ABSTRACT
Entry of herpesviruses depends on the combined action of viral glycoprotein B (gB) and the heterodimeric gH/gL complex, which are activated by binding of the virion to specific cellular receptors. While gB carries signatures of a bona fide fusion protein, efficient membrane fusion requires gH/gL. However, although gB and gH/gL are essential for entry, the alphaherpesvirus pseudorabies virus (PrV) is capable of limited cell-to-cell spread in the absence of gL. To understand gH/gL function in more detail, the limited spread of PrV-ΔgL was used for reversion analyses by serial cell culture passages. In a first experiment, an infectious gL-negative mutant in which gL function was replaced by generation of a gD-gH hybrid protein was isolated (B. G. Klupp and T. C. Mettenleiter, J Virol 73:3014–3022, 1999). In a second, independent experiment PrV-ΔgLPassB4.1, which also replicated productively without gL, was isolated. Sequence analysis revealed mutations in gH but also in gB and gD. In a transfection-based fusion assay, two amino acid substitutions in the N-terminal part of gHB4.1 (L70P and W103R) were found to be sufficient to compensate for lack of gL, while mutations present in gBB4.1 enhanced fusogenicity. Coexpression of gBB4.1 with the homologous gHB4.1 resulted in strongly increased syncytium formation, which was further augmented by truncation of the gBB4.1 C-terminal 29 amino acids. Nevertheless, gH was still required for membrane fusion. Surprisingly, coexpression of gDB4.1 blocked syncytium formation in the fusion assays, which could be attributed to a V106A substitution within the ectodomain of gDB4.1.
IMPORTANCE In contrast to many other enveloped viruses, herpesviruses rely on the concerted action of four viral glycoproteins for membrane fusion during infectious entry. Although the highly conserved gB shows signatures of a fusion protein, for fusion induction it requires the gH/gL complex, whose role is still elusive. Here we demonstrated fusion activation by gH in the absence of gL after reversion analysis of gL-deleted pseudorabies virus. This gL-independent fusion activity depended on single amino acid exchanges affecting the gL-binding domain in gH, increasing fusogenicity in gB and allowing negative fusion regulation by gD. Thus, our results provide novel information on the interplay in the fusion machinery of herpesviruses.
INTRODUCTION
Infection of susceptible cells by herpesviruses occurs by fusion of the viral envelope with the host cell plasma membrane or, after endocytic uptake, with the membrane of the endosome. For both processes a cascade of events has to be initiated, whose molecular details are still not completely understood. The conserved viral glycoprotein B (gB) and the heterodimeric gH/gL complex form the core fusion machinery and are required for fusion during virus entry and direct virus transmission to neighboring cells (reviewed in references 1 and 2).
In the alphaherpesviruses herpes simplex virus 1 (HSV-1) and pseudorabies virus (PrV), the cascade ultimately leading to membrane fusion is initiated by interaction of the essential viral attachment glycoprotein gD to specific host cell receptors. As shown for HSV-1, this results in a conformational rearrangement in gD. The C-terminal 50 amino acids (aa) of the ectodomain, which are tightly folded around the N-terminal part in the unbound state, are displaced by receptor binding, thereby opening the structure (3, 4). This conformational switch is believed to signal, in a yet-unknown manner, to the gH/gL complex, which in turn triggers membrane fusion catalyzed by gB (reviewed in references 1 and 2).
The crystal structures of HSV-1 gB and Epstein-Barr virus (EBV) gB are surprisingly similar to structures of the fusion proteins vesicular stomatitis virus (VSV) G and baculovirus gp64 (5–8). All three fusion proteins are characterized by an alpha-helical coiled-coil domain, similar to that in class I fusion proteins, and extended hairpins with fusion peptides, similar to class II fusion proteins, which led to the allocation of a new class of fusion proteins, designated class III (reviewed in references 1 and 2). In spite of the close structural similarity, in contrast to VSV G and baculovirus gp64, herpesvirus gB alone is not sufficient to induce efficient membrane fusion but depends on the presence of the gH/gL complex.
The role of the gH/gL complex, and in particular of gL, during membrane fusion is still unclear. Although gH has been proposed to act as a fusion protein (9–11), the deduced crystal structures revealed no features of viral fusion proteins, and amino acid stretches which had been suspected as potential fusion peptides are deeply buried within the molecule, arguing against a direct role in merging of lipid membranes (12). gL depends on interaction with gH for membrane association and virion incorporation due to the lack of a membrane anchor (13–16). It was long considered a chaperone for gH since it is required for correct folding, transport, and virion incorporation of gH in HSV-1 and EBV (13, 17). However, in PrV, bovine herpesvirus 4, and murid herpesvirus 4, gH is incorporated into virions also in the absence of gL. Nevertheless, gL is required for entry (18–20), pointing to a role beyond chaperoning.
While PrV gL is essential for membrane fusion during entry, direct transmission of infectivity to neighboring noninfected cells, a process which is also mediated by the core fusion machinery, occurs to a limited extent also in the absence of gL (19). The capability for restricted cell-to-cell-spread of PrV-ΔgL was used for reversion analysis by repeated passages in cell culture. This led to the isolation of a revertant virus which replicated like the wild type, demonstrating that gL function can be compensated by mutations elsewhere in the virus genome (21). The compensatory mutation in PrV-ΔgLPass could be attributed to the formation of a hybrid protein consisting of the N-terminal 271 amino acids of gD fused to the C-terminal 590 amino acids of gH, thereby deleting the gL-interaction domain (21). This gDH hybrid protein is sufficient to complement not only the absence of gL but also lack of wild-type gH and gD, singly or in different combinations, and to induce extensive syncytia after coexpression with only gB (22), indicating that gL is not directly involved in activating the fusion process.
The recently solved crystal structures of EBV gH/gL (23), HSV-2 gH/gL (24), and a core fragment of PrV gH (12), in combination with mutational analyses, shed further light on the function of the gH/gL complex. Despite low conservation of the primary amino acid sequences (25), the gH structures were surprisingly similar, and the gH ectodomains could be divided into three (24) or four (12, 23) distinct regions. Domain I, comprising the N terminus of gH, is tightly associated with gL, and its secondary structure depends on presence of both proteins (23, 24). In the PrV gH structure, which was derived from the gH core fragment present in the gDH hybrid protein (12, 21), this domain is missing. Nevertheless, PrV gH domains II to IV can be easily correlated to the gH/gL structures of EBV and HSV-2 (12). Domain II consists of a sheet of antiparallel beta-chains designated “fence” and a syntaxin-like bundle (SLB) consisting of three alpha-helices with structural similarities to cellular syntaxins (12). Integrity of the SLB and flexibility between domains II and III are important for PrV gH maturation, transport, and function (26). Domain III contains eight alpha-helices and harbors a highly conserved amino acid stretch (serine-proline-cysteine) which plays an important role in fusion (27). Domain IV, the best conserved region of gH, is composed of two four-stranded beta-sheets with connection to a region designated the “flap,” which covers a stretch of hydrophobic amino acids (12). Recent studies indicated that movement of the flap and the subsequent exposure of the hydrophobic patch are important for PrV gH function during membrane fusion (28).
