV Controllers study, funded by the Bill and Melinda Gates Foundation, the Schwartz Foundation, and the Harvard University Center for AIDS Research. A.F. is the recipient of a Canada Research Chair on Retroviral Entry. M.A.B. holds a Canada Research Chair on Viral Pathogenesis and Immunity. N.A. is the recipient of a King Abdullah scholarship for higher education from the Saudi Government. J.R. is the recipient of CIHR Fellowship Award 135349. D.E.K. is supported by a Research Scholar Career Award of the Quebec Health Research Fund (FRQS). This work was supported by a Canada Foundation for Innovation Program Leader grant, by the FRQS AIDS and Infectious Diseases Network, and by FRQS Establishment of Young Scientist grant 26702 to A.F.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We have no conflicts of interest to report.
REFERENCES
- 1.Kestler HW III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651–662. doi: 10.1016/0092-8674(91)90097-I. [DOI] [PubMed] [Google Scholar]
- 2.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, Lawson VA, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan JS, Cunningham A, Dwyer D, Dowton D, Mills J. 1995. Genomic structure of an attenuated quasi-species of HIV-1 from a blood transfusion donor and recipients. Science 270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med 332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 4.Zaunders JJ, Geczy AF, Dyer WB, McIntyre LB, Cooley MA, Ashton LJ, Raynes-Greenow CH, Learmont J, Cooper DA, Sullivan JS. 1999. Effect of long-term infection with nef-defective attenuated HIV type 1 on CD4+ and CD8+ T lymphocytes: increased CD45RO+ CD4+ T lymphocytes and limited activation of CD8+ T lymphocytes. AIDS Res Hum Retroviruses 15:1519–1527. doi: 10.1089/088922299309801. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JV, Miller AD. 1991. Serine phosphorylation-independent downregulation of cell surface CD4 by nef. Nature 350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz O, Dautry-Varsat A, Goud B, Marechal V, Subtil A, Heard JM, Danos O. 1995. Human immunodeficiency virus type 1 Nef induces accumulation of CD4 in early endosomes. J Virol 69:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 8.Munch J, Rajan D, Schindler M, Specht A, Rucker E, Novembre FJ, Nerrienet E, Muller-Trutwin MC, Peeters M, Hahn BH, Kirchhoff F. 2007. Nef-mediated enhancement of virion infectivity and stimulation of viral replication are fundamental properties of primate lentiviruses. J Virol 81:13852–13864. doi: 10.1128/JVI.00904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MD, Warmerdam MT, Gaston I, Greene WC, Feinberg MB. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med 179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwimanzi P, Markle TJ, Martin E, Ogata Y, Kuang XT, Tokunaga M, Mahiti M, Pereyra F, Miura T, Walker BD, Brumme ZL, Brockman MA, Ueno T. 2013. Attenuation of multiple Nef functions in HIV-1 elite controllers. Retrovirology 10:1. doi: 10.1186/1742-4690-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyoda M, Ogata Y, Mahiti M, Maeda Y, Kuang XT, Miura T, Jessen H, Walker BD, Brockman MA, Brumme ZL, Ueno T. 2015. Differential ability of primary HIV-1 Nef isolates to downregulate HIV-1 entry receptors. J Virol 89:9639–9652. doi: 10.1128/JVI.01548-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M Jr, Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, Johnson RP, Evans DT. 2012. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog 8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks ND, Kinsey N, Clements J, Hildreth JE. 2002. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses 18:1197–1205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 14.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol 157:2168–2173. [PubMed] [Google Scholar]
