Abstract
Hypothesis
B7-H1 is expressed in vestibular schwannomas.
Background
Little is known about how benign human vestibular schwannomas interact with antibody-mediated or cell-mediated immunity. We report on the aberrant expression of a novel T-cell coregulatory molecule, B7 homolog 1 (B7-H1), in vestibular schwannomas and discuss the implications of B7-H1 expression and tumor aggressiveness and a potential regulator of B7-H1 expression.
Methods
Immunohistochemical staining for B7-H1, CD8+, CD3+, and CD4+ lymphocytes were performed on 48 fresh-frozen vestibular schwannoma tissue specimens. A clinical review of patient presenting symptoms and tumor characteristics was performed. Real-time polymerase chain reaction was used to determine if there was differential expression of B7-H1 messenger RNA and microRNA-513, a known regulator of B7-H1, in several strongly positive and negative B7-H1 vestibular schwannomas.
Results
Nine (19%) of 48 tumors were negative, 23 (48%) tumors were 1+ mildly positive (<20% section area), and 16 (33%) stained 2+ strongly positive (≥20% section area) for B7-H1. The average number of CD8+ cells per high-power field was 2.1 for positive-staining tumors and 1.0 for negative tumors (p = 0.16). Failure of tumor control with stereotactic radiation (p = 0.029) was significantly greater in the strongly positive B7-H1 tumors. Real-time polymerase chain reaction did not show significant differential expression of microRNA-513 (p = 0.62) or B7-H1 messenger RNA (p = 0.35) between the tumors showing strong and negative immunohistochemical staining for B7-H1 protein.
Conclusion
Vestibular schwannoma tumors express B7-H1, which has been associated with immune tolerance and adverse disease characteristics in several malignancies. Growing tumors that were surgically removed after failed stereotactic radiation therapy were significantly more likely to strongly express B7-H1 protein, which lends some credibility to the hypothesis that immuno-evasion may play some role in their continued growth. Although clinical trends were seen, greater statistical power is required to evaluate whether B7-H1 expression correlates with more aggressive tumor growth or poorer hearing class. B7-H1 seems to be expressed in equal amounts at the RNA level in all vestibular schwannoma tumors that suggests that differential protein expression is occurring at the posttranscriptional level. However, microRNA-513 does not regulate B7-H1 protein expression in these tumors.
Keywords: Acoustic neuroma, Immunology, Vestibular schwannoma
Within the past several years, it has become clear that the tight regulation of the immune response to antigenic signals is controlled by a group of costimulatory pathways (1). B7 homolog 1 (B7-H1) is one such important regulator in cell-mediated immune responses. The B7-H1 protein is not commonly expressed in most normal cell types, specifically brain and neural tissues (1,2), but is frequently aberrantly expressed in a variety of malignant tumor types, such as renal, lung, ovarian, breast, and head and neck cancers. B7-H1 acts as a ligand that interacts with its counter-receptor, programmed death-1 (PD-1), on activated T cells and induces apoptosis and inhibits proliferation and cytokine production of these T cells. In turn, this can lead to diminished immune clearance of these tumor cells. B7-H1 also can interact with the cost-imulatory receptor on naive T cells to increase interleukin 10 secretion and T-cell proliferation. However, this subset of T cells displays functional impairment or exhaustion and anergy to antigen-specific responses that lead to ineffective tumor elimination (1,3,4). Furthermore, tumor-expressed B7-H1 can interact with PD-1 expressing dysfunctional T cells that stimulates the intracellular domain of B7-H1 to transmit antiapoptotic signals into the tumor cells, thereby enhancing tumor resistance to apoptosis induction (5) (Fig. 1).
FIG. 1.
This figure demonstrates B7-H1/PD-1 interaction in the tumor cell and T cell microenvironment.
Very little is known about how benign human vestibular schwannomas (VS) interact with antibody-mediated or cell-mediated immunity in the patient. Older studies demonstrated histologic presence of a moderate number of macrophages, CD8+ lymphocytes, and CD4+ lymphocytes in 96%, 87%, and 23% of 37 VS, respectively (6). Other studies used indirect leukocyte migration techniques to detect leukocyte migration inhibition in serum, cerebrospinal fluid, or perilymph in schwannoma patients versus controls. Nearly all VS patients exhibited cell-mediated reaction to acoustic neuroma extract from tumors. The general conclusions were that an unknown antigen is responsible for this (7–9).
It is these observations that have collectively implicated VS as immunogenic tumors. In the current study, we report the first investigation on the aberrant expression of a novel T-cell coregulatory molecule, B7-H1, in VS tumors. We also report on the implications of B7-H1 expression and tumor aggressiveness. Finally, we discuss a potential regulator of B7-H1 expression in these tumors.
METHODS
Upon approval from the Mayo Clinic institutional review board, we identified 48 patients who underwent resection of a VS. Surgical waste specimens from the 48 patient-excised VS were used for immunohistochemical staining and polymerase chain reaction (PCR) experiments.
