Abstract
Ethnopharmacological relevance
American ginseng is capable of ameliorating cardiac dysfunction and activating Nrf2, a master regulator of antioxidant defense, in the heart. This study was designed to isolate compounds from American ginseng and to determine those responsible for the Nrf2-mediated resolution of inflamed macrophage-induced cardiomyocyte hypertrophy.
Materials and methods
A standardized crude extract of American ginseng was supplied by the National Research Council of Canada, Institute for National Measurement Standards. A bioassay-based fractionization of American ginseng was performed to identify the putative substances which could activate Nrf2-mediated suppression of pro-inflammatory cytokine expression in macrophages and macrophage-mediated pro-hypertrophic growth in cardiomyocytes.
Results
A hexane fraction of an anti-inflammatory crude extract of American ginseng was found to be most effective in suppressing the inflammatory responses in macrophages. Preparative, reverse-phase HPLC and a comparative analysis by analytical scale LC–UV/MS revealed the hexane fraction contains predominantly C17 polyacetylenes and linolenic acid. Panaxynol, one of the major polyacetylenes, was found to be a potent Nrf2 activator. Panaxynol posttranscriptionally activated Nrf2 by inhibiting Kelch-like ECH-associated protein (Keap) 1-mediated degradation without affecting the binding of Keap1 and Nrf2. Moreover, panaxynol suppressed a selected set of cytokine expression via the activation of Nrf2 while minimally regulating nuclear factor-kappa B (NF-κB)-mediated cytokine expression in macrophages. It also dramatically inhibited the inflamed macrophage-mediated cardiomyocyte death and hypertrophy by activating Nrf2 in macrophages.
Conclusions
These results demonstrate that American ginseng-derived panaxynol is a specific Nrf2 activator and panaxynol-activated Nrf2 signaling is at least partly responsible for American ginseng-induced health benefit in the heart.
Keywords: American ginseng, Panaxynol, Nrf2, Inflammation, Macrophages, Cardiomyocytes
1. Introduction
Ginseng, the root of genus Panax of the family Araliaceae, has been used in Asian countries as a folk medicine for thousands of years (Gillis, 1997). Emerging evidence has suggested that regular use of ginseng is helpful in the treatment of human illnesses including cardiovascular disease (Wang et al., 2007; Zhou et al., 2004). However, the underlying cellular and molecular mechanisms remain largely unknown.
Recently, we have found that American ginseng is capable of suppressing lipopolysaccharide (LPS)-induced inducible nitric oxide synthase (iNOS) expression independent of NF-κB in macrophages (Ichikawa et al., 2009b) and oxidative stress-mediated cell death in H9C2 cardiomyocytes via its ability to activate Nrf2 (Li et al., 2010). On the other hand, we have demonstrated that Nrf2 activation suppresses a selected set of pro-inflammatory cytokines including iNOS, monocyte chemotactic protein-1 (MCP-1), and macrophage inflammatory protein-1 beta (MIP-1β) while minimally regulating NF-κB activity and its downstream cytokine expression, such as interleulin-6 (IL-6), IL-1β, and tumor necrosis factor alpha (TNFα) in macrophages (Li et al., 2014). These results suggest that American ginseng contains substances which may activate Nrf2-mediated resolution of inflammatory responses in macrophages.
Nrf2 is a key transcription factor that binds to cis-acting enhancer sequence known as the antioxidant response element (ARE) with a core nucleotide sequence of 5′-RTGACnnnGC-3′ to control the basal and inducible expression of more than 200 genes. These genes are functionally grouped into several categories including antioxidants, phase II detoxifying enzymes, transcriptional factors, transporters, scavenger receptors, and chaperone proteins (Kensler et al., 2007; Kobayashi and Yamamoto, 2005; Li et al., 2009a; Suzuki et al., 2013). As a result, Nrf2 appears to be a major transcription factor of the cellular defense system against a variety of environmental or intrinsic insults in different organs including lung, liver, gastrointestinal tract, bladder, kidney, brain, skin, and ovary, and heart (Li et al., 2009a; Li et al., 2009b; Wang et al., 2014). Of note, we have demonstrated that knockout of Nrf2 results in the earlier onset of cardiac maladaptive remodeling and dysfunction while cardiac specific overexpression of Nrf2 is cardioprotective (Li et al., 2009b; Wang et al., 2014). These findings indicate that Nrf2 is a potential drug target for the prevention and/or treatment of cardiovascular diseases such as heart failure. Although there is a large number of Nrf2 activating small molecules that are naturally occurring or chemically synthesized (Kumar et al., 2014; Liby et al., 2007), a therapeutic Nrf2 activator for cardiovascular disease remains to be established.
Given the historically verified safety of American ginseng and the emerging evidence of American ginseng-induced Nrf2 activation for cardioprotection, identifying the natural Nrf2 activating molecules from American ginseng may provide valuable insight into the development of novel Nrf2 activators to treat cardiac disease. Therefore, in the present study, we performed a bioassay based fractionation of American ginseng, aiming at the isolation of Nrf2 activating single compounds which are capable of specifically driving Nrf2-mediated health benefit in the heart.
2. Materials and methods
2.1. Animals
Breeding pairs of heterozygous Nrf2 knockout (Nrf2+/−/C57BL/6J) mice were purchased from Riken BioResource Center, Japan, and housed under standard conditions in the Institution’s AAALAC approved animal facility. Littermates of wild type (WT; Nrf2+/+) and homozygous Nrf2 knockout (Nrf2−/−) mice were generated using the Nrf2+/− breeding pairs as previously described (Itoh et al., 1997). Genotypes (Nrf2+/+, Nrf2−/−, and Nrf2+/−) of the animals were determined by polymerase chain reaction (PCR) amplification of genomic DNA obtained from the tail using Tissue-Direct™ PCR KIT (Cat#D300-1000, LAMDA BIOTEC, USA). The PCR products were resolved on a 1% agarose gel. The genotypes of mice were verified by examining the size of the PCR products: Nrf2+/+ (734 bp), Nrf2−/− (400 bp), Nrf2+/− (734 and 400 bp). Primers for Genotypes: 5′-TGGACGGGACTATTGAAGGCTG-3′ (sense for Nrf2+/+ and Nrf2−/−), 5′-GCCGCCTTTTCAGTAGATGGAGG-3′ (antisense for Nrf2+/+), and 5′-GCGGATTGACCGTAATGGGATAGG-3′ (antisense for LacZ). All of the animal protocols were conducted in accordance with the Guideline for Care and Use of Laboratory Animals (National Institute of Heath, USA) and were approved by the Institutional Animal Care and Use Committee at Shandong University, China, and the University of South Carolina, USA.
2.2. Preparation of crude extract of American ginseng
A standardized American ginseng crude extract reference material (GINX-1) was prepared by the National Research Council of Canada, Institute for National Measurement Standards (NRCC-INMS) as previously described (Ichikawa et al., 2009b; Li et al., 2010). This extract was derived from four year old, cultivated, ginseng roots grown by Chai-Na-Ta Farms Ltd. (Kamloops, British Columbia, Canada) and processed by Canadian Phytopharmaceuticals Corporation (Richmond, British Columbia, Canada). The identity of the roots was independently confirmed by Agriculture and Agri-Foods Canada and a voucher specimen of the Panax quinquefolius used in this study deposited with the University of Ottawa herbarium (UO 19908). Plant morphology conformed to that as previously described for P. quinquefolius (Small and Catling, 1999) and the presence of a marker compound ginsenoside F11, unique to P. quinquefolius, confirmed by liquid chromatography– mass spectrometry. Following thorough homogenization, 4000 1 g lots of the extract were bottled under argon, irradiated (5 kGy), and stored under cryogenic conditions (−80 °C). Periodic analyses of the extract over 4 years have shown no significant change in ginsenoside content which is 10.5% w/w measured as the sum of: Rg1 3.5 (0.1), Re 21.2 (0.4), Rb1 45.1 (1.6), Rc 15.5 (0.7), Rb2 2.2 (0.1), Rd 17.8 (0.4) mg/g ± one standard deviation, respectively.
