ABSTRACT
Seasonal influenza virus infections continue to cause significant disease each year, and there is a constant threat of the emergence of reassortant influenza strains causing a new pandemic. Available influenza vaccines are variably effective each season, are of limited scope at protecting against viruses that have undergone significant antigenic drift, and offer low protection against newly emergent pandemic strains. “Universal” influenza vaccine strategies that focus on the development of humoral immunity directed against the stalk domains of the viral hemagglutinin (HA) show promise for protecting against diverse influenza viruses. Here, we describe such a strategy that utilizes vesicular stomatitis virus (VSV) as a vector for chimeric hemagglutinin (cHA) antigens. This vaccination strategy is effective at generating HA stalk-specific, broadly cross-reactive serum antibodies by both intramuscular and intranasal routes of vaccination. We show that prime-boost vaccination strategies provide protection against both lethal homologous and heterosubtypic influenza challenge and that protection is significantly improved with intranasal vaccine administration. Additionally, we show that vaccination with VSV-cHAs generates greater stalk-specific and cross-reactive serum antibodies than does vaccination with VSV-vectored full-length HAs, confirming that cHA-based vaccination strategies are superior at generating stalk-specific humoral immunity. VSV-vectored influenza vaccines that express chimeric hemagglutinin antigens offer a novel means for protecting against widely diverging influenza viruses.
IMPORTANCE Universal influenza vaccination strategies should be capable of protecting against a wide array of influenza viruses, and we have developed such an approach utilizing a single viral vector system. The potent antibody responses that these vaccines generate are shown to protect mice against lethal influenza challenges with highly divergent viruses. Notably, intranasal vaccination offers significantly better protection than intramuscular vaccination in a lethal virus challenge model. The results described in this study offer insights into the mechanisms by which chimeric hemagglutinin (HA)-based vaccines confer immunity, namely, that the invariant stalk of cHA antigens is superior to full-length HA antigens at inducing cross-reactive humoral immune responses and that VSV-cHA vaccine-induced protection varies by site of inoculation, and contribute to the further development of universal influenza virus vaccines.
INTRODUCTION
On average, there are more than 200,000 hospitalizations due to influenza-associated complications each year in the United States (1), despite the widespread use of seasonal influenza vaccines. Many of these hospitalized patients had received the seasonal vaccine and yet developed disease (2). Recent meta-analysis suggests that the effectiveness of seasonal influenza vaccines is ca. 69% (3), although individual studies report much lower effectiveness for a given influenza season (3). This is in contrast to the effectiveness of other commonly utilized vaccines, including measles (93 to 97%), rotavirus (>85%), and hepatitis B virus (>90%) (4–6).
There are many explanations for the relatively low effectiveness of the seasonal influenza vaccine. Vaccine-induced immunity is largely determined by the humoral immune responses against the influenza virus hemagglutinin (HA) surface glycoprotein. The HA protein consists of two spatially and functionally distinct regions: the globular head domain, responsible for attachment to host sialic acid receptors, and the stalk domain, required for pH-dependent membrane fusion and viral entry into host cells. Current seasonal influenza vaccines elicit robust immune responses to the globular head domain, thus making it the “immunodominant” region of influenza HA. The immune pressure against the head domain of the HA, combined with ability of the HA head to tolerate mutations, results in the rapid accumulation influenza virus variants that subvert vaccine-induced immunity. In addition, certain populations, including the very young and the elderly, develop low effective immune responses to influenza vaccination, making them more vulnerable to disease (7, 8). Furthermore, newly emergent influenza viruses, which are the result of reassortment events in animal reservoirs, can cause pandemics at irregular intervals, and seasonal vaccines are not protective against these antigenic shift strains (9).
Efforts to generate “universal” influenza vaccines that induce broadly cross-reactive immune responses offer the prospect of eliminating the requirement for yearly revaccination. Annual influenza virus vaccination is burdensome for health care providers and vaccinees, and multiple strategies have been developed in the pursuit of a universal influenza vaccine. One of the most promising of these strategies uses sequential vaccination with chimeric hemagglutinins (cHAs). cHAs consist of highly divergent HA globular head domains from different subtypes positioned on top of a conserved HA stalk domain (10, 11). The goal of this strategy is to redirect the immune response toward the HA stalk domain and to create antibody responses that are reactive across divergent HAs (11–14). Due to the highly conserved nature of the HA stalk region among HAs within either group 1 or group 2, this approach has generated promising stalk-specific immune responses in animals protecting against a wide array of influenza viruses (12–16).
