Abstract
Acrylamide is a high-volume industrial chemical, a component of cigarette smoke, and a product formed in certain foods prepared at high temperatures. Previously, we compared the extent of DNA adduct formation and mutations in B6C3F1/Tk mice treated neonatally with acrylamide or glycidamide to obtain information concerning the mechanism of acrylamide genotoxicity. We have now examined the tumorigenicity of acrylamide and glycidamide in mice treated neonatally. Male B6C3F1 mice were injected intraperitoneally on postnatal days 1, 8, and 15 with 0.0, 0.14, or 0.70 mmol acrylamide or glycidamide per kg body weight per day and the tumorigenicity was assessed after one year. Survival in each of the groups was >87%, there were no differences in body weights among the groups, and the only treatment-related neoplasms involved the liver. The incidence of combined hepatocellular adenoma or carcinoma was 3.8% in the control group, 8.3% in the 0.14 mmol acrylamide and glycidamide per kg body weight groups, 4.2% in the 0.70 mmol acrylamide per kg body weight group, and 71.4% in the 0.70 mmol glycidamide per kg body weight group. Analysis of the hepatocellular tumors indicated that the increased incidence observed in mice administered 0.70 mmol glycidamide per kg body weight was associated with A→ G and A → T mutations at codon 61 of H-ras. These results, combined with our previous data on DNA adduct formation and mutation induction, suggest that the carcinogenicity of acrylamide is dependent upon its metabolism to glycidamide, a pathway that is deficient in neonatal mice.
Keywords: Acrylamide, glycidamide, tumorigenicity
INTRODUCTION
Acrylamide (Figure 1) is a low-molecular-weight industrial chemical used in the production of polyacrylamides for water and wastewater treatment, crude-oil, mineral, concrete, textile, paper, and pulp processing, soil and sand treatment, cosmetics, and coating applications.1-2 Acrylamide is also found in many foods that are prepared by high-temperature frying, baking, or roasting, as a result of Maillard reactions involving the amino acid asparagine and reducing sugars.3,4 Examples of foods containing acrylamide include fried potatoes, certain breads and other bakery products, and coffee, and it has been estimated that individuals in the U.S. have a mean daily dietary exposure of 440 ng per kg body weight.5 An additional source of acrylamide exposure is cigarettes, which are estimated to contribute an additional 3.1 μg acrylamide per kg body weight per day.6
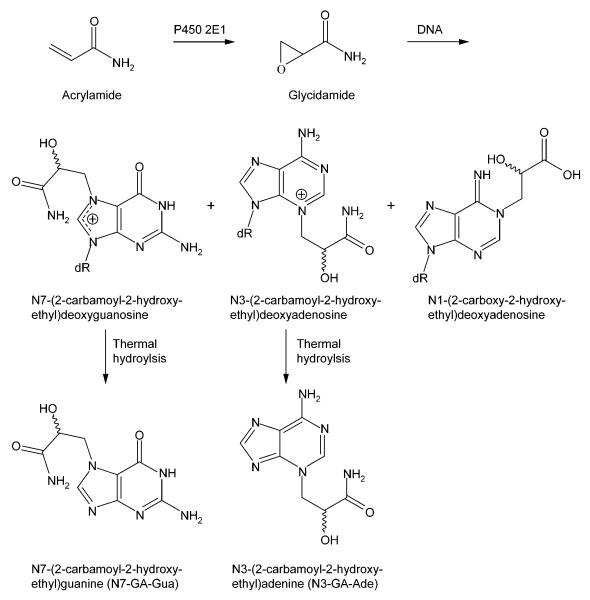
Figure 1.
Structures of acrylamide and glycidamide, and the DNA adducts resulting from the reaction of glycidamide with DNA. N7-GA-Gua and N3-GA-Ade are released from the DNA upon thermal hydrolysis.
The carcinogenicity of acrylamide has been demonstrated in a number of animal models including A/J mice, Swiss-ICR mice, B6C3F1 mice, and F344 rats.7-9 Although there is convincing evidence that acrylamide is carcinogenic in experimental animals, data regarding the carcinogenicity of acrylamide in humans are inconclusive. With the possible exception of the pancreas, occupational exposure to acrylamide has not been associated with a consistent dose-related increase in cancer incidence at any organ site.1,7,8,10 There is no evidence of increased risk of colorectal, bladder, esophageal, prostate, oropharyngeal, laryngeal, pancreatic, gastric, or lung cancer upon dietary exposure to acrylamide, while data regarding the effect of dietary acrylamide on the risk of breast, renal, ovarian, and endometrial cancer are inconsistent.11,12
Epidemiological studies subsequent to these reviews have examined the relationship between dietary exposure to acrylamide and brain, breast, endometrial, head and neck, ovarian, prostate, and thyroid cancer.13-17 None were positive, with the exception of ovarian cancer,17 and endometrial cancer, which was positive in one study,17 but not in another.15 One of the limitations of dietary acrylamide epidemiological studies is that the difference in acrylamide exposure between “low” and “high” exposure groups is relatively small, being only approximately a factor of three (see, for example, Wilson et al.17). Another complication is that acrylamide exposure assessments based upon dietary questionnaire data poorly reflect internal dose measurements of acrylamide.18
Acrylamide is metabolized to the epoxide glycidamide (Figure 1),19 primarily by cytochrome P450 2E1.20,21 Glycidamide reacts with DNA to give a number of DNA adducts, with the major ones being N7-(2-carbamoyl-2-hydroxyethyl)guanine (N7-GA-Gua), which results from the depurination of N7-(2-carbamoyl-2-hydroxyethyl)deoxyguanosine, and N3-(2-carbamoyl-2-hydroxyethyl)adenine (N3-GA-Ade), which results from the depurination of N3-(2-carbamoyl-2-hydroxyethyl)deoxyadenosine (Figure 1).22,23 Administering acrylamide to mice lacking cytochrome P450 2E1 results in much reduced levels of male germ cell mutagenicity,24 micronuclei,25 and glycidamide DNA adducts 21 compared to what is observed in cytochrome P450 2E1-proficient counterparts, which indicates the importance of glycidamide in the genotoxicity of acrylamide. Other studies have shown that adult Big Blue mice and rats treated with acrylamide have mutant frequencies comparable to those induced by glycidamide,26-28 and that the pattern of mutations induced by acrylamide in mice is consistent with the types of mutations and DNA adducts arising from glycidamide.26
In a previous study,29 we compared the extent of DNA adduct formation and the induction of micronuclei and mutations in B6C3F1/Tk mice dosed neonatally with acrylamide or glycidamide to obtain information concerning the mechanism of acrylamide genotoxicity. Neonatal mice given glycidamide had much higher DNA adduct levels than those administered acrylamide. In addition, glycidamide-treated mice had higher levels of micronuclei in reticulocytes and normochromatic erythrocytes and an increased mutant frequency at the hypoxanthine-guanine phosphoribosyltransferase gene in spleen lymphocytes. These data suggest that the genotoxicity of acrylamide is mediated through its metabolism to glycidamide. As a continuation of these studies, we have now compared the tumorigenicity of acrylamide and glycidamide in mice treated neonatally.
