ABSTRACT
Children with acute respiratory syncytial virus (RSV) infection often develop sequelae of persistent airway inflammation and wheezing. Pulmonary C fibers (PCFs) are involved in the generation of airway inflammation and resistance; however, their role in persistent airway diseases after RSV is unexplored. Here, we elucidated the pathogenesis of PCF activation in RSV-induced persistent airway disorders. PCF-degenerated and intact mice were used in the current study. Airway inflammation and airway resistance were evaluated. MMP408 and FSLLRY-NH2 were the selective antagonists for MMP-12 and PAR2, respectively, to investigate the roles of MMP-12 and PAR2 in PCFs mediating airway diseases. As a result, PCF degeneration significantly reduced the following responses to RSV infection: augmenting of inflammatory cells, especially macrophages, and infiltrating of inflammatory cells in lung tissues; specific airway resistance (sRaw) response to methacholine; and upregulation of MMP-12 and PAR2 expression. Moreover, the inhibition of MMP-12 reduced the total number of cells and macrophages in bronchiolar lavage fluid (BALF), as well infiltrating inflammatory cells, and decreased the sRaw response to methacholine. In addition, PAR2 was upregulated especially at the later stage of RSV infection. Downregulation of PAR2 ameliorated airway inflammation and resistance following RSV infection and suppressed the level of MMP-12. In all, the results suggest that PCF involvement in long-term airway inflammation and airway hyperresponsiveness occurred at least partially via modulating MMP-12, and the activation of PAR2 might be related to PCF-modulated MMP-12 production. Our initial findings indicated that the inhibition of PCF activity would be targeted therapeutically for virus infection-induced long-term airway disorders.
IMPORTANCE The current study is critical to understanding that PCFs are involved in long-term airway inflammation and airway resistance after RSV infection through mediating MMP-12 production via PAR2, indicating that the inhibition of PCF activity can be targeted therapeutically for virus infection-induced long-term airway disorders.
INTRODUCTION
Respiratory syncytial virus (RSV) remains the leading cause of viral bronchiolitis and pneumonia in infants and young children throughout the world. Among the 1% to 3% of infants hospitalized with RSV bronchiolitis, up to 90% of children have experience 2 or more wheezing episodes, and almost 50% will be given a diagnosis of asthma by the age of 6 years (1, 2). Several studies, including epidemiology studies and animal model studies, have shown that RSV persistence in the lung of the host after RSV infection was related to long-term airway hyperresponsiveness (AHR) and inflammation (3–5). However, the mechanisms of these sequelae are poorly understood.
We have reported that enhanced NGF (nerve growth factor) was partially involved in long-term airway inflammation and AHR after RSV infection (6), suggesting that neurogenic factors participate in long-term airway diseases. Elevated NGF during inflammation enhanced C fiber density and activity. Pulmonary C fibers (PCFs) are activated by various exogenous chemical substances and endogenous ligands and result in cough, dyspnea, mucus secretion, and bronchoconstriction (7, 8). Capsaicin-stimulating mucosal sensory fibers during the acute stage after RSV infection exerted important immunomodulatory effects by attracting selected lymphocyte subpopulations from the local bronchiolar lavage fluid (BALF), as well as monocytes, to the infected airways (9). Substance P (SP)-immunoreactive fibers increased during persistent RSV infection (10), indicating that persistent RSV infection induces persistent innervating fiber activation in the airways. Thus, we speculated that PCF activation was associated with long-term airway disorders in the later stage of RSV infection.
Matrix metalloproteinase 12 (MMP-12) is reported to contribute to acute airway inflammation and AHR in nude mice (11). Our pilot study had shown a persistently elevated MMP-12 level beyond the acute phase of RSV infection. Xu et al. found that cigarette smoke (CS) stimulated PCFs to release SP, promoting MMP-12-producing alveolar macrophages and causing COPD (chronic obstructive pulmonary disease) (12, 13). Thus, the persistence of elevated MMP-12 may be related to PCFs and involved in long-term airway disorders after RSV infection.
