ABSTRACT
Because the viral DNA burden correlates with disease development, we investigated the contribution of monocyte subsets (classical, intermediate, and nonclassical monocytes) to the total viral burden in 22 human T cell leukemia virus type 1 (HTLV-1)-infected individuals by assessing their infectivity status, frequency, as well as chemotactic and phagocytic functions. All three monocyte subsets sorted from HTLV-1-infected individuals were positive for viral DNA, and the frequency of classical monocytes was lower in the blood of HTLV-1-infected individuals than in that of uninfected individuals, while the expression levels of the chemokine receptors CCR5, CXCR3, and CX3CR1 in classical monocytes were higher in HTLV-1-infected individuals than uninfected individuals; the percentage of intermediate monocytes and their levels of chemokine receptor expression did not differ between HTLV-1-infected and uninfected individuals. However, the capacity of intermediate monocytes to migrate to CCL5, the ligand for CCR5, was higher, and a higher proportion of nonclassical monocytes expressed CCR1, CXCR3, and CX3CR1. The level of viral DNA in the monocyte subsets correlated with the capacity to migrate to CCL2, CCL5, and CX3CL1 for classical monocytes, with lower levels of phagocytosis for intermediate monocytes, and with the level of viral DNA in CD8+ and CD4+ T cells for nonclassical monocytes. These data suggest a model whereby HTLV-1 infection augments the number of classical monocytes that migrate to tissues and become infected and the number of infected nonclassical monocytes that transmit virus to CD4+ and CD8+ T cells. These results, together with prior findings in a macaque model of HTLV-1 infection, support the notion that infection of monocytes by HTLV-1 is likely a requisite for viral persistence in humans.
IMPORTANCE Monocytes have been implicated in immune regulation and disease progression in patients with HTLV-1-associated inflammatory diseases. We detected HTLV-1 DNA in all three monocyte subsets and found that infection impacts surface receptor expression, migratory function, and subset frequency. The frequency of nonclassical patrolling monocytes is increased in HTLV-1-infected individuals, and they have increased expression of CCR1, CXCR3, and CX3CR1. The viral DNA level in nonclassical monocytes correlated with the viral DNA level in CD4+ and CD8+ T cells. Altogether, these data suggest an increased recruitment of classical monocytes to inflammation sites that may result in virus acquisition and, in turn, facilitate virus dissemination and viral persistence. Our findings thus provide new insight into the importance of monocyte infection in viral spread and suggest targeting of monocytes for therapeutic intervention.
INTRODUCTION
Approximately 2 to 3% of human T cell leukemia virus type 1 (HTLV-1)-infected individuals develop adult T-cell leukemia/lymphoma (ATL) and another 2 to 3% develop HTLV-1-associated myelopathy (HAM)/tropical spastic paraparesis (TSP) in their lifetimes (1–4). In addition to HAM/TSP (5, 6), HTLV-1 is also associated with other inflammatory conditions, such as uveitis (6) Sjögren's syndrome (7), bronchoalveolitis and arthritis (8), and polymyositis (9). It is noteworthy that some patients present with more than one of these inflammatory conditions (10). HTLV-1 primarily infects CD4+ and CD8+ effector and memory T cells and regulatory CD4+ CD25+ T cells (11, 12). A high viral DNA burden in peripheral blood mononuclear cells (PBMCs) is a risk factor for HAM/TSP (13) and ATL development (14–16), and patients with HAM/TSP have a higher virus level in the cerebrospinal fluid (CSF) than in the peripheral blood (12). The virus level alone is not sufficient to differentiate symptomatic patients from healthy carriers, suggesting the importance of other factors, including the host immune response (16–20). HAM/TSP patients present diverse immunological alterations, such as increased levels of spontaneous lymphocyte proliferation (21, 22), tax-specific cytotoxic CD8+ T cell expansion, and the production of high levels of inflammatory cytokines (23–25). Several studies have also suggested that monocytes are involved in immune regulation and disease progression in patients with HAM/TSP. Jones et al. showed that dendritic cells (DCs) can be efficiently infected in vitro by cell-free virus (26), and Alais et al. went on to further show that the virus must be within cellular biofilms for DC infection (27). In addition, DCs beneath the epithelial barrier can be infected by cell-free virus through a transcytosis mechanism (28). Infected DCs have been shown to effectively transmit viruses to CD4+ T cells (26, 27). Moreover, HTLV-1-infected DCs can stimulate CD4+ and CD8+ T cells (29), and infection of CD14+ cells with the concomitant expression of interleukin-15 (IL-15) mediates spontaneous degranulation and gamma interferon (IFN-γ) production in CD8+ T cells (30). Furthermore, the maturation of DCs seems to be inhibited in HTLV-1-infected patients, which could contribute to the complex immune dysregulation that underlies HTLV-1 pathogenesis (31, 32).
Altogether there appears to be a deregulation of immune responses that may be associated with abnormal immune stimulation. Monocytes are precursors of tissue macrophages and dendritic cells and play a central role in the immune response to pathogens. Monocytes can be infected in vitro and in vivo by HTLV-1 (26, 29, 30, 33–40). Furthermore, studies with nonhuman primates indicate that monocyte infection, which depends on the expression of the viral orf-I-encoded p8 and p12 proteins and the viral p30 protein, is pivotal for viral infectivity and persistence in vivo (38, 40, 41). However, recent in vitro studies by others demonstrated that infection of primary monocytes is abortive due to the expression of the sterile alpha motif and histidine/aspartic acid domain-containing protein 1 (SAMHD1) restriction factor and that, by hydrolyzing endogenous deoxynucleoside triphosphates, it inhibits reverse transcription (RT) (42), calling into question the role of monocytes in viral persistence.
In humans and nonhuman primates, peripheral blood monocytes can be classified into three main subsets on the basis of the expression levels of CD14 and CD16 molecules (43, 44). CD14+ CD16− monocytes, which are known as classical monocytes, are the most prevalent subset in human blood; CD14+ CD16+ monocytes are referred to as intermediate, and CD14− CD16+ monocytes are referred to as nonclassical (44, 45). Classical monocytes express high levels of CCR2, while nonclassical monocytes express CX3CR1 (46–48). Monocytes expressing high levels of surface CD16 are expanded in elderly individuals, as well as in a variety of inflammatory and infectious states (43, 44, 47, 49–51). The CD16+ monocytes are phenotypically activated and express elevated intracellular levels of proinflammatory cytokines (47, 50, 52). Interestingly, patients with multiple sclerosis (MS), a neuroinflammatory disease, present a significant expansion in the proportion of the CD16+ monocyte subset (53, 54) that responds with a pronounced decrease after glucocorticoid (GC) treatment (55). During infection by some viruses, CD16+ monocytes tend to increase (56). In HIV infection, both nonclassical and intermediate monocytes are infected and expanded. Furthermore, HIV-infected monocytes and macrophages can be found in patients with no detectable plasma viral loads (57, 58).
While monocytes also appear to be targets of HTLV-1 infection, their role in infection and the inflammatory stages of HAM/TSP remains unknown. Here, we investigated whether monocyte subsets are infected by HTLV-1 by studying ex vivo PBMCs from HTLV-1-infected individuals with or without HAM/TSP and found that monocytes are infected and contribute to up to 15% of the total virus burden. HTLV-1 appears to alter their migratory and phagocytic functions to persist in the host.
MATERIALS AND METHODS
Primary cells.
For phenotyping and functional studies, PBMC samples were obtained from 10 healthy volunteers and 23 HTLV-1-infected patients. Among them, 15 individuals had HAM/TSP (these are referred to as HT patients) and 8 had HTLV-1-related diseases other than HAM/TSP and were designated to have no HAM/TSP (these are referred to as NHT patients) (Table 1). PBMCs were isolated from EDTA-anticoagulated blood samples by density gradient sedimentation and frozen until assayed. Samples were obtained upon informed consent according to the Helsinki Statement, and the study was approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board.
TABLE 1.
Virological and clinical status of HTLV-1-infected individuals for phenotypic analysisa
| Patient group and patient | Clinical status | Treatmentb | % PBMCs with viral DNA |
|---|---|---|---|
| No HAM/TSP | |||
| NHT-1 | Diffuse pain | Untreated | 21.89 |
| NHT-2 | Myositis | Prednisone (March 2009 to July 2009) and azathioprine (March 2009 to time of evaluation) | 4.21 |
| NHT-3 | Asymptomatic | Untreated | 1.45 |
| NHT-4 | Uveitis, pain | Prednisone eyedrops | 9.27 |
| NHT-5 | Pain | Untreated | 10.37 |
| NHT-6 | Asymptomatic | Untreated | 0.5 |
| NHT-7 | Asymptomatic | NAc | 31.37 |
| NHT-8 | Asymptomatic | NA | 0.12 |
| HAM/TSP | |||
| HT-1 | HAM/TSP | Untreated | 14.46 |
| HT-2 | HAM/TSP | Untreated | 17.85 |
| HT-3 | HAM/TSP | Untreated | 10.77 |
| HT-4 | HAM/TSP | 5 days of intravenous methylprednisolone sodium succinate (Solumedrol; December 2010) | 8.65 |
| HT-5 | HAM/TSP | Untreated | 21.4 |
| HT-6 | HAM/TSP | IFN-β1 (Rebif; since July 2008) | 14.84 |
| HT-7 | HAM/TSP | Untreated | 6.41 |
| HT-8 | HAM/TSP | Untreated | 12.15 |
| HT-9 | HAM/TSP | Untreated | 8.32 |
| HT-10 | HAM/TSP, uveitis, dermatitis | Prednisone and methotrexate (since 2009) | 31.19 |
| HT-11 | HAM/TSP, Uveitis | Prednisone (multiple courses for uveitis and asthma) | 9.05 |
| HT-12 | HAM/TSP | Danazol | 3.91 |
| HT-13 | HAM/TSP | HumikB1 trial (last infusion, April 2007) | 9.71 |
| HT-14 | HAM/TSP | HumikB1 trial (starting September 2007) | 13.15 |
Data are from the humanized anti-IL-2/IL-15 receptor beta antibody (HumikB1) trial with a monoclonal antibody against CD122.
