ABSTRACT
The gastrointestinal mucosa is the primary site where human immunodeficiency virus type 1 (HIV-1) invades, amplifies, and becomes persistently established, and cell-to-cell transmission of HIV-1 plays a pivotal role in mucosal viral dissemination. Mast cells are widely distributed in the gastrointestinal tract and are early targets for invasive pathogens, and they have been shown to have increased density in the genital mucosa in HIV-infected women. Intestinal mast cells express numerous pathogen-associated molecular patterns (PAMPs) and have been shown to combat various viral, parasitic, and bacterial infections. However, the role of mast cells in HIV-1 infection is poorly defined. In this study, we investigated their potential contributions to HIV-1 transmission. Mast cells isolated from gut mucosal tissues were found to express a variety of HIV-1 attachment factors (HAFs), such as DC-SIGN, heparan sulfate proteoglycan (HSPG), and α4β7 integrin, which mediate capture of HIV-1 on the cell surface. Intriguingly, following coculture with CD4+ T cells, mast cell surface-bound viruses were efficiently transferred to target T cells. Prior blocking with anti-HAF antibody or mannan before coculture impaired viral trans-infection. Cell-cell conjunctions formed between mast cells and T cells, to which viral particles were recruited, and these were required for efficient cell-to-cell HIV-1 transmission. Our results reveal a potential function of gut mucosal mast cells in HIV-1 dissemination in tissues. Strategies aimed at preventing viral capture and transfer mediated by mast cells could be beneficial in combating primary HIV-1 infection.
IMPORTANCE In this study, we demonstrate the role of human mast cells isolated from mucosal tissues in mediating HIV-1 trans-infection of CD4+ T cells. This finding facilitates our understanding of HIV-1 mucosal infection and will benefit the development of strategies to combat primary HIV-1 dissemination.
INTRODUCTION
Despite great advances in antiretroviral therapies, human immunodeficiency virus type 1 (HIV-1) infection still remains a major global epidemic. Sexual transmission is the principal route of HIV-1 acquisition, making the genital and rectal mucosae the major sites of viral transmission. The intestinal mucosa is also the primary site where HIV-1 amplifies to disseminate virus throughout the host and is critical in the early events in the establishment of infection and evasion of immune defenses. However, the mechanisms contributing to the establishment of HIV-1 primary infection remain largely unexplored. Cell-associated viral dissemination has been proposed to play pivotal roles in HIV-1 primary infection, and multiple cell types, such as dendritic cells (DCs) and macrophages, have been reported to be hijacked by HIV-1 for local and systemic viral spread (1–4). DCs provide one of the best-described cell models for understanding cell-mediated HIV-1 capture and dissemination (1, 5–8).
Mast cells are derived from hematopoietic progenitor cells and undergo final maturation in vascularized tissues. Mast cells are strategically in close contact with the host-environment interface, such as the skin, airway, gastrointestinal tract, and urinary tract. They express numerous pathogen-associated molecular patterns and play an important role in the early immunosurveillance for many pathogens (9, 10). Mast cells can interact with various immune cells in complex ways, including release of soluble factors and direct contact (11), and are important immune effector and modulatory cells that help to link innate and adaptive immunity in the fight against pathogens (9, 12–14). They have been shown to be important for host defense against various viruses, such as vesicular stomatitis virus, Sendai virus, hantavirus, reovirus, dengue virus, influenza virus, herpes simplex virus, and murine cytomegalovirus (15–23). Additionally, mast cells can serve as antigen-presenting cells and participate in traditional immunologic synapse formation with T cells to mediate antigen-specific T cell activation (24).
Although they are among the first cells at mucosal sites to encounter viruses, the role of mast cells in HIV-1 infection is poorly defined. The genital mucosae of HIV-infected women showed increased mast cell density, and increased numbers of mucosal mast cells were noted in men with AIDS-associated diarrhea (25, 26), suggesting a potential role for mast cells in HIV-1 infection. We hence investigated the potential contribution of mucosal mast cells to HIV-1 infection and found that mast cells isolated from gut mucosal tissues express a variety of HIV-1 attachment factors (HAFs) and mediate capture of HIV-1 and the subsequent viral trans-infection of CD4+ T cells. Our results reveal a potential function of gut mucosal mast cells in HIV-1 dissemination in tissues.
MATERIALS AND METHODS
Ethics statement.
Normal intestinal samples from sites adjacent to excised colorectal carcinomas were collected by a licensed medical doctor. Written informed consent was provided by study participants, and the study was approved by the institutional ethical committee of the First Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Isolation of mucosal mast cells.
Human colorectal mucosa tissue from a surgical operation was washed with Hanks balanced salt solution (HBSS) containing 2 mM EDTA and 7 mM β-mercaptoethanol and then cut with scissors into about 0.3-cm3 cubes. Adherent mesentery and fat were removed using tweezers and scissors prior to fragmentation. The tissue cubes were treated with 75 U/ml type I collagenase (Sigma) and 72 U/ml hyaluronidase (Worthington Biochemical) for 90 min at 37°C with gentle agitation. After filtering through sterile nylon mesh, the cell solution was subjected to a density gradient centrifugation performed with 1.088 g/ml Percoll (GE Healthcare). The granulocyte fraction was collected, and mast cells were further positively selected by using an FcεR1+ or CD117+ cell isolation kit (Miltenyi Biotec).
Generation of MDDCs and collection of PBLs.
A Ficoll-Paque density gradient medium was used to separate peripheral blood mononuclear cells (PBMCs) from fresh buffy coats collected from healthy donors. CD14+ monocytes were isolated from PBMCs by using anti-CD14 antibody-coated magnetic beads (Miltenyi Biotec) and were treated with 50 ng/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF) and recombinant human interleukin 4 (rhIL-4) for 5 days to generate monocyte-derived dendritic cells (MDDCs), as described in previous studies (2, 27). The peripheral blood lymphocytes (PBLs) harvested from PBMCs with CD14+ monocyte depletion were activated with phytohemagglutinin P (PHA-P) for 48 h in the presence of 20 IU/ml recombinant IL-2 (R&D Systems).
Toluidine blue staining.
Intestinal mast cells were evaluated by toluidine blue staining as previously described (28). Briefly, cells were fixed with 4% paraformaldehyde (PFA) (Sigma-Aldrich) for 10 min at room temperature (RT) and then stained with 1% toluidine blue (Sigma-Aldrich) for 1 h at RT, and subsequently, cells were washed in distilled water 3 times and covered by use of a coverslip and mounting medium.
HIV-1 stocks.
Pseudotyped single-cycle luciferase reporter HIV-1 particles were generated by calcium phosphate-mediated cotransfection of HEK293 T cells with pLAI-ΔEnv-Luc and an expression plasmid for the envelope protein (Env) of HIV-1 JRFL (CCR5-tropic) (29). HIV-1 virus-like particles (VLPs) were generated by cotransfection of HEK293 T cells with a plasmid encoding HIV Gag-GFP and an expression plasmid for Env of HIV-1 JRFL (29). Replication-competent HIV AD8 and NL4-3 were generated by transfection of HEK293 T cells with the proviral constructs pAD8 and pNL4-3. Cell-free supernatants were harvested, filtered, and titrated using a p24gag enzyme-linked immunosorbent assay (ELISA).