2.3. Fractionation of American ginseng crude extract
The crude extract of American ginseng was further fractionated as previously described (Poudyal et al., 2012). Briefly, 10 g of American ginseng extract was dissolved in 150 mL of water and sequentially partitioned against 3 × 50 mL aliquots of hexane, dichloromethane, ethyl acetate, water, and butanol. The fractions were reduced to near dryness on a vacuum centrifuge, freeze dried, and their respective dry weights determined: water fraction, 7.320 g (i.e., 73% of the original material); butanol fraction, 1.544 g; ethyl acetate fraction, 0.064 g; dichloromethane fraction, 0.062 g; and hexane fraction, 0.044 g. Each fraction was then redissolved in a small volume of solvent to facilitate blending with the appropriate amount of maltodextrin to give a final weight of 10 g after a second round of evaporation by vacuum centrifuge and freeze drying. Thus, the original extract was subdivided on the basis of polarity and reconstituted with maltodextrin to give an equivalent weight as the starting material for bioassay. All fractions were thoroughly vortexed to give a free flowing powder and split into two: one set was retained at National Research Council (Ottawa, ON, Canada) as a reference and the other used for bioassay. Neat maltodextrin was used as a negative control.
2.4. Large scale purification of panaxynol
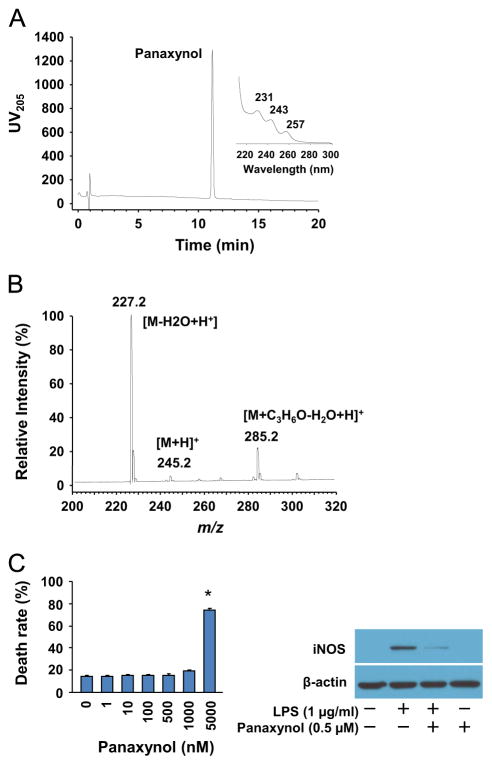
All solvents were of analytical grade and purchased from Fisher Scientific (Nepean, ON, Canada). 1 kg of American ginseng (P. quinquefolius) spray dried extract (Canadian Phytopharmaceuticals, Richmond, BC, Canada) derived from an aqueous ethanol extract of 4 year old roots was processed as 10, 100 g lots where each 100 g was dissolved/suspended in 1 L of water in a 2 L separatory funnel and extracted with 4 × 400 mL of hexane. The organic layers were concentrated by vacuum centrifuge to yield ~10 g of a free flowing dark brown oil. The extract was crudely separated using C-18 flash chromatography (1 kg packed open column, Phenomenex Sepra C-18 50 μm, Torrance, CA, USA), developed stepwise with 6 L of 55% acetonitrile (ACN), 9 L of 75% and 2 L of 100% ACN with all eluents collected in 500 mL volumes and each fraction analyzed by LC–UV. The polyacetylenes began eluting at 75% ACN over 18 fractions with the major panaxynol fractions eluting in fractions 10 through 16. These were combined and evaporated to dryness yielding ~1 g of crude panaxynol. The crude panaxynol was further purified on a Shimadzu preparative chromatography system (Canby, OR, USA) equipped with binary LC-8A pumps, SDP-10 AV controller and SCL-10A UV detector monitoring at 205 nm. Multiple 50 mg injections were made on a Waters Symmetry Shield C-18 19 × 300 mm2 column (Milford, MA, USA) operated at 40 mL min−1, using a 55–90% ACN/water gradient elution in 15 min then 90% ACN/water for 5 min and a 10 min re-equilibration under starting conditions before subsequent injections. Panaxynol eluted between 11 and 12 min and all collected fractions were pooled, evaporated to dryness on a SpeedVac vacuum concentrator (Themo-Fisher Scientific, Nepean, ON, Canada) and subjected to a second pass under identical conditions. Finally, the purified panaxynol was dissolved in 5 mL methanol, and decolorized by adding 30 mg of activated charcoal (Sigma-Aldrich, Oakville, On, Canada) and heating to 80 °C for 10 min. The solution was passed through a 0.45 μm PTFE membrane syringe filter (Chromatographic specialties, Brockville, ON, Canada) and evaporated to dryness under a gentle stream of nitrogen to yield 330 mg of a light yellow colored oil. The purified panaxynol was flame sealed in glass ampules in 5 mg aliquots under argon and stored at −80 °C until needed. Liquid chromatography with UV diode array detection (LC–UV DAD) analysis of the purified panaxynol using the same gradient conditions as above but on an Agilent 1100 system (Palo Alto, CA, USA) with a 4.6 × 150 mm2 Phenomenex Synergy C-18 column at a flow rate of 1 mL min−1 and a 5 μL injection gave a single major peak at 11.3 min with an area percent purity of 99.3% (Fig. 1). The UV spectra showed the highly characteristic UV maxima of panaxynol at 231, 243 and 257 nm and liquid chromatography–mass spectrometry (LC–MS) analysis in positive ion mode acquired on a TSQ triple-quadrupole mass spectrometer (Thermo Electron Corp., San Jose, CA, USA) fitted with an APCI source using acetone in the mobile phase gave a base peak at m/z 227.2 corresponding to the [M−H2O+H]+ ion; protonated and acetone adducts were also observed.
Fig. 1.
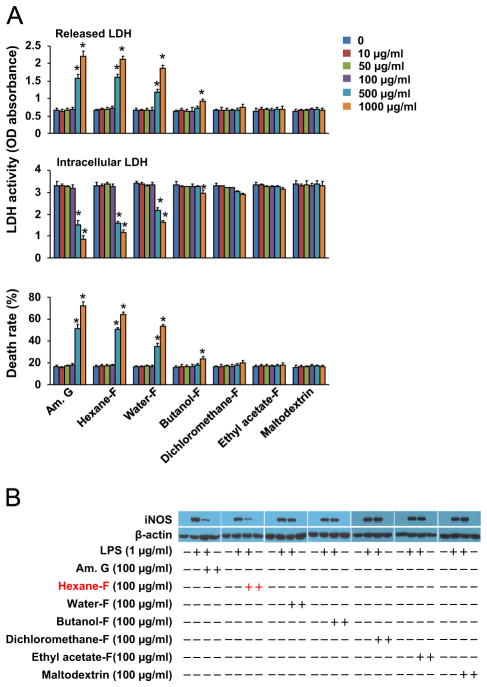
Sub-fractioning anti-inflammatory components of American ginseng crude extract. (A) Cytotoxicity of American ginseng crude extracts (Am. G), hexane fraction (Hexane-F), water-F, butanol-F, dichloremethane-F, ethyl acetate-F, and maltodextrin control in RAW264.7 cells. Sub-confluent RAW264.7 cells were treated with American ginseng crude extracts and other fractions in DMEM supplemented with 2% FBS as indicated for 24 h. n=6, *p<0.05 vs. control (0) in each group. (B) The effects of American ginseng crude extracts (Am. G), hexane fraction (Hexane-F), water-F, butanol-F, dichloremethane-F, ethyl acetate-F, and maltodextrin control on iNOS expression in LPS-inflamed RAW264.7 cells. Sub-confluent RAW264.7 cells were treated with LPS, American ginseng crude extracts and other fractions in DMEM supplemented with 2% FBS for 6 h. The immunoblotting results are representatives of three separate experiments.
