ABSTRACT
Lytic infection by herpes simplex virus 1 (HSV-1) triggers a change in many host cell programs as the virus strives to express its own genes and replicate. Part of this process is repression of host cell transcription by RNA polymerase II (Pol II), which also transcribes the viral genome. Here, we describe a global characterization of Pol II occupancy on the viral and host genomes in response to HSV-1 infection using chromatin immunoprecipitation followed by deep sequencing (ChIP-seq). The data reveal near-complete loss of Pol II occupancy throughout host cell mRNA genes, in both their bodies and promoter-proximal regions. Increases in Pol II occupancy of host cell genes, which would be consistent with robust transcriptional activation, were not observed. HSV-1 infection induced a more potent and widespread repression of Pol II occupancy than did heat shock, another cellular stress that widely represses transcription. Concomitant with the loss of host genome Pol II occupancy, we observed Pol II covering the HSV-1 genome, reflecting a high level of viral gene transcription. Interestingly, the positions of the peaks of Pol II occupancy at HSV-1 and host cell promoters were different. The primary peak of Pol II occupancy at HSV-1 genes is ∼170 bp upstream of where it is positioned at host cell genes, suggesting that specific steps in transcription are regulated differently at HSV-1 genes than at host cell mRNA genes.
IMPORTANCE We investigated the effect of herpes simplex virus 1 (HSV-1) infection on transcription of host cell and viral genes by RNA polymerase II (Pol II). The approach we used was to determine how levels of genome-bound Pol II changed after HSV-1 infection. We found that HSV-1 caused a profound loss of Pol II occupancy across the host cell genome. Increases in Pol II occupancy were not observed, showing that no host genes were activated after infection. In contrast, Pol II occupied the entire HSV-1 genome. Moreover, the pattern of Pol II at HSV-1 genes differed from that on host cell genes, suggesting a unique mode of viral gene transcription. These studies provide new insight into how HSV-1 causes changes in the cellular program of gene expression and how the virus coopts host Pol II for its own use.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) is a double-stranded DNA virus that proliferates in the nuclei of host cells during lytic infection (reviewed in reference 1). HSV-1 can cause lifelong infection by establishing asymptomatic latency in host sensory neurons, where it remains in a transcriptionally silenced state until it is stimulated by stress to reactivate and replicate in epithelial cells (2). The majority of the population are infected with HSV-1, which is largely responsible for oral cold sores; however, in rarer cases, it can cause severe conditions, such as blindness and encephalitis (3, 4). Recent experimental and epidemiological evidence also suggests a role for recurrent HSV-1 infection in Alzheimer's disease (5). Despite its clear medical significance, the relationship between the virus and its host cells is complex and is still not fully understood.
During the hours following lytic infection, host cell RNA polymerase II (Pol II) transcribes the HSV-1 genome, a step required for productive infection. HSV-1 genes are expressed through a specific cascade of events, which in the early stages is regulated primarily at the level of transcription (6). Transcribed first are the α, or immediate-early, genes, whose protein products are ICP0, ICP4, ICP22, ICP27, and ICP47; their levels peak between 2 and 4 h of infection. The immediate-early genes are activated by the combined actions of the host cell transcription factors Oct-1 and HCF-1 and the viral protein VP16, which enters the host cell with the viral particles (7). Many of the α gene protein products facilitate the expression of genes activated later in infection—the β, or early, genes and the γ, or late, genes. The mechanisms by which the β genes (maximally expressed between 4 and 7 h of infection) and the γ genes (expressed 6 h postinfection and on) are transcriptionally activated are not completely understood. Evidence shows that the transcription of early and late genes is dependent on viral factors, for example, the immediate-early protein ICP4 (8–10). Also important for early- and late-gene transcription are promoter-independent processes, such as mobilization of Pol II and host cell factors, changes in the phosphorylation state of Pol II, and/or isolation of viral DNA into nuclear compartments (11). When host cell genes are artificially embedded in the viral genome, their transcription mimics that of the viral genes, indicating that the genomic environment dictates the transcriptional outcome, as opposed to the promoter sequence (12, 13). Moreover, a viral promoter upstream of a reporter gene is activated by viral transcriptional activators, although they are differentially sensitive to protein kinase inhibitors, further suggesting that the genomic environment dictates the transcriptional outcome (14).
To establish a favorable environment for its own proliferation, HSV-1 affects a number of host cell processes, one of which is the inhibition of host cell Pol II transcription (15, 16). Nuclear run-on experiments after HSV-1 infection revealed a decrease in Pol II transcription of several mRNA genes over a 9-h infection period compared to mock-infected cells (17). This transcriptional repression required expression of HSV-1 proteins, although the molecular mechanism behind virus-mediated repression of host cell Pol II transcription remains unclear. Moreover, experiments using mutant viruses implicated the immediate-early genes in the repression of host cell transcription; however, no single immediate-early protein was required for complete inhibition of transcription of the host cell genes investigated (17).
Microarray studies have documented an overall decrease in host cell transcript levels after HSV-1 infection on a more global scale, while also identifying host mRNA genes that appear to be upregulated (18–21). Although changes in host cell mRNA levels might be attributed, at least in part, to transcriptional changes, HSV-1 also impacts other cellular processes in a manner that leads to altered mRNA levels. For example, specific RNA degradation pathways that differentially target host cell mRNAs are tightly regulated after HSV-1 infection (22, 23). In addition, splicing is inhibited, which in turn impacts nuclear export, resulting in decreased levels of cytoplasmic cellular spliced mRNAs (24). In order to ultimately uncover how host cell Pol II transcription is repressed during HSV-1 infection, fundamental knowledge of which genes are transcriptionally repressed and with what potency needs to be acquired using techniques that specifically assess transcriptional changes, not steady-state RNA levels.
We performed a global characterization of how the Pol II transcriptional program changes after HSV-1 infection using chromatin immunoprecipitation of Pol II followed by deep sequencing (ChIP-seq), which detects Pol II occupancy on the genome and therefore assesses transcriptional activity. Our data reveal that Pol II occupancy across the bodies of host cell genes is globally reduced, with near-complete loss of Pol II across the host cell genome, which is consistent with extensive transcriptional repression. Even promoter-proximally paused Pol II disappeared after HSV-1 infection, indicating loss of Pol II recruitment to the promoters of host cell genes. We compared the repression of host cell transcription following viral infection to that observed after heat shock and found the effects of the two stress responses on Pol II transcription to markedly differ. Heat shock caused repression of fewer genes and substantial activation of many genes that were repressed by viral infection. The ChIP-seq data also revealed substantial occupancy of Pol II across the HSV-1 genome at all classes of genes (i.e., immediate-early, early, and late), consistent with transcriptional activation. Interestingly, patterns of Pol II occupancy at HSV-1 promoters are different than on the host cell genome. At host genes, Pol II is regulated at a postinitiation step called promoter-proximal pausing; our data suggest that this step might not occur at HSV-1 genes.
MATERIALS AND METHODS
Cells.
NIH 3T3 cells (American Type Culture Collection) were maintained in 5% CO2 at 37°C in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamate. For infection, cells were grown to 70% confluence and then treated with HSV-1 strain KOS (provided by N. Giordani and D. Bloom) at a multiplicity of infection of 3 PFU/cell for 1 h. The inoculum was then removed, and fresh prewarmed medium was added for 3 h prior to harvesting.
Chromatin immunoprecipitation assays.
