Dietary deficiencies of zinc and iron are a major global public health problem. An estimated two billion people suffer these deficiencies 1 causing a loss of 63 million life years annually 2,3. Most of these people depend upon C3 grains and legumes as their primary dietary source of zinc and iron. We report that C3 grains and legumes have lower concentrations of zinc and iron when grown under field conditions at the elevated atmospheric CO2 concentration anticipated for the middle of this century. C3 crops other than legumes also have lower concentrations of protein, whereas C4 crops appear to be less affected. Differences among cultivars of a single crop suggest that breeding for reduced sensitivity to atmospheric CO2 concentration, i.e., [CO2], could partially address these new challenges to global health.
In the 1990s, several investigators found that elevated [CO2] decreased the concentrations of zinc, iron, and protein in grains of wheat 4–7, barley 5, and rice 8 grown in controlled environment chambers. Subsequent studies, however, failed to replicate these results when plants were grown in open top chambers and free air CO2 enrichment (FACE) experiments. Lieffering et al. (2004)9 found no [CO2] effect on the concentrations of zinc or iron in rice grains grown under FACE and suggested that the earlier findings had been influenced by “pot effects,” whereby a small rooting volume led to nutrient dilution at the root-soil interface. Of the more recent studies [10–13], most have indicated lower elemental concentrations in soybeans 10, sorghum 10, potatoes 11, wheat 12, or barley, 13 grown at elevated [CO2], but with the exception of iron in one wheat study 12, these results were statistically insignificant, perhaps because of small sample sizes.
Small sample sizes have limited the statistical power of individual studies of many aspects of plant responses to elevated [CO2], and meta-analyses involving larger samples of genotypes, environmental conditions, and experimental locations have played an important role in resolving which elements of plant function respond reliably to altered [CO2] 14,15. A recent meta-analysis of published data concluded that only sulfur is decreased in grains grown at elevated [CO2] 16.
Here we report findings from meta-analysis of newly acquired data from 143 comparisons of the edible portions of crops grown at ambient and elevated [CO2] from seven different FACE experimental locations in Japan, Australia, and the United States involving six food crops (see Table 1). We tested the nutrient concentrations of the edible portions of rice (Oryza sativa, 18 cultivars), wheat (Triticum aestivum, 8 cultivars), maize (Zea mays, 2 cultivars), soybeans (Glycine max, 7 cultivars), field peas (Pisum sativum, 4 cultivars) and sorghum (Sorghum bicolor, 1 cultivar). In all, forty genotypes were tested over 1 to 6 growing seasons at ambient and elevated [CO2], where the latter was in the range of 546–586 ppm across all seven study sites. Collectively, these experiments contribute more than 10-fold greater data regarding both zinc and iron content of the edible portions of crops grown under FACE conditions than is currently available in the literature. Consistent with earlier meta-analyses of other aspects of plant function under FACE conditions 14,15, we considered the response comparisons observed from different species, cultivars, and stress treatments and from different years to be independent. The natural log of the mean response ratio (r = response in elevated [CO2]/response in ambient [CO2] is used as the metric for all analyses. Meta-analysis is used to estimate the overall effect of elevated [CO2] on the concentration of each nutrient in a particular crop and to determine the significance of this effect (see Methods).
Table 1.
Characteristics of agricultural experiments
| Crops | Country | Treatments used | Years grown | # of Replicates* | # of Cultivars | CO2 ambient/elev (ppm) |
|---|---|---|---|---|---|---|
|
| ||||||
| Wheat | ||||||
| Site 1: | Australia | 2 water levels, 2 N treatments, 2 Sowing times | 2007–10 | 4 | 8 | 382/546-550 |
| Site 2: | Australia | 1 Water level, 1 N treatment 2 Sowing times | 2007–9 | 4 | 1 | 382/546-550 |
|
| ||||||
| Field Peas | Australia | 2 water levels | 2010 | 4 | 4 | 382/546-550 |
|
| ||||||
| Rice | ||||||
| Site 1: | Japan | 1 N treatment, 2 warming treatments | 2007–8 | 3 | 3 | 376-379/570-576 |
| Site 2: | Japan | 3 N treatments, 2 warming treatments | 2010 | 4 | 18 | 386/584 |
|
| ||||||
| Maize | U.S. | 2 N treatments | 2008 | 4 | 2 | 385/550 |
|
| ||||||
| Soybeans | U.S. | 1 treatment | 2001, 02, 04, 2006–08 | 4 | 7 | 372-385/550 |
|
| ||||||
| Sorghum | U.S. | 2 water levels, | 1998–99 | 4 | 1 | 363-373/556-579 |
“# of replicates” refers to the number of identical cultivars grown under identical conditions in the same year and location but in separate FACE rings
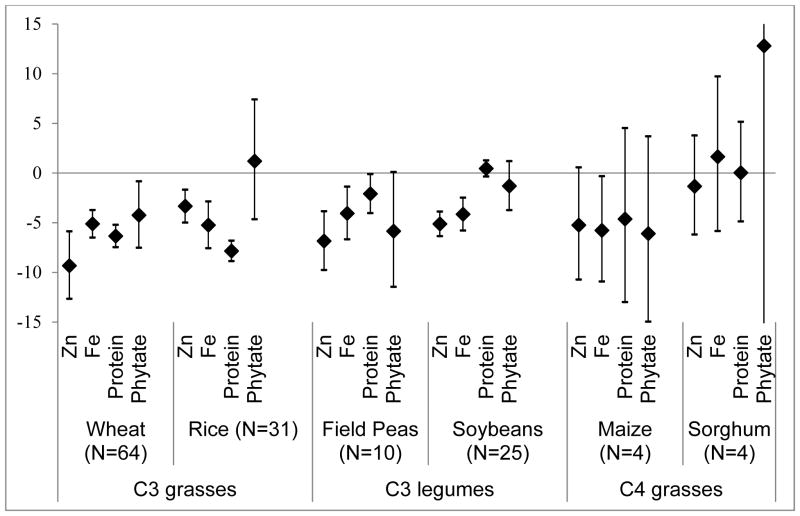
We find that elevated [CO2] is associated with significant decreases in the concentrations of zinc and iron in all C3 grasses and legumes (Figure 1, Table E1). For example, wheat grains grown at elevated [CO2] have 9.3% (95% CI: −12.7, −5.9) lower zinc and 5.1% (95% CI: −6.5, −3.7) lower iron than those grown at ambient [CO2]. We also find that elevated [CO2] is associated with lower protein in C3 grasses with a 6.3% (95% CI: −7.5, −5.2) decrease in wheat grains and a 7.8% (95% CI: −8.9, −6.8) decrease in rice grains. Elevated [CO2] is associated with a small decrease in protein in field peas and no significant effect in soybeans or C4 crops (Figure 1, Table E1).