Immunostaining
Fresh frozen tissue specimens (−140°C) were sectioned on a Leica 1850 cryostat (5 µm) and mounted on Superfrost charged slides. After sectioning, the slides were air dried and frozen at −70°C until immunostaining could be undertaken (<48 h). The slides were brought to room temperature and fixed in 1% paraformaldehyde for 10 minutes. Slides were washed with deionized water for 5 minutes and then 1× Tris-buffered saline and Tween 20 (TBST) wash buffer (Dako North American, Inc., Carpinteria, CA, USA) for 5 minutes. Up to 40 slides were then placed in the Autostainer Plus (Dako) for the following automated protocol. Slide sections were washed with wash buffer and then blocked with Endogenous Blocking Solution (Dako) for 5 minutes. The slides were washed 2 times with wash buffer and then incubated for 60 minutes in purified mouse antihuman B7-H1 (Kwon/Dong Laboratory, Mayo Foundation, Rochester, MN, USA), CD3, CD4, or CD8 antibody (Dako) diluted 1:100 in antibody diluent. Sections were then washed 2 times with wash buffer and incubated with mouse horseradish peroxidase immunoglobulin (Ig) G secondary antibody (EnVision+/HRP labeled polymer, Dako) for 30 minutes. After 2 washing steps with wash buffer, the slides were incubated for 10 minutes with 3,3’-diaminobenzidine (DAB+). The slides were removed from the Autostainer and placed in deionized water for 5 minutes and then counterstained with hematoxylin. Slides were then dehydrated in sequential 95% to 100% ethanol baths for 5 minutes and then xylene baths for 5 minutes times 3. Coverslips and permount were used for final preparation of the slides. Specimens used for negative controls underwent an identical process, except sections were incubated for 60 minutes in purified mouse antihuman IgG1 blocking antibody (Dako) diluted 1:10 in antibody diluent instead of the mouse antihuman B7-H1 antibody. A section of human vestibular nerve removed during a vestibular nerve section for Ménière’s disease was stained as a control tissue for B7-H1. Cultured mouse Schwann cells were also used as an additional B7-H1 tissue control after being cytospun and fixed onto slides. An antimouse B7-H1 antibody (Kwon/Dong Laboratory, Mayo Foundation) was diluted 1:100 for immunohistochemical staining of these cells. Human tonsil tissue was used as a known positive control for B7-H1 protein expression.
Quantification of B7-H1 and T-Cell Expression
The percentage of tumor cells that stained positive for B7-H1 was assessed and recorded as absent (0), moderate (1+; <20% of section area with positive staining), or strongly positive (2+; ≥20% of section area with positive staining) by 2 physicians blinded to patient clinical variables. Additionally, the extent of CD3, CD4, and CD8 positive T-cell infiltration was measured by averaging the number of positive-staining T cells per high-powered field of 4 randomly chosen areas within the tumor section.
Clinical Data
A blinded retrospective chart review of patient clinical features was performed on 47 patients with VS whose surgical waste tumor tissue underwent the previously described immunohistochemical staining. After statistically analyzing the data, it was found that the addition of only a few more postradiation failed tumors would likely bring about statistical significance if it was present. The authors were blinded to all clinical variables for the 48th patient tumor except that it was a postradiation schwannoma. In the absence of any identifiers, this tumor section was mixed in with 9 other tumor sections that had been used in this investigation, and all 10 sections were immunostained for B7-H1 protein expression. A third independent pathologist was asked to grade these 10 tumors in a blinded fashion to all clinical data. Additionally, they also were not told what protein was being evaluated or whether the hypothesis was attempting to distinguish overexpression or underexpression of the protein.
Real-Time PCR
Total RNA was isolated from 5 VS tumor specimens that strongly expressed B7-H1 and from 5 VS tumor specimens that had no B7-H1 expression following the RNeasy kit instructions (Qiagen, Inc., Valencia, CA, USA). Three tumors from each group were used for the miR-513 analysis; whereas, sufficient RNA from all 5 tumors in each group was available for quantifying B7-H1 messenger (mRNA) levels. For miR-513 and B7-H1, total RNA was reverse transcribed to complementary DNA by using the TaqMan microRNA reverse transcription kit and TaqMan reverse transcription reagent kit, respectively (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed for miR-513 using the TaqMan universal PCR master mix (Applied Biosystems) and for B7-H1 using the Lightcycler FastStart DNA MasterPLUS SYBR Green I kit (Roche, Indianapolis, IN, USA). The LightCycler Carousel-based, real-time PCR System (Roche) machine was used for real-time PCR reactions. Specific primers and probes for mature miR-513 (hsamiR-513; Assay ID: 001146) and small nuclear RNA RNU6B (endogenous reference) were obtained from Applied Biosystems. For B7-H1 RNA quantification, primers corresponding to the 5’ and 3’ regions of B7-H1 (forward, GGTGCCGACTACAAG CGAAT; reverse GGTGACTGGATCCACAACCAA) were designed and created by the Mayo Advanced Genomics Technology Center based on published sequences (10). All real-time PCR reactions were run in triplicate. The amount of miR-513 was obtained by normalizing to small nuclear RNA RNU6B and relative to a (untreated cell) control as reported by others (11,12). The amount of B7-H1 RNA was normalized to actin and further quantified by comparing the Ct values to a standard curve created from serial dilutions of a known copy number concentration of B7-H1 mRNA. Human cholangiocytes (H69 cells) were used as a proven positive control for miR-513 and B7-H1 mRNA. H69 cells are SV40-transformed immortalized human cholangiocytes originally derived from normal liver harvested for transplant and have been extensively characterized, and resting human cholangiocytes in culture have recently been shown to express miR-513 and B7-H1 mRNA but not B7-H1 protein (13).
