Abstract
We assessed whether influenza virus hemagglutinin stalk-based immunity protects ferrets against aerosol-transmitted H1N1 influenza virus infection. Immunization of ferrets by a universal influenza virus vaccine strategy based on viral vectors expressing chimeric hemagglutinin constructs induced stalk-specific antibody responses. Stalk-immunized ferrets were cohoused with H1N1-infected ferrets under conditions that permitted virus transmission. Hemagglutinin stalk-immunized ferrets had lower viral titers and delayed or no virus replication at all following natural exposure to influenza virus.
TEXT
Currently licensed influenza virus vaccines are proven to reduce the burden of influenza virus infections. However, epidemics of influenza still occur every year, resulting in significant morbidity (1) and mortality (2) worldwide. Humoral immune responses induced by seasonal influenza virus vaccines are typically focused on the immunodominant globular head domains of hemagglutinin (HA) and are specific for the respective vaccine strains but are suboptimal against strains that have antigenically drifted. Thus, annual vaccination is required to keep up with an antigenically “moving target” (3). This limitation of currently licensed vaccines is additionally complicated by the emergence of pandemic influenza virus strains that are difficult to predict. Upon the emergence of a pandemic, redirection of commercial vaccine manufacture is unlikely to occur in a sufficiently timely fashion to limit viral spread, as was the case during the 2009 H1N1 influenza pandemic (4, 5). HA-specific universal influenza virus vaccines shift humoral immune responses toward the antigenically conserved but immunosubdominant HA stalk region, thereby overcoming these limitations. Such a vaccine could confer protection against homologous and drifted influenza virus strains, eliminate the requirement for reformulation of annual influenza virus vaccines, and confer increased protection against influenza viruses with pandemic potential (6–8). To investigate the level of protection conferred by HA stalk-based immunity against infection by influenza viruses, we tested a universal influenza vaccine approach in the ferret model.
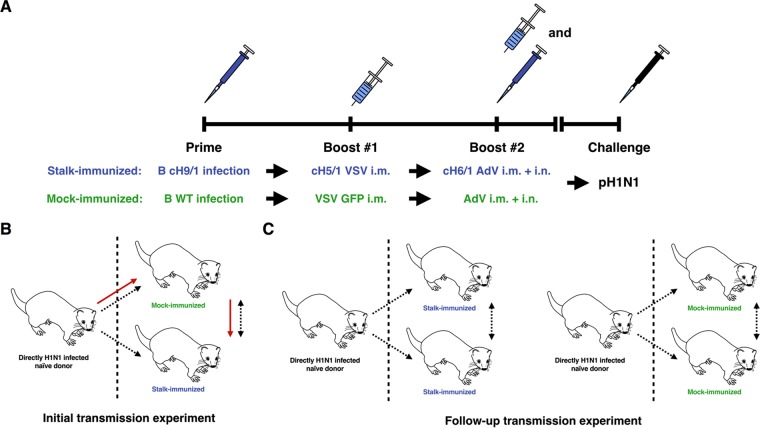
We sequentially immunized a business of 5-month-old male Fitch ferrets (Triple F Farms; Sayre, PA) with viral vectors expressing chimeric HA (cHA) as described previously (9) (Fig. 1A). Ferrets (n = 6) were primed by intranasal infection with 2 × 107 PFU of an influenza B virus vector expressing cH9/1 HA (B-cH9/1). The ferrets were then boosted by the intramuscular administration of 1 × 107 PFU of a recombinant vesicular stomatitis virus (VSV) vector expressing cH5/1 HA (VSV-cH5/1, 0.5 ml administered intramuscularly), followed by a second boost with 1 × 109 PFU of a replication-deficient recombinant adenovirus type 5 (AdV) vector expressing cH6/1 HA (AdV-cH6/1, intranasal and intramuscular administrations of 0.5 ml each) (9). By sequential vaccination with immunogens that have the same conserved stalk domain but divergent head domains, it is possible to specifically induce high levels of stalk-reactive antibodies. Control ferrets (n = 6) received the same control virus vectors (wild-type influenza B virus, VSV expressing green fluorescent protein (VSV-GFP) and AdV completely lacking an insert by the same immunization routes and the same regimen. Seroconversion of the immunized ferrets to the HA globular head expressed by the indicated viral vector was assessed by hemagglutination inhibition (HI) assays (10, 11) (Fig. 2D), as well as by neutralization assay for VSV (Fig. 2E). Although priming of ferrets with influenza B virus expressing cH9/1 resulted in detectable serum responses, no seroconversion was detected by HI assay following boosting with either VSV-cH5/1 HA or AdV-cH6/1 (Fig. 2D)—probably reflecting the redirection of the immune response to the stalk domain. Importantly, during the course of the vaccination regimen, the stalk-immunized and control-immunized ferrets did not develop HI titers against the pandemic H1 globular head domain (Fig. 2D).
