ABSTRACT
Human noroviruses are a leading cause of gastroenteritis across the globe, but the pathogenic mechanisms responsible for disease are not well established. The availability of a murine norovirus model system provides the opportunity to elucidate viral and host determinants of virulence in a natural host. For example, previous studies have revealed that the protruding domain of the murine norovirus capsid protein VP1, specifically residue 296 of VP1, regulates virulent infection. We identified a panel of nonsynonymous mutations in the open reading frame 2 (ORF2) gene encoding VP1 that arose in persistently infected mice and tested whether these mutations conferred phenotypic changes to viral replication and virulence. Consistent with previous studies, we demonstrate that a glutamic acid at position 296 results in attenuation. For the first time, we also demonstrate that a lysine at this position is sufficient to confer virulence on an otherwise attenuated murine norovirus strain. Moreover, our studies reveal a direct correlation between the efficiency of viral replication in B cells and virulence. These data are especially striking because mutations causing reduced B cell replication and attenuation had minimal effects on the ability of the virus to replicate in macrophages. Thus, norovirus infection of B cells may directly contribute to disease outcome.
IMPORTANCE Human noroviruses are a major global cause of disease, yet we know very little about their pathogenic mechanisms. The availability of a murine norovirus model system facilitates investigation of noroviruses in a natural host organism and the identification of viral and host determinants of pathogenesis. We have identified a panel of mutations arising in the viral capsid protein VP1 during persistent infection of mice. Our data reveal that the protruding domain of VP1 regulates the ability of the virus to replicate in B cells, and this directly correlates with virulence. Importantly, mutations impairing B cell infection had minimal effects on macrophage infection, revealing a potentially critical role for B cell infection in norovirus pathogenesis.
INTRODUCTION
Human noroviruses (HuNoVs) are the major cause of nonbacterial gastroenteritis worldwide, causing rapid bouts of nausea, severe vomiting, and watery diarrhea in all age groups. They are the leading cause of foodborne disease globally and of severe childhood diarrhea in parts of the world where rotavirus vaccination is being implemented (1–3). Although HuNoV infection is typically self-limited in immunocompetent adults, more severe consequences can occur in risk groups, including the elderly, immunocompromised and transplant patients, and infants (4–6). Considering the huge disease burden attributable to HuNoV infections (7) and the tremendous economic loss associated with these infections (8), development of effective therapeutics and vaccines is highly warranted. However, HuNoV studies are greatly hampered by the lack of robust culturing systems and small-animal models. Although there have been exciting advances in both of these areas in recent years (9, 10), the ability to study a virus in its natural host organism can provide unique insight into host-pathogen interactions.
Murine NoVs (MuNoVs) are well-established HuNoV surrogates that replicate efficiently in cultured cells and in their natural rodent host (11): MuNoVs infect macrophages, dendritic cells, and B cells in vitro, while they do not infect cultured epithelial cells (9, 12). Available evidence supports this cell tropism during in vivo infections as well (9, 12–14). HuNoVs also infect cultured B cells (9, 15), and available data suggest that they share cell tropism properties with MuNoV in vivo as well (recently reviewed in reference 16). While MuNoV infection of immunocompetent mice fails to cause overt disease, MuNoVs do replicate along the intestinal tract and are spread via fecal-oral transmission, indicating that they share general pathogenic features with HuNoVs. Moreover, infection of mice lacking intact type I interferon (IFN) signaling pathways leads to severe gastroenteritis, systemic disease, and even mortality (13, 17), providing a widely used model of virulence. This combination of robust culture systems and the ability to study in vivo infections in a natural host and a genetically tractable system makes the MuNoV model system an excellent tool for investigating NoV replication and pathogenesis.
NoVs are nonenveloped, positive-sense RNA viruses with three or four open reading frames (ORFs) (18). ORF1 encodes a polyprotein that is cleaved into six nonstructural proteins by the viral protease. ORF2 encodes the major capsid protein, referred to as VP1, which contains a well-conserved shell (S) domain and a protruding (P) domain that is further divided into a variable P1 stalk domain and a hypervariable surface-exposed P2 domain (Fig. 1). ORF3 encodes a minor structural protein referred to as VP2. Finally, ORF4 overlaps ORF2 in MuNoV genomes and encodes a newly described protein, referred to as virulence factor 1 (VF1), that regulates MuNoV pathogenesis and antagonizes type I IFN expression in vitro (19). The power of the MuNoV model system lies in its utility to identify viral and host determinants of virulence and immunity. For example, the P domain of the VP1 protein, specifically residue 296 within the P2 domain, has been demonstrated to regulate MuNoV virulence: a lysine at position 296 (observed naturally in the MNV-1 strain) is associated with virulence, whereas a glutamic acid results in attenuation (20, 21). The K296E mutation occurs frequently during tissue culture passaging of MNV-1, and most natural MuNoV isolates have a glutamic acid at this location (12, 20).
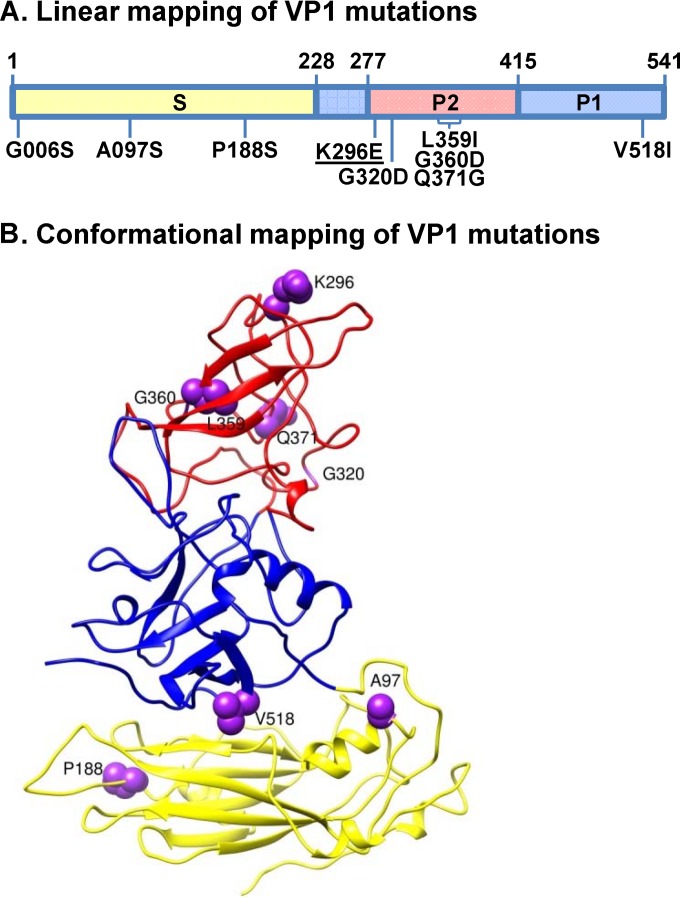
FIG 1.
VP1 mutations arising during persistent MNV-1 infection. (A) Locations of mutations along the linear VP1 protein. The shell domain (S; amino acids 1 to 218) is shaded yellow, the protruding 1 domain (P1; amino acids 229 to 277 and 416 to 541) is shaded blue, and the P2 domain (P2; amino acids 278 to 415) is shaded red. Amino acid substitutions investigated in this study are labeled based on their position in VP1 protein. The lysine-to-glutamic acid mutation at residue 296 (K296E; underlined) has been previously reported to be attenuating (20, 21). All other mutations are novel. (B) Conformational mapping of mutations on the MNV-1 VP1 protein. The predicted secondary structure of the MNV-1 VP1 protein is shown, with amino acids investigated in this study highlighted by purple balls; the G006 residue was not included in the model, so it is not indicated on this structure.
