ABSTRACT
Influenza A virus (IAV) infection frequently causes hospitalization and mortality due to severe immunopathology. Annual vaccination and antiviral drugs are the current countermeasures against IAV infection, but they have a limited efficacy against new IAV variants. Here, we show that intranasal pretreatment with Fc-fused interleukin-7 (IL-7–mFc) protects mice from lethal IAV infections. The protective activity of IL-7–mFc relies on transcytosis via neonatal Fc receptor (FcRn) in the lung and lasts for several weeks. Introduction of IL-7–mFc alters pulmonary immune environments, leading to recruitment of T cells from circulation and their subsequent residency as tissue-resident memory-like T (TRM-like) cells. IL-7–mFc-primed pulmonary TRM-like cells contribute to protection upon IAV infection by dual modes. First, TRM-like cells, although not antigen specific but polyclonal, attenuate viral replication at the early phase of IAV infection. Second, TRM-like cells augment expansion of IAV-specific cytotoxic T lymphocytes (CTLs), in particular at the late phase of infection, which directly control viruses. Thus, accelerated viral clearance facilitated by pulmonary T cells, which are either antigen specific or not, alleviates immunopathology in the lung and mortality from IAV infection. Depleting a subset of pulmonary T cells indicates that both CD4 and CD8 T cells contribute to protection from IAV, although IL-7-primed CD4 T cells have a more prominent role. Collectively, we propose intranasal IL-7–mFc pretreatment as an effective means for generating protective immunity against IAV infections, which could be applied to a potential prophylaxis for influenza pandemics in the future.
IMPORTANCE The major consequence of a highly pathogenic IAV infection is severe pulmonary inflammation, which can result in organ failure and death at worst. Although vaccines for seasonal IAVs are effective, frequent variation of surface viral proteins hampers development of protective immunity. In this study, we demonstrated that intranasal IL-7–mFc pretreatment protected immunologically naive mice from lethal IAV infections. Intranasal pretreatment with IL-7–mFc induced an infiltration of T cells in the lung, which reside as effector/memory T cells with lung-retentive markers. Those IL-7-primed pulmonary T cells contributed to development of protective immunity upon IAV infection, reducing pulmonary immunopathology while increasing IAV-specific cytotoxic T lymphocytes. Since a single treatment with IL-7–mFc was effective in the protection against multiple strains of IAV for an extended period of time, our findings suggest a possibility that IL-7–mFc treatment, as a potential prophylaxis, can be developed for controlling highly pathogenic IAV infections.
INTRODUCTION
Influenza A virus (IAV) has caused seasonal epidemics and four pandemics in the last century, which threaten the global public health (1). More recently, it has been reported that avian IAV variants, including H5N1 and H7N9, can cross-infect humans with higher mortality than other strains of human-infectious IAV (2). Although the human-to-human transmission of avian IAV variants has not been involved in the major cases yet, the potential generation of new IAV strains against humans who have no preexisting immunity raises the risk of pandemic emergence (3). Although an annual vaccination for specific IAV variants is the current primary choice for seasonal influenza control, there are limitations to this strategy, such as production problems and low efficacies, and vaccines are unlikely to be available in time to manage new antigenic variants (4). Antiviral drugs, such as neuraminidase inhibitors, reduce progression to more severe complications, especially when treatment is within 2 to 3 days of infection (5). However, new variants that are resistant to existing antiviral drugs are emerging and might limit the effectiveness of antiviral drugs in the future (6). Thus, there is an urgent need for an alternative strategy against highly pathogenic IAV.
Induction of both innate and adaptive immune responses is crucial for the control of viremia after IAV infection. However, the current understanding of IAV infection suggests that excessive host immune responses lead to immunopathology followed by respiratory dysfunction and mortality (7, 8). Previous studies of pandemic H1N1 infection have suggested that excess production of cytokines and chemokines, such as granulocyte colony-stimulating factor (G-CSF), interleukin-6 (IL-6), IL-8, IL-10, IP-10, and monocyte chemoattractant protein-1 (MCP-1), caused severe immunopathology, including excessive recruitment of neutrophils and mononuclear cells in the lungs, which resulted in severe complications leading to death (9, 10). Thus, immune-modulatory strategies as therapeutics to reduce severe complications are to be investigated for the cure against IAV infection. Accordingly, recent experimental studies showed successful reduction of IAV-induced mortality by use of immune-modulatory agents, including a Toll-like receptor 4 antagonist, the sphingosine analog AAL-R, and a membrane-associated prostaglandin E2 (PGE2) synthase-1 inhibitor (11–14). However, these approaches required repeated treatments due to their transient effectiveness. Thus, the development of long-term and universal protective agents against IAV infection is strongly needed.
Interestingly, several clinical observations suggest that severe immunopathology is frequently accompanied by defective T cell-mediated immunity (15, 16). It is well known that CD4 and CD8 T cells are involved in the viral clearance during primary and secondary IAV infection (17). Moreover, several reports have demonstrated the essential role of lung-resident IAV-specific memory CD4 and CD8 T cells, which express lung-retentive markers such as CD11a, CD49d, and CD103, in optimal disease control during IAV infection (18, 19). Meanwhile, these tissue-resident memory (TRM) cells in the lung also confer broad immunity against heterologous IAV infection. For instance, preexisting influenza virus-specific CD4 T cells in healthy humans induce a heterotypic immune response after IAV challenge, which was correlated with disease protection with less severe illness (20). These heterologous CD4 TRM cells would control the IAV infection via induction of cross-reactive IAV-specific immunity (21) and/or production of innate-like cytokines and chemokines during IAV infection (22). However, although the role of IAV-specific memory T cells in the lung is relatively well-established, the impact of bystander-activated, antigen-nonspecific memory T cells in the lung during IAV protection remains elusive (23). Since optimal protection from IAV infection requires orchestration of the immune response by T cells in the mucosal site, it would be intriguing to investigate whether augmentation of pulmonary T cell-mediated immunity without administration of cognate antigen can also provide potential benefit to the host during IAV infection.
In this study, we investigated the prophylactic effects of interleukin-7 (IL-7) against lethal IAV infection. IL-7 is known to induce expansion of T cells under lymphopenic conditions (24). In our previous studies, we demonstrated that IL-7 can be used as a vaccine adjuvant to augment antigen-specific T cell response (25, 26) and to enhance antibody responses through the induction of follicular helper T cells (27). Here, we demonstrate that a single intranasal pretreatment with Fc-fused IL-7 (IL-7–mFc), but not a native form of IL-7, completely protects mice from IAV-induced mortality for an extended period of time, even without preexisting IAV-specific immunity. IL-7–mFc treatment alters immune environments in the lung, with prolonged occupancy of lung-retentive effector/memory phenotype T (TRM-like) cells, which play an essential role in protection from IAVs by limiting viral replication and immunopathology, while helping IAV-specific cytotoxic T lymphocytes (CTLs) to propagate. Among pulmonary T cell populations expressing IL-7 receptor (CD127), CD4 TRM-like cells appeared to be crucial for protective functions generated by IL-7–mFc. Collectively, our data show a novel prophylactic function of IL-7–mFc against IAV infections, highlighting an immune-modulatory role of pulmonary T cells.
MATERIALS AND METHODS
Animals.
Female BALB/c, BALB/c-nude, C57BL/6, and FcRn−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed under specific-pathogen-free conditions in an approved animal facility at Pohang University of Science and Technology (POSTECH) Biotech Center and International Vaccine Institute (Seoul, Republic of Korea). All mouse experiments were performed in accordance with National Institutes of Health guidelines, and protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
Preparation and treatment.