Four hours after the addition of HSV-1 or medium (mock-infected sample), cells (∼2 × 107) were treated with 1% formaldehyde for 10 min at room temperature. Glycine (0.125 M) was added for 5 min. Nuclei were isolated by resuspending the cells in buffer A (80 μl/million cells; 4 mM MgCl2, 10 mM Tris-HCl [pH 7.4], 10 mM NaCl, 0.5% [vol/vol] NP-40, 0.4 mM phenylmethylsulfonyl fluoride [PMSF], and protease inhibitors [Complete cocktail tablets; Roche]), nutating for 5 min, spinning at low speed, and then washing the nuclei once in buffer A. The nuclei were resuspended in 540 μl of buffer B (50 mM Tris [pH 7.9], 10 mM EDTA, 0.4 mM PMSF, 1% SDS, protease inhibitors) and nutated for 10 min at 4°C; then, 1.08 ml of buffer C (15 mM Tris [pH 7.9], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.4 mM PMSF, protease inhibitors) was added, and samples were sonicated for 25 min using a Diagnode Bioruptor at the high setting for 30 s on followed by 30 s off. The samples were centrifuged, and the supernatants were used for immunoprecipitation. Antibody was added (sc899-x; Santa Cruz Biotechnology), and the samples were nutated at 4°C overnight. Protein A/G beads (Santa Cruz Biotechnology) were equilibrated in buffer D (15 mM Tris [pH 7.9], 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.4 mM PMSF), preblocked with Saccharomyces cerevisiae RNA and bovine serum albumin (BSA), and then added to the samples, which were then nutated for 1 to 2 h at 4°C. The beads were washed sequentially with low-salt buffer (20 mM Tris [pH 7.9], 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS), high-salt buffer (20 mM Tris [pH 7.9], 500 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS), and LiCl buffer (10 mM Tris [pH 7.9], 1 mM EDTA, 1% deoxycholate, 1% NP-40, 250 mM LiCl) and twice with TE (10 mM Tris [pH 7.9], 1 mM EDTA). Chromatin was eluted by nutation for 1 h at 37°C with 1% SDS, 50 mM Tris (pH 7.9), and 10 mM EDTA. NaCl was added to the supernatants to 200 mM, and cross-links were reversed by incubation at 65°C for 12 h. The samples were diluted with water to 0.5% SDS, treated with proteinase K, and then phenol extracted and ethanol precipitated.
High-throughput sequencing and read mapping.
Libraries for Illumina sequencing of the mock-infected and HSV-1-infected samples were prepared using the Ovation Ultralow System V2 (NuGen) and the TruSeq ChIP Sample Prep kit (Illumina) for input chromatin. Samples were sequenced using an Illumina HiSeq2000, obtaining 1- by 126-bp reads for the HSV-1 and mock-infected samples and 1- by 50-bp reads for the input chromatin. The data have been deposited in NCBI's Gene Expression Omnibus (GEO) (25). Total reads were as follows: mock-infected sample, 33,658,737; HSV-1-infected sample, 37,613,524; input chromatin, 50,341,566. The sequencing quality was assessed using FastQC. The first 50 nucleotides (nt) of reads was mapped to the mouse genome (mm10 assembly) using Bowtie (version 1.0.0) in the −v alignment mode, allowing one mismatch per read and reporting only reads with unique alignments (26). Using these parameters, 50 to 63% of the reads mapped.
Computational analysis of mapped reads.
Analysis of mapped reads was performed using the suite of tools available in HOMER (Hypergeometric Optimization of Motif EnRichment) (27). Tag directories were created from the .map files generated by Bowtie, and the autocorrelation between tag densities on the minus versus plus strands was assessed. To allow visualization of the mapped reads in the UCSC Genome Browser, bedGraph files were generated using the default makeUCSCfile command in HOMER. The analyzeRNA.pl command was used to count tags in RefSeq genes for each data set; tags were normalized to a constant number for each data set and counted from the transcription start site (TSS) to +500 (i.e., the early transcribed region) and from +500 to the 3′ end (i.e., the gene body). For each gene body, the normalized tag count was divided by the length of the gene to determine reads per kilobase (rpk). Gene body rpk values were used to determine the set of transcribed genes before and after HSV-1 infection. To do so, the values were compared to the input control, both by subtracting input rpk values and by calculating the fold increase over input for each gene. In all cases, transcribed genes were at least 2-fold above background and at least 5.2 rpk after input subtraction. Alternatively spliced variants that utilized the same start site and end site were not included, nor were genes less than 1 kb in length. Fold changes upon infection were calculated using rpk values in the absence of subtracting input.
Tag counts surrounding the TSSs and 3′ ends of RefSeq genes were binned using the annotatePeaks.pl command in HOMER, designating specific sets of genes. The data were output as tags per base pair per gene (histogramming the data after setting to a constant read number) and then normalized to 1.0 for display. For heat map histograms (see Fig. 2B), tag counts were binned per gene using the -ghist option in HOMER. The data were log10 transformed, sorted by the intensity of the promoter-proximal peak, and plotted as a heat map using JavaTreeView. The images of ChIP-seq data over individual genes were generated from the UCSC Browser using bedGraph files of the mapped reads; the HSV-1 data were smoothed over 3 pixels. Gene ontology (GO) analysis was performed using PANTHER, and genes were classified according to statistical overrepresentation by biological process.
FIG 2.
HSV-1 infection causes a global loss of Pol II occupancy across repressed mRNA genes. (A) The occupancy of promoter-proximally paused Pol II is sharply reduced after HSV-1 infection. Normalized sequence tag counts were histogrammed in 10-bp bins for 2 kb around the TSS. Shown are data from control cells (green), HSV-1-infected cells (red), and input chromatin (gray). (B) Pol II binding profiles around transcription start sites of repressed mRNA genes in mock-infected and HSV-1-infected cells. Each row of the heat map represents Pol II occupancy for one gene from −500 to +500 in 10-bp bins centered on the TSS (indicated by the arrows). The genes are ranked by the Pol II promoter-proximal signal in the mock-infected data. (C) Pol II ChIP-seq data across the Egr1 gene, viewed using the UCSC Genome Browser. The y axis for the HSV-1 data in the bottom plot has a different scale. (D) Occupancy of Pol II around the 3′ ends of repressed mRNA genes is sharply reduced after HSV-1 infection. Normalized sequence tag counts were histogrammed in 100-bp bins for 4 kb, centered on the 3′ end. Shown are data from control cells (green), HSV-1-infected cells (red), and input chromatin (gray).
Computational analysis of HSV-1 genome sequence reads.
To map the Pol II ChIP-seq data to the HSV-1 genome, a custom index in Bowtie 1.0.0 was built using the HSV-1 strain KOS genome sequence. Mapping was performed in the -v alignment mode, allowing two mismatches and reporting the best alignment per read; a requirement for uniqueness was not included to allow mapping to the repeat short (RS) and repeat long (RL) regions. A total of 901,571 reads from the HSV-10-infected samples mapped to the HSV-1 genome, which is 2.3% of the total reads (38,515,095) that mapped to either the host cell or viral genome. The Integrative Genomics Viewer (IGV) was used to visualize the data (28, 29). A custom genome track and annotation were built using the KOS genome sequence; a FASTA file was used for the genome sequence, and a .gtf file was used for the gene annotations. HOMER was used to generate a bedGraph file of the HSV-1 mapped ChIP-seq reads that could be read by the IGV. Tag counting across the HSV-1 genes and histogramming were performed using HOMER as described for the mm10 genome but using custom files to designate HSV-1 TSSs.
BrU labeling, immunoprecipitation, and RT-qPCR.