FIG 1.
Experimental setup for influenza virus transmission between ferrets. (A) Schematic of the experimental setup. Animals were primed intranasally (i.n.) with a recombinant influenza B virus expressing cH9/1 HA (B cH9/1) and then boosted intramuscularly (i.m.) with recombinant VSV expressing cH5/1 HA (cH5/1 VSV). The animals were finally boosted (intramuscularly and intranasally) with a replication-deficient Ad expressing cH6/1 (cH6/1 AdV). Control animals received wild-type influenza B virus, VSV, and AdV at the same doses and via the same routes. Finally, animals were challenged with a pandemic H1N1 influenza virus isolate. (B) Schematic of the design of the initial transmission experiment. The directly infected ferret was housed on the left side of the cage and separated from the mock- and stalk-immunized animals by a perforated divider that allowed airflow (as indicated by dashed arrows) but prevented direct contact between the animals. One control-vaccinated ferret and one stalk-vaccinated ferret were cohoused on the right side—a setting that allowed transmission by direct contact between these two ferrets (as indicated by the dashed bidirectional arrow). The most likely infection route for the stalk-vaccinated animals in this experiment is indicated by red arrows. We hypothesize that mock-immunized animals amplified the virus before the stalk-immunized animals became infected. (C) Schematic of the design of the follow-up transmission experiment. Again, the directly infected ferret was housed on the left side of the cage, separated from the other animals by a perforated divider that allowed airflow and aerosol transmission of virus. The ferrets on the right side both underwent the same vaccination regimen. All animal experiments were conducted by using protocols approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee (IACUC). Ferrets were provided access to food and water ad libitum (9, 10, 22, 23).
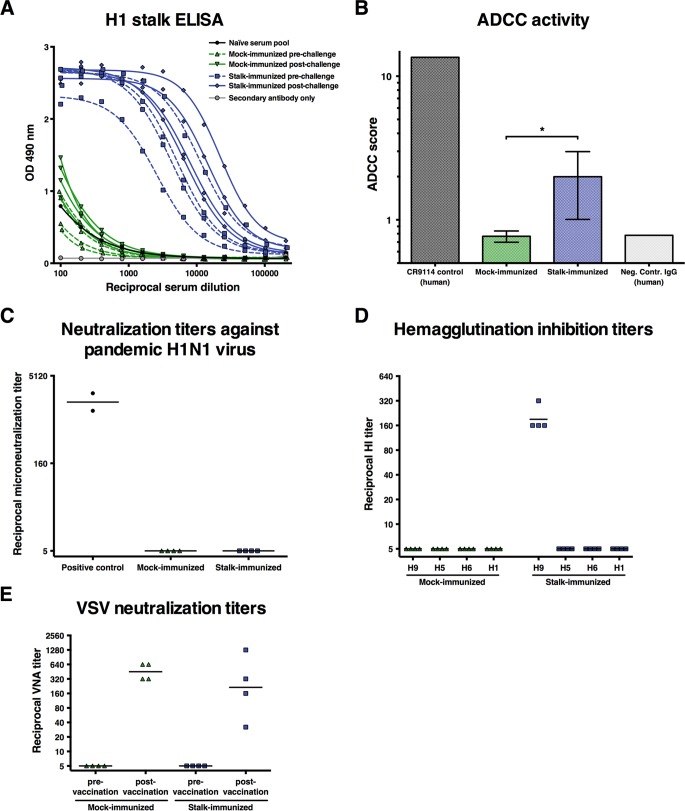
FIG 2.
Serum responses of vaccinated ferrets increased after virus challenge. The data shown were collected from ferrets included in the initial transmission experiment (four mock immunized and four stalk immunized). (A) The development of H1 stalk-reactive antibody responses was assessed by ELISAs with baculovirus-produced cH2/1 HA. ELISAs were performed as described before (9, 24). OD, optical density. (B) Activity of sera from mock- and stalk-vaccinated ferrets was measured in an ADCC reporter assay (Promega; as described in reference 12) at a dilution of 1:90. The assay was developed to measure the interaction between human Fc fragments and human FcγRIIIa. This is a caveat of the assay because ferret antibodies might be less effective than human antibodies at activating this receptor. However, no ferret-specific assay is available. *, P ≤ 0.05. Ferret influenza virus microneutralization titers (C), HI titers (D), and VSV neutralization titers (E) in sera from mock- and stalk-vaccinated animals are shown. Statistical analysis of the data in panel B was done by an unpaired t test that was performed in GraphPad Prism.