The murine nonlytic Fc-fused IL-7 and nonlytic Fc fragment were prepared as previously described (28). Recombinant human IL-7 (IL-7) was purchased from Shenandoah Biotechnology (Warwick, PA, USA). After being anesthetized with ketamine (100 mg/kg; Yuhan, Republic of Korea) and xylazine hydrochloride (10 mg/kg; Bayer, Belgium) in phosphate-buffered saline (PBS) intraperitoneally (i.p.), mice received 50 μl of the indicated doses of cytokines in PBS via the indicated routes with a micropipette or syringe. The depletion monoclonal antibodies (MAbs) against mouse CD4 (GK1.5), CD8 (2.43), Vγ2 (UC3-10A6), and polyclonal rat IgG were purchased from Bioxcell (West Lebanon, NH, USA). Mice received 200 μg of each depletion MAb i.p. at −1, 0, 1, and 4 days after IAV infection.
Virus infection and titration.
Influenza virus H1N1 (A/Puertorico/8/34) and H5N2 (A/aquatic bird/ma81/2007) strains were kindly provided by Young Ki Choi (Chungbuk National University of Medicine, Republic of Korea). Mouse-adapted H5N2 was generated by passaging the H5N2 A/Aquatic bird/Korea/W81/05 strain as described previously (29). At the indicated time point after IL-7–mFc treatment, mice were anesthetized and infected intranasally (i.n.) with 3 50% lethal doses (LD50) of PR8 or H5N2. Body weight change and survival were monitored daily following infection, and groups with more than 50% dead mice were excluded from the body weight graph. Mice that lost more than 30% of their initial body weight were euthanized.
To measure virus titers, total lung homogenate samples taken at 3 and 7 days postinfection (dpi) were added to a monolayer of Madin-Darby canine kidney (MDCK) cells in quadruplicate with 10-fold serial dilution, and the cytopathic effects were monitored daily. Virus titer was determined with a hemagglutinin test and calculated by the method of Reed and Muench, as previously described (30). Virus titer was expressed as log10 of the 50% tissue culture infective dose (TCID50) per milliliter.
BALF collection and lung homogenate preparation.
The mice were anesthetized, and bronchoalveolar lavage fluid (BALF) was collected with 1 ml of PBS. After BALF collection, the lungs were collected, minced into small pieces, and treated with type I collagenase (Gibco/Life Technology, Grand Island, NY, USA) and DNase I (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 30 to 45 min. Tissue fragments were harvested and crushed through a 70-μm strainer (BD Biosciences/Falcon, San Jose, CA, USA) to generate single cell suspensions. The cells were then washed and resuspended with RPMI 1640 (Welgene, Republic of Korea) containing 10% fetal bovine serum (FBS) (HyClone, South Logan, UT, USA), 2-mercaptoethanol (Gibco), and antibiotics (Gibco).
Quantification of cytokines, chemokines, influenza virus-specific antibodies, and total proteins in BALF and serum.
The levels of cytokines and chemokines were first screened with a Milliplex MAP mouse cytokine/chemokine kit (Millipore, Billerica, MA, USA) and further analyzed using DuoSet enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) for mouse gamma interferon (IFN-γ), IL-6, G-CSF, MCP-1, and IP-10 according to the manufacturer's protocol. Total protein concentrations in the BALF were quantified using protein assay dye reagent (Bio-Rad, Hercules, CA, USA) with bovine serum albumin (BSA) (Roche, Germany) as a standard. To quantify the level of influenza virus-specific antibodies, total IgG and IgA were analyzed by direct ELISA with inactivated H5N2 virus. Total IgG-horseradish peroxidase (IgG-HRP) and IgA-HRP were purchased from Southern Biotech (Birmingham, AL, USA).
Flow cytometry.
Single-cell suspensions of lung homogenate were incubated with Fc blocker (2.4G2; eBioscience, San Diego, CA, USA) in staining buffer (1% FBS in PBS) to prevent nonspecific antibody binding. The cells were then stained with MAbs against B220, CD3, CD4, CD8, CD11a, CD11b, CD11c, CD44, CD49d, CD62L, CD69, DX5, F4/80, Ly6C, major histocompatibility complex (MHC) II, IFN-γ, T cell receptor γδ (TCRγδ), and 7-aminoactinomycin D (7-AAD) (all from eBioscience) and with MAbs against CD19, CD45, Gr-1, and Ly6G (all from BD Biosciences). For the intracellular cytokine staining of IFN-γ-producing CD8 T cells, lung homogenates were incubated for 6 h with hemagglutinin (HA) peptide (residues 529 to 543; Peptron, Republic of Korea), brefeldin A (eBioscience), and DNase I (Sigma) and then stained using Cytofix/Cytoperm following the manufacturer's protocol (BD Bioscience). All samples were analyzed with LSR Fortessa (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR, USA).
In vivo antibody labeling.
To analyze the pulmonary residency of T cells, we treated mice with PBS or 1 μg of IL-7–mFc intranasally. At 7 and 14 days after IL-7–mFc treatment, mice were injected with 2.5 μg of anti-mouse CD3e-peridinin chlorophyll protein (PerCP)-Cy5.5 (BD bioscience) intravenously at 10 min prior to sacrifice as previously described (31). Residual antibody was removed by cardiac perfusion with PBS, and the lung resident T cell populations in single-cell suspensions of lung homogenate were analyzed by flow cytometry.
Histological analysis and inflammation score.
Mice were anesthetized, and the lungs were prepared via thoracotomy and transcardial perfusion with ice-cold PBS. Perfused lungs were immediately fixed with 4% paraformaldehyde, kept at 4°C overnight, and embedded into paraffin. Lung sections were then stained with hematoxylin and eosin (H&E) solution (Sigma-Aldrich). The images of whole lung tissues were captured with a Pannoramic MIDI slide scanner (3DHISTECH, Hungary). Pulmonary inflammation was assessed by the degree of peribronchiolar and perivascular inflammation, as previously described (32).
mRNA preparation, cDNA synthesis, and qPCR analysis of lung homogenate.
After preparation of lung homogenate, mRNAs were prepared with a Reliaprep mRNA preparation kit (Promega, Fitchburg, WI, USA), and cDNAs were synthesized with the GoScript reverse transcriptase system (Promega) according to the manufacturer's protocol. Quantitative PCR (qPCR) assay was performed using Power SYBR green master mix (Applied Biosystems, Foster City, CA). The following primers for qPCR analysis were synthesized by Genotech (Republic of Korea): NS-1 forward, TGCGGGAAAGCAGATAGTGG; NS-1 reverse, TCAGTTAGGTAGCGCGAAGC; L32 forward, GAAACTGGCGGAAACCCA; and L32 reverse, GGATCTGGCCCTTGAACCTT. Relative expression levels of H5N2 NS-1 mRNA were normalized to the level of L32 mRNA.
Statistical analysis.
A two-tailed Student t test was used to evaluate the differences between two groups. A one-way analysis of variance (ANOVA) with Bonferroni's posttest was used for more than three groups. Differences in survival rates between groups were determined by a log rank test.
RESULTS
Intranasal pretreatment of Fc-fused IL-7 protects mice from lethal IAV infection.