The neonatal mouse tumorigenicity bioassay was chosen to test critically two hypotheses in acrylamide carcinogenicity. The first hypothesis is that acrylamide tumorigenicity is mediated by its metabolism to glycidamide and glycidamide-DNA adduct formation. The second hypothesis is that hormonal dysregulation may be involved in acrylamide carcinogenicity, a proposition resulting from the observation of tumors in male and female F344 rat tissues regulated by the endocrine system (e.g., thyroid, mammary gland, peri-testicular mesothelium).7-9 The chosen animal model has two distinct advantages to clarify these conjectures. First, neonatal mice are deficient in cytochrome P450 2E1 activity relative to adult mice. This characteristic allows the comparison of neonatal mice responses to equimolar doses of the parent compound and its metabolite, in order to demonstrate the role of metabolic activation in acrylamide carcinogenesis. Second, as recognized by international risk assessment organizations (e.g., International Council on Harmonization and International Life Sciences Institute),30 the neonatal mouse assay responds only to DNA-reactive “genotoxic” compounds, based on an enhanced susceptibility of developing neonatal tissues that is manifested by tumor formation almost exclusively in liver and lung. Thus, the neonatal mouse model has the ability to discriminate between genotoxic and epigenetic mechanisms for acrylamide carcinogenesis.
MATERIALS AND METHODS
Chemicals
Acrylamide (stated purity ≥99%, CAS 79-06-1) was purchased from Sigma Chemical Co., St. Louis, MO, and glycidamide (stated purity >98%, CAS 5694-00-8) was obtained from Toronto Research Chemicals, North York, Ontario. The purity and identity of the acrylamide and glycidamide were confirmed by gas chromatography coupled with electron impact mass spectrometry, nuclear magnetic resonance spectroscopy, and gas chromatography using flame ionization detection.
Animals
The Institutional Animal Care and Use Committee at the National Center for Toxicological Research reviewed and approved the protocol for these experiments.
Male C3H/HeN MTV- mice and female C57BL/6N mice were obtained from colonies maintained at the National Center for Toxicological Research. Female C57BL/6N mice were mated with male C3H/HeN MTV- mice to produce B6C3F1 mice. On postnatal day (PND) 1, the female B6C3F1 mice were culled because they are less sensitive than male B6C3F1 mice to the induction of tumors when treated as newborns.30,31 The male B6C3F1 mice were pooled and assigned randomly to dams, with a total of 32 male pups in each treatment group.
Animal treatment
On PNDs 1, 8, and 15, the male B6C3F1 mice were dosed intraperitoneally with 0.0, 0.14, or 0.70 mmol acrylamide or glycidamide per kg body weight per day. This treatment schedule corresponds to a protocol typically used in newborn mouse bioassays.31 To minimize stress upon the pups, historical body weights for mice of this age were used to determine the amount of acrylamide and glycidamide administered. These doses are identical to those used in our previous study that examined the DNA adducts and induction of mutations in B6C3F1/Tk mice treated with acrylamide or glycidamide.29 The compounds were dissolved in deionized water and administered in 5 μL on PND 1, 10 μL on PND 8, and 20 μL on PND 15. The mice were weaned on PND 21, culled to 24-26 male B6C3F1 mice per treatment group, and monitored for one year. Throughout the study, the mice (both the dams and the pups) were fed, ad libitum, irradiated Purina 5LG6 pellets, a diet low in acrylamide (< 50 ppb) compared to other commercial formulations.32 One mouse from the 0.70 mmol glycidamide per kg body weight treatment group was mis-sexed and discarded. Two additional mice from this group were inadvertently used for microbiological surveillance. These three mice were not examined further.
Necropsy and histopathology
The animals were monitored for one year after treatment. At the termination of the study, all surviving animals were euthanized by exposure to carbon dioxide, gross examinations were performed, and all gross lesions visible at necropsy were recorded, including number, location, size, and color. Gross observations predominantly involved the liver, a primary target tissue in the neonatal mouse bioassay.33-35
The livers and lungs were removed and dorsal and ventral views of these organs were recorded using digital imagery before they were preserved in 10% neutral buffered formalin. The livers were trimmed, processed, and embedded in infiltrating media (Formula R®), sectioned at approximately 5 microns, and stained with hematoxylin and eosin for histopathological examination. Complete necropsies were also performed on animals that died naturally or that were submitted moribund prior to the scheduled terminal sacrifice.
Ras oncogene analyses
Genomic DNA was isolated from the formalin-fixed and paraffin-embedded normal and tumor tissue samples. Briefly, paraffin-embedded liver tissue sections were deparaffinized by incubation with xylene for 30 min at 45°C, further washed with 100% ethanol, and subsequently rehydrated in 90% and 70% ethanol. Tissue pellets were digested overnight with 40 μg of proteinase K (New England Biolabs, Ipswich, MA) in 400 μL of DNA extraction buffer (500 mM Tris-HCl, 1 mM EDTA, 5 mM NaCl, pH 8.0). DNA was further recovered by phenol:chloroform:isoamyl alcohol extraction.