Figure 1. Percent change in nutrient content at elevated [CO2] relative to ambient [CO2].
Percent change (95% Confidence Intervals) in nutrients at elevated [CO2] relative to ambient [CO2]. N refers to the number of comparisons where replicates of a particular cultivar grown at a specific site under one set of growing conditions in one year at elevated [CO2] have been pooled and mean nutrient values for these replicates are compared with mean values for identical cultivars under identical growing conditions except grown at ambient [CO2]. In most instances, data from four replicates were pooled for each value meaning that eight experiments were combined for each comparison (see Table 1 for details of experiments).
In addition to our own observations, we obtained data from ten of eleven previously published studies investigating nutrient changes in the edible portion of food crops (Table E6) and combined these data with our own observations in a larger meta-analysis. Analysis of our results combined with previously published FACE data (Table E2), or combined with previously published data from both FACE and chamber experiments (Table E3), is consistent with the results obtained using only our new data. Combining our data with previously published data does not alter the significance or substantially alter the effect size of the nutrient changes for any crop or any nutrient.
In addition to nutrient concentrations, we also measured phytate—a phosphate storage molecule present in most plants that inhibits the absorption of dietary zinc in the human gut 17. We had no a priori reason to assume phytate concentrations would change in response to rising [CO2]. However, formulas for calculating absorbed, or bioavailable, zinc depend both on the amount of dietary zinc and dietary phytate consumed, 17 making it important to interpret changes in zinc concentration in the context of possible changes in phytate. Phytate decreased significantly at elevated [CO2] only in wheat (P < 0.01). This decrease might offset some of the declines in zinc for this particular crop, though the decrease is fractionally less than half of the decrease in zinc. For other crops examined, however, the lack of a concurrent decrease in phytate may further exacerbate problems of zinc deficiency.
The global [CO2] in the atmosphere is expected to reach 550 ppm in the next 40–60 years, even if further actions are taken to reduce emissions 18. At these concentrations, we find that the edible portions of many of the key crops for human nutrition have decreased nutritional value when compared with the same plants grown under identical conditions but at present ambient [CO2]. Analysis of the United Nations’ Food and Agriculture Organization food balance sheets reveals that, as of 2010, roughly 2.3 billion people were living in countries whose populations receive at least 60% of their dietary zinc and/or iron from C3 grains and legumes and 1.9 billion lived in countries that receive at least 70% of one or both of these nutrients from these crops (Table E5). Reductions in the zinc and iron content of the edible portion of these food crops will increase the risk of zinc and iron deficiencies across these populations and add to the already considerable burden of disease associated with them.
The implications of reduced protein concentrations in non-leguminous C3 crops are less clear. From a study of adult men and women in the United States, there is strong evidence that the substitution of dietary carbohydrate for dietary protein increased the risk of hypertension, lipid disorders, and 10-year coronary heart disease risk 19. For the developing world, minimum protein requirements for different demographic groups are an area of active research and debate 20. For countries like India, however, where up to one third of the rural population is thought to be at risk of not meeting protein requirements 21 and where most protein comes in the form of C3 grains 21, decreased protein in non-leguminous C3 crops may have serious public health consequences.
Whereas zinc and iron were significantly decreased in all C3 crops tested, only iron in maize is observed to decrease amongst the C4 crops. No changes are found in sorghum. That zinc and iron declines are notable in C3 crops but less so in C4 crops is consistent with differences in physiology. C4 crops concentrate CO2 internally which results in photosynthesis being CO2-saturated even under ambient [CO2] conditions, leading to no stimulation of photosynthetic carbon assimilation at elevated [CO2] levels under mesic growing conditions 22. Our finding that protein is less affected in legumes than other C3 crops is also physiologically consistent with leguminous crops’ general ability to match stimulation of photosynthetic C gain at elevated [CO2] with greater N2 fixation in order to maintain tissue C:N ratios 23. In contrast, most temperate, non-legume C3 crops are generally unable to extract and assimilate sufficient N from soils to maintain tissue C:N ratios 24,25.
Little is known about the mechanism(s) responsible for the decline in nutrient concentrations associated with elevated [CO2]. Some authors have proposed “carbohydrate dilution” whereby CO2-stimulated carbohydrate production by plants dilutes the rest of the grain components 26. To test this hypothesis, we measured concentrations of additional elements for all crops except wheat (Table E4). Our findings are inconsistent with carbohydrate dilution operating alone. If only passive dilution of nutrients were occurring, we would expect to see very similar changes in the concentration of each nutrient tested for a given crop. In contrast, we find elemental changes in each given crop appear distinct from one another. For example, in rice grains (Table E4) the decrease in zinc concentrations associated with elevated [CO2] was significantly different from the decreases in the concentrations of copper (P ≤ 0.001), calcium (P ≤ 0.001), boron (P ≤ 0.001), or phosphate (P = 0.010). This heterogeneous response was also observed in recent analyses reviewing possible mechanisms for nutrient changes in both edible and non-edible plant tissues grown at elevated [CO2] 27. It also appears that the mechanism(s) causing these changes operate distinctly in different species. In one instance, for example, we find boron to be significantly decreased in soybeans (P ≤ 0.001), whereas it is significantly elevated in rice grains (P ≤ 0.001). While these differences may, in part, derive from different environmental conditions, it suggests that the mechanism is more complex than carbohydrate dilution alone. Of all the elements, changes in nitrogen content at elevated [CO2] are the most studied, and inhibition of photorespiration and malate production 24, carbohydrate dilution 26, slower root N uptake 25, and decreased transpiration-driven mass flow of N 27 may all play a role.