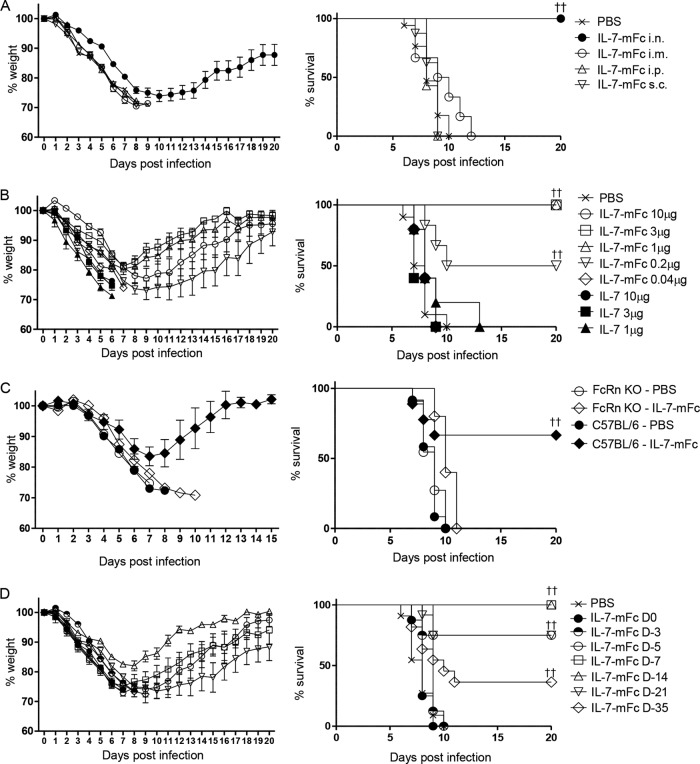
To investigate the protective effect of IL-7 on lethal IAV infection and its route dependency, BALB/c mice were treated with 1 μg of IL-7–mFc through various routes and then infected with 3 LD50 of mouse-adaptive avian influenza virus (H5N2, A/Aquatic bird/Korea/ma81/2005) at 2 weeks after IL-7–mFc treatment. Of note, intranasal IL-7–mFc treatment completely protected mice against lethal influenza virus infection, whereas IL-7–mFc treatment via an intraperitoneal, subcutaneous, or intramuscular route showed no significant protection (Fig. 1A). This finding indicated that induction of local immune responses by IL-7–mFc at the pathogen entry site, namely, the airway mucosa, may be critical for protection against lethal IAV infection.
FIG 1.
Protective effect of IL-7–mFc against lethal influenza virus infection. (A) Mice (BALB/c, n = 8 mice per group) were treated with 1 μg of IL-7–mFc via the indicated routes. At 14 days after the treatment, the mice were infected with 3 LD50 of H5N2. (B) Mice (BALB/c, n = 6 mice per group) received the indicated dose of IL-7–mFc, IL-7, or PBS intranasally and then were infected with 3 LD50 of H5N2 14 days later. (C) C57BL/6 and FcRn−/− mice (n = 8 mice per group) were administered PBS or 1 μg of IL-7–mFc intranasally. At 14 days after treatment, the mice were infected with 3 LD50 of H5N2 virus. (D) The mice (BALB/c, n = 11 mice per group) received 1 μg of IL-7–mFc intranasally at the indicated time points prior to the infection. All groups of mice were then simultaneously infected with 3 LD50 of H5N2 at day 0. The survival rate was analyzed by the Kaplan-Meier method. The results are representative of more than two independent experiments with similar results. ††, P < 0.01 by log-rank test.
To define an optimal dose of IL-7–mFc for protection against lethal IAV infection, we treated mice with IL-7–mFc at multiple doses ranging from 0.04 μg to 10 μg. We observed no significant protection with 0.04 μg, partial protection with 0.2 μg (50% of the mice survived), and complete protection with more than 1 μg of IL-7–mFc (Fig. 1B). Interestingly, treatment with recombinant human IL-7 (IL-7) did not provide protection at any tested doses (1 μg, 3 μg, and 10 μg for IL-7) (Fig. 1B). In addition, intranasal pretreatment with 1 μg of Fc fragment exhibited no significant protective effect (data not shown). Altogether, these data suggest a critical role of Fc-fused IL-7, but not Fc fragment itself, in the protection against IAV infection.
To further evaluate whether Fc fusion to IL-7 is required for the protective function, we tested IL-7–mFc in neonatal Fc receptor-deficient (FcRn−/−) mice. Remarkably, IL-7–mFc-mediated protection against IAV infection was completely abrogated in FcRn−/− mice (Fig. 1C). This finding indicates that an efficient transport of IL-7–mFc into the airway mucosa through FcRn is critical for protection from IAV infection, as intranasal delivery of Fc-fused mucosal vaccines induced protective immune responses by the FcRn-dependent transcellular transport (33). Based on these results, we administered 1 μg of IL-7–mFc intranasally at 2 weeks prior to IAV infection in subsequent experiments as a standard protocol for evaluating its protective function.
IL-7–mFc pretreatment exhibits a long-term prophylactic effect against lethal IAV infection.
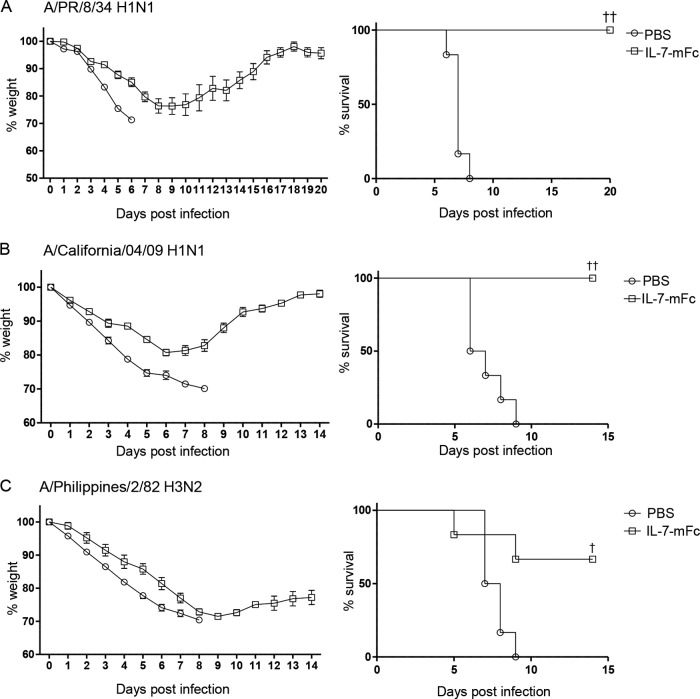
To investigate whether IL-7–mFc treatment confers a prolonged protective function against lethal IAV infection, we infected mice at different time points from 0 to 35 days after intranasal delivery of IL-7–mFc (Fig. 1D). The protective effect of IL-7–mFc was observed 5 days after IL-7–mFc treatment, but not from 0 to 3 days. Remarkably, complete protection against IAV was achieved at 7 days and even at 14 days after IL-7–mFc treatment. This protective effect was diminished over time but was still significant until 35 days, with 63% and 36% of mice surviving when infected at 21 days and 35 days after IL-7–mFc treatment, respectively. To determine whether IL-7–mFc treatment is able to exert protection against different IAV strains, we pretreated mice with PBS or IL-7–mFc and infected them with lethal doses of H1N1 (A/Puerto Rico/8/34 and A/California/04/09) and H3N2 (A/Philippines/2/82) strains. Pretreatment with IL-7–mFc at 14 days prior to challenge gave protection from a lethal infection of the tested IAVs, suggesting that IL-7–mFc-mediated IAV protection is independent of the viral strain (Fig. 2A to C).
FIG 2.
Protective effect of IL-7–mFc against infection with other strains of IAV. The mice (BALB/c, n = 6 mice per group) were infected with a lethal dose of A/PR/8/34 H1N1 (A), A/California/04/09 H1N1 (B), or A/Philippines/2/82 H3N2 (C) at 14 days after intranasal IL-7–mFc treatment. The average body weight loss is shown as a percentage of initial weight at the time of infection (mean ± standard error of the mean [SEM]). The survival rates were analyzed by the Kaplan-Meier method. The results are representative of two independent experiments with similar results. †, P < 0.05; ††, P < 0.01 (by log rank test).
IL-7–mFc treatment induces the lung-resident memory T cells required for protection from IAV infection.
CD127, the receptor for IL-7 (IL-7Rα), is important for the survival and proliferation of T cells (24). Thus, we analyzed pulmonary lymphocyte populations from 0 to 35 days after intranasal IL-7–mFc administration to address whether IL-7–mFc affects pulmonary T cells. In fact, IL-7–mFc significantly increased the absolute number of CD127-expressing immune cells, including CD4, CD8, and γδ T cells and B cells (Table 1). This overall increase of pulmonary immune cells peaked at day 7 and gradually waned over time. An expansion of pulmonary immune cells in general almost disappeared at 14 days after IL-7–mFc treatment, although an increase of γδ T cells still remained until 21 days.
