Abstract
Objective
Reduced reward responsiveness and altered response to loss of reward are observed in adults with major depressive disorder (MDD) and adolescents at increased risk for MDD based on family history. However, it is unclear whether altered behavioral responsiveness to reward/loss is a lifelong marker of MDD risk, which is evident before the normative adolescent increase in incentive responding.
Method
Healthy 7- to 10-year-old children of mothers with MDD (high risk: n = 27) or without MDD (low risk: n = 42) performed 2 signal detection tasks assessing response bias toward reward (approach) and away from loss (avoidance). Differences in approach/avoidance were related to MDD risk, child general depressive symptoms (maternal report), child-reported anhedonic symptoms, and child-reported negative mood symptoms via repeated-measures analysis of variance.
Results
MDD risk did not significantly relate to gain approach or loss avoidance. However, within high-risk children, higher numbers of maternal depressive episodes predicted blunted loss avoidance. Blunted gain approach was related to elevated anhedonic symptoms, whereas enhanced loss avoidance was related to elevated negative mood. Elevated negative mood was further related to blunted gain approach in high-risk children but related to enhanced gain approach in low-risk children.
Conclusion
In children, individual differences in specific depressive symptoms and recurrence of maternal depression significantly predicted gain approach/loss avoidance, but the presence/absence of maternal MDD did not. Child depressive symptoms characterized by low positive affect (anhedonia) were related to blunted gain responsiveness, whereas elevated depressed/negative mood was related to enhanced loss responsiveness. Findings suggest that relations between gain approach and negative mood may be an important distinction between those at high versus low risk for MDD.
Keywords: depression risk, reward, punishment, anhedonia
Behavioral and neural endophenotypes associated with risk for affective psychopathology have received much focus in recent years. Samples free of current pathology but at increased risk for developing affective disorders provide unique opportunities to parse risk-related endophenotypes from the effects of a disorder. For example, a maternal history of major depressive disorder (MDD), and, in particular, a more severe course of maternal MDD (i.e., younger age of onset and greater number of depressive episodes) confers increased risk for developing MDD.1–3 Healthy children at high risk for MDD often show elevated, subclinical levels of depressive symptoms, such as negative mood and anhedonia.4 There has also been a call for more studies investigating how specific domains of affective functioning, such as reward expectancy, learning, and loss reactivity, relate both to specific symptom constructs such as anhedonia5 and to familial MDD risk. Application of such approaches within adolescent and adult populations has yielded compelling results; however, little work has examined relations among incentive behaviors, familial MDD risk, and specific symptom domains (i.e., anhedonia and negative mood) in school-aged children.
Neuroimaging and behavioral studies have consistently reported reduced response to reward in both adults and adolescents with MDD.6–8 Across paradigms, groups with depression show reduced influence of reward feedback or contingencies on behavior/affect. Specifically, adults/ adolescents with depression are less willing to expend effort to obtain reward,9 show less response bias towards reward,10 and are less likely to seek reward under advantageous conditions (i.e., high probability and/or amount of reward)11,12 than individuals without depression. Neuro-imaging studies also report reduced response to reward in adults/adolescents with MDD, particularly within the striatum (for review of different components of reward processing in the adult literature, see Barch et al.13). Interestingly, healthy adolescents with familial MDD (i.e., at increased risk for developing MDD) also show reduced behavioral14 and striatal15,16 responses to reward. Furthermore, reduced response to reward in high-risk adolescents prospectively predicts worsening depressive symptoms and onset of depressive episodes.11,14,17
Together these lines of evidence indicate reduced reward responding not only in clinical depression, but also among adolescents at heightened risk for depression. However, given that typically developing adolescents show elevated gain approach (i.e., greater risk taking, greater social affiliation)18,19 and striatal responses to reward receipt relative to children and adults,20,21 whether behavioral response to reward is also reduced in school-aged children at high MDD risk is an important open question. The majority of studies investigating reward responses in high-risk groups do so during this normative “peak” in reward responsiveness, with very few studies focusing on childhood.22,23 As such, it is unclear whether blunted behavioral response to reward is characteristic of high-risk groups across development, or whether this group difference is less prominent in childhood and strengthens in adolescence when the normative peak in reward responsiveness occurs. The current study investigates whether the difference in behavioral responsiveness to reward observed between high- and low-risk groups is evident in children before adolescence.