H-ras codons 12, 13, and 61 mutations were analyzed using the method of Mitchell and Warshawsky.36 Briefly, primary polymerase chain reaction (PCR) amplification of DNA was performed and the PCR products were further digested with BstNI (codon 12), BglI (codon 13), and BclI (codon 61) restriction endonucleases (New England Biolabs, Ipswich, MA). Digested PCR products were subjected to a second amplification, followed by a second digestion with the restriction endonucleases used previously. The digested products were then separated by electrophoresis on a 2.5% agarose gel. Mutant bands were excised from the gel, re-amplified using nested primers, and sequenced (Retrogen Inc., San Diego, CA).
Statistical analyses
The effect of treatment on body weights was assessed using a repeated measures analysis of variance, with treatment and weeks as main effects. Body weights obtained on weeks 8, 16, 24, 32, 40, and 48 were used to conduct these analyses. Pair-wise comparisons of the mean weights of the treatment groups to the mean weights of the vehicle control group were conducted by Dunnett’s test.
Kaplan-Meier survival analysis was used to determine the effect of treatment on animal survival.
Fisher’s Exact test was used to assess the effect of treatment on the prevalence of neoplasms and non-neoplastic lesions. The reported p-values are two-sided.
H-ras codon 61 mutation spectra were compared with a hypergeometric test.37
RESULTS
Male B6C3F1 mice were treated on PNDs 1, 8, and 15 with 0.14 or 0.70 mmol acrylamide or glycidamide per kg body weight per day, or the vehicle, and the extent of tumorigenicity was monitored for a period of one year.
Body weights and animal survival
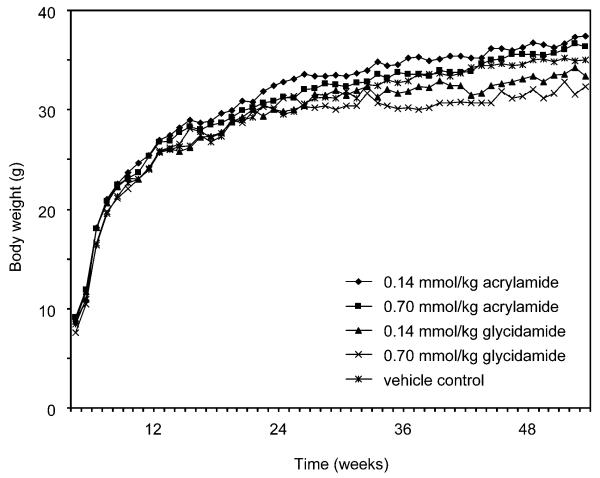
Body weights were not affected by treatment with either dose level of acrylamide or glycidamide (Figure 2). The mean body weights for each of the treatment groups were within 5% of the mean body weight of the vehicle control group throughout the one-year observation period.
Figure 2.
Mean body weights, measured weekly, in male B6C3F1 mice administered 0.14 or 0.70 mmol acrylamide or glycidamide per kg body weight, or the vehicle, intraperitoneally on PNDs 1, 8, and 15. (◆) 0.14 mmol acrylamide per kg body weight; (■) 0.70 mmol acrylamide per kg body weight; (▲) 0.14 mmol glycidamide per kg body weight; (×) 0.70 mmol glycidamide per kg body weight; (*) vehicle.
Animal survival was not affected by treatment, with >87% of the mice in each treatment group surviving until the scheduled terminal sacrifice. Two control mice, three mice dosed with 0.14 mmol acrylamide per kg body weight, one mouse given 0.70 mmol acrylamide per kg body weight, and two mice administered 0.14 mmol glycidamide per kg body weight were removed due to death or morbidity before the scheduled terminal sacrifice.
Neoplastic and non-neoplastic findings
Neoplasms related to treatment with acrylamide or glycidamide were noted only in the liver. Mice in the vehicle control group, both acrylamide dose groups, and the 0.14 mmol glycidamide per kg body weight dose group had a low incidence of hepatocellular adenoma (3.8-8.3%; Table 1). Mice treated with 0.70 mmol glycidamide per kg body weight had primarily multiple hepatocellular adenoma. In addition, hepatocellular carcinoma was observed only in this group. The incidences of singular or multiple hepatocellular adenoma and of combined hepatocellular adenoma or carcinoma were significantly increased in the group administered 0.70 mmol glycidamide per kg body weight compared to the vehicle control group. In none of the other treatment groups did the incidence of hepatocellular adenoma differ significantly from that observed in the control group.
Table 1.
Incidence of neoplasms in the livers of male B6C3F1 mice administered 0.14 or 0.70 mmol acrylamide or glycidamide per kg body weight, or the vehicle, intraperitoneally on PNDs 1, 8, and 15
| Liver neoplasm | Treatment (mmol acrylamide or glycidamide per kg body weight) |
||||
|---|---|---|---|---|---|
| Control | Acrylamide | Glycidamide | |||
| 0.0 | 0.14 | 0.70 | 0.14 | 0.70 | |
| Hepatocellular adenoma |
1/26a (3.8%)b |
2/24 (8.3%) |
1/24 (4.2%) |
2/24 (8.3%) |
2/21 (9.5%) |
| Hepatocellular adenoma, multiple |
0/26 (0.0%) |
0/24 (0.0%) |
0/24 (0.0%) |
0/24 (0.0%) |
13/21 (61.9%)* |
| Hepatocellular adenoma, singular or multiple |
1/26 (3.8%) |
2/24 (8.3%) |
1/24 (4.2%) |
2/24 (8.3%) |
15/21 (71.4%)* |
| Hepatocellular carcinoma |
0/26 (0.0%) |
0/24 (0.0%) |
0/24 (0.0%) |
0/24 (0.0%) |
2/21c (9.5%) |
| Hepatocellular adenoma or carcinoma |
1/26 (3.8%) |
2/24 (8.3%) |
1/24 (4.2%) |
2/24 (8.3%) |
15/21 (71.4%)* |
Number of mice with the specified neoplasm per number of mice examined microscopically.