HIV-1 capture and gp120 binding assays.
Freshly isolated mast cells were pulsed with an amount of HIV-1-gag-GFP/JRFL VLPs corresponding to 40 ng of p24gag for 1 h at 4°C, and VLPs/ΔEnv, which do not incorporate HIV-1 envelope proteins, were used to monitor nonspecific binding. The levels of Gag-GFP were detected by flow cytometry. To test the location of cell-associated HIV-1, virus-loaded cells were treated with 0.25% trypsin (without EDTA) (Invitrogen) for 5 min at RT. Virus binding was visualized by confocal microscopy or transmission electron microscopy (TEM) as described below. The HIV-1 gp120 binding assay was performed as previously described (29). Briefly, mast cells were incubated with 5 μg/ml gp120 (JRFL) (eEnzyme) in adherence buffer (1 mM CaCl2, 2 mM MgCl2, 5% bovine serum albumin [BSA], pH 7.4) for 1 h at 4°C and then fixed with 4% PFA for 10 min and stained with goat anti-gp120 antibody (SAB3500463; Sigma-Aldrich). Subsequently, the cells were stained with a fluorescein isothiocyanate (FITC)-conjugated secondary anti-goat antibody (sc-2356; Santa Cruz Biotechnology) and detected by flow cytometry.
HIV-1 transmission and enhancement assays.
Freshly isolated mast cells were loaded with the pseudotyped single-cycle luciferase reporter HIV-luc/JRFL (10 ng of p24gag) for 2 h at 37°C and then washed thoroughly and cocultured with Hut/CCR5 cells for 2 days. Viral infection was analyzed by measuring the luciferase activities in cell lysates by use of a commercially available kit (Promega). For the blocking experiments, mast cells were pretreated with 10 μg/ml of anti-DC-specific intercellular adhesion molecule 3 (ICAM3)-grabbing nonintegrin (anti-DC-SIGN) antibody (120507; Abcam), anti-heparan sulfate proteoglycan (anti-HSPG) antibody (A7L6; Abcam), anti-α4 (EPR1355Y; Abcam), or anti-β7 (EP5948; Abcam) or with 20 μg/ml of mannan for 1 h at 4°C prior to coculture with T cells. Where denoted, transwell plates with a 0.4-μm insert membrane were used to separate the donor cells from the target cells.
The enhancement assay was performed as previously described (29, 30). Briefly, mast cells were loaded with HIV-luc/JRFL (1 ng p24gag) for 2 h and the pulsed cells cocultured with Hut/CCR5 cells for an additional 2 days, or the virus was added directly to Hut/CCR5 cells for 2 days of infection. A commercially available kit (Promega) was used to analyze viral infection by measuring the luciferase activity in the cell lysate.
HIV-1 infection assay.
HIV-1 infection was determined mainly by real-time PCR for quantification of viral replication products. FcεR1+ mast cells, MDDCs, or PHA-P-activated PBLs were inoculated with wild-type HIV AD8 or HIV NL4-3 (5 ng of p24gag) for 2 h and then washed and further cultured for the indicated time. Total cellular DNA or RNA was extracted by use of a QIAamp DNA minikit or QIAamp RNeasy minikit (Qiagen). The integrated HIV-1 proviral DNA, HIV-1 late reverse transcription products, HIV-1 total transcribed RNA, and multiply spliced (Tat-Rev) and singly spliced (Vpu) mRNAs were quantified using the primers and probes listed in Table 1. The products were semiquantified by use of SYBR green I and normalized to β-actin. Real-time PCR was performed on an ABI 7900HT real-time PCR system. Purified FcεR1+ mast cells were infected with replication-competent HIV-1 AD8 (5 ng of p24gag) for 2 h and then washed thoroughly. The cell culture supernatant was harvested at 1 or 2 days postinfection for either HIV-1 p24gag capture ELISA to detect viral production or titration in TZMB1 indicator cells, which contain a long terminal repeat (LTR)-driven luciferase reporter.
TABLE 1.
Primers and probes used for quantitative real-time PCR
| Target | Primer name | Primer sequence (5′–3′)a |
|---|---|---|
| HIV-1 integrants | Alu-F (1st cycle) | AGCCTCCCGAGTAGCTGGGA |
| SB704 (1st cycle) | TGCTGGGATTACAGGCGTGAG | |
| Gag-R (1st cycle) | CAATATCATACGCCGAGAGTGCGCGCTTCAGCAAG | |
| LTR-F (2nd cycle) | TTGTTACACCCTATGAGCCAGC | |
| Gag-R (2nd cycle) | CAATATCATACGCCGAGAGTGC | |
| P-HUS-SS1 (probe) | FAM-TAGTGTGTGCCCGTCTGTTGTGTGAC-TAMRA | |
| HIV-1 multiply spliced RNA | Tat-Rev-F | ATGGCAGGAAGAAGCGGAG |
| Tat-Rev-R | ATTCCTTCGGGCCTGTCG | |
| HIV-1 singly spliced RNA | Vpu-F | GGCGGCGACTGGAAGAAGC |
| Vpu-R | CTATGATTACTATGGACCACAC | |
| HIV-1 unspliced total RNA | Gag-F | GTGTGGAAAATCTCTAGCAGTGG |
| Gag-R | CGCTCTCGCACCCATCTC | |
| Late reverse transcription | MH531(F) | TGTGTGCCCGTCTGTTGTGT |
| MH532(R) | GAGTCCTGCGTCGAGAGATC | |
| LTR-P (probe) | FAM-CAGTGGCGCCCGAACAGGGA-TAMRA | |
| β-Actin | Actin-F | GGGAAATCGTGCGTGACAT |
| Actin-R | GTCAGGCAGCTCGTAGCTCTT |
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Flow cytometry.
Mast cells were stained with specific monoclonal antibodies or isotype-matched IgG controls. Monoclonal antibodies for specific staining of human molecules were as follows: allophycocyanin (APC)-CD117 (A3C6E2; Biolegend), APC-FcεR1α (AER-37; eBioscience), peridinin chlorophyll protein (PerCP)-Cy5.5-CD123 (6H6; eBioscience), phycoerythrin (PE)-CD203c (NP4D6; Biolegend), FITC-DC immunoreceptor (FITC-DCIR) (50586; R&D Systems), PE–DC-SIGN (eB-h209; eBioscience), PE-CD4 (L3T4; eBioscience), APC-CXCR4 (12G5; BD Pharmingen), and APC-Cy7-CCR5 (2D7; BD Pharmingen) antibodies. Purified antibodies directed against human tryptase (G3; Merck Millipore), HSPG (A7L6; Abcam), α4 (EPR1355Y; Abcam), and β7 (EP5948; Abcam) were used in some experiments, and secondary anti-mouse IgG–FITC or anti-rat IgG–FITC was used for detection. In the appropriate experiments, human IgE protein (ab90392; Abcam) was used, followed by PE-conjugated anti-human IgE antibody (MHE-18; Biolegend). The stained cells were detected using a Fortessa flow cytometer (BD Pharmingen) and analyzed with FlowJo 7.6.1 software.