After 3.5 h of HSV-1 infection or mock treatment, NIH 3T3 cells were treated for 30 min with 2 mM 5-bromouridine (BrU). Total cellular RNA was isolated using RiboZol RNA Extraction Reagent (Amresco). The RNA from ∼7 × 105 cells was DNase treated and cleaned up over an RNeasy minicolumn (Qiagen). Murine RNase inhibitor (New England BioLabs) was added to all buffers at a final concentration of 8 units/ml, and all incubations were carried out at room temperature on a nutator unless otherwise noted. Sixty-microliter aliquots of anti-bromodeoxyuridine (BrdU) antibody-conjugated agarose beads (sc-32323; Santa Cruz Biotechnology) were washed twice in binding buffer (0.25× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, 1 mM EDTA, pH 7.7], 1 mM EDTA, 0.05% Tween 20, 37.5 mM NaCl), blocked for 1 h (0.25× SSPE, 1 mM EDTA, 0.05% Tween 20, 37.5 mM NaCl, 0.1% polyvinylpyrrolidone 40 [PVP-40], 1 μg/ml BSA), and then washed twice with binding buffer. RNA samples were heated to 65°C for 5 min, placed on ice, and then incubated with beads in binding buffer for 1 h. The beads were washed as follows: once with binding buffer, once with low-salt buffer (0.2× SSPE, 1 mM EDTA, 0.05% Tween 20), once with high-salt buffer (0.25× SSPE, 1 mM EDTA, 0.05% Tween 20, 137.5 mM NaCl), and twice with TET buffer (10 mM Tris, pH 8.0, 1 mM EDTA, 0.05% Tween 20). The RNA was eluted with four incubations in 125-μl elution buffer (50 mM Tris, pH 7.5, 20 mM dithiothreitol [DTT], 1 mM EDTA, 150 mM NaCl, and 0.1% SDS) at 42°C and then pooled. The eluted RNA was extracted with RiboZol and used for cDNA synthesis with random hexamers, and quantitative PCR (qPCR) was performed using the following primers (all 5′ to 3′): Eif3a, TGTGACAGATAAAACTTACTC and CGAGTATACTGGAGGCAGA; Myc, GCTAACTGTGATCTTCCACT and GGAGGTTTGCTGTGGC; Rpl11, CTCATGTTGGGCTACTTGAA and CCGAGAAGTTATTTTTCCGC; Tpm1, ACCTGGCACCCATTTGTTATGTGTCTCC and GCCCGCAGCAGCTTCTCTTTCG; Actb, ATGGCTACGTACATGGCTG and GTGGCTTTCTGAACTTGACAAC.
Microarray data accession numbers.
The data have been deposited in NCBI's Gene Expression Omnibus (GEO) (25) and are accessible through GEO Series accession number GSE66487. The Pol II ChIP-seq data before and after heat shock (see Fig. 5) are also in GEO (accession number GSE66513).
FIG 5.
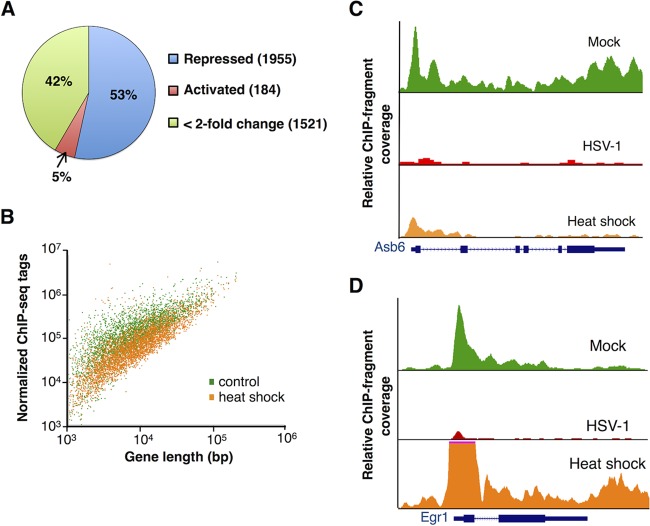
The Pol II response to heat shock shows widespread repression but is fundamentally different from the response to viral infection. (A) Response of Pol II occupancy to heat shock for genes that are repressed upon HSV-1 infection. The activated and repressed genes were those that showed a 2-fold or greater increase or decrease, respectively, in their rpk values across gene bodies upon heat shock. (B) Scatterplot of Pol II ChIP-seq gene body tag counts versus gene length revealing the magnitude of Pol II occupancy changes upon heat shock for the same set of genes as in panel A. The x and y axes are on logarithmic scales. (C) ChIP-seq data for the Asb6 gene showing repression in response to HSV-1 infection and heat shock. The data are scaled relative to the mock infection condition. (D) Pol II occupancy at the Egrl gene showing repression in response to HSV-1 infection and activation in response to heat shock. The data are scaled relative to the mock infection condition.
RESULTS
Transcription of host cell genes is globally and potently repressed after HSV-1 infection.
We used Pol II ChIP-seq to monitor polymerase occupancy on the host cell and viral genomes before and after HSV-1 infection. An overall change in Pol II occupancy throughout the transcribed region of a gene after infection provides direct evidence of a change in the level of transcription of that gene. Therefore, we chose to use Pol II ChIP-seq to evaluate transcriptional changes as opposed to monitoring levels of total RNA or newly synthesized RNA, which can require extensive normalization controls when total RNA levels are changing (30). Mouse NIH 3T3 cells were either mock infected or HSV-1 infected for 4 h, after which the cells were formaldehyde treated and chromatin was harvested. The use of NIH 3T3 cells enabled us to make direct comparisons to the transcriptional changes that occur during heat shock, another type of cell stress that triggers transcriptional reprogramming, which we have previously studied in this cell line (31). Moreover, mouse models are used to investigate reactivation of HSV-1 from latency to lytic infection (32, 33). ChIP assays were performed with an antibody that recognizes the N-terminal region of the Rpb1 subunit of Pol II. Libraries were constructed from the ChIP eluates and input chromatin, and samples were subjected to Illumina sequencing. Sequence reads were mapped to the host cell and viral genomes. Uniquely mapped reads were considered in downstream analyses and normalized to a constant number for each of the three samples (HSV-1 infected, mock infected, and input chromatin).
We first determined which host cell mRNA genes were repressed or activated in response to HSV-1 infection by identifying genes at which Pol II occupancy decreased or increased, respectively, in comparison to the mock-infected sample. At most host mRNA genes, the greatest level of Pol II occupancy is just downstream of the transcription start site due to a regulated pausing event that occurs during early transcription, referred to as promoter-proximal pausing (34). To eliminate pausing from consideration when defining transcribed genes in the mock-infected cells, we counted the rpk from +500 through the 3′ end (referred to as the gene body) for RefSeq genes. There were 3,720 genes in the mock-infected sample with Pol II gene body occupancies that conclusively reflected ongoing transcription (all had gene body rpk values of >5.2 and were minimally 2-fold above the input chromatin). To determine which of these genes were repressed, we identified those at which gene body Pol II occupancy was reduced 2-fold or more after HSV-1 infection. The data revealed that a surprising 98% (3,658 genes) showed this level of decrease in Pol II occupancy after viral infection (Fig. 1A). The mean and median fold decreases in Pol II occupancy in the bodies of repressed genes were 5.3 and 4.4, respectively. Of the 62 transcribed genes that were not repressed (∼2%), 61 exhibited moderate changes (less than 2-fold) in Pol II gene body occupancy after HSV-1 infection. Only one gene, the Mettl23 (methyltransferase like 23) gene, exhibited a greater than 2-fold increase in Pol II gene body tags after infection; however, it exhibited only a 2.08-fold increase, making it more similar to the genes with moderate changes in Pol II occupancy than to a gene highly activated due to infection.