Previous studies have examined responses to vaccination with adjuvanted cHA proteins (15), cHA DNA and protein vaccination (12, 14, 16), and mixed approaches combining different viral vectors expressing cHAs (13). In order to create a vaccination strategy that exploits the advantages of live viral vectors, while at the same time limiting exposure to a single viral vector system, vesicular stomatitis virus (VSV) vectors with different vesiculovirus envelope proteins (17) have been generated that can be used in prime-boost vaccination. We have previously shown that VSV vectors can express influenza HA molecules and that these VSV-HA constructs protect against challenge with homologous influenza viruses (18–20). Here, we show that VSV-cHA vaccines administered by different routes (intramuscular [i.m.] and intranasal [i.n.]) generate humoral responses against a variety of group 1 HAs and that these responses are largely targeted against conserved regions of the stalk portion of the HA glycoprotein. Furthermore, these vaccines provide broad protection against challenge with both homologous (H1N1 and PR8) and heterosubtypic (H5N1 and HALo [21]) influenza virus challenge strains. Interestingly, while we note only minor differences between routes of vaccination in their ability to generate stalk-specific and cross-reactive antibodies in serum, we see marked differences in the ability of VSV-cHA vaccines to protect against challenge depending on the route of vaccination. Mucosal (i.n.) vaccination provides superior protection compared to i.m. vaccination. Our results have important implications for future development of universal influenza vaccine strategies.
MATERIALS AND METHODS
Recombinant virus recovery.
Recombinant VSV-cHAs and VSV-HAs were recovered as described previously (22, 23). In brief, BHK-21 cells were infected with vTF-7.3 (24), using a multiplicity of infection of 10. The cells were transfected with pVSV-cH5/1, pVSV-cH9/1, pVSV-cH6/1, pVSV-H1HA, pVSV-H5, pVSV-H9, or pVSV-H6, together with support plasmids pBS-N, pBS-P, pBS-L, and pBS-G encoding VSV proteins. The cell supernatants were collected at 48 h and passaged onto BHK-21 cells. Supernatant containing the virus was collected after 48 h. Virus was plaque purified on BHK-21 cells and further passaged on BHK-21 cells to generate viral stocks. The stock was serially diluted and plaqued on BHK-21 cells. Plaques were stained after 48 h with crystal violet. Three different VSV G proteins were utilized for the construction of these vectored vaccines in order to allow for boosting vaccination (17). VSV G, serotype Indiana (VSV GI), was used in combination with cH5/1, H5 and H1. VSV G, serotype New Jersey (VSV GNJ), was used in combination with cH9/1 and H9. VSV G from Chandipura virus (VSV GChan), was used in combination with cH6/1 and H6.
Animal vaccination and challenge experiments.
Six- to eight-week-old BALB/c mice were obtained from Charles River Laboratories, Wilmington, MA, and housed for 1 week prior to immunization. Animals were housed in microisolator cages. Live recombinant VSV vaccine constructs were diluted in serum-free Dulbecco modified Eagle medium for immunizations. Intranasal immunizations were performed with either VSV-cHAs, VSV-HAs, or control VSV viruses (2 × 105 PFU) administered in a volume of 50 μl to animals that were lightly anesthetized with 20% isoflurane (Baxter) diluted in propylene glycol (vol/vol). For i.m. immunizations, VSV-cHAs, VSV-HAs, or control VSV viruses (4.5 × 105 PFU) were injected in a volume of 50 μl into the left hind leg muscle. Blood was collected for enzyme-linked immunosorbent assays (ELISAs) from the retro-orbital sinus.
Influenza virus challenges were performed with either a homologous mouse-adapted H1N1 influenza virus (PR8, A/Puerto Rico/8/34) or a low-pathogenicity H5N1 HALo influenza virus (6:2 reassortant influenza virus containing internal gene segments from A/Puerto Rico/8/34 and HA and NA from A/Vietnam/1203/04 from which the polybasic cleavage site in the HA was removed [21]). Mice were anesthetized as before, with 20% isoflurane (Baxter), and the challenge virus was administered in a volume of 50 μl. The Yale University Institutional Animal Care and Use Committee approved all immunization and challenge experiments (Yale University Protocol permit 2012-07680), and experiments were performed in accordance with the regulations of Yale University.
