Abstract
Background
Previous work from our group showed hypoxia can induce endoplasmic reticulum (ER) stress and block the processing of the WNT3 protein in cells engineered to express WNT3a. Acute lymphoblastic leukemia (ALL) cells with the t(1:19) translocation express the WNT16 gene, which is thought to contribute to transformation.
Results
ER-stress blocks processing of endogenous WNT16 protein in RCH-ACV and 697 ALL cells. Biochemical analysis showed an aggregation of WNT16 proteins in the ER of stressed cells. These large protein masses cannot be completely cleared by ER-associated protein degradation, and require for additional autophagic responses. Pharmacological block of autophagy significantly increased cell death in ER-stressed ALL. Furthermore, murine cells engineered to express WNT16 are similarly sensitized.
Conclusion
ALL cells expressing WNT16 are sensitive to ER stress, and show enhanced killing after addition of chloroquine. These findings suggest a potential clinical application of inducers of ER stress with inhibitors of autophagy in patients with high-risk ALL.
Keywords: Unfolded protein response, tumor hypoxia, acute lymphoblastic leukemia, WNT growth factors, ER-associated degradation
Environmental conditions or drugs can interfere with the proper functioning of the endoplasmic reticulum (ER) (1). A block to the normal flow of proteins through the ER results in ER stress and elicits the compensatory unfolded protein response (UPR) (2). Proteins can accumulate in the ER in an improperly folded state after a block of essential ER functions such as protein folding, disulfide bond formation, or glycosylation. Due to the presence of unfolded proteins in the ER, cells initiate a series of responses designed to reduce the burden of proteins in the ER through decreased translation, increased chaperone expression, and increased removal of the malfolded proteins through degradation. If the cell is unable to relieve the ER stress, then cellular death can ensue (3).
The microenvironment of solid tumors is often poorly perfused, resulting in regions of hypoxia and nutrient deprivation (4, 5). However, hypoxia has been also shown to impact cancer of the bone marrow such as aggressive leukemia (6). In addition to inducing the hypoxia-inducible factor 1 (HIF1) transcription factor, severe hypoxia induces stress in the ER (7, 8). Cells with compromised ability to respond to ER stress (due to genetic ablation of the x-box binding protein (XBP1) transcription factor or the Protein kinase RNA- like endoplasmic reticulum kinase (PERK)) grow poorly as model tumors (9, 10). Pharmacological inhibition of ER stress responses can also have anticancer effects in vitro and in model myeloma (11). Hypoxia-induced ER stress can inhibit the secretion and paracrine activity of growth factors such as WNT family members (12). WNT proteins have highly conserved series of 25–27 cysteine residues that are thought to establish a complex tertiary structure essential for their activity (13). Lack of oxygen disrupts the normal formation of disulphide bonds in the WNT proteins, leading to their retention in the ER and ultimately in their degradation through proteasomal and autophagic mechanisms (12). Activation of autophagy under ER stress conditions is therefore compensatory and leads to the degradation of unfolded/misfolded protein aggregates that are not soluble and cannot be degraded by ER associated degradation (ERAD) (14, 15).
We have previously shown that hypoxia induces autophagy via AMP activated protein kinase (AMPK) activity, in an HIF-independent process (16). We have also shown that hypoxic ER stress can inhibit the processing of the WNT family of secreted glycoproteins in engineered cancer cells (12). In the present study, we again use WNT glycoproteins as tools to explore the hypothesis that autophagy is integral to the hypoxia-induced ER stress response. Here we report studies examining expression of endogenous WNT16 protein in pre-B acute lymphoblastic leukemia (ALL) cell lines after treatment with conditions that induce ER stress.