We wondered whether formation of a gD-gH hybrid protein would be the only way to overcome absence of gL function. Thus, we repeated the passaging experiment with gL-deleted PrV and isolated additional infectious revertants which apparently did not produce the hybrid protein. Characterization of one of them, designated PrV-ΔgLPassB4.1, revealed compensatory mutations not only in gH but also in gB and gD, pointing to a tight regulatory balance between gD, gH(/gL), and gB.
MATERIALS AND METHODS
Cells and viruses.
Rabbit kidney (RK13) and RK13-gH/gL cells (29) were grown in Dulbecco's modified Eagle's minimum essential medium (MEM) supplemented with 10% fetal calf serum at 37°C. All viruses used in this study were derived from PrV strain Kaplan (PrV-Ka) (30). PrV mutants lacking gL have been described previously (19, 29).
Passaging of PrV-ΔgL.
Passaging of PrV-ΔgL was done as described before (21). Briefly, RK13 cells were incubated with PrV-ΔgL at a low multiplicity of infection (MOI), trypsinized when the cell monolayer was confluent, and reseeded until infectivity in the supernatant was stably detectable. Subsequently, the precleared supernatant was used to infect fresh RK13 cells. One single-plaque isolate derived from the supernatant of the 100th passage and designated PrV-ΔgLPassB4.1 was further characterized.
In vitro growth kinetics.
For analysis of growth kinetics, RK13 cells were infected with PrV-Ka, PrV-ΔgL, or PrV-ΔgLPassB4.1 at an MOI of 0.5 for 2 h at 4°C. The inoculum was removed, prewarmed medium was added, and cells were incubated for 2 h at 37°C. Thereafter, virus that had not penetrated was inactivated by low-pH treatment (31). Cells and supernatant were harvested at different times after inactivation and titrated on RK13 and RK13-gH/gL cells. For plaque size measurement, cells were infected with the same set of viruses under plaque assay conditions for 2 days. Cells were fixed and analyzed by indirect immunofluorescence using an anti-pUL19 serum (32). Images were taken using a Nikon Eclipse Ti-S fluorescence microscope, and plaque areas were measured using the Nikon NIS-Elements imaging software (Nikon, Düsseldorf, Germany).
Expression plasmids.
Generation of expression plasmids for PrV-Ka gB, gD, gH, and gL has been described previously (22). pcDNA-gB008 expresses a truncated gB lacking the C-terminal 29 amino acids (aa), including predicted endocytosis motifs (33). It possesses a higher fusogenic potential than full-length gB (22). The open reading frames of PrV-ΔgLPassB4.1 gB and gH were amplified by PCR and cloned as described previously (22, 33). The gDB4.1 open reading frame was PCR amplified from PrV-ΔgLPassB4.1 genomic DNA using primers gDfw and gDrv (Table 1), and truncated gBB4.1 (gBB4.1008) was obtained with primers gB ASS FW (33) and gBmutΔK883/008R (Table 1). The numbering of amino acids in gB is derived from GenBank accession number JF797218.1 (34), while the other proteins were deduced from accession number JQ809328.1 (35). PCR products were cloned into pcDNA3 (Invitrogen) via restriction sites which had been introduced by the primers. Expression plasmids for gBKa E290G, gBKa G672R, and gBKa E290G/G672R as well as for mutants gDB4.1 V25A, gDB4.1 V106A, gDB4.1 FS379 (frameshifted), gDKa A25V, gDKa A106V, and gDKa A25V/A106V were generated using the QuikChange II XL site-directed mutagenesis kit (Agilent) as described recently (27) and primers shown in Table 1. Expression plasmids for gBKa ΔK883, gB E290G/ΔK883, and gBKa G672R/ΔK883 were created by ligation of BamHI/FseI cleavage products derived from pcDNA-gB or the respective mutated plasmids and pcDNA-gBB4.1. Correct mutagenesis and cloning were verified by sequencing. Protein expression was controlled by indirect immunofluorescence and Western blotting after transfection into RK13 cells (data not shown).
TABLE 1.
Primers used in this study
| Primer | Sequence (5′→3′)a | Restriction site | Locationb |
|---|---|---|---|
| gDfw | CAC AGA ATT CAC CTG CCA GCG CCA TGC | EcoRI | 121132–121148 |
| gDrv | CAC AGA ATT CCA TCG ACG CCG GTA CTG C | EcoRI | 122370–121353 |
| gBmutΔK883/008R | CCG AAT TCC TAC CCG CTG TTC TTG CGC GC | EcoRI | 16138–16157 |
| gBmutE290G F | CGT CGA GGA GGT GGG GGC GCG CTC CG | 17943–17918 | |
| gBmutE290G R | CG GAG CGC GCC CCC ACC TCC TCG ACG | 17918–17943 | |
| gBmutG672R F | CGC TAC TTT AAG CTG AGG AGC GGG TAC G | 16799–16772 | |
| gBmutG672R R | C GTA CCC GCT CCT CAG CTT AAA GTA GCG | 16772–16799 | |
| gDmut V25A-fwd | G GAC GCC GTG CCC GCG CCG ACC TTC CCC C | 121164–121173 | |
| gDmut V25A-rev | G GGG GAA GGT CGG CGC GGG CAC GGC GTC C | 121173–121164 | |
| gDmut A25V-fwd | G GAC GCC GTG CCC GTG CCG ACC TTC CCC C | 121164–121173 | |
| gDmut A25V-rev | G GGG GAA GGT CGG CAC GGG CAC GGC GTC C | 121173–121164 | |
| gDmut V106A-fwd | GCG GAC GGG TGC GCG CAC CTG CTG TAC | 121247–121255 | |
| gDmut V106A-rev | GTA CAG CAG GTG CGC GCA CCC GTC CGC | 121255–121247 | |
| gDmut A106V-fwd | GCG GAC GGG TGC GTG CAC CTG CTG TAC | 121247–121255 | |
| gDmut A106V-rev | GTA CAG CAG GTG CAC GCA CCC GTC CGC | 121255–121247 |
Nucleotide substitutions are underlined, and added restriction sites are in bold.
Primer sequence locations in the PrV-Ka genome corresponding to accession number JF797218.1 (34) for gB primers or JQ809328.1 (35) for gD and gH primers.
Fusion assays.
Fusion activity of the different glycoprotein mutants was tested after transfection of RK13 cells as described recently (27). Approximately 1.8 × 105 RK13 cells per well were seeded into 24-well culture dishes. On the following day, cells were transfected with 125 ng each of the expression plasmids in different combinations and with pEGFP-N1 (Clontech) as a marker in 50 μl serum-free MEM using 1 μl polyethylenimine (PEI). Empty vector (pcDNA3) served as negative control. The mixture was incubated for 15 min at room temperature and then added to the cells. After 24 h at 37°C, cells were washed with phosphate-buffered saline (PBS), fixed with 3% paraformaldehyde (PFA) for 20 min, and washed two times with PBS. Syncytium formation was analyzed using a Nikon Eclipse Ti-S fluorescence microscope and the Nikon NIS-Elements imaging software (Nikon, Düsseldorf, Germany). The area of cells with three or more nuclei within 10 fields of view was measured, and the mean value was multiplied by the number of syncytia to give the total fusion activity. The experiment was repeated three times, and average percent values with respect to control transfections, as well as standard deviations, were calculated.