In a previous study, we sequenced the MNV-1 ORF2 gene encoding VP1 from the viral quasispecies present in the feces of healthy and malnourished mice at 50 days postinfection (dpi). These studies revealed greater viral diversity in the malnourished animals than in healthy controls, with an increased number of coding changes particularly in the surface-exposed P domain of the virus particle (22). Interestingly, the K296E mutation in VP1 became dominant in every infected mouse irrespective of malnutrition status. We became interested in determining the phenotypic consequences of other mutations arising in VP1 during in vivo viral replication, hypothesizing that certain mutations would provide the virus with a fitness advantage reflected in viral replication kinetics, virulence, or antigenic escape. We instead report that a panel of amino acid substitutions in the P domain of VP1 (n = 5) resulted in impaired replication in cultured B cells and attenuation in vivo, although the mutant viruses replicated comparably to parental virus in macrophages. Conversely, a panel of amino acid substitutions in the VP1 S domain (n = 3) had minimal effects on in vitro replication kinetics in either cell type and no effect on virulence. Together with previous results of the K296E mutation, these data confirm that P domain residues regulate MuNoV virulence. They also reveal that B cell infection efficiency directly correlates with virulence.
MATERIALS AND METHODS
Construction of MuNoV mutant viruses.
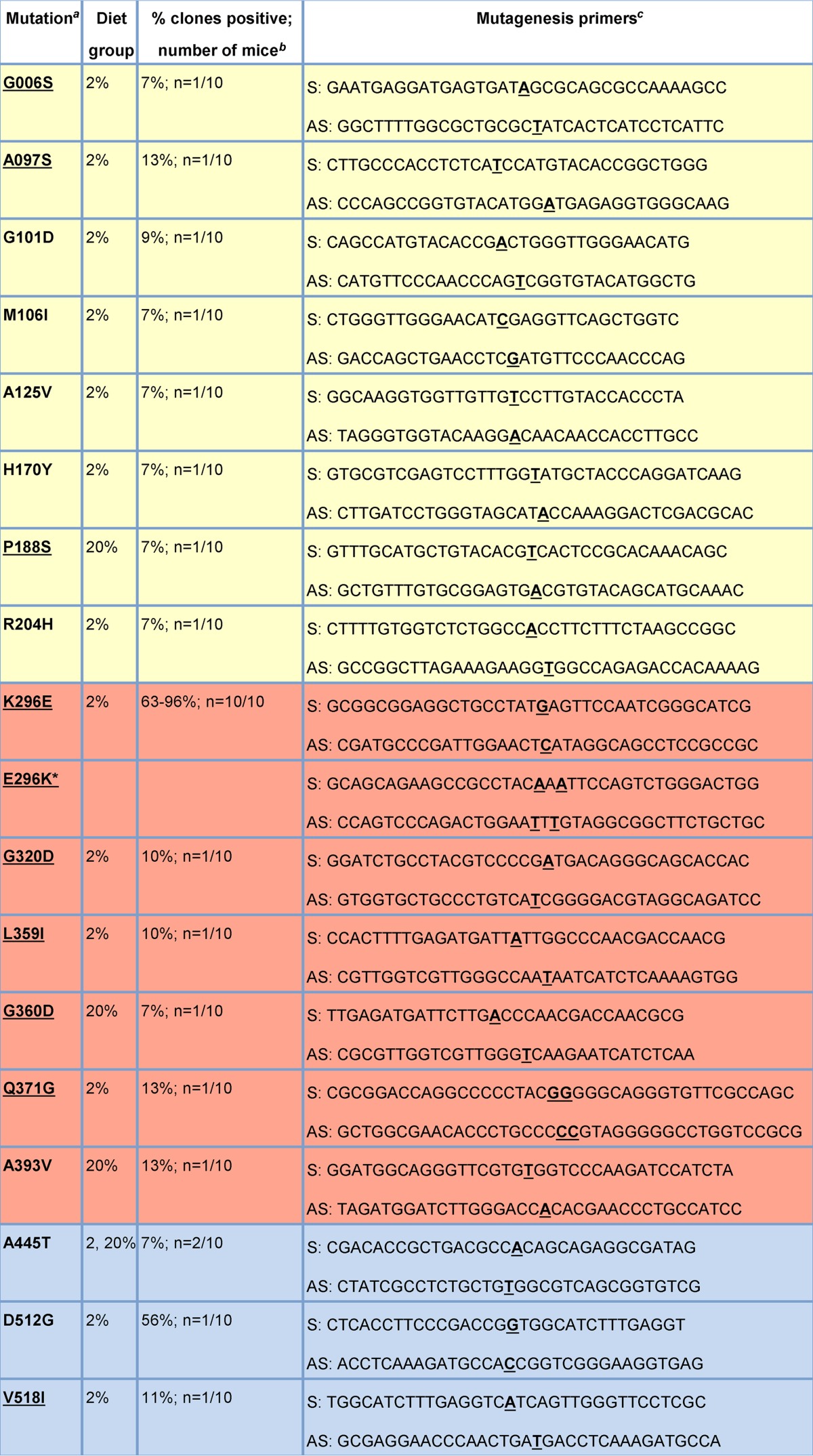
The full-length plasmid pSPMNV-1.CW3 containing MuNoV strain MNV-1.CW3 (referred to as MNV-1; GenBank accession no. KC782764) was used for constructing the recombinant MNV-1 clones (23). Specific mutations identified in our previous study (22) were introduced into the MNV-1 genome with mutagenic primers (Table 1) using a QuikChange Lightning site-directed mutagenesis kit (Agilent; catalog number 210519) according to the manufacturer's protocol. The K296E mutation became dominant in the quasispecies of every persistently infected mouse (22). To test the effect of this change on a virus isolate naturally containing a glutamic acid at this position, plasmid pSPMNV-3 carrying MuNoV strain MNV-3 (GenBank accession no. KC792553) was used to construct the MNV-3.E296K recombinant virus (23). Stocks of recombinant viruses were generated as previously described (23). Briefly, 293T cells (kindly provided by Fanxiu Zhu, Florida State University) were transfected with recombinant MuNoV clones. Culture lysates were harvested at 24 h posttransfection, freeze-thawed twice, and titrated to determine whether mutations were viable. For all viable viruses (MNV-1, MNV-1.K296E, MNV-1.G006S, MNV-1.A097S, MNV-1.P188S, MNV-1.G320D, MNV-1.L359I, MNV-1.G360D, MNV-1.Q371G, MNV-1.V518I, MNV-3, and MNV-3.E296K), the 293T lysates were inoculated onto RAW264.7 cells at a multiplicity of infection (MOI) of 0.05. At >90% cytopathic effect (CPE), cell lysates were freeze-thawed twice, clarified by centrifugation at 3,000 rpm for 15 min, and purified through a 25% sucrose cushion at 25,000 rpm for 5 h. All viable mutations were mapped onto the VP1 protein crystal structure of the human norovirus Norwalk strain (PBD 1IHM) using the UCSF Chimera program (24). All recombinant virus stocks were fully sequenced to confirm that there were no deviations from the input cDNA clones and were titrated by a standard 50% tissue culture infective dose (TCID50) assay (25, 26).
TABLE 1.
Mutagenesis primers for generating recombinant MuNoV strains
Ten to 15 clones of the ORF2 gene encoding the MNV-1 VP1 protein were sequenced from the fecal material of individual persistently infected mice fed either a 2% or 20% protein diet (n = 10 mice total), as described by Hickman et al. (22). Seventeen nonsynonymous mutations were detected in total. Rows describing S domain residues are shaded yellow, rows describing P1 domain residues are shaded blue, and rows describing P2 domain residues are shaded red. We introduced each mutation into an MNV-1 infectious clone. The underlined mutations were viable and thus investigated further in this study. The asterisk (*) indicates a mutation that was introduced into the parental MNV-3 infectious clone.
The frequency of clones in individual mice containing the specified mutation compared to inoculum; the number of mice containing this mutation.
Sense (S) and antisense (AS) primers, all listed 5′ to 3′, were used to introduce the mutations indicated by bold and underlining into the parental MNV-1 infectious clone.
Virus growth kinetics.