Confocal microscopy.
Mast cells were incubated with an amount of HIV-Gag-GFP/JRFL equivalent to 40 ng p24gag for 1 h at 37°C and then seeded onto poly-l-lysine-coated microscope slides (Polyscience) after washing. For the formation of virological synapses, VLP-loaded mast cells were cocultured with Hut/CCR5 cells for 30 min in polystyrene tubes (Falcon) at 37°C, followed by seeding onto slides. Cells were then fixed with 4% PFA for 10 min at RT. For immunostaining, purified monoclonal antibodies against human CD4 (clone Q4120; Sigma-Aldrich), HSPG (A7L6; Abcam), α4 (EPR1355Y; Abcam), β7 (EP5948; Abcam), DC-SIGN (120507; Abcam), and tryptase (G3; Merck Millipore) and a polyclonal antibody against CCR5 (ab7346; Abcam) were added first, and then an Alexa 546-labeled goat anti-mouse (or anti-rabbit) IgG (1 μg/ml; Invitrogen) secondary antibody was used. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). All slides were mounted with fluorescence mounting medium (Dako) and observed under a laser scanning confocal microscope (Leica SP5).
Transmission electron microscopy.
The morphological characteristics of freshly isolated mast cells and HIV-1 binding on cells were visualized by transmission electron microscopy as previously described (29). Mast cells were pulsed with replication-competent HIV-1 AD8 (5 ng p24gag) for 2 h and then were fixed. Thin sections were examined using a Jeol JEM-1230 TEM operating at 100 kV.
Statistical analysis.
SigmaStat software was used to perform paired and unpaired t tests to analyze the significance of differences.
RESULTS
Purification of mast cells from human intestinal mucosa.
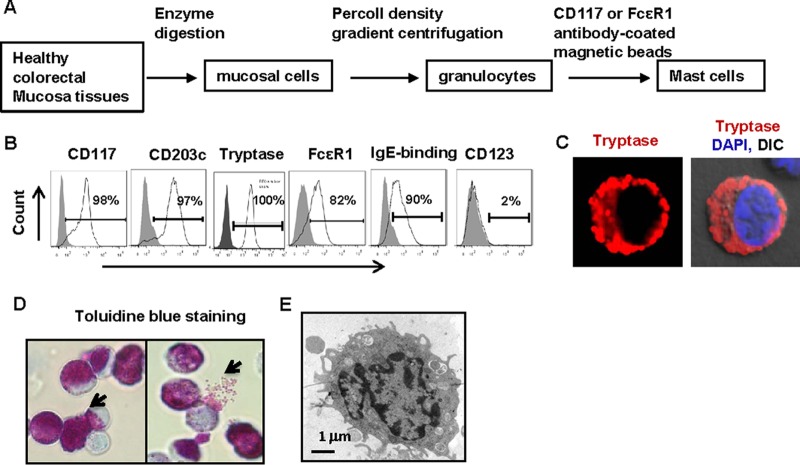
We collected normal intestinal samples from sites adjacent to excised colorectal carcinoma samples for mechanical fragmentation, enzyme digestion, and Percoll density gradient centrifugation (GE Healthcare). The granulocyte fraction was harvested, and CD117+ mast cells were positively selected using anti-CD117 or anti-FcεR1 antibody-coated magnetic beads (Fig. 1A). In the anti-CD117 antibody-enriched cells, 97% of the cells presented a CD203c+ phenotype, and no or little expression of CD123 was observed (Fig. 1B). All cells showed a tryptase-positive reaction on intracellular staining, and the majority of purified cells expressed the high-affinity IgE receptor FcεR1 and displayed binding with soluble IgE immunoglobulin (Fig. 1B). Tryptase is one of the granule components of mast cells and could be observed by confocal microscopy of intracellular staining (Fig. 1C), and ongoing degranulation of cells was also observed after toluidine blue staining (Fig. 1D). Under transmission electron microscopy, purified cells exhibited a characteristic phenotype, with the monolobed nuclei and numerous narrow, elongated folds around the cells (Fig. 1E) that are typical of mast cells (31).
FIG 1.
Characteristics of intestinal mucosal mast cells. (A) Enrichment and purification of mucosal mast cells from human healthy colorectal tissues. (B) Phenotype of purified mast cells as analyzed by immunostaining with specific antibodies and flow cytometry. (C) Intracellular immunostaining of tryptase (red) was confirmed by confocal microscopy; nuclei were stained with DAPI. DIC, differential interference contrast. (D) Positive staining of mast cells by toluidine blue. (E) Visualization of mast cells by transmission electron microscopy.
Human mucosal mast cells express HIV-1 attachment factors for viral capture.
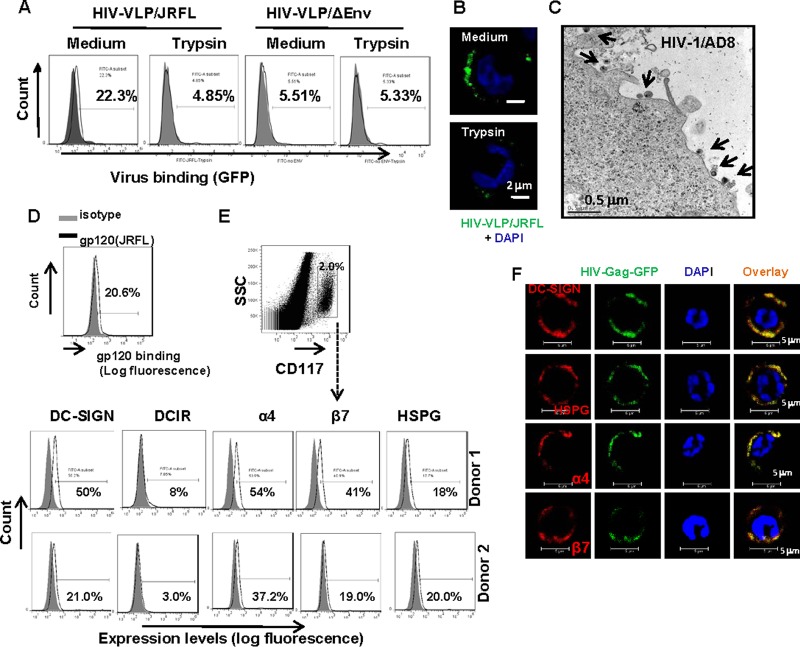
To investigate the interaction of mast cells with HIV-1, we first explored the binding of viruses to cells. Freshly isolated mast cells were pulsed with HIV-1-gag-GFP/JRFL VLPs, and VLPs/ΔEnv, which do not incorporate HIV-1 envelope proteins, were used to monitor nonspecific binding. Viral association was quantified by flow cytometry to detect green fluorescent protein (GFP) levels. At 4°C, about 22.3% of mast cells were found to capture JRFL VLPs, and no obvious binding was observed with VLPs/ΔEnv, indicating that the binding was envelope dependent and that the cell-associated HIV-1 particles could be removed by trypsin treatment (Fig. 2A). Confocal microscopy was also used to visualize and confirm viral surface binding (Fig. 2B), and replication-competent HIV-1 AD8 was used to visualize the binding of virus to mast cells by TEM (Fig. 2C). To confirm that HIV-1 binding is envelope dependent, we examined the binding of recombinant HIV-1 gp120 glycoprotein to mast cells. As shown in Fig. 2D, HIV-1 JRFL-derived gp120 glycoproteins were found to bind to mast cells.