- 15.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. 2011. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A 108:7505–7510. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, Kaplan J. 1999. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis 180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 17.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog 8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Asmal M, Lane S, Permar SR, Schmidt SD, Mascola JR, Letvin NL. 2011. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J Virol 85:6906–6912. doi: 10.1128/JVI.00326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, Devico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richard J, Veillette M, Batraville LA, Coutu M, Chapleau JP, Bonsignori M, Bernard N, Tremblay C, Roger M, Kaufmann DE, Finzi A. 2014. Flow cytometry-based assay to study HIV-1 gp120 specific antibody-dependent cellular cytotoxicity responses. J Virol Methods 208:107–114. doi: 10.1016/j.jviromet.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Veillette M, Coutu M, Richard J, Batraville LA, Desormeaux A, Roger M, Finzi A. 2014. Conformational evaluation of HIV-1 trimeric envelope glycoproteins using a cell-based ELISA assay. J Vis Exp 2014:51995. doi: 10.3791/51995:51995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veillette M, Coutu M, Richard J, Batraville LA, Dagher O, Bernard N, Tremblay C, Kaufmann DE, Roger M, Finzi A. 2015. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 89:545–551. doi: 10.1128/JVI.02868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy JF. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambotte O, Pollara J, Boufassa F, Moog C, Venet A, Haynes BF, Delfraissy JF, Saez-Cirion A, Ferrari G. 2013. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS One 8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Bredow B, Arias JF, Heyer LN, Gardner MR, Farzan M, Rakasz EG, Evans DT. 2015. Envelope glycoprotein internalization protects human and simian immunodeficiency virus infected cells from antibody-dependent cell-mediated cytotoxicity. J Virol 89:10648–10655. doi: 10.1128/JVI.01911-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, Rakasz EG, Evans DT. 2014. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 111:6425–6430. doi: 10.1073/pnas.1321507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, Fierer DS, Simon V, Chen BK. 2014. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol 88:6031–6046. doi: 10.1128/JVI.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoxie JA, Alpers JD, Rackowski JL, Huebner K, Haggarty BS, Cedarbaum AJ, Reed JC. 1986. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science 234:1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- 32.Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. 2010. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood 115:1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, Kadie CM, Carlson JM, Heckerman D, Graham RR, Plenge RM, Deeks SG, Gianniny L, Crawford G, Sullivan J, Gonzalez E, Davies L, Camargo A, Moore JM, Beattie N, Gupta S, Crenshaw A, Burtt NP, Guiducci C, Gupta N, Gao X, Qi Y, Yuki Y, Piechocka-Trocha A, Cutrell E, Rosenberg R, Moss KL, Lemay P, O'Leary J, Schaefer T, Verma P, Toth I, Block B, Baker B, Rothchild A, Lian J, Proudfoot J, Alvino DM, Vine S, Addo MM, Allen TM et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557 doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brumme ZL, Li C, Miura T, Sela J, Rosato PC, Brumme CJ, Markle TJ, Martin E, Block BL, Trocha A, Kadie CM, Allen TM, Pereyra F, Heckerman D, Walker BD, Brockman MA. 2011. Reduced replication capacity of NL4-3 recombinant viruses encoding reverse transcriptase-integrase sequences from HIV-1 elite controllers. J Acquir Immune Defic Syndr 56:100–108. doi: 10.1097/QAI.0b013e3181fe9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miura T, Brockman MA, Brumme ZL, Brumme CJ, Pereyra F, Trocha A, Block BL, Schneidewind A, Allen TM, Heckerman D, Walker BD. 2009. HLA-associated alterations in replication capacity of chimeric NL4-3 viruses carrying gag-protease from elite controllers of human immunodeficiency virus type 1. J Virol 83:140–149. doi: 10.1128/JVI.01471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, Baker B, Trocha A, Rosenberg R, Mackey E, Ueda P, Lu Z, Cohen D, Wrin T, Petropoulos CJ, Rosenberg ES, Walker BD. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 37.Miura T, Brockman MA, Brumme CJ, Brumme ZL, Carlson JM, Pereyra F, Trocha A, Addo MM, Block BL, Rothchild AC, Baker BM, Flynn T, Schneidewind A, Li B, Wang YE, Heckerman D, Allen TM, Walker BD. 2008. Genetic characterization of human immunodeficiency virus type 1 in elite controllers: lack of gross genetic defects or common amino acid changes. J Virol 82:8422–8430. doi: 10.1128/JVI.00535-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno T, Motozono C, Dohki S, Mwimanzi P, Rauch S, Fackler OT, Oka S, Takiguchi M. 2008. CTL-mediated selective pressure influences dynamic evolution and pathogenic functions of HIV-1 Nef. J Immunol 180:1107–1116. doi: 10.4049/jimmunol.180.2.1107. [DOI] [PubMed] [Google Scholar]