For virus growth curves, 1.6 × 105 RAW264.7 (ATCC) or M12 (kindly provided by Scott Tibbetts, University of Florida) cells were cultured overnight in 48-well plates and infected at an MOI of either 0.05 (multistep growth curves) or 5 (single-step growth curves). The cells were incubated with virus for 1 h at 4°C, washed, and incubated at 37°C, and supernatants were harvested at the desired times postinfection. The M12 cultures were maintained for 3 days, whereas the RAW264.7 cultures were maintained for 2 days based on the known kinetics of viral replication in these cell types (9). Virus titers were determined using a standard TCID50 assay (26).
Mice, infections, tissue harvest, and titration.
Wild-type C57BL/6J mice (Jackson no. 000664; referred to as B6) and Ifnar1tm1Agt mice (referred to as IFNAR−/−; kindly provided by Herbert W. Virgin at Washington University School of Medicine) were bred and housed in animal facilities at the University of Florida under specific-pathogen-free conditions. All animal experiments were performed in strict accordance with federal and university guidelines. The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Florida (study number 201507538). For all experiments, 6- to 8-week old, sex-matched mice were inoculated perorally (p.o.) with 25 μl of virus inoculum. For virus load detection in specific tissues, mice were euthanized at the desired time points, tissues were dissected, weighed, and homogenized in medium by bead beating using 1.0-mm zirconia/silica beads (BioSpec Products, Inc.), and tissue homogenates were tested for virus titers using a standard virus plaque assay (12, 25).
Neutralization assays.
Virus neutralization assays were performed as previously described (23). In brief, serum collected from B6 mice immunized with 107 TCID50 units of parental MNV-1 or MNV-3 for 6 weeks was incubated at 56°C for 30 min to heat inactivate complement. This serum was serially diluted, and each dilution was incubated with 3 × 104 TCID50 units of a parental or mutant virus at 37°C for 1 h. The infectivity of each sample was then determined by a standard TCID50 assay. The neutralization titer was determined for each virus using the Reed-Muench method.
Statistical analysis.
All data analyses were performed using GraphPad Prism software. In all graphs, standard errors of means were used to define error bars. P values were determined for virus growth curves using paired two-tailed t tests, for virus loads in mouse tissues using unpaired two-tailed t tests, and for survival curves using log rank (Mantel-Cox) tests. In the figures, one asterisk represents P values of 0.01 to 0.05, two asterisks represent P values of 0.001 to 0.01, and three asterisks represent P values of less than 0.001.
RESULTS
Residue 296 of the VP1 P2 domain is necessary and sufficient for MuNoV virulence.
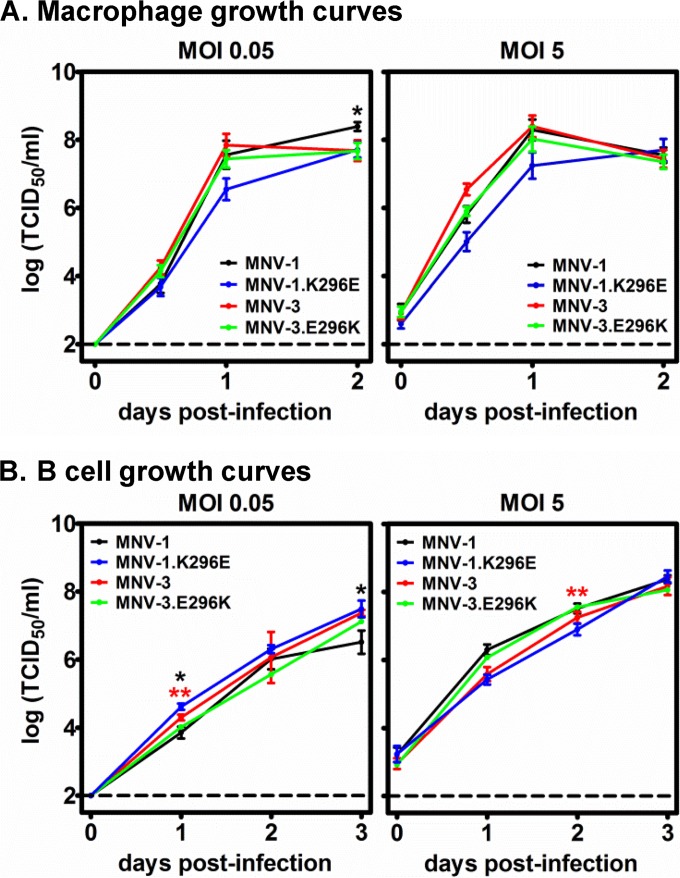
We previously reported that MNV-1 evolves more readily in malnourished mice than in healthy controls, as indicated by an increased divergence of the ORF2 sequence, and an increased number of nonsynonymous VP1 substitutions, at 50 dpi compared to the inoculating viral swarm (22). In the present study, we were interested in determining whether the specific VP1 amino acid changes arising in persistently infected mice had phenotypic consequences. We first focused on the K296E mutation (Fig. 1), which has been previously reported to be attenuating when introduced into the MNV-1 strain (20, 21). Consistent with previous studies, this mutation in the MNV-1 backbone had only a modest effect on viral replication in the murine macrophage cell line RAW264.7 (Fig. 2A). While the K296E mutation has been described to be attenuating in the MNV-1 strain, most natural MuNoV isolates (e.g., MNV.CR6 and MNV-3) have a glutamic acid at this position and are attenuated relative to MNV-1 (20, 21, 25). Thus, it is of interest whether a lysine at this position is sufficient to confer virulence on an otherwise attenuated strain. Although efforts to engineer the E296K mutation into the MNV.CR6 strain were unsuccessful (21), we were able to engineer this change into the MNV-3 backbone. The chimeric MNV-3.E296K virus replicated comparably to parental MNV-3 virus in RAW264.7 cells (Fig. 2A). We recently identified B cells as a target of MuNoV and HuNoV infection (9), so we also tested whether residue 296 regulated viral infection of this cell type. As observed in RAW264.7 cells, all four viruses replicated with similar kinetics in the murine B cell line M12 (Fig. 2B).
FIG 2.
VP1 P2 domain residue 296 does not affect viral replication in cultured cells. RAW264.7 macrophages (A) or M12 B cells (B) were infected with MNV-1, MNV-1.K296E, MNV-3, or MNV-3.E296K at an MOI of 0.05 or 5. Supernatants were collected from duplicate wells per condition at the indicated days postinfection, and virus titers were determined by TCID50 assay. The entire experiment was repeated three times, and data from all experiments were averaged. The limit of detection is indicated by the dashed line. Titers for each parental virus were compared to those of their respective residue 296 mutant viruses at each time point for statistical purposes.