RESULTS
Isolation of PrV-ΔgLPassB4.1.
In the first passaging experiment, a PrV-ΔgL revertant which efficiently entered cells in the absence of gL after formation of a hybrid gene expressing a gDH hybrid protein was isolated (21). Since the formation of this hybrid gene was unexpected, we tested whether it would be repeated in an independent passaging experiment or whether other compensatory mutations inducing membrane fusion in the absence of gL could be unraveled. From the supernatant of an independent passage experiment with RK13 cells, one isolate, designated PrV-ΔgLPassB4.1, was further investigated.
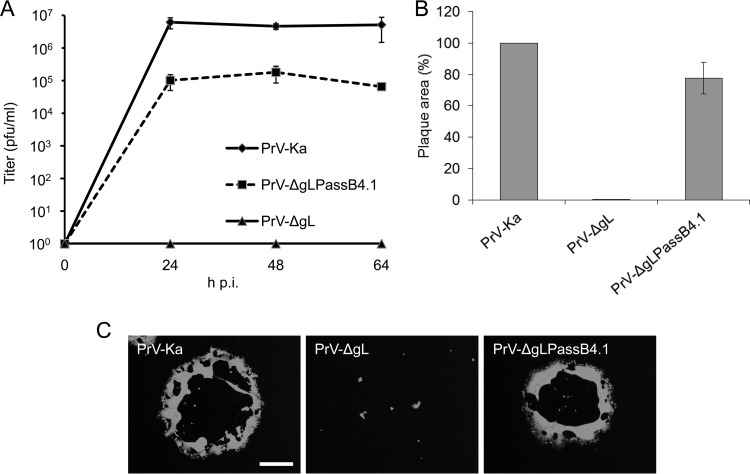
Characterization of PrV-ΔgLPassB4.1 in cell culture showed, in contrast to PrV-ΔgL, productive virus replication in noncomplementing cells, with plaque areas reaching approximately 80% and 20- to 80-fold-lower titers than PrV-Ka (Fig. 1). Virus propagation on gL-expressing cells had no influence on the in vitro replication properties (data not shown), corroborating the gL-independent phenotype.
FIG 1.
In vitro growth properties of PrV-ΔgLPassB4.1. (A) For growth kinetics, RK13 cells were infected with PrV-Ka, PrV-ΔgL, or PrV-ΔgLPassB4.1 at an MOI of 0.5. Total cell lysates were harvested after 24, 48, and 64 h and titrated on RK13 cells. Given are mean values from three independent experiments with the corresponding standard deviations. (B) Cells were infected with PrV-Ka, PrV-ΔgL, or PrV-ΔgLPassB4.1 under plaque assay conditions and fixed at 2 days postinfection. Infected cells were visualized by indirect immunofluorescence with a monospecific anti-pUL19 serum, and plaque areas were measured. Plaque areas formed by PrV-Ka were set as 100%, and values for PrV-ΔgL and PrV-ΔgLPassB4.1 were calculated accordingly. Shown are the mean values and standard deviations from three independent experiments. (C) Representative images. Bar, 400 μm.
Sequencing of genes encoding glycoproteins involved in fusion in PrV-ΔgLPassB4.1.
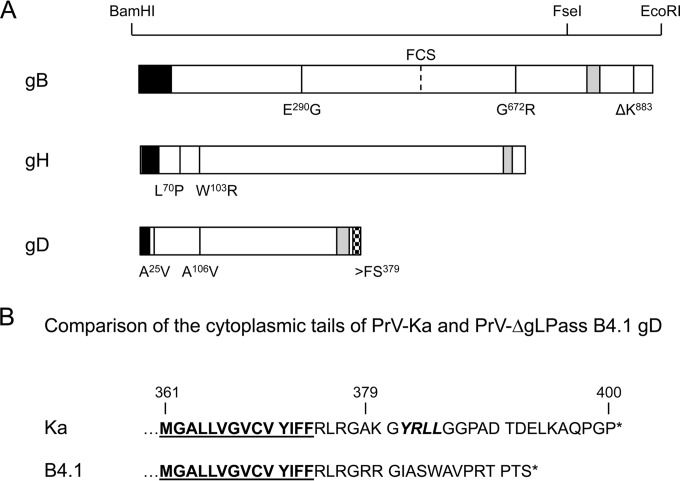
To test whether changes in the components of the herpesvirus fusion machinery account for the gL-independent entry and membrane fusion mechanism, the complete open reading frames encoding gH, gB, and gD were amplified from genomic PrV-ΔgLPassB4.1 DNA and cloned into the eukaryotic expression vector pcDNA3 (Invitrogen). Sequencing of the gHB4.1 open reading frame revealed two amino acid changes, L70P and W103R, which are both located in the predicted gL-binding domain. PrV-ΔgLPassB4.1 gB contains two amino acid changes in the ectodomain (E290G and G672R) and a deletion of a lysine residue at position 883 (ΔK883) in the cytoplasmic domain, while gD specifies two point mutations in the ectodomain (A25V and A106V) and a frameshift mutation after codon 378 leading to an altered and truncated cytoplasmic tail (Fig. 2).
FIG 2.
Mutations in PrV-ΔgLPassB4.1. (A) Amino acid changes found in PrV-ΔgLPassB4.1 gB, gH, and gD compared to PrV-Ka are indicated at the corresponding positions in one-letter code. Restriction enzyme recognition sites used for cloning of gB mutants are indicated above the diagram. The predicted signal sequences are shown in black and the transmembrane domains in gray. The location of the furin cleavage site (FCS) in gB is given. The frameshift mutation (>FS379) in gDB4.1, which results in an altered cytoplasmic tail, is indicated by a checkered box. (B) Amino acid sequences of the gDKa and gDB4.1 C termini. Residues predicted to reside within the membrane are underlined, and the C-terminal ends are marked by asterisks. Indicated by italics is the predicted endocytosis motif in gDKa which is lost in gDB4.1 (58).
Mutations in the predicted gL-binding domain of gHB4.1 substitute for gL in transient cell-cell fusion assays.
As shown before, cotransfection of expression plasmids encoding PrV glycoproteins gB, gH, and gL results in the formation of multiple syncytia (22). In contrast to a similar assay reported for HSV-1 (36), addition of the plasmid encoding PrV gD only moderately enhances fusion, while omission of gL significantly reduces fusion (22). In both systems, however, in the absence of gB or gH expression, syncytium formation was reduced to background levels.