FIG 1.
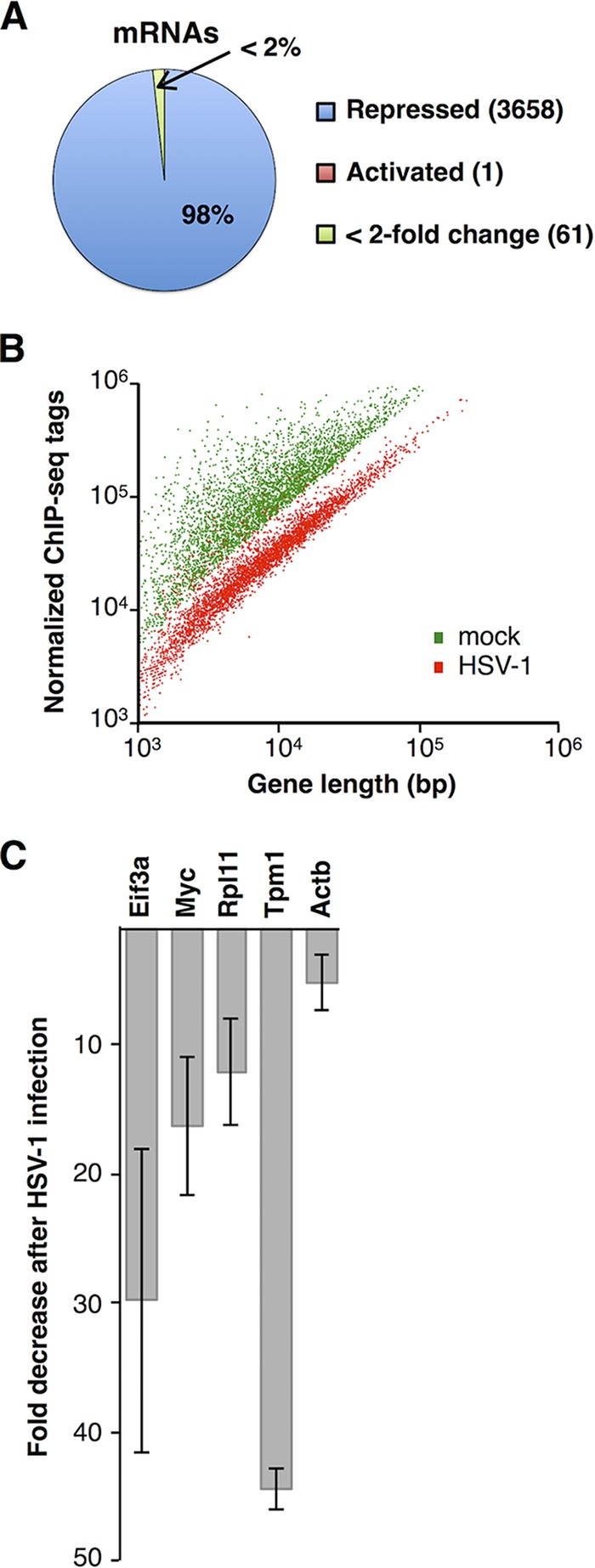
Transcription by Pol II is globally repressed in response to HSV-1 infection. (A) Pol II occupancy changes upon HSV-1 infection for 3,720 genes. Cells were infected for 4 h at a multiplicity of infection (MOI) of 3. (B) Scatterplot of Pol II ChIP-seq tag counts versus gene length revealing widespread repression in response to HSV-1 infection. (C) Levels of newly transcribed mRNAs decrease after HSV-1 infection. The plot shows the fold decrease in mRNA levels detected by RT-qPCR after immunopurification of BrU-labeled RNA. The bars represent the averages of two replicate immunoprecipitations, and the error bars indicate the ranges of the measurements.
We considered that genes activated upon infection might not be transcribed prior to infection and would therefore be missed in the analysis described above. To identify such genes, we used a similar strategy, except we started with the HSV-1-infected samples and looked for genes showing Pol II occupancy consistent with ongoing transcription after infection, applying the same criteria we applied to the mock-infected sample. Only 86 genes were identified using this approach, and all were present among the 3,720 considered transcribed prior to infection in the analysis above. We also investigated the noncoding RNA genes present in the RefSeq database and found that 99% of these genes were repressed after infection and none showed increases in Pol II gene body tags.
The global nature of the loss in Pol II across mRNA genes is illustrated by the scatterplot in Fig. 1B. Here, the sequence tags across gene bodies are plotted versus gene length for all mRNA genes deemed to be transcribed under either the mock-infected or infected condition. HSV-1 infection (Fig. 1B, red points) caused a significant downward shift in the cloud of points compared to the control condition (green points), consistent with widespread loss of Pol II occupancy. To validate the repression of host cell transcription we observed by Pol II ChIP-seq, we performed experiments monitoring newly synthesized RNA using pulse-labeling with BrU, immunoprecipitation against the BrU, and reverse transcription (RT)-qPCR of five mRNA transcripts (35). Myc was chosen because it was previously observed to decrease after infection in nuclear run-on experiments (17), and the other four are representative constitutively transcribed mRNAs. Figure 1C shows that transcriptional repression due to HSV-1 infection was potent for these five genes, ranging from 5- to 44-fold. These data corroborate the Pol II ChIP-seq data showing that transcription is strongly repressed during HSV-1 infection.
Our evaluation of Pol II occupancy changes after infection focused on RefSeq genes. To address the possibility that regions of the host genome outside those in the RefSeq collection might show significant increases in Pol II occupancy during HSV-1 infection, we applied a peak-calling algorithm to the ChIP-seq data sets. In the HSV-1 data, there were ∼400 non-RefSeq peaks enriched over the input chromatin and unique to the infected cells. Most of the peaks, however, had very small tag counts compared to peaks identified in the mock-infected samples. Moreover, no distinct pattern of occupancy emerged from manually inspecting the strongest peaks. For example, none of the peaks showed patterns consistent with localization in promoter regions upstream of genes or profiles that suggested transcription of intergenic noncoding RNAs (ncRNAs). Together, our data support the conclusion that massive loss of Pol II occupancy on the host cell genome is the dominant outcome of HSV-1 infection.
HSV-1 infection causes loss of Pol II occupancy at the transcriptional start sites and 3′ ends of the majority of host cell genes.
To gain insight into how transcription is repressed, we examined the effect of HSV-1 infection on Pol II occupancy patterns in the promoter regions of mRNA genes. For example, if Pol II occupancy in the promoter-proximal region remained after infection, it would indicate that Pol II still initiates transcription but cannot productively elongate beyond the promoter-proximal pause. Alternatively, if Pol II occupancy in the promoter region decreased after infection, it would indicate that recruitment of Pol II to promoters is repressed. We histogrammed sequence reads by position around the TSS for the repressed mRNA genes before and after HSV-1 infection (Fig. 2A). The Pol II density in the mock-infected cells (Fig. 2A, green line) showed a strong promoter-proximal peak centered just downstream of the TSS. HSV-1 infection caused a near-complete loss of the promoter-proximal peak (red line). The loss of Pol II surrounding TSSs of repressed genes is also apparent in the heat map shown in Fig. 2B. These data are most consistent with a model in which Pol II either is not recruited to promoters after HSV-1 infection or does not stably associate and initiate transcription.