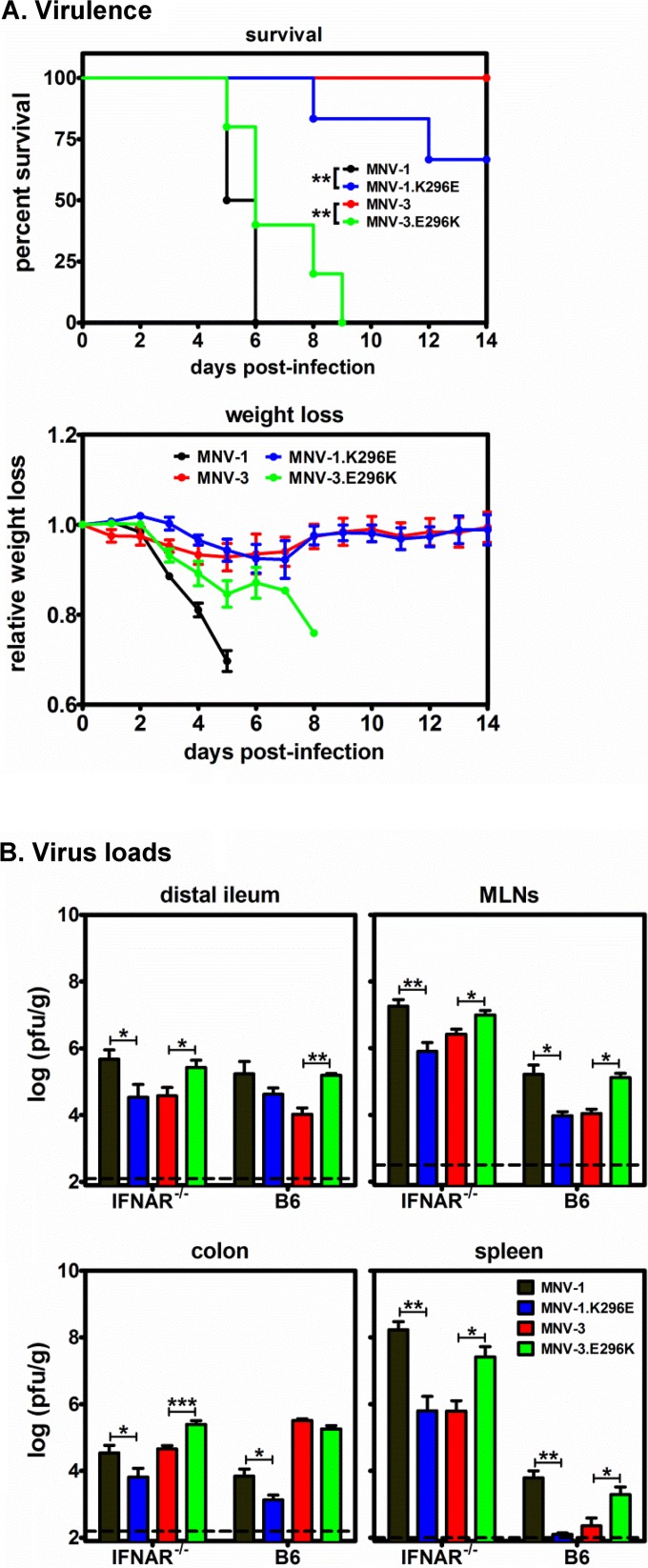
We next tested whether E296K was sufficient for virulence by infecting IFNAR−/− mice p.o. with 5 × 105 TCID50 units of MNV-1, MNV-1.K296E, MNV-3, or MNV-3.E296K. As expected (20, 21), MNV-1.K296E was less virulent than parental MNV-1 as measured by survival kinetics and weight loss (Fig. 3A, compare black and blue lines). Conversely, MNV-3.E296K was more virulent than its parental MNV-3 counterpart (Fig. 3A, compare red and green lines), indicating that, indeed, this single amino acid in the VP1 protein is sufficient to confer a degree of virulence in a type I IFN-deficient background. To determine whether the differences in virulence between parental and mutant viruses correlated with altered viral replication efficiency in vivo, IFNAR−/− mice were inoculated with 5 × 105 TCID50 units of MNV-1, MNV-1.K296E, MNV-3, or MNV-3.E296K, and viral loads were determined in the distal ileum, mesenteric lymph nodes (MLNs), colon, and spleen at 3 dpi. Consistent with virulence data, MNV-1.K296E titers were significantly lower than parental MNV-1 titers in all tissues, whereas MNV-3.E296K titers were significantly higher than parental MNV-3 titers (Fig. 3B). To test whether VP1 residue 296 altered replicative efficiency in immunocompetent mice, groups of B6 wild-type mice were infected with 107 TCID50 units of MNV-1, MNV-1.K296E, MNV-3, or MNV-3.E296K, and virus loads were determined at 1 dpi, the expected peak of infection (25). As observed in IFNAR−/− mice, MNV-1.K296E titers in the MLNs, colon, and spleen (but not distal ileum) were significantly lower than MNV-1 titers, while MNV-3.E296K titers in the distal ileum, MLNs, and spleen (but not colon) were significantly higher than MNV-3 titers (Fig. 3B). Together, these data provide novel insight into the contribution of VP1 residue 296 to MuNoV pathogenesis by revealing that (i) a lysine at this position is sufficient to confer virulence to an otherwise attenuated strain, and (ii) this residue regulates the efficiency of in vivo viral replication in the presence and absence of functional type I IFN responses.
FIG 3.
VP1 P2 domain residue 296 is necessary and sufficient for MuNoV virulence. (A) Groups of IFNAR−/− mice (n = 2 or 3) were inoculated with 5 × 105 TCID50 units of MNV-1 (black), MNV-1.K296E (blue), MNV-3 (red), or MNV-3.E296K (green) p.o. and observed for survival and weight loss daily for 14 days. The daily weights were compared to day 0 weights to calculate the relative weight loss. The experiment was repeated twice, and data for both experiments are included. (B) Groups of IFNAR−/− mice (n = 3) were infected with 5 × 105 TCID50 units of MNV-1, MNV-1.K296E, MNV-3, or MNV-3.E296K, and the indicated tissues harvested at 3 dpi were subjected to plaque assay to determine the virus loads. Groups of B6 mice (n = 3) were infected with 107 TCID50 units of each virus, and tissue virus titers were determined at 1 dpi. MLNs, mesenteric lymph nodes. The experiment was repeated twice, and all data were averaged. The data are reported as PFU per gram of tissue. The limit of detection is indicated by the dashed line. Titers for each parental virus were compared to those of their respective residue 296 mutant viruses in each mouse strain for statistical purposes.
The P domain of VP1 regulates viral replication efficiency in cultured B cells but not macrophages.
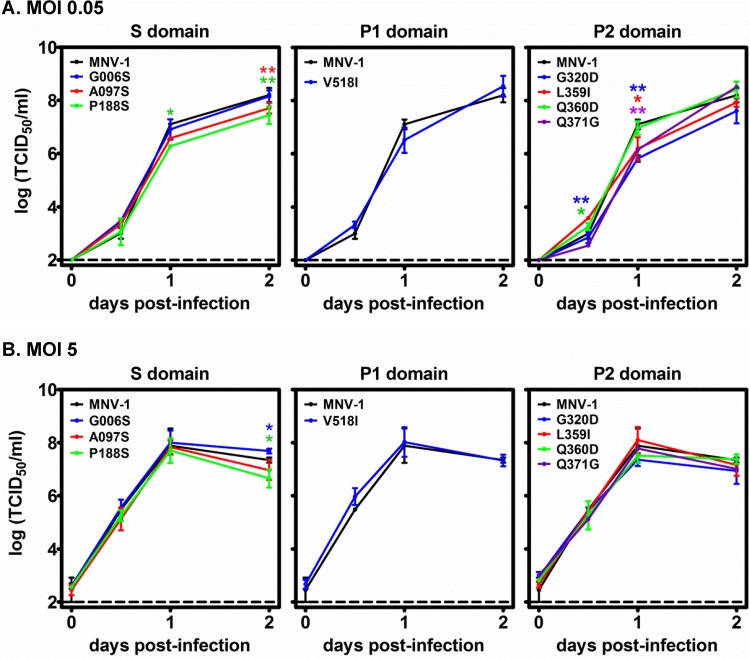
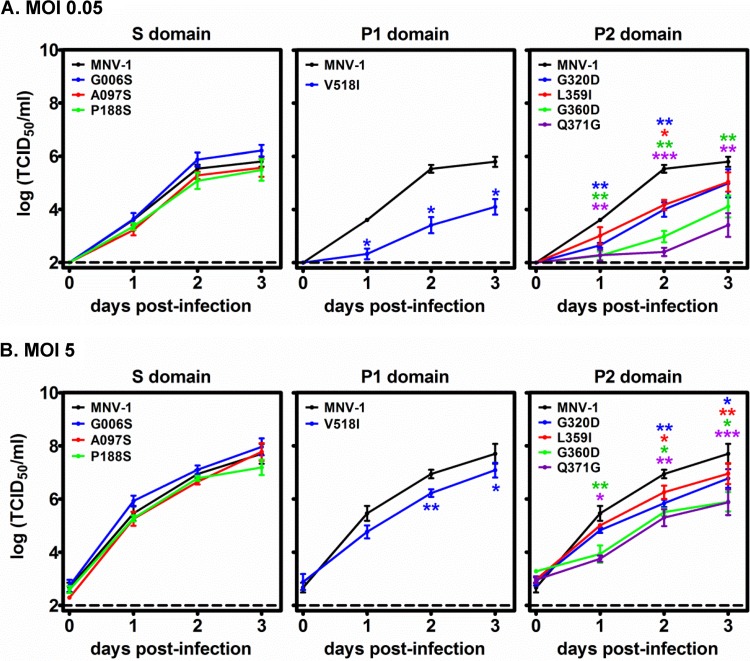
In addition to K296E, we detected 16 other nonsynonymous VP1 mutations in the viral swarm being shed from malnourished mice at 50 dpi (Table 1). Importantly, no mutations were detected in the VF1 coding sequence, which overlaps the VP1 coding sequence. We engineered each of the ORF2 mutations individually into an established MNV-1 infectious clone (23). Eight of the mutations—G006S, A097S, and P188S in the S domain, V518I in the P1 domain, and G320D, L359I, Q360D, and Q371D in the P2 domain—were nonlethal and thus amenable to further analysis. These are indicated on the linear structure of VP1 (Fig. 1A) and a model of the secondary