TABLE 1.
Kinetics of pulmonary immune cells after intranasal IL-7–mFc treatment
| Cells | Absolute cell numbers on day after IL-7–mFc treatmenta: |
|||||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | 35 | |
| Total CD4 T (×106) | 0.68 ± 0.07 | 0.76 ± 0.03 | 2.08 ± 0.28** | 0.89 ± 0.19 | 0.79 ± 0.14 | 0.70 ± 0.07 |
| CD62Llo CD44high CD4 T (×105) | 0.58 ± 0.08 | 1.44 ± 0.08** | 7.80 ± 1.24** | 1.61 ± 0.25** | 0.93 ± 0.05** | 0.69 ± 0.05 |
| Total CD8 T (×106) | 0.31 ± 0.07 | 0.32 ± 0.01 | 0.75 ± 0.11** | 0.42 ± 0.08 | 0.36 ± 0.08 | 0.28 ± 0.03 |
| CD62Llo CD44high CD8 T (×105) | 0.18 ± 0.03 | 0.29 ± 0.02* | 1.22 ± 0.14** | 0.50 ± 0.12* | 0.25 ± 0.03 | 0.26 ± 0.04 |
| γδ T (×105) | 0.24 ± 0.02 | 0.39 ± 0.01** | 4.71 ± 0.59** | 0.54 ± 0.16** | 0.40 ± 0.06* | 0.38 ± 0.02** |
| B (×106) | 0.84 ± 0.17 | 0.97 ± 0.03 | 2.27 ± 0.38* | 1.13 ± 0.10 | 0.83 ± 0.18 | ND |
| NK (×106) | 0.36 ± 0.03 | 0.58 ± 0.03* | 0.71 ± 0.07** | 0.39 ± 0.04 | 0.29 ± 0.05 | ND |
Mice received 1 μg of IL-7–mFc intranasally at 0, 3, 7, 14, and 21 days prior to sacrifice. Absolute numbers of immune cells in the total lung homogenates at each indicated time point were calculated based on the percentage of total cell number with flow cytometry. The results are representative of two independent experiments and are expressed as the mean ± SEM for four mice per group. ND, not determined. *, P < 0.05; **, P < 0.01 (by Student's t test, compared with cell numbers on day 0).
Of note, the numbers of CD4 and CD8 T cells displaying an effector/memory T cell phenotype (CD62Llow CD44high) were significantly increased at day 7 after IL-7–mFc treatment (Table 1). In addition, those CD4, but not CD8, T cells remained at the lung until day 21. Interestingly, those T cells express CD11a and CD49d (data not shown), both of which are known as lung-retentive markers for T cells (34). Furthermore, we conducted in vivo antibody labeling experiments to clarify that the pulmonary T cells are actually lung retentive (35). At 7 and 14 days after PBS or IL-7–mFc treatment, a fluorescence-conjugated antibody against CD3 was administered intravenously to mice 10 min before perfusion and harvest of the lung tissue. We found that intranasal treatment with IL-7–mFc could significantly increase the pulmonary CD4 and CD8 T cells that “protected” against in vivo antibody labeling (data not shown), indicating that treatment with IL-7–mFc increased lung-resident T cells. Interestingly, the number of “labeled” T cells returned to the basal level, while that of “protected” T cells still showed a significant increase at 14 days after IL-7–mFc treatment compared to control values. Moreover, the protected CD4 T cells after IL-7–mFc treatment preferentially exhibited an effector/memory phenotype, while both naive and effector/memory phenotype CD8 T cells were protected by antibody labeling (data not shown). Altogether, these characteristics of pulmonary T cells recruited by IL-7–mFc treatment are reminiscent of tissue-resident memory T (TRM) cells, albeit a polyclonal population. Thus, we here describe those cells as TRM-like cells. We also found that both CD4 and CD8 T cells were significantly increased in the mediastinal lymph nodes, but not in the spleen, at 7 days after IL-7–mFc treatment (data not shown). Interestingly, a retention of IL-7-primed T cells, in particular CD4 T cells, in the lung correlated with a prolonged protective activity of IL-7–mFc against a lethal IAV infection (Fig. 1D), which provides a possibility that TRM-like cells residing in the local pulmonary site may contribute to IL-7–mFc-mediated protection.
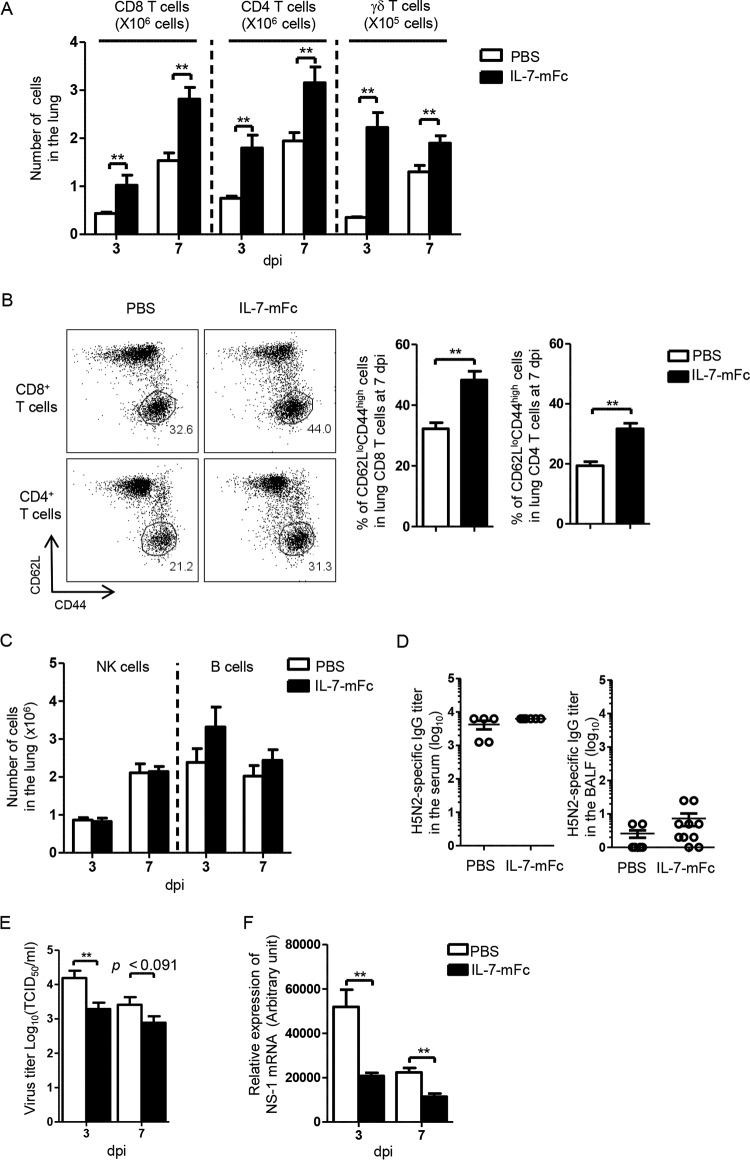
Analogously, the absolute numbers of pulmonary T cells, such as CD8, CD4, and γδ T cells, were increased after IAV infection by IL-7–mFc pretreatment compared to PBS treatment (Fig. 3A). Moreover, activated CD8 and CD4 T cells having an effector/memory phenotype were significantly increased in IL-7–mFc-treated mice (Fig. 3B). In contrast, there was no significant difference in the number of pulmonary NK cells (CD3− panNK+) between PBS- and IL-7–mFc-treated mice (Fig. 3C). Although pulmonary B cells were slightly increased by IL-7–mFc pretreatment, IAV-specific IgG production in the BALF and serum was not further upregulated (Fig. 3C and D). This indicates that NK cells and IAV-specific IgG might not be associated with IL-7–mFc-mediated protection from lethal IAV infection.