Statistical Analysis
Comparisons among the clinical variables and B7-H1 expression were evaluated by using χ2, Fisher’s exact, and Kruskal-Wallis tests. For continuously scaled features (e.g., age, tumor size), the study had an 80% power to detect an effect size (difference in group means/pooled standard deviation) of 0.91. For dichotomous features (e.g., sex, presence/absence of symptoms), the study had an 80% power to detect differences. These calculations were based on the 2-sided t test and χ2 test, respectively, and assuming a type I error level of 5%.
RESULTS
Primary Tumor–Associated B7-H1 Expression
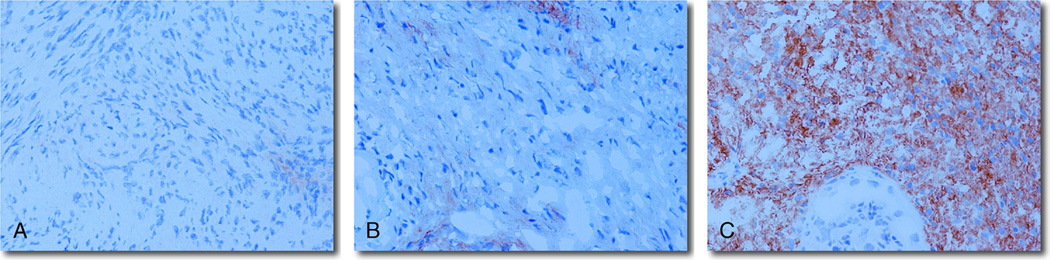
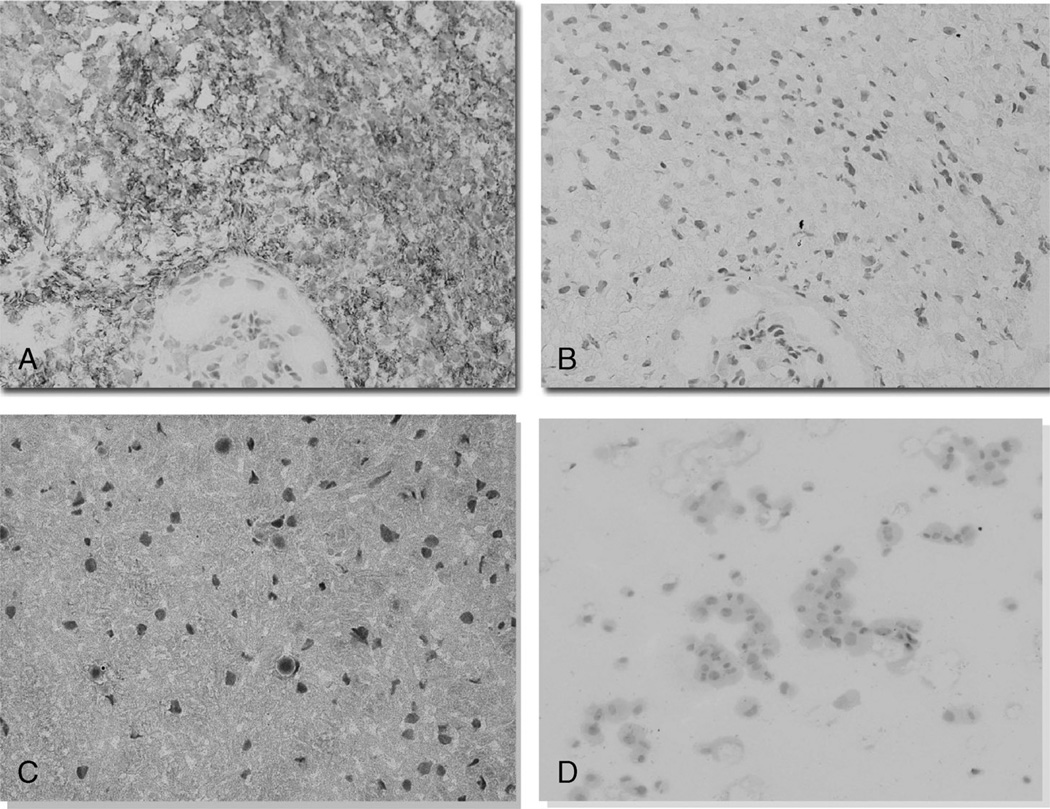
Immunohistochemical staining of the 48 VS specimens revealed no B7-H1 expression, moderate expression (1+), or marked degrees (2+) of B7-H1 expression by tumor cells (Fig. 2). Nine (19%) of the 48 tumors were negative, 23 (48%) tumors were moderately positive, and 16 (33%) stained markedly for B7-H1. A human vestibular nerve section and mouse Schwann cells were both negative for B7-H1 expression (Fig. 3).
