ABSTRACT
HIV-1 Vpu decreases the exposure of epitopes within the viral envelope glycoprotein (Env) on the surface of infected cells by downregulating both BST2 and CD4. To test the hypothesis that inhibiting Vpu activity would increase the exposure of these epitopes and sensitize infected cells to antibody-dependent cellular cytotoxicity (ADCC), we treated cells with the Nedd8 activation enzyme (NAE) inhibitor MLN4924, which inhibits the cullin1-based ubiquitin ligase complex coopted by Vpu to degrade cellular targets. Treatment of HeLa cells with MLN4924 or expression of a dominant negative mutant of cullin1 inhibited the Vpu-mediated downregulation of CD4 but not the downregulation of BST2. NAE inhibition also increased the surface exposure of CD4-induced epitopes within Env on HEK293 cells containing an inducible HIV genome, on infected CEM T cells, and on infected primary T cells. In contrast, the Vpu-mediated downregulation of BST2 was substantially inhibited by MLN4924 only when T cells were treated with alpha interferon (IFN-α) to induce high levels of BST2 expression. As reported previously, the absence of vpu or nef and even more so the combined absence of these two genes sensitized infected cells to ADCC. However, NAE inhibition affected ADCC minimally. Paradoxically, even in infected, IFN-treated cells in which NAE inhibition substantially rescued the surface level of BST2, the surface level of Env detected with an antibody recognizing a CD4-independent epitope (2G12) was minimally increased. Mutation of the C-terminal Vpu residue W76, which supports the ability of Vpu to stimulate virion release by displacing BST2 from assembly sites on the plasma membrane by a cullin1-independent mechanism, increased the exposure of Env detected by 2G12 on infected T cells. Thus, inhibiting the displacement function of Vpu together with its ability to degrade CD4 and BST2 may be required to sensitize infected cells to ADCC.
IMPORTANCE Pathogenic viruses encode gene products that enable evasion of host immune surveillance mechanisms. One such mechanism is antibody-dependent cellular cytotoxicity (ADCC), whereby host antibodies bind envelope glycoproteins of the virus that are inserted into the cellular membrane and direct the destruction of infected cells. Targeting pharmacologically the activity of HIV-1 Vpu, which contributes to evasion of ADCC, could potentially sensitize infected cells to this immune surveillance mechanism, an outcome that would have therapeutic implications with respect to the goal of curing HIV-1 infection. The Nedd8 activation enzyme inhibitor MLN4924 blocks the activity of the host ubiquitin ligase that Vpu coopts to direct the degradation of CD4 and BST2. We observed that while MLN4924 partially reverses the activity of Vpu and could become part of a therapeutic approach by virtue of CD4-induced epitope exposure, sufficient Vpu activity as an antagonist of BST2 persists despite this drug to allow escape from ADCC.
INTRODUCTION
The accessory proteins of HIV-1 remain undeveloped drug targets whose inhibition could sensitize infected cells to immunological clearance. The accessory proteins Nef and Vpu independently downregulate the host cofactor CD4 (1, 2), whereas the Vpu protein of group M strains downregulates the host antiviral factor BST2 (CD317; tetherin) (3, 4). Recent observations indicate that the absence of CD4− and BST2 downregulation increases the exposure of HIV-1 envelope glycoprotein (Env) molecules on the surface of the infected cell (5–9). The increase in cell surface Env is presumably due to the retention of virions on the cell surface by BST2 (3, 10), although CD4 can also contribute to virion retention (11). In addition, when in complex with CD4, the conformation of Env is changed and CD4-induced (CD4i) epitopes are exposed (12). These effects yield an increase in the sensitivity of infected cells to antibody-dependent cellular cytotoxicity (ADCC) (5–9). Thus, inhibiting Vpu and/or Nef should increase the sensitivity of infected cells to ADCC and could facilitate immunologic clearance of the infection.
While Nef-mediated counteraction of CD4 relies primarily on the interaction with the clathrin adaptor complex AP-2 (13), Vpu-mediated counteraction of CD4 and BST2 relies partly on the interaction with β-TrCP, a subunit of a cullin1-based ubiquitin ligase complex (14–16). This E3 ubiquitin ligase is part of the host protein degradation machinery. Its role in the ability of Vpu to direct the degradation of CD4 via a mechanism similar to the endoplasmic reticulum-associated degradation (ERAD) pathway is well established (14, 17). In contrast, the role of the β-TrCP/cullin1 complex in the downregulation and degradation of BST2 by Vpu is more subtle. The Vpu-stimulated degradation of BST2 occurs primarily within the endolysosomal system and is mediated by the β-TrCP/cullin1 complex as well as by components of the ESCRT (endosomal sorting complexes required for transport) pathway, but the degradation process and β-TrCP itself are dispensable for the virologic counteraction of BST2 by Vpu under certain conditions (15, 16, 18–20).
The activity of cullin-based E3 ligase complexes, and specifically the β-TrCP/cullin1 complex, requires posttranslational modification by the ubiquitin-like molecule Nedd8 (21). Before covalent attachment to the cullins, Nedd8 must be preactivated by Nedd8 activation enzyme (NAE) (22). NAE can be potently and selectively inhibited by the small-molecule drug MLN4924, a structural relative of adenosine 5′-monophosphate (23). This drug is being tested in clinical trials targeting various cancers due to its ability to deregulate S-phase DNA synthesis and trigger apoptosis, as well as its ability to inhibit the activation of NF-κB, which occurs in part via the degradation of IκB by the β-TrCP/cullin1 complex (24–26). MLN4924 has recently been reported to inhibit HIV accessory proteins that rely on cullin-mediated degradation of their targets, namely, the degradation of apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3B (APOBEC3) proteins by Vif, the degradation of UNG2 by Vpr, and the degradation of SAM domain and HD domain-containing protein 1 (SAMHD1) by Vpx (27–29).
We hypothesized that the NAE inhibitor MLN4924 would inhibit the Vpu-mediated counteraction of CD4 and possibly BST2 and as a consequence sensitize infected cells to ADCC. We evaluated MLN4924 as an inhibitor of the Vpu-mediated downregulation of CD4 and BST2 from the cell surface. We also examined whether MLN4924 could increase the exposure of Env epitopes at the surface of infected cells. Finally, we tested MLN4924 for the ability to increase the susceptibility of infected cells to killing by ADCC. The results indicate that MLN4924 inhibits the downregulation of CD4 by Vpu and triggers exposure of CD4i epitopes in Env at the cell surface. In contrast, MLN4924 increases cell surface BST2 in cells expressing Vpu only when the cells are also treated with alpha interferon (IFN-α) to maximize BST2 expression. MLN4924 did not substantially sensitize infected cells to the ADCC activity of natural killer (NK) cells under the conditions tested. Moreover, MLN4924 did not increase cell surface Env measured with an antibody that recognizes a CD4-independent epitope even in IFN-treated cells in which it upregulated BST2. We conclude that inhibiting this single Vpu cofactor, the β-TrCP/cullin1 E3 ubiquitin ligase complex, is insufficient to sensitize infected cells to ADCC, probably because the exposure of CD4i epitopes is insufficient and because total surface Env is not effectively increased.
MATERIALS AND METHODS
Cells, antibodies, plasmids, and reagents.
HeLa P4.R5 cells were obtained from Ned Landau and maintained in Dulbecco's modified Eagle medium (DMEM) plus 10% fetal bovine serum (FBS) and puromycin. The 293-CD4-tetHIV-WT and -ΔNef cells are HEK293 cell lines (originating from ATCC 1573) engineered to constitutively express CD4 as well as the reverse tetracycline-responsive transactivator protein by stable transfection. They also contain an HIV-1 genome inserted by stable transfection in which the 5′ long terminal repeat (LTR) upstream of the TATA box has been replaced with the Clontech tet-responsive sequence, rendering viral expression inducible by doxycycline. The ΔNef version contains a deletion and frameshift in the 5′ region of the nef gene, as previously described (30). These cells were maintained in DMEM supplemented with 10% FBS, G418, puromycin, and zeocin. CEM.NKR.luc and NK-CD16 cells were obtained from David Evans (31) and maintained in RPMI medium supplemented with 10% FBS, 2 mM l-glutamine, and 1 μg/ml cyclosporine (only for NK cells). Peripheral blood mononuclear cells (PBMCs) were purified from deidentified uninfected donors by using density gradient centrifugation in Lymphoprep (Stemcell Technologies). CD4-positive cells were isolated from PBMCs using the EasySep CD4+ T-cell enrichment kit and RoboSep (Stemcell Technologies). The antibodies A32, G12, b12, 17b, HIV-1 IgG, and human recombinant interleukin-2 (rIL-2) were obtained from the NIH AIDS Reagent Repository. Human rIL-2 was deposited by Maurice Gately, Hoffman La Roche Inc. (32). IL-15 was from R&D Systems. Secondary anti-human-Alexa Fluor 647 was from Jackson ImmunoResearch. Anti-BST2-Alexa Fluor 647 and anti-CD4-APC (OKT4) were from Biolegend. Anti-CD3 and anti-CD28 were from BD Biosciences. IFN-α was from Cell Signaling (catalog number 8927LC). MLN4924 was from Active Biochem. pVphu (encoding codon-optimized Vpu), pCINL (expressing Nef), pCG-GFP (expressing green fluorescent protein [GFP]), pNL4-3, and related plasmids lacking intact vpu, nef, or vpu and nef genes or encoding vpu mutants have been described previously (33–40). The plasmid expressing dominant negative (DN) cullin1 (pcDNA3-DN-hCUL1-FLAG; Addgene plasmid 15818) was deposited by Wade Harper (41).