FIG 3.
Effect of intranasal IL-7–mFc pretreatment on pulmonary T cells and viral titer in the BALF after IAV infection. BALB/c mice were treated with IL-7–mFc intranasally and infected with 3 LD50 of H5N2 after 14 days. (A) Absolute numbers of T cells from total lung homogenates at 3 dpi and 7 dpi were measured. (B) Frequencies of the CD62Llow CD44high population of CD8 and CD4 T cells were analyzed at 7 dpi. (C) Absolute numbers of pulmonary B and NK cells were measured at 3 dpi and 7 dpi. (D) H5N2-specific IgG titers in the BALF and serum were analyzed at 7 dpi. (E and F) Virus titers (E) and relative expression of H5N2 NS-1 mRNA (normalized by housekeeping gene L32) in total lung homogenates (F) were analyzed at 3 dpi and 7 dpi. The bars indicate the mean ± SEM for 6 mice per group. Representative data from more than two independent experiments are shown. *, P < 0.05; **, P < 0.01 (by Student's t test). White bars, PBS control group; black bars, IL-7–mFc-treated group.
Next, we measured virus titers in the total lung homogenate to determine whether IL-7-primed pulmonary T cells are associated with clearance of virus. Viral titers in the total lung homogenates of the IL-7–mFc-treated group were decreased at both 3 and 7 dpi compared to those in the PBS control group, although statistical significance was observed at 3 dpi (Fig. 3E). In parallel, the expression level of H5N2 NS-1 mRNA was significantly reduced in the total lung homogenates of IL-7–mFc-treated mice (Fig. 3F). Collectively, these results demonstrate that IL-7–mFc pretreatment generates TRM-like cells in the lung, which subsequently accelerates pulmonary T-cell responses upon infection, leading to protection from IAV.
IL-7–mFc-primed mice show reduced pulmonary immunopathology after IAV infection.
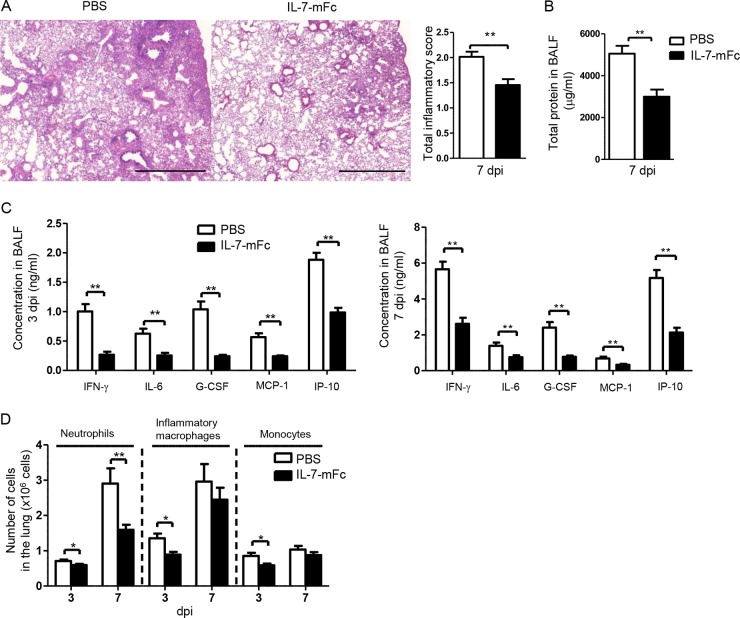
Because we observed a profound survival benefit from a single intranasal pretreatment of IL-7–mFc and with early control of viral infection, we next evaluated the pulmonary immunopathology induced by infection. The histopathological parameters for airway inflammation at 7 dpi were significantly reduced in the lungs of mice given IL-7–mFc pretreatment compared to PBS-treated mice (Fig. 4A). Accordingly, the total protein concentration in the BALF, which is one of the hallmarks of airway inflammation and tissue destruction, was also decreased in IL-7–mFc-treated mice (Fig. 4B).
FIG 4.
Effect of IL-7–mFc on IAV-induced pulmonary pathology. BALB/c mice were treated intranasally with IL-7–mFc and then infected with 3 LD50 of H5N2 after 14 days. (A) H&E staining of lung sections at a magnification of ×50 (left) and total inflammatory scores (right) at 7 dpi. Scale bars, 1 mm. (B) Total protein concentration in the BALF at 7 dpi. (C) Levels of inflammatory cytokines and chemokines in the BALF at 3 dpi and 7 dpi. (D) Numbers of neutrophils, monocytes, and macrophages analyzed by flow cytometry at 3 dpi and 7 dpi. The bars indicate the mean ± SEM for 8 mice per group. Representative data from more than three independent experiments are shown. *, P < 0.05; **, P < 0.01 (by Student's t test). H&E, hematoxylin and eosin. White bars, PBS control group; black bars, IL-7–mFc-treated group.
Because an excessive production of cytokines and chemokines after IAV infection correlates with severe pulmonary pathology following mortality (9, 36), we examined the levels of various inflammatory cytokines and chemokines in the BALF after infection. The levels of IFN-γ, IL-6, G-CSF, MCP-1, and IP-10 in the BALF were significantly reduced in IL-7–mFc-treated mice compared to PBS-treated controls at both 3 and 7 dpi (Fig. 4C). More importantly, the number of pulmonary neutrophils (Gr-1high Ly6clow CD11b+) was sharply decreased in IL-7–mFc-treated mice at 3 and 7 dpi (Fig. 4D). In addition, the numbers of monocytes (CD11b+ CD11c− Ly6c+) and inflammatory macrophages (CD11b+ CD11c+ MHCII+ Ly6c+ F4/80+) were also decreased at 3 dpi in IL-7–mFc-treated mice (Fig. 4D). These results agree well with a previous report that an increase in myeloid-lineage cells, including neutrophils, monocytes, and inflammatory macrophages, in the lung directly correlates with the severity of IAV-induced pathology (10). Of note, a dramatic decrease of neutrophils at 7 dpi in the IL-7–mFc-treated group likely contributes to reduced IAV-induced mortality (37). Thus, we focused on neutrophil infiltration as a main feature of IAV-induced pathology in further studies.
T cells are required for IL-7–mFc-mediated protection from IAV infection.
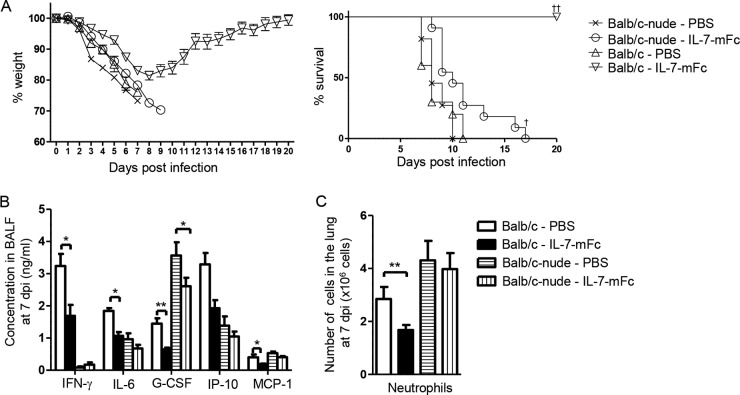
To investigate whether T cells directly contribute to reduced pulmonary pathology and subsequent protection from IAV infection by IL-7–mFc pretreatment, we used athymic nude (BALB/c-nude) mice. In an obvious contrast to wild-type (WT) mice, IL-7–mFc treatment in BALB/c-nude mice could not confer protection from lethal IAV infection, although it provided a slight increase in median survival for 2 days, compared to that in PBS-treated controls (Fig. 5A). In parallel, the levels of cytokines and chemokines in the BALF were not significantly changed by IL-7–mFc pretreatment in nude mice, except for G-CSF, albeit the expression scale of individual molecules in nude mice appeared somewhat different from that in WT mice, in particular for IFN-γ, which is a typical T cell-mediated cytokine (Fig. 5B). Consistently, IL-7–mFc pretreatment did not reduce the number of pulmonary neutrophils in nude mice upon infection (Fig. 5C). Together, these results suggest that T cells are required for IL-7–mFc-mediated protection against IAV infection and that pulmonary T cells stimulated with IL-7–mFc seem to reduce the severity of immunopathology.