Pol II occupancy before and after infection at a representative repressed gene (encoding Egr1) is shown in Fig. 2C. In the upper two plots, the y axes are the same, and very little sequence tag density is observed in the HSV-1 data (red) compared to the mock-infected-cell data (green). The lower plot shows the HSV-1 data with the magnitude of the y axis decreased 8-fold. Here, a typical pattern of Pol II occupancy is observed, albeit at a significantly reduced level compared to the mock-infected cells.
To determine the effect of HSV-1 infection on Pol II occupancy near the 3′ ends of mRNA genes, we histogrammed sequence tags for the HSV-1 repressed genes across 4 kb, centered on the 3′ end. In the mock infection data (Fig. 2D, green line), a peak of Pol II occupancy is observed just downstream of the 3′ end, which is typical of actively transcribed genes (34). Pol II occupancy across the entire region disappeared after HSV-1 infection (red line), which is again consistent with widespread transcriptional repression. Together, the data in Fig. 2 are most consistent with a model in which HSV-1 infection caused global repression of mRNA transcription at a point prior to transcription initiation.
The level of repression does not correlate with gene function or the level of promoter-proximal pausing.
We next asked whether a correlation exists between the level at which a gene is transcriptionally repressed upon HSV-1 infection and its function. If so, this would suggest that repression of host cell mRNA transcription is regulated in a gene-specific manner. We divided the repressed mRNA genes into three groups based on their levels of repression: top 25% (i.e., greatest repression), middle 50%, and bottom 25%. We considered the GO classifications according to biological process for each set of genes. We found that more than 2/3 of the enriched GO terms in the three groups of repressed genes overlapped, suggesting that repression of transcription does not correlate with gene function.
We then investigated whether transcriptional repression might correlate with the level of either promoter-proximal pausing or transcription prior to infection. The sequence tags for genes in the three groups were binned and histogrammed around their TSSs and 3′ ends, as shown in Fig. 3A. The TSS plot on the left shows that there is not a substantial difference in the promoter-proximally paused Pol II between the three groups of genes prior to infection. Differences in the three groups of genes, however, can be seen in the 3′-end plot shown on the right. If we consider the tags in the 4 kb flanking the 3′ end as a reflection of ongoing transcription prior to infection, then these data indicate that the level of repression due to infection simply reflects the level at which a given gene was transcribed prior to infection. Hence, our data are most consistent with a single mechanism of transcriptional repression that is not gene specific. We also investigated whether there was a relationship between the level of promoter-proximal pausing at a gene and loss of Pol II occupancy within the gene upon viral infection. The degree of promoter-proximal pausing varies across genes, which is reflected by the ratio of promoter-proximal to gene body Pol II density, termed the pause index (34). As can be seen in the scatterplot in Fig. 3B, there is no correlation between the pause index prior to infection and the fold decrease in Pol II body occupancy after HSV-1 infection.
FIG 3.
At repressed genes, the level at which Pol II occupancy decreases correlates with the level of transcription prior to infection. (A) Genes were categorized according to their fold repression: the top 25% of the genes decreased an average of 9.0-fold with a median of 7.7-fold; the middle 50% of the genes decreased an average of 4.5-fold with a median of 4.4-fold; the bottom 25% of the genes decreased an average of 3.0-fold with a median of 3.0-fold. Histograms of Pol II occupancy surrounding the TSSs and 3′ ends of genes in each group are shown. The gray lines represent the input chromatin. Sequence tags were binned as described for Fig. 2. (B) There is no relationship between the extent of promoter-proximal pausing and the extent of repression of Pol II gene body occupancy after HSV-1 infection. Shown is a plot of the pause index versus fold repression for the ∼3,600 mRNA genes whose Pol II gene body occupancy decreased >2-fold after HSV-1 infection.
Genes that exhibit moderate changes in Pol II occupancy after HSV-1 infection lose their promoter-proximal Pol II.
We investigated the patterns of Pol II occupancy at the 61 host cell mRNA genes that changed less than 2-fold after HSV-1 infection. Histograms of sequence tags surrounding both the TSS and 3′ ends for these genes are shown in Fig. 4A. The 3′ end plot (right) is consistent with these genes being classified as having less than a 2-fold change in Pol II occupancy across their gene bodies after HSV-1 infection. Interestingly, there was a much greater decrease in Pol II occupancy surrounding the TSSs of the genes (left). A representative example of a gene (encoding Calr) in this category is shown in Fig. 4B. In the plots on the left, the y axes for mock and HSV-1 infection data were independently set to the heights of their respective promoter-proximal peaks. In the plots on the right, the y axes are equal to allow visualization of the data sets on the same scale. These data show that occupancy of Pol II surrounding the TSS was significantly reduced upon HSV-1 infection but occupancy in the body of the gene and around the 3′ end remained fairly constant. It is possible that this small set of 61 genes is still transcribed after infection, but in a manner that does not involve a substantial promoter-proximal pause. We performed a GO analysis on this set of genes and found there is statistically significant enrichment (P < 0.05) of biological processes involving mRNA metabolism (e.g., splicing, processing, and localization, including negative regulation of these processes). Perhaps mechanisms are in place to keep transcription of the genes ongoing after HSV-1 infection, albeit with a different mechanism than before infection.
FIG 4.
Analysis of genes at which Pol II occupancy changed less than 2-fold after HSV-1 infection. (A) Occupancy of Pol II around the TSSs and 3′ ends for the 61 genes whose rpk values across their bodies changed less than 2-fold after HSV-1 infection. Normalized sequence tags were histogrammed in 10-bp bins for 2 kb centered on the TSS or in 100-bp bins for 4 kb centered on the 3′ end. (B) Representative ChIP-seq data for the Calr gene showing that promoter-proximal occupancy of Pol II decreased significantly after HSV-1 infection while Pol II occupancy across the body of the gene changed less than 2-fold. The mock infection data are shown with two different y axes. The graphs were created from the UCSC Genome Browser.
HSV-1 infection results in much broader and more potent repression of Pol II transcription than heat shock.
Heat shock is another cellular stress that causes global changes in Pol II transcription in eukaryotic cells. Although some genes, such as those encoding heat shock proteins, are significantly activated in response to heat shock, most mRNA genes are thought to be transcriptionally repressed (36–39). We were interested in learning whether the genes repressed during HSV-1 infection also showed a profound loss in Pol II occupancy in response to heat shock, which would suggest similarities in how Pol II transcription is downregulated in response to the two different stresses. For the ∼3,600 mRNA genes repressed after HSV-1 infection, we asked what happened to Pol II occupancy at the genes in response to heat shock, using Pol II ChIP-seq data. As shown in Fig. 5A, the set of genes repressed after HSV-1 infection are not all repressed after heat shock. Although repression remains the dominant outcome in response to heat shock (53% of the genes), a large portion of the gene bodies show only small (<2-fold) changes in Pol II occupancy (42%). Moreover, 184 genes repressed upon viral infection were activated 2-fold or more upon heat shock. Figure 5B shows a scatterplot of the sequence tags across gene bodies versus gene length before heat shock (green points) and after heat shock (orange points). Comparison of this scatterplot with that for HSV-1 infection (Fig. 1B) shows that the magnitude of the loss in Pol II occupancy across genes bodies of repressed genes is smaller after heat shock.