FIG 2.
Intestinal mucosal mast cell-mediated HIV-1 capture. (A) Detection of HIV-1 VLP binding on mast cells by flow cytometry. VLPs containing Gag-GFP were pulsed with mast cells at 4°C, and VLPs/ΔEnv were used as the control to monitor nonspecific binding. Trypsin treatment was used to remove surface-bound viruses. (B) HIV-1 VLP association with cells was observed by confocal microscopy. (C) Binding of replication-competent HIV-1 AD8 on mast cells as visualized by TEM. Arrows indicate viruses. (D) Binding of gp120 on mast cells. Purified mast cells were cultured with recombinant gp120 glycoproteins for 1 h at 4°C and then fixed for immunostaining and detected by flow cytometry. (E) Expression of HIV-1 attachment factors as detected by immunostaining with specific antibodies and flow cytometry. (F) Colocalization of HIV VLPs with DC-SIGN, HSPG, or α4β7 integrin. Purified mast cells were incubated with HIV-Gag-GFP/JRFL VLPs (40 ng p24gag) for 1 h at 4°C and then seeded onto poly-l-lysine-coated microscope slides. Cells were fixed and immunostained with specific antibodies against human DC-SIGN, HSPG, α4, or β7, followed by secondary Alexa 546-labeled goat anti-mouse IgG antibodies. Nuclei were stained with DAPI, and cells were observed by confocal microscopy.
In addition to entry receptors, viruses subvert a wide variety of molecules expressed on the cell surface as viral attachment receptors; among these, HSPG, α4β7 integrin, and the C-type lectins DC-SIGN and DCIR (also known as CLEC4A) have been shown to bind to HIV-1 gp120 (8, 32–34). Heparan sulfate was recently demonstrated to be a novel attachment receptor for Nipah virus to mediate viral binding and spread (35). We found that mast cells expressed multiple HIV-1 attachment factors (HAFs), including DC-SIGN, α4β7 integrin, and HSPG, and also expressed low levels of DCIR (Fig. 2D). Using confocal microscopy, we observed the colocalization of HIV-1 with the tested HAFs DC-SIGN, HSPG, and α4β7 integrin (Fig. 2F), indicating the role of HAFs in viral capture. Collectively, these data demonstrate that human mucosal mast cells express HIV-1 attachment factors for viral capture.
Human mucosal mast cells mediate HIV-1 trans-infection of CD4+ T cells.
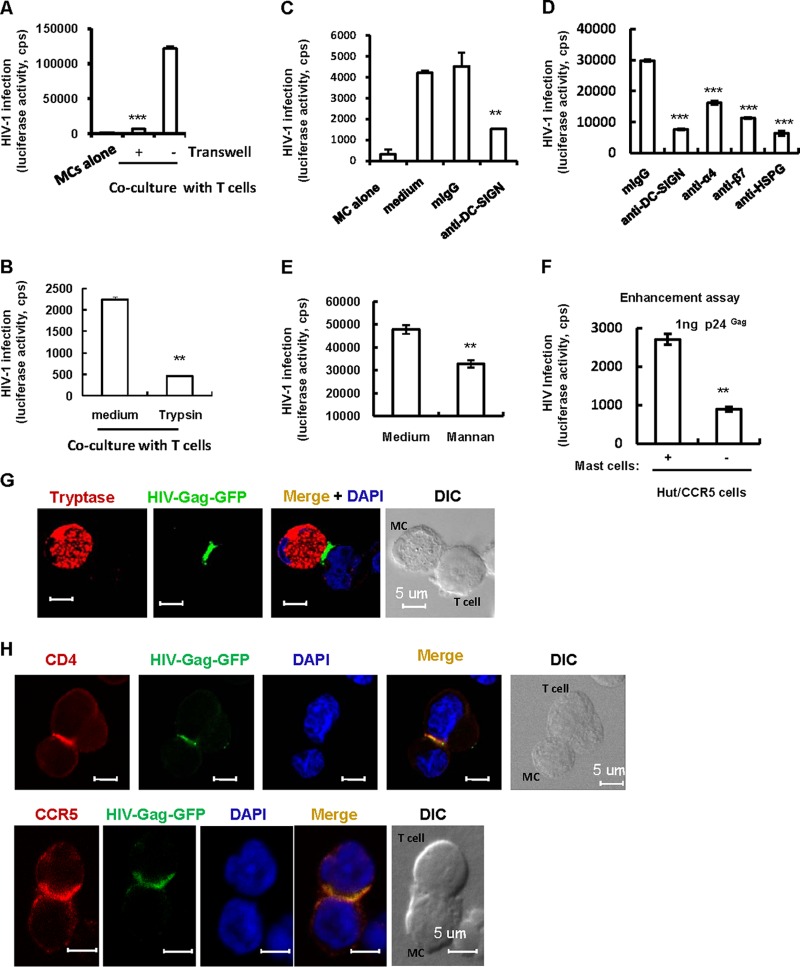
To investigate whether mast cells are capable of transferring surface-bound viruses to CD4+ T cells, freshly purified mucosal mast cells were pulsed with pseudotyped single-cycle HIV-luc/JRFL viruses and then cocultured with Hut/CCR5 CD4+ T cells for 48 h. Infection caused by the transfer of HIV-1 to the Hut/CCR5 cells was monitored by measuring luciferase activity. Mast cells were found to efficiently transfer HIV-1 to cocultured Hut/CCR5 cells, leading to robust infection (Fig. 3A).
FIG 3.