The listed drugs are immunomodulatory/immunosuppressive. All of the patients were on some other drug for unrelated issues or for symptomatic treatment.
NA, not available.
Phenotyping and sorting of PBMCs.
PBMCs were thawed and stained with the antibodies anti-CD3 (Alexa Fluor 700), anti-CD4 (peridinin chlorophyll protein [PerCP]-Cy5.5), anti-CD8 (allophycocyanin [APC]-Cy7), anti-CD28 (fluorescein isothiocyanate [FITC]), anti-CD20 (Qdot 650), anti-CD14 (phycoerythrin [PE]), anti-CD16 (Pacific Blue), anti-CD95 (PE-Cy7), and anti-CD163 (APC), all of which were obtained from BD Biosciences, as well as anti-SAMHD1 (Proteintech Group). Antibody isotype controls were purchased from Life Technologies. The cells were also stained with LIVE/DEAD fixable yellow dead cell stain (Molecular Probes). The purity of each monocyte subset was confirmed directly following the sorting procedure on a FACSAria I cell sorter. CD4+ and CD8+ cells were isolated from the CD3+ population using a Dynabeads CD4-positive isolation kit (Invitrogen), as described by the manufacturer.
DNA and RNA extraction.
PBMCs and CD4+, CD8+, CD14+ CD16−, and CD14+ CD16+ sorted cells were submitted to genomic DNA and total RNA extraction. Genomic DNA was isolated using a DNeasy blood and tissue kit (Qiagen). PCR amplification was performed with 100 ng of DNA and first-round primers gag-F1 (5′-GGCCAAATCCTTTCCCGTAG-3′) and gag-R1 (5′-GTTGTGGATTGTTGGCTTGG-3′) and second-round primers gag-F2 (5′-GTCCCTCCAGTTACGATTTCC-3′) and gag-R2 (5′-AGGGAGGAGCAAAGGTACTG-3′). Correctly sized amplicons were identified by 1% agarose gel electrophoresis.
Total RNA was extracted from CD3+ cells and classical, intermediate, and nonclassical monocytes isolated from PMBCs from two uninfected individuals and one HTLV-1-infected individual using an RNeasy minikit (Qiagen, Valencia, CA) and retrotranscribed using a QuantiTect reverse transcription kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The quantity and quality of the RNA were assessed on a NanoDrop spectrophotometer. Quantitative real-time RT-PCR (qPCR) of cDNA samples was performed with primers GAPDH-forward (5′-GGTCTCCTCCGACTTCAACA-3′) and GAPDH-reverse (5′-TGCTGTAGCCAAATTCGTTG-3′) to obtain the relative amounts of the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping gene and with primers CD3-forward (5′-CTGGACCTGGGAAACGCATC-3′) and CD3-reverse (5′-GTACTGAGCATCATCGATC-3′) to obtain the relative amounts of the target gene.
The relative level of expression of target genes was calculated, using delta delta threshold cycles (ΔΔCT), as the ratio between the level of expression of the target gene and the level of expression of the GAPDH housekeeping gene. The level of CD3 expression in Jurkat T cells was set equal to 1, and the remaining values are the fold change in expression compared to that in Jurkat T cells.
Quantitative HTLV-1 DNA detection.
The level of HTLV-1 DNA (the viral load [VL]) in PBMCs and in sorted CD4+ cells, CD8+ cells, and monocyte subsets was determined using a QX100 digital droplet PCR (ddPCR) system (Bio-Rad). The assay was performed as described before (59). Briefly, primers specific for HTLV-1 tax and the RNase P protein subunit P30 (RPP30) or the β-actin housekeeping gene were used. The standard curve for HTLV-1 tax was derived from the DNA of the TARL-2 murine cell line (60), which carries one copy of the tax gene (pX) per cell. For qPCR analysis, Via7 (version 1.2.1) software (Applied Biosystems, Foster City, CA) was used. All samples were run in triplicate, and the average quantity was used to calculate the VL. The VL was calculated as the percentage of infected cells using the following formula: [(quantity of HTLV-1 tax)/(quantity of housekeeping gene/2)] × 100.
Phagocytosis assay.
The phagocytic activity of the monocyte subsets was measured by using a pHrodo Red Escherichia coli BioParticles phagocytosis kit for flow cytometry (Molecular Probes). First, CD3+ cells were depleted from PBMCs derived from 9 HTLV-1-infected individuals (1 NHT and 8 HT individuals) and 8 uninfected individuals, as described above, using human CD3 microbeads (Miltenyi Biotec) for positive selection on an autoMACS separator. The flowthrough cells (CD3-depleted PBMCs) were used for the phagocytosis assay, and 5 × 106 cells were incubated with 2 ml of 5% RPMI at 37°C in 5% CO2 for 45 min for adherence. The pHrodo Red E. coli BioParticles, whether they were opsonized or not, were added to the PBMCs at a ratio of 1:20, and the cells were incubated at 37°C for an additional 1 h. The opsonization was carried out by treating 3 × 108 pHrodo Red E. coli BioParticles with 100 μl of an pHrodo Red E. coli BioParticle opsonizing reagent (Molecular Probes) at 37°C for 1 h. The cells were then lifted using the StemProAccutase cell dissociation reagent (Gibco) at 37°C for 20 min, recovered by centrifugation, and washed once in phosphate-buffered saline (PBS). Next, the cells were stained with anti-CD14 (FITC), anti-CD16 (Pacific Blue), and anti-CD3 (Alexa 700), all of which were from BD Biosciences. Percent phagocytosis was measured using an LSR II flow cytometer (BD Biosciences) and FlowJo (version 9.4) software (TreeStar).
Chemotaxis assay.
The chemotaxis assay was performed as previously reported (61) with some modifications. Briefly, 600 μl RPMI medium containing 0.5% fetal bovine serum (FBS) with no chemokine or with chemokine (R&D Systems) was added to a 12-well plate. The chemokines included 25 ng/ml CCL2, 50 ng/ml CCL5, 150 ng/ml CXCL9, or 20 ng/ml CXC3L1 or all chemokines (mix) in the same well (12.5 ng/ml CCL2, 25 ng/ml CCL5, 75 ng/ml CXCL9, 10 ng/ml CX3CL1). The concentrations of the chemokines were determined by dose-response experiments or as described previously (62–68). Cell culture inserts with 5.0-μm-pore-size membranes (12-well Millicell cell culture inserts; Millipore) were placed in each well, and 5 × 106 PBMCs suspended in 300 μl of 0.5% FBS-RPMI medium were added to the insert. The cells were incubated at 37°C in 5% CO2 for 3 h, after which the inserts were moved to new wells containing 1 ml of enzyme-free, PBS-based dissociation buffer (Life Technologies, Gibco) to remove cells that went through the pore but that were still bound to the insert membrane. After a 30-min incubation at 37°C in 5% CO2, the two populations were combined and all migrated cells were recovered by centrifugation and stained with the following antibodies: anti-CD14 (PE), anti-CD16 (Pacific Blue), anti-CD191 (Alexa Fluor 647), anti-CD197 (Alexa Fluor 700), anti-CD3 (PE-CF594), anti-CD183 (PE-Cy5), and anti-CD195 (PE-Cy7) from BD Pharmingen and anti-CX3CR1 (PerCP-Cy5.5), anti-CCR2 (Brilliant Violet 605), and anti-HLA-DR (APC-Cy7) from BioLegend. The cells were then transferred to Trucount bead tubes (BD Trucount/BD Biosciences), and the volume was adjusted. The cells and 10,000 events with beads were acquired on an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo (version 9.4) software (TreeStar). The absolute number of migrated cells was calculated using the following formula according to the instructions of BD: (number of events in the region containing cells/number of events in absolute count bead region) × (number of beads per test/test volume) = absolute cell count.
RESULTS
HTLV-1 infection is associated with a decreased frequency of classical monocytes and an increased frequency of nonclassical monocytes.