Figure 5C and D show representative Pol II occupancy traces across two genes before and after heat shock and HSV-1 infection. The Asb6 gene (Fig. 5C) was repressed in response to both viral infection and heat shock, although the potency was not as significant in response to heat shock. In contrast, the Egr1 gene (Fig. 5D), which is also shown in Fig. 2C as an example of a virus-repressed gene, is activated in response to heat shock. The Pol II occupancy profile shows a large increase in the early transcribed region after heat shock, consistent with a high level of new initiation; there is also a substantial increase in Pol II throughout the body of the gene. The figure also illustrates a canonical Pol II occupancy pattern for strong transcriptional activation in response to a stimulus, which we did not observe in response to HSV-1 infection. The data in Fig. 5 show that the global repression of Pol II transcription in response to HSV-1 infection is different from the response to the stress of heat shock.
Pol II occupancy is abundant across the HSV-1 genome and occurs in a pattern that is different from that for the host genome.
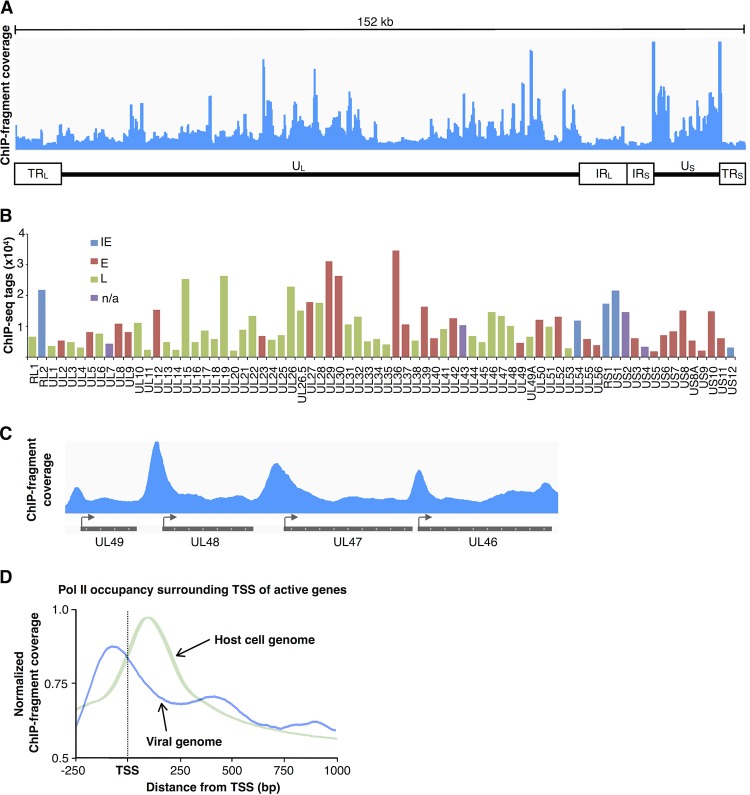
We investigated Pol II occupancy at viral genes by mapping ChIP-seq reads to the HSV-1 genome (∼900,000 reads obtained from the HSV-1-infected sample mapped); Fig. 6A shows an overview. Regions of higher and lower Pol II occupancy can be observed; however, the entire genome contains Pol II, showing robust polymerase recruitment. Pol II tag counts per HSV-1 gene are shown as a bar plot in Fig. 6B. The bars are colored according to a gene's designation as immediate-early, early, or late (18). Interestingly, Pol II occupancy levels do not strongly correlate with the gene classification.
FIG 6.
Pol II occupancy is high across the entire HSV-1 genome. (A) A broad view of Pol II occupancy on the 152-kb HSV-1 genome. The graph was generated using the Integrative Genomics Viewer (28, 29). The genome is comprised of unique long (UL) and unique short (US) sequences, flanked by repeat sequences: terminal long (TRL), terminal short (TRS), internal long (IRL), and internal short (IRS). (B) The distribution of Pol II over HSV-1 genes does not strongly correlate with their kinetic classification. The bar plot shows the ChIP-seq tag counts per gene. IE, immediate-early; E, early; L, late; n/a, unknown. Gene classifications are as described previously (18). (C) Representative Pol II occupancy profiles over four genes in the HSV-1 genome. The arrows indicate the TSSs and direction of transcription. (D) Pol II does not exhibit a promoter-proximal-pause peak at HSV-1 genes. Shown is the Pol II occupancy around the TSSs of HSV-1 genes 1 kb or longer (blue line). Normalized sequence tag counts were histogrammed in 10-bp bins from 250 bp upstream of the TSS through 1 kb downstream. The plot also shows Pol II density around the TSSs of the host cell genome prior to infection (light-green line) as a point of comparison.
Occupancy profiles of Pol II at four genes are shown in Fig. 6C. Peaks are observed just upstream of the TSS of each gene, which is different than the peaks of promoter-proximally paused Pol II observed at host cell genes. The histogram in Fig. 6D shows the summed Pol II occupancies flanking TSSs at all viral genes after infection (blue line) and host cell genes in mock-treated cells (light-green line). The primary peak of Pol II occupancy at the HSV-1 genes is approximately 70 bp upstream of the transcription start site (dashed line). This is in sharp contrast to the promoter-proximal Pol II peak at host cell genes, which is approximately 100 bp downstream of the TSS. These data show that the early steps of transcription (preinitiation complex formation through promoter-proximal pausing) are regulated differently on viral genes than on host cell genes.
DISCUSSION
We used ChIP-seq to evaluate the fate of Pol II 4 h after HSV-1 infection. The data revealed a global and near-complete loss of Pol II on the host cell genome and no increases in Pol II occupancy, consistent with robust transcriptional activation of any mRNA gene. The loss of Pol II occurred throughout the lengths of genes, including the promoter-proximal pause, the gene body, and the polymerase peak found downstream of the 3′ end. The repression of Pol II transcription in response to HSV-1 infection was far more potent and widespread than that occurring in response to heat shock, another cellular stress that generally downregulates Pol II transcription. Concomitant with the loss of host cell Pol II occupancy after HSV-1 infection was a high level of Pol II occupancy over the entire HSV-1 genome, reflecting abundant viral gene transcription. Interestingly, the general pattern of Pol II occupancy at viral genes is different than at host cell genes, suggesting that specific steps in the Pol II transcription reaction are regulated differently on the two genomes.
Our observation that HSV-1 infection causes loss of Pol II over the entirety of host cell genes, including the promoter-proximal regions, is most consistent with a model where repression occurs at the point of Pol II assembling into preinitiation complexes at host cell promoters. It is also possible that Pol II is recruited to promoters but does not stably associate in a manner that allows the polymerase to be cross-linked to the DNA or to be captured by ChIP. Recently, 4-thiouridine labeling and deep sequencing (4sU-seq) was used to evaluate ongoing transcription in 1-h windows during HSV-1 infection of human cells (40). Similar to what we observed by Pol II ChIP-seq, the transcriptional changes were dominated by repression; the vast majority of mRNA genes exhibited decreased transcription, and little activation was observed. Interestingly, at later time points of infection, 4sU-seq reads mapped well beyond the 3′ ends of thousands of genes, indicating a transcriptional termination defect that was most pronounced 7 to 8 h postinfection (40). At time points correlating with our work, only a subtle increase in the fraction of 4sU-seq reads mapping to intergenic regions was observed. Consistent with this, our ChIP-seq data do not show Pol II occupancy patterns indicative of a widespread termination defect.
The localization of Pol II within nuclei is known to change during HSV-1 infection. Indirect immunofluorescence experiments showed that, 6 h after HSV-1 infection, much of the cellular Pol II localizes to discrete viral replication compartments within the nucleus (11, 41), along with several general Pol II transcription factors (42). Similar associations were also detected by iPOND, a technique to label and purify replicating HSV-1 genomes and identify associated proteins (43). Sequestration of Pol II into replication compartments could contribute to the shift in transcription away from the host cell genome to the viral genome at later times of infection but cannot by itself explain the near-complete loss of host cell Pol II occupancy we observed at 4 h of infection.