2.5. Cell cultures
RAW264.7 and H9C2 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, 4.5 g/L glucose) supplemented with 10% fetal bovine serum (Cat#: s11150, Atlanta Biologicals, USA) and 100 U/ml penicillin and 100 μg/ml streptomycin (Cat#: 30-002-CI, Mediatech, USA) as previously described (Ichikawa et al., 2009b; Li et al., 2010). Sub-confluent RAW264.7 cells were treated with or without LPS (Cat#: L4391, Sigma, USA) and either the crude extract of American ginseng, the fractions of hexane extract, or panaxynol for different time periods in DMEM supplemented with 1% FBS prior to the cell viability assay, quantitative real-time PCR (qPCR) and Western blot analyses.
Mouse L929 cell (American Type Culture Collection, ATCC) conditioned medium was prepared as a source of colony-stimulating factor (CSF) as previously described with a minor modification (Boltz-Nitulescu et al., 1987). Briefly, mouse L929 cells were cultured for 3 days in the DMEM medium with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. The supernatant was then collected and centrifuged for 20 min at 500 × g to remove cells or cell debris, pooled, aseptically filtered, aliquoted, and frozen at −80 °C.
Bone marrow was isolated as previously described (Weischenfeldt and Porse, 2008). Briefly, 8–16 week old male mice were euthanized by cervical dislocation. After sterilizing the mice with 70% ethanol, an incision in the midline of the abdomen was made, and clipped outward to expose the hind legs. All muscle tissue from the bones of hind legs was removed and the femur and tibia were separated by cutting at the knee joint. The femur was sterilized with 70% ethanol and washed twice with 1 × PBS. In a hood, both ends of the femur were cut and the bone marrow was then flushed out with 1 × PBS using a 5 mL syringe with a 25-gauge needle. The cells were passed through a 70 μm cell strainer. The filtered cells were further centrifuged for 5min at 1000 rpm/min. The cell pellets were resuspended in 10% FBS contained DMEM medium supplemented with 20% L929 cell-conditioned medium, 100 U/ml penicillin and 100 μg/ml streptomycin, and the cells from each mouse were plated in a 6-well plate for 8 days. Culture medium was changed every 3 days using the 10% FBS contained DMEM medium supplemented with 20% L929 cell-conditioned medium. Fluorescence activated cell sorting (FACS) using a flow cytometer (BD Accuri™ C6, BD Biosciences, USA) showed that almost 100% of these differentiated cells are double positive with macrophage specific biomarkers CD11b (Cat#: 14-0112, eBioscience, USA) and F4/80 (Cat#: 123109, Biolegend, USA). The double positive cells were stimulated with the crude extract of American ginseng, the fractions of crude extract, and panaxynol for different time periods in DMEM supplemented with 2% FBS.
Cell viability was determined by the monitoring of release of the cytoplasmic enzyme lactate dehydrogenase (LDH) utilizing a cytotoxicity detection kit (Cat#: 04744926001, Roche Applied Science, USA).
2.6. Transfection and transcription reporter assay
RAW264.7 cells were transfected with ARE-Luc (kindly provided by Dr. Donna D. Zhang at The University of Arizona, Tucson, USA), Neh2-Luc (kindly provided by Dr. Irina G. Gazaryan at Weil Medical College of Cornell University) (Smirnova et al., 2011), or pNF-κB-TA-Luc (Promega, USA) together with pRL-TK (Promega, USA) for normalization of transfection efficiency using ROCHE X-tremeGENE HP DNA transfection reagent (Cat#: 06366236001, Roche Diagnostics, USA) as previously described (Ichikawa et al., 2009b). The NF-κB and Nrf2 transcriptional activity was determined using a luminometer (Victor II; Perkin-Elmer, USA) and a dual luciferase assay kit (Cat#: E1960, Promega, USA) according to the manufacturer’s instructions. In addition, RAW264.7 cells were transfected with the scramble siRNA (Cat#: sc-37007, Santa Cruz Biotechnology, USA) or Nrf2 siRNA (Cat#: sc-37049, Santa Cruz Biotechnology, USA) using ROCHE X-tremeGENE HP DNA transfection reagent. For the transcriptional reporter assay, RAW164.7 cells were seeded in 24-well plates (1 × 105 cells per well) overnight. The cells were transfected with plasmids of ARE-Luc, Neh2-Luc, pNF-κB-TA-Luc, and pRL-TK as indicated for 6 h and cultured with full growth medium for an additional 18 h prior to the treatments. The ratio of reporters and internal control is 9:1 with the total amount of plasmids being 0.5 μg per well. For the knocking down of Nrf2, RAW264.7 cells were transfected with control or Nrf2 siRNA twice as described above.
2.7. Conditioned medium preparation and [3H]-Leucine uptake assay
RAW264.7 cells (1 × 106) were seeded with DMEM supplemented with 10% FBS into a 10-cm diameter culture dish overnight, and then treated with or without LPS (1 μg/ml), the crude extract of American ginseng (100 μg/ml), the fractions of hexane extract (100 μg/ml), and panaxynol (1 μM) in plain DMEM for 48 h. The culture medium was collected and centrifuged at 3000 × g for 5 min, and the supernatant was used as conditioned medium. H9C2 cells (4 × 104) were seeded with DMEM supplemented with 10% FBS into each well of 24-well plates, and then cultured with 5 or 15% of the conditioned medium in DMEM supplemented with 2% FBS for 48 h. During the last 4 h, the cells were pulsed with 1 μCi/ml [3H]-Leucine and subjected to [3H]-Leucine uptake assay as previously described (Li et al., 2009b).
2.8. Immunochemical staining and confocal microscopic analysis
Cells cultured on Lab-Tek™ Chamber Slides (Cat#: 12-565-22, Thermo Fisher Scientific, USA) were stained using rabbit polyclonal anti-Nrf2 (Cat#: sc-722, C20, Santa Cruz Biotechnology, Inc., USA) as previously described (Li et al., 2009b). Nuclei were labeled using blue dye of 4′,6-Diamidino-2-phenylindole (DAPI, Cat#: D9542, Sigma-Aldrich, USA). F-actin was labeled using green dye of Alexa Fluor 488 phalloidin (Cat#: A12379, Invitrogen, USA). Images were acquired using a confocal microscope (LSM510META, Carl Zeiss Inc., USA).