ELISAs.
Flat-bottom Immuno nonsterile 96-well plates 4 HBX (Thermo Scientific) were coated with 50 μl of recombinant protein (25) diluted in ELISA coating buffer (pH 9.4) at a concentration of 2 μg/ml per well and refrigerated at 4°C overnight. Coating buffer was discarded, and the wells were blocked for 1 h at room temperature with 100 μl of blocking solution (phosphate-buffered saline containing 0.1% Tween 20 [T-PBS], 3% goat serum [Gibco], and 0.5% milk powder). Another 50 μl of blocking solution was added to the first column of wells, as well as 1.5 μl of mouse serum (starting concentration of 1:100). The samples were 3-fold serially diluted and incubated at room temperature for 2 h. The plates were washed six times with T-PBS, and 50 μl of blocking solution containing anti-mouse IgG (Fab specific)-peroxidase antibody (Sigma) at a concentration of 1:3,000 was added. After 1 h of incubation at room temperature, the plates were washed six times with T-PBS and developed with 100 μl of SigmaFast OPD (Sigma) per well. The developing process was stopped after 10 min with 3 M hydrochloric acid (HCl) and read at an absorbance of 490 nm with a Synergy H1 Hybrid Multi-Mode microplate reader (BioTek). The average plus 3 times the standard deviation of all blanks was calculated and used as cutoff for endpoint titer analysis.
Statistical analysis.
Data were compiled and graphs created using Prism software (GraphPad Software, San Diego, CA). For weight loss graphs, error bars include the 95% confidence interval (CI). Statistical significance between curves was determined using a one-way ANOVA with Tukey's multiple-comparison test. For survival curves, significance was determined using the log-rank (Mantel-Cox) test.
RESULTS
Sequential vaccination with VSV-cHAs results in antibody responses directed against the HA stalk.
Previous studies have shown that the vaccination of mice (12, 14–16) and ferrets (13) with cHA constructs results in the production of stalk-specific antibodies that are broadly cross-reactive across multiple HAs within the same phylogenetic group. These strategies relied on multiple boosting vaccinations with either DNA and/or protein with potent adjuvants (12, 14–16), or a combination of DNA and multiple different virally vectored cHAs (13). Here, we determined whether a prime-boost vaccination strategy utilizing a single viral vector system expressing different cHAs could produce potent stalk-specific, cross-reactive responses to group 1 HAs. Vaccine constructs consisting of different VSV-cHA combinations were recovered, as previously described (22, 23), and used to inoculate BALB/c mice. These constructs all had the same VSV Indiana serotype genome but the G protein genes were derived either from the Indiana serotype (GI) or the New Jersey serotype (GNJ) (17).
Groups of BALB/c mice were vaccinated either i.m. or i.n. with VSV(GI)-cH9/1 and then boosted 1 month later with VSV(GNJ)-cH5/1 (Fig. 1A) or immunized initially with VSV(GI)-H1 and then boosted 1 month later with VSV(GNJ)-cH9/1 (Fig. 2A). Control groups of mice primed and boosted with the same vectors expressing irrelevant antigens were also included. To determine whether boosting with cHA was necessary to achieve an increase in antibody titer, an additional group of mice was primed with VSV(GI)-cH9/1 and then boosted with an irrelevant VSV control virus. Boosting was performed by the same route as the initial prime. The rationale for including a group of mice immunized with VSV-H1 in place of VSV-cH5/1 was to avoid the induction of H5 head-specific antibodies in anticipation of challenge with a heterosubtypic H5N1 influenza virus.
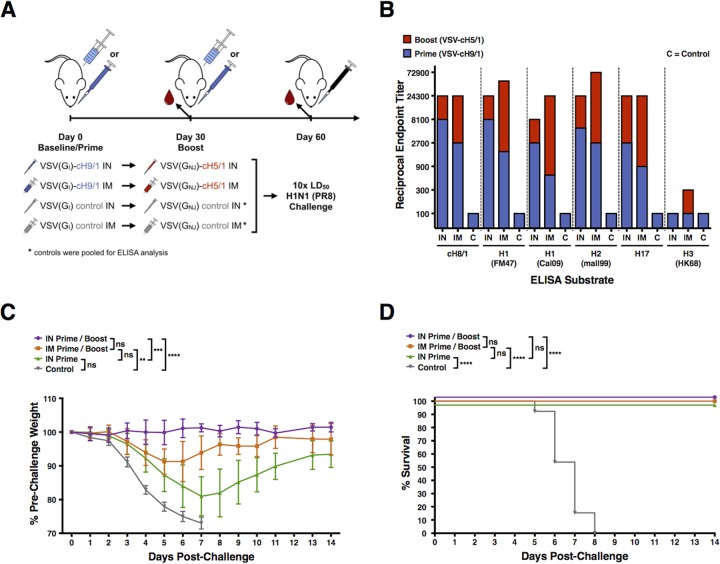
FIG 1.