Another important underexplored question is whether the reduced responsiveness discussed above is specific to reward or whether it reflects a more general blunting of responsiveness to incentives. The adult literature regarding responses to loss (of reward) or negative affective stimuli has been quite mixed. Some studies report blunted behavioral/ neural response to negative feedback/stimuli in MDD,24–27 whereas others report enhanced neural responses to negative feedback/stimuli in MDD.8,28 Far fewer studies have focused on loss in adolescent MDD risk; however, the few studies that do focus on this tend to report elevated neural responses to loss/aversive stimuli in high-risk groups.15,29
In addition to risk status, individual differences in depression-related symptoms are also relevant to understanding incentive responsiveness. For example, elevated levels of specific depressive symptoms such as anhedonia (reduced pleasure) or melancholy have been linked to blunted gain approach in healthy30 adults and those with depression.10 However, only a few studies have linked blunted gain approach to elevated anhedonia in adolescents/children.14,31 Interestingly, there is growing evidence in the adult literature also linking elevated anhedonia to blunted neural and behavioral responses to loss/negative affective stimuli,24–26,32 and some preliminary evidence links reduced hedonic capacity to blunted loss avoidance in children, as well.31 Given the lack of studies relating specific depressive symptoms, such as anhedonia, to both gain approach and loss avoidance behaviors in children at high and low risk for MDD, it is unclear whether anhedonia will relate to both types of behavior. Furthermore, it is unclear whether such relations would be specific to anhedonia or would also be found for other depressive symptoms, such as negative mood.
In the current study, we used age-appropriate positive and negative incentive tasks that have been well studied in the adult literature,10,30 along with dimensional measures of depressive (including anhedonia), other internalizing, and externalizing symptoms to test 2 hypotheses within a sample of healthy 7- to 10-year-old children at high risk (maternal depressive episode history) or low risk (no maternal psychopathology) for developing MDD. First, we hypothesized that high-risk children would show reduced gain approach behavior and altered loss avoidance behavior relative to low-risk children, although it is unclear whether we should expect blunted or enhanced loss avoidance in the high-risk group. Second, we hypothesized that children with elevated levels of anhedonic symptoms would show blunted responses to both gain and loss, and that this pattern of relations would be specific to anhedonic symptom level.
METHOD
Participants and Procedure
A total of 119 mothers with or without a history of depression and their 7-to 10-year-old children from the St. Louis, Missouri metropolitan area were enrolled in the study. Families were recruited via flyers/brochures distributed through schools and posted in the community as well as via the Research Participant Registry at Washington University School of Medicine. Before enrollment, mothers completed a phone screen to help determine eligibility. Children who had begun menstruation (female), could not consume candy, were born before 35 weeks’ gestation, or were diagnosed previously with a psychiatric, learning, or other major medical disorder were excluded.
Data presented here were collected during the first session of a multi-session protocol. Mothers provided written informed consent, and children provided written assent. Mothers then completed clinical interviews and questionnaires about themselves and their child in a separate room. Children completed a “tasty task” (see Supplement 1, available online) in which candy was tasted and rated. In unpublished pilot work, this task improved attention/ compliance during task instructions and practice. Children then completed 2 versions of a Probabilistic Incentive Learning Task (PILT), a clinical interview, and self-report questionnaires. The Washington University in St. Louis Institutional Review Board approved all study procedures.
Assessment of Psychopathology and Risk
Diagnostic Interviews. Child Diagnostic Information
Given our questions regarding risk for depression, analyses focus on the psychiatrically healthy offspring of women either with or without a history of at least 1 depressive episode. To assess child mental health, children and mothers both completed the Kiddie–Structured Assessment for Affective Disorders–Present and Lifetime Version (K-SADS),33 administered by masters-level clinicians trained to reliability. Data from dyads for whom only 1 reporter completed the K-SADS (n = 5; 2 high-risk) or who were missing behavioral/self-report data (n = 4; 1 high-risk) were excluded. Based on combined reports,34 12 children met criteria for externalizing or internalizing disorders and were excluded from analyses. Children with a disorder affecting their ability to respond during the task (i.e., tic disorder or dyslexia: n = 5) or whose mother reported using illicit drugs during pregnancy (n = 3) were also excluded.
Maternal Diagnostic Information
Depression risk was defined by maternal depressive episode history, established via the Structured Clinical Interview for DSM Disorders (SCID).35 Children of mothers without any lifetime psychiatric diagnoses were considered low risk (n = 42). Children of mothers who had experienced at least 1 depressive episode (n = 27) were considered high risk. The remaining 21 mothers did not meet inclusion criteria for either group (see Table S1, available online, for maternal diagnoses). Of the 27 high-risk mothers, 23 had experienced recurrent depressive episodes. Ten high-risk mothers had experienced “too many episodes to count” and were coded as having experienced “20” episodes. The remaining mothers reported experiencing between 2 and 6 lifetime episodes. Table 1 provides descriptive statistics regarding the number of maternal MDD episodes and age of maternal MDD onset, and Table S2, available online, lists correlations between maternal and child symptom measures.
TABLE 1.