Mucosal mast cells mediate HIV-1 trans-infection of CD4+ T cells. (A) Viral trans-infection. Freshly isolated mucosal mast cells were incubated with single-cycle infectious HIV-luc/JRFL for 2 h. Virus-harboring cells were washed and cocultured with or without CD4+ Hut/CCR5 T cells, and HIV trans-infection was determined after 2 days by measuring the luciferase activity. Where indicated, a transwell culture plate with a 0.4-μm insert membrane was used to separate the virus-loaded mast cells from the target cells. (B) Treatment with trypsin to remove surface-bound viral particles before coculture diminished viral trans-infection. (C to E) Pretreatment with antibodies against DC-SIGN, HSPG, or α4β7 integrin or with mannan prior to viral inoculation diminished mast cell-mediated transmission. (F) Enhancement assay. HIV-luc/JRFL-pulsed mast cells were cocultured with Hut/CCR5 cells, or the same amounts of cell-free viruses were added directly to T cells, and viral infection was measured as described above after 2 days of culture. (G) Visualization of the cell-cell conjunction between mucosal mast cells and T cells by confocal microscopy. After 2 h of exposure to HIV-Gag-GFP/JFRL, mast cells were washed and cocultured with Hut/CCR5 cells for 1 h. Cells were fixed and immunostained for intracellular tryptase (red); nuclei were stained with DAPI (blue). (H) Recruitment of viruses, CD4, and CCR5 to conjunction sites. Data in graphs are means and standard deviations (SD). Results are representative of three independent experiments. cps, counts per second. **, P < 0.01; ***, P < 0.001 (paired t test).
To determine whether viral transfer requires direct contact between mast cells and CD4+ T cells, a transwell culture plate with a 0.4-μm insert membrane was used to separate the virus-loaded mast cells from the Hut/CCR5 target cells. Notably, mast cell-mediated transmission of HIV-luc/JRFL ceased in the transwell assay (Fig. 3A), suggesting that contact between mast cells and CD4+ T cells is required for viral transfer.
Moreover, treating the virus-harboring mast cells with trypsin before coculturing them with T cells was found to significantly diminish HIV-1 transmission (Fig. 3B), suggesting that surface-bound HIV-1 particles play the greatest role in viral transfer. Incubation of mast cells with antibodies against DC-SIGN, HSPG, or α4β7 integrin or with mannan before viral inoculation was found to significantly diminish mast cell-mediated HIV-1 trans-infection (Fig. 3C to E), suggesting a role for these HAFs in mediating viral spread. We also performed an enhancement experiment to show the significantly increased viral infection mediated by cell-associated HIV-1 relative to that with an equivalent amount of free viruses (Fig. 3F).
Direct cell-cell contact to form infectious synapses appears to be essential for cell-associated viral trans-infection (1, 2, 30). Recruitment of HIV-1-gag-GFP/JRFL VLPs to the mast cell-CD4+ T cell contact sites that form infectious synapses was visualized by confocal microscopy (Fig. 3G). Analysis of infectious synapses between dendritic cells and T cells revealed the recruitment of HIV-1 receptor and coreceptors to conjugate sites (30), and we also observed that the CD4 and CCR5 molecules on T cells were recruited to the interface whereon mast cells concentrated viral particles (Fig. 3H). Together, these data demonstrate that mast cells isolated from the human gut mucosa can mediate HIV-1 trans-infection of CD4+ T cells through viral attachment factor-dependent viral binding on the cell surface.
Human gut mucosal mast cells support HIV-1 infection.
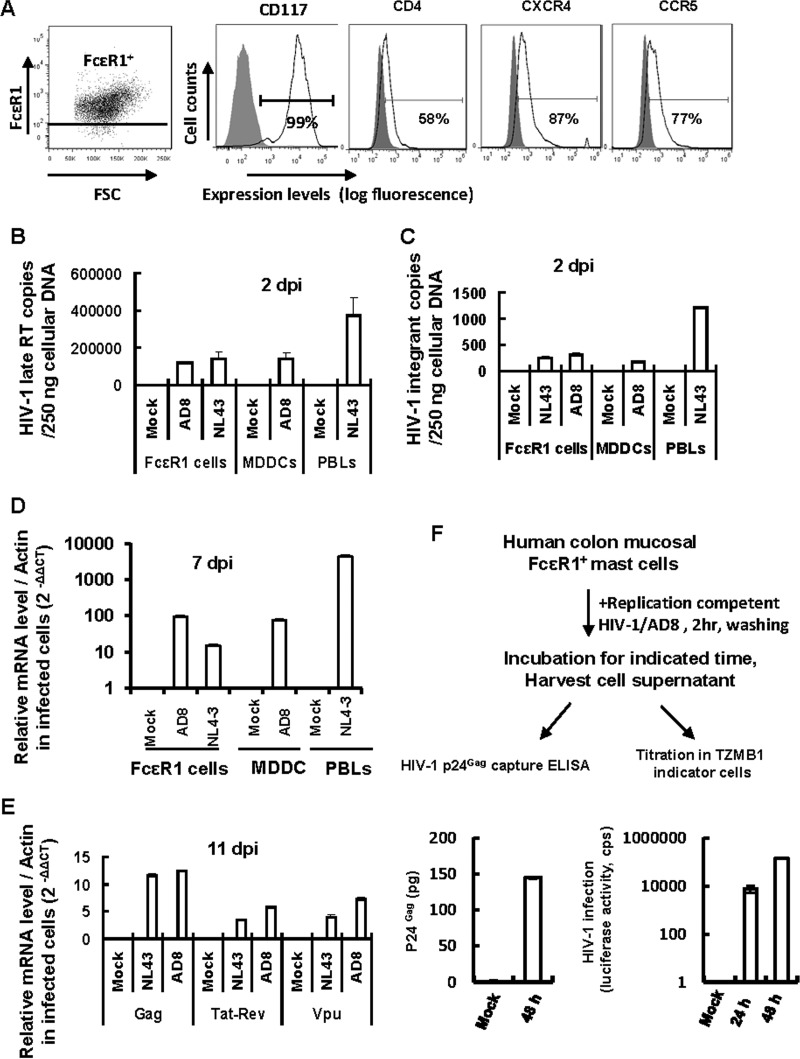
To assess the expression of HIV-1 (co)receptors, anti-FcεR1 antibody-enriched mucosal mast cells were immunostained with specific antibodies and detected by flow cytometry. Mast cells expressed CD4 and the coreceptors CXCR4 and CCR5 (Fig. 4A).
FIG 4.
HIV-1 infection of gut mucosal mast cells. (A) Expression of HIV-1 (co)receptors as detected by immunostaining with specific antibodies and flow cytometry. (B to E) HIV-1 infection of mast cells was quantified by real-time PCR. Freshly isolated mucosal mast cells, autologous MDDCs, and PHA-P-activated PBLs were infected with wild-type HIV-1 AD8 or NL4-3 for 2 h, washed, cultured, and harvested at the indicated time. Mock infection was used as a control. Cellular DNA was extracted for quantification of late reverse transcriptase (late RT) products (B) and viral integrants (by Alu-PCR) (C), using 250 ng of cellular DNA for each sample, or total RNA was extracted after 7 or 11 days of infection, and the levels of HIV-1 total Gag RNA, multiply spliced RNA (Tat-Rev), and singly spliced RNA (Vpu) were quantified by real-time PCR and normalized to β-actin (D and E). (F) Mast cells are susceptible to productive HIV-1 infection. Purified FcεR1+ mast cells were infected with an amount of wild-type replication-competent HIV-1 AD8 equivalent to 5 ng p24gag for 2 h and then washed thoroughly. The cell culture supernatant was harvested at the indicated time for either HIV-1 p24gag capture ELISA to detect viral production or titration in TZMB1 indicator cells. Data are means and SD. Results are representative of four independent experiments.