FIG. 2.
Immunohistochemical staining of the 48 VS specimens revealed either no (0) (A), moderate (1+) (B), or marked (2+) (C) degrees of B7-H1 expression by tumor cells (original magnification, ×40).
FIG. 3.
VS tumor specimens were stained with antihuman B7-H1 (A), and negative tissue controls were incubated with purified mouse anti-human IgG1 blocking antibody (B; original magnification, ×40). Human vestibular nerve removed from a Ménière re’s disease patient was negative for B7-H1 immunostaining (C; original magnification, ×40). Schwann cells are the likely progenitor cell of schwannoma tumors (D). Cytospun mouse Schwann cells were negative for B7-H1 immunostaining (original magnification, ×10). These cells were stained after passage 1 and, in culture, showed their typical bipolar, spindle-shaped morphology. The cells lost their typical projecting arms because the trypsinization and cytospinning procedure.
Associations of Tumor B7-H1 Expression and Lymphocyte Infiltration
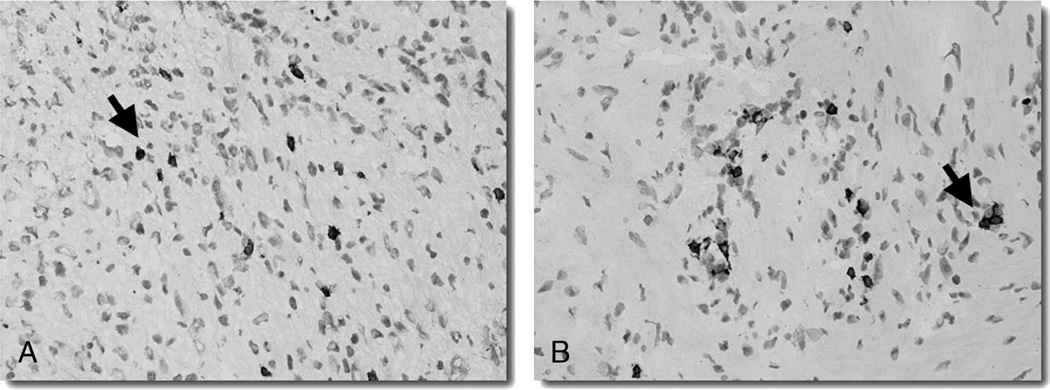
The degree of T-lymphocyte infiltration was compared with B7-H1 strongly positive- and B7-H1 negative-staining VS specimens. The average number of CD8+ cells per high-power field was 2.1 for the strongly positive-staining tumors and 1.0 for the negative tumors (p = 0.16). The average number of CD3+ cells per high-power field was 3.6 for the strongly positive-staining tumors and 2.3 for the negative tumors (p = 0.48). The average number of CD4+ cells per high-power field was 0.5 for the strongly positive-staining tumors and 0.2 for the negative tumors (p = 0.80) (Fig. 4).
FIG. 4.
The degree of T-lymphocyte infiltration in the VS specimens was assessed via immunohistochemical staining (original magnification, ×10) for CD3+ (A) or CD8+ (B) lymphocytes (black arrow).
Associations of Tumor B7-H1 Expression, Patient Symptoms, and Tumor Characteristics
Among the 48 patients who underwent surgery for treatment of their VS, the mean age was 49.6 years (47% male and 53% female). Patients presenting symptoms and tumor characteristics were compared with B7-H1 strongly positive (2+) and B7-H1 negative staining VS specimens (Table 1). Failure of tumor control with stereotactic radiation (p = 0.029) was found to be highly statistically correlated with marked (2+) B7-H1 expression. Poor word discrimination (p = 0.16) and poorer presenting hearing class (American Academy of Otolaryngology–Head and Neck Surgery Class A–D; p = 0.11) tended to be greater in the strongly positive B7-H1 tumors, although these did not reach statistical significance. No difference was found in symptoms of tinnitus (p = 0.23), dysequilibrium (p = 0.30), facial numbness (p = 0.21), or headache (p = 0.83) between the 2 groups. There also was no difference in the patient and tumor characteristics with the following p values: age (p = 0.36), sex (p = 0.30), preoperative House-Brackmann facial nerve scores (p = 0.63), cystic tumor pathology (p = 0.24), presence of NF2 syndrome (p = 1.00), surgeon perception of tumor adherence to the facial nerve (p = 0.17), surgical length/time (p = 0.59), or average maximal cerebellopontine angle or internal auditory canal (IAC) diameter of tumor on magnetic resonance imaging (MRI; p = 0.36). The ideal measurement for correlating tumor growth with B7-H1 expression is serial increases of tumor volume on MRI. We had data for 8 of 16 strongly positive (2+) tumors including all of the post radiation tumor failures, but we had tumor growth measurements on only one of the negative tumors. Unfortunately, there were too many missing data points (only one preoperative MRI) for us to make any valid analysis of these data.