Transfection and infection.
HeLaP4.R5 and 293-CD4-tetHIV cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol and incubated overnight. Viral stocks were made from 293T cells transfected with proviral plasmid DNA, wild type or lacking one or more genes encoding accessory proteins, using BioT transfection reagent (Bioland Scientific). CEM.NKR.luc and primary CD4-positive T cells were infected using 200 ng of p24 antigen, as determined by p24 antigen capture enzyme-linked immunosorbent assay (ELISA; Advanced Bioscience Laboratories), per 500,000 cells by spinoculation in 5-ml polystyrene tubes for 2 h at 2,000 × g for the CEM.NKR.luc cells or 1,000 × g for the primary cells. Infected cells were incubated for 4 days before staining for flow cytometry (CEM.NKR.luc or primary T cells) or use in the ADCC assay (CEM.NKR.luc). Primary cells were also activated immediately after spinoculation by incubating in anti-CD3/28-coated plates for 3 days and additionally incubated in IL-2 and/or IL-15 without CD3/28 overnight.
Flow cytometry.
Cells were incubated with 2 μg/ml of each of primary antibodies for A32, 2G12, b12, or 17b, with 10 μg/ml for HIV IgG, with 1 μg/ml for CD4, or with 4 μg/ml for BST2, followed by incubation with 6 μg/ml Alexa Fluor 647-conjugated donkey anti-human IgG as appropriate. The cells were fixed, permeabilized (BD Biosciences fixation/permeabilization kit), and stained where indicated for intracellular p24 capsid using anti-p24(KC-57)-fluorescein isothiocyanate (FITC) (Beckman Coulter). The cells were refixed with 1% paraformaldehyde in phosphate-buffered saline (PBS) and analyzed by flow cytometry using an Accuri C6 flow cytometer (BD Biosciences). The cells were first gated using forward- and side-scatter characteristics, before gating for GFP or p24 positivity as indicated.
Antibody-dependent cellular cytotoxicity.
The assay was performed largely as described previously (31). Briefly, 4 days after infection with HIV-1 clone NL4-3 or related isogenic mutants, CEM.NKR.luc cells were preincubated with 200 nM MLN4924 in dimethyl sulfoxide (DMSO) or with DMSO alone for 4 h and then mixed with NK-CD16 effector cells at a 10:1 effector-to-target (E/T) ratio, in the presence or absence of antibodies as indicated in figure legends. Cells were incubated for an additional 8 h, again in the presence of 200 nM MLN4924 in DMSO or DMSO alone in wells of a round-bottom 96-well plate. The cell suspensions were then transferred to white-walled, clear-bottom 96-well plates (Corning) and mixed with BriteLite luciferase substrate/buffer (PerkinElmer). Luminescence was determined using a SpectraMax plate reader (Molecular Devices).
Ethics statement.
Blood was obtained from human volunteers using a protocol approved by the UCSD Human Subjects Committee; all subjects provided written informed consent.
RESULTS
MLN4924 and a cullin1 dominant negative mutant inhibit Vpu-mediated downregulation of CD4 but not BST2 in HeLa cells.
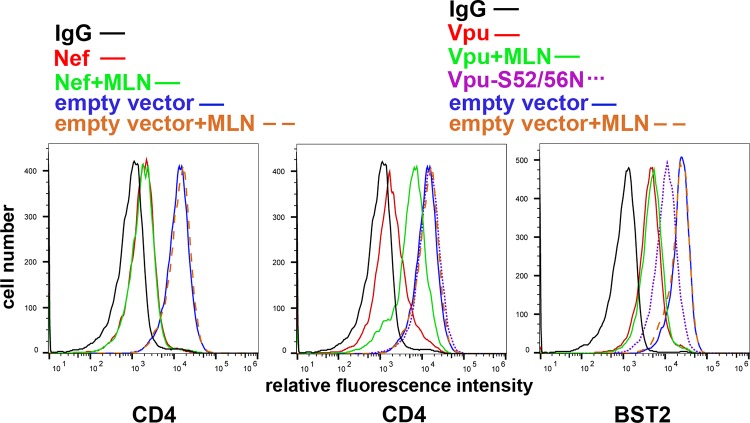
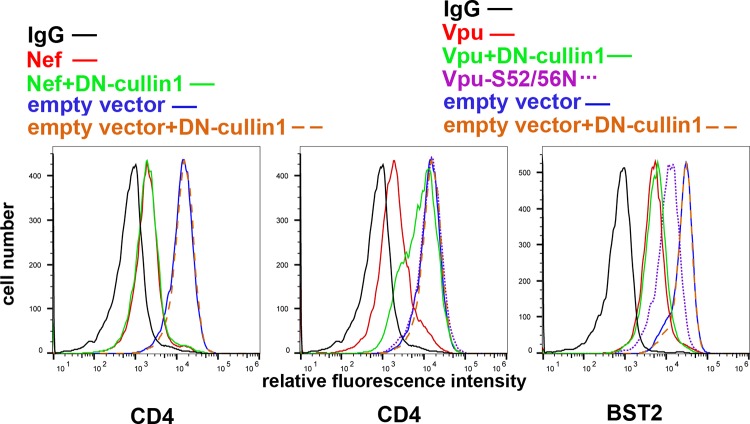
To test the effects of MLN4924 on the Vpu-mediated downregulation of CD4 and BST2, HeLa P4.R5 cells, which express CD4 and BST2 constitutively, were transfected to express Vpu using a plasmid encoding a human codon-optimized Vpu or to express Nef, along with a plasmid expressing GFP as a transfection marker. The next day, the cells were incubated with 500 nM MLN4924 in DMSO or with DMSO alone for 4 h. As expected, Nef downregulated CD4, and this was unaffected by MLN4924 (Fig. 1). In the absence of MLN4924, Vpu downregulated both CD4 and BST2 from the cell surface. MLN4924 inhibited Vpu-mediated CD4 downregulation substantially but not completely. MLN4924 had no effect on Vpu-mediated downregulation of BST2, even though replacement of Vpu serines 52 and 56 (Vpu-52/56N), which are required for the interaction of Vpu with the β-TrCP containing cullin1-based ubiquitin ligase complex, caused a partial loss of this activity. Vpu-52/56N was, as expected, devoid of CD4 downregulation activity. MLN4924 did not affect the surface levels of CD4 or BST2 in the absence of expression of the viral accessory proteins, suggesting that these cellular proteins are not regulated by cullin complexes under basal conditions and that the effect of MLN4924 is Vpu specific. To validate the results obtained using MLN4924, we coexpressed Vpu with a dominant negative version of cullin1 (41). DN-cullin1 substantially inhibited the Vpu-mediated downregulation of CD4 from the cell surface, but it had no effect on the downregulation of BST2, again even though the replacement of Vpu serines 52 and 56 caused a partial loss of this activity (Fig. 2). Expression of DN-cullin1 did not affect the surface levels of CD4 or BST2 in the absence of Vpu, nor did it affect the activity of Nef. Thus, Vpu-mediated downregulation of CD4 relies on cullin1 neddylation and is inhibited by the neddylation inhibitor MLN4924, whereas the Vpu-mediated downregulation of BST2, at least in HeLa cells under conditions of basal expression, is not. This conclusion is consistent with a recent study of Vpu targets: of CD4, BST2, NTB-A, and CCR7, only the downregulation of CD4 was cullin1 dependent (42). The observation that the Vpu-S52/56N mutant is nonetheless partially impaired with respect to BST2 downregulation supports the notion that these Vpu residues support an interaction in addition to the recruitment of the cullin1 complex that contributes to the modulation of BST2 (18, 43, 44). With respect to the modulation of CD4 by Vpu, we note that although the inhibitory activity of MLN4924 appears to be less than that of DN-cullin1, this is likely due to the relatively short (4-hour) exposure of the cells to the drug prior to analysis by flow cytometry; DN-cullin1, in contrast, was expressed by transfection concomitantly with Vpu.
FIG 1.
The NAE inhibitor MLN4924 inhibits Vpu-mediated downmodulation of CD4, but not Nef-mediated downmodulation of CD4 or Vpu-mediated downmodulation of BST2 in HeLa cells. HeLa cells were transfected to express Vpu or Nef, along with GFP as a transfection marker. The next day, cells were incubated with either DMSO alone or 500 nM MLN4924 in DMSO for 4 h, stained for surface CD4 or BST2, and analyzed by flow cytometry, gating on the GFP-positive populations. The results shown are representative of three experiments.
FIG 2.
A dominant negative mutant of cullin1 inhibits Vpu-mediated downmodulation of CD4 but not Vpu-mediated downmodulation of BST2 in HeLa cells. HeLa cells were transfected to express Vpu or Nef, either with or without a dominant negative version of cullin1, along with GFP as a transfection marker. The next day, cells were analyzed for surface CD4 or BST2 by flow cytometry, gating on the GFP-positive populations. The results shown are representative of three experiments.
Inhibition of Vpu-mediated downmodulation of CD4 by MLN4924 is associated with increased expression of CD4-induced epitopes in Env at the cell surface.