Demographic and Clinical Characteristics of Healthy Children at Low and High Risk for Developing Depression
| Characteristic | Low Risk (n = 42) | High Risk (n = 27) | t/χ2 |
|---|---|---|---|
| Gender (% male)a | 53.3 | 53.6 | 0.98 |
| Age (y)b | 8.99 (1.12) 7.02–10.68 | 8.69 (1.21) 7.01–10.83 | 1.05 |
| Pubertal Development Scaleb | 1.53 (0.53) | 1.54 (0.43) | −0.13 |
| Ethnicity (% white)a | 48.9 | 50.0 | 1.17 |
| Family incomeb | 12.02 (7.09) 1–21 | 11.18 (7.30) 1–21 | 0.49 |
| CDI-C b | |||
| Total t score | 49.02 (13.66) 37–83 | 53.70 (14.59) 37–77 | −1.37 |
| Anhedonia Subscale t score | 48.19 (10.45) 37–83 | 52.44 (10.59) 37–75 | −1.67 |
| Negative Mood Subscale t score | 53.67 (15.49) 39–91 | 55.52 (15.92) 39–80 | −0.49 |
| CDI-Pb,c | |||
| Total t score | 41.36 (5.54) 34–61 | 47.79 (8.12) 35–67 | 3.69*** |
| CBCLb,c | |||
| Anxiety Subscale t score | 51.35 (2.98) 50–63 | 55.75 (6.19) 50–70 | −3.51*** |
| ADHD Subscale t score | 52.44 (5.45) 50–78 | 54.93 (6.83) 50–75 | −1.62 |
| Current maternal depressive symptoms, BDIc | 3.05 (4.15) 0–16 | 15.41 (12.69) 0–49 | −4.88*** |
| Age (y) of maternal MDD onset | 20.04 (7.97) 8–41 | ||
| Number of maternal MDD episodesc,d | 0 | 9.15 (8.57) 1–20 | −5.55*** |
Note: Family income level coded in 21 increments of $5,000 starting with 1 = $1,000 to $5,000 and ending with 21 = >$100,000. ADHD = attention-deficit/ hyperactivity disorder; BDI = Beck Depression Inventory; CBCL = Child Behavior Checklist; CDI-C = Children’s Depression Inventory–Child; CDI-P = Children’s Depression Inventory–Parent; MDD = major depressive disorder.
χ2 Statistic presented.
Mean (and standard deviation) minimum – maximum values are reported along with t-statistic.
Equal variance assumption not met; thus the t statistic was computed based on unequal variances.
Mothers reporting “too many episodes to count” were coded as having “20” episodes.
p ≤ .001.
Symptom Measures
Children and mothers completed a variety of dimensional self-report measures designed to assess depressive symptomology, affect, mood regulation, and sensitivity to rewards/ punishments. Maternal report of child depressive symptoms was obtained from the Child Depression Inventory–Parent Version (CDI-P).36 Child self-report was obtained from the Child Depression Inventory–Child Version (CDI-C).36 Maternal report of child anxiety and attention-deficit/hyperactivity disorder (ADHD) symptoms were obtained from the Child Behavior Checklist (CBCL anxiety and ADHD subscales).37 Maternal self-report of current depressive symptoms was obtained using the Beck Depression Inventory (BDI).38
The CDI-C consists of 27 items assessing various components of depressive symptomology. We focus on the anhedonia and negative mood subscales of the CDI-C and use age-/gender-normalized t scores for all analyses.36 The CDI-C anhedonia subscale “reflects “endogenous depression,” including impaired ability to experience pleasure, loss of energy, problems with sleeping and appetite, and a sense of isolation.”39 Thus it should be noted that although this measure of “anhedonic depressive symptoms” is similar to the symptom measures used in the adult PILT literature,10,30,40 it goes beyond reduced pleasure and includes symptoms such as sleep and appetite.41 The CDI-C negative mood subscale “reflects feeling sad, feeling like crying, worrying about ‘bad things,’ being bothered or upset by things, and being unable to make up one’s mind.”39 Both subscales and the total score show adequate internal consistency in previous studies.39,42
The CDI-P has 17 items and asks parents to rate on a scale from 0 to 3 how often each item has been true of their child in the past 2 weeks. The CDI-P shows adequate internal consistency and test–retest reliability.43 It is not uncommon for there to be little agreement between child self-reports (CDI-C) and maternal reports (CDI-P) of child depressive symptoms.44 Thus, we include both child (CDI-C Negative Mood and Anhedonia subscales) and parent report (CDI-P total score) of child depressive symptomology as separate predictors of behavior in the current analyses.
The CBCL ADHD and anxiety problem subscales were also used to assess dimensional parent-rated measures of these constructs. As ADHD and anxiety have been related to reward behaviors in pediatric groups, these measures were included in current analyses.45,46 The CBCL has shown adequate internal consistency and test–retest reliability.37
Pubertal Development
Mothers rated the pubertal development of their children using the Pubertal Development Scale (PDS).47 The PDS assesses whether changes in general factors such as body hair/ skin as well as sex-specific factors such as breast development, voice changes, and facial hair have not yet begun (1), are underway (2), or have completed (3). Higher scores indicate more advanced puberty.
Probabilistic Incentive Learning Task
Task Design and Data Processing
To assess gain approach and loss avoidance behavior, we used 2 versions of the probabilistic reward task used/developed by Pizzagalli et al.30 and Tripp and Alsop45 and previously modified for use in child populations.31 In 1 version of the PILT (PILT-Positive or PILT-P), children gain 1 piece of candy for every gain feedback instance. In the other task (PILT-Negative or PILT-N), children lose 1 piece of candy from a 70-piece allotment for every loss feedback instance. At the end of the behavioral session, children receive the net amount of candy won or not lost during the 2 tasks.