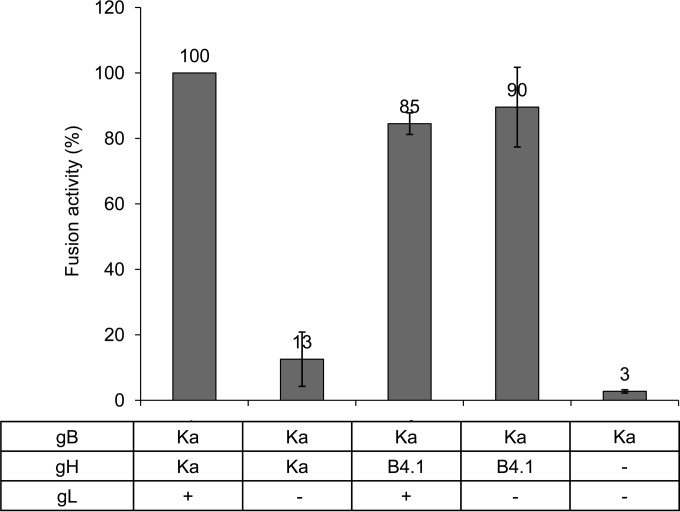
To analyze the effects of the mutations in the predicted gL-binding domain of gHB4.1, RK13 cells were transfected with plasmids coding for gBKa and gHKa or gHB4.1 in the presence or absence of gL expression. At 1 day posttransfection, the area of cells containing more than two nuclei was measured and multiplied by the total number of syncytia. Mean values from three independent assays with corresponding standard deviations are shown in Fig. 3. Results from assays with gBKa, gHKa, and gL were set as 100%. Substitution of gHKa by gHB4.1 resulted in an only slightly reduced total fusion activity, but in contrast to assays with gHKa, expression of gL had no enhancing effect, demonstrating gL-independent function.
FIG 3.
Mutations in gH substitute for gL function in transient-transfection fusion assays. To test whether the mutations present in gHB4.1 are sufficient to compensate for lack of gL, RK13 cells were cotransfected with expression plasmids for gBKa and gHKa or gHB4.1 in the presence or absence of gL expression as indicated. Results from assays with gBKa, gHKa, and gL were set as 100%. As a negative control, an assay with the gBKa expression plasmid only (the DNA amount was adjusted by addition of empty vector) was used. The relative fusion activity (number of syncytia × syncytium area) with corresponding standard deviation from three independent experiments is depicted.
Mutations present in gBB4.1 enhance cell-cell fusion.
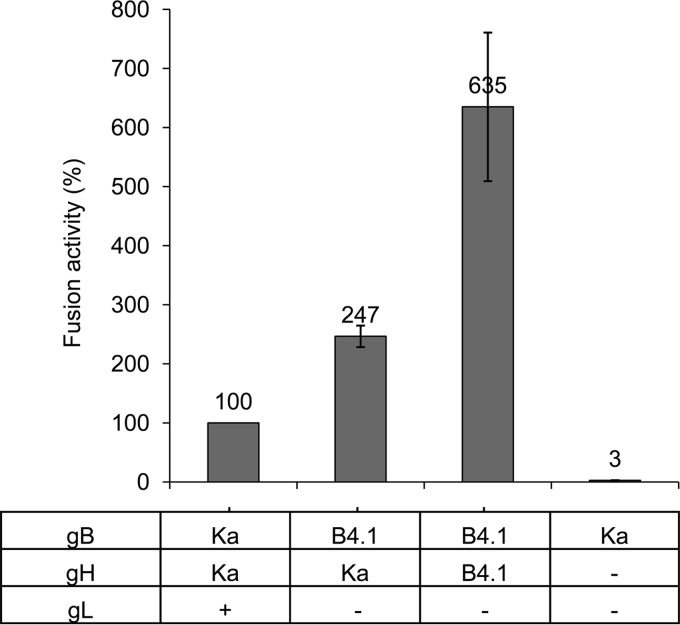
To test for the influence of the mutations in gBB4.1, it was coexpressed with gHKa. Coexpression increased fusion activity by approximately 2.5-fold compared to that in assays with gBKa, gHKa, and gL. Syncytium formation was further augmented by coexpression of gBB4.1 with the homologous gHB4.1, which resulted in approximately 6.5-fold-higher values (Fig. 4). Additional expression of gL had no effect (results not shown).
FIG 4.
gBB4.1 contains fusion-enhancing mutations. To test for fusion activity of gBB4.1, RK13 cells were cotransfected with combinations of plasmids expressing the different glycoproteins as indicated. Results from assays with gBKa, gHKa, and gL were set as 100%, and those with the gBKa expression plasmid only (together with empty vector) served as negative control. Shown are the mean values and corresponding standard deviations from three independent experiments (these data, originating from a larger data set testing multiple variables, are also included in Fig. 7).
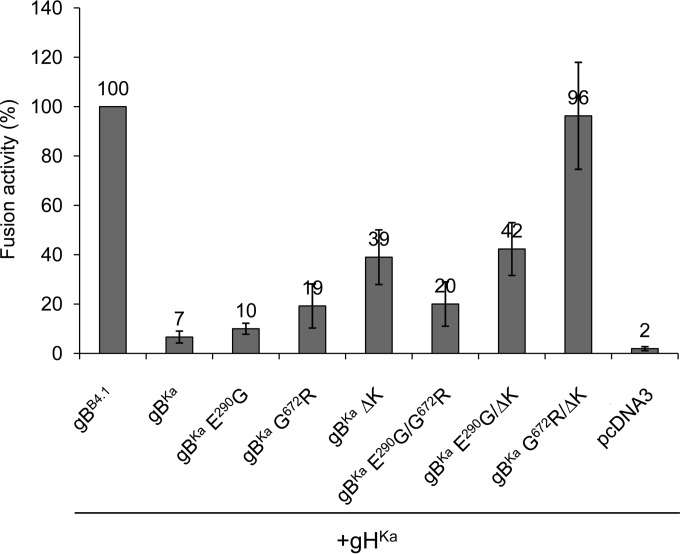
To elucidate which of the three amino acid changes in gBB4.1 account for the higher fusion activity, the mutations were introduced into gBKa singly or in combinations. Fusion activity was tested by coexpression of gHKa and the different gB mutants, and fusion activity observed with gBB4.1 and gHKa was set as 100%. The single-amino-acid substitution G672R and the deletion of lysine at position 883 (ΔK883) increased fusion activity compared to that of gBKa (Fig. 5), while pronounced syncytium formation similar to that of gBB4.1 was achieved by the combination of both mutations. The replacement of glutamate with glycine at position 290 had no significant effect on fusion (Fig. 5).
FIG 5.
The G672R mutation and the deletion of K883 in the cytoplasmic tail of gBB4.1 account for enhanced fusion activity. To test which changes in gBB4.1 are responsible for the higher fusion activity, mutations were introduced singly or in combinations into gBKa. Fusion activity in combination with gHKa was measured in cotransfected RK13 cells, and results from assays with gBB4.1 and gHKa were set as 100%. Given are mean values from three independent experiments with corresponding standard deviations.