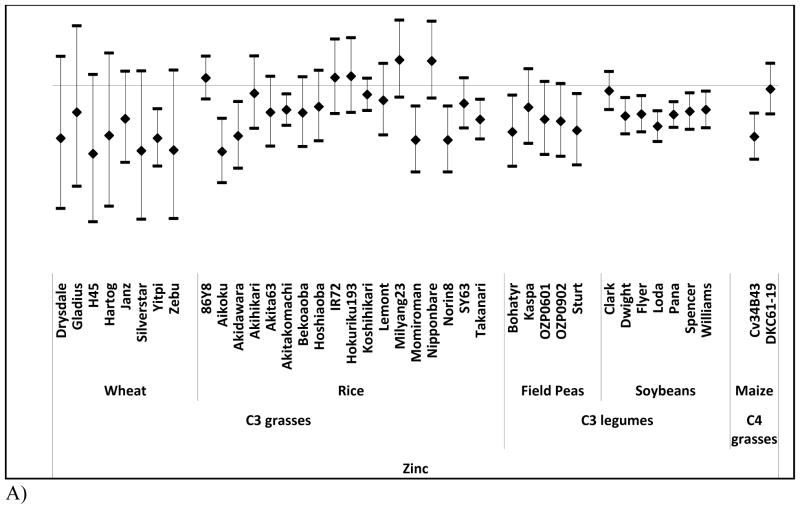
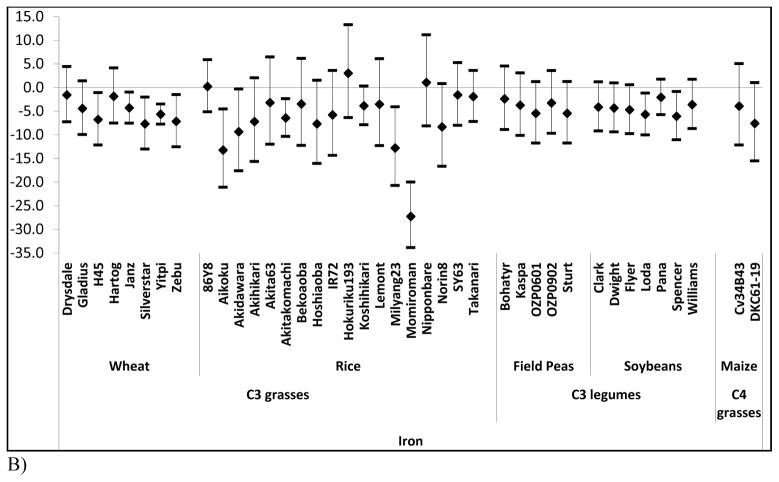
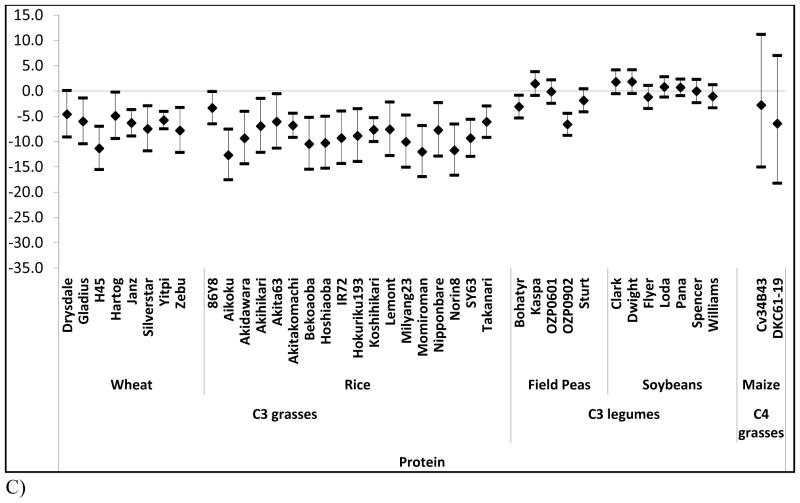
We examined the effects of elevated [CO2] on zinc, iron, and protein as a function of cultivar when data were available (Figure 2). Whereas most crops show negligible differences across cultivars, concentrations of zinc and iron across rice cultivars substantially vary (P = 0.04, and P = 0.03 respectively) (Figure 2a, and 2b).
Figure 2. Percent change in nutrient content at elevated [CO2] relative to ambient [CO2] by cultivar for each of three nutrients.
Percent change (95% Confidence Intervals) in zinc (A) iron (B) and protein (C) at elevated [CO2] relative to ambient [CO2] by cultivar.
Such cultivar differences suggest a basis for breeding rice cultivars whose micronutrient levels are less vulnerable to rising [CO2]. Similar effects may hold in other crops given that the statistical power of many of our other inter-cultivar tests is limited by sample size. We note, however, that such breeding programs will not be a panacea for many reasons including affordability of improved seeds and the numerous criteria used by farmers in making planting decisions that include taste, tradition, marketability, growing requirements, and yield. In addition, as has been noted previously, there are likely to be tradeoffs with respect to yield and other performance characteristics when breeding for increased zinc and iron content 28.
The public health implications of global climate change are difficult to anticipate, and we expect there will be many surprises. The finding that raising atmospheric [CO2] lowers the nutritional value of C3 food crops is one such surprise that we can now anticipate and prepare for. In addition to efforts to retard the elevation of future [CO2], it may be important to develop breeding programs designed to reduce the vulnerability of key crops to these changes. Nutritional analysis of which human populations are most vulnerable to decreased dietary zinc, iron, and protein from C3 crops could help target response efforts including breeding reduced sensitivity to elevated [CO2], biofortification, and supplementation.
Methods
We examine the response of nutrient levels to elevated atmospheric [CO2] for the edible portions of rice (Oryza sativa, 18 cultivars), wheat (Triticum aestivum, 8 cultivars), maize (Zea mays, 2 cultivars), soybeans (Glycine max, 7 cultivars), field peas (Pisum sativum, 4 cultivars) and sorghum (Sorghum bicolor, 1 cultivar). The six crops are grown under FACE conditions, and, in all six experiments, the elevated [CO2] is in the range of 546–586 ppm (see the Agricultural Methods section below for details associated with individual trials).
Statistics
In accordance with methods described by Ainsworth and Long 14 and Curtis and Wang 15, the natural log of the response ratio (r = response in elevated [CO2]/response in ambient [CO2]) is used as the metric for analyses and is reported as the mean percentage change [(r-1) X 100] at elevated [CO2]. Consistent with these earlier analyses of multiple species grown under FACE conditions, the responses of different species, cultivars, and stress treatments and from different years of the FACE experiments are considered to be independent and suited to meta-analytic analysis 14.
The meta-analysis is designed to estimate the overall effect of elevated [CO2] on the concentration of each nutrient in a particular crop and to determine the significance of this effect relative to a null hypothesis of no change. All tests are conducted as two-sided---not specifying which direction the nutrient concentrations are expected to change under elevated [CO2]---in order to make the analysis as general as possible. Meta-analysis is conducted using a linear mixed model. A random intercept is included for each comparison, representing nutrient level variability unrelated to [CO2] that is common to both treatment groups. Additional analyses indicate that the [CO2] effect on zinc concentration in rice is modified by cultivar and amount of nitrogen application, suggesting systematic variations across the pooled analysis of rice, and for these samples it is shown that the effect on zinc concentration is still significant when including interactions terms for cultivar and nitrogen. No other significant modifications of the [CO2] effect are identified. We tested whether changes in different nutrients for particular crops were statistically different from each other as has been described 30. To address the issue of multiple comparisons when testing for differences among cultivars within a crop, we multiplied the P-value by the number of independent comparisons. This approach follows the so-called Bonferroni correction and is conservative in the sense of biasing the P-values high, but it is nonetheless sufficient in our case to demonstrate that individual test results are significant despite their having been selected from amongst multiple tests.