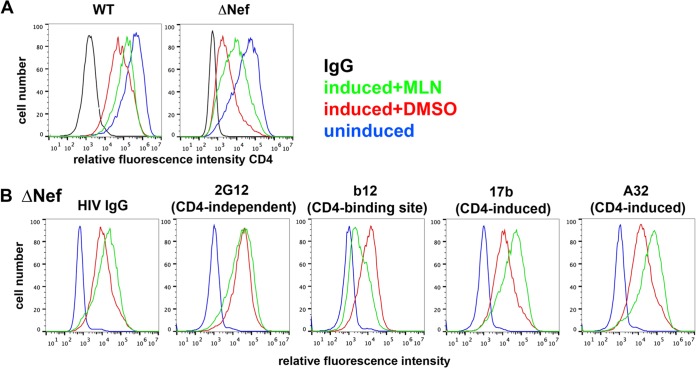
The formation of CD4-Env complexes causes the exposure of CD4i epitopes in Env and reactivity with specific monoclonal antibodies such as A32 and 17b (5–7). Therefore, we investigated whether the inhibition of Vpu-mediated downregulation of CD4 by MLN4924 increased the exposure of CD4i epitopes of Env. To avoid any potential confounding effects from virion retention by BST2 and for experimental convenience, we used cells of the HEK293 derivative line 293-CD4-tetHIV or 293-CD4-tetHIVΔNef, both of which stably express CD4 and express HIV under the control of a doxycycline-inducible promoter. Cells of these lines express very little BST2 (data not shown). They are independent clones, however, and express different levels of CD4 in the uninduced state (Fig. 3A; blue curves). The cells were treated with doxycycline for 16 h to induce HIV gene expression and then incubated with 500 nM MLN4924 in DMSO or with DMSO alone for 4 h. MLN4924 substantially inhibited the downregulation of CD4, both in the case of the wild-type HIV genome encoding both vpu and nef and in the case of the HIV genome lacking nef (Fig. 3A). In the latter cells, the surface levels of CD4i epitopes in Env as measured by staining with A32 and b17 antibodies were also increased by MLN4924, while the exposure of the CD4 binding site epitope measured by the b12 antibody was decreased and the exposure of the CD4-independent epitope measured by the 2G12 antibody remained unchanged (Fig. 3B). All of these results are consistent with the hypothesis that the upregulation of CD4 by MLN4924 directly affects CD4-related epitopes in Env, masking the CD4 binding site and revealing the CD4i sites while having no effect on the surface levels and exposure of Env epitopes unrelated to CD4. How these different effects are summarized in patient-derived IgG, which presumably contains a mixture of antibodies potentially recognizing all of these sites, was also tested; MLN4924 modestly increased the surface staining of these cells using a preparation of pooled HIV-1 IgG from infected individuals (Fig. 3B). Thus, MLN4924 specifically increases the exposure of CD4i epitopes in Env on cells expressing the HIV-1 genome; moreover, it causes a net increase in the exposure of HIV-1 Env as recognized by patient-derived HIV-1 IgG.
FIG 3.
MLN4924 inhibits Vpu-mediated downmodulation of CD4 and increases the surface expression of CD4-induced epitopes in Env. (A) 293-CD4-tetHIV-WT and -Δnef cells were treated with doxycycline to induce the expression of HIV-1 and incubated with either DMSO alone or 500 nM MLN4924 in DMSO for 4 h. The surface CD4 and internal HIV-1 p24 levels were determined by flow cytometry. Histograms represent “live” populations based on forward- and side-scatter; over 90% of live cells expressed p24 (not shown). (B) 293-CD4-tetHIV-Δnef cells were treated with doxycycline to induce the expression of HIV-1, and surface CD4 and specific Env epitopes were analyzed by flow cytometry, gating on the “live” populations. The color code applies to both panels A and B. The results shown are representative of two experiments.
MLN4924 inhibits Vpu-mediated downmodulation of CD4 and increases the expression of Env epitopes on the surface of primary CD4-positive T cells.
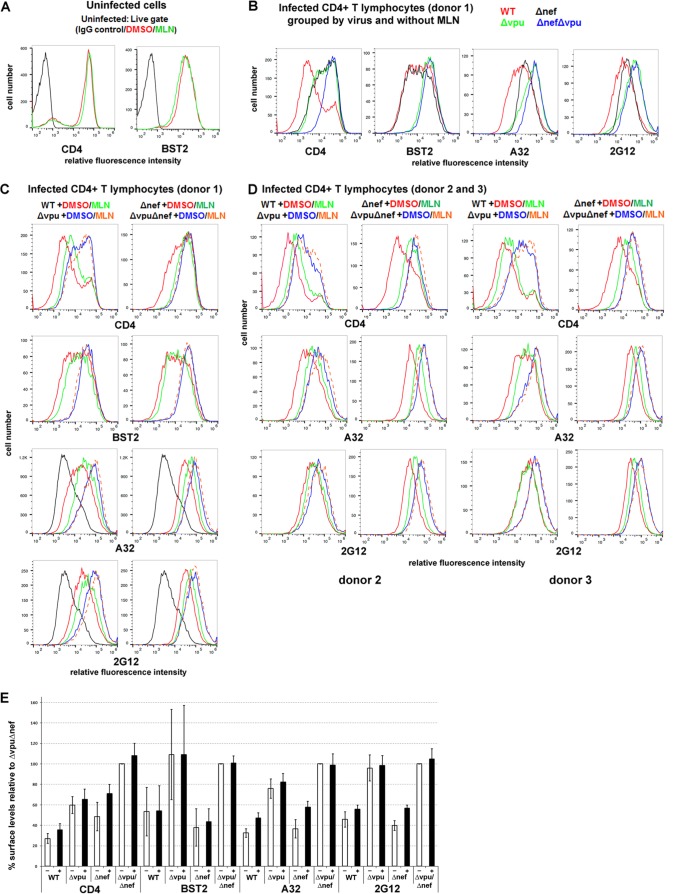
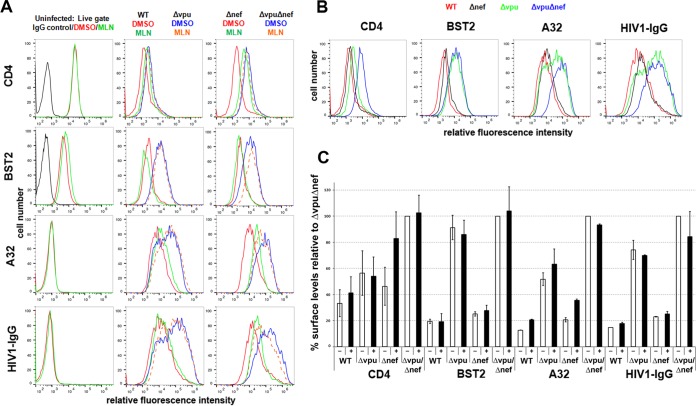
To extend our findings to a more physiologically relevant system, we evaluated the effects of MLN4924 using primary cultures of CD4-positive T cells infected with either wild-type virus or accessory gene mutants (Fig. 4 and Table 1, which shows a statistical analysis of the data). CD4-positive T cells were purified from HIV-negative donors, infected with the various viruses, and treated or not with MLN4924, and the surface levels of CD4, BST2, CD4i, and CD4-independent Env epitopes were measured (Fig. 4). The concentration of MLN4924 was decreased from the previously used 500 nM to 200 nM to avoid toxicity; 200 nM was well tolerated by the primary cells during the longer, 12-hour incubation (compared to the 4 h used in the experiments described above using HeLa and HEK293 cells), with no apparent excess toxicity compared to the DMSO control as observed by forward- and side-scatter characteristics during flow cytometry (data not shown).
FIG 4.
MLN4924 inhibits Vpu-mediated downmodulation of CD4 and increases the surface expression of CD4-induced Env epitopes in primary CD4+ T cells. (A) Uninfected primary CD4+ T cells were treated with either DMSO alone or 200 nM MLN4924 in DMSO for 12 h and then stained for surface CD4 or BST2 and analyzed by flow cytometry. (B and C) Primary CD4+ T cells were infected with either wild-type HIV-1 or the indicated mutants. Four days later, the cells were incubated with either DMSO alone or 200 nM MLN4924 in DMSO for 12 h and then stained for surface CD4, BST2, and Env with the indicated antibodies and analyzed by flow cytometry, gating on the p24-positive cells. Black lines in the panels for A32 and 2G12 staining are the results for uninfected cells. Panel B shows a subset of the data displayed in panel C selected to show the effects of Nef and Vpu in the absence of MLN4924. (D) Independent data from two additional donors for CD4 and Env (detected by A32 and 2G12); the cells were infected either with wild-type HIV-1 or with the indicated mutants and treated or not with MLN4924 as indicated. (E) For the three preceding independent donors, the mean fluorescence intensities for CD4, BST2, A32, and 2G12 were averaged and normalized to Δvpu in the absence of MLN4924, which was set to 100%. −, open bars indicate the absence of MLN4924; +, filled bars indicate treatment with MLN4924. Error bars indicate ±standard deviations.