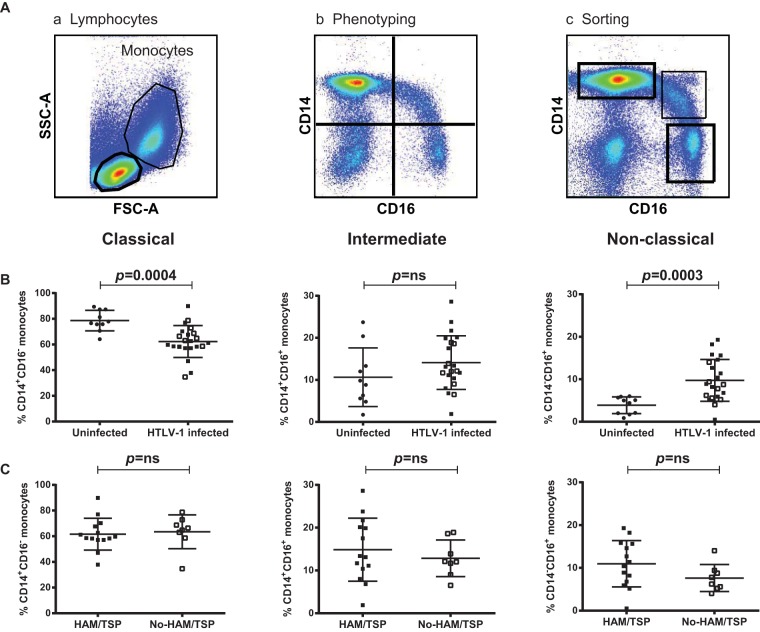
Monocyte subsets with different functions have been phenotypically defined and classified into three main subsets by their surface expression of CD14 and CD16 (44). Because of the association between HTLV-1 infection and inflammation, we studied monocyte frequency and function in 22 HTLV-1-infected individuals, 14 of whom had HAM/TSP (HT patients) and 8 of whom were either asymptomatic or had HTLV-1-associated conditions and were designated to have no HAM/TSP (NHT patients) (Table 1). At the time of blood collection, all these patients had a detectable virus burden in their PBMCs (Table 1). The gating strategy of the phenotypic analysis is outlined in Fig. 1A (panels a and b). This strategy allowed us to define the frequencies of CD14+ CD16− (classical), CD14+ CD16+ (intermediate), and CD14− CD16+ (nonclassical) monocytes. The staining profiles of 10 uninfected healthy donors were used for comparison. We found no significant difference in the overall frequency of monocytes in the blood of HTLV-1-infected individuals and the blood of uninfected healthy donors (data not shown). However, when we analyzed monocyte subsets, we saw a significant reduction in the frequency of the classical monocytes (Fig. 1B; P = 0.0004) and an increase in the frequency of the nonclassical monocytes (Fig. 1B; P = 0.0003) in HTLV-1-infected individuals compared to their frequencies in the controls. No significant difference in the frequency of intermediate monocytes was observed between the two groups (Fig. 1B; P = 0.13). The frequency of the monocyte subsets did not differ between HTLV-1-infected individuals with HAM/TSP and HTLV-1-infected individuals without HAM/TSP (Fig. 1C). This pattern shows that infected patients had lower levels of classical monocytes, comparable levels of intermediate monocytes, and higher levels of nonclassical monocytes relative to the levels in uninfected patients. By repeated-measures regression analysis of the arcsine-transformed percentages, this trend was significant at a P value of <0.0001. The estimated mean differences between uninfected and infected patients were 16% (95% confidence interval [CI], 9% to 24%) for classical monocytes, −3% (95% CI, −9% to 2%) for the intermediate monocytes, and −6% (95% CI, −8% to −3%) for the nonclassical monocytes. Together these results suggest that HTLV-1 infection alters the physiological ratio of peripheral CD14+ and CD16+ monocytes.
FIG 1.
Monocyte subset frequencies in HTLV-1-infected and uninfected individuals. (A) Gating strategy for monocytes. PBMCs from uninfected and HTLV-1-infected individuals were stained with anti-CD3, anti-CD14, and anti-CD16 antibodies. (a) Side scatter (SSC-A) and forward scatter (FSC-A) define two distinct clusters of monocytes and lymphocytes. (b) The monocytes were gated according to the levels of CD14 and CD16 expression in the quadrants that included classical monocytes (top left), intermediate monocytes (top right), and nonclassical monocytes (bottom right). (c) The boxes indicate the populations collected during sorting. (B) Frequency of monocyte subsets in HTLV-1-infected and uninfected individuals. Open symbols, HTLV-1-infected patients with no symptoms of HAM/TSP. (C) Frequency of monocyte subsets in HTLV-1-infected individuals with (closed symbols) or without (open symbols) HAM/TSP. The statistical analysis was performed using the Wilcoxon rank sum test. ns, no statistically significant difference.
Contribution of monocyte subsets to viral burden in PBMCs.
HTLV-1 is primarily found in CD4+ T cells but has also been detected in CD8+ T cells and B cells. In addition, HTLV-1 can infect monocytes in vitro, and viral DNA has been found in monocytes from infected individuals (26, 29, 30, 33, 35, 39). Because the viral burden correlates with HAM/TSP development (13, 69), we wished to investigate whether all monocyte subsets are susceptible to HTLV-1 infection and to what extent the viral DNA in monocytes contributes to the total virus burden. To address this, we sorted monocyte subsets from a total of 8 HTLV-1-infected individuals using the gating strategy depicted in Fig. 1A (panel c). We excluded the possibility of T cell contamination of the sorted monocyte subsets by performing a qPCR to measure the expression of the CD3 mRNA. The CD4+ Jurkat cell line and the monocytoid THP-1 cell line were used as a positive control and a negative control, respectively. The level of expression of the PCR product was normalized to the level of expression of the housekeeping GAPDH gene, and the level of CD3 mRNA expression was compared to that detected in Jurkat T cells. As expected, CD3 mRNA was readily detected in the Jurkat T cells and in the sorted CD3+ T cells but not in any monocyte subset from either of the uninfected patients (Fig. 2A, black and dark gray bars). To further confirm the reproducibility of our sorting approach, we also tested the PBMCs from patient HT-6 (Table 2) by performing two independent sorting and RNA analyses. As demonstrated in Fig. 2A (light gray bar), CD3 mRNA was readily detected in T cells but not the monocytoid THP-1 cell line or in monocyte subsets. Next, we extracted genomic DNA from patients HT-3 and HT-10 (Table 2) and performed nested PCR using primers that detect the HTLV-1 gag gene (Fig. 2B). We found viral sequences in the positive-control MT-2 cell line, in the patients' CD4+ and CD8+ T cells, as well as in all the monocyte subsets. As expected, PBMC DNA from an uninfected individual was negative for viral sequences. In order to assess the contribution of the monocyte subsets to the total viral burden in PBMCs, we quantified by digital droplet PCR (59) the viral copy number in each subset from seven patients with HAM/TSP and one asymptomatic individual (Table 2). The highest virus burden was observed in CD4+ cells followed by CD8+ cells, with mean values of 35.11% and 8.45%, respectively (Wilcoxon rank sum test; Fig. 2C). Interestingly, among the monocyte subsets, the nonclassical monocytes had the highest virus burden with a mean value of 2.88%, whereas the mean values for the classical and intermediate subsets were 0.94% and 0.50%, respectively (Fig. 2C). The contribution to the total viral burden of CD4+ T cells was 73.55%, and that of CD8+ T cells was 18.55%. These values were significantly higher than those found in monocytes (Wilcoxon rank sum test). The monocyte contribution to the viral burden ranged from 0% to 15.13% (Fig. 2D).
FIG 2.
HTLV-1 DNA in sorted cell populations from HTLV-1-infected individuals. The monocyte subsets were sorted according to the specified area depicted in Fig. 1A, panel c. (A) The relative amount of CD3 RNA in all three monocyte subsets was determined and is expressed as the fold change (log2) on the basis of the value for the positive control, the CD3+ Jurkat T cell line (for which the value was set equal to 1). The CD3− THP-1 monocytoid cell line was used as a negative control. RNA was obtained from sorted CD3+ cells and monocytes from two uninfected individuals and from HTLV-1-infected patient HT-6. (B) A nested PCR for gag was performed with genomic DNA from HTLV-1-infected individuals. DNA from the PBMCs of an uninfected healthy donor (ND) and DNA from the HTLV-1-positive MT2 cell line were used as a negative control and a positive control, respectively. (C) HTLV-1 DNA in the sorted cell populations of eight HTLV-1-infected individuals was examined using digital droplet PCR. The mean viral DNA loads (100 cells) in each cell population were as follows: 35.11% for CD4+ cells, 8.45% for CD8+ cells, 0.94% for CD14+ cells, 0.50% for CD14+ CD16+ cells, and 2.88% for CD16+ cells. (D) CD4+ T cells are the main contributors to the total VL (73.55%), followed by CD8+ T cells (18.55%). The contribution of all three monocyte subsets, mainly the nonclassical subset, to the viral load varied from 1.44% to 15.13%.
TABLE 2.