Protease-activated receptors (PARs) are G protein-coupled receptors containing four PARs, and they play an important role in hemostasis, thrombosis, and inflammation (14). PAR1 or PAR2 activation enhanced the production of inflammatory mediators and cytokines and participated in chronic airway diseases, such as asthma, COPD, and IPF (idiopathic pulmonary fibrosis) (15). Virus infection also activated PAR1 and PAR2 to mediate innate immune, airway inflammation, and lung function (16–18). We have found that PAR2, but not PAR1, was obviously upregulated in the later stage of RSV infection in our preliminary data. Moreover, it was reported that the ablation of sensory neurons markedly decreased the PAR2-mediated airway constriction and virtually abolished PAR2-mediated pulmonary inflammation and edema (19). Therefore, in this study, the activation of PAR2 might be associated with PCFs modulating MMP-12 production, contributing to the development of long-term airway diseases.
To demonstrate our hypothesis in this study, we developed the following examination. (i) We examined airway disorders in PCF-degenerated mice and intact mice with RSV infection. (ii) We provided evidence for the overexpression of MMP-12 in the later stage of RSV infection, which was modulated by PCFs, and its involvement in long-term airway disorders. (iii) We further implied that PAR2 activation participated in PCFs modulating MMP-12 production. Thus, PCFs modulated MMP-12 production in persistent airway inflammation and AHR after RSV infection partially via PAR2 action.
MATERIALS AND METHODS
Ethics statement.
All experiments involving animals were in accordance with the Guide for the Care and Use of Laboratory Animals (20) and were approved by the Institutional Animal Care and Use Committee (IACUC), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (FY14-006).
Virus preparation.
RSV strain A2 (VR-1540; ATCC) was propagated in HEp-2 cells (ATCC) with Dulbecco's modified Eagle's medium (DMEM; GIBCO, CA) plus 5% fetal bovine serum (FBS; GIBCO), and the viral titer was determined by plaque assay.
Animals and treatment.
(i) Female BALB/c mice at 6 to 8 weeks of age were purchased from Charles River Laboratories, Inc. (Wilmington, MA), and housed in an animal biosafety level 2 (ABSL2) facility at Lovelace Respiratory Research Institute. The mice were infected intranasally (i.n.) with 100 μl of 1.8 × 107 PFU/ml RSV. Mock-infected mice were treated with 100 μl of mock viral stocks under 2 to 5% isoflurane anesthesia. (ii) Mice were given capsaicin (50 mg/kg of body weight, subcutaneously) or vehicle alone (1:1:8, vol/vol/vol, ethanol-Tween 80-saline) at postnatal day 2 as previously described (21, 22). Adult animals (6 to 8 weeks after capsaicin pretreatment) then were instilled with RSV or received mock treatment as described above. (iii) MMP408, a selective antagonist of MMP-12, was given intragastrically to intact mice as previously described (23) from day 14 to day 20 after RSV infection. (iv) To investigate the role of PAR2 in MMP-12 production induced by RSV, leading to airway inflammation and AHR, the PAR2 antagonist FSLLRY-NH2 (500 μM per mouse; Tocris, Unite Kingdom) was administered intranasally each day from day 14 to day 20.
Cell counts in BALF.
Mice were euthanized with Euthasol (150 mg/kg intraperitoneally), and we collected BALF to perform cell counts as previously described (24).
Histopathology staining.
The lung tissue was fixed in 10% (vol/vol) neutral buffered formalin for 24 h, embedded in paraffin, and cut into 4-μm-thick sections. Paraffin sections were dewaxed in xylene twice and rehydrated first in absolute ethanol once and then once each in 95% ethanol, 90% ethanol, 80% ethanol, and 70% ethanol. Hematoxylin and eosin (H&E) staining was processed as described previously (24). For immunofluorescence staining, sections were blocked in blocking buffer (3% bovine serum albumin [BSA], 0.2% Triton X-100 in phosphate-buffered saline [PBS]) for 1 h. Polyclonal rabbit anti-MMP-12 and monoclonal mouse anti-PAR2 primary antibody (Santa Cruz, CA) were used at a 1:100 dilution. Incubation was performed at 4°C overnight in blocking buffer. Secondary Alexa Fluor 594-labeled goat anti-rabbit antibody and donkey anti-mouse antibody (Invitrogen) were used at a 1:200 dilution. Incubation was performed at room temperature for 1 h. Coverslips were mounted on stained sections with antifade reagent containing 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen).