Parameter estimates are obtained using the restricted maximum likelihood method, a standard approach for analyzing repeated measurement data 29 that, in our case, are of nutrient concentrations at time of harvest. Results for all analyses are reported as the best estimate of percent changes in the concentration of nutrients along with the 95% confidence intervals associated with each estimate. Two-tailed P-values are also reported.
Agricultural Methods
Rice (Oryza sativa, 18 cultivars), wheat (Triticum aestivum, 8 cultivars), maize (Zea mays, 2 cultivars), soybeans (Glycine max, 7 cultivars), field peas (Pisum sativum, 4 cultivars) and sorghum (Sorghum bicolor, 1 cultivar) were grown under FACE conditions during daylight hours. The experiments were conducted in Australia, Japan, and the United States between 1998 and 2010. Ambient [CO2] ranges between 363 and 386 ppm while elevated [CO2] is between 546 and 584 ppm. With the exception of soybeans, each experiment involves multiple cultivars of each crop and more than one set of growing conditions. Each experiment for each cultivar and set of treatments is replicated four times with the exception of one of the rice sites where three replicates are performed. These data are summarized in Table 1, and additional details of the soil and growing conditions, FACE methods, and experimental designs have been published for rice 31, wheat 32, maize 33, soybeans 34, field peas 32, and sorghum 35.
Laboratory Methods
Minerals Method
Samples were analyzed for minerals by heated closed vessel digestion/dissolution with nitric acid and hydrogen peroxide followed by quantitation using an inductively coupled plasma atomic emission spectrometer 36. Nitrogen content was measured using flash combustion of the sample coupled with thermal conductivity/IR detection of the combustion gases (N2, NOx, CO2) using a LECO TruSpec CN Analyzer 37. Protein values are based on measurement of nitrogen and conversion to protein per the equation below where k=5.36 38:
For phytic acid determination, a modified version of the method of Huag and Lantzsch 39 was used. The method’s accuracy was monitored by inclusion of tissue standards of known and varying levels of phytic acid 40.
Dietary Calculations
The United Nations Food and Agriculture Organization (UNFAO) publishes annual Food Balance Sheets (FBS), which provide country-specific data on the quantities of 95 ‘standardized’ food commodities available for human consumption. Data, expressed in terms of dietary energy (kcal per capita per day) were downloaded for 210 countries and territories with available information for the period from 2003–2007 (Available at http://faostat.fao.org). The percentage of dietary energy available from C3 grasses (wheat, barley, rye, oats, rice, “cereals, other” (excluding teff)) was calculated globally with estimates weighted by national population size (188 countries available; UN 2011. {Available at: http://esa.un.org/wpp/}).
Dietary intake data from the UNFAO FBS (through year 2000) and food composition data from the United States Department of Agriculture National Nutrient Database for Standard Reference were used to calculate per capita nutrient intake for 95 food items and shared with us by permission 41. This dataset was used to calculate the contribution of each food item to total dietary zinc and iron intake, and the proportions of all food items derived from C3 grains and legumes were summed to identify countries highly dependent on plant sources of iron and zinc (Table E5).
Extended Data
Extended Data Table E1.
Percent change in nutrient content at elevated [CO2] relative to ambient [CO2]
| N* (number of pairs) | Zn (μg/g) | Fe (μg/g) | Protein (mg/g) | Phytate (g/100g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| % | 95% CI | P-value | % | 95% CI | P-value | % | 95% CI | P-value | % | 95% CI | P-value | ||
| C3 grasses | |||||||||||||
| Wheat | 64 | −9.3 | (−12.7, −5.9) | <.0001 | −5.1 | (−6.5, −3.7) | <.0001 | −6.3 | (−7.5, −5.2) | <.0001 | −4.2 | (−7.5, −0.8) | 0.009 |
| Rice | 31 | −3.3 | (−5.0, −1.7) | <.0001 | −5.2 | (−7.6, −2.9) | <.0001 | −7.8 | (−8.9, −6.8) | <.0001 | 1.2 | (−4.6,7.4) | 0.697 |
| C3 legumes | |||||||||||||
| Field peas | 10 | −6.8 | (−9.8, −3.8) | 0.002 | −4.1 | (−6.7, −1.4) | 0.003 | −2.1 | (−4.0, −0.1) | 0.039 | −5.8 | (−11.5,0.1) | 0.055 |
| Soybeans | 25 | −5.1 | (−6.4, −3.9) | <.0001 | −4.1 | (−5.8, −2.5) | <.0001 | 0.5 | (−0.4,1.3) | 0.267 | −1.3 | (−3.7,1.2) | 0.303 |
| C4 grasses | |||||||||||||
| Maize | 4 | −5.2 | (−10.7,0.6) | 0.077 | −5.8 | (−10.9, −0.3) | 0.038 | −4.6 | (−13.0,4.5) | 0.312 | −6.1 | (−15.0,3.7) | 0.215 |
| Sorghum | 4 | −1.3 | (−6.2,3.8) | 0.603 | 1.6 | (−5.8,9.7) | 0.674 | 0.0 | (−4.9,5.2) | 0.993 | 12.8 | (−15.8,51.1) | 0.418 |
number of pairs refers to the number of comparisons where replicates of a particular cultivar grown at a specific site under one set of growing conditions in one year at elevated [CO2] have been pooled and mean nutrient values for these replicates are compared with mean values for identical cultivars under identical growing conditions except grown at ambient [CO2]. In most instances, data from four replicates were pooled for each value meaning that eight experiments were combined for each comparison (see Table 1 for details of experiments).
Extended Data Table E2.