2.9. qPCR and western blot
qPCR was performed as previously described (Ichikawa et al., 2009b; Li et al., 2009b). The primers for qPCR are shown in Supplementary table 1. Western blot was performed as previously described (Ichikawa et al., 2009b) using the following primary antibodies: anti-Nrf2 (Cat#: sc-722, 1:200, Santa Cruz Biotechnology Inc., USA); anti-iNOS (Cat#: 610431, 1:5000, BD, USA); anti-IB (Cat#: sc-371, 1:500, Santa Cruz Biotechnology Inc., USA); and anti-β-actin (Cat#: A1978, 1:10,000, Sigma-Aldrich, USA),
2.10. Statistics
Values are expressed as mean ± SD in the text and figures. Differences between 2 groups were evaluated for statistical significance with the Student t test when the sample size was appropriate and the population was distributed normally. When differences among >3 groups were evaluated, results were compared by one way ANOVA followed by the Bonferroni test for multiple comparisons. Differences were considered significant at p<0.05.
3. Results
3.1. Bioassay-based fractionating anti-inflammatory components of American ginseng in macrophages—identifying American ginseng-derived panaxynol as a potent and unique anti-inflammatory compound
We have demonstrated that a crude extract of American ginseng differentially suppresses iNOS expression in macrophages via a mechanism independent of NF-κB (Ichikawa et al., 2009b). We have also found that Nrf2 activation specifically suppresses iNOS expression independent of NF-κB in macrophages (Li et al., 2014). Thus, we used iNOS expression as a biomarker for screening anti-inflammatory components of American ginseng, which may contain the putative Nrf2 activators. As shown in Fig. 1A, a cytotoxicity assay showed that the crude extract of American ginseng, hexane fraction, and water fraction at doses of 500 μg/ml and 1000 μg/ml are toxic, and butanol fraction exerted a minor toxic effect, while the others at doses tested are non-toxic in RAW264.7 macrophages. Thus, we determined the effect of the crude extract and other fractions using a non-toxic dose of 100 μg/ml on LPS-induced iNOS in RAW264.7 macrophages. Interestingly, we found that LPS-induced iNOS expression was suppressed by the crude extract and the hexane fraction (Fig. 1B).
To identify the nature of anti-inflammatory components in the hexane fraction, we separated it into 5 sub-fractions by preparative, reverse-phase HPLC. Using a comparative analysis by analytical scale LC–UV, we found that the only sub-fractions containing major polyacetylenes including panaxynol, panaxydiol, and panaxynol suppressed iNOS expression in inflamed macrophages, and of the three panaxynol was the most abundant one (data not shown). Accordingly, we purified panaxynol from the hexane fraction (Fig. 2A and B) and found that panaxynol at a non-toxic dose of 0.5 μM dramatically inhibited iNOS expression in LPS-inflamed RAW264.7 cells (Fig. 2C).
Fig. 2.
Isolation of panaxynol from hexane fraction of American ginseng. (A) LC–UV DAD analysis of purified panaxynol from hexane fraction of American ginseng. (B) LC–MS analysis of purified panaxynol from hexane fraction of American ginseng. (C) Left panel: Cytotoxicity of panaxynol in RAW264.7 cells. Sub-confluent RAW264.7 cells were treated with or without panaxynol in DMEM supplemented with 2% FBS as indicated for 24 h. n=6, *p<0.05 vs. control (0). Right panel: The effect of panaxynol on iNOS expression in LPS-inflamed RAW24.7 cells. Sub-confluent RAW264.7 cells were treated with LPS and panaxynol in DMEM supplemented with 2% FBS as indicated for 6 h. The immunoblotting results are representative of three separate experiments.
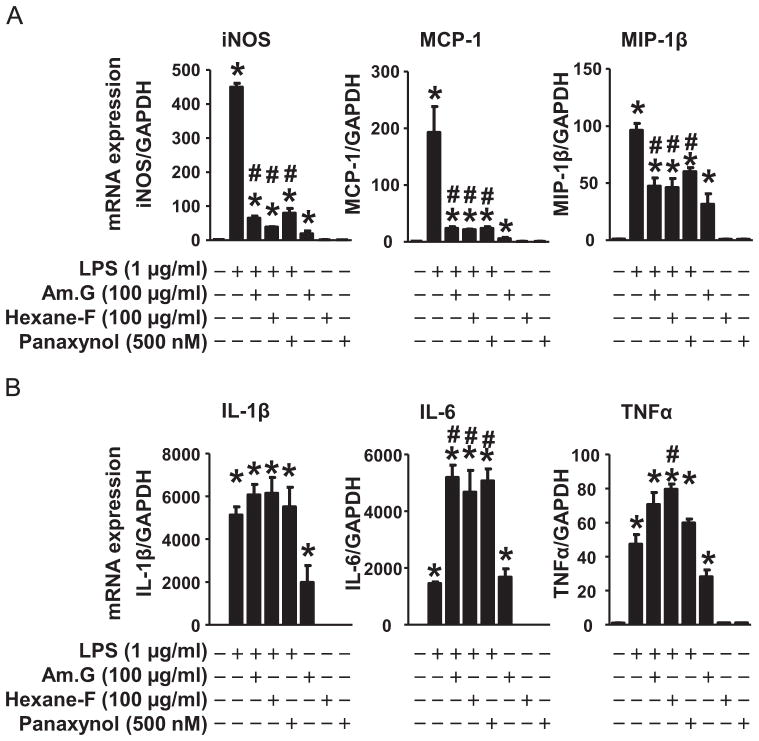
To further characterize the anti-inflammatory property of panaxynol, we compared the effects of American ginseng crude extract, hexane fraction, and panaxynol on pro-inflammatory cytokine expression in LPS-inflamed RAW264.7 cells. American ginseng crude extract suppressed LPS-induced expression of iNOS, monocyte chemotactic protein 1 (MCP-1), and macrophage inflammatory protein-1 (MIP-1)β and minimally regulated LPS-induced expression of interleukin-1 (IL-1)β, IL-6, and tumor necrosis factor (TNF)α (Fig. 3). Interestingly, American ginseng crude extract per se slightly upregulated the basal expression of aforementioned pro-inflammatory cytokines (Fig. 3). Like American ginseng crude extract, the hexane fraction and panaxynol inhibited the expression of iNOS, MCP-1, and MIP-1β and did not affect the expression of IL-1β, and IL-6, and TNFα in LPS-inflamed cells (Fig. 3A). However, they had no impact on the basal expression of aforementioned pro-inflammatory cytokines (Fig. 3B). In addition, American ginseng crude extract suppressed the rapid degradation of iκB while minimally regulating NF-κB activity in inflamed RAW264.7 cells (Supplementary Fig. 1). However, American ginseng crude extract was capable of activating basal NF-κB activity in RAW264.7 cells (Supplementary Fig. 1). On the other hand, the hexane fraction and panaxynol did not affect iκB degradation and NF-κB activity in inflamed RAW264.7 cells (Supplementary Fig. 1).
Fig. 3.
The effects of American ginseng crude extract and sub-fractions on cytokine expression in RAW264.7 cells. Sub-confluent RAW264.7 cells were treated with LPS, American ginseng crude extracts (Am. G), hexane fraction (Hexane-F), and panaxynol in DMEM supplemented with 2% FBS for 4 h and then subjected to qPCR analysis of mRNA expression of (A) iNOS, MCP-1, and MIP-1β, as well as (B) IL-1β, IL-6, and TNFα. n=6, *p<0.05 vs. control (−), #p<0.05 vs. LPS (+).