Materials and Methods
Cells, cell culture and reagents
ProB leukemic cell lines RCH-ACV and 697 cells were cultured in RPMI with 20% fetal bovine serum (FBS), Murine fibroblast L cells were cultured in Dulbecco’s modified eagle medium (DMEM) with 10% FBS. For moderate hypoxia, cells were treated in a variable-oxygen Invivo2 humidified hypoxia workstation (Ruskinn Technologies, Bridgend, UK). Severe hypoxia was generated in an anaerobic workstation gassed with 5% CO2, 5% H2 and 95% N2 containing a palladium catalyst (Sheldon Co., Cornelius, OR, USA). Transient and stable transfections were performed using Lipofectamine (Invitrogen, Carlsbad, CA, USA). MG-132, tunicamycin, thapsigargin, chloroquine, E64 and pepstatin were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Dithiothreitol (DTT) was purchased from Invitrogen. The pLPC-Wnt16 and pLPC empty retroviral vectors were a kind gift from Dr. Amato Giaccia.
Retroviral transduction
WNT16 expressing cells were generated by retroviral transduction. A WNT16-expressing retroviral vector (pLPC-WNT16) was transfected into HEK 293 Phoenix cells using Lipofectamine as directed by the manufacturer (Life Technologies, Grand Island NY USA). After 48 h, the supernatant containing the retrovirus was collected, filtered and used to transduce the indicated cell lines in the presence of 5 µg/ml polybrene (Sigma Aldrich, St Louis MO, USA). WNT16-positive clones were selected using puromycin, and expression confirmed by immunoblot.
Western blotting
In brief, treated cells were harvested in RIPA buffer, lysates were sonicated, cleared by centrifugation, and protein concentrations were quantitated by BCA reagent (Life Technologies, Grand Island NY USA). Proteins (25–50 µg) were electrophoresed on a reducing Tris-Tricine gel, and electroblotted to polyvinyl difluoride membrane. Antibodies used were mouse anti-β-catenin (BD Biosciences Pharmingen San Diego CA USA), mouse anti-human WNT16 (BD Biosciences Pharmingen), LC3 (MBL International Woburn MA USA), and mouse anti-β-actin (Abcam Hong Kong). Primary antibodies were detected with species-specific horseradish peroxidase secondary antibodies (DAKO Carpenteria CA USA) and visualized with ECL (Amersham Piscataway NJ USA) on a Storm 860 phosphoimager (Molecular Devices San Francisco CA USA).
Thiol modification blocking assay (treatment with N-ethylmaleimide)
Cells were cultured for 24 h and then lysed in RIPA buffer (1% Triton X-100, 150 mM NaCl, 20 mM Hepes (pH 7.5), 10% glycerol, 1 mM EDTA, 100 mM NaF, 17.5 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 4 µg/ml aprotinin, 2 µg/ml pepstatin A) supplemented with fresh 20 mM N-ethylmaleimide (NEM; Sigma-Aldrich). Cell lysates (30 µg) were analyzed on an 8% sodium dodecyl sulfate-polyacrylamide gel under reducing (125 mM DTT) and non-reducing (0 mM DTT) conditions.
Statistical analysis
Pairwise comparisons were made by two-tailed Students t-test on primary data points. Statistical significance was accepted at p<0.05.
Results
Expression of WNT16 is compromised by ER stress in ALL cells
Previous work from our group examined the processing of WNT3A in cells engineered to express the protein after ER stress. We engineered murine L cells and human RKO colorectal cancer cells to express exogenous WNT3A and found that WNT processing was impaired during ER stress from hypoxia, and this down-regulated β-catenin signaling. We now wished to extend these studies to determine if tumor cells that naturally expressed WNT family members were also sensitive to ER stress. Pre-B ALL cells that contain the fusion protein which joins the transactivation domain of the E2A protein with the DNA binding domain of the homeoprotein PBX express WNT16 due to the activity of the fusion protein (17, 18). We therefore obtained RCH-ACV and 697 ALL cells and examined WNT16 expression and biological activity by western blot after treatment with ER stressing agents. Figure 1 shows that treatment with reduced oxygen tension causes reduced expression of endogenous WNT16 in both cell lines (Figure 1A and B). Furthermore, we also show that there is decreased WNT signaling because there is less stabilized β-catenin in these cells after treatment with hypoxia (Figure 1A and B).