HTLV-1 DNA levels in PBMCs and sorted cells from HTLV-1-infected individuals with HAM/TSP
| Cell population | Viral DNA level (%) in the following patient: |
|||||||
|---|---|---|---|---|---|---|---|---|
| HT-1 | HT-3 | HT-6 | HT-9 | HT-10 | HT-11 | HT-12 | NHT-2 | |
| PBMCs | 14.48 | 8.40 | 11.44 | 6.75 | 24.15 | 8.33 | 3.44 | 5.31 |
| CD4+ cells | 49.04 | 55.95 | 22.28 | 40.71 | 67.86 | 22.9 | 14.16 | 7.94 |
| CD8+ cells | 5.13 | 14.03 | 4.65 | 5.70 | 25.32 | 4.5 | 3.72 | 4.58 |
| Classical monocytes | 0.49 | 0.14 | 1.15 | 0.75 | 1.22 | 0.99 | 2.81 | 0.00 |
| Intermediate monocytes | 0.00 | 0.00 | 1.46 | 0.00 | 1.48 | 0.44 | 0.13 | 0.48 |
| Nonclassical monocytes | 3.56 | 0.88 | 3.65 | 3.34 | 10.08 | 0.52 | 0.17 | 0.80 |
HTLV-1 infection is associated with increased chemokine receptor expression on monocyte subsets but does not affect their phagocytic function.
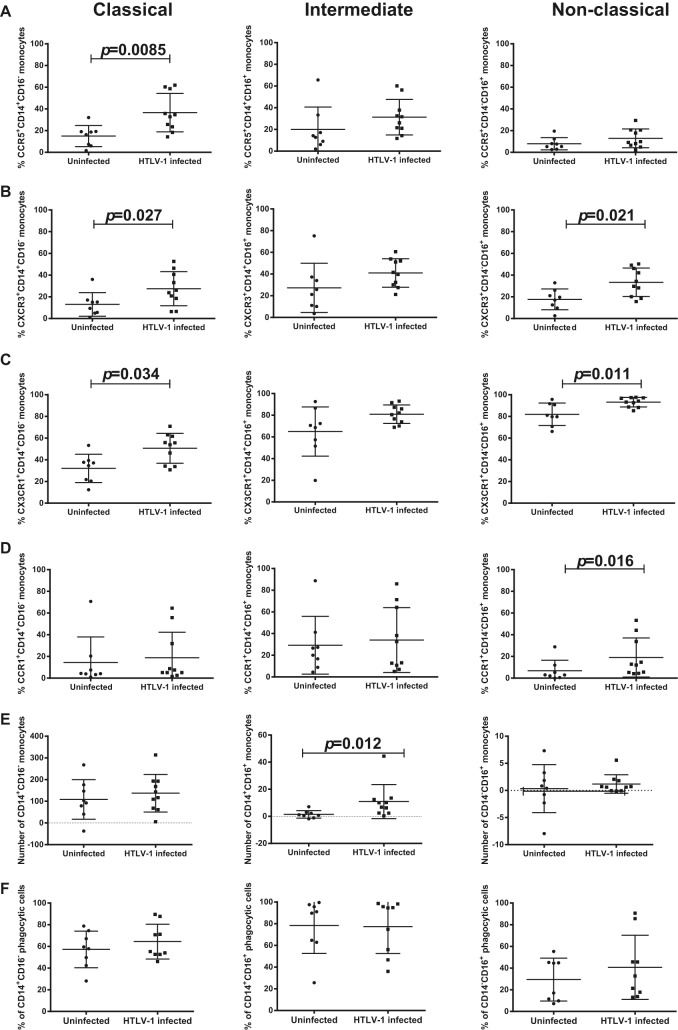
The surface expression of chemokine receptors on monocytes influences their responsiveness to cytokines/chemokines and, in turn, their function. Therefore, we evaluated the expression of CCR1, CCR2, CCR5, CXCR3, and CX3CR1 in each monocyte subset from 8 uninfected and 10 HTLV-1-infected individuals. We observed a higher percentage of classical monocytes expressing CCR5 (P = 0.0085), CXCR3 (P = 0.027), and CX3CR1 (P = 0.0.034) in the HTLV-1-infected group than in the uninfected group of individuals (Fig. 3A to C). A higher percentage of nonclassical monocytes from HTLV-1-infected individuals than from uninfected individuals expressed CCR1 (P = 0.016), CXCR3 (P = 0.021), and CX3CR1 (P = 0.011) but not CCR5. No significant difference in surface marker expression on intermediate monocytes was seen between the two groups (Fig. 3A to D). While CCR2 surface marker expression varied among the different subsets, the levels within a subset did not change between infected and uninfected individuals (data not shown). Next, we investigated whether HTLV-1 infection affected the migration of monocyte subsets by using the Boyden chamber assay (70). PBMCs from 8 uninfected individuals and 10 HTLV-1-infected patients were used to evaluate the chemotaxis of monocytes for the chemokines CCL2, CCL5, CXCL9, and CX3CL1. The absolute number of migrated cells was obtained using BD Trucount beads. The number of migrated cells was determined by finding the absolute number of cells in the chamber with a given chemokine minus the absolute number of cells in the chamber without that chemokine. While no difference in monocyte migration in response to CCL2, CXCL9, or CX3CL1 was found between HTLV-1-infected and uninfected individuals (data not shown), intermediate monocytes from infected individuals had higher levels of migration in response to CCL5 (P = 0.012). This was observed at two different concentrations of CCL5 (Fig. 3E and data not shown).
FIG 3.
Chemokine receptor expression and chemotactic and phagocytic activity of monocyte subsets. Chemokine receptor expression was investigated in each monocyte subset from 8 uninfected individuals and 10 HTLV-1-infected individuals. Expression of CCR5 (A), CXCR3 (B), CX3CR1 (C), and CCR1 (D) in the indicated monocyte subsets was graphed. The chemotaxis assay was performed using a Boyden chamber model transwell system (70). (E) PBMCs from 8 uninfected individuals and 10 HTLV-1-infected individuals (1 NHT patient and 9 HT patients) were used to evaluate the chemotactic ability of monocytes toward CCL5. (F) Phagocytic activity was measured in PBMC samples from 8 uninfected individuals and 9 HTLV-1-infected individuals (1 NHT patients and 8 HT patients). After incubation with opsonized E. coli, PBMCs were stained with anti-CD3, anti-CD14, and anti-CD16 to assess the uptake activity of each monocyte subset. The data were analyzed by the Wilcoxon sum test.
Next, we investigated whether there was a correlation between HTLV-1 infection and the phagocytic activity of monocyte subsets. PBMCs from eight uninfected individuals and nine HTLV-1-infected individuals (eight individuals with TSP and one asymptomatic individual) were depleted of CD3+ cells to enrich for monocytes. Using pHrodo Red E. coli BioParticles and anti-CD14 and anti-CD16 antibodies, we were able to monitor by flow cytometry the phagocytic ability of specific cell populations. The phagocytic activity of both nonopsonized (data not shown) and opsonized BioParticles was measured. No significant difference in the phagocytic activity of the three monocyte subsets from the HTLV-1-infected and uninfected individuals was found (Wilcoxon rank sum test; Fig. 3F).
Correlations between monocyte subset function and virus burden.
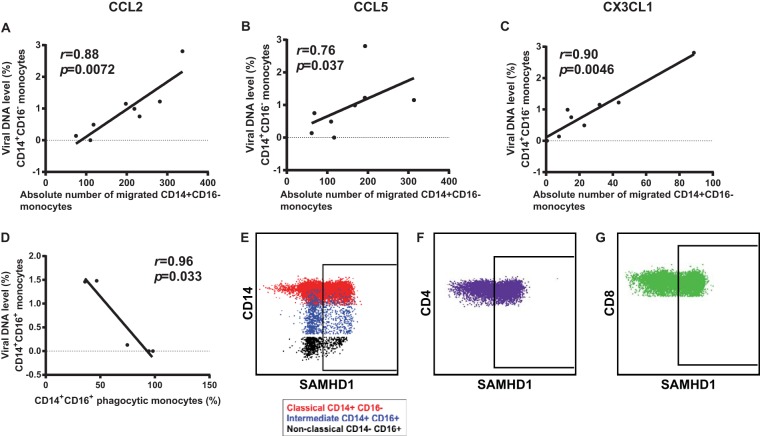
We assessed the association between the viral DNA level in the monocyte subsets and their function as well as the viral DNA levels in CD4+ T cells, CD8+ T cells, and total PBMCs. We observed a significant positive correlation between classical monocyte migration to CCL2, CCL5, and CX3CL1 and the viral DNA level (r = 0.88 and P = 0.0072, r = 0.76 and P = 0.037, and r = 0.90 and P = 0.0046, respectively; Fig. 4A to C). We did not find a correlation between the viral DNA level and CCL5 chemotaxis in intermediate monocytes (data not shown). There was an inverse correlation between the virus burden in intermediate monocytes (CD14+ CD16+) and their phagocytic function (r = −0.96, P = 0.033), (Fig. 4D). We observed positive trends between the viral DNA burden in nonclassical monocytes (CD14− CD16+) and that in CD4+ and CD8+ T cells (r = 0.69, P = 0.069 for both comparisons) (data not shown).
FIG 4.
Correlative analyses of virus burden and function in monocyte subsets. Direct correlations between the viral DNA level in classical monocytes and their migration to CCL2 (A), CCL5 (B), and CX3CL1 (C) are shown. (D) Inverse correlation between the viral DNA level in intermediate monocytes and their phagocytic ability. (E) PBMCs from uninfected individuals were stained with antibodies to CD14, CD16, CD3, CD4, CD8, and SAMHD1. The isotype control for SAMHD1 was used to distinguish expressing versus nonexpressing cell populations. SAMHD1 expression was measured in monocyte subsets (E), CD4+ T cells (F), and CD8+ T cells (G).