Percentage of mice with the specified neoplasm.
Two mice had both an adenoma and carcinoma.
Significantly different (p < 0.0001) from the incidence observed in the vehicle control group.
Non-neoplastic lesions observed in the livers of the mice included basophilic, eosinophilic, and mixed cell foci (Table 2). Of these lesions, the incidence of basophilic foci was significantly increased in the mice administered 0.70 mmol glycidamide per kg body weight compared to the control group. The incidence of this lesion did not differ significantly in the other treatment groups; likewise, the incidence of eosinophilic and mixed cell foci was not elevated significantly in any of the treatment groups.
Table 2.
Incidence of non-neoplastic lesions in the livers of male B6C3F1 mice administered 0.14 or 0.70 mmol acrylamide or glycidamide per kg body weight, or the vehicle, intraperitoneally on PNDs 1, 8, and 15
| Non-neoplastic liver lesion |
Treatment (mmol acrylamide or glycidamide per kg body weight) |
||||
|---|---|---|---|---|---|
| Control | Acrylamide | Glycidamide | |||
| 0.0 | 0.14 | 0.70 | 0.14 | 0.70 | |
| Basophilic focus, singular or multiple |
2/26a (7.7%)b |
1/24 (4.2%) |
2/24 (8.3%) |
3/24 (12.5%) |
14/21 (66.7%)* |
| Eosinophilic focus, multiple |
0/26 (0.0%) |
0/24 (0.0%) |
1/24 (4.2%) |
0/24 (0.0%) |
0/21 (0%) |
| Mixed cell focus, singular or multiple |
0/26 (0.0%) |
1/24 (4.2%) |
0/24 (0.0%) |
3/24 (12.5%) |
3/21 (14.3%) |
| Basophilic, eosinophilic, or mixed cell focus, combined |
2/26 (7.7%) |
2/24 (8.3%) |
3/24 (12.5%) |
6/24 (25.0%) |
16/21 (76.2%)* |
Number of mice with the specified non-neoplastic lesion per number of mice examined microscopically.
Percentage of mice with the specified non-neoplastic lesion.
Significantly different (p < 0.0001) from the incidence observed in the vehicle control group.
Ras oncogene analyses
DNA from the liver tissue sections containing hepatocellular adenoma or carcinoma was extracted and screened for mutations in H-ras codons 12 and 13 (exon 1) and codon 61 (exon 2) (Figure 3). A total of 51 hepatocellular neoplasms was screened from the 21 mice bearing tumors. No mutations were detected in the 5 liver tumors from mice treated with 0.14 mmol acrylamide per kg body weight, 0.14 mmol glycidamide per kg body weight, or 0.70 mmol acrylamide per kg body weight (Table 3). A CAA → CTA transversion mutation at codon 61 was observed in the single control animal bearing a hepatocellular adenoma.
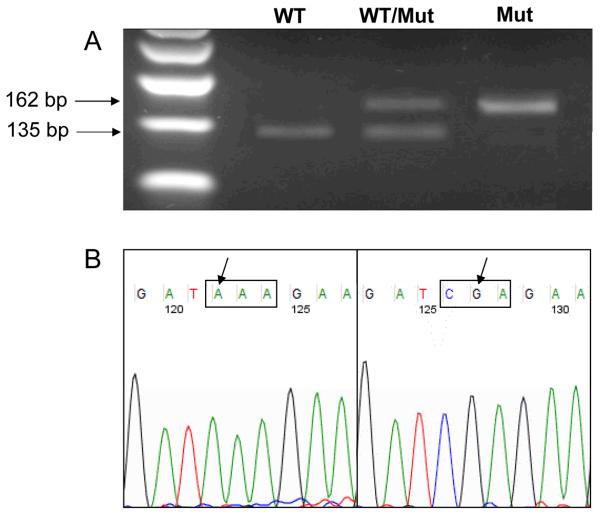
Figure 3.
Analysis of H-ras gene codon 61 mutations in acrylamide- and glycidamide-induced hepatocellular tumors. A). Representative image of an agarose gel showing the presence of mutations in H-ras codon 61 as detected by the enriched PCR screening.36 The wild type (WT) allele of H-ras corresponds to the 135 bp band, and the mutant (Mut) H-ras allele corresponds to the 162 bp band. Positive bands were excised, purified, and sequenced. B). Representative sequencing chromatograms of CAA → AAA (left) and CAA → CGA (right) mutations in H-ras codon 61.
Table 3.
Number and types of H-ras mutations in the livers of male B6C3F1 mice administered 0.14 or 0.70 mmol acrylamide or glycidamide per kg body weight, or the vehicle, intraperitoneally on PNDs 1, 8, and 15
| Treatment | H-ras codon 61 mutations |
H-ras codon 61 mutation | ||
|---|---|---|---|---|
| CAA → AAA | CAA → CGA | CAA → CTA | ||
| Historical controla | 235/444b (53%) |
139/229 (61%) |
68/229 (30%) |
22/229 (10%) |
| Control | 1/1 (100%) |
0 (0%) |
0 (0%) |
1 (100%) |
| 0.14 mmol acrylamide per kg body weight |
0/2 (0%) |
0/2 (0%) |
0/2 (0%) |
0/2 (0%) |
| 0.70 mmol acrylamide per kg body weight |
0/1 (0%) |
0/1 (0%) |
0/1 (0%) |
0/1 (0%) |
| 0.14 mmol glycidamide per kg body weight |
0/2 (0%) |
0/2 (0%) |
0/2 (0%) |
0/2 (0%) |
| 0.70 mmol glycidamide per kg body weightc |
21/45 (47%) |
1/21 (5%)* |
14/21 (67%)* |
5/21 (24%)* |
Historical data for H-ras mutation in hepatocellular adenoma and carcinoma of control B6C3F1 mice were compiled from literature data.38-43
The data are expressed as the number of H-ras mutations per number of tumors examined, with the percentage being given in parentheses.