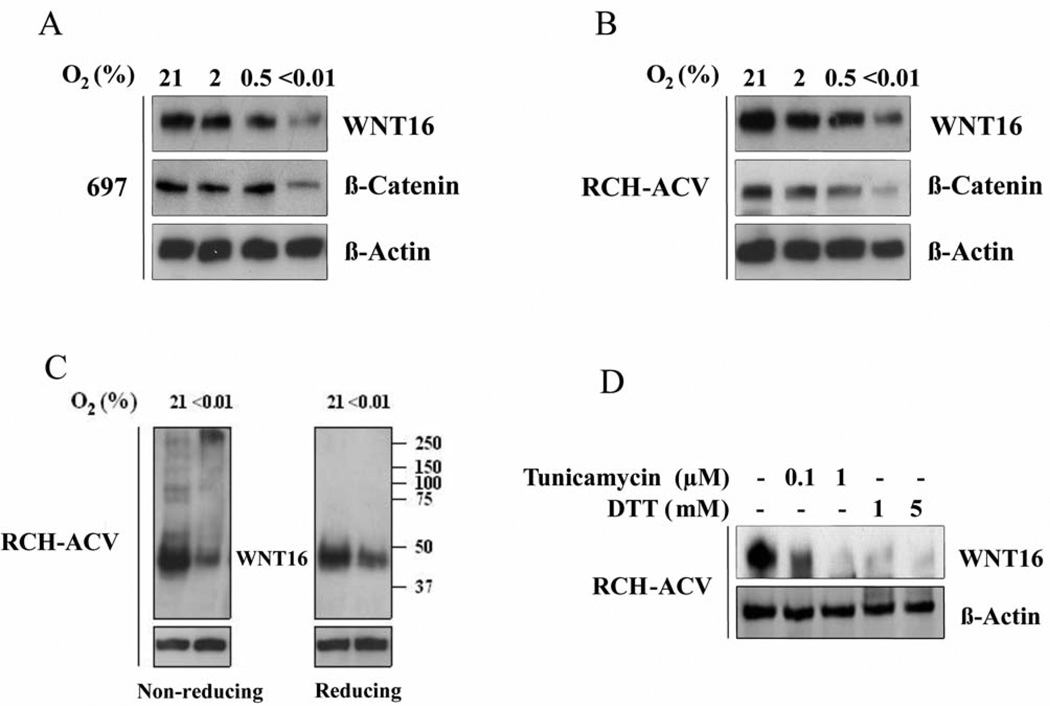
Figure 1.
ER stress causes WNT16 aggregation and destruction. A: 697 Leukemic cells expressing WNT16 were treated for 24 h with increasingly severe levels of hypoxia as indicated, and WNT16 protein levels were then detected by western blot. WNT16 activity was determined by detection of the level of stabilized β-catenin. Note that as WNT levels decrease, β-catenin levels decrease accordingly. B: RCH-ACV leukemic cells were treated similarly and WNT16 levels and activity detected as in (A). C: Determination of WNT protein aggregates in cells treated with severe hypoxia by means of the thiol protection assay and migration through non-reducing and reducing polyacrylamide gels. Under non-reducing conditions, a large fraction of WNT16 from hypoxia-treated cells migrated as a slow aggregate, with apparent mass well above 200 kDa. When these samples were reduced and disulphide bonds broken, monomeric WNT16 was detected. Comparison of left and right panels indicates WNT16 aggregates were due to aberrant disulphide bond formation. D: WNT16 protein expression is also sensitive to classical drug-induced ER stresses. WNT levels were determined after treatment with tunicamycin (to inhibit glycosylation) or DTT (to directly inhibit disulphide bond formation).