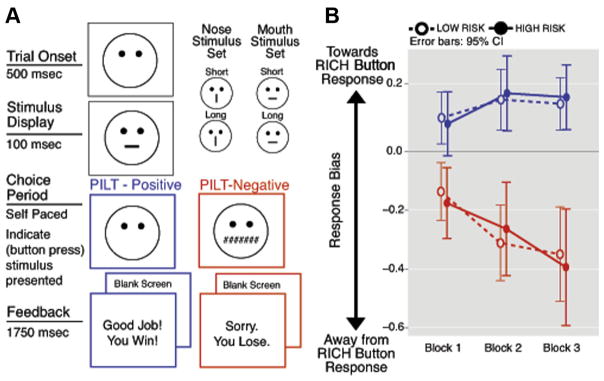
During both PILT versions, either a “short” or a “long” stimulus (Figure 1A gives examples from the mouth and nose stimulus sets) is briefly presented. Participants then must indicate whether a short or long stimulus was displayed, via 1 of 2 response buttons corresponding to long and short. During the PILT-P, feedback (Good Job! You Win!) was presented after a portion of button responses that correctly identified whether a short or long stimulus was presented, whereas the remaining correct and incorrect responses received no feedback. Conversely, during the PILT-N, feedback (Sorry. You Lose.) was presented after a portion of incorrect button responses, whereas the remaining incorrect and correct responses received no feedback. Importantly, in both PILT versions, feedback is delivered unequally between the 2 button responses. Either the long or short button response was randomly selected to receive approximately 3 times as much feedback as the other button response; this was fixed within a task for each participant and was counterbalanced across participants. The button response assigned to receive more feedback is termed the “RICH” button response (i.e., rich in feedback), and the alternative button response is termed “LEAN.” Thus, in the PILT-P, correct RICH button responses received approximately 3 times as much gain feedback as correct selection of the LEAN button. In the PILT-N, incorrect selection of the RICH button received approximately 3 times as much loss feedback as incorrectly selecting the LEAN button (see Supplement 1, available online, for more detailed discussion of task structure). In healthy children/adults, the asymmetry in feedback across the 2 button responses leads individuals to preferentially select (approach) the RICH button response that is paired with more frequent candy gain feedback during the PILT-P and to avoid selecting the RICH button response that is paired with more frequent candy loss feedback during the PILT-N.30,31
FIGURE 1.
Probabilistic Incentive Learning Task (PILT) diagram and response bias. Note: (A) Schematic diagrams of negative and positive PILT versions. (B) Response bias during each block of 40 trials for the PILT-Positive (blue; top) and PILT-Negative (red; bottom), by risk group. Open circles/dotted lines denote low risk; closed circles/solid lines denote high risk. RICH = rich in feedback.
The level of response bias (gain approach during the PILT-P and loss avoidance during the PILT-N) is calculated using signal detection statistics across a block of trials and serves as the dependent measure in behavioral analyses. Response bias indicates the extent to which a participant preferentially selects the RICH button response, which receives more frequent feedback. More positive response bias indicates greater approach of the RICH button response (expected during the PILT-P), whereas more negative response bias indicates greater avoidance of the RICH button response (expected during the PILT-N). Given that RICH and LEAN button responses should initially be selected with relatively equal frequency (bias near 0), the extent to which general response bias and/or changes in response bias from the beginning to the end of a task differ from 0 reflects the influence of gain/loss on choice behavior.
Data Analysis
We used independent-sample t tests to characterize group differences in symptom levels. To characterize relations between symptom measures used as covariates/predictors in subsequent analyses, we conducted correlations between parent-reported child depressive symptoms (CDI-P), parent-reported child ADHD symptoms (CBCL), parent-reported child anxiety symptoms (CBCL), and child-reported anhedonia and negative mood (CDI-C). To characterize behavior on the PILTs, 2 repeated measures analyses of variance (ANOVAs) were conducted. One ANOVA investigated how response bias changed as a function of task type (PILT-P, PILT-N), block (first, last), and stimulus set (mouth, nose). The second ANOVA investigated effects of task type and stimulus set on mean discriminability.
To test our main hypotheses, a repeated-measures ANOVA was conducted in which response bias served as the dependent variable. Analyses focused on effects of task type (PILT-P, PILT-N) and the interaction of task type and block (first, last). Risk group (high, low) and PILT-P stimulus set (mouth, nose) were included as between-subjects factors. Covariates of interest included general depressive symptoms (CDI-P total t score), anhedonia (CDI-C subscale t score), and negative mood (CDI-C subscale t score); interactions between covariates of interest and risk group were also investigated. Anxiety and ADHD symptom levels (CBCL subscale t scores) were also included as covariates, although we did not have specific hypotheses regarding these measures.