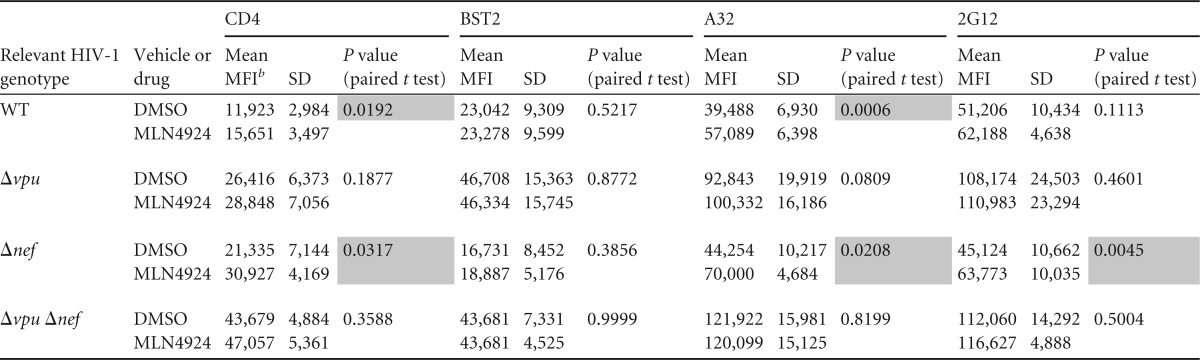
TABLE 1.
Statistical analysis of the effects of MLN4924 on the expression of cellular proteins and HIV-1 Env epitopes on the surface of primary CD4-positive lymphocytes shown in Fig. 4a
Shading indicates statistically significant upregulation by MLN4924 (P < 0.05).
b MFI, mean fluorescence intensity.
As seen in the case of HeLa cells, MLN4924 did not affect the expression of either CD4 or BST2 on the surface of uninfected primary T cells (Fig. 4A). As expected, the effects of Nef and Vpu on the surface expression of CD4 were additive, and cells infected with the mutant unable to express both Nef and Vpu expressed near-normal levels of CD4 (Fig. 4B to E). These data are consistent with previous studies, with the exception that the role of Vpu relative to that of Nef shown here appears somewhat greater than expected (45, 46). In contrast to CD4, BST2 downregulation was solely due to the expression of Vpu, with no contribution from Nef (Fig. 4B, C, and E).
The expression of cell surface Env as detected by either A32 (CD4 induced) or 2G12 (CD4 independent) was increased in the absence of Vpu (Fig. 4B to E). These results are consistent with the downregulation of both CD4 and BST2 by Vpu; lack of CD4 downregulation presumably triggers the exposure of CD4i epitopes, whereas the lack of BST2 downregulation presumably increases the levels of all Env epitopes, including those independent of CD4, due to the retention of Env-containing virions on the cell surface. Although donor variation is evident (Fig. 4B to D), on average (Fig. 4E), the lack of Nef contributed relatively little to the exposure of the CD4i epitope recognized by A32, although the surface levels of CD4 were increased. The lack of Nef also contributed relatively little to the exposure of the CD4-independent epitope recognized by 2G12, (Fig. 4B to E).
MLN4924 inhibited the Vpu-mediated downregulation of CD4 but affected minimally if at all the downregulation of BST2 in primary CD4 T cells, both in the background of wild-type virus and in the background of virus lacking nef (Fig. 4C and E). In cells from one of three donors infected with nef-negative virus, the effect of MLN4924 on CD4 was complete; the Vpu-mediated downregulation of CD4 was inhibited to the same or nearly the same extent as when the virus lacked both vpu and nef (Fig. 4C, top right panel). In the other two donors, the effect of MLN4924 on the Vpu-mediated downregulation of CD4 was incomplete but substantial. MLN4924 did not affect the levels of CD4 in the absence of Vpu expression, as seen in the case of infection with virus lacking vpu or both vpu and nef (Fig. 4C to E); this confirms that the effect of MLN4924 on CD4 is Vpu specific.
MLN4924 increased the exposure of the CD4i epitope recognized by A32 in a manner that paralleled its effects on the surface expression of CD4, and this effect was seen both in the presence and in the absence of nef (Fig. 4C to E). Interestingly, the cell surface exposure of the epitope recognized by 2G12 was also increased slightly by MLN4924, and this was also vpu dependent (Fig. 4C to E). This result could be due to the virion retention activity of CD4 (11), which, like the virion retention activity of BST2, would presumably increase the amount of Env on the cell surface. Notably, the lack of an effect of MLN4924 on the expression of the 2G12 epitope on the surfaces of the 293-CD4-tetHIVΔNef cell line when induced to express virus (Fig. 3B) is inconsistent with CD4-mediated virion retention. One potential explanation for this inconsistency is the relatively low median CD4 levels on cells of the 293-CD4 line relative to the primary T lymphocytes, which might not support virion retention (data not shown).
In summary, these data indicate that MLN4924 increases the exposure of both CD4i and CD4-independent Env epitopes on the surface of infected primary T lymphocytes, but these effects are relatively modest compared to those of the genetic absence of vpu and nef (Fig. 4B and D). When the data shown in Fig. 4E were analyzed statistically (Table 1), MLN4924 significantly upregulated CD4 and the Env epitope recognized by A32 in the case of cells infected with wild-type or nef-negative viruses (i.e., in cells expressing Vpu), but it had no effect on BST2; statistically significant upregulation of the Env epitope recognized by 2G12 was detected only in the case of cells infected with virus lacking nef (see shaded panels of Table 1, in which P is <0.05).
MLN4924 does not sensitize cells to ADCC-mediated killing in a Vpu-specific manner.
The increased exposure of Env molecules and Env epitopes on the surface of infected cells in the absence of Vpu and/or Nef renders such cells more susceptible to killing by ADCC (5, 7–9). Having shown that MLN4924 inhibits Vpu activity with respect to CD4 and consequently increases the display of Env epitopes, we tested the hypothesis that it also increases the susceptibility of infected cells to ADCC-mediated clearance. For this, we used a previously reported system in which infected CEM.NKR.luc cells are the targets for ADCC mediated by an NK cell line (31). CEM.NKR.luc cells are a derivative of the CD4-positive T cell leukemia line CEM, in which the cells were selected for resistance to NK-mediated killing in the absence of antibody and then engineered to express luciferase under the control of a Tat-inducible promoter. These cells express a quantifiable amount of luciferase at baseline, but luciferase expression is markedly increased with infection and Tat-mediated trans-activation (31; data not shown). The cells were infected with either wild-type HIV-1 or virus lacking vpu, or nef, or both vpu and nef.
Before measuring ADCC, we determined whether the effects of Vpu, Nef, and MLN4924 were similar in the CEM.NKR.luc cells and in primary T lymphocytes. In the absence of MLN4924, the four viruses displayed a hierarchy similar to that observed in primary T cells (Fig. 5A to C). Cells infected with virus lacking both nef and vpu displayed the highest levels of surface CD4, BST2, and Env as recognized by either A32 or HIV IgG. The lack of vpu contributed to each of these increases, whereas the lack of nef contributed primarily to increases in the levels of CD4 and the CD4i epitope recognized by A32, with a relatively minor effect on staining with HIV1-IgG (Fig. 5A to C). Notably, the lack of vpu increased the cell surface expression of the Env epitope recognized by A32 to an extent out of proportion to the increase in surface CD4, a result consistent with a combined effect of virion retention by BST2 together with triggering of epitope exposure by CD4.
FIG 5.
MLN4924 inhibits Vpu-mediated downmodulation of CD4 and increases the surface expression of a CD4-induced Env epitope in CEM.NKR.luc cells. (A) CEM.NKR.luc cells were infected with wild-type or mutant HIV-1 as indicated. Four days later, the cells were incubated with either DMSO alone or 200 nM MLN4924 for 12 h, and surface CD4, BST2, and Env expression was determined by flow cytometry, gating on p24-positive cells. (B) Subset of histograms from panel A regrouped to show the effects of Nef and Vpu in the absence of MLN4924. (C) For two independent experiments, the results for one of which are shown in panels A and B, the mean fluorescence intensities for CD4, BST2, A32, and HIV-IgG were averaged and normalized to ΔvpuΔnef in the absence of MLN4924, which was set to 100%. −, open bars indicate the absence of MLN4924; +, filled bars indicate treatment with MLN4924. Error bars indicate ±average deviations.
MLN4924 increased the surface levels of CD4 in the context of both wild-type virus and virus lacking nef, while the surface levels of CD4 in the cells infected with virus lacking vpu or both vpu and nef remained relatively unchanged (Fig. 5A and C). The surface levels of BST2 were unaffected by MLN4924 (Fig. 5A and C). These effects resemble the results obtained using primary T lymphocytes, and they confirm that the effects of MLN4924 are Vpu specific and relatively CD4 specific.
MLN4924 increased the exposure of the CD4i Env epitope recognized by A32 in a manner that paralleled its ability to increase the surface expression of CD4, although the effect of the drug was not equivalent to the genetic absence of vpu (Fig. 5A and C). In contrast, MLN4924 had little or no effect on the staining of cells with HIV-1 IgG (Fig. 5A and C); these results are consistent with the inability of MLN4924 to inhibit the downregulation of BST2.
When the data shown in Fig. 5C were analyzed statistically (Table 2), MLN4924 significantly upregulated the Env epitope recognized by A32 in the case of cells infected with wild-type or nef-negative viruses (i.e., in cells expressing Vpu), but it had no effect on BST2 or the recognition of the cells by HIV-1 IgG; statistically significant upregulation of CD4 was detected only in the case of cells infected with virus lacking nef, although the results from cells infected with the wild-type virus just missed formal significance (P = 0.0554).