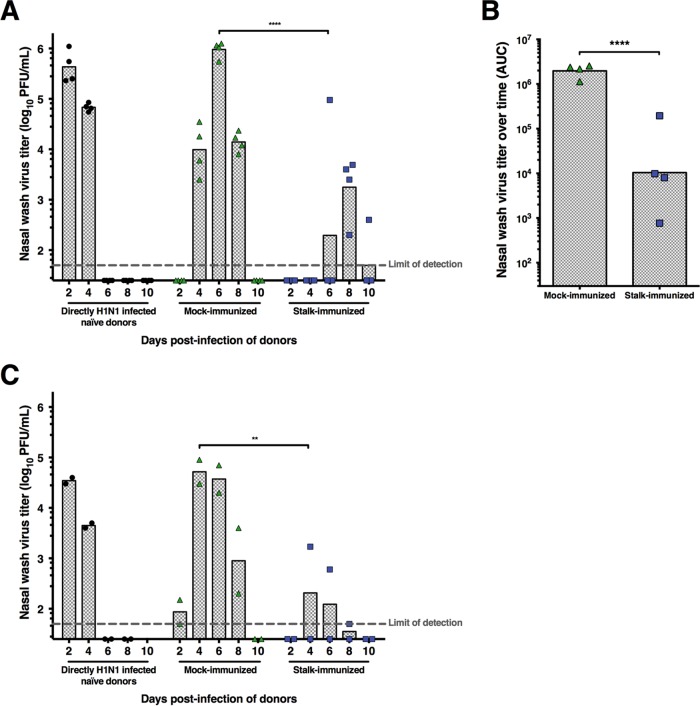
Following prime-boost vaccination, a stalk-immunized ferret and a control-immunized ferret were cohoused with a ferret directly infected with 106 PFU of pandemic H1N1 influenza virus A/California/4/2009 under conditions that permitted only aerosol transmission to occur (Fig. 1B). Importantly, sets of one stalk-immunized and one mock-immunized ferret were kept in the same chamber and contact transmission between these two animals was possible (Fig. 1B). On days 2, 4, 6, 8, and 10 postinfection, nasal washes were taken from the directly infected ferret and aerosol contacts for determination of virus titers by plaque assay. Direct intranasal infection of naive ferrets with the pandemic H1N1 influenza virus resulted in high virus titers at day 2 postinfection that declined to below detectable limits by day 6 postinfection (Fig. 3A). All mock-immunized ferrets became infected and uniformly shed virus between days 4 and 8 postinfection (between days 3 and 7 after aerosol contact), with peak nasal wash titers on day 6 postinfection. All stalk-immunized ferrets became infected but shed virus less uniformly. Importantly, the virus titers detected in the nasal wash samples from the stalk-immunized ferrets were significantly lower (mean peak of 1.8 × 103 PFU/ml on day 8 postinfection) than those of the control-immunized ferrets (Fig. 3A and B). Additionally, the time at which the stalk-immunized ferrets shed influenza virus (days 6 to 10 postinfection) was delayed compared to that of the control-vaccinated ferrets. The experimental design (Fig. 1B) had the caveat that virus could potentially be transmitted to mock-immunized animals that amplified the virus before the stalk-vaccinated animals became infected. Close direct contact with their virus-shedding cagemates might have facilitated transmission to the stalk-vaccinated animals.
FIG 3.
Vaccination with cHA vaccine constructs leads to reduced susceptibility to virus transmission. Ferrets (n = 6) were immunized with influenza B virus expressing cH9/1 HA, boosted with VSV-cH5/1 HA, and boosted a second time with an AdV vector expressing the cH6/1 protein. Control ferrets (n = 6) were immunized with corresponding viral vectors (influenza B virus, VSV-GFP, and empty AdV). Immunized ferrets were then exposed to ferrets directly infected with pandemic H1N1 virus under conditions that specifically allowed aerosol transmission. (A) Initial experiment. On day 0, a ferret was directly infected by the intranasal route with pandemic H1N1 influenza virus. On day 1 after direct infection, stalk-immunized and control-immunized ferrets were housed adjacently to the directly infected ferret under conditions that permitted only aerosol transmission to occur between the directly infected and mock- or stalk-immunized animals. However, direct-contact transmission was possible between control- and stalk-vaccinated ferrets. On days 2, 4, 6, 8, and 10 postinfection (days 1, 3, 5, 7, and 9 after aerosol contact), all of the ferrets were anesthetized with ketamine and xylazine for collection of nasal wash samples to determine virus titers by plaque assay. Virus titers are presented in PFU per milliliter, and each symbol represents one animal. Each column shows the geometric mean titer of each group. (B) Area-under-the-curve (AUC) analysis (days 2 to 10) of virus shedding by mock- and stalk-vaccinated ferrets. Differences between the two groups are highly significant (****, P ≤ 0.0001). (C) The experimental setup for the follow-up transmission experiment was similar to that for the initial experiment, but importantly, two stalk- or mock-immunized ferrets were cohoused and therefore amplification of the virus in mock-vaccinated ferrets and subsequent transmission to stalk-vaccinated ferrets by close contact were not possible. All of the mock-immunized ferrets but only one stalk-immunized ferret shed virus in this experiment. Furthermore, the virus titers of the one stalk-immunized ferret were lower than those of the ferrets in the mock-immunized group. Titers in panels A and C were analyzed by using multiple t tests corrected for multiple comparisons by the Holm-Sidak method. The data in panel B were analyzed by one-way analysis of variance with Tukey's posttest. Statistical analysis was performed in GraphPad Prism. **, P ≤ 0.01.