The main effect of risk group and/or interactions including risk group and task type/task-type × block (and associated post hoc tests) evaluated our hypothesis that approach/avoidance behavior would differ based on risk group. Interactions of task type and CDI-C Anhedonia subscale (and associated post hoc tests) evaluated our hypothesis that elevated levels of anhedonic symptoms would be related to blunted gain approach and loss avoidance behavior. Post hoc regressions were conducted to determine the direction of significant effects within the full sample and within each risk group separately. For regressions involving the full sample, all between-subjects factors and covariates were entered as a first step, followed by the interaction of risk group and covariates of interest in the second step; within-group regressions included 1 step with stimulus type and covariates as predictors.
RESULTS
Participant Characteristics and Individual Difference Measures
Descriptive statistics and symptom measure intercorrelations are displayed in Table 1 and Table S2, available online, respectively. High- and low-risk groups did not significantly differ in sex, ethnicity, age, pubertal development, or family income. Child self-report of general depressive symptoms, anhedonia, and negative mood did not significantly differ across risk groups (Table 1). Maternal report of child ADHD symptoms also did not differ significantly across groups. However, high-risk mothers did report significantly higher levels of depressive symptoms and anxiety symptoms in their children relative to low-risk mothers.
Behavioral Task Results
There were significant effects of task type and block on response bias (task type effect, F1,67 = 50.41, p < .001; task type × block interaction, F1,67 = 20.10, p < .001; Figure 1B). However, effects of PILT-P stimulus set on response bias were not significant (all p > .10). During the PILT-P, response bias was greater than 0 during all blocks (all p < .01), that is, children approached the more frequently rewarded response. Bias did not increase significantly from the first to the last block (main effect of block: F1,67 = 2.98, p = .089). During the PILT-N, response bias was less than 0 during all blocks (all p < .001), that is, children avoided the response more frequently paired with loss feedback. Bias became significantly more negative from the first to last block of the PILT-N (main effect of block: F1,67 = 17.87, p < .001). Discriminability did not significantly differ based on risk group (p > .37 for all effects of risk group), indicating similar difficulty across risk groups, but discriminability did differ based on PILT-P stimulus set (task type interaction F1,67 = 25.38, p < .001) with lower PILT-N discriminability when mouth stimuli were used during the PILT-N.
Gain Approach or Loss Avoidance and Risk Group Status
No significant effects of risk group on approach/avoidance behavior were observed (risk group main effect F1,58 = 0.37, p = .547; task type × risk group interaction F1,58 = 0.01, p = .941; task type × block × risk group interaction, F1,58 = 0.03, p = .870; Table S3, available online; Figure 1B). Risk group effects remained nonsignificant when the high-risk group was restricted to children of mothers with recurrent depressive episodes (all p > .81).
We conducted an additional exploratory repeated-measures ANOVA within the high-risk group to test whether age of onset of maternal MDD and number of maternal MDD episodes related to child approach/avoidance behavior (factors included as additional covariates). There was a significant task type × block × number of maternal MDD episodes interaction (F1,22 = 5.60, p = .027. All other main effects and interactions were nonsignificant (all p > .17). Post hoc regressions indicated a greater number of depressive episodes, predicted blunted loss avoidance (β = 0.77, t = 3.17, p = .006; see Table S4, available online, Model 2; Figure S1, available online) beyond child symptom levels, current maternal depressive symptoms (BDI), and comorbid maternal anxiety and substance abuse/dependence.
Relations Between Incentive Responsiveness and Symptom Levels
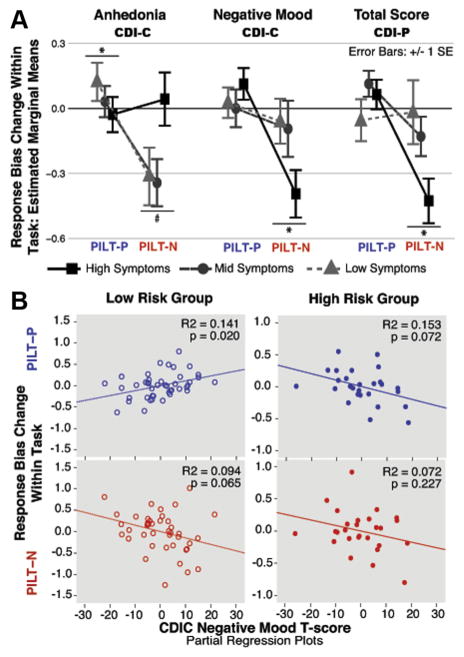
The difference across task types in response bias change within a task (i.e., interaction of task type and block) differed based on child-reported anhedonic symptoms (F1,58 = 5.34, p = .024), child-reported negative mood symptoms (F1,58 = 4.19, p = .045), and maternal report of child general depressive symptoms (F1,58 = 6.08, p = .017) (Figure 2A). Furthermore, the interaction of task type, block, and negative mood differed based on risk group (F1,58 = 5.39, p = .024; Figure 2B, Table S3, available online). Post hoc hierarchical regression analyses were conducted to determine effect directions and whether interactions reflected relations within 1 or both PILT versions. Post hoc regression results for each task are discussed below. No significant interactions with task type alone or effects/interactions of ADHD or anxiety symptoms were observed (Table S3, available online).