- 39.Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, Lovo E, Wu X, Robinson JE, Seaman MS, Fouts TR, Gallo RC, DeVico AL, Lewis GK. 2013. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci U S A 110:E69–E78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson JE YH, Holton D, Elliott S, Ho DD. 1992. Distinct antigenic sites on HIV gp120 identified by a panel of human monoclonal antibodies. J Cell Biochem 16E(Suppl):Q449. [Google Scholar]
- 41.Zhang W, Godillot AP, Wyatt R, Sodroski J, Chaiken I. 2001. Antibody 17b binding at the coreceptor site weakens the kinetics of the interaction of envelope glycoprotein gp120 with CD4. Biochemistry 40:1662–1670. doi: 10.1021/bi001397m. [DOI] [PubMed] [Google Scholar]
- 42.Fontaine J, Chagnon-Choquet J, Valcke HS, Poudrier J, Roger M, Montreal Primary HIVI, Long-Term Non-Progressor Study G. 2011. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood 117:145–155. doi: 10.1182/blood-2010-08-301887. [DOI] [PubMed] [Google Scholar]
- 43.Fontaine J, Coutlee F, Tremblay C, Routy JP, Poudrier J, Roger M, Montreal Primary HIVI, Long-Term Nonprogressor Study G. 2009. HIV infection affects blood myeloid dendritic cells after successful therapy and despite nonprogressing clinical disease. J Infect Dis 199:1007–1018. doi: 10.1086/597278. [DOI] [PubMed] [Google Scholar]
- 44.Peretz Y, Ndongala ML, Boulet S, Boulassel MR, Rouleau D, Cote P, Longpre D, Routy JP, Falutz J, Tremblay C, Tsoukas CM, Sekaly RP, Bernard NF. 2007. Functional T cell subsets contribute differentially to HIV peptide-specific responses within infected individuals: correlation of these functional T cell subsets with markers of disease progression. Clin Immunol 124:57–68. doi: 10.1016/j.clim.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Kamya P, Boulet S, Tsoukas CM, Routy JP, Thomas R, Cote P, Boulassel MR, Baril JG, Kovacs C, Migueles SA, Connors M, Suscovich TJ, Brander C, Tremblay CL, Bernard N, Canadian Cohort of HIVISP . 2011. Receptor-ligand requirements for increased NK cell polyfunctional potential in slow progressors infected with HIV-1 coexpressing KIR3DL1*h/*y and HLA-B*57. J Virol 85:5949–5960. doi: 10.1128/JVI.02652-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veillette M, Coutu M, Richard J, Batraville L-A, Désormeaux A, Roger M, Finzi A. 2014. Conformational evaluation of HIV-1 trimeric envelope glycoproteins using a cell-based ELISA assay. J Vis Exp 2014:51995. doi: 10.3791/51995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desormeaux A, Coutu M, Medjahed H, Pacheco B, Herschhorn A, Gu C, Xiang SH, Mao Y, Sodroski J, Finzi A. 2013. The highly conserved layer-3 component of the HIV-1 gp120 inner domain is critical for CD4-required conformational transitions. J Virol 87:2549–2562. doi: 10.1128/JVI.03104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, Pazgier M, Schon A, Freire E, Routy JP, Smith AB 3rd, Park J, Jones DM, Courter JR, Melillo BN, Kaufmann DE, Hahn BH, Permar SR, Haynes BF, Madani N, Sodroski JG, Finzi A. 2015. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 112:E2687–E2694. doi: 10.1073/pnas.1506755112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mwimanzi P, Markle TJ, Ueno T, Brockman MA. 2012. Human leukocyte antigen (HLA) class I down-regulation by human immunodeficiency virus type 1 negative factor (HIV-1 Nef): what might we learn from natural sequence variants? Viruses 4:1711–1730. doi: 10.3390/v4091711 (Erratum, 4: 2014–2015. doi:.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mwimanzi P, Markle TJ, Ogata Y, Martin E, Tokunaga M, Mahiti M, Kuang XT, Walker BD, Brockman MA, Brumme ZL, Ueno T. 2013. Dynamic range of Nef functions in chronic HIV-1 infection. Virology 439:74–80. doi: 10.1016/j.virol.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Ding S, Veillette M, Coutu M, Prevost J, Scharf L, Bjorkman PJ, Ferrari G, Robinson JE, Sturzel C, Hahn BH, Sauter D, Kirchhoff F, Lewis GK, Pazgier M, Finzi A. 2015. A highly-conserved residue of the HIV-1-gp120 inner domain is important for ADCC responses mediated by anti-cluster A antibodies. J Virol doi: 10.1128/JVI.02779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]