TABLE 1.
Summary of the association between B7-H1 expression (absent [0] and marked [2+]) and patient/tumor characteristics
| Absent B7-H1 expression (n = 9) |
Marked B7-H1 expression (n = 16) |
p value |
|
|---|---|---|---|
| Mean patient age (yr) | 55.7 | 48.8 | 0.36 (KW) |
| Cystic | 0.24 (F) | ||
| Yes | 2 (22.2%) | 1 (6.3%) | |
| No | 7 (77.8%) | 15 (93.8%) | |
| Failed stereotactic radiation | 0.029 (F) | ||
| Yes | 0 | 5 (31.3%) | |
| No | 9 (100%) | 11 (68.8%) | |
| Neurofibromatosis type II status | 1.00 (F) | ||
| Yes | 1 (11.1%) | 2 (12.5%) | |
| No | 8 (88.9%) | 14 (87.5%) | |
| Dysequilibrium | 0.30 (F) | ||
| Yes | 7 (77.8%) | 7 (43.8%) | |
| No | 2 (22.2%) | 9 (56.3%) | |
| Tinnitus | 0.23 (F) | ||
| Yes | 4 (44.4%) | 11 (68.8%) | |
| No | 5 (55.6%) | 5 (31.3%) | |
| Preoperative facial nerve function (House-Brackmann Scale) | 0.63 (KW) | ||
| 1 | 8 (88.9%) | 14 (87.4%) | |
| 2 | 1 (11.1%) | 0 | |
| 3 | 0 | 1 (6.3%) | |
| 4 | 0 | 1 (6.3%) | |
| 5 | 0 | 0 | |
| 6 | 0 | 0 | |
| Mean preoperative word recognition (%) | 70.6 | 49.6 | 0.16 (F) |
| Preoperative hearing class | 0.12 (KW) | ||
| Unknown | 1 (0.11%) | 0 | |
| A | 3 (37.5%) | 5 (31.2%) | |
| B | 3 (37.5%) | 3 (18.8%) | |
| C | 0 | 0 | |
| D | 2 (25%) | 8 (50%) | |
| Average preoperative magnetic resonance imaging size—maximal cerebellopontine angle diameter (cm) or maximal Internal auditory canal length (cm) | 1.8 | 1.6 | 0.36 (F) |
The comparisons of nominal variables were evaluated using the Fisher’s exact test (F). The comparisons of ordinal or continuously scaled variables were evaluated using the nonparametric Kruskal-Wallis (KW) test.
Bold values are those that reach statistical significance (p < 0.05).
Regulation of B7-H1 Expression by VS Tumors
Similar to previous studies of cell lines and tissues, our real-time PCR results showed that B7-H1 mRNA seems to be equally expressed in the 10 VS tumor (5 tumors [2+] versus 5 tumors [0]) samples (p = 0.35) irrespective of their B7-H1 protein expression (Table 2). In an effort to explore the regulation of B7-H1 protein expression in VS tumors, a known translational repressor of B7-H1 in cholangiocytes, microRNA-513 (miR-513), was investigated. Real-time PCR did not show significant differential expression of microRNA-513 in the VS tumor specimens (p = 0.62). Three tumors that strongly expressed B7-H1 had similar levels of miRNA-513 as 3 B7-H1 negative tumors (Table 2).
TABLE 2.