FIGURE 2.
Response bias change relations with anhedonia, negative mood, and general depressive symptom levels. Note: (A) Interactions of task type and symptom level (anhedonia, negative mood, general depressive symptoms CDI-P [Children’s Depression Inventory–Parent Report])—estimated marginal means for response bias change (from the repeated-measures analysis of variance reported in Table S3, available online)—are depicted using tertile splits of symptom measures for graphical depiction only. (B) Partial regression plots depicting prediction of response bias change for each task type by CDI-Child Report (C) Negative Mood for each risk group (from the regression reported in Table S6, available online). Covariates/ factors for all models include CDI-C anhedonia, CDI-C negative mood, CDI-P total score, Probabilistic Incentive Learning Task-Positive (PILT-P; blue; top) stimulus type, attention-deficit/ hyperactivity disorder (ADHD) symptoms, anxiety symptoms, and risk group. PILT-N = PILT-Negative (red; bottom); SE = standard error. *p < .05; #p < .10.
PILT-Positive (Gain)
Elevated anhedonic symptoms were significantly related to reduced gain approach behavior (β = −0.38, t = −2.03, p = .046; Table S5, available online, Model 1). Although the further interaction of task type, block, and anhedonia (discussed above) with risk group was not significant (F1,58 = 3.57, p = .064; Table S3, available online), the relation between blunted gain approach and anhedonia was significant only in the low-risk group (β = −0.63, t = −2.70, p = .010; Table S6, available online). Blunted gain approach behavior also related to elevated negative mood, but only among high-risk children. This relation was trend level within the main post hoc regression (β = −0.59, t = −1.90, p = .072; Table S6, available online; Figure 2B), but significant when maternal depressive episode number and comorbid maternal diagnoses were also included in the model (β = −0.68, t = −2.26, p = .038; Table S4, available online). Low-risk children with elevated negative mood showed enhanced gain approach (β = 0.55, t = 2.43, p = .020; Table S6, available online; Figure 2B). Negative mood and general depressive symptoms (CDI-P) did not significantly predict PILT-P bias change (all p > .25; Table S5, available online, Model 1).
PILT-Negative (Loss)
Elevated negative mood symptoms and elevated general depressive symptoms (CDI-P) were significant and independent predictors of enhanced loss avoidance (negative mood β = −0.41, t = −2.29, p = .026; CDI-P β = −0.33, t = −2.01, p = .049; Table S5, available online, Model 1; Figure 2A). Conversely, anhedonic symptoms weakly predicted blunted loss avoidance (β = 0.31, t = 1.75, p = .085; Table S5, available online, Model 1; Figure 2A). None of these relations interacted further with risk group (all p > .63; Table S5, available online).
DISCUSSION
The aim of the current study was to investigate relations between behavioral responsiveness to gain and loss feedback, MDD risk, and severity of specific depressive symptoms within healthy school-aged children. First, in contrast to adolescent behavioral and neuroimaging findings, children’s gain approach and loss avoidance behavior did not differ based on risk for MDD. However, within the high-risk group, higher numbers of maternal depressive episodes were associated with blunted loss avoidance. Second, higher levels of anhedonic symptoms were associated with reduced gain approach behavior in low-risk children. Third, higher levels of negative mood symptoms were associated with enhanced gain approach behavior in low-risk children and with reduced gain approach behavior in high-risk children. Fourth, both elevated negative mood and elevated maternal-report of child depressive symptoms were associated with enhanced loss avoidance behavior.
Depression Risk and Gain Approach/Loss Avoidance Behavior
Contrary to our hypotheses, MDD risk did not significantly predict gain approach or loss avoidance. This was notable, given the evidence of reduced behavioral and neural responses to gain/positive stimuli with elevated MDD risk in the adolescent literature.11,14–16,29,48 Although power is a concern when interpreting these null results, the current high-risk sample (n = 27) is roughly twice the size of high-risk groups in previous adolescent neuroimaging studies reporting significant group differences.15,16,23,48 Although it is possible that risk-related effect sizes are larger for neuro-imaging than for behavioral tasks, it is also possible that healthy high-risk children are able to use compensatory strategies eliminating differences in behavior despite potential differences in neural function.
Another possibility is that the PILT indexes a different component of reward processing than tasks used in the adolescent literature. For example, the neuroimaging studies cited above focus on neural response to viewing positive facial expressions or anticipating/receiving reward feedback after an instrumental response, whereas the PILT indexes the extent to which gain/loss feedback influences subsequent behavior. This complex process requires integrating responses and outcomes over a number of trials and application of that history to motivate response selection. Although this process is blunted in depressed adults,10 it may not be sensitive to MDD risk. However, we did observe blunted loss avoidance behavior in high-risk children of mothers who had experienced greater numbers of depressive episodes. Given that the severity of maternal depression, including number of lifetime episodes, confers increased risk for depression in offspring, this relation suggests that processes engaged during the PILT are affected by degree of MDD risk within high-risk samples.