FIG 5.
Effect of intranasal IL-7–mFc pretreatment on protection against IAV infection in BALB/c-nude mice. BALB/c-nude and WT BALB/c mice were treated with PBS or IL-7–mFc and infected with 3 LD50 of H5N2. (A) Weight loss and survival. Average body weight loss is shown as the mean ± SEM for 10 mice per group, and the survival rate was analyzed by the Kaplan-Meier method. The data are representative of two independent experiments. †, P < 0.05; ††, P < 0.01 (by log rank test). (B) The levels of inflammatory cytokines and chemokines in the BALF were analyzed by ELISA. (C) The numbers of pulmonary neutrophils were analyzed at 7 dpi by flow cytometry. The results are expressed as the mean ± SEM for 8 mice per group. The data are representative of two independent experiments with similar results. *, P < 0.05; **, P < 0.01 (by Student's t test). White bars, PBS-treated WT mice; black bars, IL-7–mFc-treated WT mice; white bars with horizontal lines, PBS-treated BALB/c-nude mice; white bars with vertical lines, IL-7–mFc-treated BALB/c-nude mice.
CD4 TRM-like cells are essential for the protective function of IL-7–mFc against IAV infection.
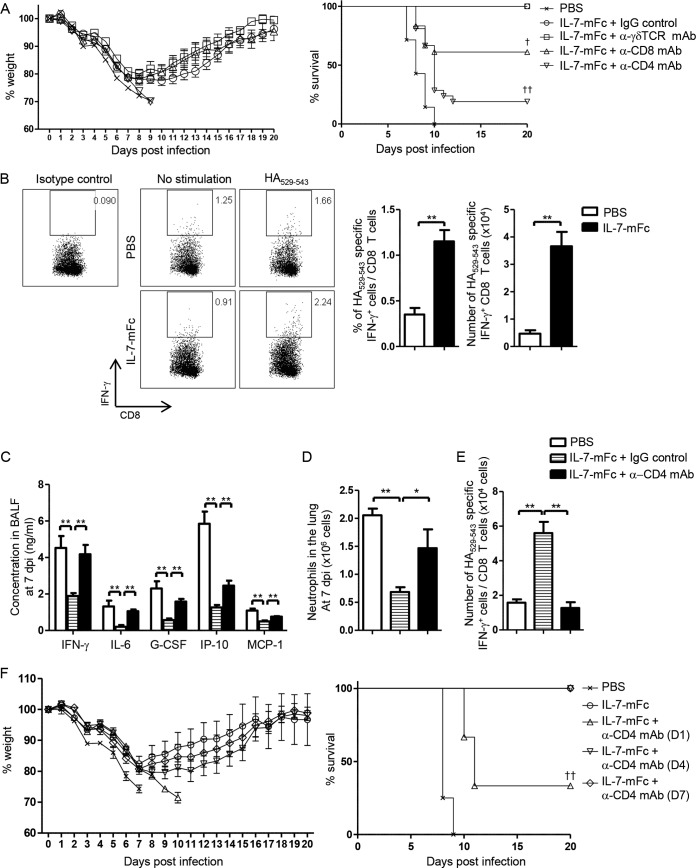
To further determine the role of specific T cell subsets in IL-7–mFc-mediated protection against IAV infection, depletion antibodies for CD4, CD8, and γδ T cells were used (Fig. 6A). While depleting γδ T cells with an anti-TCRγδ (Vγ2, GV3S1) MAb had no impact on protection, treatment with anti-CD8 MAb significantly impeded IL-7–mFc-mediated protection (61% of mice survived at 20 dpi). Indeed, we observed a significant increase of IAV-specific CD8 T cells, measured by IFN-γ production upon stimulation with HA529–543 peptide, in the lungs of IL-7–mFc-treated mice (Fig. 6B). Since virus-specific CD8 T cells play an essential role in protection from IAV infection (38), our data suggest that IAV-specific pulmonary CD8 T cells, augmented by IL-7–mFc pretreatment, contribute to protection by clearance of IAV to some extent. More importantly, depleting CD4 T cells with an anti-CD4 MAb markedly impaired the protective activity of IL-7–mFc (22% of mice survived at 20 dpi) compared to depleting CD8 T cells, with the differences being almost statistically significant (P = 0.0575 by the log rank test) (Fig. 6A). We confirmed that treatment with depletion antibodies against CD4 and CD8 resulted in nearly complete depletion of pulmonary T cells (data not shown). Again, decreases of inflammatory mediators and pulmonary neutrophils caused by IL-7–mFc were nullified by anti-CD4 treatment (Fig. 6C and D). Furthermore, the enhanced number of IAV-specific CD8 T cells induced by IL-7–mFc returned to the basal level after anti-CD4 treatment (Fig. 6E), suggesting a CD4 T cell help for CD8 T cell responses in the lung. Notably, a single treatment with anti-CD4 at 1 dpi abolished the protective activity of IL-7–mFc, while treating with anti-CD4 at later time points, such as 4 dpi and 7 dpi, showed no effects on protection (Fig. 6F). Together, these data suggest that lung-retentive CD4 TRM-like cells play a main role in the IL-7–mFc-mediated protective function against IAV infection by modulation of IAV-induced immunopathology as well as augmenting CD8 T cell responses and that IL-7-primed CD4 TRM-like cells exert their immune-modulatory roles at an early stage of IAV infection.
FIG 6.
Involvement of CD4 T cells in IL-7–mFc-mediated regulation of immunopathology after lethal IAV infection. (A) Mice (BALB/c, n = 18) were treated with IL-7–mFc and infected with 3 LD50 of H5N2 after 14 days. Then, 200 μg of anti-CD4, anti-CD8, anti-TCRγδ, or isotype MAb was injected i.p. four times at −1 dpi, 0 dpi, 1 dpi, and 4 dpi. Weight loss and survival curves were analyzed by combining two independent experiment with 9 mice per group. (B) Intracellular cytokine staining for HA529–543-specific IFN-γ production of CD8 T cells from total lung homogenates was analyzed at 7 dpi. HA-specific IFN-γ production was calculated as the difference in IFN-γ+ CD8 T cells between groups with HA529–543 and no stimulation. Representative dot plots (left) and summary graphs (right) are shown. **, P < 0.01 by Student's t test. White bars, PBS control; black bars, IL-7–mFc-treated group. (C to E) Levels of inflammatory cytokines and chemokines in BALF (C), number of pulmonary neutrophils (D), and HA529–543-specific IFN-γ production by CD8 T cells (E) in mice treated with IL-7–mFc after administration of anti-CD4 and IgG control at 7 dpi. The results are representative of three independent experiments and are expressed as the mean ± SEM for 8 mice per group. **, P < 0.01 by one-way ANOVA with Bonferroni's posttest. White bars, IL-7–mFc with IgG control; white bars with horizontal lines, IL-7–mFc with IgG control; black bars, IL-7–mFc with anti-CD4 MAb treatment. (F) Mice (BALB/c, n = 6 per group) were intranasally treated with IL-7–mFc and infected with a lethal dose of H5N2 after 14 days. Mice were treated with anti-CD4 MAb at 1 day, 4 days, or 7 days after IAV infection. Weight loss and survival rates are shown. The data are representative of two independent experiments. ††, P < 0.01 by log rank test.