In addition to the H-ras mutations indicated, one CAA → CCA codon 61 mutation and one GGA → GAA codon 12 mutation were detected.
The spectrum of codon 61 mutations in the hepatocellular adenoma and carcinoma of B6C3F1 mice administered 0.70 mmol glycidamide per kg body weight was significantly different (p = 0.0001) from the historical control spectrum of H-ras codon 61 mutations, as determined by a hypergeometric test.37
Hepatocellular adenoma or carcinoma were observed in fifteen mice treated with 0.70 mmol glycidamide per kg body weight (Table 1); 12 of these mice had a total of 22 H-ras mutations in the 45 tumors that were screened (Table 3). Twenty-one of the 22 H-ras mutations occurred at codon 61; the remaining mutation, a GGA → GAA transition, was detected at codon 12. Of the 21 codon 61 H-ras mutations, 14 (67%) were CAA → CGA transitions, 5 (24%) were CAA → CTA transversions, 1 (5%) was a CAA → CCA transversion, and 1 (5%) was a CAA → AAA transversion (Table 3).
Fifteen mice had multiple hepatocellular tumors (adenoma or adenoma and carcinoma); one mouse with 5 tumors had 3 codon 61 H-ras mutations, each of which was a CAA → CTA transversion, and another mouse with 5 tumors had 2 codon 61 H-ras mutations, each of which was a CAA → CGA transition. Other mice with multiple hepatocellular neoplasms had more than one type of codon 61 H-ras mutation. Two mice, for example, each with 4 liver tumors, had 2 CAA → CGA mutations and one CAA → CTA mutation, and another mouse with 5 liver tumors had 2 CAA → CGA mutations and 1 CAA → AAA mutation.
DISCUSSION
The carcinogenicity of acrylamide has been demonstrated in rats and mice. When given chronically to male and female F344 rats, there is clear evidence for the induction of thyroid gland follicular cell adenoma and carcinoma, peri-testicular mesothelioma, mammary gland adenoma, heart malignant schwannoma, oral cavity squamous cell papilloma and carcinoma, and skin mesenchymal neoplasms.7-9 Likewise, in male and female B6C3F1 mice treated chronically with acrylamide, there is clear evidence for the induction of harderian gland adenoma, lung alveolar/bronchiolar adenoma or carcinoma, forestomach squamous cell papilloma or carcinoma, mammary gland adenocarcinoma or adenoacanthoma, and skin mesenchymal neoplasms.9 In other bioassays, male and female A/J mice and female Swiss-ICR mice administered acrylamide have had increased incidences and/or multiplicities of lung neoplasms.7 While these experiments indicate clearly the potent carcinogenicity of acrylamide, they do not provide information concerning the mechanism of tumor induction.
Recently, the effect of perinatal exposure to acrylamide and glycidamide was investigated in C57BL/6J Min/+ mice and their wild type littermates.44 In C57BL/6J Min/+ mice administered glycidamide neonatally, there was a slight but significant dose-related induction of small intestinal tumors, with the increase being significant at a dose of 50 mg glycidamide per kg body weight. In wild-type C57BL/6J mice treated as neonates with glycidamide, there was also a dose-related increase in the frequency of mice having intestinal lesions, with the increase being significant at 50 mg glycidamide per kg body weight. These effects were not observed in mice dosed with acrylamide.
In the current study, we also used neonatal mice to elucidate mechanisms for the tumorigenicity of acrylamide. This model has two distinct advantages: first, neonatal mice are sensitive to the induction of tumors by genotoxic carcinogens,33-35 and second, neonatal mice are deficient in cytochrome P-450 activity compared to adult mice.34-35 This latter characteristic suggests that if metabolism to glycidamide is required for the carcinogenic action of acrylamide, glycidamide should be a more potent carcinogen than acrylamide in this model. Experimental support for limited metabolic oxidation of acrylamide in neonatal mice comes from the observation that the hepatic DNA adduct levels of N7-GA-Gua and N3-GA-Ade in male B6C3F1/Tk+/+ mice treated on PNDs 1, 8, and 15 with 0.70 mmol glycidamide per kg body weight were two- to three-fold higher than in male B6C3F1/Tk+/+ mice treated in an identical manner with 0.70 mmol acrylamide per kg body weight.29 Likewise, DNA adduct levels of N7-GA-Gua in three-day-old B6C3F1 mice administered 50 mg glycidamide per kg body weight were five- to six-fold higher than in mice given the same amount of acrylamide.23 In contrast, when adult male C3H/HeN MTV- mice were injected with a single dose of either 50 mg acrylamide or glycidamide per kg body weight, the hepatic DNA adduct levels of N7-GA-Gua were similar with both compounds.23
Consistent with the higher hepatic DNA adduct levels of N7-GA-Gua and N3-GA-Ade in neonatal male mice treated with 0.70 mmol glycidamide per kg body weight is the observation that the neonatal male B6C3F1 mice administered 0.70 mmol glycidamide per kg body weight (approximately 50 mg glycidamide per kg body weight) had a 71.4% incidence of combined hepatocellular adenoma or carcinoma compared to an 8.3% incidence in mice treated with an equimolar quantity of acrylamide (Table 1). The hepatic tumor incidence in neonatal male B6C3F1/Tk+/+ mice administered 0.14 mmol acrylamide or glycidamide per kg body weight or 0.70 mmol acrylamide per kg body weight did not differ from that found in the control mice (Table 1). Likewise, mice treated with 0.70 mmol glycidamide per kg body weight had a significant increase in the mutant frequency at the hypoxanthine-guanine phosphoribosyltransferase gene in spleen lymphocytes, a response not observed with the other treatments.29 Thus, while administering 0.14 mmol acrylamide or glycidamide per kg body weight or 0.70 mmol acrylamide per kg body weight resulted in detectable adduct levels of N7-GA-Gua and N3-GA-Ade when measured one day after the last treatment,29 the DNA adduct levels appeared to be insufficient to result in an increased mutant frequency or hepatic tumor incidence in this animal model.