To investigate the susceptibility of mucosal mast cells to HIV-1 infection, mast cells were purified with anti-FcεR1 antibody-coated magnetic beads and then pulsed for 2 h with wild-type replication-competent viruses, including HIV-1 AD8 (CCR5-tropic) and HIV-1 NL4-3 (CXCR4-tropic), in amounts equivalent to 5 ng p24gag. Monocyte-derived dendritic cells (MDDCs) and PHA-P-activated autologous PBLs were used as controls. Viral replication was assessed by quantitative real-time PCR-based methods at the indicated culture times (36). HIV-1 AD8 and NL4-3 exhibited integrants and late reverse transcriptase products in mast cells 2 days after viral exposure (Fig. 4B and C). Viral infection was further detected by measuring HIV-1 transcription after 7 or 11 days of culture (Fig. 4D and E). HIV-1 total transcribed RNA, multiply spliced RNA of tat-rev, and singly spliced RNA of vpu were monitored and normalized to the housekeeping β-actin gene. Mast cells supported HIV-1 transcription in exposed cells compared to mock-infected controls (Fig. 4D and E). The PCR primers and probes are listed in Table 1. The products were semiquantified with SYBR green I and normalized to β-actin.
To further confirm the susceptibility of mast cells to productive HIV-1 infection, freshly purified FcεR1+ mast cells were infected with replication-competent HIV-1 AD8 for 2 h, and after washing, the cells were further cultured and the supernatants were harvested for either p24gag capture ELISA to monitor viral production or titration in TZMB1 cells, which contain an LTR-driven luciferase reporter (Fig. 4F). The results showed that infected mast cells actually released infectious HIV-1 particles into the culture supernatant (Fig. 4F). Taken together, these data show that HIV-1 can integrate and replicate in mucosal mast cells, confirming a role for mast cells as a reservoir for persistent viral infection (37).
DISCUSSION
In this study, we showed for the first time that colorectal mucosal mast cells express multiple HAFs and mediate efficient viral binding and trans-infection of CD4+ T cells. This finding increases our understanding of the role of mast cells during HIV-1 mucosal infection. During the invasion of pathogens, mast cells can recruit diverse cell types, such as T cells, macrophages, dendritic cells, neutrophils, epithelial cells, and endothelial cells, to the site of infection for clearance of invading pathogens (9). The wide communication of mast cells with other types of cells during HIV-1 infection could provide more chances for viral dissemination. We recently showed that peripheral blood circulating basophils could also capture HIV-1 through viral binding to HAFs and could transfer bound viruses to adjacent CD4+ T cells (29). Strategies to prevent granulocyte-mediated viral capture and transfer may be developed into a new form of therapy.
Both surface-associated and intracellularly internalized viruses contributed to cell-mediated viral spread in studies of DCs (1, 2, 30). The transfer of viruses mediated by mast cells is dependent on the surface-bound majority of viral particles, as the removal of surface-bound viruses by trypsin treatment dramatically impairs viral spread. Immature DC-mediated HIV-1 transfer has been demonstrated to be dependent mainly on the surface-bound viruses bound to DC-SIGN (1, 2). The formation of a cell-cell conjunction to which numerous intact viral particles and viral receptors can be recruited appears to be required for efficient viral transfer, and this was proved previously for other types of cells during mediation of viral spread (1, 2, 29, 30). Similarly, CD4 and CCR5 molecules were also recruited to the cell contact sites formed between mast cells and T cells in this study.
DC-SIGN, α4β7, and heparan sulfate proteoglycan are principle host cell HAFs with high binding affinities for HIV-1 gp120, and they have been shown to mediate HIV-1 capture and trans-infection (1, 8, 32, 33, 38–42). The administration of an anti-α4β7 monoclonal antibody reduced mucosal transmission of simian immunodeficiency virus (SIV) in macaques (43). Further studies and confirmation of the potential role of viral attachment factors in viral capture and transmission might be helpful in the design of antiviral strategies.
The HIV-1 gp120 glycoprotein, which acts as a viral superantigen, induces the release of histamine, cysteinyl leukotrienes, and TH2 cytokines (interleukin-4 [IL-4], IL-5, IL-10, and IL-13) from healthy human FcεR1+ basophils and mast cells, thereby contributing to the creation of an allergy-like and Th2-biased condition (44–46). The binding of the gp120 glycoprotein to these FcεR1+ innate system cells is bridged by its interaction with the heavy chain variable 3 region of IgE (47, 48). Enhanced serum IgE levels have been observed in HIV-1-infected adults and children, which is believed to be due to a shift from Th1 to Th2 cytokine production (49–51). Thus, the elucidation of the interaction of HIV-1 with these FcεR1+ granulocytes might facilitate the understanding of the Th2 polarization and allergic disorders observed during HIV-1 infection.
Mast cells have been shown to take crucial roles in immunosurveillance for defense against invading pathogens. Productive infection with murine cytomegalovirus (CMV) triggers mast cell degranulation, resulting in the release of CCL5, which attracts protective CD8 T cells to control viral infection (13, 23). The Toll-like receptor 3-mediated signaling that triggers mast cell activation may play a role in CD8+ T cell recruitment (14). In response to dengue virus, mast cells trigger the activation of antiviral intracellular host response pathways and induce the de novo transcription of cytokines, including tumor necrosis factor alpha (TNF-α) and alpha interferon (IFN-α), and multiple chemokines, and cellular sensors for viral RNA, such as melanoma differentiation-associated gene 5 (MDA5) and retinoic acid-inducible gene 1 (RIG-I), were proved to contribute to the observed transcriptional response (18). These dengue virus-activated mast cells recruit natural killer and natural killer T cells to infection sites for viral clearance (18). Mast cells have been demonstrated to play a role in the inflammatory pathology induced by influenza A virus (IAV), as IAV-induced mast cells trigger RIG-I signaling-dependent production of cytokines and chemokines, resulting in a cytokine storm and systemic disease in mice during viral infection (52). Further investigations into whether HIV-1 or other retroviruses can also trigger mast cell activation could be very helpful for the elucidation of host immune responses during primary viral infection.
Several works have described the susceptibility of mast cells to HIV-1 infection. Placental tissue-isolated mast cells (PLMCs) express HIV-1 (co)receptors and show susceptibility to infection with CCR5-tropic HIV-1 in vitro (37, 53, 54). In contrast, progenitor mast cells (PrMCs) differentiated from bone marrow CD34+ pluripotent progenitors resist viral infection until full maturation (55), probably due to the diminished expression of viral receptors (37). Intriguingly, mast cells have been proposed to serve as long-lived inducible reservoirs for persistent HIV-1 infection (37, 55, 56). HIV-1-harboring PLMCs have been isolated from HIV-infected pregnant women in the late third trimester even during highly active antiretroviral treatment (HAART), which suggests that the placenta may be a sanctuary site for virus and may play a major role in mother-to-child virus transmission.