DISCUSSION
In this study, we demonstrated that intranasal introduction of IL-7–mFc into naive mice modulates immune environments of the airway mucosa, which protects them from a lethal infection with IAV. IL-7–mFc, prior to infection, induces a prolonged maintenance of TRM-like cells in the lung, which are subsequently responsible for reducing viral replication and enhancing antiviral T cell responses postinfection, leading to reduction of immunopathology and mortality from IAV. Our data provide a novel way of immune modulation in the lung, suggesting intranasal delivery of IL-7–mFc as a prophylaxis against highly pathogenic IAV infection.
The recruitment and residency of pulmonary T cells by IL-7–mFc, in particular CD4 T cells, play an essential role in the long-lasting protection against IAV. In naive mice before IAV infection, intranasal treatment with IL-7–mFc increased pulmonary T cells displaying phenotypic attributes of naive (CD62Lhigh CD44low) or effector/memory (CD62Llow CD44high) T cells. Although both cell types were increased in the lung by IL-7–mFc, the extent of expansion and long-term maintenance was more prominent in effector/memory phenotype T cells, which we designate TRM-like cells in this study. It is unlikely that IL-7–mFc preferentially affects TRM-like cells, since previous reports have shown that both naive and memory T cells express CD127, with their proliferative capacities by IL-7 being comparable (39, 40). Since we found that TRM-like cells, but not naïve cells, exhibited expression of CD11a and CD49d and protected against in vivo antibody labeling (data not shown), it is tempting to speculate that both naive and effector/memory phenotype populations are expanded and recruited by IL-7–mFc treatment, but that only effector/memory phenotype cells may reside as TRM-like cells in the lung for an extended period of time by elevated cell adhesion molecules, while naive T cells simply pass by the lung within the microvasculature, returning to circulation. Longer pulmonary residency of CD4 TRM-like cells than of CD8 cells is also notable, albeit the expression levels of lung-retentive markers in both cell types appeared to be comparable. Given that the protective function of IL-7–mFc against IAV was observed only with intranasal delivery, these changes of T cell composition in the local immune environment at the airway mucosa seem to be critical for its prophylactic effects.
Preoccupied pulmonary TRM-like cells after IL-7–mFc treatment, although not antigen specific, help IAV-specific T cells thrive upon infection. An increase of effector CD8 T cells in the lung was prominent postinfection to clear viruses, and furthermore, preexisting CD4 TRM-like cells appeared to be essential for expansion of newly generated IAV-specific CD8 cells. Those expanded antigen-specific CTLs may contribute to directly control viral clearance in the end, as shown previously (41). Indeed, the number of IAV-specific CTLs was reversely correlated with all attributes of IAV-induced pulmonary pathology at 7 dpi, such as airway inflammation, production of inflammatory mediators, and infiltration of neutrophils. However, depletion of CD8 cells displayed less profound effects on protection from IAV than that of CD4 cells. Therefore, both CD4 and CD8 T cells are necessary for the IL-7–mFc-mediated prophylaxis, but CD4 T cells play a dominant role. It is likely that antigen-specific T cells are not responsible for early control of viral replication at 3 dpi, since we failed to observe IAV-specific CD8 T cells in the lung at this time point (data not shown). Rather, we speculated that the IL-7-primed TRM-like cells, in particular CD4 cells, played an important role in primary control of viruses during the early stage of IAV infection. This might explain a correlation between preoccupancy of pulmonary CD4 TRM-like cells achieved by IL-7–mFc treatment before infection and a prolonged survival benefit against lethal IAV postinfection.
It is well known that IAV-specific memory CD4 T cells contribute to develop protective immune responses against respiratory viral infection (42). On the one hand, typical secondary recall responses to antigens by memory CD4 T cells in the lung participate in direct viral clearance (43). On the other hand, memory CD4 T cells have been shown to induce innate immune responses, producing inflammatory cytokines and chemokines in the lung, for early control of viral replication (22, 44). Cognate viral antigen was required for those innate-like functions of memory CD4 T cells in one study, but antigen independency was also observed in the other. In this regard, we speculate that pulmonary CD4 TRM-like cells induced by IL-7–mFc may also direct innate responses for early control of IAV in our study. Furthermore, those CD4 TRM-like cells are a naturally occurring polyclonal population, but not IAV specific, since IL-7–mFc was introduced prior to IAV challenge. Thus, IL-7-primed TRM-like cells would be different from the IAV-specific TRM cells described in previous studies, but they might share some functional characteristics. This suggests that cognate antigen may be dispensable for their innate functions, while bystander activation of polyclonal CD4 TRM-like cells via IAV infection can play a role. One possible limitation of our study is that depletion with antibodies to T cells to investigate the role of TRM-like cells could also abrogate the normal primary T cell responses against IAV, which unarguably contribute to protection. Thus, an ideal experimental condition should be abrogating T cell compartments during the period after posttreatment with IL-7–mFc and prior to IAV challenge to dissect the relative contribution of IL-7-primed TRM-like cells more specifically. However, the recovery of the normal CD4 T cell compartment after depletion with antibody needs approximately 100 days (45). Therefore, it would be technically difficult to achieve a removal of T cells within a specific window of time by using depletion antibodies. However, developing alternative methods for proving the relative contribution of preoccupied IL-7-primed TRM-like cells with intact primary T cell responses will be valuable for future study.
A full prophylactic effect of IL-7–mFc against IAV is achieved when it is introduced 1 or 2 weeks a prior to infection. Since we infused IL-7–mFc in the lung in otherwise unprimed naive mice, it may take time for IL-7–mFc to alter the immune environment in the lung by recruiting and modulating pulmonary T cells, which subsequently exert protective functions postinfection. Further, IL-7–mFc treatment along with IAV infection simultaneously did not confer protection (Fig. 1D), raising an issue for using it as a therapeutic modality. However, humans likely have lung-resident T cells in the airway mucosa which are generated by previous episodes of vaccination and infection, including with seasonal influenza viruses (20). Therefore, further study is needed to address the therapeutic potential of IL-7–mFc against IAV infection in mice having preexisting pulmonary T cells that are specific to homologous or heterologous viruses or even environmental antigens. In this study, we fused IL-7 to the murine Fc portion, which confers an increased half-life in vivo through FcRn-mediated recycling (46). Moreover, expression of FcRn in the lung epithelium enhances transcytosis of IL-7–mFc (47), which is indispensable for immune modulation in the lung. Thus, this approach with Fc fusion to immune-modulatory molecules would be promising for immune regulation at the mucosal tissues in which FcRn is expressed.
Collectively, intranasal treatment of IL-7–mFc could be a feasible and promising prophylaxis for reducing influenza-induced mortality in future pandemics and potentially could be used as a mucosal adjuvant for IAV vaccines.