To test this hypothesis, we designed a follow-up transmission experiment in which a directly infected ferret was cohoused with two stalk- or mock-immunized ferrets. In this setup, only infection via aerosol transmission of virus could occur from the physically separated and directly infected ferret (Fig. 1C). Again, the directly infected and mock-vaccinated ferrets showed kinetics similar to those seen in the previous experiment, with peak virus titers on days 2 and 4, respectively (Fig. 3C). Importantly, only one of the stalk-immunized ferrets in the follow-up experiment shed infectious virus at a low titer (1,700 PFU/ml).
As shown in Fig. 2A for the ferrets in the initial transmission experiment, the cHA-based universal influenza virus vaccine strategy stimulated readily detectable levels of H1 stalk-reactive antibody responses (stalk immunized prechallenge). As expected, H1N1 infection following infection by aerosol transmission had a boosting effect on these H1 stalk-reactive antibody responses (stalk immunized postchallenge). This boost in stalk-reactive antibodies (approximately 3-fold) might further enhance broad protection against future infections. Importantly, serum from naive ferrets or from control-immunized ferrets lacked detectable levels of H1 stalk-reactive antibodies. As mentioned above, all animals were negative for the H1N1 challenge virus by HI assay (Fig. 2D). Interestingly, sera from the stalk-vaccinated animals—despite robust enzyme linked immunosorbent assay (ELISA) titers—showed no activity in a standard microneutralization assay (Fig. 2C). This finding is in agreement with two recent reports that showed that, despite solid protection against viral disease, low or undetectable neutralization titers developed after stalk vaccination (12, 13). However, sera from vaccinated animals showed activity in an antibody-dependent cell-mediated cytotoxicity (ADCC) reporter assay (Fig. 2B). ADCC has been shown to be an important protective mechanism for stalk-reactive antibodies, specifically at subneutralizing concentrations (14–16). Of note, an assay with cells expressing the human Fc receptor FcγRIIIa was used because of the lack of ferret-specific reagents. The ADCC activity might therefore be lower than it would be with the corresponding ferret Fc receptor.
In this study, we used the ferret model of influenza virus transmission to assess the level of protection conferred by group 1 HA stalk-specific antibodies against natural infection with pandemic H1N1 influenza virus. Ferrets were immunized by using a universal influenza virus vaccine strategy in which the animals were vaccinated with viral vectors expressing cHAs that induce stalk-reactive antibodies. The results revealed that group 1 stalk-specific antibodies can protect ferrets from infection (up to 50% in an aerosol-only transmission model) or at least reduce the magnitude and duration of influenza virus shedding from the nasal cavity. Importantly, HA stalk-immunized ferrets did not exhibit any clinical signs of antibody-enhanced disease, a complication that has been reported upon vaccination with inactivated influenza virus vaccines in pigs (17). Collectively, our present findings, along with previous observations (9, 18–20), provide compelling evidence that a universal influenza virus vaccine strategy that stimulates robust HA stalk-focused immunity would reduce the severity of influenza virus replication and the disease burden following virus infection by natural transmission routes. The novelty and significance of the findings presented in this report support the development of universal influenza virus vaccines and the transition from research laboratories to clinical settings (21).
ACKNOWLEDGMENTS
This work was supported by PATH, the Centers for Excellence for Influenza Research and Surveillance (CEIRS, HHSN272201400008C to R.A.A., A.G.S., P.P., and F.K.), and NIH grant U19 AI109946 (P.P. and F.K.).