Combined, our data indicate a novel role for mucosal mast cells in viral capture and local dissemination in mucosal tissues. Although confirmatory studies using macaque models or ex vivo explant studies are needed, our findings have identified gut mucosal mast cells as potential novel “gatekeeper” cells which may facilitate local HIV-1–host cell interaction and viral spread during primary infection. These studies provide new insights into novel mechanisms for combating viral infection.
ACKNOWLEDGMENTS
This study was supported by research funding to J.-H.W. from the Interdisciplinary and Collaboration Team of the Chinese Academy of Sciences, the Natural Science Foundation of China (grant 81572001), and the National Grand Program on Key Infectious Disease (grant 2014ZX10001003).
REFERENCES
- 1.Wu L, KewalRamani VN. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol 6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JH, Janas AM, Olson WJ, Wu L. 2007. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol 81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin N, Sattentau Q. 2009. Cell-to-cell HIV-1 spread and its implications for immune evasion. Curr Opin HIV AIDS 4:143–149. doi: 10.1097/COH.0b013e328322f94a. [DOI] [PubMed] [Google Scholar]
- 4.Shen R, Smythies LE, Clements RH, Novak L, Smith PD. 2010. Dendritic cells transmit HIV-1 through human small intestinal mucosa. J Leukoc Biol 87:663–670. doi: 10.1189/jlb.0909605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piguet V, Steinman RM. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol 28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puigdomenech I, Casartelli N, Porrot F, Schwartz O. 2013. SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells. J Virol 87:2846–2856. doi: 10.1128/JVI.02514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed Z, Kawamura T, Shimada S, Piguet V. 2015. The role of human dendritic cells in HIV-1 infection. J Invest Dermatol 135:1225–1233. doi: 10.1038/jid.2014.490. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575–585. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 9.Abraham SN, St John AL. 2010. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urb M, Sheppard DC. 2012. The role of mast cells in the defence against pathogens. PLoS Pathog 8:e1002619. doi: 10.1371/journal.ppat.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll-Portillo A, Surviladze Z, Cambi A, Lidke DS, Wilson BS. 2012. Mast cell synapses and exosomes: membrane contacts for information exchange. Front Immunol 3:46. doi: 10.3389/fimmu.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galli SJ, Nakae S, Tsai M. 2005. Mast cells in the development of adaptive immune responses. Nat Immunol 6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 13.Podlech J, Ebert S, Becker M, Reddehase MJ, Stassen M, Lemmermann NA. 2015. Mast cells: innate attractors recruiting protective CD8 T cells to sites of cytomegalovirus infection. Med Microbiol Immunol 204:327–334. doi: 10.1007/s00430-015-0386-1. [DOI] [PubMed] [Google Scholar]
- 14.Orinska Z, Bulanova E, Budagian V, Metz M, Maurer M, Bulfone-Paus S. 2005. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood 106:978–987. doi: 10.1182/blood-2004-07-2656. [DOI] [PubMed] [Google Scholar]
- 15.Guhl S, Franke R, Schielke A, Johne R, Kruger DH, Babina M, Rang A. 2010. Infection of in vivo differentiated human mast cells with hantaviruses. J Gen Virol 91:1256–1261. doi: 10.1099/vir.0.019505-0. [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama K. 1977. Histamine release from rat mast cells induced by Sendai virus. Nature 270:614–615. doi: 10.1038/270614a0. [DOI] [PubMed] [Google Scholar]
- 17.Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. 2008. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood 111:5467–5476. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
- 18.St John AL, Rathore AP, Yap H, Ng ML, Metcalfe DD, Vasudevan SG, Abraham SN. 2011. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc Natl Acad Sci U S A 108:9190–9195. doi: 10.1073/pnas.1105079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MG, McAlpine SM, Huang YY, Haidl ID, Al-Afif A, Marshall JS, Anderson R. 2012. RNA sensors enable human mast cell anti-viral chemokine production and IFN-mediated protection in response to antibody-enhanced dengue virus infection. PLoS One 7:e34055. doi: 10.1371/journal.pone.0034055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham AC, Temple RM, Obar JJ. 2015. Mast cells and influenza A virus: association with allergic responses and beyond. Front Immunol 6:238. doi: 10.3389/fimmu.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki R, Kawamura T, Goshima F, Ogawa Y, Nakae S, Nakao A, Moriishi K, Nishiyama Y, Shimada S. 2013. Mast cells play a key role in host defense against herpes simplex virus infection through TNF-α and IL-6 production. J Invest Dermatol 133:2170–2179. doi: 10.1038/jid.2013.150. [DOI] [PubMed] [Google Scholar]
- 22.Becker M, Lemmermann NA, Ebert S, Baars P, Renzaho A, Podlech J, Stassen M, Reddehase MJ. 2015. Mast cells as rapid innate sensors of cytomegalovirus by TLR3/TRIF signaling-dependent and -independent mechanisms. Cell Mol Immunol 12:192–201. doi: 10.1038/cmi.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebert S, Becker M, Lemmermann NA, Buttner JK, Michel A, Taube C, Podlech J, Bohm V, Freitag K, Thomas D, Holtappels R, Reddehase MJ, Stassen M. 2014. Mast cells expedite control of pulmonary murine cytomegalovirus infection by enhancing the recruitment of protective CD8 T cells to the lungs. PLoS Pathog 10:e1004100. doi: 10.1371/journal.ppat.1004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valitutti S, Espinosa E. 2010. Cognate interactions between mast cells and helper T lymphocytes. Self Nonself 1:114–122. doi: 10.4161/self.1.2.11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop PE, McMillan A, Gilmour HM. 1987. Immunological study of the rectal mucosa of men with and without human immunodeficiency virus infection. Gut 28:1619–1624. doi: 10.1136/gut.28.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guimaraes JV, Costa FB, Andrade WM, Vencio EF, Salge AK, Siqueira KM, Texeira Vde P. 2011. Quantification of mast cells in the uterine cervix of women infected with human immunodeficiency virus. Ann Diagn Pathol 15:318–322. doi: 10.1016/j.anndiagpath.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Dong C, Janas AM, Wang JH, Olson WJ, Wu L. 2007. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J Virol 81:11352–11362. doi: 10.1128/JVI.01081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmetzer O, Valentin P, Smorodchenko A, Domenis R, Gri G, Siebenhaar F, Metz M, Maurer M. 2014. A novel method to generate and culture human mast cells: peripheral CD34+ stem cell-derived mast cells (PSCMCs). J Immunol Methods 413:62–68. doi: 10.1016/j.jim.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Jiang AP, Jiang JF, Guo MG, Jin YM, Li YY, Wang JH. 2015. Human blood-circulating basophils capture HIV-1 and mediate viral trans-infection of CD4+ T cells. J Virol 89:8050–8062. doi: 10.1128/JVI.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 31.Dvorak AM. 2005. Ultrastructural studies of human basophils and mast cells. J Histochem Cytochem 53:1043–1070. doi: 10.1369/jhc.5R6647.2005. [DOI] [PubMed] [Google Scholar]