Deletion of the C-terminal 29 amino acids in PrV-ΔgLPassB4.1 gB results in excessive cell-cell fusion.
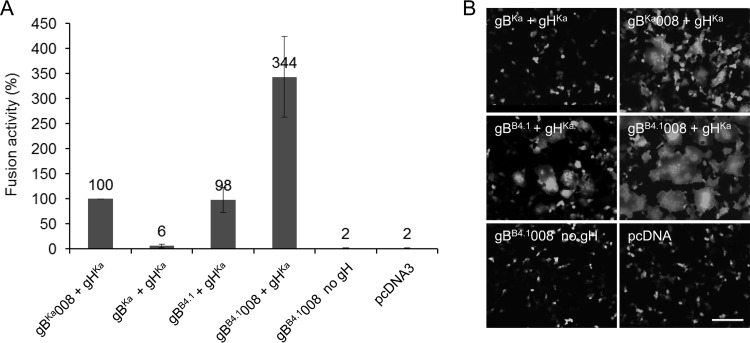
Deletion of 29 amino acids from the C terminus of gBKa, including predicted dileucine- and tyrosine-based sorting signals, resulted in the accumulation of gB at the cell surface and significantly enhanced fusion activity (22, 33). To analyze whether the same C-terminal truncation further increases fusion activity of gBB4.1 and whether this might be sufficient for gB-only-mediated membrane fusion, gHKa was coexpressed with either full-length gBKa, C-terminally truncated gBKa008, gBB4.1, or C-terminally truncated gBB4.1008 (Fig. 6). Mean values from three independent experiments, with results from gBKa008 coexpressed with gHKa set as 100%, and corresponding standard deviations are shown in Fig. 6A. Full-length gBB4.1 already showed levels of syncytium formation comparable to those of the highly fusogenic gBKa008. However, coexpression of gBB4.1008 with gHKa resulted in excessive syncytium formation, with 3.5-fold-higher values. However, despite this extreme fusion activity with gHKa, gBB4.1008 alone was not sufficient to induce cell-cell fusion above background levels. Representative images are shown in Fig. 6B.
FIG 6.
Deletion of the C-terminal 29 amino acids from gBB4.1 leads to excessive cell-cell fusion. (A) To analyze the influence of the deletion of the 29 C-terminal amino acids in gBB4.1, RK13 cells were cotransfected with the corresponding gB expression plasmids and pcDNA-gHKa. Results from assays with gBKa008 and gHKa were set as 100%. Transfection fusion assays were repeated three times, and mean values and corresponding standard deviations were calculated. (B) Representative images. Bar, 100 μm.
PrV-ΔgLPassB4.1 gD inhibits cell-cell fusion.
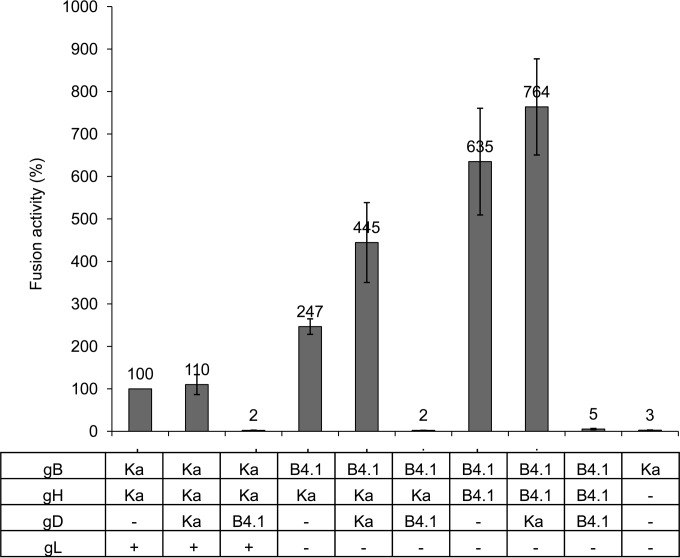
Although PrV-ΔgLPassB4.1 showed a syncytial phenotype in cell culture (Fig. 1), hyperfusion as observed after coexpression of gBB4.1 and gHB4.1 in transient assays was not obvious. To investigate the influence on fusion of gDB4.1, which carries two amino acid substitutions (A25V and A106V) in the ectodomain and a frameshift mutation affecting the cytoplasmic tail (Fig. 2), fusion assays were repeated in the presence of either gDKa or gDB4.1. As shown in Fig. 7, coexpression of gDKa slightly enhanced the number and size of syncytia not only with the wild-type glycoproteins but, even more significantly, also with gBB4.1 and gHB4.1. In striking contrast, coexpression of gDB4.1 completely blocked syncytium formation in assays with the homologous PrV-ΔgLPassB4.1-derived proteins and also with the wild-type proteins (Fig. 7).
FIG 7.
PrV-ΔgLPassB4.1 gD inhibits cell fusion. RK13 cells were cotransfected with the indicated expression plasmids. Fusion activity in assays including gBKa, gHKa, and gL but without gD was set at 100%. Shown are mean relative values from three independent experiments with standard deviations.
The A106V mutation is responsible for the fusion inhibition of gDB4.1.
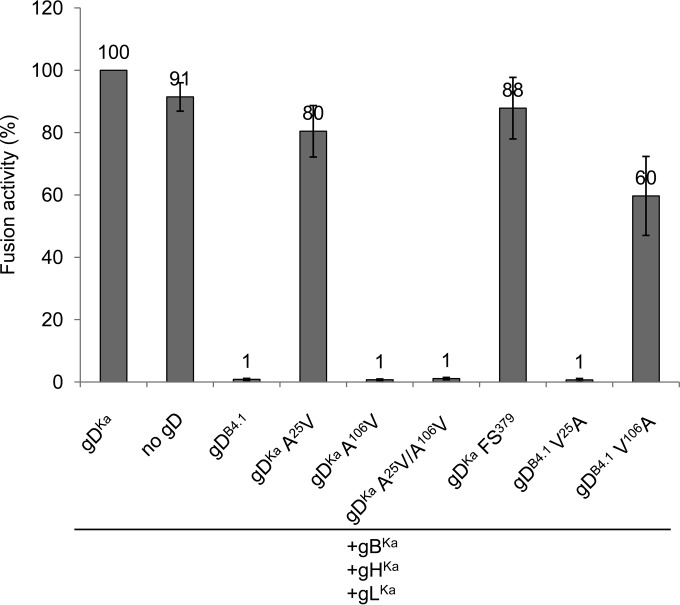
To test whether the amino acid substitutions in the ectodomain (A25V and A106V) or the frameshift mutation (FS379) affecting the C terminus is responsible for the inhibitory effect of gDB4.1, the different mutations were introduced into gDKa or repaired in gDB4.1 separately or in combinations. While the mutation A25V or the nucleotide insertion after codon 378 in gDKa affected syncytium formation only slightly, the A106V substitution resulted in a complete abrogation of cell-cell fusion. Consistently, restoration of alanine at position 106 in gDB4.1 rescued fusion activity (Fig. 8). The other mutations, either repaired in gDB4.1 or introduced into gDKa, affected syncytium formation only marginally.