Airway resistance detection.
Specific airway resistance (sRaw) in the conscious mice was assessed by two-chamber whole-body plethysmography (Buxco Electronics, Inc., Wilmington, NC) according to a previously reported method. Double-flow plethysmography is a noninvasive technology that calculates sRaw by analyzing breathing patterns at nasal and thoracic airflows (25). For the determination of sRaw in mice, inhalations of saline and methacholine were administered. Aerosols were delivered into the nasal cavity for 1 min in a dose-response manner: 0 (saline), 3.125, 6.25, 12.5, 25, and 50 mg of methacholine per milliliter. The reliability and reproducibility of measurements made using noninvasive double-flow plethysmography were increased by ensuring that all measurements were made in an air-conditioned environment controlled for temperature (22 to 23°C) and humidity (50 to 60%).
qPCR analysis.
Quantitative PCR (qPCR) was performed on an ABI PRISM 7900 HT system (Applied Biosystems, Foster City, CA). The total RNA was extracted from lung tissues using TRIzol reagents (Invitrogen, CA), and cDNA was generated with a PrimeScript RT reagent kit (TaKaRa, Japan). The expression of MMP-12 and PAR2 genes was detected using TaqMan real-time PCR assays from Life Technologies (MMP-12, Mm00500554_m1; PAR2, Mm00433160_m1). The endogenous control gene (β-actin, Mm00607939_s1) also was monitored for each assay. All qPCR results are represented as relative quantification (RQ).
Western blot analysis.
Total protein extracts from lung tissues were obtained using M2 buffer (20 mM Tris-HCl, pH 7.6, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 2 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, 20 mM β-glycerophosphate, 1 mM sodium vanadate, and 1 μg/ml leupeptin), and protein concentrations were determined using the bicinchoninic acid (BCA) assay reagent (BioTeke) according to the manufacturer's protocol. Respective lung protein extracts containing equal quantities of protein were separated on an 8% SDS-PAGE and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Membranes were probed with primary antibodies against MMP-12 (1:500; rabbit; Santa Cruz, CA), PAR2 (1:1,000; mouse; Santa Cruz, CA), or β-actin (1:3,000; rabbit; Sigma-Aldrich, St. Louis, MO). Alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (1:5,000; Santa Cruz, CA) and goat anti-mouse antibody (1:5,000; Santa Cruz, CA) were used to detect the presence of the respective protein bands. Signals were quantified using Quantity One software (Bio-Rad, Hercules, CA) and normalized relative to β-actin.
ELISA analysis.
The levels of MMP-12 in BALF were determined with a commercial enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions. ELISA kits used for the measurement of MMP-12 were purchased from Boster Immunoleader (Pleasanton, CA).
Statistical analysis.
GraphPad Prism 6.0 software was used for data analysis. The results are expressed as the means ± standard errors of the means (SEM). Statistical significance was assessed by one- or two-way analysis of variance (ANOVA). One-way ANOVA was used to analyze the significant differences between three or more groups. Two-way ANOVA was used to analyze significant differences between two variables. If an overall test was significant, Tukey's test was utilized for specific comparisons between individual groups. Differences were considered significant at P < 0.05.
RESULTS
Degenerated PCFs ameliorated long-term airway inflammation and AHR after RSV infection.