Regulation of B7-H1 expression in vestibular schwannoma tumors: real-time polymerase chain reaction cycle-thresholds (Ct) for 5 strongly positive (2+) immunohistochemistry-stained B7-H1 tumors compared with 5 B7-H1 absent (0) vestibular schwannoma specimens
| Vestibular schwannoma specimen |
miR-513 Ct | U6 Ct | miR-513 Ct/U6 Ct | B7-H1 mRNA Ct | B7-H1 copy/µl | Actin copy/µl | B7-H1/actin ratio |
|---|---|---|---|---|---|---|---|
| B7-H1 (2+) A | 59.6 | 29.88 | 1.99 | 35.0 | 1440 | 4300 | 0.33 |
| B7-H1 (2+) B | 46.7 | 32.11 | 1.45 | 35.1 | 1380 | 1790 | 0.77 |
| B7-H1 (2+) C | 43.31 | 31.71 | 1.37 | 37.2 | 716 | 751 | 0.95 |
| B7-H1 (2+) D | ND | ND | ND | 34.8 | 1580 | 3200 | 0.49 |
| B7-H1 (2+) E | ND | ND | ND | 33.1 | 4060 | 4250 | 0.96 |
| Average (2+) | 1.60 ± 0.34 (p = 0.62) | 0.70 ± 0.28 (p = 0.35) | |||||
| B7-H1 (0) F | 46.84 | 33.13 | 1.41 | 35.9 | 1020 | 1140 | 0.89 |
| B7-H1 (0) G | 49.47 | 30.29 | 1.63 | 35.9 | 1020 | 2310 | 0.44 |
| B7-H1 (0) H | 41.51 | 30.31 | 1.37 | 36.3 | 900 | 880 | 1.02 |
| B7-H1 (0) I | ND | ND | ND | 34.7 | 1690 | 1130 | 1.50 |
| B7-H1 (0) J | ND | ND | ND | 36.3 | 900 | 1130 | 0.80 |
| Average (0) | 1.47 ± (p = 0.62) | 0.93 ± 0.38 (p = 0.35) | |||||
| H69 | 47.15 | 27.84 | 1.69 | ND | ND | ND | ND |
| Tonsil | 0 | 30.21 | 0 | 32.1 | 2750 | 13200 | 0.21 |
RNA was available for only 3 positive and negative tumors for miR-513, which were normalized with small nuclear RNA RNU6 (U6). All 10 tumors were used to evaluate B7-H1 mRNA expression. Ct values were normalized to a standard curve of serial dilutions of a known B7-H1 RNA concentration (copy/µl). This was then normalized against actin mRNA copy/µl in the last column. Human cholangiocyte cells (H69) were used as a positive control, and human tonsil tissue was used as a negative control. No difference in the quantity of miR-513 or B7-H1 mRNA was detected between groups of strongly positive and negative B7-H1 protein immunostained VS (p = 0.62 and p = 0.35, respectively).
Bold values are the average miR-513 Ct/U6 Ct and B7-H1/actin ratio values for the B7-H1 2+ and negative (0) immunostaining schwannoma tumors.
ND indicates not determined.
DISCUSSION
Previous Studies
The immune system functions in tumor immuno-editing, which is a dynamic process of 3 phases: elimination (immunosurveillance), equilibrium, and escape (tumor growth). The latter refers to the final outgrowth of tumors that have overcome immunologic pressure by reducing their immunogenicity or by altering subsequent inflammatory reactions to promote tumor cell survival. One such protein involved in immuno-editing is B7-H1. B7-H1 is the ligand for PD-1, a transmembrane receptor of the Ig superfamily expressed on thymocytes, T cells, and B cells (14). Recently, investigators have shown that B7-H1 possesses dual functions in regulating T-cell homeostasis. B7-H1 activates positive signals in naive T cells to stimulate early T-cell priming and differentiation but initiates a negative response in activated T cells to inhibit T-cell function and survival (14). Many research groups have observed that B7-H1 positive tumor cells are much more resistant to CD8+ cytolytic T-cell–mediated destruction in cell culture studies. Additionally, this resistance is correlated with decreased efficacy of immunotherapy in mouse cancer models (15,16). This led to the concept of “molecular shielding,” in that ablation of the B7-H1/PD-1 interaction by neutralizing antibodies was shown to restore cytotoxic lymphocytes (CTL)-mediated tumor cell lysis in vitro (15,16).
There have not been any studies that have addressed whether benign schwannoma cells can evade the immune system detection and elimination; however, a few investigators have performed studies with malignant Schwann cell tumors or cells. Altenschmidt et al. (17) showed that malignant schwannoma cells transplanted into syngeneic BDIX rats secrete transforming growth factor (TGF) β1, TGF-β2, and TGF-β3 to inhibit the growth of T and B lymphocytes while enhancing the proliferation of tumor cells. Another study showed increased numbers of ED2+ macrophages in areas rich in premalignant Schwann precursor cells in carcinogen-sensitive BDIX rats. These BDIX rats went on to develop fatal malignant schwannomas, in contrast to carcinogen-resistant BDIV rats that demonstrated clearance of the premalignant cells. Both animal lines showed a robust immune response as measured by increased numbers of CD4, CD8, natural killer (NK) cells, and ED1+ macrophages in areas with high densities of premalignant Schwann cells. However, cancer-prone BDIX rats demonstrated significantly higher ED2+ macrophage response to premalignant cells that led to the hypothesis that ED2+ cells could support malignant progression of initiated Schwann cells by an unknown mechanism (18). Both experiments highlight the early understanding that malignant schwannoma cells and immune cells interact during the establishment of these malignant tumors.