ACKNOWLEDGMENTS
We thank Young Ki Choi (Chungbuk National University of Medicine, Republic of Korea) for providing influenza virus and Man Ki Song for organizing the challenge experiment at the International Vaccine institute.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Tumpey TM, Belser JA. 2009. Resurrected pandemic influenza viruses. Annu Rev Microbiol 63:79–98. doi: 10.1146/annurev.micro.091208.073359. [DOI] [PubMed] [Google Scholar]
- 2.Cowling BJ, Jin L, Lau EH, Liao Q, Wu P, Jiang H, Tsang TK, Zheng J, Fang VJ, Chang Z, Ni MY, Zhang Q, Ip DK, Yu J, Li Y, Wang L, Tu W, Meng L, Wu JT, Luo H, Li Q, Shu Y, Li Z, Feng Z, Yang W, Wang Y, Leung GM, Yu H. 2013. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet 382:129–137. doi: 10.1016/S0140-6736(13)61171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubenberger JK, Morens DM. 2010. Influenza: the once and future pandemic. Public Health Rep 125(Suppl 3):S16–S26. [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer SC, Bansal S. 2015. Assessing the use of antiviral treatment to control influenza. Epidemiol Infect 143:1621–1631. doi: 10.1017/S0950268814002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunning J, Baillie JK, Cao B, Hayden FG. 2014. Antiviral combinations for severe influenza. Lancet Infect Dis 14:1259–1270. doi: 10.1016/S1473-3099(14)70821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurt AC, Holien JK, Parker M, Kelso A, Barr IG. 2009. Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J Virol 83:10366–10373. doi: 10.1128/JVI.01200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damjanovic D, Small CL, Jeyanathan M, McCormick S, Xing Z. 2012. Immunopathology in influenza virus infection: uncoupling the friend from foe. Clin Immunol 144:57–69. doi: 10.1016/j.clim.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Kuiken T, Riteau B, Fouchier RA, Rimmelzwaan GF. 2012. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol 2:276–286. doi: 10.1016/j.coviro.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 9.To KK, Hung IF, Li IW, Lee KL, Koo CK, Yan WW, Liu R, Ho KY, Chu KH, Watt CL, Luk WK, Lai KY, Chow FL, Mok T, Buckley T, Chan JF, Wong SS, Zheng B, Chen H, Lau CC, Tse H, Cheng VC, Chan KH, Yuen KY. 2010. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis 50:850–859. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. 2008. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog 4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, Chen WH, Ernst RK, Rossignol DP, Gusovsky F, Blanco JC, Vogel SN. 2013. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, Kawaoka Y, Rosen H, Oldstone MB. 2009. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci U S A 106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulombe F, Jaworska J, Verway M, Tzelepis F, Massoud A, Gillard J, Wong G, Kobinger G, Xing Z, Couture C, Joubert P, Fritz JH, Powell WS, Divangahi M. 2014. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity 40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Darwish I, Mubareka S, Liles WC. 2011. Immunomodulatory therapy for severe influenza. Expert Rev Anti Infect Ther 9:807–822. doi: 10.1586/eri.11.56. [DOI] [PubMed] [Google Scholar]
- 15.Maines TR, Szretter KJ, Perrone L, Belser JA, Bright RA, Zeng H, Tumpey TM, Katz JM. 2008. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol Rev 225:68–84. doi: 10.1111/j.1600-065X.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 16.Ji H, Gu Q, Chen LL, Xu K, Ling X, Bao CJ, Tang FY, Qi X, Wu YQ, Ai J, Shen GY, Dong DJ, Yu HY, Huang M, Cao Q, Xu Y, Zhao W, Xu YT, Xia Y, Chen SH, Yang GL, Gu CL, Xie GX, Zhu YF, Zhu FC, Zhou MH. 2014. Epidemiological and clinical characteristics and risk factors for death of patients with avian influenza A H7N9 virus infection from Jiangsu Province, Eastern China. PLoS One 9:e89581. doi: 10.1371/journal.pone.0089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TS, Sun J, Braciale TJ. 2011. T cell responses during influenza infection: getting and keeping control. Trends Immunol 32:225–231. doi: 10.1016/j.it.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. 2011. Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, Staples KJ, Dong T, Douek DC, McMichael AJ, Xu XN. 2012. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 21.McMaster SR, Gabbard JD, Koutsonanos DG, Compans RW, Tripp RA, Tompkins SM, Kohlmeier JE. 2015. Memory T cells generated by prior exposure to influenza cross react with the novel H7N9 influenza virus and confer protective heterosubtypic immunity. PLoS One 10:e0115725. doi: 10.1371/journal.pone.0115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. 2010. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med 16:558–564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swain SL, McKinstry KK, Strutt TM. 2012. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol 12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackall CL, Fry TJ, Gress RE. 2011. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SH, Song MY, Nam HJ, Im SJ, Sung YC. 2010. Codelivery of IL-7 augments multigenic HCV DNA vaccine-induced antibody as well as broad T cell responses in cynomolgus monkeys. Immune Netw 10:198–205. doi: 10.4110/in.2010.10.6.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam HJ, Song MY, Choi DH, Yang SH, Jin HT, Sung YC. 2010. Marked enhancement of antigen-specific T-cell responses by IL-7-fused nonlytic, but not lytic, Fc as a genetic adjuvant. Eur J Immunol 40:351–358. doi: 10.1002/eji.200939271. [DOI] [PubMed] [Google Scholar]
- 27.Seo YB, Im SJ, Namkoong H, Kim SW, Choi YW, Kang MC, Lim HS, Jin HT, Yang SH, Cho ML, Kim YM, Lee SW, Choi YK, Surh CD, Sung YC. 2014. Crucial roles of interleukin-7 in the development of T follicular helper cells and in the induction of humoral immunity. J Virol 88:8998–9009. doi: 10.1128/JVI.00534-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim ES, Jang do S, Yang SY, Lee MN, Jin KS, Cha HJ, Kim JK, Sung YC, Choi KY. 2013. Controlled release of human growth hormone fused with a human hybrid Fc fragment through a nanoporous polymer membrane. Nanoscale 5:4262–4269. doi: 10.1039/c3nr00474k. [DOI] [PubMed] [Google Scholar]
- 29.Song MS, Pascua PN, Lee JH, Baek YH, Lee OJ, Kim CJ, Kim H, Webby RJ, Webster RG, Choi YK. 2009. The polymerase acidic protein gene of influenza A virus contributes to pathogenicity in a mouse model. J Virol 83:12325–12335. doi: 10.1128/JVI.01373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim EH, Lee JH, Pascua PN, Song MS, Baek YH, Kwon HI, Park SJ, Lim GJ, Decano A, Chowdhury MY, Seo SK, Song MK, Kim CJ, Choi YK. 2013. Prokaryote-expressed M2e protein improves H9N2 influenza vaccine efficacy and protection against lethal influenza A virus in mice. Virol J 10:104. doi: 10.1186/1743-422X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nature Protoc 9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JP, Kim YS, Tae YM, Choi EJ, Hong BS, Jeon SG, Gho YS, Zhu Z, Kim YK. 2010. A viral PAMP double-stranded RNA induces allergen-specific Th17 cell response in the airways which is dependent on VEGF and IL-6. Allergy 65:1322–1330. doi: 10.1111/j.1398-9995.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 33.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. 2011. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol 29:158–163. doi: 10.1038/nbt.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodland DL, Kohlmeier JE. 2009. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol 9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 35.Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. 2014. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutigliano JA, Sharma S, Morris MY, Oguin TH 3rd, McClaren JL, Doherty PC, Thomas PG. 2014. Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells. J Virol 88:1636–1651. doi: 10.1128/JVI.02851-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandes M, Klauschen F, Kuchen S, Germain RN. 2013. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell 154:197–212. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Gruta NL, Turner SJ. 2014. T cell mediated immunity to influenza: mechanisms of viral control. Trends Immunol 35:396–402. doi: 10.1016/j.it.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. 2003. Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J Immunol 171:61–68. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Geginat J, Lanzavecchia A, Sallusto F. 2003. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 41.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. 2006. Cell-mediated protection in influenza infection. Emerg Infect Dis 12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner DL, Farber DL. 2014. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol 5:331. doi: 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. 2012. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J Clin Invest 122:2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. 2010. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol 84:9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice JC, Bucy RP. 1995. Differences in the degree of depletion, rate of recovery, and the preferential elimination of naive CD4+ T cells by anti-CD4 monoclonal antibody (GK1.5) in young and aged mice. J Immunol 154:6644–6654. [PubMed] [Google Scholar]
- 46.Czajkowsky DM, Hu J, Shao Z, Pleass RJ. 2012. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 4:1015–1028. doi: 10.1002/emmm.201201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumont JA, Bitonti AJ, Clark D, Evans S, Pickford M, Newman SP. 2005. Delivery of an erythropoietin-Fc fusion protein by inhalation in humans through an immunoglobulin transport pathway. J Aerosol Med 18:294–303. doi: 10.1089/jam.2005.18.294. [DOI] [PubMed] [Google Scholar]