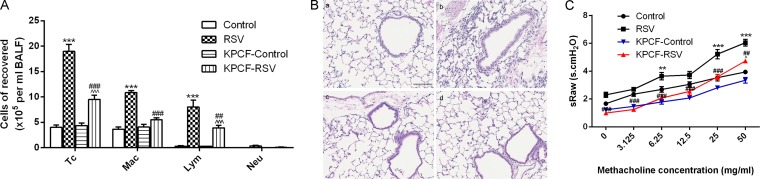
To determine the effect of PCFs on long-term airway inflammation and AHR after RSV infection, we examined PCF-degenerated mice by using capsaicin treatment. We determined the number of total cells (lymphocytes, macrophages, and neutrophils) and types in BALF after RSV infection (Fig. 1A), and the data showed that the total number of cells and macrophages was significantly decreased in PCF-degenerated mice infected with RSV compared to those of intact mice infected with RSV (P < 0.001). Lymphocytes also were reduced in PCF-degenerated mice with RSV infection, but the level still was significantly increased compared to that of PCF-degenerated mice that received mock treatment (P < 0.001). Compared to the levels for RSV-infected intact mice, few infiltrating inflammatory cells were found at the peribronchial and perivascular locations of lung tissues in the PCF-degenerated mice with RSV infection (Fig. 1B). The value of sRaw after aerosolizing different concentrations of methacholine was significantly decreased in PCF-degenerated mice infected with RSV compared to that of intact mice infected with RSV (Fig. 1C).
FIG 1.
Degenerated PCFs reduced number of cells counted in BALF and infiltrating inflammatory cells at peribronchial and perivascular regions and ameliorated airway resistance after RSV infection. (A) By day 21 of virus infection, the total number of cells, macrophages, and lymphocytes was reduced in PCF-degenerated mice infected with RSV (KPCF-RSV) compared to that of intact mice infected with RSV (RSV). (B) Inflammatory cells infiltrating around the bronchioles were decreased in PCF-degenerated mice with RSV infection (magnification, ×200). (a) Control; (b) RSV; (c) KPCF control; (d) KPCF-RSV. (C) Degenerated PCFs depressed airway responses to aerosol methacholine according to double-chamber plethysmography. Results are representative of two independent experiments, with a total of 5 to 7 mice/group, and all data are presented as means ± SEM. P < 0.01 (**) and P < 0.001 (***) compared to the control group; P < 0.01 (##) and P < 0.001 (###) compared to the RSV group; P < 0.05 (^) and P < 0.001 (^^^) compared to the KPCF control group. Tc, total cells; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils.
PCFs modulated MMP-12 production in the later stage of RSV infection.
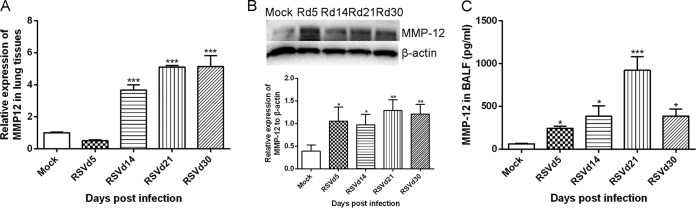
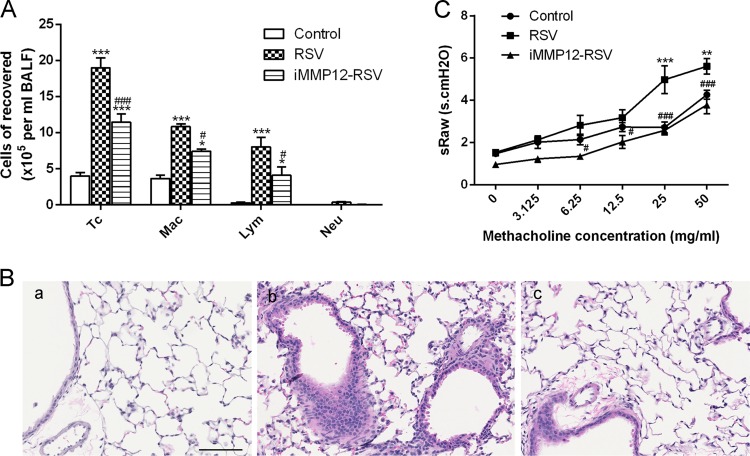
We first determined the level of MMP-12 after RSV infection. As illustrated in Fig. 2, RSV infection dramatically enhanced MMP-12 gene and protein expression in lung tissues in the later stage of RSV infection, with a peak level on day 21 after virus infection, and increased the level of MMP-12 in BALF compared to that of mock-treated mice (P < 0.001). In order to determine the role of MMP-12 in long-term airway inflammation and AHR, MMP408, a selective antagonist of MMP-12, was administered. MMP408 administration reduced the total number of cells, macrophages, and lymphocytes in BALF and also decreased the infiltrating inflammatory cells around the bronchioles, as shown in Fig. 3A and B. MMP-408 treatment decreased sRaw after using an aerosolizing concentration of 6.25 mg/ml to 50 mg/ml methacholine on day 21 following RSV infection (P < 0.001) (Fig. 3C).