3.2. Characterizing the potentials of American ginseng-derived components on Nrf2 activation in macrophages—identifying American ginseng-derived panaxynol as a potent Nrf2 activator
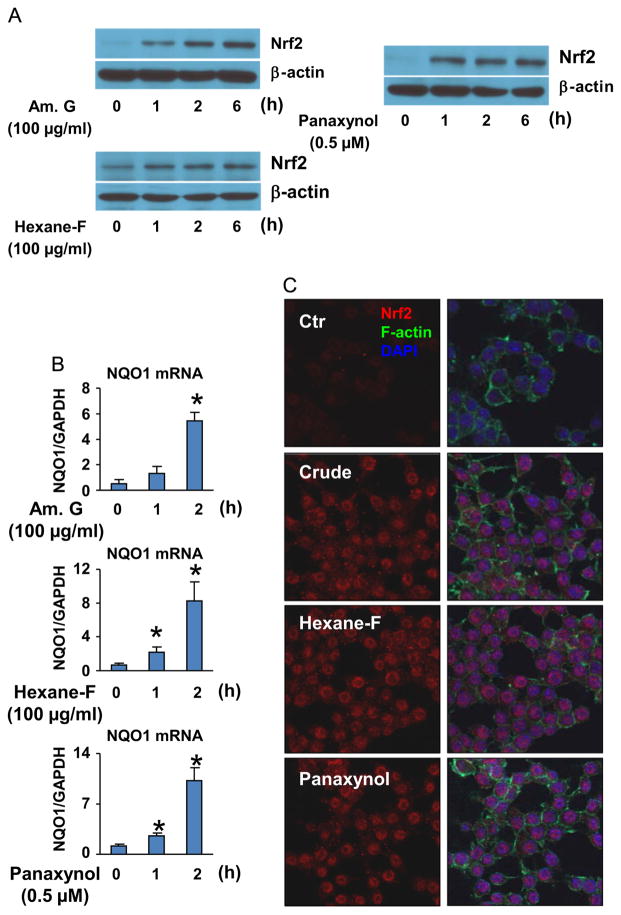
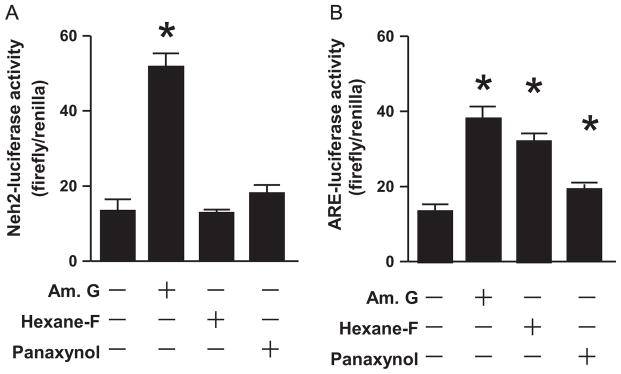
To determine panaxynol as an Nrf2 activator contributing to American ginseng-induced resolution of inflammatory responses in macrophages, we first examined the effects of American ginseng crude extract, hexane fraction, and panaxynol on Nrf2 activation in Raw264.7 cells. All of them upregulated Nrf2 protein expression and nuclear translocation while activating the expression of NQO1, a typical Nrf2 target gene (Fig. 4). Next, we measured the effects of American ginseng crude extract, hexane fraction, and panaxynol on the ARE-Luc activity which indicates Nrf2-driven transcription (Wasserman and Fahl, 1997) as well as the Neh2-Luc activity that reflects the amount of Nrf2 escaped from Keap1-mediated degradation (Smirnova et al., 2011). We found that American ginseng crude extract enhanced both ARE-luc and Neh2-Luc activities, whereas the hexane fraction and panaxynol activated ARE-Luc activity without affecting Neh2-Luc activity (Fig. 5).
Fig. 4.
The effects of American ginseng crude extract and sub-fractions on Nrf2 activation in RAW264.7 cells. (A) The effect of American ginseng crude extracts, hexane fraction, and panaxynol on Nrf2 expression in RAW264.7 cells. Sub-confluent RAW264.7 cells were treated with American ginseng crude extracts (Am. G), hexane fraction (Hexane-F), and panaxynol in DMEM supplemented with 2% FBS as indicated. The immunoblotting results are representative of three separate experiments. (B) The effect of American ginseng crude extracts, hexane fraction, and panaxynol on NQO-1 expression in RAW264.7 cells. Sub-confluent RAW264.7 cells were treated with American ginseng crude extracts (Am. G), hexane fraction (Hexane-F), and panaxynol in DMEM supplemented with 2% FBS. n=4, *p<0.05 vs. control (0). (C) The effect of American ginseng crude extracts, hexane fraction, and panaxynol on Nrf2 expression and nuclear translocation in RAW264.7 cells. Sub-confluent RAW264.7 cells were treated with American ginseng crude extracts (Am. G, 100 μg/ml), hexane fraction (Hexane-F, 100 μg/ml), and panaxynol (0.5 μM) in DMEM supplemented with 2% FBS for 1 h. The images of confocal analysis of Nrf2 expression are representative of three separate experiments.
Fig. 5.
American ginseng crude extract- and sub-fractions-operated Nrf2 transcriptional activity in RAW264.7 cells. (A) The effects of American ginseng crude extracts, hexane fraction, and panaxynol on Nrf2 Neh2-luciferase (Luc) activity in RAW264.7 cells. Sub-confluent RAW264.7 cells transfected with Nrf2 Neh2-Luc and TK-Luc were treated with American ginseng crude extracts (Am. G, 100 μg/ml), hexane fraction (Hexane-F, 100 μg/ml), and panaxynol (1 μM) in DMEM supplemented with 2% FBS for 6 h. n=6, *p<0.05 vs. control (−). (B) The effects of American ginseng crude extracts, hexane fraction, and panaxynol on Nrf2 ARE-luciferase (Luc) activity in RAW264.7 cells. Sub-confluent RAW264.7 cells transfected with Nrf2 ARE-Luc and TK-Luc were treated with American ginseng crude extracts (Am. G, 100 μg/ml), hexane fraction (Hexane-F, 100 μg/ml), and panaxynol (1 μM) in DMEM supplemented with 2% FBS for 6 h. n=6, *p<0.05 vs. control (−).
3.3. An essential role of Nrf2 in mediating panaxynol-induced suppression of inflammatory responses in macrophages
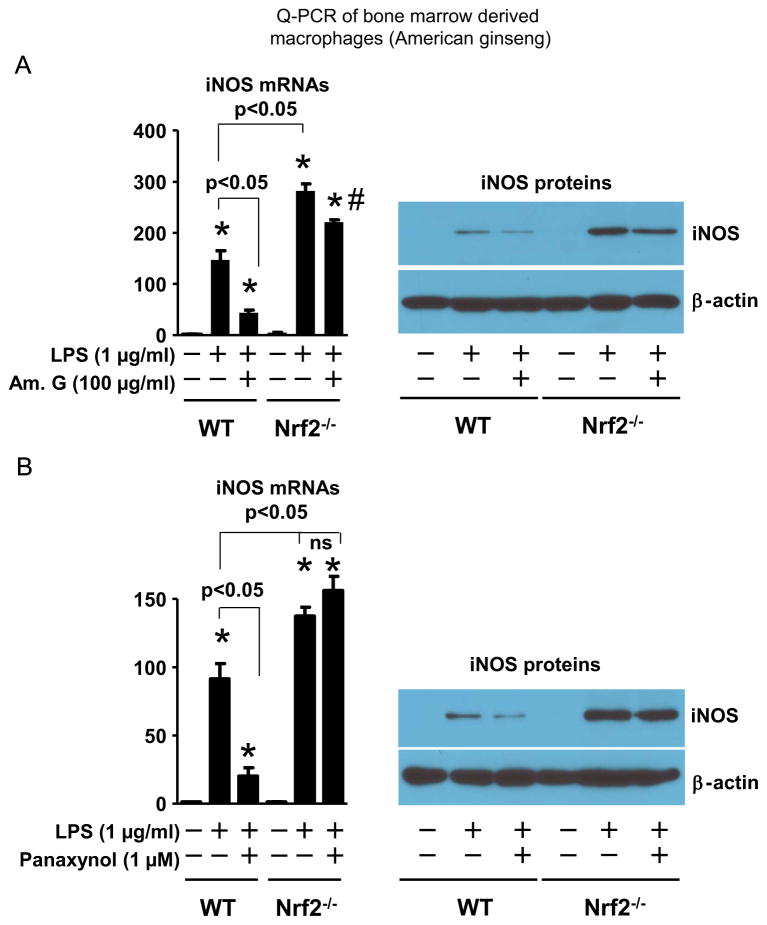
To determine a mediator role of Nrf2 in American ginseng crude extract- and panaxynol-induced suppression of inflammatory cytokine expression in macrophages, we utilized the Nrf2 gene loss of function approach in macrophages. The LPS-induced iNOS expression in bone marrow-derived macrophages of Nrf2−/− mice was enhanced compared with that of WT mice (Fig. 6), demonstrating an anti-inflammatory role of Nrf2 in macrophages as previously reported (Thimmulappa et al., 2006). American ginseng crude extract and panaxynol inhibited iNOS expression in LPS-inflamed bone marrow-derived macrophages of WT mice, (Fig. 6). However, the inhibitory effects of American ginseng crude extract and panaxynol were attenuated and blocked, respectively, in bone marrow-derived macrophages of Nrf2−/− mice (Fig. 6). Similarly, American ginseng crude extract and panaxynol significantly suppressed the expression of MCP-1 and MIP-1β in bone marrow-derived macrophages from WT but not in the cells from Nrf2−/− mice (Supplementary Fig. 2).