We confirmed that the decreased WNT16 protein is due to incomplete processing in the ER using the thiols protection assay. Extracts were collected in N-ethylmaleimide and this stabilized the thiols and disulphide bonds in the WNT16 protein. The samples were electrophoresed either on a non-reducing (Figure 1C left) or reducing (Figure 1C right) SDS polyacrylamide gel. Figure 1C shows that without addition of DTT to break disulphide bonds, a high fraction of the WNT16 proteins extracted from hypoxia-treated cells migrate as very large protein aggregates with apparent velocity indicating sizes above 250 kDa. Addition of DTT to these extracts breaks all disulfide bonds and collapses the WNT16 reactive material to an apparent monomeric size of 45 kDa. We interpret these results to indicate that severe hypoxia interferes with correct disulphide bond formation of WNT16 proteins in the ER, because oxygen is the terminal electron acceptor in the reaction forming disulphide bonds (7). Without correct 3-dimensional structure, WNT proteins aggregate, and this signals for their destruction. We also directly tested cellular processing of WNT16 to classical ER stressor tunicamycin which inhibits N-linked glycosylation and DTT which inhibits disulphide bond formation in the cell. Western blot analysis of lysates from these cells shows that both these stresses had a profound effect, and reduced WNT16 protein expression in RCH-ACV cells (Figure 1D).
Reduced WNT16 expression after ER stress is due to proteolysis
We have shown that severe hypoxia can stimulate autophagy in carcinoma cells (16). We, therefore, examined WNT16-expressing leukemia cells for markers of autophagy after treatment with ER stress. Figure 2A shows that 697 cells treated with either chemical ER stress or severe hypoxia displayed an increased processing of myosin-associated light chain protein 3 (LC3-II) that is a marker of active autophagy (19). Similarly, there was increased turnover of the adaptor protein p62/sequestrosome 1 in 697 and RCH-ACV cells treated with hypoxia, indicating autophagic turnover of protein aggregates (20).
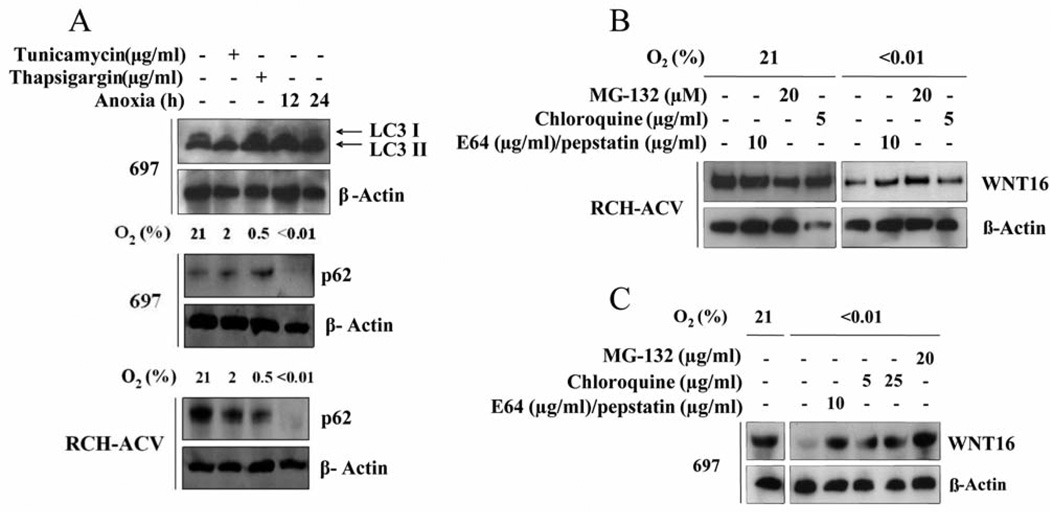
Figure 2.