VSV-cH9/1 and VSV-cH5/1 vaccination and H1N1 challenge experiment. (A) Schematic of VSV-cHA prime-boost-challenge vaccination experiments: i.m. and i.n. vaccinations, PR8 homologous influenza challenge. Intranasal vaccinations were performed with 2 × 105 PFU, and i.m. vaccinations were performed with 4.5 × 105 PFU. (B) Postprime (VSV-cH9/1) and postboost (VSV-cH5/1) sera were tested in ELISAs against a panel of HA substrates, which are listed on the x axis. Blue bars represent the reciprocal endpoint ELISA titer of pooled postprime serums, and red bars represent the total reciprocal endpoint ELISA titer after prime and boosting vaccination. Intranasal vaccination results in greater postprime titers. The overall serum antibody titers postboost are similar between i.m.- and i.n.-vaccinated animals. (C) Vaccinated mice were challenged i.n. 1 month postboost with 10 LD50 of homologous H1N1 (PR8) influenza virus from which the stalk of the chimeric HA antigens was derived. Weights were recorded daily for 14 days postchallenge and are graphically displayed as the mean percentage of the prevaccination weight. Error bars represent the 95% CI of the mean weight. All control mice died or required euthanasia per protocol by day 7 postchallenge. Significant differences between groups are indicated in the graph. (D) Kaplan-Meier survival curves. Significant differences between groups, identified by a log-rank (Mantel-Cox) test, are indicated on the graph. ns, not significant; *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001.
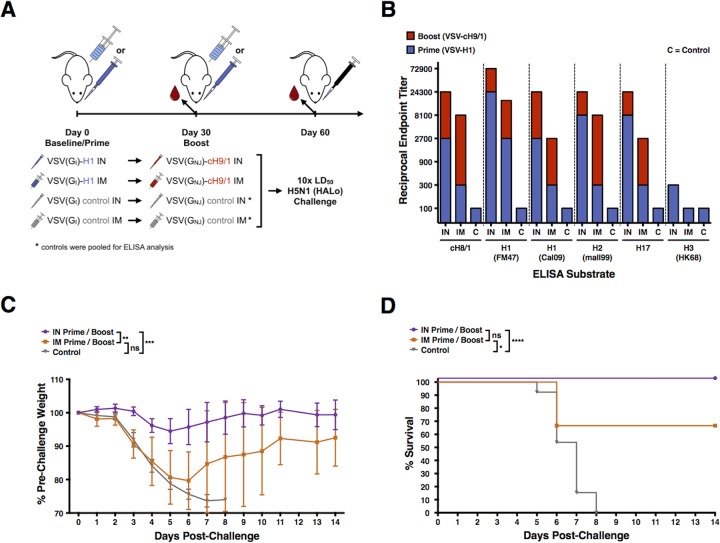
FIG 2.
VSV-H1 and VSV-cH9/1 vaccination and H5N1 challenge experiment. (A) Schematic of the prime-boost-challenge vaccination experiments: i.m. and i.n. vaccinations, H5N1 heterologous influenza challenge. Intranasal vaccinations were performed with 2 × 105 PFU, and i.m. vaccinations were performed with 4.5 × 105 PFU. (B) Serum reciprocal endpoint ELISA titers of mice vaccinated with VSV-H1 (prime) and VSV-cH9/1 (boost). Intramuscular vaccination results in greater boost, but overall serum antibody titers postboost are greater for i.n.-vaccinated animals. (C) Vaccinated mice were challenged i.n. 1 month postboost with 10 LD50 of heterosubtypic H5N1 (HALo) influenza virus. Weights were recorded daily for 14 days postchallenge and are graphically displayed as the mean percentage of the prevaccination weight. Error bars represent the 95% CI of the mean weight. All control mice died or required euthanasia per protocol by day 8 postchallenge. Significant differences between groups are indicated in the graph. (D) Kaplan-Meier survival curves. Significant differences between groups, as identified by a log-rank (Mantel-Cox) test, are indicated in the graph. Two mice from the i.m.-vaccinated group challenged with HALo H5N1 influenza virus died on day 6 postchallenge (66% overall survival). ns, not significant; *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001.