FIG 2.
RSV infection upregulated levels of MMP-12. MMP-12 gene and protein expression and the concentration of MMP-12 in BALF were determined by qPCR, Western blotting, and ELISA, respectively, at early and later stages of RSV infection. (A) MMP-12 gene expression; (B) MMP-12 protein expression; (C) level of MMP-12 in BALF. Results are representative of two independent experiments, with a total of 5 to 7 mice/group, and all data are presented as means ± SEM. P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) compared to mock treatment.
FIG 3.
MMP408 administration reduced number of cells in BALF and inflammatory cells infiltrating around bronchioles and attenuated airway resistance induced by RSV infection. MMP408 was given after RSV infection (iMMP12-RSV) to inhibit MMP-12 expression, reducing the total number of cells, macrophages, and lymphocytes (A) and inflammatory cells (B) infiltrating around the bronchioles induced by RSV infection (magnification, ×200). (a) Control; (b) RSV; (c) iMMP12-RSV. (C) MMP408 administration attenuated AHR induced by RSV infection. Results are representative of two independent experiments, with a total of 5 to 7 mice/group, and all data are presented as means ± SEM. P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) compared to the control group; P < 0.05 (#) and P < 0.001 (###) compared to the RSV group. Tc, total cells; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils.
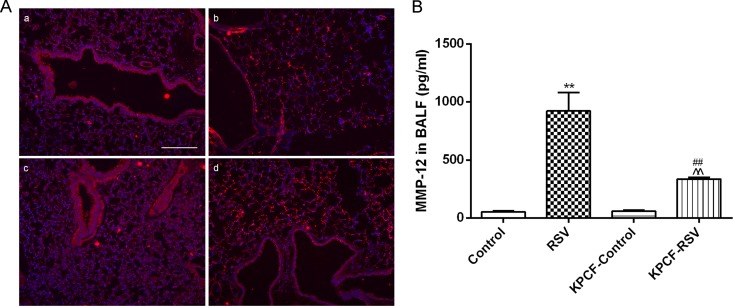
Furthermore, we detected MMP-12 expression in lung tissues and the level in BALF in PCF-degenerated mice infected with RSV to investigate if PCFs modulated MMP-12 to contribute to airway disorders. Compared to that of intact mice infected with RSV, MMP-12 protein expression around the bronchioles was downregulated in PCF-degenerated mice infected with RSV (Fig. 4A). The levels of MMP-12 in BALF also were reduced in PCF-degenerated mice infected with RSV (P < 0.01) (Fig. 4B).
FIG 4.
Degenerated PCFs suppressed MMP-12 production induced by RSV infection. MMP-12 expression around the bronchioles was suppressed in PCF-degenerated mice infected with RSV (KPCF-RSV) compared to that of intact mice infected with RSV (RSV) (magnification, ×100) and reduced the level of MMP-12 in BALF. (a) Control; (b) RSV; (c) KPCF control; (d) KPCF-RSV. (B) Level of MMP-12 in BALF. Results are representative of two independent experiments, with a total of 5 to 8 mice/group, and all data are presented as means ± SEM. **, P < 0.01 compared to the control; ##, P < 0.01 compared to the RSVd21 group; ^^, P < 0.01 compared to the KPCF control group.