FIG 8.
The amino acid substitution A106V is responsible for fusion inhibition by gDB4.1. Mutations found in gDB4.1 were introduced into gDKa or repaired in gDB4.1 and tested in transfection fusion assays with gBKa, gHKa, and gLKa. Shown are mean values and standard deviations from three independent experiments, with results from cotransfections with gBKa, gDKa, gHKa, and gLKa set as 100%.
DISCUSSION
Most enveloped viruses use only one or two viral proteins for receptor binding and membrane fusion, and the molecular details are well understood for several of them (reviewed in reference 37). In contrast, the molecular basis for membrane fusion induced by herpesviruses, which engage four or more different proteins, is still largely enigmatic. We demonstrate here that the absence of a herpesvirus protein, gL, which normally forms a heterodimeric complex with gH and is essential for entry, can be compensated by mutations (i) within the gL-binding domain of gH resulting in loss of gL interaction, (ii) within gB enhancing its fusogenicity, and (iii) within gD to allow negative regulation of excess fusion.
Elucidation of the crystal structures of the key players in fusion from different herpesviruses has sparked studies on membrane fusion using targeted mutagenesis (26, 28, 38–41). Here, we used a different approach, designated reversion analysis, to select for spontaneous mutations which compensate for defects induced by the absence of a crucial component of the fusion-inducing machinery. Repeated passaging of respective deletion mutants in cultured cells resulted in the isolation of revertants which were able to productively infect cells in the absence of either gD, gL, or C-terminally truncated gB (21, 42, 43). The absence of gL was compensated by the formation of a hybrid gene encoding the N-terminal 271 amino acids of gD fused to the 590 C-terminal amino acids of gH, thereby lacking the first 96 gH codons, which encompass the predicted gL interaction domain (21). This gDH hybrid protein was able to complement viruses lacking gD, gH, or gL singly or in combination (29) and was sufficient to induce efficient cell-cell fusion in transient-transfection fusion assays in combination with only gB (22), reducing the number of proteins required for herpesvirus-induced membrane fusion to two.
From an independent passage experiment with gL-deleted PrV, we isolated another revertant, designated PrV-ΔgLPassB4.1. In this revertant, no genetic rearrangement comprising the gH or gD gene region was observed, but sequence analyses revealed two amino acid changes in the N-terminal gL-binding domain of gH (L70P and W103R). To test the impact of these mutations on membrane fusion, we used our transfection-based cell-cell fusion assay (27). This assay partially reflects fusion during entry but more closely resembles fusion during direct cell-to-cell spread, for which gD is not required in PrV (44, 45). Coexpression of PrV gBKa, gHKa, and gLKa is sufficient to induce multiple syncytia in rabbit kidney cells without the need for activation of the core fusion machinery by binding of gD to cellular receptors (22). This contrasts the situation found in HSV-1 (36). Here, we show that PrV gHB4.1 in combination with gBKa induced cell-cell fusion in the absence of gD and gL to an extent comparable to that observed after coexpression of all four wild-type proteins. This indicates that the two mutations in the N-terminal part of gHB4.1 are sufficient to compensate for the absence of gL and its function in membrane fusion. As a consequence, addition of the gL expression plasmid had no fusion promoting effect (Fig. 3), and coprecipitation studies demonstrated that gHB4.1 is unable to interact with gL (data not shown). Unfortunately, gH domain I comprising the two amino acid substitutions was not present in the recently solved crystal structure of PrV gH (12) and is the least conserved region within herpesvirus gH proteins, so the impact of the amino acid substitutions on gH structure cannot be predicted. However, elucidation of the crystal structure of gHB4.1 might shed light on the conformation of a gH primed for fusion activation without gL.
Cell lines stably expressing gHB4.1 complemented plaque formation by mutants lacking gL, gH, or both proteins simultaneously to approximately 80% of wild-type virus diameters, but titers derived from these cells reached only approximately 104 PFU/ml (data not shown). This indicates that the two amino acid substitutions in gHB4.1 compensate for gL function during direct cell-cell spread but are not sufficient to compensate for all gL functions during the viral replication cycle. Since, therefore, additional mutations must have occurred, we focused on the other components of the fusion machinery as the most auspicious candidates. In contrast to the first PrV-ΔgLPass revertant (21), analysis of PrV-ΔgLPassB4.1 disclosed additional mutations in gB and gD.
gBB4.1 specifies two amino acid substitutions in the ectodomain and a deletion of one of two consecutive lysine residues present at positions 883 and 884 in the cytoplasmic tail (ΔK883). Fusion assays showed that gBB4.1 in combination with gHKa resulted in fusion activity 2- to 3-fold-higher than that of PrV-Ka gB and gH/gL (Fig. 4). Coexpression of gBB4.1 with the homologous gHB4.1 further increased fusion by 6.5-fold (Fig. 4), indicating that mutations present in both proteins act cooperatively on membrane fusion. To test which mutation(s) is/are relevant for the enhanced fusogenicity of gBB4.1, they were introduced singly and in combinations into gBKa. Simultaneous replacement of glycine 672 by arginine (G672R) and deletion of lysine at position 883 (ΔK883) resulted in gBB4.1-like fusion activity, while the substitution E290G had no significant influence (Fig. 5). Glycine 672 in PrV gB corresponds to the well-conserved G644 in HSV-1 gB, which is located in domain IV (5). This domain of HSV-1 gB is postulated to undergo extensive conformational rearrangements during the conversion of the pre- to the postfusion form (5, 46). It is conceivable that the replacement of the small and neutral glycine by the bulky and charged arginine decreases the necessary energy for this conformational change.
The cytoplasmic domain of gB plays an important role in fusion regulation (33, 47–53). Cell-cell fusion in transfection-based assays could be significantly enhanced by deleting the most C-terminal amino acids, including the predicted endocytosis motifs (33, 52–56). Although it was originally speculated that enhanced presence of gB at the plasma membrane increases cell-cell fusion, recent data showed that the degree of surface localization is not necessarily causally related to increased fusion (52).
For EBV and HSV-1 gB, it could be shown that the cytoplasmic tail interacts with lipid membranes (48, 49, 57). Recent data indicate that predicted alpha-helical domains in the cytoplasmic tail of HSV-1 gB, designated h3 and h2b, function as clamps for stabilization of the prefusion form (53). For PrV gB, two helical regions are predicted in the cytoplasmic tail (33). The absence of helical domain II in PrV gB008 resulted in significantly enhanced fusion (22). Lysine residues at position 883/884 are located at the C-terminal end of helical domain I. Deletion of one of the basic amino acids may weaken this interaction with the membrane, thereby reducing the constraint on fusion. Whether electrostatic interactions with the membrane indeed play a role in fusion regulation has yet to be verified.