We thank Ariana Hirsh (Department of Microbiology, Icahn School of Medicine at Mount Sinai) for technical assistance with cH2/1 protein expression.
REFERENCES
- 1.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. 2007. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). 2010. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep 59:1057–1062. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5933a1.htm. [PubMed] [Google Scholar]
- 3.Wang TT, Palese P. 2011. Biochemistry. Catching a moving target. Science 333:834–835. doi: 10.1126/science.1210724. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). 2009. Update on influenza A (H1N1) 2009 monovalent vaccines. MMWR Morb Mortal Wkly Rep 58:1100–1101. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5839a3.htm. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). 2009. Swine influenza A (H1N1) infection in two children—southern California, March-April 2009. MMWR Morb Mortal Wkly Rep 58:400–402. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5815a5.htm. [PubMed] [Google Scholar]
- 6.Yewdell JW. 2013. To dream the impossible dream: universal influenza vaccination. Curr Opin Virol 3:316–321. doi: 10.1016/j.coviro.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subbarao K, Matsuoka Y. 2013. The prospects and challenges of universal vaccines for influenza. Trends Microbiol 21:350–358. doi: 10.1016/j.tim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado VH, Ryder AB, Miller MS, Rose JK, Palese P, Garcia-Sastre A, Albrecht RA. 2014. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol 88:3432–3442. doi: 10.1128/JVI.03004-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker SF, Guo H, Albrecht RA, Garcia-Sastre A, Topham DJ, Martínez-Sobrido L. 2013. Protection against lethal influenza with a viral mimic. J Virol 87:8591–8605. doi: 10.1128/JVI.01081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seibert CW, Rahmat S, Krause JC, Eggink D, Albrecht RA, Goff PH, Krammer F, Duty JA, Bouvier NM, Garcia-Sastre A, Palese P. 2013. Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J Virol 87:7793–7804. doi: 10.1128/JVI.00979-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, de Man M, Ding Z, Apetri A, Kükrer B, Sneekes-Vriese E, Tomkiewicz D, Laursen NS, Lee PS, Zakrzewska A, Dekking L, Tolboom J, Tettero L, van Meerten S, Yu W, Koudstaal W, Goudsmit J, Ward AB, Meijberg W, Wilson IA, Radošević K. 2015. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349:1301–1306. doi: 10.1126/science.aac7263. [DOI] [PubMed] [Google Scholar]
- 13.Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, Gallagher JR, Wang L, Zhang Y, Joyce MG, Lingwood D, Moin SM, Andersen H, Okuno Y, Rao SS, Harris AK, Kwong PD, Mascola JR, Nabel GJ, Graham BS. 2015. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 21:1065–1070. doi: 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- 14.DiLillo DJ, Tan GS, Palese P, Ravetch JV. 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med 20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. 2013. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol 87:5512–5522. doi: 10.1128/JVI.03030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, Kent SJ. 2013. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 190:1837–1848. doi: 10.4049/jimmunol.1201574. [DOI] [PubMed] [Google Scholar]
- 17.Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, Vincent AL, Golding H. 2013. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 5:200ra114. doi: 10.1126/scitranslmed.3006366. [DOI] [PubMed] [Google Scholar]
- 18.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krammer F, Pica N, Hai R, Tan GS, Palese P. 2012. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J Virol 86:10302–10307. doi: 10.1128/JVI.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallajosyula VV, Citron M, Ferrara F, Lu X, Callahan C, Heidecker GJ, Sarma SP, Flynn JA, Temperton NJ, Liang X, Varadarajan R. 2014. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A 111:E2514–E2523. doi: 10.1073/pnas.1402766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxford JS. 2013. Towards a universal influenza vaccine: volunteer virus challenge studies in quarantine to speed the development and subsequent licensing. Br J Clin Pharmacol 76:210–216. doi: 10.1111/bcp.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Romero C, de Vries E, Belicha-Villanueva A, Mena I, Tscherne DM, Gillespie VL, Albrecht RA, de Haan CA, Garcia-Sastre A. 2013. Substitutions T200A and E227A in the hemagglutinin of pandemic 2009 influenza A virus increase lethality but decrease transmission. J Virol 87:6507–6511. doi: 10.1128/JVI.00262-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seibert CW, Kaminski M, Philipp J, Rubbenstroth D, Albrecht RA, Schwalm F, Stertz S, Medina RA, Kochs G, Garcia-Sastre A, Staeheli P, Palese P. 2010. Oseltamivir-resistant variants of the 2009 pandemic H1N1 influenza A virus are not attenuated in the guinea pig and ferret transmission models. J Virol 84:11219–11226. doi: 10.1128/JVI.01424-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]