- 32.Ceballos A, Remes Lenicov F, Sabatte J, Rodriguez Rodrigues C, Cabrini M, Jancic C, Raiden S, Donaldson M, Agustin Pasqualini R Jr, Marin-Briggiler C, Vazquez-Levin M, Capani F, Amigorena S, Geffner J. 2009. Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells. J Exp Med 206:2717–2733. doi: 10.1084/jem.20091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol 9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 34.Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. 2008. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood 112:1299–1307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathieu C, Dhondt KP, Chalons M, Mely S, Raoul H, Negre D, Cosset FL, Gerlier D, Vives RR, Horvat B. 2015. Heparan sulfate-dependent enhancement of henipavirus infection. mBio 6:e02427. doi: 10.1128/mBio.02427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian RR, Guo HX, Wei JF, Yang CK, He SH, Wang JH. 2012. IFN-lambda inhibits HIV-1 integration and post-transcriptional events in vitro, but there is only limited in vivo repression of viral production. Antiviral Res 95:57–65. doi: 10.1016/j.antiviral.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Sundstrom JB, Ellis JE, Hair GA, Kirshenbaum AS, Metcalfe DD, Yi H, Cardona AC, Lindsay MK, Ansari AA. 2007. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood 109:5293–5300. doi: 10.1182/blood-2006-11-058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel M, Yanagishita M, Roderiquez G, Bou-Habib DC, Oravecz T, Hascall VC, Norcross MA. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses 9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 39.Vives RR, Imberty A, Sattentau QJ, Lortat-Jacob H. 2005. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J Biol Chem 280:21353–21357. doi: 10.1074/jbc.M500911200. [DOI] [PubMed] [Google Scholar]
- 40.Vidricaire G, Gauthier S, Tremblay MJ. 2007. HIV-1 infection of trophoblasts is independent of gp120/CD4 interactions but relies on heparan sulfate proteoglycans. J Infect Dis 195:1461–1471. doi: 10.1086/515576. [DOI] [PubMed] [Google Scholar]
- 41.Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, Ogundare O, Pascuccio M, Patel N, Wei D, Fauci AS, Arthos J. 2011. The genotype of early-transmitting HIV gp120s promotes alpha(4) beta(7)-reactivity, revealing alpha(4) beta(7)+/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog 7:e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura GR, Fonseca DP, O'Rourke SM, Vollrath AL, Berman PW. 2012. Monoclonal antibodies to the V2 domain of MN-rgp120: fine mapping of epitopes and inhibition of alpha4beta7 binding. PLoS One 7:e39045. doi: 10.1371/journal.pone.0039045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, Kersh EN, McNicholl JM, Hanson D, Reimann KA, Brameier M, Walter L, Rogers K, Mayne AE, Dunbar P, Villinger T, Little D, Parslow TG, Santangelo PJ, Villinger F, Fauci AS, Ansari AA. 2014. Targeting α4β7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med 20:1397–1400. doi: 10.1038/nm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Secor WE, Sundstrom JB. 2007. Below the belt: new insights into potential complications of HIV-1/schistosome coinfections. Curr Opin Infect Dis 20:519–523. doi: 10.1097/QCO.0b013e3282e9ac03. [DOI] [PubMed] [Google Scholar]
- 45.Min B, Paul WE. 2008. Basophils and type 2 immunity. Curr Opin Hematol 15:59–63. doi: 10.1097/MOH.0b013e3282f13ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker Y. 2004. HIV-1 induced AIDS is an allergy and the allergen is the Shed gp120—a review, hypothesis, and implications. Virus Genes 28:319–331. doi: 10.1023/B:VIRU.0000025778.56507.61. [DOI] [PubMed] [Google Scholar]
- 47.Patella V, Florio G, Petraroli A, Marone G. 2000. HIV-1 gp120 induces IL-4 and IL-13 release from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J Immunol 164:589–595. doi: 10.4049/jimmunol.164.2.589. [DOI] [PubMed] [Google Scholar]
- 48.Marone G, Florio G, Petraroli A, Triggiani M, de Paulis A. 2001. Human mast cells and basophils in HIV-1 infection. Trends Immunol 22:229–232. doi: 10.1016/S1471-4906(01)01903-2. [DOI] [PubMed] [Google Scholar]
- 49.Vigano A, Principi N, Crupi L, Onorato J, Vincenzo ZG, Salvaggio A. 1995. Elevation of IgE in HIV-infected children and its correlation with the progression of disease. J Allergy Clin Immunol 95:627–632. doi: 10.1016/S0091-6749(95)70326-8. [DOI] [PubMed] [Google Scholar]
- 50.Marone G, Florio G, Triggiani M, Petraroli A, de Paulis A. 2000. Mechanisms of IgE elevation in HIV-1 infection. Crit Rev Immunol 20:477–496. [PubMed] [Google Scholar]
- 51.Marone G, Florio G, Petraroli A, Triggiani M, de Paulis A. 2001. Role of human FcepsilonRI+ cells in HIV-1 infection. Immunol Rev 179:128–138. doi: 10.1034/j.1600-065X.2001.790113.x. [DOI] [PubMed] [Google Scholar]
- 52.Graham AC, Hilmer KM, Zickovich JM, Obar JJ. 2013. Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling. J Immunol 190:4676–4684. doi: 10.4049/jimmunol.1202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Li L, Wadley R, Reddel SW, Qi JC, Archis C, Collins A, Clark E, Cooley M, Kouts S, Naif HM, Alali M, Cunningham A, Wong GW, Stevens RL, Krilis SA. 2001. Mast cells/basophils in the peripheral blood of allergic individuals who are HIV-1 susceptible due to their surface expression of CD4 and the chemokine receptors CCR3, CCR5, and CXCR4. Blood 97:3484–3490. doi: 10.1182/blood.V97.11.3484. [DOI] [PubMed] [Google Scholar]
- 54.Bannert N, Farzan M, Friend DS, Ochi H, Price KS, Sodroski J, Boyce JA. 2001. Human mast cell progenitors can be infected by macrophagetropic human immunodeficiency virus type 1 and retain virus with maturation in vitro. J Virol 75:10808–10814. doi: 10.1128/JVI.75.22.10808-10814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundstrom JB, Little DM, Villinger F, Ellis JE, Ansari AA. 2004. Signaling through Toll-like receptors triggers HIV-1 replication in latently infected mast cells. J Immunol 172:4391–4401. doi: 10.4049/jimmunol.172.7.4391. [DOI] [PubMed] [Google Scholar]
- 56.Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J, Persaud D, Gallant JE, Cofrancesco J Jr, Quinn TC, Wilke CO, Ray SC, Siliciano JD, Nettles RE, Siliciano RF. 2006. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]