Analysis of H-ras mutations in the hepatocellular adenomas and carcinomas from mice treated with 0.70 mmol glycidamide per kg body weight indicated a substantial increase in the frequency of CAA → CGA (Gln → Arg) and CAA → CTA (Gln → Leu) mutations at codon 61 compared to what has been found in spontaneous hepatocellular tumors in control B6C3F1 mice, where CAA → AAA mutations predominate (Table 3). This indicates that the tumors arising from glycidamide are not due to the promotion of spontaneous mutations. A → G transition mutations have been reported in Big Blue mouse embryonic fibroblasts treated with acrylamide and glycidamide,45,46 and these were attributed to the miscoding properties of N1-(2-carboxy-2-hydroxyethyl)deoxyadenosine (Figure 1),47 a glycidamide DNA adduct found at high levels in in vitro modified DNA.23 A → T transversion mutations have been ascribed to apurinic sites in DNA resulting from the spontaneous depurination of N3-(2-carbamoyl-2-hydroxyethyl)deoxyadenosine.47 Similar types of A → G transition and A → T transversion mutations have been observed within H-ras codon 61 in B6C3F1 mice treated with urethane and vinyl carbamate,48-50 both of which are thought to be metabolized to vinyl carbamate epoxide, a small electrophilic epoxide with chemical properties similar to glycidamide. These results, combined with our previous data, indicate that the carcinogenicity of acrylamide is dependent upon acrylamide being metabolized to glycidamide, with the resultant formation of glycidamide-DNA adducts and mutations. This metabolic pathway is deficient in neonatal mice.
ACKNOWLEDGEMENTS
We thank Michelle Vanlandingham, Carey Nobles, Sherry Smith, and Tina Glover for helping in the treatment and care of the mice. This work was supported by an Interagency Agreement between the National Institute of Environmental Health Sciences, National Toxicology Program, and the U.S. Food and Drug Administration, National Center for Toxicological Research (NCTR/FDA IAG #224-07-007; NIH/NTP IAG #Y1ES1027), and by a research grant from Fundação para a Ciência e a Tecnologia (PTDC/SAU-TOX/111663/2009), Portugal. The opinions expressed in this paper do not necessarily represent those of the U.S. Food and Drug Administration.
Abbreviations
- N3-GA-Ade
N3-(2-carbamoyl-2-hydroxyethyl)adenine
- N7-GA-Gua
N7-(2-carbamoyl-2-hydroxyethyl)guanine
- PCR
polymerase chain reaction
- PND
postnatal day
Footnotes
Brief statements (Novelty and impact statements): The authors have assessed the carcinogenicity of acrylamide, a product formed in certain foods prepared at high temperatures, and of its oxidized metabolite glycidamide in neonatal mice. Their data indicate that the carcinogenicity of acrylamide is dependent upon its metabolism to glycidamide, with the resultant formation of glycidamide-DNA adducts and mutations.
REFERENCES
- 1.International Agency for Research on Cancer . Some Industrial Chemicals. Vol. 60. International Agency for Research on Cancer; Lyon: 1994. Acrylamide. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; pp. 389–433. [Google Scholar]
- 2.Cosmetic Ingredient Review Expert Panel. Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int J Toxicol. 2005;24(Suppl. 2):21–50. doi: 10.1080/10915810590953842. [DOI] [PubMed] [Google Scholar]
- 3.Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- 4.Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert M-C, Riediker S. Acrylamide from Maillard reaction products. Nature. 2002;419:449–450. doi: 10.1038/419449a. [DOI] [PubMed] [Google Scholar]
- 5.Doerge DR, Young JF, Chen JJ, DiNovi MJ, Henry SH. Using dietary exposure and physiologically based pharmacokinetic/pharmacodynamic modeling in human risk extrapolations for acrylamide toxicity. J Agric Food Chem. 2008;56:6031–6038. doi: 10.1021/jf073042g. [DOI] [PubMed] [Google Scholar]
- 6.Bergmark E. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem Res Toxicol. 1997;10:78–84. doi: 10.1021/tx960113p. [DOI] [PubMed] [Google Scholar]
- 7.Rice JM. The carcinogenicity of acrylamide. Mutat Res. 2005;580:3–20. doi: 10.1016/j.mrgentox.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C. Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36:481–608. doi: 10.1080/10408440600851377. [DOI] [PubMed] [Google Scholar]
- 9.Toxicology and carcinogenesis studies of acrylamide . F344/N rats and B6C3F1 mice (drinking water study) NTP TR 575. National Toxicology Program; Research Triangle Park, NC: CAS No. 79-06-1. in press. [Google Scholar]
- 10.Erdreich LS, Friedman MA. Epidemiologic evidence for assessing the carcinogenicity of acrylamide. Regul Toxicol Pharmacol. 2004;39:150–157. doi: 10.1016/j.yrtph.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Mucci LA, Wilson KM. Acrylamide intake through diet and human cancer risk. J Agric Food Chem. 2008;56:6013–6019. doi: 10.1021/jf703747b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mucci LA, Adami H-O. The plight of the potato: is dietary acrylamide a risk factor for human cancer? J Natl Cancer Inst. 2009;101:618–621. doi: 10.1093/jnci/djp080. [DOI] [PubMed] [Google Scholar]