Fig. 6.
Nrf2 deficiency on American ginseng crude extract- and subfraction-mediated suppression of iNOS expression in macrophages. Bone-derived macrophages of WT and Nrf2−/− mice were treated with LPS, (A) American ginseng crude extracts (Am. G), and (B) panaxynol in DMEM supplemented with 2% FBS as indicated for 6 h. Left panel: qPCR analysis. n=6, *p<0.05 vs. control (−) in each group. Right panel: Representative immunoblotting of 3 separate experiments.
3.4. A crucial role of Nrf2 in mediating panaxynol-induced resolution of adverse inflammatory interaction of macrophages with cardiomyocytes
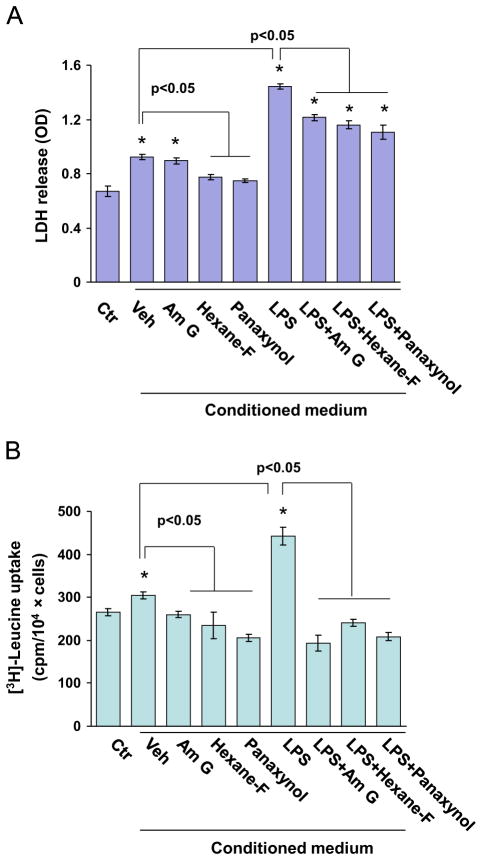
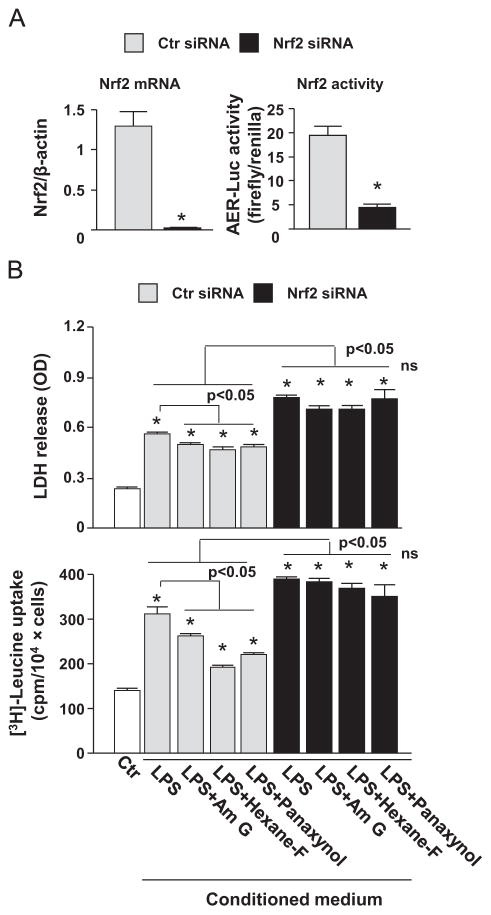
To establish a pathophysiological relevance of panaxynol-activated Nrf2 in macrophages, we examined the effects of American ginseng crude extract, the hexane fraction, and panaxynol on inflamed macrophage-mediated cell death and on hypertrophic growth in cardiomyocytes. As shown in Fig. 7, conditioned medium of unstimulated RAW264.7 cells slightly increased cell death and induced hypertrophic growth in H9C2 cardiomyocytes, whereas conditioned medium of LPS-inflamed RAW264.7 cells caused dramatic augmentation of cell death and hypertrophic growth (Fig. 7). However, the RAW264.7 cell-mediated H9C2 cell death and hypertrophy were strongly suppressed by treatment with American ginseng crude extract, the hexane fraction, and panaxynol in Raw264.7 cells (Fig. 7). Notably, the inhibitor effect of panaxynol was even stronger than that of American ginseng crude extract (Fig. 7). These results indicate that panaxynol is one of the potent effectors in the American ginseng-induced resolution of pro-inflammatory responses in macrophages that lead to cardiomyocyte death and hypertrophy. To determine a crucial role of Nrf2 in mediating American ginseng- and panaxynol-induced resolution of the adverse pro-inflammatory response in macrophages, we applied Nrf2 knockdown approach using Nrf2 siRNA in RAW264.7 cells. After establishing the efficacy of Nrf2 siRNA in knocking down of Nrf2 in RAW264.7 cells (Fig. 8A), we determined the effects of conditioned medium of scramble siRNA and Nrf2 siRNA transfected RAW264.7 cells treated with or without American ginseng crude extract and panaxynol (Fig. 8B). As expected, the knockdown of Nrf2 in RAW264.7 cells wiped out the inhibitory effects of American ginseng crude and panaxynol on RAW264.7 cell conditioned medium-induced H9C2 cell death and hypertrophic growth (Fig. 8B).
Fig. 7.
American ginseng crude extract- and subfraction-conditioned medium of RAW264.7 cells on cardiomyocyte death and hypertrophy. (A) H9C2 cardiomyocytes were treated with 5% of plain DMEM as the control (Ctr) and 5% of conditioned medium of RAW264.7 cells treated with American ginseng crude extract, hexane fraction (Hexane-F), or panaxynol in DMEM as indicated for 24 h. (B) H9C2 cardiomyocytes were treated with 5% of plain DMEM as the control (Ctr) and 5% of conditioned medium of RAW264.7 cells treated with American ginseng crude extract, hexane fraction (Hexane-F), or panaxynol in DMEM supplemented with 2% FBS as indicated for 48 h.