MNV-1 capsid structure, with the exception of G006S because this position was not included in the model (Fig. 1B). We first tested whether each mutation altered in vitro replication kinetics in RAW264.7 or M12 cells. All eight mutant viruses replicated efficiently in RAW264.7 macrophages in both multistep (Fig. 4A) and single-step (Fig. 4B) growth curves, although modest differences were noted at certain time points. The three viruses with mutations in the VP1 S domain also replicated with kinetics similar to those of parental MNV-1 in M12 B cells (Fig. 5). In contrast, the V518I, G320D, L359I, G360D, and Q371G P domain mutations each resulted in decreased viral replication in M12 B cells in multistep (Fig. 5A) and single-step (Fig. 5B) growth curves. The P domain phenotype in M12 cells was especially notable in multistep growth curves, where G320D- and L359I-containing viruses each had ∼1.5-log reductions, V518I-containing virus had a 2-log reduction, G360D-containing virus had a 2.5-log reduction, and Q371G-containing virus had a 3-log reduction in virus titers at 2 dpi compared to those of parental MNV-1. In conclusion, mutations in the VP1 P domain had a marked effect on the ability of MNV-1 to replicate in cultured B cells, although they did not substantially impair replication in macrophages.
FIG 4.
Mutations in the MNV-1 VP1 S and P domains do not influence replication kinetics in macrophages. RAW264.7 macrophages were infected with parental MNV-1 or the remaining S, P1, and P2 domain mutant viruses at an MOI of 0.05 (A) or 5 (B), and viral growth curves were determined as described in the legend to Fig. 2. Data from two independent experiments were averaged. Each mutant virus was compared to parental MNV-1 for statistical purposes.
FIG 5.
Mutations in the MNV-1 VP1 P domain, but not the S domain, regulate replication kinetics in B cells. M12 B cells were infected with parental MNV-1 or S, P1, and P2 domain mutant viruses at an MOI of 0.05 (A) or 5 (B), and viral growth curves were determined as described in the legend to Fig. 2. Data from two independent experiments were averaged. Each mutant virus was compared to parental MNV-1 for statistical purposes.
VP1 P domain mutations result in MNV-1 attenuation.
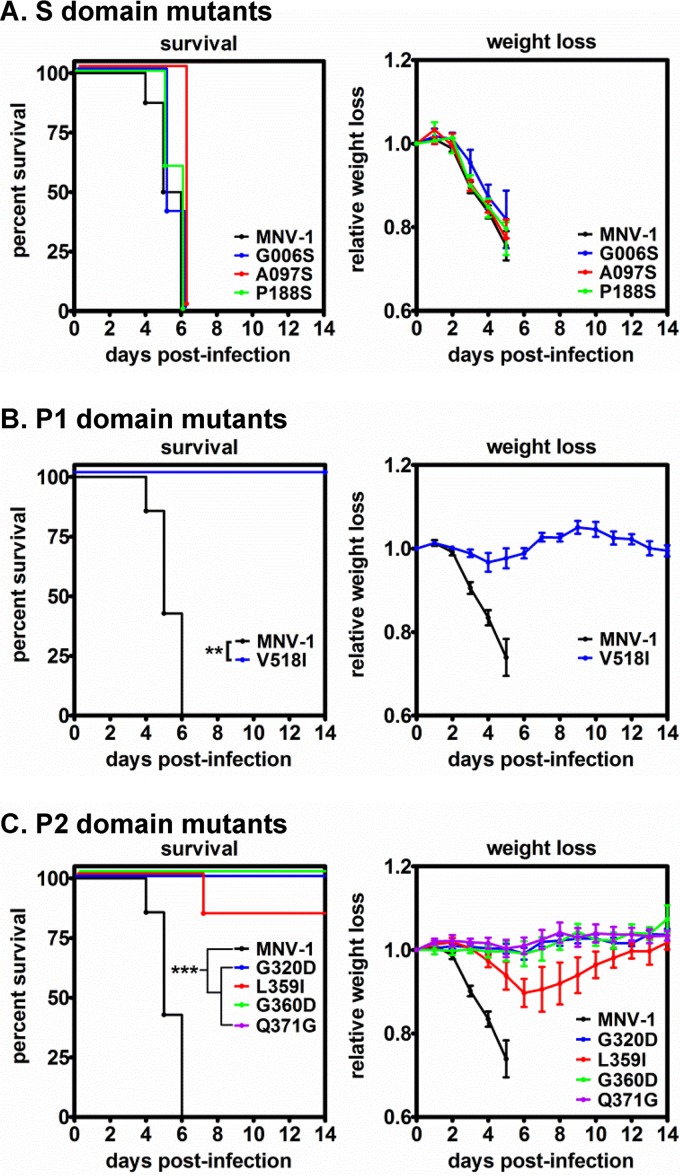
To next test whether this panel of VP1 mutations regulated virulence, we infected IFNAR−/− mice with 5 × 105 TCID50 units of parental MNV-1 or mutant viruses by the p.o. route and monitored them for survival and weight loss. Mice infected with the three S domain mutations exhibited survival kinetics and weight loss similar to those of animals infected with parental MNV-1 (Fig. 6A). In contrast, all IFNAR−/− mice survived infection with the V518I P1 domain mutant virus and presented with only a slight (∼5%) weight loss at 2 to 3 dpi, fully rebounding by 7 dpi (Fig. 6B). For IFNAR−/− mice inoculated with VP1 P2 domain mutant viruses, only 1 of 6 L359I-infected mice succumbed to infection at 7 dpi, while all animals infected with the G320D, G360D, or Q371G mutant viruses survived (Fig. 6C). Consistent with this, L359I-infected animals lost approximately 10% of their body weight by 7 dpi, whereas mice infected with the other P2 domain mutant viruses did not lose weight over the 14-day course of the experiment (Fig. 6C). Collectively, these data revealed that VP1 P domain mutations caused significant attenuation of MNV-1 in IFNAR−/− mice, while S domain mutant viruses retained virulence, directly correlating with B cell infection efficiency.
FIG 6.
Mutations in the MNV-1 VP1 P domain, but not the S domain, regulate virulence. Groups of IFNAR−/− mice (n = 3 or 4) were inoculated p.o. with 5 × 105 TCID50 units of parental virus MNV-1 (data included in all panels for clarity) or recombinant viruses carrying S domain (A), P1 domain (B), or P2 domain (C) mutations. The infected animals were monitored for survival and weight loss daily for 14 days, as described for Fig. 3A. The experiment was repeated twice, and all data are presented.
VP1 P domain mutations cause reduced replication in wild-type mice.