SAMHD1 expression.
Recently, the sterile alpha motif and histidine/aspartic acid domain-containing protein 1 (SAMHD1), a deoxynucleoside triphosphate triphosphohydrolase and 3′-5′ exonuclease, was shown to act as a viral restriction factor that prevents HTLV-1 reverse transcription in primary monocytes in vitro (42). In this study, we detected HTLV-1 DNA in all three monocyte subsets, suggesting either that in vivo the virus overcomes SAMHD1 restriction to infect monocytes or that not all monocytes express SAMHD1. We therefore investigated the level of SAMHD1 expression by fluorescence-activated cell sorting in different subsets of PBMCs from five uninfected individuals and five infected individuals following staining with antibodies to CD3, CD4, CD8, CD14, CD16, and SAMHD1. A representative example for an uninfected individual is shown in Fig. 4E to G. This allowed us to evaluate the percentage of positive cells as well as the level of intracellular SAMHD1 in individual cell populations. Within each monocyte subset as well as in CD4+ and CD8+ cells from uninfected individuals, we observed the proportion of SAMHD1-negative cells to range from approximately 30% for T cells to from 40 to 70% for monocytes (Table 3). Interestingly, analyses of the mean fluorescent intensity of SAMHD1 staining in SAMHD1-positive cells showed that the mean fluorescence intensity was the lowest in CD4+ T cells and the highest in the nonclassical monocytes (Table 3). When HTLV-1-infected CD4+ and CD8+ cells were compared to uninfected cells, we detected a shift to a higher level of expression of SAMHD1 in CD4+ and CD8+ cells, resulting in an increase in the percentage of SAMHDI-positive CD4+ and CD8+ cells (Table 3). More strikingly, we saw a shift in the percentage of SAMHD1-positive cells in the monocyte subsets of HTLV-1-infected samples (Table 3). Thus, while all cell types appeared to contain two populations of SAMHD1-expressing cells, HTLV-1 infection appeared to cause a shift to increased SAMHD1 expression.
TABLE 3.
Percentage and mean intensity of SAMHD1 expression in PBMCs
| Cell type | % of cells expressing SAMHD1 | Mean SAMHD1 intensity | Mean SAMHD1 intensity/cell |
|---|---|---|---|
| Uninfected control | |||
| CD3+ CD4+ | 64 ± 16 | 2,690 ± 243 | 0.2 |
| CD3+ CD8+ | 50 ± 19 | 2,274 ± 346 | 0.5 |
| CD14+ CD16− | 49 ± 9 | 3,837 ± 394 | 0.4 |
| CD14+ CD16− | 58 ± 11 | 5,068 ± 437 | 7.2 |
| CD14− CD16+ | 32 ± 6 | 3,236 ± 472 | 14.6 |
| HTLV-1-infected | |||
| CD3+ CD4+ | 69.9 ± 11 | 3,004 ± 342 | 0.1 |
| CD3+ CD8+ | 76 ± 4 | 2,604 ± 418 | 0.3 |
| CD14+ CD16− | 95 ± 2 | 4,840 ± 1,255 | 0.6 |
| CD14+ CD16− | 96 ± 2 | 6,417 ± 1,195 | 4.4 |
| CD14− CD16+ | 94 ± 2 | 5,165 ± 705 | 4.1 |
DISCUSSION
Monocytes bridge innate and adaptive immune functions and are thought to be involved in several inflammatory conditions. Monocyte subsets with distinct functions can be classified according to the expression of CD14 (the lipopolysaccharide receptor), CD16 (the FcyRIII receptor), and CCR2 (the receptor for the CCL2 chemokine). Both the CD14+ CD16− classical monocytes and CD14+ CD16+ intermediate monocytes are phagocytic, and the latter are also thought to mediate antigen presentation and produce high levels of cytokines that modulate immune responses. The CD14− CD16+ nonclassical monocytes also mediate adaptive immune responses and patrol the blood/endothelial border, but because of their low level of CCR2 expression, they are limited in their transendothelial migration ability. Nonclassical monocytes have anti-inflammatory functions. We hypothesized that monocyte numbers and functions may be affected by HTLV-1 infection and studied monocyte subset frequencies and functions and the contribution of monocytes to the viral burden in 23 HTLV-1-infected individuals. Using sorted populations, we demonstrated that all monocyte subsets are infected by HTLV-1. Consistent with the findings of Amorim et al. (71), we found a decrease in the frequency of classical monocytes in the blood of HTLV-1-infected individuals compared to that in the blood of uninfected individuals. Further, we found that within the classical monocyte population, a higher percentage of cells expressed CCR5, CXCR3, and CX3CR1 chemokine receptors. However, we did not observe an increase in the chemotactic responses of classical monocytes to their ligands, CCL2 (CCR2), CCL5 (CCR5), CXCL9 (CXCR3), or CX3CL1 (CX3CR1), among infected individuals. Rabin et al. showed that receptor expression is often not the sole determinant of the responses to chemokines in T cell subsets (72). This may suggest that viral infection primes the cells but further stimulation is needed to induce chemotaxis. This is supported by the findings of Li et al., who showed that extracellular signal-regulated kinase (ERK) activation influences the chemotaxis of mature human cord blood monocyte-derived dendritic cells (73). Interestingly, we did find a significant positive correlation with classical monocyte migration to CCL2, CCL5, and CX3CL1 and the viral burden. This raises the possibility that classical monocyte migration into tissues may result in their infection in vivo. CCL2 has been suggested to circulate in the lymph and bind to high endothelial venules in lymph nodes, thereby guiding monocytes into the lymph nodes (74).
In contrast to the findings of Amorim et al. (71), we observed no differences in the frequency of intermediate monocytes in HTLV-1-infected and uninfected individuals. Possible explanations for the difference in subset frequencies found in our study and those found in the study of Amorim et al. (71) are variations in the gating strategies used and/or the HAM/TSP patient populations tested. The HAM/TSP individuals in our study had viral DNA levels ranging from 39,100 to 311,900 copies/106 cells, whereas in the study of Amorim et al. (71) the range was 932 to 1,186,254 copies/106 cells. In addition, Amorim and colleagues (71) did not determine the viral burden in the monocytes or the individual monocyte subsets from infected individuals. This may also contribute to the differences between their findings and ours.
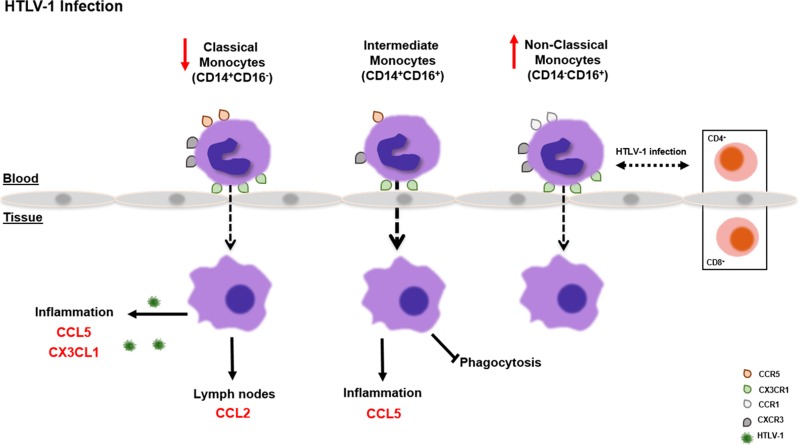
Although we found no change in the frequency of intermediate monocytes, we did find that this population had a greater capacity to migrate in response to CCL5 (a chemokine that recruits T cells, monocytes, and eosinophils to inflammatory sites) in infected individuals than in uninfected individuals. In addition, there was a negative correlation with their viral burden and their phagocytic activity, suggesting an impairment of this function. A study with macrophages infected with respiratory syncytial virus showed that CCR5 activation upon CCL5 interaction initiates dual signals, MEK-ERK and phosphatidylinositol 3-kinase–AKT, which are essential for inhibition of apoptosis of virus-infected cells (75). The increased migration of intermediate monocytes toward CCL5 could be a mechanism by which HTLV-1 drives monocytes to the inflammation site, where the infected cells would be resistant to apoptosis. The authors also showed that “CCR5 mRNA expression is influenced by viral infection so that expression of CCR5 protein may depend on increased translation or accelerated transport of CCR5 to the cell surface” (75). These regulatory mechanisms could explain the increased migration of intermediate monocytes toward CCL5, despite unchanged levels of CCR5. Interestingly, the HTLV-1 Tax protein can induce the expression of CC chemokines, including CCL5, by PBMCs (76). Lastly, the frequency of nonclassical monocytes (CD14− CD16+) was augmented in HTLV-1-infected individuals, and within this population, the percentage of cells expressing the CCR1, CXCR3, and CX3CR1 chemokine receptors was higher. Importantly, we found strong trends between the viral burden in nonclassical monocytes and that in both CD8+ and CD4+ T cells. Collectively, these data suggest a model whereby the inflammatory milieu associated with HTLV-1 infection results in the upregulation of chemokine receptor expression on monocytes and favors the extravasation of classical monocytes, the most abundant monocyte population in blood, which in turn may become infected. Similarly more intermediate monocytes may be recruited to inflammatory tissue by CCL5, become infected, and be impaired in their phagocytic capacity. Finally, nonclassical monocytes, whose frequency is augmented in blood, may be a conduit for the transmission of HTLV-1 to T cells (Fig. 5).