It is possible HSV-1 repurposes Pol II to viral DNA by targeting the polymerase itself. Changes in Pol II have been observed after infection, and it is possible these changes reduce Pol II occupancy on the host cell genome and favor transcription of the viral genome. For example, HSV-1 infection changes the phosphorylation state of the Pol II C-terminal domain (CTD). The hyperphosphorylated form of the CTD that is characteristic of transcript elongation and the hypophosphorylated form recruited to preinitiation complexes are both replaced by a virus-induced intermediately phosphorylated form in a process that involves the viral protein ICP22 (11, 41, 44, 45). HSV-1 also induces changes in the Pol II “holoenzyme,” a large macromolecular complex containing Pol II and several of its regulatory factors. After HSV-1 infection, purified holoenzyme complexes lack the general transcription factor TFIIE (42) and gain viral proteins (46). Evidence suggests that viral proteins could participate in the transcriptional repression of host cell genes. In nuclear run-on experiments, efficient transcriptional repression of select host cell genes required expression of the HSV-1 genome, and the immediate-early gene products were implicated (17). In other studies, infection with virus containing a deletion of ICP27 resulted in an increase in the levels of many cellular transcripts (18). ICP27 and ICP8 coimmunoprecipitate with Pol II in the hours after HSV-1 infection (46), and ICP27 and ICP22 biochemically purify with Pol II after infection (46).
Comparison of the data from HSV-1-infected cells and heat-shocked cells shows that global transcriptional reprogramming in response to the two different types of stress is not the same. Many of the genes repressed by HSV-1 infection either do not change significantly or are activated during heat shock. Moreover, the general potency of transcriptional repression is lower in response to heat shock than to HSV-1 infection. Another major difference is that robust increases in Pol II occupancy do not occur in response to viral infection, whereas multiple genes are strongly activated after heat shock. Although our ChIP-seq data do not support transcriptional activation of host genes after infection, previous microarray studies have reported that tens to hundreds of host transcripts increase after HSV-1 infection (18–21). It is possible that the transcripts from these genes are stabilized postinfection, allowing their detection by microarrays in the absence of ongoing transcription. Studies using immune system cells (e.g., leukocytes, macrophages, and dendritic cells) have shown that some interleukins are upregulated in response to HSV-1 infection (47, 48) and are thought to play a role in latency (49). It is possible that observing increases in Pol II occupancy at specific host genes after infection is cell type or time point specific.
Our experiments suggest that Pol II transcription of the HSV-1 genome is regulated differently than on the host cell genome. Specifically, Pol II occupancy profiles at HSV-1 genes lack a promoter-proximally paused peak; rather, the highest peaks of Pol II occupancy are ∼70 bp upstream of the TSS. This upstream position is consistent with the expected location of preinitiation complexes prior to initiation. Therefore, initiation might serve as a kinetic block during viral transcription, which is not observed at the majority of host cell genes. Two factors, DSIF and NELF, trigger promoter-proximal pausing at host cell genes by associating with Pol II just downstream of the start site (34). It is possible that NELF and DSIF do not participate in transcription of HSV-1 genes. Alternatively, it is possible that pausing occurs during viral transcription but the paused Pol II is released quickly and therefore is not captured by Pol II ChIP-seq. The roles of DSIF and NELF in transcription of HSV-1 genes have not been reported, although these factors help control the transcription of other herpesviruses (Kaposi's sarcoma-associated herpesvirus [KSHV] and Epstein-Barr virus [EBV] [50, 51]).
We observed that 4 h after infection the viral genome was completely occupied by Pol II, and there was no apparent correlation between Pol II occupancy levels and the kinetic classes of HSV-1 genes (i.e., immediate-early, early, and late). When microarray data (18) and more recent RNA-seq data (52) from HSV-1-infected cells are viewed at a single time point, results similar to ours are observed. However, in these studies, differences between the transcript levels of genes were more meaningful with respect to their classification when data across a time course of infection were compared. Future ChIP-seq studies of Pol II across a time course of infection will be informative for tracking the switch of Pol II from the host genome to the HSV-1 genome and observing how the occupancy patterns of Pol II change. Our data illustrate that ChIP-seq can provide positional resolution that lends novel insight into models of how viral transcription is regulated.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Taylor TJ, Brockman MA, McNamee EE, Knipe DM. 2002. Herpes simplex virus. Front Biosci 7:d752–d764. doi: 10.2741/taylor. [DOI] [PubMed] [Google Scholar]
- 2.Jones C. 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin Microbiol Rev 16:79–95. doi: 10.1128/CMR.16.1.79-95.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schillinger JA, Xu F, Sternberg MR, Armstrong GL, Lee FK, Nahmias AJ, McQuillan GM, Louis ME, Markowitz LE. 2004. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976-1994. Sex Transm Dis 31:753–760. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- 4.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 5.Piacentini R, De Chiara G, Li Puma DD, Ripoli C, Marcocci ME, Garaci E, Palamara AT, Grassi C. 2014. HSV-1 and Alzheimer's disease: more than a hypothesis. Front Pharmacol 5:97. doi: 10.3389/fphar.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weir JP. 2001. Regulation of herpes simplex virus gene expression. Gene 271:117–130. doi: 10.1016/S0378-1119(01)00512-1. [DOI] [PubMed] [Google Scholar]
- 7.Wysocka J, Herr W. 2003. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem Sci 28:294–304. doi: 10.1016/S0968-0004(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 8.Lester JT, DeLuca NA. 2011. Herpes simplex virus 1 ICP4 forms complexes with TFIID and mediator in virus-infected cells. J Virol 85:5733–5744. doi: 10.1128/JVI.00385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampath P, DeLuca NA. 2008. Binding of ICP4, TATA-binding protein, and RNA polymerase II to herpes simplex virus type 1 immediate-early, early, and late promoters in virus-infected cells. J Virol 82:2339–2349. doi: 10.1128/JVI.02459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner LM, Bayer A, DeLuca NA. 2013. Requirement of the N-terminal activation domain of herpes simplex virus ICP4 for viral gene expression. J Virol 87:1010–1018. doi: 10.1128/JVI.02844-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice SA, Long MC, Lam V, Spencer CA. 1994. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J Virol 68:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smibert CA, Smiley JR. 1990. Differential regulation of endogenous and transduced beta-globin genes during infection of erythroid cells with a herpes simplex virus type 1 recombinant. J Virol 64:3882–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smiley JR, Smibert C, Everett RD. 1987. Expression of a cellular gene cloned in herpes simplex virus: rabbit beta-globin is regulated as an early viral gene in infected fibroblasts. J Virol 61:2368–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan R, Schang L, Schaffer PA. 1999. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J Virol 73:8843–8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston CM, Newton AA. 1976. The effects of herpes simplex virus type 1 on cellular DNA-dependent RNA polymerase activities. J Gen Virol 33:471–482. doi: 10.1099/0022-1317-33-3-471. [DOI] [PubMed] [Google Scholar]