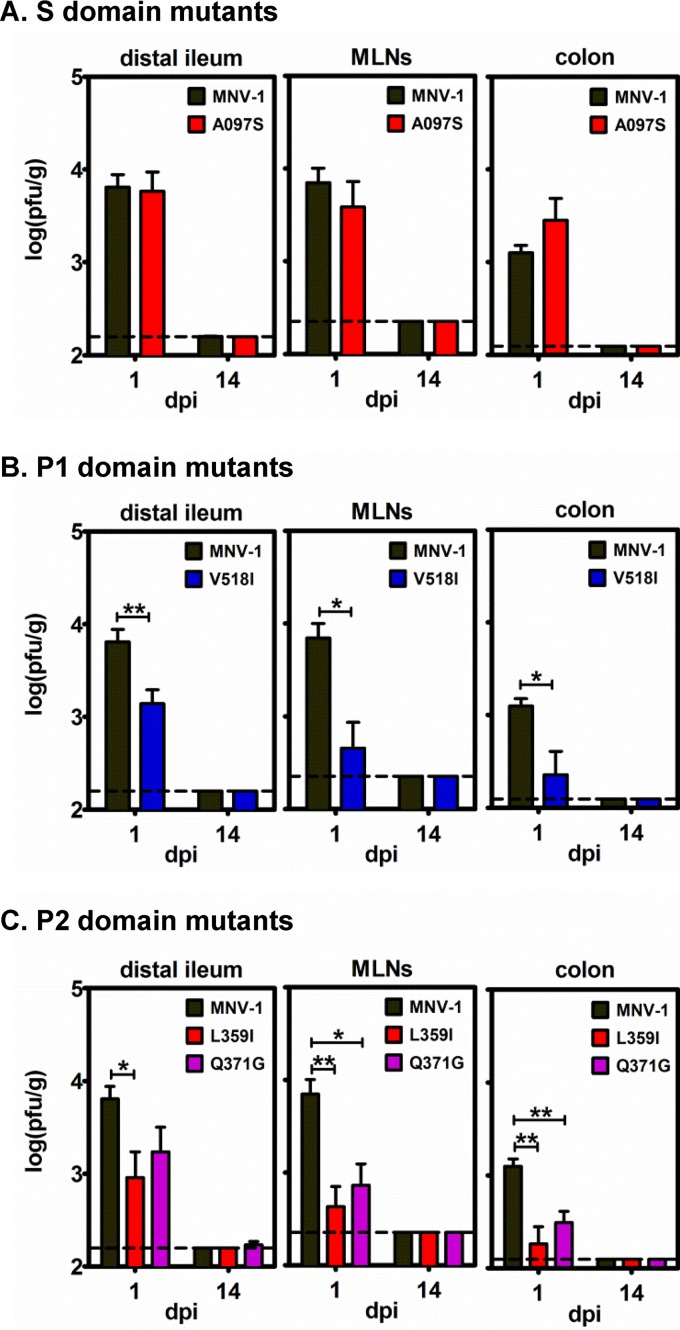
We next examined whether VP1 mutations influenced viral replication efficiency in wild-type mice. Since the subdomain mutations displayed similar phenotypes in viral growth curve determinations and virulence assays, we selected the A097S mutant virus as a representative S domain mutation and the L359I and Q371G mutant viruses as representative P2 domain mutations. Wild-type B6 mice were infected with 106 TCID50 units of parental MNV-1, A097S, V518I, L359I, or Q371G mutant viruses by the p.o. route, and virus loads were determined at 1 dpi, the expected peak of infection for parental MNV-1 at this inoculum dose (25). Consistent with virulence data, the S domain mutant virus displayed virus titers in the distal ileum, MLNs, and colon comparable to parental MNV-1 titers (Fig. 7A), whereas the P1 domain mutant virus (Fig. 7B) and the P2 domain mutant viruses (Fig. 7C) each displayed reduced titers in all three tissues. Because these mutations arose during persistent MNV-1 infection, it was possible that they enhanced persistence establishment. To test this, we also collected tissues and determined virus loads in parallel groups of mice infected for 14 days. No infectious virus was detected in any tissue of mice infected with any of the viruses used in these studies (Fig. 7). In conclusion, mutations in the MNV-1 P domain reduced viral replication efficiency in wild-type mice. Moreover, none of the identified mutations enhanced persistence establishment.
FIG 7.
Mutations in the MNV-1 VP1 P domain, but not the S domain, regulate replication efficiency in wild-type mice. Groups of B6 mice (n = 3 or 4) were infected p.o. with 106 TCID50 units of parental MNV-1 (data included in each panel for clarity) or recombinant viruses carrying S domain (A), P1 domain (B), or P2 domain (C) mutations. The indicated tissues were collected at 1 or 14 dpi, and viral loads were determined by plaque assay. The entire experiment was repeated twice, and all data were averaged. The limit of detection is indicated by the dashed line. Each mutant virus titer was compared to parental MNV-1 titers at each time point for statistical purposes.
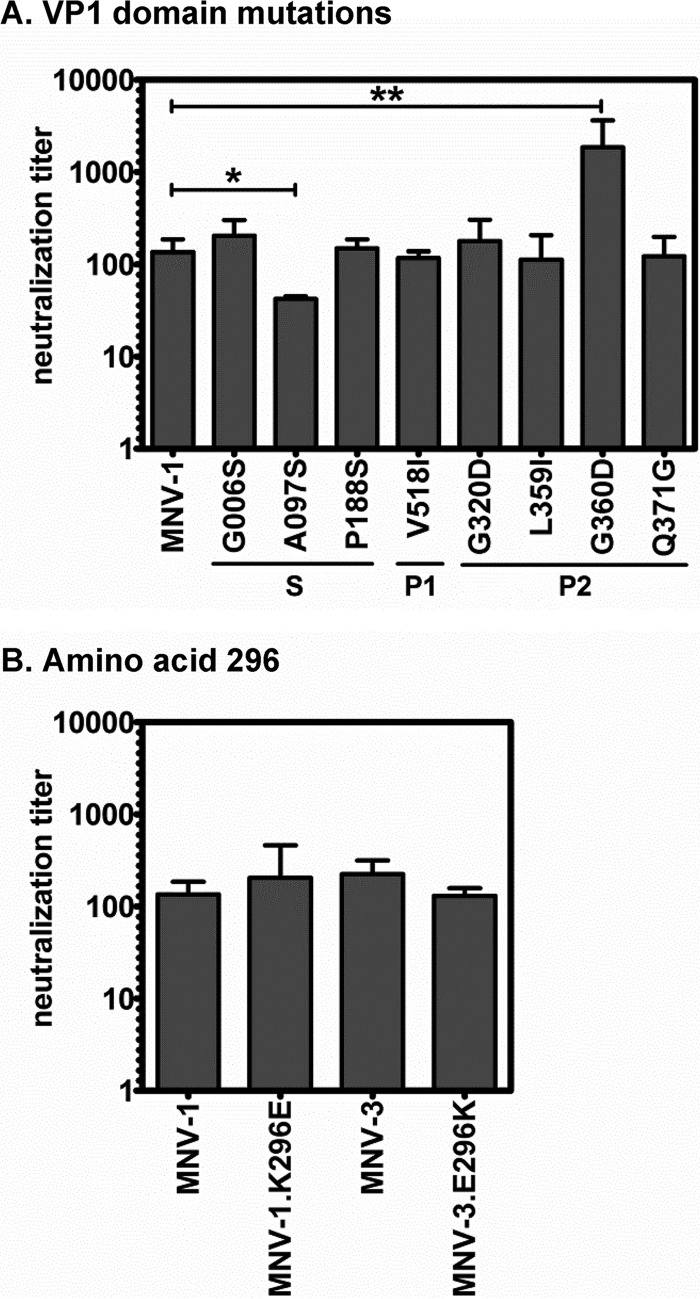
Certain VP1 mutations affect antibody neutralization.