TABLE 2.
Statistical analysis of the effects of MLN4924 on the expression of cellular proteins and HIV-1 Env epitopes on the surface of CEM.NKR lymphoid cells shown in Fig. 5a
Shading indicates statistical significance (P < 0.05).
b MFI, mean fluorescence intensity.
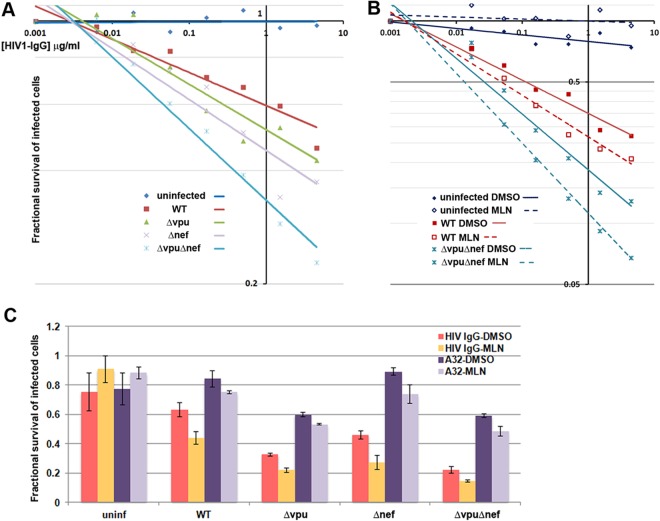
To measure ADCC, the killing of the infected CEM.NKR.luc cells by cells of a CD16-positive NK line was determined by measuring the luminescence generated after the cells in the coculture were lysed and incubated with luciferase substrate; this value decreases when the CEM.NKR.luc cells die. This ADCC assay has been used previously to show the effect of Vpu on ADCC using HIV-1 IgG as the antibody (8). First, we extended those results by analyzing our panel of four viruses and testing A32 as well as HIV-1 IgG (Fig. 6). Preliminary experiments confirmed that killing measured as a decrease in the luciferase activity of these cocultures is dependent on the presence of HIV-specific antibody and the presence of NK cells (data not shown). The dose-dependent killing of infected cells by HIV IgG in the presence of NK cells is shown in Fig. 6A and B. The fractional survival (luciferase signal) of uninfected cells in the presence of NK cells was unaffected by increasing concentrations of HIV IgG, whereas infected cells were killed in a manner directly related to the concentration of antibody (Fig. 5A). These results weigh against a substantial contribution of killing due to nonspecific effects of either the antibody mixture or the NK cells. Cells infected with virus lacking vpu and nef were killed most efficiently, whereas cells infected with wild-type virus were killed least efficiently, and cells infected with virus lacking either vpu or nef were killed with an intermediate efficiency (Fig. 5A). This hierarchy of killing corresponded imperfectly with the hierarchy of staining with HIV-1 IgG (compare Fig. 5A and C); the increased sensitivity to ADCC caused by the lack of nef was similar to that caused by the lack of vpu, whereas the cell surface staining with HIV IgG was much more dramatically increased by the lack of vpu. Nonetheless, these data confirmed that vpu and nef each contribute to the evasion of ADCC mediated by a mixture of antibodies to HIV-1. The data also confirmed that virus lacking both of these accessory genes is rendered maximally sensitive to ADCC.
FIG 6.
The absence of nef and/or vpu, or treatment with MLN4924, sensitizes infected cells to ADCC, but the effect of MLN is not specific to cells expressing Vpu. (A and B) CEM.NKR.luc cells, which contain a luciferase indicator gene under the control of an HIV LTR, were either uninfected or infected for 4 days with the indicated viruses and then mixed with cells of an NK line at a 10:1 effector/target (E/T) ratio in the presence of increasing concentrations of HIV IgG and incubated for 8 h. The amount of viable cells was determined by luminescence and normalized to the signal of a no-IgG sample for each indicated condition, which was set to 1. Fractional cell survival (y axis) is graphed versus the concentration of HIV-IgG (x axis) on a log-log plot. Trend lines were determined using Excel. (A) Sensitivities of the various viruses; (B) effects of 200 nM MLN4924 (used as described below) on the sensitivity of uninfected cells and cells infected with either wild-type virus or virus lacking vpu and nef. (C) CEM.NKR.luc cells, either uninfected or infected for 4 days with the indicated viruses, were preincubated with 200 nM MLN4924 in DMSO or with DMSO alone for 4 h and then mixed with cells of an NK line at a 10:1 E/T ratio in the presence of 0.75 μg/ml HIV IgG or 2.5 μg/ml A32 and incubated for 8 h either with 200 nM MLN in DMSO or with DMSO alone, in triplicate. The amount of viable cells was determined by luminescence and normalized to the signal of a no-IgG sample for each indicated condition, which was set to 1 as described for panels A and B. Error bars indicate ±standard deviations of triplicates.
To test the effect of MLN4924 on ADCC, the infected CEM.NKR.luc cells were incubated either with 200 nM MLN4924 in DMSO or with DMSO alone for a total of 12 h, 4 h before and 8 h after the addition of antibodies and the NK cells (Fig. 6B and C). Figure 6B shows results of a dose-response experiment using HIV-1 Ig that is similar to that in Fig. 6A, but in which the effect of MLN4924 is evaluated on the killing of cells infected with either wild-type virus or, as a maximally sensitive control, virus lacking both vpu and nef. MLN4924 modestly sensitized cells infected with the wild-type virus to ADCC-mediated killing, but surprisingly it similarly sensitized to killing cells infected with virus lacking vpu and nef (Fig. 6B). This effect was not likely due to toxicity of the drug, since MLN4924 did not cause a decrease in the survival of uninfected cells (Fig. 6B and C). Since MLN4924 increased the surface staining of infected cells by A32 much more than it did the staining by HIV IgG (Fig. 5A and C), we tested this antibody at a single concentration in comparison to HIV-1 IgG (Fig. 6C). Again, MLN4924 had no adverse effect on the signal from uninfected cells, which indicated a lack of toxicity of the drug as used. At the single concentrations of antibodies tested and in the absence of MLN4924, the killing of the infected cells again correlated imperfectly with the surface exposure of epitopes recognized by either A32 or HIV IgG. Specifically, cells infected with virus lacking nef were more sensitized to ADCC using HIV IgG than might have been expected based on the barely detectable increase in surface staining with this antibody mixture, whereas they were less sensitized to ADCC using A32 than might have been expected based on the relatively greater increase in surface staining detected with this antibody. In contrast, the lack of vpu sensitized cells to ADCC mediated by either A32 or HIV IgG, and these effects correlated directly with the increased cell surface staining obtained using these antibodies. Again, MLN4924 modestly enhanced the apparent killing of all infected cells, regardless of whether the virus was wild type, lacked vpu or nef, or lacked both vpu and nef (Fig. 6C). When the data shown in Fig. 6C were analyzed statistically (Table 3), MLN4924 significantly sensitized infected cells to killing regardless of the genotype of the virus, whereas it had no significant effect on uninfected cells. These results suggested that despite its ability to specifically inhibit the Vpu-mediated downregulation of CD4 and expose CD4i epitopes, MLN4924 appeared to enhance ADCC by a mechanism that was not specific to Vpu.
TABLE 3.
Statistical analysis of the effect of MLN4924 on ADCC using either pooled HIV-1 IgG or the monoclonal antibody A32 on either uninfected cells or cells infected with wild-type HIV-1 (clone NL4-3) or isogenic mutants containing deletions in vpu, nef, or vpu and nefa
Shading indicates statistical significance (P < 0.05).
b Mean fractional survival values are the same as those graphed in Fig. 6C.
c CI, confidence interval.
MLN4924 inhibits Vpu-mediated downmodulation of BST2 in interferon-treated cells.