Original data combined with previously published FACE data from studies 3, 4, 6, and 7. (See Extended Data Table E6 for list of experiments). Percent change in nutrient content at elevated [CO2] relative to ambient [CO2]
| N* (number of pairs) | Zn (μg/g) | Fe (μg/g) | Protein (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| % | 95% CI | P-value | % | 95% CI | P-value | % | 95% CI | P-value | ||
| C3 grasses | ||||||||||
| Wheat | 70 | −8.8 | (−11.9, −5.6) | <.0001 | −5.5 | (−6.8, −4.1) | <.0001 | −6.5 | (−7.5, −5.4) | <.0001 |
| Rice | 32 | −3.1 | (−4.8, −1.5) | <.0001 | −4.9 | (−7.3, −2.6) | <.0001 | −8.0 | (−9.0, −6.9) | <.0001 |
| Barley | 4 | −11.4 | (−19.3, −2.7) | 0.012 | −10.5 | (−12.2, −8.7) | <.0001 | −11.9 | (−13.1, −10.7) | <.0001 |
| C3 legumes | ||||||||||
| Field peas | 10 | −6.8 | (−9.8, −3.8) | 0.002 | −4.1 | (−6.7, −1.4) | 0.003 | −2.1 | (−4.0, −0.1) | 0.039 |
| Soybeans | 25 | −5.1 | (−6.4, −3.9) | <.0001 | −4.1 | (−5.8, −2.5) | <.0001 | 0.5 | (−0.4,1.3) | 0.267 |
| C3 Tuber | ||||||||||
| Potato | 2 | −3.9 | (−12.9,6.2) | 0.440 | 2.3 | (−3.8,8.7) | 0.472 | −4.6 | (−7.7, −1.4) | <.0001 |
| C4 grasses | ||||||||||
| Maize | 4 | −5.2 | (−10.7,0.6) | 0.077 | −5.8 | (−10.9, −0.3) | 0.038 | −4.6 | (−13.0,4.5) | 0.312 |
| Sorghum | 4 | −1.3 | (−6.2,3.8) | 0.603 | 1.6 | (−5.8,9.7) | 0.674 | 0.0 | (−4.9,5.2) | 0.993 |
number of pairs refers to the number of comparisons where replicates of a particular cultivar grown at a specific site under one set of growing conditions in one year at elevated [CO2] have been pooled and mean nutrient values for these replicates are compared with mean values for identical cultivars under identical growing conditions except grown at ambient [CO2]. In most instances, data from four replicates were pooled for each value meaning that eight experiments were combined for each comparison (see Table 1 for details of experiments).
Extended Data Table E3.
Original data combined with previously published FACE and chamber data from studies 1–10. (See extended data Table E6 for list of experiments). Percent change in nutrient content at elevated [CO2] relative to ambient [CO2]
| N* (number of pairs) | Zn (μg/g) | Fe (μg/g) | Protein (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| % | 95% CI | P-value | % | 95% CI | P-value | % | 95% CI | P-value | ||
| C3 grasses | ||||||||||
| Wheat | 78 | −9.1 | (−12.1, −6.1) | <.0001 | −5.9 | (−7.8, −4.0) | <.0001 | −7.2 | (−8.6, −5.8) | <.0001 |
| Rice | 32 | −3.1 | (−4.8, −1.5) | <.0001 | −4.9 | (−7.3, −2.6) | <.0001 | −8.0 | (−9.0, −6.9) | <.0001 |
| Barley | 6 | −13.6 | (−19.3, −7.6) | <.0001 | −10.0 | (−12.4, −7.4) | <.0001 | −15.0 | (−19.1, −10.7) | <.0001 |
| C3 legumes | ||||||||||
| Field peas | 10 | −6.8 | (−9.8, −3.8) | <.0001 | −4.1 | (−6.7, −1.4) | 0.003 | −2.1 | (−4.0, −0.1) | 0.039 |
| Soybeans | 28 | −5.0 | (−6.1, −3.9) | <.0001 | −5.2 | (−7.9, −2.5) | <.0001 | 0.1 | (−0.8,0.9) | 0.865 |
| C3 Tuber | ||||||||||
| Potato | 5 | −10.0 | (−20.9,2.4) | 0.110 | −4.1 | (−16.6,10.3) | 0.555 | −9.7 | (−15.9, −3.1) | 0.005 |
| C4 grasses | ||||||||||
| Maize | 4 | −5.2 | (−10.7,0.6) | 0.077 | −5.8 | (−10.9, −0.3) | 0.038 | −4.6 | (−13.0,4.5) | 0.312 |
| Sorghum | 7 | −0.6 | (−4.5,3.4) | 0.764 | 33.8 | (−10.2,99.3) | 0.153 | −5.6 | (−12.7,2.1) | 0.150 |
number of pairs refers to the number of comparisons where replicates of a particular cultivar grown at a specific site under one set of growing conditions in one year at elevated [CO2] have been pooled and mean nutrient values for these replicates are compared with mean values for identical cultivars under identical growing conditions except grown at ambient [CO2]. In most instances, data from four replicates were pooled for each value meaning that eight experiments were combined for each comparison (see Table 1 for details of experiments).
Extended Data Table E4.
Percent change in nutrient content at elevated [CO2] compared with ambient [CO2] for all nutrients
| C3 grasses | C3 legumes | C4 grasses | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | Rice | Field Peas | Soybean | Maize | Sorghum | |||||||||||||
| % | 95% CI | P-value | % | 95% CI | P-value | % | 95% CI | P-value | % | 95% CI | P-value | % | 95% CI | P-value | % | 95% CI | P-value | |
| Zinc (ppm) | −9.3 | (−12.7, −5.9) | <.0001 | −3.3 | (−5.0, −1.7) | <.0001 | −6.8 | (−9.8, −3.8) | <.0001 | −5.1 | (−6.4, −3.9) | <.0001 | −5.2 | (−10.7,0.6) | 0.077 | −1.3 | (−6.2,3.8) | 0.603 |
| Iron (ppm) | −5.1 | (−6.5, −3.7) | <.0001 | −5.2 | (−7.6, −2.9) | <.0001 | −4.1 | (−6.7, −1.4) | <.0001 | −4.1 | (−5.8, −2.5) | <.0001 | −5.8 | (−10.9, −0.3) | 0.038 | 1.6 | (−5.8,9.7) | 0.674 |
| Phytate (mg/g) | −4.2 | (−7.5, −0.8) | 0.009 | 1.2 | (−4.6,7.4) | 0.70 | −5.8 | (−11.5,0.1) | 0.055 | −1.3 | (−3.7,1.2) | 0.303 | −6.1 | (−15.0,3.7) | 0.215 | 12.8 | (−15.8,51.1) | 0.418 |
| Protein | −6.3 | (−7.5, −5.2) | <.0001 | −7.8 | (−8.9, −6.8) | <.0001 | −2.1 | (−4.0, −0.1) | 0.039 | 0.5 | (−0.4,1.3) | 0.267 | −4.6 | (−13.0,4.5) | 0.312 | 0.0 | (−4.9,5.2) | 0.993 |
| Mn (ppm) | −7.5 | (−12.0, −2.8) | 0.00 | −2.5 | (−4.2, −0.8) | 0.005 | −1.4 | (−3.5,0.8) | 0.204 | −4.2 | (−10.5,2.5) | 0.215 | 1.7 | (−4.5,8.3) | 0.596 | |||
| Mg (%) | −0.9 | (−2.3,0.6) | 0.24 | 0.0 | (−1.3,1.4) | 0.960 | −3.5 | (−4.3, −2.8) | <.0001 | −5.7 | (−9.9, −1.3) | 0.011 | −0.2 | (−5.1,4.9) | 0.944 | |||
| Cu (ppm) | −10.6 | (−13.8, −7.1) | <.0001 | −2.7 | (−5.1, −0.3) | 0.025 | −5.7 | (−8.0, −3.4) | <.0001 | −9.9 | (−19.3,0.7) | 0.066 | −2.9 | (−7.1,1.5) | 0.190 | |||
| Ca (%) | 2.0 | (−0.8,4.9) | 0.16 | −0.5 | (−4.2,3.3) | 0.787 | −5.8 | (−7.3, −4.2) | <.0001 | −2.7 | (−16.9,13.9) | 0.734 | 11.2 | (−5.2,30.3) | 0.190 | |||
| S (ppm) | −7.8 | (−8.8, −6.8) | <.0001 | −2.2 | (−3.6, −0.7) | 0.003 | −2.9 | (−3.5, −2.2) | <.0001 | 2.1 | (−2.2,6.7) | 0.342 | −0.2 | (−5.4,5.2) | 0.936 | |||
| K (%) | 1.1 | (−0.3,2.5) | 0.13 | 2.2 | (0.6,3.8) | 0.008 | 0.1 | (−0.8,1.0) | 0.857 | −2.7 | (−3.1, −2.2) | <.0001 | 3.0 | (−2.7,9.1) | 0.308 | |||
| B (ppm) | 5.1 | (1.9,8.4) | 0.002 | −1.9 | (−3.9,0.1) | 0.057 | −6.4 | (−9.1, −3.6) | <.0001 | 4.9 | (−1.0,11.1) | 0.107 | −0.3 | (−9.3,9.6) | 0.952 | |||
| P (%) | −1.0 | (−2.4,0.4) | 0.16 | −3.7 | (−6.8, −0.5) | 0.023 | −0.7 | (−2.2,0.9) | 0.379 | −7.1 | (−9.0, −5.1) | <.0001 | 0.3 | (−4.0,4.9) | 0.881 | |||
Extended Data Table E5.