Turnover of WNT16 after ER stress is partly through autophagy A: Analysis of myosin associated light chain 3 processing in 697 cells exposed to chemical ER stress or severe hypoxia for 12 or 24 h (top panel). Increased LC3-II indicates processing of LC3 at the autophagolysosome. Decrease of adaptor protein p62 was noted in both 697 cells (middle panel) and RCH-ACV cells (bottom panel) exposed to hypoxia. Note that a decrease in p62 indicates turnover of protein aggregates in the autophagolysosome. B: Partial rescue of WNT16 protein levels in RCH-ACV cells exposed to severe hypoxia for 24 h by addition of inhibitors of the proteasome, or the lysosomal proteases for the final 2 h. Note that treatment of normoxic cells with protease inhibitors did not alter WNT16 level. C: Partial rescue of WNT16 protein expression in ER-stressed 697 cells treated similarly to the RCH-ACV cells shown in (B).
In order to determine if the reduction of WNT16 protein was due to proteolysis by the proteasome or the autophagasome, we used inhibitors of each of these processes in ER-stressed cells. Figures 2B and C show that under normoxia, neither inhibitor had an effect on WNT16 expression level. However, in hypoxia, both inhibitors resulted in a partial restoration of WNT protein levels. These results suggest that both proteasome and autophagasome/cathepsins contribute to reduced WNT16 protein expression after ER stress from hypoxia.
ER stress-induced toxicity can be potentiated in ALL cells with autophagy inhibitors
We next treated ALL cells with either ER stress-inducing agents, autophagy inhibitors, or both, and determined the dead fraction by trypan blue exclusion. Figure 3A shows that when RCH-ACV cells are treated with hypoxia there was very modest toxicity. Similarly, the autophagy inhibitors E64/pepstatin and chloroquine were not very toxic to normoxic cells. However, when the autophagy inhibitors were combined with hypoxia, there was significant cellular death (Figure 3A). These experiments were repeated with chemical inducers of ER stress and resulted in qualitatively similar results, although the drugs themselves did have some toxicity such that the increased toxicity with autophagy inhibitors led to a smaller fold increase (Figure 3B). Thapsigargin and DTT led to significant increase in cell death with the addition of chloroquine, while tunicamycin tended to increase toxicity (p=0.06).
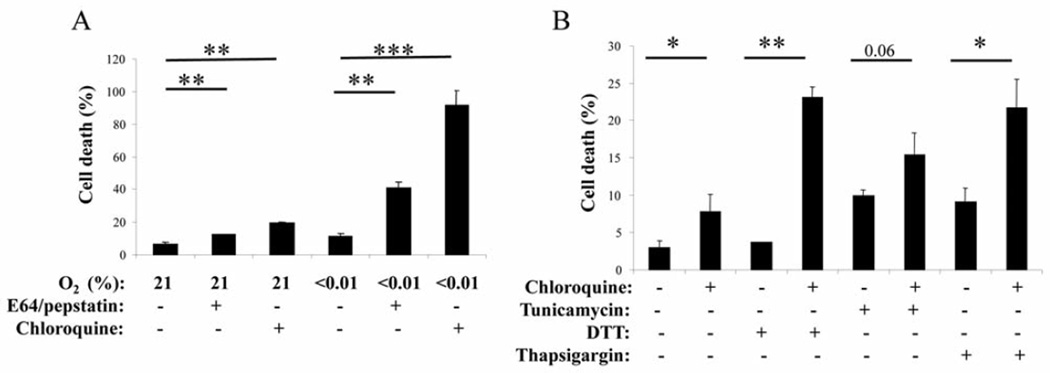
Figure 3.
Toxicity due to ER stress can be potentiated by the addition of autophagy inhibitors. A: Killing of RCH-ACV cells exposed to 72 h of severe hypoxia or hypoxia with the autophagy inhibitors. There was very modest toxicity in the cells treated with hypoxia alone, but addition of the autophagy inhibitors greatly enhanced cell killing as measured by trypan blue exclusion. N=3 independent experiments performed in triplicate. B: Killing of RCH-ACV cells by exposure to 24 h of the chemical ER stresses tunicamycin, Thapsigargin or DTT as indicated, and the potentiation of the killing from the ER stress by the addition of chloroquine. N=3 Independent experiments performed in triplicate. Statistical significance: *0.05>p>0.01, **0.01>p>0.001, and ***p<0.001.