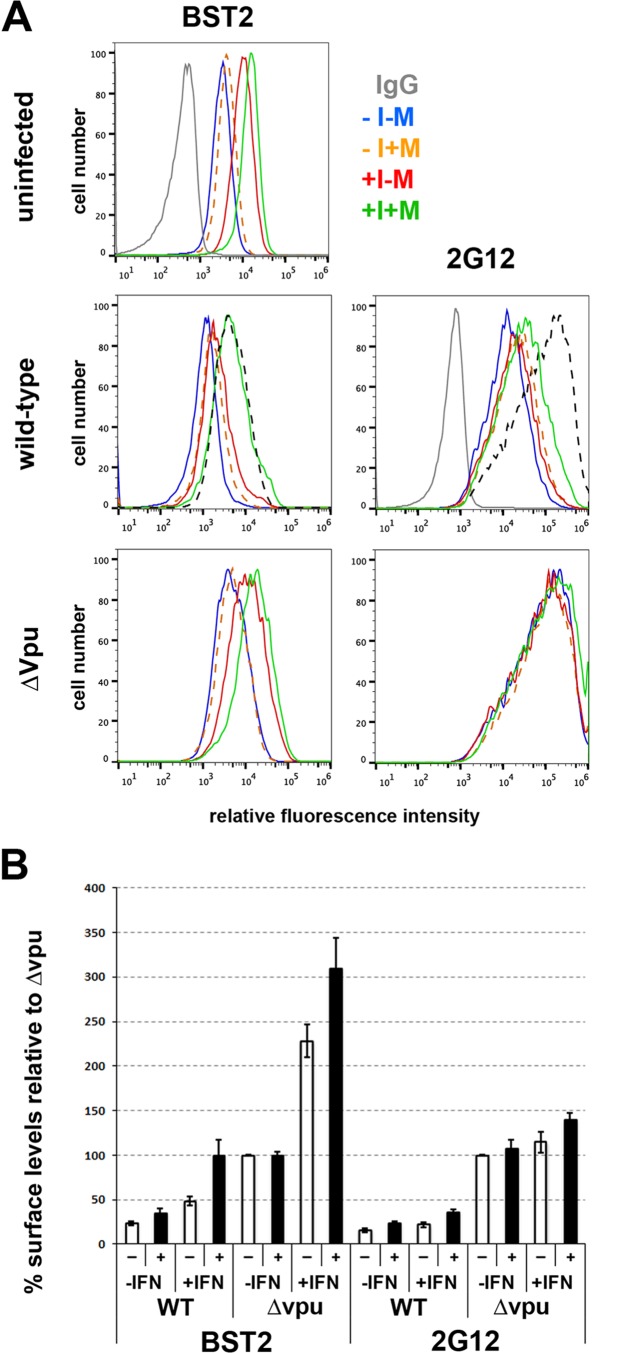
We considered that the minimal effects of NAE inhibition on the Vpu-mediated downregulation of BST2 and consequently on the cell surface levels of Env and on ADCC might be due to relatively low levels of basal BST2 expression. Under such conditions, ubiquitin-mediated degradation of BST2 is potentially dispensable for Vpu's activity; Vpu can mediate surface downregulation of BST2 via endosomal trafficking (19, 47), and mutational analyses suggest that Vpu can displace BST2 from sites of virion assembly to stimulate virion release without substantial BST2 downregulation (40, 48). To test the hypothesis that NAE inhibition can upregulate BST2 and Env in cells infected with the wild-type virus when BST2 expression is relatively high, we treated CEM.NKR.luc cells with alpha interferon (here IFN), infected the cells with wild-type or vpu-negative virus, and measured the surface levels of BST2 and Env as detected by the 2G12 antibody (Fig. 7 and Table 4). IFN upregulated BST2 on uninfected cells, and MLN4924 upregulated BST2 slightly further. In IFN-treated and infected cells, BST2 was downregulated in a vpu-dependent manner, but this downregulation was substantially and significantly reversed by MLN4924 (Fig. 7B and Table 4). The surface levels of BST2 on cells infected with wild-type virus and treated with IFN and MLN4924 in most experiments were similar to those of cells infected with vpu-negative virus in the absence of IFN (Fig. 7A, dashed black line; Fig. 7B, averages). These data support the hypothesis that ubiquitin- and Vpu-mediated cell surface downregulation of BST2 is revealed by IFN treatment, presumably due to the induction of high levels of BST2 expression. Surprisingly, MLN4924 increased surface BST2 substantially and significantly in interferon-treated cells infected with vpu-negative virus (Fig. 7 and Table 4); these data, together with the data from uninfected cells, suggest that in interferon-treated cells a cullin-dependent mechanism might be involved in the turnover of BST2. The MLN4924-induced increase in BST2 on the surface of cells infected with wild-type virus was associated with only a modest increase in cell surface Env detected by 2G12, and this did not achieve statistical significance (Table 4). In contrast, in the absence of vpu, staining by 2G12 was markedly increased. Thus, although published data indicate a strong positive correlation between ADCC, the levels of cell surface Env, and the levels of BST-2 based on the examination of cells infected with a subset of vpu mutants (8), the data here suggest that cell surface Env is not necessarily a direct correlate of BST2 levels.
FIG 7.
MLN4924 upregulates BST2 in interferon-treated cells expressing wild-type virus but minimally upregulates surface Env as measured by 2G12. (A) CEM.NKR.luc cells were infected with wild-type or vpu-negative (ΔVpu) HIV-1 as indicated. Three days later, the cells were treated or not with 30 ng/ml IFN-α, MLN4924, or both and then stained and analyzed the next day for surface BST2 and Env (using antibody 2G12), gating on p24-positive cells. The black dashed lines in the “wild-type” panels are the curves for ΔVpu in the absence of both interferon and MLN4924 for comparison. (B) For three independent experiments, the results for one of which are shown in panel A, the mean fluorescence intensities for BST2 and 2G12 were averaged and normalized to Δvpu in the absence of interferon or MLN4924, which was set to 100%. −, open bars indicate the absence of MLN4924; +, filled bars indicate treatment with MLN4924. Error bars indicate ±standard deviations.
TABLE 4.
Statistical analysis of the effects of MLN4924 on cell surface BST2 and Env recognized by 2G12 in interferon-treated or -untreated CEM.NKR cells shown in Fig. 7a
Shading indicates statistical significance (P < 0.05).
b MFI, mean fluorescence intensity.
Vpu W76 modulates the display of Env on infected cells.
We suspected that even when NAE inhibition together with IFN treatment increases BST2 on the surface of infected cells, Vpu can stimulate virion release via the displacement activity noted above such that cell surface Env remains relatively low (40, 48). The ability of Vpu to displace BST2 from sites of virion assembly is independent of serines 52 and 56 and thus the β-TrCP containing cullin1-based ubiquitin ligase complex, but it depends on a C-terminal tryptophan (W76) in clade B virus (40). We tested whether this residue was important for the display of Env in infected CEM.NKR cells (Fig. 8 and Table 5). Whether the cells were treated or not with IFN and relative to cells expressing the wild-type protein, the W76G substitution in Vpu increased the levels of surface Env detected by 2G12 at least as much if not more than the S52/56N substitution, although not all of these comparisons reached statistical significance (Table 5). These data are consistent with the previously described role of Vpu W76 in virion release (40, 49). The data thus provide a second reason, in addition to the inability of NAE inhibition to mimic the phenotype of the Vpu 52/56N substitution as shown in Fig. 1, why MLN4924 does not effectively increase surface Env in the presence of Vpu.
FIG 8.
W76 in Vpu contributes to the modulation Env in infected T cells. CEM.NKR.luc cells were infected with wild-type, vpu-negative, or the indicated vpu mutant viruses as indicated. Three days later, the cells were treated or not with 30 ng/ml IFN-α, MLN4924, or both and then stained and analyzed the next day for surface Env (using antibody 2G12), gating on p24-positive cells. (B) For two independent experiments, the results for one of which are shown in panel A, the mean fluorescence intensities for 2G12 were averaged and normalized to Δvpu in the absence of interferon or MLN4924, which was set to 100%. −, open bars indicate the absence of MLN4924; +, filled bars indicate treatment with MLN4924. Error bars indicate ±standard deviations.
TABLE 5.
Statistical analysis of the effect of Vpu amino acid substitutions W76G and S52/56N on cell surface Env recognized by 2G12 in CEM.NKR cells as shown in Fig. 8a
Shading indicates statistical significance (P < 0.05).
DISCUSSION
Recent reports have revealed that the HIV-1 accessory proteins Vpu and Nef provide evasion of ADCC by at least two mechanisms, one related to the downregulation of CD4 and the other related to the downregulation of BST2 (5, 7–9). The downregulation of CD4 prevents the surface exposure of CD4i epitopes in Env that are particularly good targets for ADCC (5–7). The downregulation of BST2 prevents the entrapment of Env-containing virions on the cell surface, which also increases the available targets for ADCC (5, 8, 9). These findings invigorated us to consider approaches to the pharmacologic inhibition of these accessory proteins, toward the goal of rendering infected cells more effectively killed by ADCC. Such pharmacologically enhanced immune surveillance could become a key adjunct in HIV eradication strategies. Many of these strategies are variants of the “shock and kill” model, in which latent infection is reversed in cells harboring transcriptionally inactive provirus, and these cells, once producing viral proteins, are recognized and killed by host immune surveillance mechanisms (50, 51). ADCC is an immune surveillance mechanism that could be enhanced by inhibiting Vpu and/or Nef. Currently, no small-molecule drugs are available to directly inhibit these accessory proteins, but we hypothesized that a key cellular cofactor of Vpu, the β-TrCP/cullin1 E3 ubiquitin ligase complex, would be inhibited by the NAE inhibitor MLN4924. This small molecule, which blocks the neddylation of cullin1 required for E3 ubiquitin ligase activity, has advanced to clinical trials for various cancers (24). Here we show that NAE inhibition inhibits Vpu's ability to modulate CD4 and BST2. The inhibition of CD4 downregulation occurs under basal conditions, whereas the inhibition of BST2 downregulation is apparent in IFN-treated cells, in which the expression of BST2 is induced above basal levels. Although NAE inhibition substantially reverses the downregulation of CD4 in infected cells and triggers increased exposure of CD4i epitopes in Env on the cell surface, this was insufficient to yield increased susceptibility to ADCC under the conditions tested. Moreover, even in IFN-treated cells, in which NAE inhibition partially reverses the downregulation of BST2 by Vpu, the levels of cell surface Env were not increased substantially. Thus, the data suggest that inhibiting only Vpu's ability to stimulate ubiquitin-mediated degradation of its targets is insufficient to sensitize infected cells to ADCC.