- 13.Hogervorst JGF, Schouten LJ, Konings EJM, Goldbohm RA, van den Brandt PA. Dietary acrylamide intake and brain cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1663–1666. doi: 10.1158/1055-9965.EPI-08-1133. [DOI] [PubMed] [Google Scholar]
- 14.Larsson SC, Åkesson A, Wolk A. Dietary acrylamide intake and prostate cancer risk in a prospective cohort of Swedish men. Cancer Epidemiol Biomarkers Prev. 2009;18:1939–1941. doi: 10.1158/1055-9965.EPI-09-0280. [DOI] [PubMed] [Google Scholar]
- 15.Larsson SC, Håkansson N, Åkesson A, Wolk A. Long-term dietary acrylamide intake and risk of endometrial cancer in a prospective cohort of Swedish women. Int J Cancer. 2009;124:1196–1199. doi: 10.1002/ijc.24002. [DOI] [PubMed] [Google Scholar]
- 16.Schouten LJ, Hogervorst JGF, Konings EJM, Goldbohm RA, van den Brandt PA. Dietary acrylamide intake and the risk of head-neck and thyroid cancers: results from the Netherlands cohort study. Amer J Epidemiol. 2009;170:873–884. doi: 10.1093/aje/kwp213. [DOI] [PubMed] [Google Scholar]
- 17.Wilson KM, Mucci LA, Rosner BA, Willett WC. A prospective study on dietary acrylamide intake and the risk for breast, endometrial, and ovarian cancers. Cancer Epidemiol Biomarkers Prev. 2010;19:2503–2515. doi: 10.1158/1055-9965.EPI-10-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirfält E, Paulsson B, Törnqvist M, Axmon A, Hagmar L. Associations between estimated acrylamide intakes, and hemoglobin AA adducts in a sample from the Malmö Diet and Cancer cohort. Eur J Clin Nutr. 2008;62:314–323. doi: 10.1038/sj.ejcn.1602704. [DOI] [PubMed] [Google Scholar]
- 19.Calleman CJ, Bergmark E, Costa LG. Acrylamide is metabolized to glycidamide in the rat: evidence from hemoglobin adduct formation. Chem Res Toxicol. 1990;3:406–412. doi: 10.1021/tx00017a004. [DOI] [PubMed] [Google Scholar]
- 20.Sumner SCJ, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem Res Toxicol. 1999;12:1110–1116. doi: 10.1021/tx990040k. [DOI] [PubMed] [Google Scholar]
- 21.Ghanayem BI, McDaniel LP, Churchwell MI, Twaddle NC, Snyder R, Fennell TR, Doerge DR. Role of CYP2E1 in the epoxidation of acrylamide to glycidamide and formation of DNA and hemoglobin adducts. Toxicol Sci. 2005;88:311–318. doi: 10.1093/toxsci/kfi307. [DOI] [PubMed] [Google Scholar]
- 22.Segerbäck D, Calleman CJ, Schroeder JL, Costa LG, Faustman EM. Formation of N-7-(2-carbamoyl-2-hydroxyethyl)guanine in DNA of the mouse and the rat following intraperitoneal administration of [14C]acrylamide. Carcinogenesis. 1995;16:1161–1165. doi: 10.1093/carcin/16.5.1161. [DOI] [PubMed] [Google Scholar]
- 23.Gamboa da Costa G, Churchwell MI, Hamilton LP, Von Tungeln LS, Beland FA, Marques MM, Doerge DR. DNA adduct formation from acrylamide via conversion to glycidamide in adult and neonatal mice. Chem Res Toxicol. 2003;16:1328–1337. doi: 10.1021/tx034108e. [DOI] [PubMed] [Google Scholar]
- 24.Ghanayem BI, Witt KL, El-Hadri L, Hoffler U, Kissling GE, Shelby MD, Bishop JB. Comparison of germ cell mutagenicity in male CYP2E1-null and wild-type mice treated with acrylamide: evidence supporting a glycidamide-mediated effect. Biol Reprod. 2005;72:157–163. doi: 10.1095/biolreprod.104.033308. [DOI] [PubMed] [Google Scholar]
- 25.Ghanayem BI, Witt KL, Kissling GE, Tice RR, Recio L. Absence of acrylamide-induced genotoxicity in CYP2E1-null mice: evidence consistent with a glycidamide-mediated effect. Mutat Res. 2005;578:284–297. doi: 10.1016/j.mrfmmm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Manjanatha MG, Aidoo A, Shelton SD, Bishop ME, McDaniel LP, Lyn-Cook LE, Doerge DR. Genotoxicity of acrylamide and its metabolite glycidamide administered in drinking water to male and female Big Blue mice. Environ Mol Mutagen. 2006;47:6–17. doi: 10.1002/em.20157. [DOI] [PubMed] [Google Scholar]
- 27.Mei N, McDaniel LP, Dobrovolsky VN, Guo X, Shaddock JG, Mittelstaedt RA, Azuma M, Shelton SD, McGarrity LJ, Doerge DR, Heflich RH. The genotoxicity of acrylamide and glycidamide in Big Blue rats. Toxicol Sci. 2010;115:412–421. doi: 10.1093/toxsci/kfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R-S, McDaniel LP, Manjanatha MG, Shelton SD, Doerge DR, Mei N. Mutagenicity of acrylamide and glycidamide in the testes of Big Blue mice. Toxicol Sci. 2010;117:72–80. doi: 10.1093/toxsci/kfq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Tungeln LS, Churchwell MI, Doerge DR, Shaddock JG, McGarrity LJ, Heflich RH, Gamboa da Costa G, Marques MM, Beland FA. DNA adduct formation and induction of micronuclei and mutations in B6C3F1/Tk mice treated neonatally with acrylamide or glycidamide. Int J Cancer. 2009;124:2006–2015. doi: 10.1002/ijc.24165. [DOI] [PubMed] [Google Scholar]
- 30.McClain RM, Keller D, Casciano D, Fu P, MacDonald J, Popp J, Sagartz J. Neonatal mouse model: review of methods and results. Toxicol Pathol. 2001;29(Suppl):128–137. doi: 10.1080/019262301753178537. [DOI] [PubMed] [Google Scholar]