Countries whose populations receive at least 60% of dietary iron and/or zinc from C3 grains and legumes per United Nations Food and Agriculture Organization food balance sheets and 2010 United Nations estimated population
| Country | % Iron from C3 grains & legumes | % Zinc from C3 grains & legumes | Population (in thousands) |
|---|---|---|---|
| Afghanistan | 78% | 78% | 31,412 |
| Algeria | 76% | 79% | 35,468 |
| Iraq | 74% | 83% | 31,672 |
| Bangladesh | 72% | 88% | 148,692 |
| Iran, Islamic Rep of | 72% | 77% | 73,974 |
| Pakistan | 70% | 72% | 173,593 |
| Tunisia | 70% | 77% | 10,481 |
| Jordan | 69% | 73% | 6,187 |
| Morocco | 69% | 78% | 31,951 |
| Syrian Arab Republic | 67% | 71% | 20,411 |
| Libya | 67% | 71% | 6,355 |
| Yemen | 66% | 75% | 24,053 |
| Myanmar | 65% | 81% | 47,963 |
| Tajikistan | 62% | 56% | 6,879 |
| India | 59% | 71% | 1,224,614 |
| Egypt | 54% | 65% | 81,121 |
| Indonesia | 52% | 65% | 239,871 |
| Sierra Leone | 51% | 70% | 5,868 |
| Cambodia | 49% | 68% | 14,138 |
| Sri Lanka | 46% | 69% | 20,860 |
| Laos | 44% | 66% | 6,201 |
| Viet Nam | 43% | 61% | 87,848 |
| Total | 2,329,612 |
Extended Data Table E6.
Literature reporting nutrient changes in the edible portion of crops grown at elevated and ambient [CO2]
| Study | Experimental Method | Associated Citations |
|---|---|---|
| 1 | Growth Chambers | Conroy, J., Seneweera, S. P., Basra, A., Rogers, G. & Nissen-Wooller, B. Influence of rising atmospheric CO2 concentrations and temperature on growth, yield and grain quality of cereal crops. Australian Journal of Plant Physiology 21, 741–758 (1994). |
| Seneweera, S., Milham, P. & Conroy, J. Influence of elevated CO2 and phosphorus nutrition on the growth and yield of a short-duration rice. Australian Journal of Plant Physiology 21, 281–292 (1994). | ||
| Seneweera, S. P. & Conroy, J. P. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition (Reprinted from Plant nutrition for sustainable food production and environment, 1997). Soil Sci. Plant Nutr. 43, 1131–1136 (1997). | ||
| 2 | Temperature Gradient Tunnels | De la Puente, L. S., Perez, P. P., Martinez-Carrasco, R., Morcuende, R. M. & Del Molino, I. M. M. Action of elevated CO2 and high temperatures on the mineral chemical composition of two varieties of wheat. Agrochimica 44, 221–230 (2000). |
| 3 | Open Top Chambers & FACE | De Temmerman L et al. Effect of climatic conditions on tuber yield (Solanum tuberosum L.) in the European ‘CHIP’ experiments. European Journal of Agronomy 17, 243–255 (2002). |
| De Temmerman, L., Hacour, A. & Guns, M. Changing climate and potential impacts on potato yields and quality ‘CHIP’: introduction, aims and methodology. European Journal of Agronomy 17, 233–242 (2002). | ||
| Fangmeier, A., De Temmerman, L., Black, C., Persson, K. & Vorne, V. Effects of elevated CO2 and/or ozone on nutrient concentrations and nutrient uptake of potatoes. European Journal of Agronomy 17, 353–368 (2002). | ||
| Högy, P. & Fangmeier, A. Atmospheric CO2 enrichment affects potatoes: 2. Tuber quality traits. European Journal of Agronomy 30, 85–94 (2009). | ||
| 4 | FACE | Erbs, M. et al. Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agriculture, Ecosystems and Environment 136, 59–68 (2010). |
| 5 | Open Top Chambers | Fangmeier, A. et al. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat. I. Growth and yield. Environmental Pollution 91, 381–390 (1996). |
| Fangmeier, A., Grüters, U., Högy, P., Vermehren, B. & Jäger, H.-J. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat – II. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environmental Pollution 96, 43–59 (1997). | ||
| Fangmeier, A. et al. Effects on nutrients and on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment ‘ESPACE-wheat’. European Journal of Agronomy 10, 215–229 (1999). | ||
| Jäger, H.-J., Hertstein, U. & Fangmeier, A. The European Stress Physiology and Climate Experiment – project 1: wheat (ESPACE-wheat): introduction, aims and methodology. European Journal of Agronomy 10, 155–162 (1999). | ||
| 6 | FACE | Högy, P. & Fangmeier, A. Effects of elevated atmospheric CO2 on grain quality of wheat. Journal of Cereal Science 48, 580–591 (2008). |
| Högy, P. et al. Does elevated atmospheric CO2 allow for sufficient wheat grain quality in the future?. Journal of Applied Botany and Food Quality 82, 114–121 (2009). | ||
| Högy, P. et al. Effects of elevated CO2 on grain yield and quality of wheat: results from a 3-year free-air CO2 enrichment experiment. Plant Biology 11, 60–69 (2009). | ||
| Högy, P., Zörb, C., Langenkämper, G., Betsche, T. & Fangmeier, A. Atmospheric CO2 enrichment changes the wheat grain proteome. Journal of Cereal Science 50, 248–254 (2009). | ||