FIG 5.
Summary of the contribution of monocyte subsets to HTLV-1 persistence. This cartoon summarizes the results of our correlative analysis of the HTLV-1 DNA burden in the three monocyte subsets and either their functions (migration and phagocytosis) or the level of viral DNA in CD8+ and CD4+ T cells. Briefly, the hypothesis is that in HTLV-1 infection, more classical monocytes leave the blood and go to tissues, where they acquire HTLV-1 infection. Similarly intermediate monocytes more often migrate to inflammatory sites in tissue in response to CCL5, but their phagocytic function decreases and their virus burden increases. Lastly, nonclassical monocyte levels increase in blood, and the virus burden in nonclassical monocytes reflects that in CD4+ and CD8+ T cells.
Whether the altered numbers of classical and nonclassical monocytes found in this study is the cause or the result of abnormal immune stimulation and the release of chemokines and cytokines in tissues remains unclear. HTLV-1 infection is characterized by the production of high levels of Th1 inflammatory cytokines in blood and cerebrospinal fluid and increased levels of IFN-γ, tumor necrosis factor alpha (TNF-α), IL-2, and proinflammatory IL-6, particularly in patients with HAM/TSP (77, 78). CX3CR1 is the receptor for fractalkine and is highly expressed on CD16+ cells, which migrate in response to fractalkine, which is known to induce high levels of transendothelial migration of CD16+ monocytes (50). Importantly, the membrane-bound form of fractalkine is expressed on endothelial cells after stimulation with the inflammatory cytokine TNF-α or IL-1 (79).
The levels of chemokines and their receptors are altered in patients with neurodegenerative disorders. CX3CR1+ nonclassical monocytes are recruited to the inflamed central nervous system (CNS) in patients with multiple sclerosis (MS) (80), and the CX3CR1 ligand, CX3CL1, serves as a neuronal regulatory protein controlling microglia activation under physiological conditions. Depending on the type of CNS injury, the CX3CL1/CX3CR1 axis plays a different role in neurodegeneration compared with the role that it plays in neuroprotection (81). The levels of the chemokine receptors CXCR3 and CCR5 expressed on Th1 cells are increased in the CSF and brain lesions of patients with active demyelinating MS (82). The levels of the serum chemokines CCL5, CXCL8, CXCL9, and CXCL10 are altered in HTLV-1 infection (83), and a correlation with high levels of CXCL9 and CXCL10 in the CSF (84) and low levels of CCL2 in HAM/TSP in serum (85) has been described.
Although it was previously demonstrated that HTLV-1 infection of primary monocytes in vitro is nonproductive (42), we believe that our data show that all three monocyte subsets are infected in vivo. Consistent with our results, ex vivo monocytes were shown to express HTLV-1 antigens when cultured for a short period of time (36). Analyses of uninfected cell populations demonstrated that within each subset of cells there are SAMHD1-positive and -negative populations. However, in HTLV-1-infected individuals, these populations shifted such that we detected an increase in the percentage of SAMHD1-positive monocyte populations. It is known that HTLV-1 infection causes local and systemic inflammation, and perhaps this inflammatory response alters SAMHD1 expression. In addition, SAMHD1 expression has been shown to be regulated by interferons (86–89). We recently showed that expression of the HTLV-1 p30 protein dampens interferon responses in monocytes and dendritic cells by blocking the expression of interferon-responsive genes (40). Thus, more studies are needed to determine whether HTLV-1 preferentially infects SAMHD1-negative monocytes in vivo, what role HTLV-1 regulatory proteins play, and how SAMHD1 is regulated in different cell types.
How and under which conditions the infected monocytes transmit HTLV-1 to T cells or vice versa remain unclear, but it is tempting to speculate that viral transmission may occur during the process of antigen presentation and T cell activation. Our data point to a key role of monocytes in perpetuating viral infection in vivo and imply that therapeutic approaches aimed at decreasing the viral burden and the risk of disease development in HTLV-1-infected individuals should include a strategy that inhibits infection of monocytes.
ACKNOWLEDGMENTS
This work was entirely supported by the NIH Intramural Program.
We are grateful to David Abram for editorial work. We also thank Thessika Hialla A. Araújo, Ph.D. student at Fiocruz-BA-CPqGM, for editorial assistance with the figures.
M.F.D.C.-A. designed the study and performed the experiments with the assistance of R.W.P., M.O., V.A., and V.G. R.M., G.B., and B.C. performed the viral DNA-level experiments. K.M. performed flow cytometric staining, cell sorting, and analysis with the assistance of M.F.D.C.-A. S.J. provided patient samples and valuable discussion. C.A.P.-M. and G.F. designed the study, interpreted the results, and wrote the paper with the assistance of M.F.D.C.-A. D.V. performed the statistical analysis of the data.
We declare no competing financial interests.
Funding Statement
This work was funded entirely by the NIH intramural program.
REFERENCES
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A 77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The G. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407–410. [DOI] [PubMed] [Google Scholar]
- 3.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031–1032. [DOI] [PubMed] [Google Scholar]
- 4.Yamano Y, Sato T. 2012. Clinical pathophysiology of human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Front Microbiol 3:389. doi: 10.3389/fmicb.2012.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umehara F, Izumo S, Nakagawa M, Ronquillo AT, Takahashi K, Matsumuro K, Sato E, Osame M. 1993. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol 52:424–430. doi: 10.1097/00005072-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kamoi K, Mochizuki M. 2012. HTLV-1 uveitis. Front Microbiol 3:270. doi: 10.3389/fmicb.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguchi K, Matsuoka N, Ida H, Nakashima M, Sakai M, Sakito S, Kawakami A, Terada K, Shimada H, Kawabe Y. 1992. Primary Sjogren's syndrome with antibodies to HTLV-I: clinical and laboratory features. Ann Rheum Dis 51:769–776. doi: 10.1136/ard.51.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. 1989. Chronic inflammatory arthropathy associated with HTLV-I. Lancet i:441. [DOI] [PubMed] [Google Scholar]
- 9.Morgan OS, Rodgers-Johnson P, Mora C, Char G. 1989. HTLV-1 and polymyositis in Jamaica. Lancet ii:1184–1187. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa M, Izumo S, Ijichi S, Kubota H, Arimura K, Kawabata M, Osame M. 1995. HTLV-I-associated myelopathy: analysis of 213 patients based on clinical features and laboratory findings. J Neurovirol 1:50–61. doi: 10.3109/13550289509111010. [DOI] [PubMed] [Google Scholar]
- 11.Araya N, Sato T, Yagishita N, Ando H, Utsunomiya A, Jacobson S, Yamano Y. 2011. Human T-lymphotropic virus type 1 (HTLV-1) and regulatory T cells in HTLV-1-associated neuroinflammatory disease. Viruses 3:1532–1548. doi: 10.3390/v3091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demontis MA, Hilburn S, Taylor GP. 2013. Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res Hum Retroviruses 29:359–364. doi: 10.1089/AID.2012.0132. [DOI] [PubMed] [Google Scholar]
- 13.Furtado Mdos S, Andrade RG, Romanelli LC, Ribeiro MA, Ribas JG, Torres EB, Barbosa-Stancioli EF, Proietti AB, Martins ML. 2012. Monitoring the HTLV-1 proviral load in the peripheral blood of asymptomatic carriers and patients with HTLV-associated myelopathy/tropical spastic paraparesis from a Brazilian cohort: ROC curve analysis to establish the threshold for risk disease. J Med Virol 84:664–671. doi: 10.1002/jmv.23227. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Higuchi I, Arimura K, Kubota H, Izumo S, Akiba S, Osame M. 2001. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J Neurovirol 7:228–234. doi: 10.1080/13550280152403272. [DOI] [PubMed] [Google Scholar]
- 15.Yamano Y, Nagai M, Brennan M, Mora CA, Soldan SS, Tomaru U, Takenouchi N, Izumo S, Osame M, Jacobson S. 2002. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood 99:88–94. doi: 10.1182/blood.V99.1.88. [DOI] [PubMed] [Google Scholar]
- 16.Iwanaga M, Watanabe T, Utsunomiya A, Okayama A, Uchimaru K, Koh KR, Ogata M, Kikuchi H, Sagara Y, Uozumi K, Mochizuki M, Tsukasaki K, Saburi Y, Yamamura M, Tanaka J, Moriuchi Y, Hino S, Kamihira S, Yamaguchi K, Joint Study on Predisposing Factors of ATL Development Investigators. 2010. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood 116:1211–1219. doi: 10.1182/blood-2009-12-257410. [DOI] [PubMed] [Google Scholar]