Coexpression of full-length gBB4.1 with gHKa and gL already resulted in fusion activity similar to that with gBKa008 lacking the C-terminal 29 amino acids (Fig. 6). An identical C-terminal deletion increased fusion activity of gBB4.1 further, by approximately 3-fold compared to that of gBKa008 and gBB4.1. Restoration of lysine 883 of gBB4.1008 did not affect syncytium formation (data not shown), supporting the hypothesis that the gB cytoplasmic tail modulates fusion and that both changes, deletion of several amino acids from the C terminus and deletion of a single basic residue, affect the fusion process similarly by altering gB membrane interactions.
Considering the hyperfusogenic activity of gBB4.1 with gHB4.1 in transient-transfection fusion assays, it is surprising that PrV-ΔgLPassB4.1 is competent to replicate at all and does not exhibit an excessive syncytial phenotype (Fig. 1). This could be due to the mutations present in the putative receptor-binding domain in gDB4.1 and/or the frameshift in the cytoplasmic tail, which shortens the protein and deletes a functional endocytosis motif (YRLL) (Fig. 2) (58). Coexpression of gDB4.1 in fusion assays completely inhibited syncytium formation (Fig. 7), demonstrating a dominant negative effect on membrane fusion, which was also observed in the simultaneous presence of gDKa (data not shown). The A106V mutation seems to play the major role, since its introduction into gDKa (gDKa A106V) inhibited fusion completely. However, introduction of A25V into gDKa also moderately reduced fusion to 80%, and repair of V106A in gDB4.1 resulted in only approximately 60% of wild-type fusion activity (Fig. 8). Further studies including insertion of the mutated and wild-type versions of gD into the wild-type and passaged virus backgrounds are needed to investigate the molecular basis for the fusion-inhibiting function of gDB4.1.
In summary, our results show that absence of PrV gL can be functionally compensated not only by formation of a gDH hybrid protein but also by a combination of point mutations in gH, gB, and gD. The mutations present in gHB4.1 are located in the gL-binding domain and might mimic the gD-activated gH/gL complex. Mutations present in the ectodomain and in the cytoplasmic tail of gBB4.1 could facilitate the conformational change from the pre- to the postfusion form and resulted in a hyperfusogenic phenotype. Excessive fusogenicity, however, seems to be counteracted by mutations in gDB4.1 which inhibit fusion in transient-transfection assays to restrict it to a level compatible with productive virus replication.
ACKNOWLEDGMENTS
We thank Cindy Meinke for expert technical assistance and Britta Möhl, Northwestern University, Chicago, IL, for help in the interpretation of the influence of mutations on the structure.
REFERENCES
- 1.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol 84:12292–12299. doi: 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fusco D, Forghieri C, Campadelli-Fiume G. 2005. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc Natl Acad Sci U S A 102:9323–9328. doi: 10.1073/pnas.0503907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, Cohen GH, Wiley DC, Carfi A. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J 24:4144–4153. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 6.Backovic M, Longnecker R, Jardetzky TS. 2009. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A 106:2880–2885. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche S, Bressanelli S, Rey FA, Gaudin Y. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187–191. doi: 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 8.Kadlec J, Loureiro S, Abrescia NG, Stuart DI, Jones IM. 2008. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat Struct Mol Biol 15:1024–1030. doi: 10.1038/nsmb.1484. [DOI] [PubMed] [Google Scholar]
- 9.Duus KM, Hatfield C, Grose C. 1995. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy. Virology 210:429–440. doi: 10.1006/viro.1995.1359. [DOI] [PubMed] [Google Scholar]
- 10.Galdiero S, Falanga A, Vitiello M, Browne H, Pedone C, Galdiero M. 2005. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J Biol Chem 280:28632–28643. doi: 10.1074/jbc.M505196200. [DOI] [PubMed] [Google Scholar]
- 11.Galdiero S, Falanga A, Vitiello M, D'Isanto M, Collins C, Orrei V, Browne H, Pedone C, Galdiero M. 2007. Evidence for a role of the membrane-proximal region of herpes simplex virus type 1 glycoprotein H in membrane fusion and virus inhibition. Chembiochem 8:885–895. doi: 10.1002/cbic.200700044. [DOI] [PubMed] [Google Scholar]
- 12.Backovic M, DuBois RM, Cockburn JJ, Sharff AJ, Vaney MC, Granzow H, Klupp BG, Bricogne G, Mettenleiter TC, Rey FA. 2010. Structure of a core fragment of glycoprotein H from pseudorabies virus in complex with antibody. Proc Natl Acad Sci U S A 107:22635–22640. doi: 10.1073/pnas.1011507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson AC, Johnson DC. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol 66:2240–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaye JF, Gompels UA, Minson AC. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J Gen Virol 73:2693–2698. doi: 10.1099/0022-1317-73-10-2693. [DOI] [PubMed] [Google Scholar]
- 15.Klupp BG, Baumeister J, Karger A, Visser N, Mettenleiter TC. 1994. Identification and characterization of a novel structural glycoprotein in pseudorabies virus, gL. J Virol 68:3868–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu DX, Gompels UA, Nicholas J, Lelliott C. 1993. Identification and expression of the human herpesvirus 6 glycoprotein H and interaction with an accessory 40K glycoprotein. J Gen Virol 74:1847–1857. doi: 10.1099/0022-1317-74-9-1847. [DOI] [PubMed] [Google Scholar]
- 17.Roop C, Hutchinson L, Johnson DC. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol 67:2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillet L, May JS, Colaco S, Stevenson PG. 2007. Glycoprotein L disruption reveals two functional forms of the murine gammaherpesvirus 68 glycoprotein H. J Virol 81:280–291. doi: 10.1128/JVI.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klupp BG, Fuchs W, Weiland E, Mettenleiter TC. 1997. Pseudorabies virus glycoprotein L is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J Virol 71:7687–7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lete C, Machiels B, Stevenson PG, Vanderplasschen A, Gillet L. 2012. Bovine herpesvirus type 4 glycoprotein L is nonessential for infectivity but triggers virion endocytosis during entry. J Virol 86:2653–2664. doi: 10.1128/JVI.06238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klupp BG, Mettenleiter TC. 1999. Glycoprotein gL-independent infectivity of pseudorabies virus is mediated by a gD-gH fusion protein. J Virol 73:3014–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klupp BG, Nixdorf R, Mettenleiter TC. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J Virol 74:6760–6768. doi: 10.1128/JVI.74.15.6760-6768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuura H, Kirschner AN, Longnecker R, Jardetzky TS. 2010. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc Natl Acad Sci U S A 107:22641–22646. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol 17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klupp BG, Mettenleiter TC. 1991. Sequence and expression of the glycoprotein gH gene of pseudorabies virus. Virology 182:732–741. doi: 10.1016/0042-6822(91)90614-H. [DOI] [PubMed] [Google Scholar]