We used HeLa, HEK293, and a CD4-positive T cell line as well as primary cultures of CD4-positive T lymphocytes in an attempt to dissect the multifaceted mechanism by which Vpu contributes to the evasion of ADCC. The observation that pharmacologic NAE inhibition impaired only the downregulation of CD4 by Vpu in HeLa cells was supported by experiments in which a dominant negative mutant of cullin1 was expressed; the downregulation of CD4 was again substantially inhibited, whereas the downregulation of BST2 was unaffected. How can these results be reconciled with the putative role of the Vpu-β-TrCP interaction in the downregulation of BST2 reported by us and others (15, 16, 52)? This role was supported primarily by two lines of evidence: (i) the mutation of serines in Vpu that when phosphorylated mediate binding to β-TrCP impairs the downregulation of surface BST2 as well as the degradation of total cellular BST2; and (ii) expression of a dominant negative mutant of β-TrCP that can bind Vpu but cannot participate in the cullin1 complex also impairs the downregulation of BST2. In contrast, suppression of the expression of β-TrCP (specifically, isoform 2) by RNA interference did not block Vpu-mediated downregulation of BST2 from the cell surface, although it did inhibit the degradation of total cellular BST2 (18). These and other lines of evidence (43), including the experiments here in which NAE inhibition had little or no effect on BST2 downregulation, lead to the hypothesis that Vpu can influence the trafficking of BST2 within the endosomal system in a manner independent of the β-TrCP/cullin1 E3 ubiquitin ligase complex. Since the key phosphoserines noted above are in close proximity to a recently well-characterized clathrin adaptor protein (AP) binding motif in Vpu (19, 53), one possibility is that, at least under conditions of relatively low BST2 expression, AP-mediated protein sorting within the endosomal system is sufficient for the downregulation of BST2, while the interaction with the β-TrCP/cullin1 E3 ubiquitin ligase complex is dispensable. This possibility has been further supported by the recent finding that the phosphoserines have a dual role in binding to clathrin adaptors in addition to the β-TrCP/cullin1 E3 ubiquitin ligase complex (44). Nonetheless, BST2 is an interferon-inducible protein, and the data here indicate that treatment of cells of a CD4-positive T lymphocyte line with IFN yields a condition in which the downregulation of surface BST2 by Vpu is indeed sensitive to NAE inhibition. This sensitivity is presumably due to the induction of BST2 expression to levels that require β-TrCP/cullin1-mediated degradation for fully effective surface downregulation. Notably, a recent study describing the cullin1 independence of Vpu-mediated downregulation of BST2 in primary cultures of T cells did not evaluate IFN-mediated induction of expression, as done here (42).
Remarkably, even in IFN-treated cells in which NAE inhibition partly reversed the downregulation of BST2 by Vpu, the surface levels of Env as measured by the antibody 2G12, which detects a CD4-independent glycan epitope on gp120 (54), were increased only slightly and remained much lower than the levels of Env associated with the genetic absence of vpu. The reason for this is not fully clear, but we suspect that it relates to the ability of Vpu to stimulate virion release by displacing BST2 from sites of virion assembly within the plane of the plasma membrane (40, 48). This activity is seemingly independent of BST2 downregulation, and it depends on C-terminal sequences in Vpu that are distinct from those required for the interaction of Vpu with the β-TrCP/cullin1 E3 ubiquitin ligase complex, in particular W76 in clade B Vpu (40). Indeed, we show here that W76 contributes substantially to the ability of Vpu to decrease the surface levels of Env. Thus, even when BST2 levels are partially restored by NAE inhibition, this cullin1-independent activity of Vpu presumably stimulates release such that virions are not effectively entrapped by BST2 and cell surface Env is minimally increased. In other words, while cell surface Env may yet be a direct correlate of the degree of restricted virion release, it is not a simple and direct correlate of BST2 surface levels.
Although several seminal studies have made a strong case for Vpu in the evasion of ADCC (5, 7–9), the data regarding Nef are somewhat less clear. Two studies support a role of Nef via CD4 downregulation (5, 7), which as noted above prevents the exposure of CD4i epitopes in Env. For reasons yet unclear, such CD4i epitopes appear to be especially good targets for ADCC (6). However, another study that focused on Vpu showed no effect of suppressing the expression of CD4 by RNA interference on the susceptibility of cells to ADCC (8). The data here confirm a complex interplay between the downregulation of CD4 by Vpu and Nef together with the downregulation of BST2 by Vpu. This interplay appears to allow both accessory proteins to influence the exposure of multiple distinct epitopes in Env on the cell surface. The contribution of Nef and Vpu to the exposure of CD4i epitopes shown here is consistent with previously reported data and the ability of these proteins to independently downregulate CD4 (5, 7). Also consistent with previously reported data is the contribution of Vpu to the exposure of CD4-independent epitopes (5, 8, 9), which as discussed above is likely a consequence of Vpu's ability to stimulate virion release, preventing virion entrapment by BST2 on the cell surface. The data here further confirm that the high levels of the CD4i epitope recognized by A32 on the surface of cells infected with vpu-negative virus is due to the combined effect of virion retention by BST2 together with triggering of epitope exposure by CD4.
The net effect of these various influences on the display of Env epitopes at the cell surface to the efficiency of ADCC is probably best assessed here by considering the experiments using patient-derived HIV-1 IgG, which presumably contains a mixture of antibodies recognizing multiple epitopes. In the experiments using the CEM.NKR.luc cells, the lack of nef caused a very minor increase in surface staining with HIV-1 IgG, whereas the lack of vpu caused a striking increase. Nonetheless, nef and vpu each contributed to the evasion of ADCC mediated by HIV-1 IgG, suggesting that the modulation of both CD4 and BST2 are components of the approach evolved by the virus to evade this host defense.
The lack of a Vpu-specific effect of NAE inhibition on ADCC was, as discussed above, potentially due to the dispensability of the β-TrCP/cullin 1 E3 ubiquitin ligase complex for Vpu-mediated stimulation of virion release. Nonetheless, NAE inhibition modestly upregulated the expression of the Env epitope recognized by A32 on the surface of the CEM.NKR.luc cells used as targets in the ADCC assay in a Vpu-dependent manner, and this was detectable in cells infected with either wild-type or nef-negative virus. Why this did not translate into enhancement of ADCC mediated by A32 is not clear, but the degree of upregulation of the epitope might simply have been insufficient to yield an effect in the ADCC assay. Increased sensitivity to ADCC might be manifest only with the much higher levels of epitope exposure that are associated with the genetic absence of vpu and the consequent virion retention by BST2. On the other hand, whether higher concentrations of A32, the use of other antibodies with better ADCC activity, or the use of more potent NAE inhibitors could yield sensitization to ADCC mediated by antibodies recognizing CD4i epitopes remains an open question. The potential for this strategy to work as envisioned is supported by the ability of CD4 mimetics to effectively sensitize infected cells to ADCC (55).
Finally, the small but consistent sensitization to ADCC induced by MLN4924 that occurred in the case of all viral genotypes tested remains unexplained. We note that Vpr is another viral accessory protein that acts by coopting a cullin-based E3 ligase complex and presumably would be inhibited by MLN4924 (28). Moreover, Vpr has been associated with evasion of NK-mediated killing, although not specifically with evasion of ADCC (56). The possibility that the apparently nonspecific effects of NAE inhibition in the ADCC assay are due to inhibition of Vpr remains to be tested. Alternatively, the broad activity of MLN4924 as an inhibitor of all cullin-based ubiquitin ligases could enhance a cryptic cellular process required for ADCC.
In summary, we evaluated the ability of the NAE inhibitor MLN4924 to inhibit Vpu activity and sensitize infected cells to ADCC. This drug inhibited the downregulation of CD4 by Vpu, but it substantially inhibited the downregulation of BST2 by Vpu only in IFN-treated cells. Although the inhibition of Vpu-mediated downregulation of CD4 by MLN4924 was sufficient to increase the exposure of CD4i epitopes in Env at the cell surface, this alone was insufficient to sensitize infected cells to ADCC under the conditions tested. We suspect that more-potent inhibitors of Vpu-mediated counteraction of BST2-mediated restriction will be necessary to enhance the killing of infected cells by this immune surveillance mechanism.
ACKNOWLEDGMENTS
We thank David Evans for the CEM.NKR.luc and NK-CD16 cell lines and the NIH AIDS Reagent Program for antibodies and reagents as noted in Materials and Methods.
The work was supported in part by The James B. Pendleton Charitable Trust and the University of California San Diego Center for AIDS Research (CFAR).