PAR2 activation was involved in PCFs modulating MMP-12.
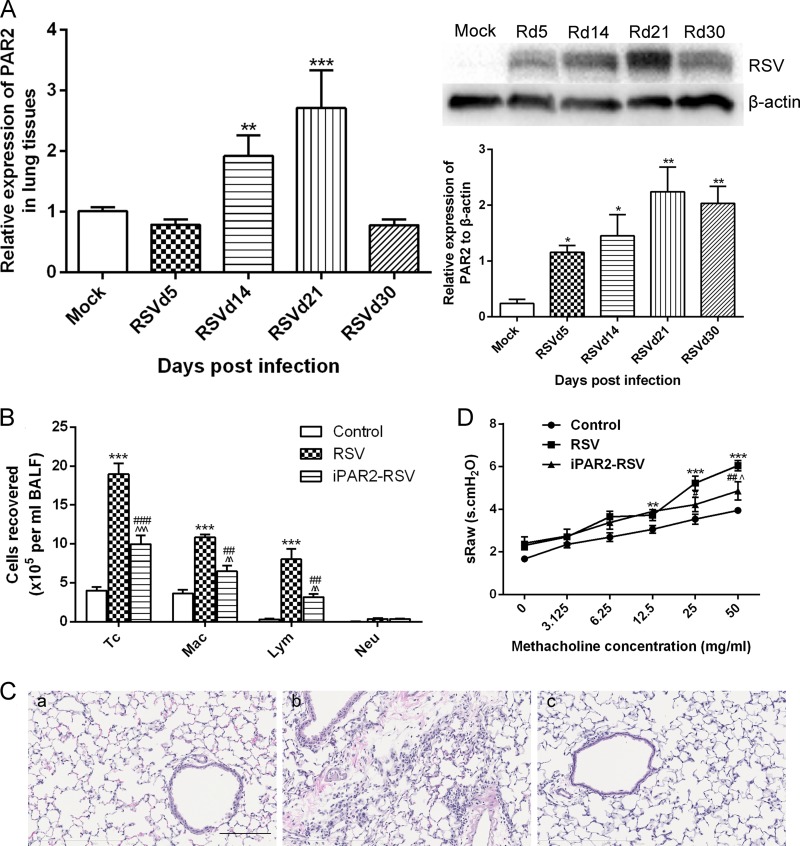
Levels of PAR1 gene and protein expression in lung tissues of mice infected with RSV were not dramatically altered (data not shown). However, the level of PAR2 gene was increased by 3-fold compared to the level for the control group on day 21, and the level gradually decreased from day 30 (Fig. 5A). Interestingly, PAR2 protein was upregulated during the acute stage and later stages after RSV infection, and this upregulation persisted for at least 30 days (Fig. 5A). In order to determine the effect of PAR2 on persistent airway inflammation and AHR, a PAR2 selective antagonist was used. PAR2 antagonist administration decreased total cells counts and the numbers of lymphocytes and macrophages in BALF (Fig. 5B); a few infiltrating cells were found around the bronchioles (Fig. 5C). Moreover, PAR2 antagonist administration decreased the airway response to methacholine at 25 mg/ml to 50 mg/ml (Fig. 5D). These results implied that the activation of PAR2 at least in part contributed to persistent airway inflammation and AHR after RSV infection.
FIG 5.
Activation of PAR2 was involved in long-term airway inflammation and AHR after RSV infection. PAR2 gene and protein expression in lung tissues were determined by qPCR and Western blotting, respectively. (A) PAR2 gene and protein expression. (B) PAR2 antagonist was given after RSV infection (iPAR2-RSV) to reduce the total number of cells, macrophages, and lymphocytes. (C) Inflammatory cells infiltrating around the bronchioles induced by RSV infection (magnification, ×200). (a) Control; (b) RSV; (c) iPAR2-RSV. (D) PAR2 antagonist administration attenuated AHR induced by RSV infection. Results are representative of two independent experiments, with a total of 5 to 7 mice/group, and all data are presented as means ± SEM. P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) compared to mock-infected or control groups; P < 0.05 (#) and P < 0.001 (###) compared to the RSV group, and P < 0.05 (^), P < 0.01 (^^), and P < 0.001 (^^^) compared to control groups. Tc, total cells; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils.