Expression of WNT16 in mouse L cells sensitizes them to ER stress in combination with autophagy inhibitors
In order to molecularly connect WNT16 expression with cellular sensitivity to the two-drug cocktail, we needed a genetically-matched system that only differed by the expression of the one gene. However, the ALL cells are dependent on WNT signaling for survival (18), hence it is not possible to knockdown WNT16 in these cells. As an alternative, we expressed WNT16 in murine L cells that are commonly used to produce WNT proteins in a cell that does not rely on the biological effects of the growth factor for survival (21). Figure 4A shows the stable expression of the engineered WNT16 in L cells after transduction with recombinant retrovirus. Figure 4B shows that the expression of WNT16 in these cells is significantly reduced after treatment with hypoxia, and this reduction can be reversed with treatment of chloroquine or E64/pepstatin. Finally, Figure 4C and D show that cells expressing WNT16 protein are specifically sensitized to the disulphide bond disrupter DTT when it was combined with the autophagy inhibitor chloroquine. Figure 4C shows the acute toxicity after 72 h treatment, and Figure 4D shows the colony formation after 9 days growth of the cells in the indicated media. Both measures indicate that there was significant death only of the WNT16-expressing cells, and only in the combination treatment with ER stress and chloroquine.
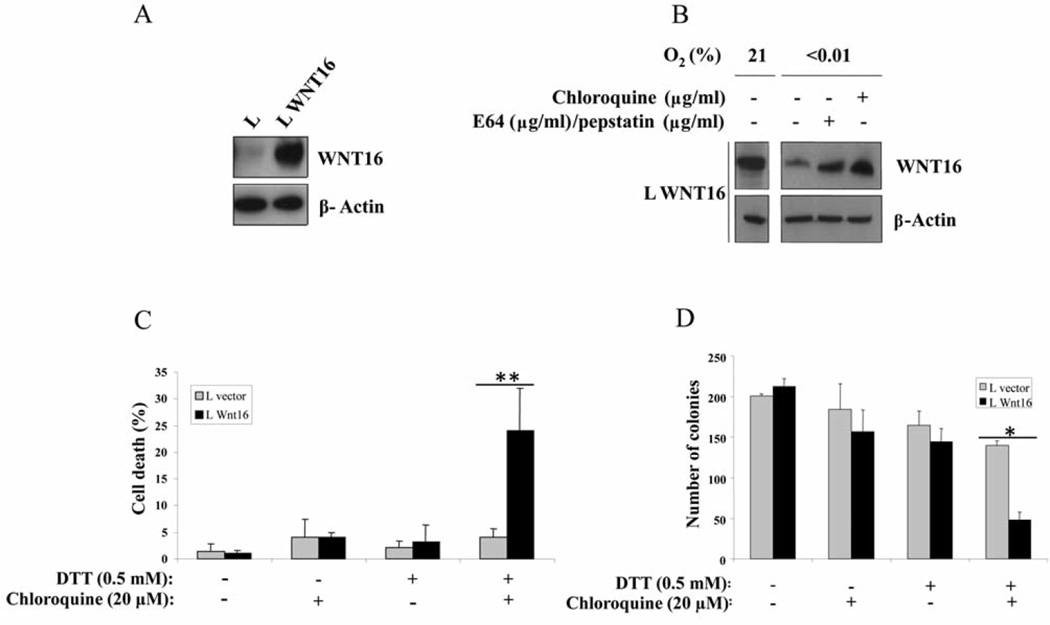
Figure 4.