Since these VP1 mutations arose during persistent MNV-1 infection and several of them are in surface-exposed residues in the hypervariable and immunogenic P2 domain (27), we next tested whether they resulted in antibody escape. Specifically, parental MNV-1 and each mutant virus were incubated with serum from MNV-1-immunized mice and subjected to TCID50 assay to measure infectivity. Surprisingly, only the S domain A097S mutant displayed reduced neutralization by immune serum, whereas the G006S, P188S, V518I, G320D, L359I, and Q371G mutant neutralization titers were comparable to that of parental MNV-1 (Fig. 8A). The G360D mutant virus actually had an elevated neutralization titer compared to that of parental MNV-1, demonstrating that it was more sensitive to antibody-mediated neutralization using parental MNV-1 immune sera. Because residue 296 is predicted to reside at the tips of the P2 arches (Fig. 1B), we also tested whether a mutation at this site conferred escape activity toward antibodies raised to parental virus. However, MNV-1.K296E was neutralized comparably to parental MNV-1 using MNV-1 immune sera, and MNV-3.E296K was neutralized comparably to parental MNV-3 using MNV-3 immune sera (Fig. 8B). These overall data indicate that certain VP1 mutations arising in persistently infected mice do regulate antibody neutralizing activity but in unexpected ways, with only one S domain mutation impeding antibody neutralization and one P2 domain mutation surprisingly enhancing antibody-mediated neutralization.
FIG 8.
MNV-1 VP1 mutations arising during persistence have minimal effects on antibody neutralization. (A) A total of 3 × 104 TCID50 units of MNV-1 or VP1 mutant viruses indicated on the x axis were incubated with 2-fold serially diluted MNV-1 immune serum. (B) A total of 3 × 104 TCID50 units of MNV-1 or MNV-1.K296E were incubated with serial dilutions of MNV-1 immune serum, and 3 × 104 TCID50 units of MNV-3 or MNV-3.E296K were incubated with serial dilutions of MNV-3 immune serum. For both panels, the viral infectivity of each sample was determined by TCID50 assay after 1 h of incubation of virus and immune serum. Neutralization titers were calculated using the Reed-Muench method. All data from three independent experiments were averaged.
DISCUSSION
In this study, we tested whether amino acid substitutions in the MNV-1 VP1 capsid protein that arose during persistent infection of mice had phenotypic consequences in vitro and in vivo. A summary of all results is presented in Table 2. We first examined the effect of a K296E mutation in the P2 domain of VP1 since it became dominant in the viral quasispecies of all 10 persistently infected mice analyzed in our previous study (22) and it arises during tissue culture passaging of MNV-1 (12, 20). Considering the frequency with which MNV-1 acquires a glutamic acid at this position and the fact that nearly all natural isolates have a glutamic acid at position 296 (20), it is logical to predict that this residue provides a fitness advantage to MuNoV strains. Yet two previous studies revealed that the K296E change results in attenuation of MNV-1 in STAT1−/− mice (20, 21). Our results are entirely consistent with this since MNV-1.K296E caused significantly less weight loss and showed less lethality than parental MNV-1 in IFNAR−/− mice (Fig. 3A). Furthermore, we demonstrated that the K296E change impaired the ability of the virus to replicate in both IFNAR−/− and wild-type B6 mice (Fig. 3B). Moreover, our studies demonstrate for the first time that a lysine at position 296 is sufficient to confer a degree of virulence on an otherwise attenuated strain: MNV-3.E296K caused significantly more weight loss and showed more lethality than parental MNV-3 (Fig. 3A), and it reached higher titers in IFNAR−/− and B6 mice (Fig. 3B). It should be noted that MNV-3.E296K was still not as virulent as parental MNV-1, so there must be additional viral determinants of virulence yet to be discovered. Considering that changes to residue 296 had only modest effects on replication kinetics in macrophages (Fig. 2A) and B cells (Fig. 2B), and they did not impact antibody neutralization (Fig. 8B), it seems likely that this residue is critical for an in vivo property of viral pathogenesis. For example, it could regulate an immune evasion or antagonism function encoded by VP1, or it could alter virion structure in a manner that regulates host sensing pathways that drive innate immune responses. Alternatively, it is formally possible that attenuation itself provides increased fitness by facilitating the establishment or maintenance of persistence.
TABLE 2.
Summary of phenotypes for viable mutantsa
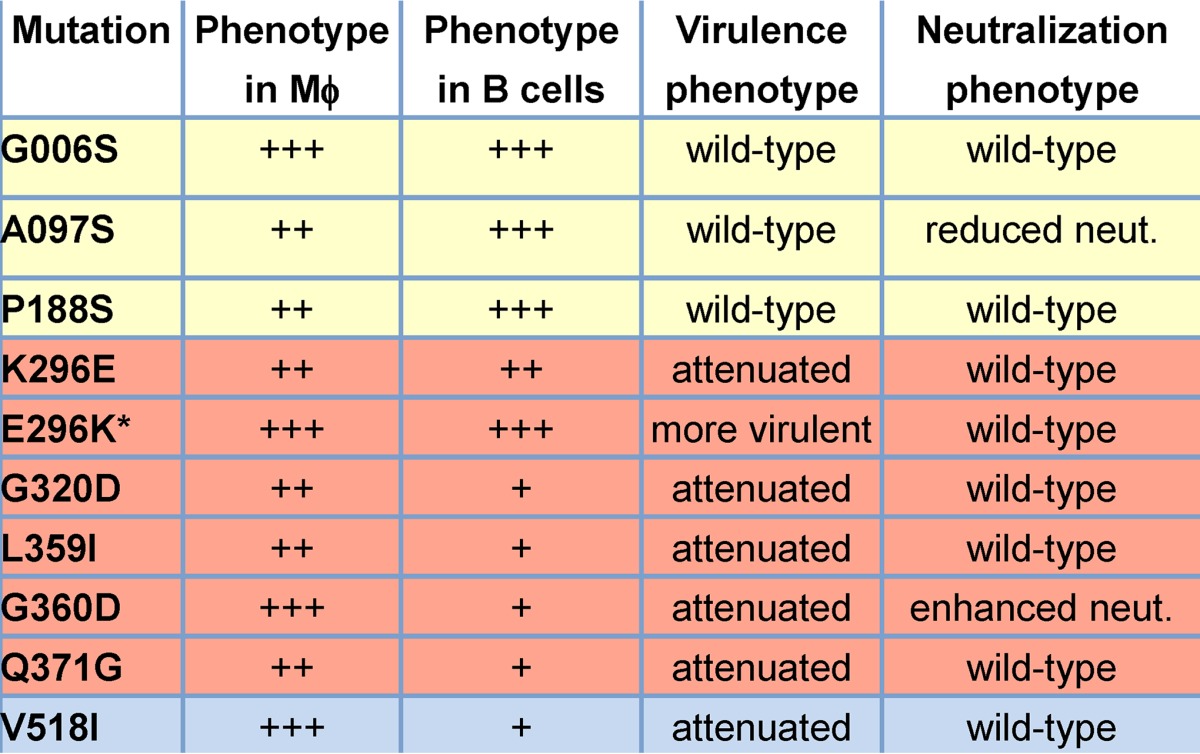
Mϕ, macrophages; +++, wild type; ++, modest replication defect; +, severe replication defect. *, mutation introduced into the MNV-3 backbone, whereas all other mutations were introduced into the MNV-1 backbone.
Of the eight additional ORF2 mutations that were detected in persistently infected mice and were viable when introduced into a MNV-1 infectious clone, three were located in the S domain of VP1 (Fig. 1). This is the most conserved domain of the protein, forming the shell of the virus particle (28). These three mutations—G006S, A097S, and P188S—had only minimal effects on replication kinetics in macrophages (Fig. 4) and no effect in B cells (Fig. 5), no effect on virulence (Fig. 6A), and no effect on viral tissue titers at 1 or 14 dpi (Fig. 7A). One S domain mutation, the A097S mutation, did reduce the ability of MNV-1 immune sera to neutralize viral infectivity (Fig. 8A). Considering that this virus was not impaired in its ability to infect cells or mice, it is unlikely that the mutation in the conserved shell domain of the virus particle caused a major structural change. However, it may result in a subtle conformational change that interferes with antibody binding.