- 26.Böhm SW, Eckroth E, Backovic M, Klupp BG, Rey FA, Mettenleiter TC, Fuchs W. 2015. Structure-based functional analyses of domains II and III of pseudorabies virus glycoprotein H. J Virol 89:1364–1376. doi: 10.1128/JVI.02765-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schröter C, Klupp BG, Fuchs W, Gerhard M, Backovic M, Rey FA, Mettenleiter TC. 2014. The highly conserved proline at position 438 in pseudorabies virus gH is important for regulation of membrane fusion. J Virol 88:13064–13072. doi: 10.1128/JVI.01204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs W, Backovic M, Klupp BG, Rey FA, Mettenleiter TC. 2012. Structure-based mutational analysis of the highly conserved domain IV of glycoprotein H of pseudorabies virus. J Virol 86:8002–8013. doi: 10.1128/JVI.00690-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klupp B, Altenschmidt J, Granzow H, Fuchs W, Mettenleiter TC. 2008. Glycoproteins required for entry are not necessary for egress of pseudorabies virus. J Virol 82:6299–6309. doi: 10.1128/JVI.00386-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan AS, Vatter AE. 1959. A comparison of herpes simplex and pseudorabies viruses. Virology 7:394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 31.Mettenleiter TC. 1989. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology 171:623–625. doi: 10.1016/0042-6822(89)90635-1. [DOI] [PubMed] [Google Scholar]
- 32.Klupp BG, Granzow H, Mettenleiter TC. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J Virol 74:10063–10073. doi: 10.1128/JVI.74.21.10063-10073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nixdorf R, Klupp BG, Karger A, Mettenleiter TC. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J Virol 74:7137–7145. doi: 10.1128/JVI.74.15.7137-7145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szpara ML, Tafuri YR, Parsons L, Shamim SR, Verstrepen KJ, Legendre M, Enquist LW. 2011. A wide extent of inter-strain diversity in virulent and vaccine strains of alphaherpesviruses. PLoS Pathog 7:e1002282. doi: 10.1371/journal.ppat.1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm KS, Klupp BG, Granzow H, Müller FM, Fuchs W, Mettenleiter TC. 2012. Analysis of viral and cellular factors influencing herpesvirus-induced nuclear envelope breakdown. J Virol 86:6512–6521. doi: 10.1128/JVI.00068-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner A, Bruun B, Minson T, Browne H. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol 72:873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison SC. 2015. Viral membrane fusion. Virology 479–480:498–507. doi: 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atanasiu D, Saw WT, Gallagher JR, Hannah BP, Matsuda Z, Whitbeck JC, Cohen GH, Eisenberg RJ. 2013. Dual split protein-based fusion assay reveals that mutations to herpes simplex virus (HSV) glycoprotein gB alter the kinetics of cell-cell fusion induced by HSV entry glycoproteins. J Virol 87:11332–11345. doi: 10.1128/JVI.01700-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Jardetzky TS, Longnecker R. 2013. The large groove found in the gH/gL structure is an important functional domain for Epstein-Barr virus fusion. J Virol 87:3620–3627. doi: 10.1128/JVI.03245-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Möhl BS, Sathiyamoorthy K, Jardetzky TS, Longnecker R. 2014. The conserved disulfide bond within domain II of Epstein-Barr virus gH has divergent roles in membrane fusion with epithelial cells and B cells. J Virol 88:13570–13579. doi: 10.1128/JVI.02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vleck SE, Oliver SL, Brady JJ, Blau HM, Rajamani J, Sommer MH, Arvin AM. 2011. Structure-function analysis of varicella-zoster virus glycoprotein H identifies domain-specific roles for fusion and skin tropism. Proc Natl Acad Sci U S A 108:18412–18417. doi: 10.1073/pnas.1111333108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt J, Klupp BG, Karger A, Mettenleiter TC. 1997. Adaptability in herpesviruses: glycoprotein D-independent infectivity of pseudorabies virus. J Virol 71:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nixdorf R, Klupp BG, Mettenleiter TC. 2001. Role of the cytoplasmic tails of pseudorabies virus glycoproteins B, E and M in intracellular localization and virion incorporation. J Gen Virol 82:215–226. doi: 10.1099/0022-1317-82-1-215. [DOI] [PubMed] [Google Scholar]
- 44.Rauh I, Mettenleiter TC. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol 65:5348–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol 66:894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol 9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruel N, Zago A, Spear PG. 2006. Alanine substitution of conserved residues in the cytoplasmic tail of herpes simplex virus gB can enhance or abolish cell fusion activity and viral entry. Virology 346:229–237. doi: 10.1016/j.virol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Silverman JL, Greene NG, King DS, Heldwein EE. 2012. Membrane requirement for folding of the herpes simplex virus 1 gB cytodomain suggests a unique mechanism of fusion regulation. J Virol 86:8171–8184. doi: 10.1128/JVI.00932-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia NJ, Chen J, Longnecker R. 2013. Modulation of Epstein-Barr virus glycoprotein B (gB) fusion activity by the gB cytoplasmic tail domain. mBio 4:e00571–12. doi: 10.1128/mBio.00571-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bold S, Ohlin M, Garten W, Radsak K. 1996. Structural domains involved in human cytomegalovirus glycoprotein B-mediated cell-cell fusion. J Gen Virol 77:2297–2302. doi: 10.1099/0022-1317-77-9-2297. [DOI] [PubMed] [Google Scholar]
- 51.Gage PJ, Levine M, Glorioso JC. 1993. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B. J Virol 67:2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan Z, Grantham ML, Smith MS, Anderson ES, Cardelli JA, Muggeridge MI. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J Virol 76:9271–9283. doi: 10.1128/JVI.76.18.9271-9283.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogalin HB, Heldwein EE. 23 September 2015. The interplay between the HSV-1 gB cytodomains and the gH cytotail during cell-cell fusion. J Virol doi: 10.1128/JVI.02391-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baghian A, Huang L, Newman S, Jayachandra S, Kousoulas KG. 1993. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J Virol 67:2396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdary TK, Heldwein EE. 2010. Syncytial phenotype of C-terminally truncated herpes simplex virus type 1 gB is associated with diminished membrane interactions. J Virol 84:4923–4935. doi: 10.1128/JVI.00206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Zhang X, Jardetzky TS, Longnecker R. 2014. The Epstein-Barr virus (EBV) glycoprotein B cytoplasmic C-terminal tail domain regulates the energy requirement for EBV-induced membrane fusion. J Virol 88:11686–11695. doi: 10.1128/JVI.01349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park SJ, Seo MD, Lee SK, Lee BJ. 2008. Membrane binding properties of EBV gp110 C-terminal domain; evidences for structural transition in the membrane environment. Virology 379:181–190. doi: 10.1016/j.virol.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 58.Ficinska J, Van Minnebruggen G, Nauwynck HJ, Bienkowska-Szewczyk K, Favoreel HW. 2005. Pseudorabies virus glycoprotein gD contains a functional endocytosis motif that acts in concert with an endocytosis motif in gB to drive internalization of antibody-antigen complexes from the surface of infected monocytes. J Virol 79:7248–7254. doi: 10.1128/JVI.79.11.7248-7254.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]