Sensitization of mouse L cells to ER stress and autophagy inhibitors by the introduction of WNT16 gene. A: Western blot of lysates from L cells transduced with empty vector, or those transduced with retrovirus expressing WNT16 as indicated. B: Reduction of WNT16 protein expression in L cells exposed to 24 h of severe hypoxia and rescue of the expression by addition of either MG132 to inhibit the proteasome, or chloroquine to inhibit the lysosomal cathepsins for the final 2 h. C: L cells expressing WNT16 are specifically sensitized to killing by the combination of DTT to induce ER stress and chloroquine to block autophagy. Cells were treated for 72 h and the dead fraction determined by trypan blue exclusion. N=2 independent experiments performed in triplicate. D: ER-stress dependent killing of L cells expressing WNT16 was also measured by colony-formation assay after plating of 300 cells. This assay also shows specific killing of the WNT16-expressing variant line. N=2 independent experiments performed in triplicate. Statistical significance: *0.05>p>0.01, and **0.01>p>0.001.
Discussion
These results support our previous findings and the concept that hypoxia is a negative regulator of WNT processing and signaling because it blocks WNT protein in the ER, and leads to its ultimate destruction. The WNT protein that accumulates in the ER has improper disulfide bonds and generates toxic protein aggregates. These aggregates must be cleared by an autophagic process because the insoluble aggregates are resistant to ERAD.
Accumulation of malfolded proteins in the ER elicits the cell to respond with the UPR and the activation of the three arms of the UPR: PERK-dependent induction of activating transcription factor 4 (ATF4), endoplasmic reticulum to nucleus signaling 1 (IRE1/ERN1)-dependent XBP1 splicing and proteolytic activation of ATF6. Together these three arms of the response attempt to decompress the ER by slowing the introduction of new proteins into the ER, and enhancing proper folding of those proteins already in the ER through increased chaperone expression, and removal of the proteins deemed unrepairable. It is well established that the ERAD pathway is an efficient means of removal of proteins that can be transported across the ER membrane and into the cytoplasm for proteasomal destruction (22). However, if these proteins become aggregated into insoluble tangles, then the ERAD pathway is not efficient, and an autophagic process must compensate for their removal (23).
Paracrine and autocrine WNT signaling has been suggested as a mechanism for maintaining 'stemness' and aggressiveness of cancer cells (24, 25). However, these findings suggest that it may not be quite as simple in vivo for cells in hypoxic regions of tumors or bone marrow to produce and secrete WNT growth factors to simulate canonical and non-canonical β-catenin signaling. Furthermore, WNT gene expression may even be detrimental to the growth of tumor cells growing in microenvironmental hypoxia, as compared to activation of β-catenin by mutation (12).
The ability of cells to adapt to environmental stress appears to be essential to the growth of cells in solid tumors (9, 10). However, hypoxia exists in the bone marrow in health and disease and influences leukemic progression (26–28). These studies indicate that hypoxia is an area for additional study to establish its importance in the natural history of diseases of the bone marrow. These findings also support the concept that rational design of combination chemotherapy may include drugs that influence ER stress (29). If microenvironmental hypoxia can initiate a condition of ER stress, perhaps addition of chemotherapeutic agents that enhance ER stress, such as bortezomib, could be combined with anti-autophagy agents, such as chloroquine, in order to achieve therapeutic gain.
WNT proteins have a complex three-dimensional structure. Expression of WNT proteins in cancer cells can promote aggressive cancer growth but can also sensitize them to agents or conditions that cause ER stress. This sensitivity can be further exploited in a fraction of acute leukemia by the addition of inhibitors of autophagy. Identifying cancer types that express WNT proteins (especially those addicted to WNT protein signaling) may identify those that would be sensitive to such a therapeutic approach.
Acknowledgments
The Authors would like to thank Drs Roel Nusse and Michael Cleary for cell lines, Dr Amato Giaccia for his gift of the pLC constructs, and Dr Albert Koong for his helpful discussions.