- 17.Satoh M, Kiyuna S, Shiroma Y, Toma H, Kokaze A, Sato Y. 2003. Predictive markers for development of strongyloidiasis in patients infected with both Strongyloides stercoralis and HTLV-1. Clin Exp Immunol 133:391–396. doi: 10.1046/j.1365-2249.2003.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy EL, Lee TH, Chafets D, Nass CC, Wang B, Loughlin K, Smith D, HTLV Outcomes Study Investigators. 2004. Higher human T lymphotropic virus (HTLV) provirus load is associated with HTLV-I versus HTLV-II, with HTLV-II subtype A versus B, and with male sex and a history of blood transfusion. J Infect Dis 190:504–510. doi: 10.1086/422398. [DOI] [PubMed] [Google Scholar]
- 19.Yakova M, Lezin A, Dantin F, Lagathu G, Olindo S, Jean-Baptiste G, Arfi S, Cesaire R. 2005. Increased proviral load in HTLV-1-infected patients with rheumatoid arthritis or connective tissue disease. Retrovirology 2:4. doi: 10.1186/1742-4690-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adaui V, Verdonck K, Best I, Gonzalez E, Tipismana M, Arevalo J, Vanham G, Campos M, Zimic M, Gotuzzo E. 2006. SYBR green-based quantitation of human T-lymphotropic virus type 1 proviral load in Peruvian patients with neurological disease and asymptomatic carriers: influence of clinical status, sex, and familial relatedness. J Neurovirol 12:456–465. doi: 10.1080/13550280601039634. [DOI] [PubMed] [Google Scholar]
- 21.Sakai JA, Nagai M, Brennan MB, Mora CA, Jacobson S. 2001. In vitro spontaneous lymphoproliferation in patients with human T-cell lymphotropic virus type I-associated neurologic disease: predominant expansion of CD8+ T cells. Blood 98:1506–1511. doi: 10.1182/blood.V98.5.1506. [DOI] [PubMed] [Google Scholar]
- 22.Pinto LA, Galvao Castro B, Soares MB, Grassi MF. 2011. An evaluation of the spontaneous proliferation of peripheral blood mononuclear cells in HTLV-1-infected individuals using flow cytometry. ISRN Oncol 2011:326719. doi: 10.5402/2011/326719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 24.Kubota R, Nagai M, Kawanishi T, Osame M, Jacobson S. 2000. Increased HTLV type 1 tax specific CD8+ cells in HTLV type 1-asociated myelopathy/tropical spastic paraparesis: correlation with HTLV type 1 proviral load. AIDS Res Hum Retroviruses 16:1705–1709. doi: 10.1089/08892220050193182. [DOI] [PubMed] [Google Scholar]
- 25.Hanon E, Goon P, Taylor GP, Hasegawa H, Tanaka Y, Weber JN, Bangham CR. 2001. High production of interferon gamma but not interleukin-2 by human T-lymphotropic virus type I-infected peripheral blood mononuclear cells. Blood 98:721–726. doi: 10.1182/blood.V98.3.721. [DOI] [PubMed] [Google Scholar]
- 26.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. 2008. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med 14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 27.Alais S, Mahieux R, Dutartre H. 2015. Viral source-independent high susceptibility of dendritic cells to human T-cell leukemia virus type 1 infection compared to that of T lymphocytes. J Virol 89:10580–10590. doi: 10.1128/JVI.01799-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Latil S, Gnadig NF, Mallet A, Desdouits M, Guivel-Benhassine F, Jeannin P, Prevost MC, Schwartz O, Gessain A, Ozden S, Ceccaldi PE. 2012. Transcytosis of HTLV-1 across a tight human epithelial barrier and infection of subepithelial dendritic cells. Blood 120:572–580. doi: 10.1182/blood-2011-08-374637. [DOI] [PubMed] [Google Scholar]
- 29.Makino M, Shimokubo S, Wakamatsu SI, Izumo S, Baba M. 1999. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J Virol 73:4575–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enose-Akahata Y, Oh U, Grant C, Jacobson S. 2008. Retrovirally induced CTL degranulation mediated by IL-15 expression and infection of mononuclear phagocytes in patients with HTLV-I-associated neurologic disease. Blood 112:2400–2410. doi: 10.1182/blood-2008-02-138529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nascimento CR, Lima MA, de Andrada Serpa MJ, Espindola O, Leite AC, Echevarria-Lima J. 2011. Monocytes from HTLV-1-infected patients are unable to fully mature into dendritic cells. Blood 117:489–499. doi: 10.1182/blood-2010-03-272690. [DOI] [PubMed] [Google Scholar]
- 32.Inagaki S, Takahashi M, Fukunaga Y, Takahashi H. 2012. HTLV-I-infected breast milk macrophages inhibit monocyte differentiation to dendritic cells. Viral Immunol 25:106–116. doi: 10.1089/vim.2011.0069. [DOI] [PubMed] [Google Scholar]
- 33.Koralnik IJ, Lemp JF Jr, Gallo RC, Franchini G. 1992. In vitro infection of human macrophages by human T-cell leukemia/lymphotropic virus type I (HTLV-I). AIDS Res Hum Retroviruses 8:1845–1849. doi: 10.1089/aid.1992.8.1845. [DOI] [PubMed] [Google Scholar]
- 34.Macatonia SE, Cruickshank JK, Rudge P, Knight SC. 1992. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses 8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman PM, Dhib-Jalbut S, Mikovits JA, Robbins DS, Wolf AL, Bergey GK, Lohrey NC, Weislow OS, Ruscetti FW. 1992. Human T-cell leukemia virus type I infection of monocytes and microglial cells in primary human cultures. Proc Natl Acad Sci U S A 89:11784–11788. doi: 10.1073/pnas.89.24.11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koyanagi Y, Itoyama Y, Nakamura N, Takamatsu K, Kira J, Iwamasa T, Goto I, Yamamoto N. 1993. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology 196:25–33. doi: 10.1006/viro.1993.1451. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi H, Takahashi M, Norose Y, Takeshita T, Fukunaga Y, Takahashi H. 2010. Transformation of breast milk macrophages by HTLV-I: implications for HTLV-I transmission via breastfeeding. Biomed Res 31:53–61. doi: 10.2220/biomedres.31.53. [DOI] [PubMed] [Google Scholar]
- 38.Valeri VW, Hryniewicz A, Andresen V, Jones K, Fenizia C, Bialuk I, Chung HK, Fukumoto R, Parks RW, Ferrari MG, Nicot C, Cecchinato V, Ruscetti F, Franchini G. 2010. Requirement of the human T-cell leukemia virus p12 and p30 products for infectivity of human dendritic cells and macaques but not rabbits. Blood 116:3809–3817. doi: 10.1182/blood-2010-05-284141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enose-Akahata Y, Matsuura E, Tanaka Y, Oh U, Jacobson S. 2012. Minocycline modulates antigen-specific CTL activity through inactivation of mononuclear phagocytes in patients with HTLV-I associated neurologic disease. Retrovirology 9:16. doi: 10.1186/1742-4690-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenizia C, Fiocchi M, Jones K, Parks RW, Ceribelli M, Chevalier SA, Edwards D, Ruscetti F, Pise-Masison CA, Franchini G. 2014. Human T-cell leukemia/lymphoma virus type 1 p30, but not p12/p8, counteracts Toll-like receptor 3 (TLR3) and TLR4 signaling in human monocytes and dendritic cells. J Virol 88:393–402. doi: 10.1128/JVI.01788-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pise-Masison CA, de Castro-Amarante MF, Enose-Akahata Y, Buchmann RC, Fenizia C, Washington Parks R, Edwards D, Fiocchi M, Alcantara LC Jr, Bialuk I, Graham J, Walser JC, McKinnon K, Galvao-Castro B, Gessain A, Venzon D, Jacobson S, Franchini G. 2014. Co-dependence of HTLV-1 p12 and p8 functions in virus persistence. PLoS Pathog 10:e1004454. doi: 10.1371/journal.ppat.1004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sze A, Belgnaoui SM, Olagnier D, Lin R, Hiscott J, van Grevenynghe J. 2013. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe 14:422–434. doi: 10.1016/j.chom.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler-Heitbrock L. 2007. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81:584–592. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. 2010. Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 45.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. 2011. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 46.Weber C, Weber KS, Klier C, Gu S, Wank R, Horuk R, Nelson PJ. 2001. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and T(H)1-like/CD45RO(+) T cells. Blood 97:1144–1146. doi: 10.1182/blood.V97.4.1144. [DOI] [PubMed] [Google Scholar]
- 47.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. 2002. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol 168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 48.Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW. 2000. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol 67:699–704. [DOI] [PubMed] [Google Scholar]
- 49.Passlick B, Flieger D, Ziegler-Heitbrock HW. 1989. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74:2527–2534. [PubMed] [Google Scholar]
- 50.Ancuta P, Weiss L, Haeffner-Cavaillon N. 2000. CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics. Eur J Immunol 30:1872–1883. doi:. [DOI] [PubMed] [Google Scholar]
- 51.Shi C, Pamer EG. 2011. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merino A, Buendia P, Martin-Malo A, Aljama P, Ramirez R, Carracedo J. 2011. Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J Immunol 186:1809–1815. doi: 10.4049/jimmunol.1001866. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Moratalla N, Gonzalez A, Aymerich MS, Lopez-Zabalza MJ, Pio R, de Castro P, Santiago E. 1997. Monocyte inducible nitric oxide synthase in multiple sclerosis: regulatory role of nitric oxide. Nitric Oxide 1:95–104. doi: 10.1006/niox.1996.0111. [DOI] [PubMed] [Google Scholar]
- 54.Chuluundorj D, Harding SA, Abernethy D, La Flamme AC. 2014. Expansion and preferential activation of the CD14(+)CD16(+) monocyte subset during multiple sclerosis. Immunol Cell Biol 92:509–517. doi: 10.1038/icb.2014.15. [DOI] [PubMed] [Google Scholar]