Blood was collected from mice following the prime and following the boost and used to determine the level of serum antibody that reacted with a panel of HAs by ELISA at both time points (Fig. 1B and 2B). Sera from control mice were tested against the same panel of HAs, and gave ELISA titers of 100 (limit of detection) or below for every substrate. We noted that both i.m. and i.n. vaccination (Fig. 1B and 2B) resulted in a boost in stalk-specific (as measured with a cH8/1 HA substrate) and cross-reactive serum antibody responses within group 1 HAs, but little to no reactivity with group 2 HAs, represented by an H3 HA substrate. We also saw that the animals vaccinated with VSV-cH9/1 and boosted with a control “sham” VSV virus showed no change in serum antibody titer between 30 and 60 days postprime (data not shown). This result indicates that exposure to a second cHA construct is necessary to achieve a boost in the humoral immune response.
In both vaccination schemes (Fig. 1A and 2A), priming via the i.n. route was superior to priming via the i.m. route. In contrast, i.m. vaccination resulted in a more potent boost in antibody titer, with increases in serum antibody titers of 6- to 81-fold (average, 27.6-fold) versus 3- to 9-fold (average, 5-fold) for i.n. vaccination (Fig. 1B and 2B). It should be noted that the final serum antibody titers after prime and boost vaccinations were similar for both i.m. and i.n. routes.
VSV-cHA vaccination protects mice against homologous and heterosubtypic influenza challenges.
In order to determine whether vaccinated mice were protected against influenza challenge, we infected the groups described above (Fig. 1A and 2A) with ten times the median lethal dose (LD50) of either a homologous PR8 influenza virus (H1N1) or a heterosubtypic H5N1 influenza virus (HALo, 6:2 reassortant influenza virus containing the HA and NA segments of the H5N1 A/Vietnam/1203/04 [VN1203]) (21).
Mice that had been primed and boosted with VSV-cH9/1 and VSV-cH5/1 were challenged with the homologous PR8 (H1N1) influenza virus, as were mice that had been primed with VSV-cH9/1 and boosted with an irrelevant control virus. Mice that had been primed and boosted with VSV-H1 and VSV-cH9/1 were challenged with the heterosubtypic HALo (H5N1) influenza virus. Control mice were primed and boosted with VSV constructs expressing irrelevant genes and challenged with the same influenza viruses as the cHA-vaccinated mice.
Weight loss and survival curves of the challenge experiments indicate that both i.m. and i.n. vaccination with VSV-cHAs provided protection against homologous (Fig. 1C and D) and heterosubtypic (Fig. 2C and D) influenza virus challenge. However, i.n. vaccination was superior to i.m. vaccination at achieving protection from weight loss. Mice that received an i.n. “sham” boosting vaccination with a control VSV virus lost significantly more weight after homologous challenge than mice that received an i.n. VSV-cH5/1 boost, indicating that there is increased protection from a prime-boost strategy over a prime-alone (Fig. 1C and D). When the weight loss data for mice vaccinated i.n. are compared directly to those vaccinated i.m. in a two-way t test, i.n. vaccination is superior for both homologous and heterosubtypic challenges.
Two mice in the i.m. prime-boost group died when challenged with the heterosubtypic HALo virus (66% overall survival, Fig. 2D). No other vaccinated mice died, with the exception of control animals. Each vaccinated group showed significantly improved survival over control mice.
Vectored chimeric hemagglutinins are superior to full-length hemagglutinins at inducing stalk-specific antibodies.