We also detected one mutation in the P1 domain (V518I) and four mutations in the P2 domain (G320D, L359I, G360D, and Q371G) of VP1 in persistently infected mice (Fig. 1). In contrast to the absence of phenotypic changes associated with mutations in the S domain, all P domain mutations had significant consequences on MNV-1 replication in vitro and in vivo. While these mutations had no to a minimal effect on viral replication kinetics in macrophages (Fig. 4), they all substantially reduced viral replication in B cells (Fig. 5). Our previous work demonstrated that the nature of macrophage and B cell infection by MuNoV strains is distinct in that macrophage infection is lytic and of high infectivity, whereas B cell infection occurs in the absence of apparent cytopathic effect and is of low infectivity (9). Thus, it is not altogether surprising that there are viral determinants regulating infection in a cell-type-specific manner. These findings should inform future studies of the unique nature of B cell versus macrophage infection. Moreover, the fact that multiple mutations arose in persistently infected mice that resulted in impaired B cell infection while maintaining replication competency in macrophages may support macrophages as a persistent reservoir of MuNoVs. This is, however, in apparent contradiction to viral replication properties in cultured cells where B cells, but not macrophages, can become persistently infected (9). Future studies will be required to confirm the cell type(s) supporting persistent infection in mice.
All of the P domain mutations caused attenuation of MNV-1 in IFNAR−/− mice (Fig. 6B and C) and decreased virus titers in wild-type mice (Fig. 7B and C), revealing a direct correlation between B cell infection efficiency and virulence. These data are consistent with findings of Strong et al., who revealed that the VP1 P domain of MNV-1 conferred virulence on the MNV.CR6 strain, and, conversely, the P domain of MNV.CR6 caused attenuation of MNV-1 in STAT1−/− mice (21). Our findings further advance this observation by revealing specific residues associated with virulence and suggesting that virulence directly correlates with efficient infection of B cells.
In conclusion, we predicted that mutations arising in persistently infected mice would provide a fitness advantage to the virus, but our results instead indicate that they either had no measurable effect on viral replication and virulence (S domain mutations) or impaired viral replication and caused attenuation (P domain mutations). It is possible that they (i) enhance fitness in certain combinations but not individually, (ii) enhance fitness in facets of pathogenesis not illuminated by our assays, or (iii) were simply neutral mutations resulting from the error-prone nature of viral RNA replication. Nonetheless, a majority of the mutations identified in our original study (22) had significant effects on viral replication and virulence that advance our understanding of NoV pathogenesis. First, we revealed for the first time that a lysine at residue 296 in the P2 domain of VP1 is sufficient to confer virulence on an otherwise attenuated MuNoV strain and that it regulates viral replication in wild-type mice as it does in IFN-deficient hosts. Second, we have identified five residues in the P domain of the MuNoV VP1 protein that regulate infection of B cells and virulence. NoVs are known to infect a variety of immune cell types (16), but the biological relevance of each target cell type is unknown. Moreover, the mediators of pathogenesis during NoV infections are not well established. Thus, linking infection of a specific cell type to virulence should advance our understanding of NoV disease mechanisms.
ACKNOWLEDGMENTS
We thank Scott A. Tibbetts, Herbert W. Virgin, and Fanxiu Zhu for providing valuable reagents.
Molecular graphics and analyses were performed with the UCSF Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS grant P41-GM103311).
Funding Statement
This work was funded by NIH under grants R01AI116892 and R01AI081921.
REFERENCES
- 1.Koo HL, Ajami N, Atmar RL, DuPont HL. 2010. Noroviruses: the principal cause of foodborne disease worldwide. Discov Med 10:61–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Koo HL, Neill FH, Estes MK, Munoz FM, Cameron A, Dupont HL, Atmar RL. 2013. Noroviruses: the most common pediatric viral enteric pathogen at a large university hospital after introduction of rotavirus vaccination. J Pediatr Infect Dis Soc 2:57–60. doi: 10.1093/jpids/pis070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD. 2013. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green KY. 2014. Norovirus infection in immunocompromised hosts. Clin Microbiol Infect 20:717–723. doi: 10.1111/1469-0691.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bok K, Green KY. 2012. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med 367:2126–2132. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata T, Katsushima N, Mizuta K, Muraki Y, Hongo S, Matsuzaki Y. 2007. Prolonged norovirus shedding in infants under 6 months of age with gastroenteritis. Pediatr Infect Dis J 26:46–49. doi: 10.1097/01.inf.0000247102.04997.e0. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA. 2014. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 14:725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharff RL. 2010. Health-related costs from foodborne illness in the United States. Produce Safety Project. Georgetown University, Washington, DC. [Google Scholar]
- 9.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinjé J, Tibbetts SA, Wallet SM, Karst SM. 2014. Enteric bacteria promote human and murine norovirus infection of B cells. Science 346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taube S, Kolawole AO, Höhne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE. 2013. A mouse model for human norovirus. mBio 4:e00450-13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. 2014. Advances in norovirus biology. Cell Host Microbe 15:668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wobus CE, Karst SM, Thackray LB, Chang K-O, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. 2007. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol 81:3251–3263. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward JM, Wobus CE, Thackray LB, Erexson CR, Faucette LJ, Belliot G, Barron EL, Sosnovtsev SV, Green KY. 2006. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol Pathol 34:708–715. doi: 10.1080/01926230600918876. [DOI] [PubMed] [Google Scholar]
- 15.Jones MK, Grau KR, Costantini V, Kolawole AO, de Graaf M, Freiden P, Graves CL, Koopmans M, Wallet SM, Tibbetts SA, Schultz-Cherry S, Wobus CE, Vinjé J, Karst SM. 2015. Human norovirus culture in B cells. Nat Protoc 10:1939–1947. doi: 10.1038/nprot.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karst SM, Wobus CE. 2015. A working model of how noroviruses infect the intestine. PLoS Pathog 11:e1004626. doi: 10.1371/journal.ppat.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 18.Green KY. 2013. Caliciviridae:the noroviruses, p 582–608. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 19.McFadden N, Bailey D, Carrara G, Benson A, Chaudhry Y, Shortland A, Heeney J, Yarovinsky F, Simmonds P, Macdonald A, Goodfellow I. 2011. Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLoS Pathog 7:e1002413. doi: 10.1371/journal.ppat.1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey D, Thackray LB, Goodfellow IG. 2008. A single amino acid substitution in the murine norovirus capsid protein is sufficient for attenuation in vivo. J Virol 82:7725–7728. doi: 10.1128/JVI.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strong DW, Thackray LB, Smith TJ, Virgin HW. 2012. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J Virol 86:2950–2958. doi: 10.1128/JVI.07038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickman D, Jones MK, Zhu S, Kirpatrick E, Ostrov DA, Wang X, Ukhanova M, Sun Y, Mai V, Salemi M, Karst SM. 2014. The effect of malnutrition on norovirus infection. mBio 5:e01032-13. doi: 10.1128/mBio.01032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu S, Regev D, Watanabe M, Hickman D, Moussatche N, Jesus DM, Kahan SM, Napthine S, Brierley I, Hunter RN, Devabhaktuni D, Jones MK, Karst SM. 2013. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog 9:e1003592. doi: 10.1371/journal.ppat.1003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 25.Kahan SM, Liu G, Reinhard MK, Hsu CC, Livingston RS, Karst SM. 2011. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology 421:202–210. doi: 10.1016/j.virol.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thackray LB, Wobus CE, Chachu KA, Liu B, Alegre ER, Henderson KS, Kelley ST, Virgin HW. 2007. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J Virol 81:10460–10473. doi: 10.1128/JVI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katpally U, Wobus CE, Dryden K, Virgin HW, Smith TJ. 2008. Structure of antibody-neutralized murine norovirus and unexpected differences from viruslike particles. J Virol 82:2079–2088. doi: 10.1128/JVI.02200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad BVV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]