It is also possible that differences in responsiveness to gain/loss between groups at high/low risk for MDD are simply small during childhood and increase across adolescence, with low-risk individuals showing the developmentally normative increase in reward responding. Longitudinal studies that follow participants from early childhood through adolescence are needed to explicitly test this hypothesis. However, consistent with this hypothesis, a cross-sectional study investigating extreme early life stress/ neglect as a risk factor for MDD observed reduced ventral striatal responses to happy faces within high-risk adolescents (11–15 years), but not in children (5–10 years).23
Factors Relating to Gain Approach Behavior
As hypothesized, children who reported elevated anhedonic symptoms also showed reduced gain approach behavior, particularly in the low-risk group. This result is conceptually consistent with previous PILT-P studies in nonclinical adult30,40 and nonclinical low-risk child31 samples. Reduced gain approach behavior was also observed in high-risk children who reported elevated negative mood. The direction of this relationship is not surprising, given the extant literature pointing to reduced striatal response to positive feedback/stimuli with elevated depressive symptoms in adolescents.12,16,49 However, the opposite pattern of enhanced gain approach behavior was observed in low-risk children reporting elevated negative mood. This interesting and unexpected finding could suggest that low-risk children display an adaptive response to elevated negative mood by actively seeking out reward, in contrast to high-risk children who, with similar elevations in negative mood, show reward avoidance. Given that high- and low-risk groups endorsed similar levels of negative mood symptoms, differences in behavioral relations cannot be interpreted as being based on negative mood severity. However, in addition to the interpretation above, other factor(s) not examined here that may differ across groups, such as parenting style or the relationship between levels of positive and negative mood, may mediate the group difference in this relationship. As no other studies have compared relations between gain approach behavior and negative mood symptoms in similar populations, future studies are needed to replicate this group difference and to examine potential mediators.
Factors Relating to Loss Avoidance Behavior
Elevated loss avoidance was related to both elevated child-reported negative mood and maternal report of child general depressive symptoms. These relations are consistent with the negative potentiation theory of emotion reactivity in MDD, in which current negative mood is thought to potentiate responses to negative stimuli.50,51 It is interesting that both negative mood and CDI-P were related to enhanced loss avoidance and explained unique variance in loss-related behavior. Future studies are needed to replicate this finding and to explore the mechanisms of these unique predictions.
Given prior adult and child work relating elevated anhedonia to blunted gain approach and loss avoidance behavior,24,25,31 we expected to observe reduced loss avoidance in children reporting elevated anhedonic symptoms. Although we did observe a negative relationship between anhedonic symptoms and loss avoidance, it was trend level. However, given the extant literature supporting blunted responses with elevated anhedonia, and the fact that the direction of the relationship that we observed between loss avoidance and anhedonia was in the opposite direction of that with negative mood and CDI-P, we suggest that future studies use anhedonia and negative mood as separate predictors, particularly of loss-related behavior.
We focused on maternal history of psychopathology to define MDD risk. There are other sources of risk that we did not investigate, such as trauma/stress and paternal psycho-pathology. Future studies defining “risk” in different ways are needed to replicate the current null result of risk status and the significant dimensional relations between symptoms and behavior. The generalizability of the current results is also somewhat limited by our exclusion of children with any type of past/current pathology, given that onset of disorders such as generalized anxiety disorder (GAD) and ADHD often predates MDD diagnosis and that maternal MDD also confers increased risk for these disorders. Thus, although excluding such children is necessary for investigating true effects of risk versus pathology, future studies are needed to determine whether MDD risk relates to incentive processing in children with different types of pathology.
MDD risk based on a maternal history of depression was not significantly related to either gain approach or loss avoidance in healthy school-aged children. However, the number of maternal depressive episodes and children’s depressive symptom severity predicted both types of behavior. The current results show continuity with the extant adult literature using the PILT, as anhedonic depressive symptoms related to blunted gain approach behavior. This suggests that mechanisms subserving relations between anhedonia and incentive-related behaviors may be conserved across development. However, high- and low-risk children showed differing directions in the relation between negative mood and gain approach behavior. If this finding reflects a true difference in behavior, maintaining an elevated gain approach despite negative mood may indicate resilience and may be a proactive target for intervention. This unexpected finding would be an important issue for future study.
Supplementary Material
Acknowledgments
Grants from the Sidney R. Baer Jr. Foundation (J.L.; D.B.) and the Brain and Behavior Research Foundation (D.B.; J.L.) funded the current study. Katherine Luking’s work on this manuscript was supported by a grant from the National Institute of Mental Health (F31 MH097335).
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Dr. Luby has received research support from the National Institute of Mental Health and has received royalties from Guilford Press. Dr. Barch has received research support from the National Institute of Mental Health. Drs. Luking and Pagliaccio report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Katherine R. Luking, Neuroscience Program at Washington University in St. Louis.