In the present study, we report the first investigation of B7-H1 expression in benign VS tumors. The immunohistochemical staining of 48 patient-excised VS for B7-H1 expression levels using an anti-B7-H1 monoclonal antibody revealed that 81% (33% 2+ and 48% 1+) of these specimens express B7-H1. This finding in itself is novel, as B7-H1 expression has not been reported in VS or other benign peripheral nerve tumors. The observation that VS cells acquire the ability to express surface B7-H1 protein suggests the possibility that B7-H1 may modulate the immunogenicity of this tumor. In human studies, there has been a strong positive correlation between high levels of B7-H1 protein with a poor prognosis in patients with renal cell, gastric, and esophageal carcinoma. This has been hypothesized to be related to B7-H1’s ability to suppress activated T cells and costimulate naive T cells into reduced tumor cell recognition. It also may be due to tumor expressed B7-H1 acting as a receptor for PD-1 expressing dysfunctional T cells that stimulate the intracellular domain of B7-H1 to transmit antiapoptotic signals into the tumor cells, thereby enhancing its resistance to apoptosis induction (15) (Fig. 1). Although VS are benign tumors, it also is possible that they could be evading immune system checkpoints because of the expression of B7-H1.
Clinical Characteristics in B7-H1–Expressing VSs
In the present study, we hypothesized that patients with high levels of B7-H1, present on their VS tumor cells, would be more likely to exhibit adverse patient or tumor pathology features. This statement is true when considering that surgically salvaged VS tumors that had failed stereotactic irradiation were statistically more likely to immunostain strongly (2+) for B7-H1 than negatively (0) (p = 0.029). Of a total of 6 tumors, 5 of them stained 2+, one stained 1+, and no tumors were negative for B7-H1. All of these patients had received stereotactic radiation (LINAC or gamma knife) within the last 10 years with a marginal tumor dose of 12 to 14 Gy, and all tumors demonstrated serial MRI growth for 25 to 51 months after treatment. The mechanism by which stereotactic radiation causes clinical tumor control is not clearly defined. Hypotheses based on radiobiology principles have suggested that tumor growth arrest occurs because of interruption of tumor vascularity (hyalinization/fibrosis) or tumor cell apoptosis; however, the data to support these hypotheses in VS patients are sparse (19,20). Our clinical findings support the testing of a new hypothesis where tumor growth after radiation may be, in part, due to B7-H1–mediated immunotolerance. Unfortunately, these data do not support B7-H1 as a predictor of radio responsiveness, and future experiments should focus on whether this clinical observation can bring forward a useful intervention. We also found that poor word discrimination and hearing class correlated with strongly positive B7-H1 tumors, although these came close to but did not reach statistical significance. In this initial study, power calculations predict that another 40 strongly positive and negative tumors would be needed to see if these trends in hearing will reach statistical significance. The ultimate measure of tumor aggressiveness would be increased rates of tumor growth. Unfortunately, we did not have enough patients (13/48) who had undergone serial MRI scans to assess whether tumor growth correlated with B7-H1 expression. Future studies should prospectively recruit patients with tumor growth information; however, we predict that patient accrual will be difficult because of patients electing for treatment without an observation period.
B7-H1 Regulation in VSs
Although expression of B7-H1 mRNA is common in many cells, B7-H1 protein is usually undetectable, suggesting posttranscriptional suppression (14). Comparably, this study found that human VS specimens expressed similar amounts of B7-H1 mRNA irrespective of whether B7-H1 protein expression was present or absent. One such posttranscriptional regulatory mechanism involves micro-RNAs (miRNAs), which are thought to potentially control 20% to 30% of human genes and are likely involved in the fine tuning for cellular responses to external influences (21,22). miRNAs are single-stranded regulatory RNA molecules of approximately 21 to 23 nt (21). miRNAs regulate gene expression based on their complementary base pairs to the 3’-untranslated region of target mRNAs resulting in mRNA degradation or translational suppression (21). It has recently been reported that a cellular miRNA, microRNA-513 (miR-513), is expressed in non-stimulated resting human cholangiocytes and is capable of targeting a predicted site in the B7-H1 3’-untranslated region, resulting in translational repression (13). In an effort to understand the regulation of B7-H1 expression in VS tumor cells, we hypothesized that tumors may express low levels of B7-H1 protein because of miR-513–mediated suppression of B7-H1 RNA translation. Our data showed equal miR-513 expression in B7-H1–positive and B7-H1–negative tumors. Consequently, although miR-513 is present in minute quantities in VS cells, it does not seem to regulate B7-H1 expression. The mechanism of differential expression of B7-H1 in this study’s tumor samples is still not defined, and future studies should focus on other potential posttranscriptional regulatory mechanisms, such as alternate miRNAs, alternative splicing, or cytokine induction.