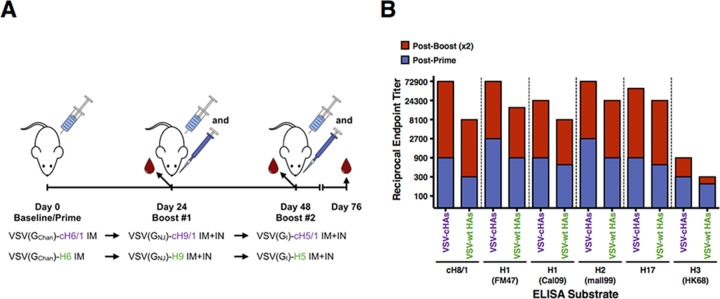
Sequential vaccination with cHA antigens exposes animals to an invariant stalk region at each round of vaccination. This will generate a recall response which results in significant boosts in stalk-specific antibody production. To determine whether maintaining an invariant stalk is necessary to achieve this boost, we conducted an experiment (Fig. 3A) to compare stalk-specific and cross-reactive serum antibody responses in mice vaccinated with VSV-cHAs or VSV expressing full-length HAs (VSV-HAs). Full-length HAs were derived from influenza virus strains closely related to those from which the heads of the chimeric antigens were derived. Mice were primed i.m. with either a VSV-cHA or VSV-HA, then boosted 3 weeks later by both the i.m. and the i.n. routes with a different VSV-cHA or VSV-HA, and then boosted a second time 3 weeks thereafter (Fig. 3A). Sera were collected prior to each boost and 1 month after the final boost and subjected to ELISA using a panel of HA substrates, as before (Fig. 3B).
FIG 3.
Vaccination with VSV-cHAs is superior to VSV-HAs at generating broadly reactive and stalk-specific serum HA antibodies. (A) Schematic of VSV-cHA versus VSV-HA prime-boost-boost experiment. Vaccinations were performed with 2 × 106 PFU in each case. (B) Groups of BALB/c mice were primed (i.m.) and boosted (combined i.m. and i.n.) after 3 weeks, and again after another 3 weeks, with either VSV-cHAs or VSV-HAs. Serum was collected prior to each boost and 1 month after the final boost and then subjected to ELISA against a panel of HA substrates. Blue bars represent the reciprocal endpoint ELISA titer of pooled postprime serums, and red bars represent the total reciprocal endpoint ELISA titer after prime two boosting vaccinations. Both VSV-cHA and VSV-HA vaccination results in stalk-specific and cross-reactive serum antibody, although VSV-cHAs generate higher titer responses and improved boosting of these responses.
For both groups of animals, boosting resulted in increased HA stalk-specific antibody titers, but vaccination with VSV-cHAs generated both higher-titer HA-specific serum antibody and a greater increase in this titer in response to boosting vaccination. Interestingly, for both VSV-cHA- and VSV-HA-vaccinated mice, a second boost did not result in a large increase in serum antibody titer (data not shown). The lack of increase in antibody titers after the third vaccination might be caused by increasing immune responses against the internal proteins of the vector. This immunity might limit the in vivo replication and expression of the target antigen.
Compared to mice vaccinated with VSV-HAs, those receiving VSV-cHAs had between 1.5- and 3-fold (average, 2.4-fold) greater HA-specific serum antibody titers postpriming. After the second boosting vaccination, mice receiving VSV-cHAs experienced a 27- to 81-fold (average, 43.2-fold) increase in HA-specific serum antibody titers compared to a 13.5- to 40.5-fold (average, 25.2-fold) increase for mice vaccinated with VSV-HAs. Overall, after priming and two boosting vaccinations, VSV-cHA vaccinated mice had 2- to 9-fold (average, 4.3-fold) greater serum antibody reactivity against the panel of HA substrates. The stalk-specific reactivity was 9-fold greater for the VSV-cHA group over the VSV-HA group. This indicates that VSV-cHAs are more potent at inducing cross-reactive stalk-specific antibodies than VSV-HAs. Still, VSV-HAs induced such antibodies, presumably because there is a high degree of epitope similarity in the stalk regions of group 1 HAs.
DISCUSSION
The goal of achieving “universal,” or at the very least, more broadly cross-reactive and cross-protective, influenza virus vaccines has inspired new and innovative approaches to promote desirable immune responses. The incorporation of cHA molecules into vaccine strategies has shown great promise with regard to generating broadly protective immunity (12–16). Here, we show that such HA stalk-specific immunity can be produced utilizing a VSV vector system. By altering the VSV G serotype at each round of vaccination, vector immunity is minimized, and boosting potential is maximized (17). The approaches outlined here show that a VSV-cHA vaccine is a viable candidate for producing broadly protective influenza vaccines. The advantages of such an approach include exploiting the adjuvant properties of a live viral vector and achieving a high level of protection against both homologous and highly divergent heterosubtypic influenza virus challenges after just one boost.