| 7 | FACE | Kim, H., Lieffering, M., Miura, S., Kobayashi, K. & Okada, M. Growth and nitrogen uptake of CO2-enriched rice under field conditions. New Phytologist 150, 223–229 (2001). |
| Kim, H. et al. Effects of free-air CO2 enrichment and nitrogen supply on the yield of temperate paddy rice crops. Field Crops Research 83, 261–270 (2003). | ||
| Lieffering, M., Kim, H.-Y., Kobayashi, K. & Okada, M. The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crops Research 88, 279–286 (2004). | ||
| 8 | Open Top Chambers | Pleijel, H. et al. Effects of elevated carbon dioxide, ozone and water availability on spring wheat growth and yield. Physiologia Plantarum 108, 61–70 (2000). |
| Pleijel, H. & Danielsson, H. Yield dilution of grain Zn in wheat grown in open-top chamber experiments with elevated CO2 and O3 exposure. Journal of Cereal Science 50, 278–282 (2009). | ||
| 9 | Open Top Chambers | Prior, S. A., Runion, G. B., Rogers, H. H., Torbert, H. A. Effects of atmospheric CO2 enrichment on crop nutrient dynamics under no-till conditions. Journal of Plant Nutrition 31, 758–773 (2008). |
| 10 | Open Top Chambers | Weigel, H., Manderscheid, R., Jäger, H.-J. & Mejer, G. Effects of season-long CO2 enrichment on cereals. I. Growth performance and yield. Agriculture, Ecosystems and Environment 48, 231–240 (1994). |
| Manderscheid, R., Bender, J., Jager, H., J & Weigel, H., J. Effects of season long CO2 enrichment on cereals. II. Nutrient concentrations and grain quality. Agriculture, Ecosystems & Environment 54, 175–185 (1995). | ||
| 11 | FACE | Yang, L., Wang, Y., Dong, G., Gu, H., Huang, J., Zhu, J., Yang, H., Liu, G., Han, Y. The impact of free-air CO2 enrichment (FACE) and nitrogen supply on grain quality of rice. Field Crops Research 102, 128–140 (2007). |
| Meta-Analyses | Loladze, I. Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends in Ecology and Evolution 17 (10), 457–461 (2002). [Uses data from studies 1, 2, 5, and 10 as well as numerous other studies on non-edible tissues and plants other than food crops]. | |
| McGrath, J. M. and Lobell, D. B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant, Cell, & Environment 36, 697–705 (2013). [Uses data from studies 1, 5, and 10 as well as numerous other studies on non-edible tissues and plants other than food crops]. | ||
| Duval, B.D., Blankinship, J. C., Dijkstra, P., Hungate, B. A. CO2 effects on plant nutrient concentration depend on plant functional group and available nitrogen: a meta-analysis. Plant Ecology 213, 505–521 (2012). [Uses data from studies 1,2, 3, 5, 6, and 9 as well as numerous other studies on non-edible tissues and plants other than food crops]. |
Acknowledgments
We thank the following for financial support of this work: the Bill & Melinda Gates Foundation; the Winslow Foundation; the Commonwealth Department of Agriculture, Fisheries and Forestry (Australia), the International Plant Nutrition Institute, (Australia), the Grains Research and Development Corporation (Australia), the Ministry of Agriculture, Forestry and Fisheries, (Japan), the National Science Foundation: NSF IOS-08-18435, the US Department of Agriculture Agricultural Research Service (SoyFACE) and the US Department of Energy (SoyFACE). Early stages of this work received support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05. We thank the following investigators for sharing data from their groups with us: L.S. De la Puente, M. Erbs, A. Fangmeier, P. Högy, M. Lieffering, R. Manderscheid, H. Pleijel, and S. Prior. The National Agricultural Research Organization (Japan) provided the grain samples of some rice cultivars. Contributions of H. Nakamura, T. Tokida, Z. Chunwu, and S. Yoshinaga to the rice FACE project are acknowledged. Raboy thanks Amanda Lewis (USDA-ARS, Aberdeen ID) for her efforts in producing the phytate data included herein. We also thank the following individuals for their informal reviews of earlier drafts or conceptual contributions to this project: Michael Hambidge, Walter Willett, Daniel Schrag, Kenneth Brown, Ryan Wessells, Nimesha Fernando, Jan Peerson, and Bruce Kimball.
Footnotes
Author Contributions: SSM conceived the overall project and drafted the manuscript. AZ, IK, JS, and PH performed statistical analyses. PH and ADBL provided substantial input into methods descriptions. AB, EC, and VR analyzed grain samples for nutrient content. GF, TH, ADBL, RLN, MJO, HS, SS, MT, and YU conducted FACE experiments and supplied grain for analysis. NMH, and PH assisted with elements of experimental design. KS and LD assisted with data collection and analysis. All authors contributed to manuscript preparation.
The authors declare no competing financial interests.