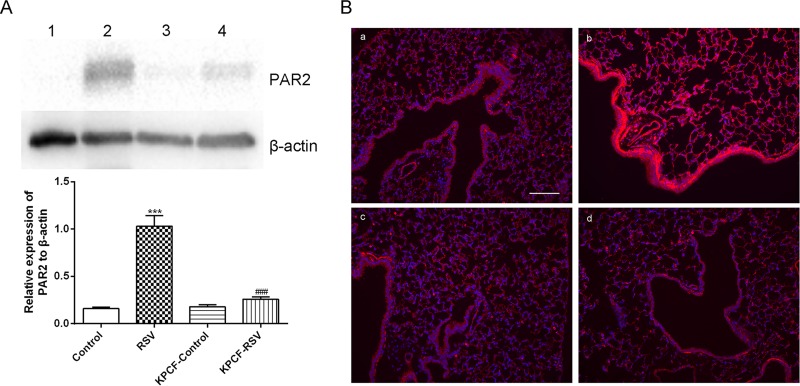
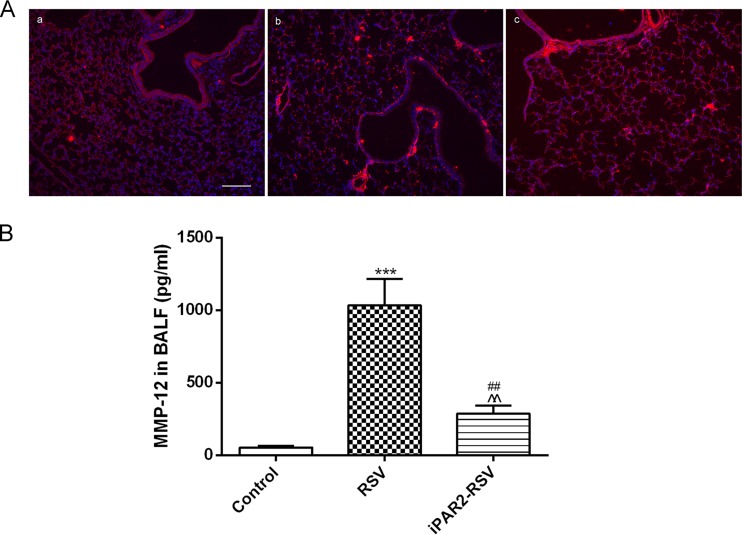
To determine whether PAR2 activation could participate in PCF modulation of MMP-12 production, we detected PAR2 expression in PCF-degenerated mice infected with RSV. Compared to RSV-infected intact mice, PAR2 protein expression in lung tissues was significantly downregulated, as determined by Western blotting (Fig. 6A) and immunofluorescence (Fig. 6B). In addition, MMP-12 protein expression in lung tissues was suppressed (Fig. 7A), and the level of MMP-12 in BALF decreased after PAR2 antagonist treatment (P < 0.01) (Fig. 7B).
FIG 6.
Degenerated PCFs inhibited PAR2 expression induced by RSV infection. (A) PAR2 expression in the lung tissues was inhibited in PCF-degenerated mice infected with RSV (KPCF-RSV) compared to that of intact mice infected with RSV (RSV). (B) PAR2 around the bronchioles was reduced in KPCF-RSV according to immunofluorescence (magnification, ×100). (a) Control; (b) RSV; (c) KPCF control; (d) KPCF-RSV. Results are representative of two independent experiments, with a total of 5 to 7 mice/group, and all data are presented as means ± SEM. ***, P < 0.001 compared to the control group; ###, P < 0.001 compared to the RSV group. 1, control; 2, RSV; 3, KPCF control; 4, KPCF-RSV.