Fig. 8.
American ginseng-activated Nrf2 in RAW264.7 cell-mediated cardiomyocyte cell death and hypertrophy. (A) Left panel: RAW264.7 cells transfected with control scramble siRNA (Ctr siRNA) and Nrf2 siRNA for 48 h were subjected to qPCR analysis of Nrf2 mRNA expression. n=6, *p<0.05 vs. Ctr siRNA. Right panel: RAW264.7 cells transfected with Ctr siRNA and Nrf2 siRNA were further transfected with ARE-Luc reporters for 48 h and then subjected to dual luciferase assay. n=4, *p<0.05 vs. Ctr siRNA. (B) Upper panel: H9C2 cells were treated with 15% of plain DMEM as the control (Ctr) and 15% of conditioned medium of RAW264.7 cells treated with American ginseng crude extract, hexane fraction (Hexane-F), or panaxynol in DMEM as indicated for 24 h. Lower panel: H9C2 cells were treated with 15% of plain DMEM as the control (Ctr) and 15% of conditioned medium of RAW264.7 cells treated with American ginseng crude extract, hexane fraction (Hexane-F), or panaxynol in DMEM supplemented with 2% FBS as indicated for 48 h. The cells were pulsed with 1 μCi/ml [3H]-leucine during the last 4 h of culture.
4. Discussion
Potentially active components from ginseng include ginsenosides, polysaccharides, peptides, polyacetylenes, and fatty acids. Historically, the pharmacological actions of ginseng have focused on ginsenosides (Schlag and McIntosh, 2006). Indeed, the medicinal potentials of ginsenosides on cardiovascular disease have been documented (Lee and Kim, 2014; Lu et al., 2009). At a molecular level, the beneficial effects of ginsenosides and other components on the cardiovascular system may be coupled with the component-specific mechanisms, whereas the precise mediators for individual ginseng component-mediated cardiac protection remain to be established. Nevertheless, the suppression of myocardial oxidative stress appears to be a common mechanism by which different ginseng extracts and individual components protect against cardiac dysfunction. In this regard, we have demonstrated an essential role of Nrf2 in mediating American ginseng-induced suppression of oxidative stress and cell death in cardiomyocytes, indicating that Nrf2 is a crucial mediator of the ginseng-induced suppression of oxidative stress and cardioprotection (Li et al., 2010). In the present study, we identified that panaxynol, a polyacetylene isolated from American ginseng (Figs. 1 and 2), is a potent Nrf2 activator and capable of specifically activating Nrf2-mediated resolution of pathological inflammatory responses in macrophages (Figs. 3–6), which cause cardiomyocyte death and hypertrophy (Figs. 7 and 8). Collectively, these findings suggest that selective activation of Nrf2 by panaxynol may be responsible for the documented beneficial effects of ginseng in the heart.
It has been well demonstrated that oxidative stress plays a causative role in cardiac pathological remodeling and dysfunction (Murdoch et al., 2006; Takimoto and Kass, 2007). In addition, uncontrolled inflammatory responses also contribute to cardiac adverse remodeling and dysfunction (Heymans et al., 2009; Nian et al., 2004; Sasayama et al., 1999). While the precise role of inflammatory responses in the heart remains to be dissected, an intimate link between inflammation and oxidative stress has been noted (Sasayama et al., 1999; Sekiguchi et al., 2004). Uncontrolled production of inflammatory cytokines from inflammatory cells such as macrophages could cause oxidative stress in cardiomyocytes thereby leading to cardiomyocyte death, which, in turn, stimulates inflammatory responses in macrophages. Such a vicious cycle paves a way for the development of heart failure. However, a therapeutic target for the treatment of oxidative stress and inflammation in the heart remains to be established. In this context, our studies have revealed that Nrf2 is capable of providing protection against cardiac adverse remodeling and dysfunction via suppression of oxidative stress in the heart (Ichikawa et al., 2009a; Li et al., 2009b; Li et al., 2011; Tan et al., 2011; Wang et al., 2014; Xing et al., 2012). Given the crucial role of Nrf2 in negatively regulating inflammatory responses in macrophages (Thimmulappa et al., 2006), the Nrf2-mediated cardioprotection may be not only owing to its ability to suppress oxidative stress, but also due to its capability to inhibit inflammatory responses in the heart. This notion is further supported by our findings that Nrf2 is a critical negative regulator of inflammatory responses in macrophages, which cause cardiomyocyte death and hypertrophy. Accordingly, Nrf2 appears to be a promising drug target for the treatment of cardiac pathological remodeling and dysfunction.
Previous studies have shown that activation of Nrf2 by genetic knockout or knockdown of Keap1 is not always beneficial (Komatsu et al., 2010; Wakabayashi et al., 2003; Xu et al., 2012). Accordingly, it is likely that activation of Nrf2 due to Keap1 loss-of-function is detrimental in some specific pathological settings. Also, it is plausible that the maximal beneficial effects of Nrf2 might be achieved by targeting Nrf2 while keeping Keap1 function intact. Therefore, as a possible pharmacological target of Nrf2, our finding of panaxynol-induced activation of Nrf2 which is independent of Keap1 (Fig. 5) is particularly interesting. Further investigation of the molecular mechanism by which panaxynol activates Nrf2 will reveal the nature of Nrf2-mediated protection. Furthermore, the unique anti-inflammatory profile of panaxynol which is Nrf2 dependent while independent of NF-κB (Supplementary Figs. 1 and 2, and Fig. 6) is also intriguing. That is, despite the well-documented mediator role of NF-κB in promoting inflammatory responses, the NF-κB pathway also plays a critical role in the suppression of the pro-inflammatory response in macrophage and the resolution of inflammation in vivo (Fong et al., 2008; Greten et al., 2007). Inappropriate suppression of NF-κB signaling results in adverse consequences. For example macrophage specific knockout of NF-κB kinase (IKK)β, which is essential for NF-κB activation and NF-κB-driven expression of inflammatory cytokines, increases atherosclerosis in LDL receptor-deficient mice (Kanters et al., 2003). Also, the loss of IKKβ or the NF-κB essential modulator IKKγ/NEMO in murine cardiomyocytes exaggerates or results in dysregulated myocardial inflammatory responses, dilated cardiomyopathy and cardiac dysfunction (Hikoso et al., 2009; Kratsios et al., 2010). Also of interest, is the fact that increased oxidative stress is a common feature in cardiomyocyte specific IKKβ and IKKγ knockout mice and is also the causative mechanism for the dilated cardiomyopathy and heart failure in these mice. In this context, we observed that panaxynol minimally regulates the IKK/NF-κB pathway and specifically activates Nrf2 signaling, which presumably mimics the IKK-mediated antioxidant defense and resolution of inflammation. Taken together, panaxynol is positioned to be a promising drug for the maximal activation of Nrf2-driven cytoprotection. Given the aforementioned cardioprotective role of Nrf2, the specific targeting of Nrf2 by panaxynol may have a unique pharmacological potential for the treatment of cardiac disease and heart failure. However, whether the unique feature of panaxynol-induced Nrf2 activation is critical for its pharmacological efficacy in vivo over the other Nrf2 activators regarding anti-oxidative stress, inflammation resolution, and side effects requires further investigation.