- 55.Fingerle-Rowson G, Angstwurm M, Andreesen R, Ziegler-Heitbrock HW. 1998. Selective depletion of CD14+ CD16+ monocytes by glucocorticoid therapy. Clin Exp Immunol 112:501–506. doi: 10.1046/j.1365-2249.1998.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwissa M, Nakaya HI, Onlamoon N, Wrammert J, Villinger F, Perng GC, Yoksan S, Pattanapanyasat K, Chokephaibulkit K, Ahmed R, Pulendran B. 2014. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell Host Microbe 16:115–127. doi: 10.1016/j.chom.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lambotte O, Taoufik Y, de Goer MG, Wallon C, Goujard C, Delfraissy JF. 2000. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J Acquir Immune Defic Syndr 23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 58.Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L. 2002. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol 76:707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunetto GS, Massoud R, Leibovitch EC, Caruso B, Johnson K, Ohayon J, Fenton K, Cortese I, Jacobson S. 2014. Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations. J Neurovirol 20:341–351. doi: 10.1007/s13365-014-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tateno M, Kondo N, Itoh T, Chubachi T, Togashi T, Yoshiki T. 1984. Rat lymphoid cell lines with human T cell leukemia virus production. I. Biological and serological characterization. J Exp Med 159:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krinninger P, Ensenauer R, Ehlers K, Rauh K, Stoll J, Krauss-Etschmann S, Hauner H, Laumen H. 2014. Peripheral monocytes of obese women display increased chemokine receptor expression and migration capacity. J Clin Endocrinol Metab 99:2500–2509. doi: 10.1210/jc.2013-2611. [DOI] [PubMed] [Google Scholar]
- 62.Amatschek S, Lucas R, Eger A, Pflueger M, Hundsberger H, Knoll C, Grosse-Kracht S, Schuett W, Koszik F, Maurer D, Wiesner C. 2011. CXCL9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through CXCR3-mediated activation of melanoma cells. Br J Cancer 104:469–479. doi: 10.1038/sj.bjc.6606056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. 2001. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol 167:5381–5385. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- 64.Vomaske J, Varnum S, Melnychuk R, Smith P, Pasa-Tolic L, Shutthanandan JI, Streblow DN. 2010. HCMV pUS28 initiates pro-migratory signaling via activation of Pyk2 kinase. Herpesviridae 1:2. doi: 10.1186/2042-4280-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM. 2006. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain 129:212–223. [DOI] [PubMed] [Google Scholar]
- 66.Johnson LA, Jackson DG. 2013. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J Cell Sci 126:5259–5270. doi: 10.1242/jcs.135343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lauro C, Catalano M, Trettel F, Mainiero F, Ciotti MT, Eusebi F, Limatola C. 2006. The chemokine CX3CL1 reduces migration and increases adhesion of neurons with mechanisms dependent on the beta1 integrin subunit. J Immunol 177:7599–7606. doi: 10.4049/jimmunol.177.11.7599. [DOI] [PubMed] [Google Scholar]
- 68.Beck G, Ludwig F, Schulte J, van Ackern K, van der Woude FJ, Yard BA. 2003. Fractalkine is not a major chemoattractant for the migration of neutrophils across microvascular endothelium. Scand J Immunol 58:180–187. doi: 10.1046/j.1365-3083.2003.01298.x. [DOI] [PubMed] [Google Scholar]
- 69.Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M. 1998. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol 4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 70.Boyden S. 1962. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amorim CF, Souza AS, Diniz AG, Carvalho NB, Santos SB, Carvalho EM. 2014. Functional activity of monocytes and macrophages in HTLV-1 infected subjects. PLoS Negl Trop Dis 8:e3399. doi: 10.1371/journal.pntd.0003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabin RL, Park MK, Liao F, Swofford R, Stephany D, Farber JM. 1999. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol 162:3840–3850. [PubMed] [Google Scholar]
- 73.Li G, Basu S, Han MK, Kim YJ, Broxmeyer HE. 2007. Influence of ERK activation on decreased chemotaxis of mature human cord blood monocyte-derived dendritic cells to CCL19 and CXCL12. Blood 109:3173–3176. doi: 10.1182/blood-2006-04-014753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. 2001. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med 194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O'Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, Holtzman MJ. 2005. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med 11:1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barrios CS, Abuerreish M, Lairmore MD, Castillo L, Giam CZ, Beilke MA. 2011. Recombinant human T-cell leukemia virus types 1 and 2 Tax proteins induce high levels of CC-chemokines and downregulate CCR5 in human peripheral blood mononuclear cells. Viral Immunol 24:429–439. doi: 10.1089/vim.2011.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goncalves DU, Proietti FA, Barbosa-Stancioli EF, Martins ML, Ribas JG, Martins-Filho OA, Teixeira-Carvalho A, Peruhype-Magalhaes V, Carneiro-Proietti AB. 2008. HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) inflammatory network. Inflamm Allergy Drug Targets 7:98–107. doi: 10.2174/187152808785107642. [DOI] [PubMed] [Google Scholar]
- 78.Greten TF, Slansky JE, Kubota R, Soldan SS, Jaffee EM, Leist TP, Pardoll DM, Jacobson S, Schneck JP. 1998. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci U S A 95:7568–7573. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature 385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 80.Ashhurst TM, Vreden CV, Niewold P, King NJ. 2014. The plasticity of inflammatory monocyte responses to the inflamed central nervous system. Cell Immunol 291:49–57. doi: 10.1016/j.cellimm.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferretti E, Pistoia V, Corcione A. 2014. Role of fractalkine/CX3CL1 and its receptor in the pathogenesis of inflammatory and malignant diseases with emphasis on B cell malignancies. Mediators Inflamm 2014:480941. doi: 10.1155/2014/480941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uzawa A, Mori M, Hayakawa S, Masuda S, Nomura F, Kuwabara S. 2010. Expression of chemokine receptors on peripheral blood lymphocytes in multiple sclerosis and neuromyelitis optica. BMC Neurol 10:113. doi: 10.1186/1471-2377-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coelho-dos-Reis JG, Passos L, Duarte MC, Araujo MG, Campi-Azevedo AC, Teixeira-Carvalho A, Peruhype-Magalhaes V, Trindade BC, Dos Santos Dias R, Martins ML, Carneiro-Proietti AB, Guedes AC, Goncalves DU, Martins-Filho OA. 2013. Immunological profile of HTLV-1-infected patients associated with infectious or autoimmune dermatological disorders. PLoS Negl Trop Dis 7:e2328. doi: 10.1371/journal.pntd.0002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato T, Coler-Reilly A, Utsunomiya A, Araya N, Yagishita N, Ando H, Yamauchi J, Inoue E, Ueno T, Hasegawa Y, Nishioka K, Nakajima T, Jacobson S, Izumo S, Yamano Y. 2013. CSF CXCL10, CXCL9, and neopterin as candidate prognostic biomarkers for HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS Negl Trop Dis 7:e2479. doi: 10.1371/journal.pntd.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guerreiro JB, Santos SB, Morgan DJ, Porto AF, Muniz AL, Ho JL, Teixeira AL Jr, Teixeira MM, Carvalho EM. 2006. Levels of serum chemokines discriminate clinical myelopathy associated with human T lymphotropic virus type 1 (HTLV-1)/tropical spastic paraparesis (HAM/TSP) disease from HTLV-1 carrier state. Clin Exp Immunol 145:296–301. doi: 10.1111/j.1365-2249.2006.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin F, Taylor GP, Jacobson S. 2014. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev Clin Immunol 10:1531–1546. doi: 10.1586/1744666X.2014.966690. [DOI] [PubMed] [Google Scholar]
- 87.Dragin L, Nguyen LA, Lahouassa H, Sourisce A, Kim B, Ramirez BC, Margottin-Goguet F. 2013. Interferon block to HIV-1 transduction in macrophages despite SAMHD1 degradation and high deoxynucleoside triphosphates supply. Retrovirology 10:30. doi: 10.1186/1742-4690-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.St Gelais C, de Silva S, Amie SM, Coleman CM, Hoy H, Hollenbaugh JA, Kim B, Wu L. 2012. SAMHD1 restricts HIV-1 infection in dendritic cells (DCs) by dNTP depletion, but its expression in DCs and primary CD4+ T-lymphocytes cannot be upregulated by interferons. Retrovirology 9:105. doi: 10.1186/1742-4690-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berger A, Sommer AF, Zwarg J, Hamdorf M, Welzel K, Esly N, Panitz S, Reuter A, Ramos I, Jatiani A, Mulder LC, Fernandez-Sesma A, Rutsch F, Simon V, Konig R, Flory E. 2011. SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutieres syndrome are highly susceptible to HIV-1 infection. PLoS Pathog 7:e1002425. doi: 10.1371/journal.ppat.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]