While a single priming vaccination with VSV-cHAs generates HA stalk-specific and cross-reactive antibodies (Fig. 1B) and protects animals challenged with a homologous PR8 influenza virus against death (Fig. 1C and D), the added benefit of a boosting vaccination, both to HA-specific antibody production (Fig. 1B and 2B) and protection against challenge (Fig. 1C and D and Fig. 2C and D), makes this strategy desirable.
Furthermore, these experiments show that protection is improved when the VSV-cHA vaccines are administered i.n. as opposed to i.m.. Although both vaccination routes induce robust serum antibody titers, i.n. vaccination results in improved protection from viral challenge (Fig. 1C and 2C). We suspect that there is a contribution from other immune mechanisms than serum IgG involved in the improved protection seen with i.n. vaccination. Specifically, we hypothesize that i.n. vaccination with VSV-cHAs promotes the development of mucosal antibodies that provide an additional level of protection at sites of exposure to influenza challenge viruses, including the nasal and respiratory mucosa. Although we did not measure mucosal HA-specific antibody titers in these experiments, it has been shown that i.n. administration of influenza virus vaccines induces mucosal IgA antibody production, whereas i.m. vaccination does not (14, 26). It is assumed that these antibodies contribute to protection and that both serum and mucosal antibodies play a role in protection against influenza virus challenge.
We did not assess the possibility that vaccine-induced T cells, specifically those generated in response to i.n. vaccination, may contribute to protection. Earlier studies have indicated that CD8+ T cells do not significantly contribute to cHA-induced protection against a homologous influenza challenge (12), but their role may be more important in the context of divergent challenge viruses.
Our data indicate that i.m. vaccination did result in a robust boost in serum antibody titers (Fig. 1B and 2B). This improved boosting ability of i.m. vaccination, combined with the improved protection afforded by i.n. vaccination, may be utilized in the future to augment immune responses and protection by influenza virus vaccination.
The comparison between prime-boost-boost vaccination with VSV-vectored cHAs and full-length HAs provides evidence that the cHA antigens, presumably due to their 100% amino acid conservation of the stalk region, are superior to full-length HAs at inducing HA-stalk specific antibodies. Interestingly, we still noted robust boosting of HA stalk-specific and cross-reactive antibody titers in response to VSV-HA vaccination (Fig. 3B), despite the relatively low amino acid conservation among the stalk regions of these antigens (60 to 76% amino acid conservation among the H5, H6, and H9 stalks and 62 to 80% amino acid conservation between these stalks and that of H1). This may be due to the fact that antibodies are induced against specific epitopes that are conserved within group 1 HA stalks.
It has recently been shown that vaccination of humans with an avian influenza H5N1 vaccine results in significant increases in HA stalk-specific antibodies, presumably due to recognition of conserved stalk epitopes in the presence of an HA head to which recipients were naive (27, 28). Both the results of that study and those described in the present study indicate that HA stalk-specific antibodies can be generated using naturally occurring HA antigens. Our data indicate that the added value of maintaining an invariant stalk region with a cHA strategy is to produce greater titers of stalk-specific antibodies. However, it needs to be taken into consideration that expression levels of different VSV-vectored HA and cHA proteins in vivo might vary and that variation could influence the generated immune response.
The data from our experiments indicate that a VSV-cHA vaccination strategy is effective at producing broadly cross-reactive HA stalk-specific antibody responses and that these responses can protect against lethal challenges with different influenza viruses. We believe these virally vectored vaccines represent a viable means for achieving a universal influenza vaccination strategy.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health (NIH) grants R01-AI080781 (J.K.R.), P01-AI097092 (P.P.), and U19-AI109946 (P.P. and F.K.) and by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, NIH. A.B.R. was supported by a Yale School of Medicine NRSA training grant 5T32HL007974-13 and by CTSA grant UL1 TR000142.