5. Conclusion
The edible panaxynol, isolated from American ginseng, specifically activates Nrf2-mediated resolution of inflammatory responses in macrophages that are responsible for cardiomyocyte death and hypertrophy. Because panaxynol also exists in a variety of other foods (Christensen, 2011), a comprehensive understanding of the dietary component-mediated Nrf2 signaling in cardiovascular system will provide novel insight into the adoption of desirable dietary behaviors as well as the development of unique therapeutic approaches against cardiovascular disease.
Supplementary Material
Acknowledgments
This work was supported by Shandong University National Qianren Scholar Fund (XLW), PO20 GM103641 (NP and TC), and 2PO1AT003961-06A1 (PN, MN, LJH, and TC).
Abbreviations
- Nrf2
nuclear factor erythroid-2 related factor 2
- Am. G
American ginseng
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MCP-1
monocyte chemotactic protein-1
- MIP-1β
macrophage inflammatory protein-1 beta
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- TNFα
tumor necrosis factor alpha
- ARE
antioxidant response element
- Atg7
autophagy related gene 7
- CSF
colony-stimulating factor
- FACS
fluorescence activated cell sorting
- LDH
lactate dehydrogenase
- NQO-1
NAD(P)H dehydrogenase, quinone 1
- WT
wild type
- LC–UV DAD
Liquid chromatography with UV diode array detection
- LC–MS
Liquid chromatography mass spectrometry
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jep.2015.04.004.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BL, CQ, LY, and HL participated in data collection; AW and LJH provided key reagents; MN and PN secured the research funding; XLW and DT provided research materials; JSJ played a major role in writing and editing the manuscript; TC and XT designed the study, secured the research funding and finalized the manuscript. All authors have read and approved the final manuscript.
References
- Boltz-Nitulescu G, Wiltschke C, Holzinger C, Fellinger A, Scheiner O, Gessl A, Forster O. Differentiation of rat bone marrow cells into macrophages under the influence of mouse L929 cell supernatant. J Leukoc Biol. 1987;41:83–91. doi: 10.1002/jlb.41.1.83. [DOI] [PubMed] [Google Scholar]
- Christensen LP. Aliphatic C(17)-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat Food, Nutr Agric. 2011;3:64–77. doi: 10.2174/2212798411103010064. [DOI] [PubMed] [Google Scholar]
- Fong CH, Bebien M, Didierlaurent A, Nebauer R, Hussell T, Broide D, Karin M, Lawrence T. An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. J Exp Med. 2008;205:1269–1276. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O’Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschope C, Van Bilsen M, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the translational research committee of the heart failure association of the European society of cardiology Eur J Heart Fail. 2009;11:119–129. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikoso S, Yamaguchi O, Nakano Y, Takeda T, Omiya S, Mizote I, Taneike M, Oka T, Tamai T, Oyabu J, Uno Y, Matsumura Y, Nishida K, Suzuki K, Kogo M, Hori M, Otsu K. The I{kappa}B kinase {beta}/nuclear factor {kappa}B signaling pathway protects the heart from hemodynamic stress mediated by the regulation of manganese superoxide dismutase expression. Circ Res. 2009;105:70–79. doi: 10.1161/CIRCRESAHA.108.193318. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One. 2009a;4:e8391. doi: 10.1371/journal.pone.0008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Li J, Nagarkatti P, Nagarkatti M, Hofseth LJ, Windust A, Cui T. American ginseng preferentially suppresses STAT/iNOS signaling in activated macrophages. J Ethnopharmacol. 2009b;125:145–150. doi: 10.1016/j.jep.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Investig. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Ann Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- Kratsios P, Huth M, Temmerman L, Salimova E, Al Banchaabouchi M, Sgoifo A, Manghi M, Suzuki K, Rosenthal N, Mourkioti F. Antioxidant amelioration of dilated cardiomyopathy caused by conditional deletion of NEMO/IKKgamma in cardiomyocytes. Circ Res. 2010;106:133–144. doi: 10.1161/CIRCRESAHA.109.202200. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kim IS, More SV, Kim BW, Choi DK. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep. 2014;31:109–139. doi: 10.1039/c3np70065h. [DOI] [PubMed] [Google Scholar]
- Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Abdalrahman A, Lai Y, Janicki JS, Ward KW, Meyer CJ, Wang XL, Tang D, Cui T. Dihydro-CDDO-trifluoroethyl amide suppresses inflammatory responses in macrophages via activation of Nrf2. Biochem Biophys Res Commun. 2014;444:555–561. doi: 10.1016/j.bbrc.2014.01.101. [DOI] [PubMed] [Google Scholar]
- Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009a;13:785–794. doi: 10.1517/14728220903025762. [DOI] [PubMed] [Google Scholar]
- Li J, Ichikawa T, Jin Y, Hofseth LJ, Nagarkatti P, Nagarkatti M, Windust A, Cui T. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J Ethnopharmacol. 2010;130:222–230. doi: 10.1016/j.jep.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009b;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, Tang DQ, Cui T. Up-regulation of p27(kip1) contributes to Nrf2-mediated protection against angiotensin II-induced cardiac hypertrophy. Cardiovasc Res. 2011;90:315–324. doi: 10.1093/cvr/cvr010. [DOI] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- Poudyal D, Le PM, Davis T, Hofseth AB, Chumanevich A, Chumanevich AA, Wargovich MJ, Nagarkatti M, Nagarkatti PS, Windust A, Hofseth LJ. A hexane fraction of American ginseng suppresses mouse colitis and associated colon cancer: anti-inflammatory and proapoptotic mechanisms. Cancer Prev Res. 2012;5:685–696. doi: 10.1158/1940-6207.CAPR-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama S, Matsumori A, Kihara Y. New insights into the pathophysiological role for cytokines in heart failure. Cardiovasc Res. 1999;42:557–564. doi: 10.1016/s0008-6363(99)00050-4. [DOI] [PubMed] [Google Scholar]
- Schlag EM, McIntosh MS. Ginsenoside content and variation among and within American ginseng (Panax quinquefolius L.) populations. Phytochemistry. 2006;67:1510–1519. doi: 10.1016/j.phytochem.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K, Li X, Coker M, Flesch M, Barger PM, Sivasubramanian N, Mann DL. Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc Res. 2004;63:433–442. doi: 10.1016/j.cardiores.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Small E, Catling Paul M. Canddian medicinal crops. NRC Press; Ottawa, Ontario, Canada: 1999. [Google Scholar]
- Smirnova NA, Haskew-Layton RE, Basso M, Hushpulian DM, Payappilly JB, Speer RE, Ahn YH, Rakhman I, Cole PA, Pinto JT, Ratan RR, Gazaryan IG. Development of Neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of Nrf2 activators. Chem Biol. 2011;18:752–765. doi: 10.1016/j.chembiol.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L, Cui T. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–633. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Investig. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li S, Wang H, Li B, Shao L, Lai Y, Horvath G, Wang Q, Yamamoto M, Janicki JS, Wang XL, Tang D, Cui T. Nrf2 enhances myocardial clearance of toxic ubiquitinated proteins. J Mol Cell Cardiol. 2014;72:305–315. doi: 10.1016/j.yjmcc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008 doi: 10.1101/pdb.prot5080. pdb prot5080. [DOI] [PubMed] [Google Scholar]
- Xing Y, Niu T, Wang W, Li J, Li S, Janicki JS, Ruiz S, Meyer CJ, Wang XL, Tang D, Zhao Y, Cui T. Triterpenoid dihydro-CDDO-trifluoroethyl amide protects against maladaptive cardiac remodeling and dysfunction in mice: a critical role of Nrf2. PLoS One. 2012;7:e44899. doi: 10.1371/journal.pone.0044899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61:3208–3218. doi: 10.2337/db11-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen CJ. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit. 2004;10:RA187–192. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.