References
- 1.Sovolyova N, Healy S, Samali A, Logue SE. Stressed to death - mechanisms of ER stress-induced cell death. Biol Chem. 2014;395:1–13. doi: 10.1515/hsz-2013-0174. [DOI] [PubMed] [Google Scholar]
- 2.Merksamer PI, Papa FR. The UPR and cell fate at a glance. J Cell Sci. 2010;123:1003–1006. doi: 10.1242/jcs.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends in cell biology. 2014;24:472–478. doi: 10.1016/j.tcb.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 6.Benito J, Zeng Z, Konopleva M, Wilson WR. Targeting hypoxia in the leukemia microenvironment. Int J Hematol Oncol. 2013;2:279–288. doi: 10.2217/IJH.13.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3:597–605. doi: 10.1158/1541-7786.MCR-05-0221. [DOI] [PubMed] [Google Scholar]
- 8.Koumenis C, Bi M, Ye J, Feldman D, Koong AC. Hypoxia and the unfolded protein response. Methods in enzymology. 2007;435:275–293. doi: 10.1016/S0076-6879(07)35014-3. [DOI] [PubMed] [Google Scholar]
- 9.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M, Bell JC. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le QT, Koong AC. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 11.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, Koong AC. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verras M, Papandreou I, Lim AL, Denko NC. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Molecular and cellular biology. 2008;28:7212–7224. doi: 10.1128/MCB.00947-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho SJ, Valles Y, Giani VC, Jr, Seaver EC, Weisblat DA. Evolutionary dynamics of the wnt gene family: a lophotrochozoan perspective. Mol Biol Evol. 2010;27:1645–1658. doi: 10.1093/molbev/msq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 15.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Molecular and cellular biology. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–1581. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- 17.Casagrande G, te Kronnie G, Basso G. The effects of siRNA-mediated inhibition of E2A-PBX1 on EB-1 and Wnt16b expression in the 697 pre-B leukemia cell line. Haematologica. 2006;91:765–771. [PubMed] [Google Scholar]
- 18.Mazieres J, You L, He B, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene. 2005;24:5396–5400. doi: 10.1038/sj.onc.1208568. [DOI] [PubMed] [Google Scholar]
- 19.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 20.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 21.Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Merulla J, Fasana E, Solda T, Molinari M. Specificity and regulation of the endoplasmic reticulum-associated degradation machinery. Traffic. 2013;14:767–777. doi: 10.1111/tra.12068. [DOI] [PubMed] [Google Scholar]
- 23.Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol. 2012;34:286–297. [PubMed] [Google Scholar]
- 24.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng KP, Manjeri A, Lee KL, Huang W, Tan SY, Chuah CT, Poellinger L, Ong ST. Physiologic hypoxia promotes maintenance of CML stem cells despite effective BCR-ABL1 inhibition. Blood. 2014;123:3316–3326. doi: 10.1182/blood-2013-07-511907. [DOI] [PubMed] [Google Scholar]
- 27.Silveira VS, Freire BM, Borges KS, Andrade AF, Cruzeiro GA, Sabino JP, Glass ML, Yunes JA, Brandalise SR, Tone LG, Scrideli CA. Hypoxia-related gene expression profile in childhood acute lymphoblastic leukemia: prognostic implications. Leuk Lymphoma. 2014;55:1751–1757. doi: 10.3109/10428194.2013.858812. [DOI] [PubMed] [Google Scholar]
- 28.Velasco-Hernandez T, Hyrenius-Wittsten A, Rehn M, Bryder D, Cammenga J. HIF-1alpha can act as a tumor suppressor gene in murine acute myeloid leukemia. Blood. 2014;124:3597–3607. doi: 10.1182/blood-2014-04-567065. [DOI] [PubMed] [Google Scholar]
- 29.Kharabi Masouleh B, Geng H, Hurtz C, Chan LN, Logan AC, Chang MS, Huang C, Swaminathan S, Sun H, Paietta E, Melnick AM, Koeffler P, Muschen M. Mechanistic rationale for targeting the unfolded protein response in pre-B acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2014;111:E2219–E2228. doi: 10.1073/pnas.1400958111. [DOI] [PMC free article] [PubMed] [Google Scholar]