References and Notes
- 1.Tulchinsky TH. Micronutrient Deficiency Conditions: Global Health Issues. Public Health Reviews. 2010;32:243–255. doi: 10.1186/s40985-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caulfield LE, Black RE. In: Comparative quantification of health risks: global and regional burden of disease attribution to selected major risk factors. Ezzati Majid, Lopez Alan D, Rodgers Anthony, Christopher Murray JL., editors. Vol. 1. World Health Organization; 2004. Ch. 5. [Google Scholar]
- 3.Stoltzfus RJ, Mullany L, Black RE. In: Comparative quantification of health risks: global and regional burden of disease attribution to selected major risk factors. Ezzati Majid, Lopez Alan D, Rodgers Anthony, Christopher Murray JL., editors. Vol. 1. World Health Organization; 2004. Ch. 3. [Google Scholar]
- 4.De la Puente LS, Perez PP, Martinez-Carrasco R, Morcuende RM, Del Molino IMM. Action of elevated CO2 and high temperatures on the mineral chemical composition of two varieties of wheat. Agrochimica. 2000;44:221–230. [Google Scholar]
- 5.Manderscheid R, Bender J, Jager HJ, Weigel HJ. Effects of season long CO2 enrichment on cereals. II. Nutrient concentrations and grain quality. Agriculture, Ecosystems & Environment. 1995;54:175–185. [Google Scholar]
- 6.Fangmeier A, Grüters U, Högy P, Vermehren B, Jäger HJ. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat – II. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn) Environmental Pollution. 1997;96:43–59. doi: 10.1016/s0269-7491(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 7.Pleijel H, et al. Effects of elevated carbon dioxide, ozone and water availability on spring wheat growth and yield. Physiologia Plantarum. 2000;108:61–70. [Google Scholar]
- 8.Seneweera SP, Conroy JP. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition (Reprinted from Plant nutrition for sustainable food production and environment, 1997) Soil Sci Plant Nutr. 1997;43:1131–1136. [Google Scholar]
- 9.Lieffering M, Kim HY, Kobayashi K, Okada M. The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crops Research. 2004;88:279–286. [Google Scholar]
- 10.Prior SA, Runion GB, Rogers HH, Torbert HA. Effects of Atmospheric CO2 Enrichment on Crop Nutrient Dynamics under No-Till Conditions. Journal of Plant Nutrition. 2008;31:758–773. [Google Scholar]
- 11.Högy P, Fangmeier A. Atmospheric CO2 enrichment affects potatoes: 2. Tuber quality traits. European Journal of Agronomy. 2009;30:85–94. [Google Scholar]
- 12.Högy P, et al. Effects of elevated CO2 on grain yield and quality of wheat: results from a 3-year free-air CO2 enrichment experiment. Plant Biology. 2009;11:60–69. doi: 10.1111/j.1438-8677.2009.00230.x. [DOI] [PubMed] [Google Scholar]
- 13.Erbs M, et al. Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agriculture, Ecosystems and Environment. 2010;136:59–68. [Google Scholar]
- 14.Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties, and plant production to rising CO2. New Phytologist. 2005;172:283–663. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 15.Curtis PS, Wang X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. [DOI] [PubMed] [Google Scholar]
- 16.Duval BD, Blankinship JC, Dijkstra P, Hungate BA. CO2 effects on plant nutrient concentration depend on plant functional group and available nitrogen: a meta-analysis. Plant Ecol. 2012;213:505–521. [Google Scholar]
- 17.Miller LV, Krebs NF, Hambidge MK. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. The Journal of Nutrition. 2007;137:135–141. doi: 10.1093/jn/137.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher BS, et al. In: Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Inter-governmental Panel on Climate Change. Metz B, et al., editors. Cambridge University Press; 2007. [Google Scholar]
- 19.Appel LJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. Journal of the American Medical Association. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 20.Millward D, Joe Identifying recommended dietary allowances for protein and amino acids: a critique of the 2007 WHO/FAO/UNU report. British Journal of Nutrition. 2012;108:S3–S21. doi: 10.1017/S0007114512002450. [DOI] [PubMed] [Google Scholar]
- 21.Swaminathan S, Vaz M, Kurpad AV. Protein intakes in India. British Journal of Nutrition. 2012;108:S50–S58. doi: 10.1017/s0007114512002413. [DOI] [PubMed] [Google Scholar]
- 22.Leakey A. Rising atmospheric carbon dioxide concentration and the future of C-4 crops for food and fuel. Proceedings of the Royal Society B-Biological Sciences. 2009;276:2333–2343. doi: 10.1098/rspb.2008.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers A, Ainsworth EA, Leakey AD. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiology. 2009;151:1009–1016. doi: 10.1104/pp.109.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloom AJ, et al. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology. 2012;93:355–367. doi: 10.1890/11-0485.1. [DOI] [PubMed] [Google Scholar]
- 25.Leakey AD, et al. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany. 2009;60:2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- 26.Gifford R, Barrett D, Lutze J. The effects of elevated [CO2] on the C:N and C:P mass ratios of plant tissues. Plant and Soil. 2000;224:1–14. doi: 10.1023/A:1004790612630. [DOI] [Google Scholar]
- 27.McGrath JM, Lobell DB. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant, Cell & Environment. 2013;36:697–705. doi: 10.1111/pce.12007. [DOI] [PubMed] [Google Scholar]
- 28.Monasterio I, Graham RD. Breeding for trace minerals in wheat. Food and Nutrition Bulletin. 2000;21:392–396. [Google Scholar]
- 29.Searle SR, Casella G, McCulloch CE. Variance Components. John Wiley & Sons; 1992. [Google Scholar]
- 30.Schenker N, Gentleman JF. On judging the significance of differences by examining the overlap between confidence intervals. The American Statistician. 2001;55:182–186. [Google Scholar]
- 31.Hasegawa T, et al. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Functional Plant Biology. 2013 doi: 10.1071/FP12357. [DOI] [PubMed] [Google Scholar]
- 32.Mollah M, Norton R, Huzzey J. Australian Grains Free Air Carbon dioxide Enrichment (AGFACE) facility: design and performance. Crop & Pasture Science. 2009;60:697–707. [Google Scholar]
- 33.Markelz R, Strellner R, Leakey A. Impairment of C-4 photosynthesis by drought is exacerbated by limiting nitrogen and ameliorated by elevated CO2 in maize. Journal of Experimental Botany. 2011;62:3235–3246. doi: 10.1093/jxb/err056. [DOI] [PubMed] [Google Scholar]
- 34.Gillespie K, et al. Greater antioxidant and respiratory metabolism in field-grown soybean exposed to elevated O3 under both ambient and elevated CO2. Plant Cell and Environment. 2012;35:169–184. doi: 10.1111/j.1365-3040.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- 35.Ottman MJ, et al. Elevated CO2 increases sorghum biomass under drought conditions. New Phytologist. 2001;150:261–273. [Google Scholar]
- 36.Sah RN, Miller RO. Spontaneous reaction for acid dissolution of biological tissues in closed vessels. Anal Chem. 1992;64:230–233. doi: 10.1021/ac00026a026. [DOI] [PubMed] [Google Scholar]
- 37.972.43, A. O. M. Official Methods of Analysis of AOAC International. 18. Vol. 12. AOAC International; 2006. pp. 5–6. Revision 1, 2006. [Google Scholar]
- 38.Mosse J. Nitrogen to protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and to seed protein content. Journal of Agricultural and Food Chemistry. 1990;38:18–24. [Google Scholar]
- 39.Haug W, Lantzsch HJ. Sensitive method for the rapid determination of phytate in cereals and cereal products. Journal of the Science of Food and Agriculture. 1983;34:3. [Google Scholar]
- 40.Raboy V, et al. Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiology. 2000;124:355–368. doi: 10.1104/pp.124.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wuehler SE, Peerson JM, Brown KH. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Public Health Nutrition. 2005;8:812–819. doi: 10.1079/phn2005724. [DOI] [PubMed] [Google Scholar]