Dr. David Pagliaccio, Neuroscience Program at Washington University in St. Louis.
Dr. Joan L. Luby, Washington University in St. Louis.
Dr. Deanna M. Barch, Washington University in St. Louis.
References
- 1.Lieb R, Isensee B, Hofler M, Pfister H, Wittchen HU. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry. 2002;59:365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- 2.Mars B, Collishaw S, Smith D, et al. Offspring of parents with recurrent depression: which features of parent depression index risk for offspring psychopathology? J Affect Disord. 2012;136:44–53. doi: 10.1016/j.jad.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Hammen C, Brennan PA. Severity, chronicity, and timing of maternal depression and risk for adolescent offspring diagnoses in a community sample. Arch Gen Psychiatry. 2003;60:253–258. doi: 10.1001/archpsyc.60.3.253. [DOI] [PubMed] [Google Scholar]
- 4.Angold A. Parent and child reports of depressive symptoms in children at low and high risk of depression. J Child Psychol Psychiatry. 1987;28:901–915. doi: 10.1111/j.1469-7610.1987.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 5.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 6.Forbes EE. fMRI studies of reward processing in adolescent depression. Neuropsychopharmacology. 2011;36:372–373. doi: 10.1038/npp.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: a systematic review. Neuro-Image. 2014;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawal A, Collishaw S, Thapar A, Rice F. ‘The risks of playing it safe’: a prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychol Med. 2013;43:27–38. doi: 10.1017/S0033291712001158. [DOI] [PubMed] [Google Scholar]
- 12.Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Barch DM, Pagliaccio D, Luking KR. Mechanisms of motivational deficits in psychopathology. In: Simpson EH, Balsam P, editors. Current Topics in Behavioral Neurosciences. Behavioral Neuroscience of Motivation. Heidelberg: Springer; 2015. pp. 1–45. [Google Scholar]
- 14.Rawal A, Riglin L, Ng-Knight T, Collishaw S, Thapar A, Rice F. A longitudinal high-risk study of adolescent anxiety, depression and parent-severity on the developmental course of risk-adjustment. J Child Psychol Psychiatry Allied Disc. 2014;55:1270–1278. doi: 10.1111/jcpp.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olino TM, McMakin DL, Morgan JK, et al. Reduced reward anticipation in youth at high-risk for unipolar depression: a preliminary study. Dev Cogn Neurosci. 2014;8:55–64. doi: 10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 20.Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J Abnorm Psychol. 2014;123:287–297. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goff B, Gee DG, Telzer EH, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chase HW, Frank MJ, Michael A, Bullmore ET, Sahakian BJ, Robbins TW. Approach and avoidance learning in patients with major depression and healthy controls: relation to anhedonia. Psychol Med. 2010;40:433–440. doi: 10.1017/S0033291709990468. [DOI] [PubMed] [Google Scholar]
- 25.Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367–2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- 26.Stoy M, Schlagenhauf F, Sterzer P, et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26:677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- 27.Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Schiller CE, Minkel J, Smoski MJ, Dichter GS. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J Affect Disord. 2013;151:756–762. doi: 10.1016/j.jad.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe C, Woffindale C, Harmer CJ, Cowen PJ. Neural processing of reward and punishment in young people at increased familial risk of depression. Biol Psychiatry. 2012;72:588–594. doi: 10.1016/j.biopsych.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 30.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luking KR, Neiman JS, Luby JL, Barch DM. Reduced hedonic capacity/approach motivation relates to blunted responsivity to gain and loss feedback in children [epub ahead of print] J Clin Child Adolesc Psychol. 2015;53:1–13. doi: 10.1080/15374416.2015.1012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. J Am Acad Child Adolesc Psychiatry. 1992;31:78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 35.First MB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. New York: New York State Psychiatric Institute: Bimetrics Research Department; 2007. [Google Scholar]
- 36.Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 37.Achenbach T. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 39.Sitarenious G, Stein S. Use of the Children’s Depression Inventory. In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Children and Adolescents. Vol. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. pp. 1–38. [Google Scholar]
- 40.Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3:12. doi: 10.1186/2045-5380-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.APA. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 42.Kovacs KM. The Children’s Depression Inventory (CDI) Software Manual. Toronto: Multi-Health Systems; 1995. [Google Scholar]
- 43.Kovacs KM. The Children’s Depression Inventory–Parent Version (CDI-P) Toronto: Multi-Health Systems; 1997. [Google Scholar]
- 44.Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol Bull. 1987;101:213–232. [PubMed] [Google Scholar]
- 45.Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28:366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- 46.Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an anti-saccade task. Biol Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 48.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 49.Forbes EE, Ryan ND, Phillips ML, et al. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010;49:162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck AT. Cognitive Therapy and the Emotional Disorders. Madison, CT: International Universities Press; 1976. [Google Scholar]
- 51.Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clin Psychol Rev. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.