REFERENCES
- 1.Guy B, Kieny MP, Riviere Y, Le Peuch C, Dott K, Girard M, Montagnier L, Lecocq JP. 1987. HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature 330:266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- 2.Willey RL, Maldarelli F, Martin MA, Strebel K. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol 66:7193–7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauter D, Schindler M, Specht A, Landford WN, Munch J, Kim KA, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, Takehisa J, Ogando Y, Ochsenbauer C, Kappes JC, Ayouba A, Peeters M, Learn GH, Shaw G, Sharp PM, Bieniasz P, Hahn BH, Hatziioannou T, Kirchhoff F. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pham TN, Lukhele S, Hajjar F, Routy JP, Cohen EA. 2014. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology 11:15. doi: 10.1186/1742-4690-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veillette M, Coutu M, Richard J, Batraville LA, Dagher O, Bernard N, Tremblay C, Kaufmann DE, Roger M, Finzi A. 2015. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 89:545–551. doi: 10.1128/JVI.02868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, Rakasz EG, Evans DT. 2014. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 111:6425–6430. doi: 10.1073/pnas.1321507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, Fierer DS, Simon V, Chen BK. 2014. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol 88:6031–6046. doi: 10.1128/JVI.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 11.Ross TM, Oran AE, Cullen BR. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol 9:613–621. doi: 10.1016/S0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- 12.Sattentau QJ, Moore JP, Vignaux F, Traincard F, Poignard P. 1993. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol 67:7383–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhuri R, Lindwasser OW, Smith WJ, Hurley JH, Bonifacino JS. 2007. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J Virol 81:3877–3890. doi: 10.1128/JVI.02725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. 1998. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell 1:565–574. doi: 10.1016/S1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, Stephens EB, Margottin-Goguet F, Benarous R, Guatelli JC. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog 5:e1000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douglas JL, Viswanathan K, McCarroll MN, Gustin JK, Fruh K, Moses AV. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism. J Virol 83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magadan JG, Perez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. 2010. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog 6:e1000869. doi: 10.1371/journal.ppat.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tervo HM, Homann S, Ambiel I, Fritz JV, Fackler OT, Keppler OT. 2011. beta-TrCP is dispensable for Vpu's ability to overcome the CD317/Tetherin-imposed restriction to HIV-1 release. Retrovirology 8:9. doi: 10.1186/1742-4690-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kueck T, Neil SJ. 2012. A cytoplasmic tail determinant in HIV-1 Vpu mediates targeting of tetherin for endosomal degradation and counteracts interferon-induced restriction. PLoS Pathog 8:e1002609. doi: 10.1371/journal.ppat.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janvier K, Pelchen-Matthews A, Renaud JB, Caillet M, Marsh M, Berlioz-Torrent C. 2011. The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST-2/tetherin down-regulation. PLoS Pathog 7:e1001265. doi: 10.1371/journal.ppat.1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Read MA, Brownell JE, Gladysheva TB, Hottelet M, Parent LA, Coggins MB, Pierce JW, Podust VN, Luo RS, Chau V, Palombella VJ. 2000. Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha. Mol Cell Biol 20:2326–2333. doi: 10.1128/MCB.20.7.2326-2333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong L, Yeh ET. 1999. Identification of the activating and conjugating enzymes of the NEDD8 conjugation pathway. J Biol Chem 274:12036–12042. doi: 10.1074/jbc.274.17.12036. [DOI] [PubMed] [Google Scholar]
- 23.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. 2009. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 24.Swords RT, Erba HP, DeAngelo DJ, Bixby DL, Altman JK, Maris M, Hua Z, Blakemore SJ, Faessel H, Sedarati F, Dezube BJ, Giles FJ, Medeiros BC. 2015. Pevonedistat (MLN4924), a First-in-Class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol 169:534–543. doi: 10.1111/bjh.13323. [DOI] [PubMed] [Google Scholar]
- 25.Lin JJ, Milhollen MA, Smith PG, Narayanan U, Dutta A. 2010. NEDD8-targeting drug MLN4924 elicits DNA rereplication by stabilizing Cdt1 in S phase, triggering checkpoint activation, apoptosis, and senescence in cancer cells. Cancer Res 70:10310–10320. doi: 10.1158/0008-5472.CAN-10-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milhollen MA, Traore T, Adams-Duffy J, Thomas MP, Berger AJ, Dang L, Dick LR, Garnsey JJ, Koenig E, Langston SP, Manfredi M, Narayanan U, Rolfe M, Staudt LM, Soucy TA, Yu J, Zhang J, Bolen JB, Smith PG. 2010. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood 116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 27.Stanley DJ, Bartholomeeusen K, Crosby DC, Kim DY, Kwon E, Yen L, Cartozo NC, Li M, Jager S, Mason-Herr J, Hayashi F, Yokoyama S, Krogan NJ, Harris RS, Peterlin BM, Gross JD. 2012. Inhibition of a NEDD8 cascade restores restriction of HIV by APOBEC3G. PLoS Pathog 8:e1003085. doi: 10.1371/journal.ppat.1003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nekorchuk MD, Sharifi HJ, Furuya AK, Jellinger R, de Noronha CM. 2013. HIV relies on neddylation for ubiquitin ligase-mediated functions. Retrovirology 10:138. doi: 10.1186/1742-4690-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei W, Guo H, Liu X, Zhang H, Qian L, Luo K, Markham RB, Yu XF. 2014. A first-in-class NAE inhibitor, MLN4924, blocks lentiviral infection in myeloid cells by disrupting neddylation-dependent Vpx-mediated SAMHD1 degradation. J Virol 88:745–751. doi: 10.1128/JVI.02568-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spina CA, Kwoh TJ, Chowers MY, Guatelli JC, Richman DD. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med 179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alpert MD, Heyer LN, Williams DE, Harvey JD, Greenough T, Allhorn M, Evans DT. 2012. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol 86:12039–12052. doi: 10.1128/JVI.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lahm HW, Stein S. 1985. Characterization of recombinant human interleukin-2 with micromethods. J Chromatogr 326:357–361. doi: 10.1016/S0021-9673(01)87461-6. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen KL, llano M, Akari H, Miyagi E, Poeschla EM, Strebel K, Bour S. 2004. Codon optimization of the HIV-1 vpu and vif genes stabilizes their mRNA and allows for highly efficient Rev-independent expression. Virology 319:163–175. doi: 10.1016/j.virol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Craig HM, Pandori MW, Guatelli JC. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci U S A 95:11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg ME, Bronson S, Lock M, Neumann M, Pavlakis GN, Skowronski J. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J 16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klimkait T, Strebel K, Hoggan MD, Martin MA, Orenstein JM. 1990. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J Virol 64:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chowers MY, Spina CA, Kwoh TJ, Fitch NJ, Richman DD, Guatelli JC. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol 68:2906–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homann S, Smith D, Little S, Richman D, Guatelli J. 2011. Upregulation of BST-2/Tetherin by HIV infection in vivo. J Virol 85:10659–10668. doi: 10.1128/JVI.05524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewinski MK, Jafari M, Zhang H, Opella SJ, Guatelli J. 2015. Membrane anchoring by a C-terminal tryptophan enables HIV-1 Vpu to displace bone marrow stromal antigen 2 (BST2) from sites of viral assembly. J Biol Chem 290:10919–10933. doi: 10.1074/jbc.M114.630095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin J, Ang XL, Shirogane T, Wade Harper J. 2005. Identification of substrates for F-box proteins. Methods Enzymol 399:287–309. doi: 10.1016/S0076-6879(05)99020-4. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez PW, DePaula-Silva AB, Szaniawski M, Barker E, Bosque A, Planelles V. 2015. HIV-1 Vpu utilizes both cullin-RING ligase (CRL) dependent and independent mechanisms to downmodulate host proteins. Retrovirology 12:65. doi: 10.1186/s12977-015-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickering S, Hue S, Kim EY, Reddy S, Wolinsky SM, Neil SJ. 2014. Preservation of tetherin and CD4 counter-activities in circulating Vpu alleles despite extensive sequence variation within HIV-1 infected individuals. PLoS Pathog 10:e1003895. doi: 10.1371/journal.ppat.1003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kueck T, Foster TL, Weinelt J, Sumner JC, Pickering S, Neil SJ. 2015. Serine phosphorylation of HIV-1 Vpu and its binding to tetherin regulates interaction with clathrin adaptors. PLoS Pathog 11:e1005141. doi: 10.1371/journal.ppat.1005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wildum S, Schindler M, Munch J, Kirchhoff F. 2006. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J Virol 80:8047–8059. doi: 10.1128/JVI.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen BK, Gandhi RT, Baltimore D. 1996. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J Virol 70:6044–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lau D, Kwan W, Guatelli J. 2011. Role of the endocytic pathway in the counteraction of BST-2 by human lentiviral pathogens. J Virol 85:9834–9846. doi: 10.1128/JVI.02633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNatt MW, Zang T, Bieniasz PD. 2013. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS Pathog 9:e1003299. doi: 10.1371/journal.ppat.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jafari M, Guatelli J, Lewinski MK. 2014. Activities of transmitted/founder and chronic clade B HIV-1 Vpu and a C-terminal polymorphism specifically affecting virion release. J Virol 88:5062–5078. doi: 10.1128/JVI.03472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamer DH. 2004. Can HIV be cured? Mechanisms of HIV persistence and strategies to combat it. Curr HIV Res 2:99–111. [DOI] [PubMed] [Google Scholar]
- 51.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. 2012. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. 2009. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog 5:e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia X, Weber E, Tokarev A, Lewinski M, Rizk M, Suarez M, Guatelli J, Xiong Y. 2014. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. Elife 3:e02362. doi: 10.7554/eLife.02362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 55.Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, Pazgier M, Schon A, Freire E, Routy JP, Smith AB III, Park J, Jones DM, Courter JR, Melillo BN, Kaufmann DE, Hahn BH, Permar SR, Haynes BF, Madani N, Sodroski JG, Finzi A. 2015. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 112:E2687–E2694. doi: 10.1073/pnas.1506755112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majumder B, Venkatachari NJ, O'Leary S, Ayyavoo V. 2008. Infection with Vpr-positive human immunodeficiency virus type 1 impairs NK cell function indirectly through cytokine dysregulation of infected target cells. J Virol 82:7189–7200. doi: 10.1128/JVI.01979-07. [DOI] [PMC free article] [PubMed] [Google Scholar]