Future Study Considerations
The blockade of tumor-associated B7-H1 has been shown to promote in vivo tumor regression in several murine cancer models (14, 16). A potential monoclonal antibody blockade of B7-H1 activity has theoretical advantages because B7-H1 is not expressed in many healthy tissues, and this may translate into minimal toxicity (23). This is important because many current cytotoxic drugs are of limited use because of unacceptable side effects in the face of benign tumor pathology. Our data show that B7-H1 was strongly expressed in many patient-excised VS in the setting of CD8 immunopositive cell infiltration. Anti–B7-H1 mAb (5H1) (Kwon/Dong Laboratory) has been developed to block the B7-H1/PD-1 interaction, and we think that this study supports further investigation of whether anti–B7-H1 mAb can induce schwannoma cell apoptosis in experiments with cocultured T cells.
CONCLUSION
We report on the first investigation revealing that B7-H1 is aberrantly expressed in VS tumors. Increased B7-H1 expression is significantly linked with postradiation VS tumor progression. Greater statistical power is required to assess trends seen in hearing loss and CD8+ T cell infiltration. B7-H1 mRNA is equally expressed in VS specimens irrespective of differential protein expression. MiRNA-513 does not seem to regulate the expression of B7-H1 in VS tumors, and thus, this mechanism remains to be elucidated.
Acknowledgments
The authors thank Susan M. Harrington for expertise and assistance with immunohistochemical staining. The authors also thank Amy L. Weaver for assistance with the statistical analysis of the data. Finally, the authors also thank Fausto J. Rodriguez, M.D., neuropathologist, for expert and blinded review of immunostaining intensity.
REFERENCES
- 1.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 2.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, Chen X. Immunoregulatory role of B7-H1 in chronicity of inflammatory responses. Cell Mol Immunol. 2006;3:179–187. [PMC free article] [PubMed] [Google Scholar]
- 5.Peng A, Koffman BM, Malley JD, et al. Disease progression in sporadic inclusion body myositis: observations in 78 patients. Neurology. 2000;55:296–298. doi: 10.1212/wnl.55.2.296. [DOI] [PubMed] [Google Scholar]
- 6.Rossi ML, Jones NR, Esiri MM, et al. Mononuclear cell infiltrate, HLA-DR expression and proliferation in 37 acoustic schwannomas. Histol Histopathol. 1990;5:427–432. [PubMed] [Google Scholar]
- 7.Rasmussen N, Bendtzen K, Thomsen J, et al. Patient-specific cell-mediated immunity against human acoustic neuroma extract. Acta Otolaryngol. 1982;94:261–265. doi: 10.3109/00016488209128912. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen N, Bendtzen K, Thomsen J, et al. Specific cellular immunity in acoustic neuroma patients. Otolaryngol Head Neck Surg. 1983;91:532–536. doi: 10.1177/019459988309100511. [DOI] [PubMed] [Google Scholar]
- 9.Harker LA, Nysather J, Katz A. Immunologic detection of acoustic neuroma: preliminary report. Laryngoscope. 1978;88:802–807. doi: 10.1002/lary.1978.88.5.802. [DOI] [PubMed] [Google Scholar]
- 10.Gong A, Zhou R, Hu G, et al. Cryptosporidium pavum induces B7-H1 expression in cholangiocytes by down-regulating microRNA-513. J Infect Dis. 2010;201:160–169. doi: 10.1086/648589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, Neilson JR, Kumar P, et al. miRNA profiling of naive, effector and memory CD8 T cells. PLoS One. 2007;2:e1020. doi: 10.1371/journal.pone.0001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller TE, Ghoshal K, Ramaswamy B, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong AY, Zhou R, Hu G, et al. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol. 2009;182:1325–1333. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 15.Azuma T, Yao S, Zhu G, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 17.Altenschmidt U, Kahl R, Klundt E, et al. Schwannoma cells induce a tumor-cell–specific cytotoxic-T-cell response upon transplantation into syngeneic rats but escape elimination through the secretion of immunosuppressive factors. Int J Cancer. 1997;70:542–550. doi: 10.1002/(sici)1097-0215(19970304)70:5<542::aid-ijc9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Gering KM, Marx JA, Lennartz K, et al. The interaction mode of premalignant Schwann and immune effector cells during chemically induced carcinogenesis in the rat peripheral nervous system is strongly influenced by genetic background. Cancer Res. 2006;66:4708–4714. doi: 10.1158/0008-5472.CAN-05-3780. [DOI] [PubMed] [Google Scholar]
- 19.Friedman RA, Brackmann DE, Hitselberger WE, et al. Surgical salvage after failed irradiation for vestibular schwannomas. Laryngoscope. 2005;115:1827–1832. doi: 10.1097/01.mlg.0000175063.76945.75. [DOI] [PubMed] [Google Scholar]
- 20.Niranjan A, Flickinger JC. Radiobiology, principle and technique of radiosurgery. Prog Neurol Surg. 2008;21:32–42. doi: 10.1159/000156557. [DOI] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 22.Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26:133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]