- 31.Wislocki PG, Bagan ES, Lu AYH, Dooley KL, Fu PP, Han-Hsu H, Beland FA, Kadlubar FF. Tumorigenicity of nitrated derivatives of pyrene, benz[a]anthracene, chrysene and benzo[a]pyrene in the newborn mouse assay. Carcinogenesis. 1986;7:1317–1322. doi: 10.1093/carcin/7.8.1317. [DOI] [PubMed] [Google Scholar]
- 32.Twaddle NC, Churchwell MI, McDaniel LP, Doerge DR. Autoclave sterilization produces acrylamide in rodent diets: implications for toxicity testing. J Agric Food Chem. 2004;52:4344–4349. doi: 10.1021/jf0497657. [DOI] [PubMed] [Google Scholar]
- 33.Flammang TJ, Von Tungeln LS, Kadlubar FF, Fu PP. Neonatal mouse assay for tumorigenicity: alternative to the chronic rodent bioassay. Regul Toxicol Pharmacol. 1997;26:230–240. doi: 10.1006/rtph.1997.1125. [DOI] [PubMed] [Google Scholar]
- 34.Fu PP, Von Tungeln LS, Yi P, Xia Q, Casciano DA, Flammang TJ, Kadlubar FF. Neonatal mouse tumorigenicity bioassay. Drug Information J. 1998;32:711–728. [Google Scholar]
- 35.Fu PP, Von Tungeln LS, Hammons GJ, McMahon G, Wogan G, Flammang TJ, Kadlubar FF. Metabolic activation capacity of neonatal mice in relation to the neonatal mouse tumorigenicity bioassay. Drug Metab Rev. 2000;32:241–266. doi: 10.1081/dmr-100100575. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell KR, Warshawsky D. Mutational analysis using enriched PCR and cycle sequencing. BioTechniques. 1998;24:1028–1031. doi: 10.2144/98246cr02. [DOI] [PubMed] [Google Scholar]
- 37.Cariello NF, Piegorsch WW, Adams WT, Skopek TR. Computer program for the analysis of mutational spectra: application to p53 mutations. Carcinogenesis. 1994;15:2281–2285. doi: 10.1093/carcin/15.10.2281. [DOI] [PubMed] [Google Scholar]
- 38.Maronpot RR, Fox T, Malarkey DE, Goldsworthy TL. Mutations in the ras proto-oncogene: clues to etiology and molecular pathogenesis of mouse liver tumors. Toxicology. 1995;101:125–156. doi: 10.1016/0300-483x(95)03112-s. [DOI] [PubMed] [Google Scholar]
- 39.Hong H-HL, Devereux TR, Roycroft JH, Boorman GA, Sills RC. Frequency of ras mutations in liver neoplasms from B6C3F1 mice exposed to tetrafluoroethylene for two years. Toxicol Pathol. 1998;26:646–650. doi: 10.1177/019262339802600508. [DOI] [PubMed] [Google Scholar]
- 40.Sills RC, Boorman GA, Neal JE, Hong HL, Devereux TR, McGregor DB, Rice JM, Venitt S. The Use of Short- and Medium-term Tests for Carcinogens and Data on Genetic Effects in Carcinogenic Hazard Evaluation. International Agency for Research on Cancer; Lyon: 1999. Mutations in ras genes in experimental tumours of rodents; pp. 55–86. IARC Scientific Publications No. 146. [Google Scholar]
- 41.Iida M, Iwata H, Inoue H, Enomoto M, Horie N, Takeishi K. Correlation between Bcl-2 overexpression and H-ras mutation in naturally occurring hepatocellular proliferative lesions of the B6C3F1 mouse. Toxicol Sci. 2000;56:297–302. doi: 10.1093/toxsci/56.2.297. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi S-m, Ton TV, Hong H-HL, Irwin RD, Haseman JK, Devereux TR, Sills RC. Genetic alterations in the Catnb gene but not the H-ras gene in hepatocellular neoplasms and hepatoblastomas of B6C3F1 mice following exposure to diethanolamine for 2 years. Chem Biol Interact. 2003;146:251–261. doi: 10.1016/j.cbi.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Jeannot E, Pogribny IP, Beland FA, Rusyn I. Chronic administration of ethanol leads to an increased incidence of hepatocellular adenoma by promoting H-ras-mutated cells. Cancer Lett. 2011;301:161–167. doi: 10.1016/j.canlet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ølstørn HBA, Paulsen JE, Alexander J. Effects of perinatal exposure to acrylamide and glycidamide on intestinal tumorigenesis in Min/+ mice and their wild-type litter mates. Anticancer Res. 2007;27:3855–3864. [PubMed] [Google Scholar]
- 45.Besaratinia A, Pfeifer GP. Weak yet distinct mutagenicity of acrylamide in mammalian cells. J Natl Cancer Inst. 2003;95:889–896. doi: 10.1093/jnci/95.12.889. [DOI] [PubMed] [Google Scholar]
- 46.Besaratinia A, Pfeifer GP. Genotoxicity of acrylamide and glycidamide. J Natl Cancer Inst. 2004;96:1023–1029. doi: 10.1093/jnci/djh186. [DOI] [PubMed] [Google Scholar]
- 47.Besaratinia A, Pfeifer GP. DNA adduction and mutagenic properties of acrylamide. Mutat Res. 2005;580:31–40. doi: 10.1016/j.mrgentox.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Wiseman RW, Stowers SJ, Miller EC, Anderson MW, Miller JA. Activating mutations of the c-Ha-ras protooncogene in chemically induced hepatomas of the male B6C3 F1 mouse. Proc Natl Acad Sci USA. 1986;83:5825–5829. doi: 10.1073/pnas.83.16.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dragani TA, Manenti G, Colombo BM, Falvella FS, Gariboldi M, Pierotti MA, Della Porta G. Incidence of mutations at codon 61 of the Ha-ras gene in liver tumors of mice genetically susceptible and resistant to hepatocarcinogenesis. Oncogene. 1991;6:333–338. [PubMed] [Google Scholar]
- 50.Watson MA, Devereux TR, Malarkey DE, Anderson MW, Maronpot RR. H-ras oncogene mutation spectra in B6C3F1 and C57BL/6 mouse liver tumors provide evidence for TCDD promotion of spontaneous and vinyl carbamate-initiated liver cells. Carcinogenesis. 1995;16:1705–1710. doi: 10.1093/carcin/16.8.1705. [DOI] [PubMed] [Google Scholar]