REFERENCES
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.McLean HQ, Meece JK, Belongia EA. 2014. Influenza vaccination and risk of hospitalization among adults with laboratory confirmed influenza illness. Vaccine 32:453–457. doi: 10.1016/j.vaccine.2013.11.060. [DOI] [PubMed] [Google Scholar]
- 3.Osterholm MT, Kelley NS, Sommer A, Belongia EA. 2012. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2015. Measles vaccination. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/measles/vaccination.html. [Google Scholar]
- 5.Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. 2012. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev 11:CD008521. [DOI] [PubMed] [Google Scholar]
- 6.Zajac BA, West DJ, McAleer WJ, Scolnick EM. 1986. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect 13(Suppl A):39–45. doi: 10.1016/S0163-4453(86)92668-X. [DOI] [PubMed] [Google Scholar]
- 7.Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, Colborn DK, Kurs-Lasky M, Haralam MA, Byers CJ, Zoffel LM, Fabian IA, Bernard BS, Kerr JD. 2003. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 290:1608–1616. [DOI] [PubMed] [Google Scholar]
- 8.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. 2002. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol 37:427–439. doi: 10.1016/S0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 9.Wright PF, Kawaoka Y. 2007. Orthomyxoviruses, p 1691–1740. In Knipe DM, Howley PM (ed), Fields virology, 5th ed Lippincott/Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 10.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, Garcia-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 86:5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado VH, Ryder AB, Miller MS, Rose JK, Palese P, Garcia-Sastre A, Albrecht RA. 2014. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol 88:3432–3442. doi: 10.1128/JVI.03004-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, Runstadler JA, Wilson PC, Albrecht RA, Garcia-Sastre A, Palese P. 2013. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J Virol 87:10435–10446. doi: 10.1128/JVI.01715-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff PH, Eggink D, Seibert CW, Hai R, Martinez-Gil L, Krammer F, Palese P. 2013. Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One 8:e79194. doi: 10.1371/journal.pone.0079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P. 2014. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol 88:2340–2343. doi: 10.1128/JVI.03183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose NF, Roberts A, Buonocore L, Rose JK. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J Virol 74:10903–10910. doi: 10.1128/JVI.74.23.10903-10910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz JA, Buonocore L, Roberts A, Suguitan A Jr, Kobasa D, Kobinger G, Feldmann H, Subbarao K, Rose JK. 2007. Vesicular stomatitis virus vectors expressing avian influenza H5 HA induce cross-neutralizing antibodies and long-term protection. Virology 366:166–173. doi: 10.1016/j.virol.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz JA, Buonocore L, Suguitan AL Jr, Silaghi A, Kobasa D, Kobinger G, Feldmann H, Subbarao K, Rose JK. 2010. Potent vesicular stomatitis virus-based avian influenza vaccines provide long-term sterilizing immunity against heterologous challenge. J Virol 84:4611–4618. doi: 10.1128/JVI.02637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryder AB, Buonocore L, Vogel L, Nachbagauer R, Krammer F, Rose JK. 2015. A viable recombinant rhabdovirus lacking its glycoprotein gene and expressing influenza virus hemagglutinin and neuraminidase is a potent influenza vaccine. J Virol 89:2820–2830. doi: 10.1128/JVI.03246-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steel J, Lowen AC, Pena L, Angel M, Solorzano A, Albrecht R, Perez DR, Garcia-Sastre A, Palese P. 2009. Live attenuated influenza viruses containing NS1 truncations as vaccine candidates against H5N1 highly pathogenic avian influenza. J Virol 83:1742–1753. doi: 10.1128/JVI.01920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson ND, Stillman EA, Whitt MA, Rose JK. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci U S A 92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnell MJ, Johnson JE, Buonocore L, Rose JK. 1997. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell 90:849–857. doi: 10.1016/S0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 24.Fuerst TR, Niles EG, Studier FW, Moss B. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A 83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margine I, Palese P, Krammer F. 2013. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J Vis Exp 81:e51112. doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Patil HP, de Vries-Idema J, Wilschut J, Huckriede A. 2013. Evaluation of mucosal and systemic immune responses elicited by GPI-0100-adjuvanted influenza vaccine delivered by different immunization strategies. PLoS One 8:e69649. doi: 10.1371/journal.pone.0069649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachbagauer R, Wohlbold TJ, Hirsh A, Hai R, Sjursen H, Palese P, Cox RJ, Krammer F. 2014. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J Virol 88:13260–13268. doi: 10.1128/JVI.02133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, Chang CY, Davis CW, McCausland M, Elbein R, Edupuganti S, Spearman P, Andrews SF, Wilson PC, Garcia-Sastre A, Mulligan MJ, Mehta AK, Palese P, Ahmed R. 2014. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A 111:13133–13138. doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]