- 16.Wagner EK, Roizman B. 1969. Ribonucleic acid synthesis in cells infected with herpes simplex virus. I. Patterns of ribonucleic acid synthesis in productively infected cells. J Virol 4:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer CA, Dahmus ME, Rice SA. 1997. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J Virol 71:2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stingley SW, Ramirez JJ, Aguilar SA, Simmen K, Sandri-Goldin RM, Ghazal P, Wagner EK. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J Virol 74:9916–9927. doi: 10.1128/JVI.74.21.9916-9927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khodarev NN, Advani SJ, Gupta N, Roizman B, Weichselbaum RR. 1999. Accumulation of specific RNAs encoding transcriptional factors and stress response proteins against a background of severe depletion of cellular RNAs in cells infected with herpes simplex virus 1. Proc Natl Acad Sci U S A 96:12062–12067. doi: 10.1073/pnas.96.21.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taddeo B, Esclatine A, Roizman B. 2002. The patterns of accumulation of cellular RNAs in cells infected with a wild-type and a mutant herpes simplex virus 1 lacking the virion host shutoff gene. Proc Natl Acad Sci U S A 99:17031–17036. doi: 10.1073/pnas.252588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamakura M, Nawa A, Ushijima Y, Goshima F, Kawaguchi Y, Kikkawa F, Nishiyama Y. 2008. Microarray analysis of transcriptional responses to infection by herpes simplex virus types 1 and 2 and their US3-deficient mutants. Microbes Infect 10:405–413. doi: 10.1016/j.micinf.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Taddeo B, Esclatine A, Roizman B. 2004. Post-transcriptional processing of cellular RNAs in herpes simplex virus-infected cells. Biochem Soc Trans 32:697–701. doi: 10.1042/BST0320697. [DOI] [PubMed] [Google Scholar]
- 23.Taddeo B, Zhang W, Roizman B. 2013. The herpes simplex virus host shutoff RNase degrades cellular and viral mRNAs made before infection but not viral mRNA made after infection. J Virol 87:4516–4522. doi: 10.1128/JVI.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandri-Goldin RM. 2008. The many roles of the regulatory protein ICP27 during herpes simplex virus infection. Front Biosci 13:5241–5256. [DOI] [PubMed] [Google Scholar]
- 25.Edgar R, Domrachev M, Lash AE. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. 2011. Integrative genomics viewer. Nat Biotechnol 29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorvaldsdottir H, Robinson JT, Mesirov JP. 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovén J, Orlando DA, Sigova AA, Lin CY, Rahl PB, Burge CB, Levens DL, Lee TI, Young RA. 2012. Revisiting global gene expression analysis. Cell 151:476–482. doi: 10.1016/j.cell.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen TA, Von Kaenel S, Goodrich JA, Kugel JF. 2004. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nat Struct Mol Biol 11:816–821. doi: 10.1038/nsmb813. [DOI] [PubMed] [Google Scholar]
- 32.Kollias CM, Huneke RB, Wigdahl B, Jennings SR. 2015. Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol 21:8–23. doi: 10.1007/s13365-014-0302-2. [DOI] [PubMed] [Google Scholar]
- 33.Webre JM, Hill JM, Nolan NM, Clement C, McFerrin HE, Bhattacharjee PS, Hsia V, Neumann DM, Foster TP, Lukiw WJ, Thompson HW. 2012. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J Biomed Biotechnol 2012:612316. doi: 10.1155/2012/612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adelman K, Lis JT. 2012. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet 13:720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulsen MT, Veloso A, Prasad J, Bedi K, Ljungman EA, Magnuson B, Wilson TE, Ljungman M. 2014. Use of Bru-Seq and BruChase-Seq for genome-wide assessment of the synthesis and stability of RNA. Methods 67:45–54. doi: 10.1016/j.ymeth.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sass H. 1982. RNA polymerase B in polytene chromosomes: immunofluorescent and autoradiographic analysis during stimulated and repressed RNA synthesis. Cell 28:269–278. doi: 10.1016/0092-8674(82)90345-2. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez J, Pauli D, Tissieres A. 1993. Transcriptional regulation in Drosophila during heat shock: a nuclear run-on analysis. Chromosoma 102:233–248. doi: 10.1007/BF00352397. [DOI] [PubMed] [Google Scholar]
- 38.Mok EH, Smith HS, DiBartolomeis SM, Kerrebrock AW, Rothschild LJ, Lange TS, Gerbi SA. 2001. Maintenance of the DNA puff expanded state is independent of active replication and transcription. Chromosoma 110:186–196. doi: 10.1007/s004120000119. [DOI] [PubMed] [Google Scholar]
- 39.Sonna LA, Fujita J, Gaffin SL, Lilly CM. 2002. Effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92:1725–1742. doi: 10.1152/japplphysiol.01143.2001. [DOI] [PubMed] [Google Scholar]
- 40.Rutkowski AJ, Erhard F, L'Hernault A, Bonfert T, Schilhabel M, Crump C, Rosenstiel P, Efstathiou S, Zimmer R, Friedel CC, Dölken L. 2015. Widespread disruption of host transcription termination in HSV-1 infection. Nat Commun 6:7126. doi: 10.1038/ncomms8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice SA, Long MC, Lam V, Schaffer PA, Spencer CA. 1995. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J Virol 69:5550–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins HL, Spencer CA. 2001. RNA polymerase II holoenzyme modifications accompany transcription reprogramming in herpes simplex virus type 1-infected cells. J Virol 75:9872–9884. doi: 10.1128/JVI.75.20.9872-9884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dembowski JA, DeLuca NA. 2015. Selective recruitment of nuclear factors to productively replicating herpes simplex virus genomes. PLoS Pathog 11:e1004939. doi: 10.1371/journal.ppat.1004939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastian TW, Rice SA. 2009. Identification of sequences in herpes simplex virus type 1 ICP22 that influence RNA polymerase II modification and viral late gene expression. J Virol 83:128–139. doi: 10.1128/JVI.01954-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long MC, Leong V, Schaffer PA, Spencer CA, Rice SA. 1999. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J Virol 73:5593–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou C, Knipe DM. 2002. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J Virol 76:5893–5904. doi: 10.1128/JVI.76.12.5893-5904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paludan SR. 2001. Requirements for the induction of interleukin-6 by herpes simplex virus-infected leukocytes. J Virol 75:8008–8015. doi: 10.1128/JVI.75.17.8008-8015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melchjorsen J, Siren J, Julkunen I, Paludan SR, Matikainen S. 2006. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J Gen Virol 87:1099–1108. doi: 10.1099/vir.0.81541-0. [DOI] [PubMed] [Google Scholar]
- 49.Baker M, Noisakran S, Gebhardt BM, Kriesel JD, Carr DJ. 1999. The relationship between interleukin-6 and herpes simplex virus type 1: implications for behavior and immunopathology. Brain Behav Immun 13:201–211. doi: 10.1006/brbi.1999.0572. [DOI] [PubMed] [Google Scholar]
- 50.Palermo RD, Webb HM, West MJ. 2011. RNA polymerase II stalling promotes nucleosome occlusion and pTEFb recruitment to drive immortalization by Epstein-Barr virus. PLoS Pathog 7:e1002334. doi: 10.1371/journal.ppat.1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toth Z, Brulois KF, Wong LY, Lee HR, Chung B, Jung JU. 2012. Negative elongation factor-mediated suppression of RNA polymerase II elongation of Kaposi's sarcoma-associated herpesvirus lytic gene expression. J Virol 86:9696–9707. doi: 10.1128/JVI.01012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harkness JM, Kader M, DeLuca NA. 2014. Transcription of the herpes simplex virus 1 genome during productive and quiescent infection of neuronal and nonneuronal cells. J Virol 88:6847–6861. doi: 10.1128/JVI.00516-14. [DOI] [PMC free article] [PubMed] [Google Scholar]