FIG 7.
Inhibition of PAR2 decreased the level of MMP-12 after RSV infection. Inhibition of PAR2 by FSLLRY-NH2 treatment decreased MMP-12 expression around the bronchioles (magnification, ×100) (A) and the level of MMP-12 in BALF (B). Results are representative of two independent experiments, with a total of 5 to 7 mice/group, and all data are presented as means ± SEM. ***, P < 0.001 compared to the control group; ##, P < 0.01 compared to the RSV group; ^^, P < 0.01 compared to the control group.
DISCUSSION
In this study, we have shown for the first time that degenerated PCFs with capsaicin ameliorated long-term airway inflammation and AHR after RSV infection. Furthermore, we found that increased MMP-12 in the later stage of RSV infection was involved in long-term airway disorders. The production of MMP-12 was modulated at least in part by PCFs. In addition, PAR2 expression was dramatically upregulated after RSV infection, which was associated with long-term airway inflammation and AHR. Levels of PAR2 were depressed in PCF-degenerated mice infected with RSV, and the activation of PAR2 after RSV infection was partially involved in PCFs mediating MMP-12 production.
Li et al. found that RSV could infect sensory neurons in lungs by a process associated with RSV G protein, implicating a mechanism and intervention strategy of chronic airway diseases after acute RSV bronchiolitis (26). During persistent RSV infection, the airway showed signs of chronic inflammation, and the level of substance P- and CGRP-positive fibers increased (27), suggesting a link between chronic inflammation and fibers in lungs. Substance P and CGRP are present mainly in sensory nerves, and PCFs in the vagus nerve constitute 75 to 90% of the sensory fibers innervating the airways and lungs. Although it is well established that the PCFs are involved in the generation of airway resistance and inflammation (28–31), their role in persistent AHR after RSV previously was unexplored. In the current study, we used PCF-degenerated mice infected with RSV. In the current study, we demonstrated for the first time that long-term airway inflammation and AHR were attenuated in PCF-degenerated mice infected with RSV. Our findings indicated that PCF degeneration reduced the total number of cells, macrophages, and lymphocytes in BALF, but lymphocyte numbers still were increased in PCF-degenerated mice infected with RSV compared to the level for the uninfected group. The data imply that PCF degeneration mainly ameliorated the inflammation of macrophages induced by RSV infection; however, there was no obvious explanation for the mechanism of PCFs influencing macrophages after RSV infection. Moreover, PCF degeneration decreased airway responses to aerosolized methacholine and attenuated long-term AHR after RSV infection, suggesting that PCF degeneration weakens the response to irritants. The results were consistent with those of other reports. Blocking the activation of single C-fiber afferents could inhibit cough, AHR, and, later, asthma (32–34).
We also showed that MMP-12 represented one possible candidate for a PCF effector, consistent with Xu and Xu (13). Elevated levels of MMP-12 were induced by RSV infection and persisted for at least 30 days. In addition, an increased level of MMP-12 also was demonstrated in the nasopharyngeal airway (NPA) in infants infected with RSV (data not shown). MMP-12 has been shown to play a critical role in airway remodeling and mucus hypersecretion, contributing to chronic airway diseases such as COPD and asthma (35, 36). The inhibition of MMP-12 obviously attenuated long-term airway inflammation and AHR, suggesting that MMP-12 was involved in long-term airway diseases induced by RSV infection. This result advanced our previous study and reports of others, which mainly found that MMP-12 was associated with acute airway inflammation and AHR after RSV infection (11, 37). Furthermore, the level of MMP-12 was decreased in PCF-degenerated mice infected with RSV. The data demonstrated that PCFs could modulate the production of MMP-12 at the later stage of RSV infection.
How PCFs modulate MMP-12 induced by RSV infection is unclear. PAR1, PAR2, and PAR4 are known to play important roles in signaling. We have shown that the elevated expression of PAR2 was induced by RSV infection, with a peak level on day 21, and the activation of PAR2 was associated long-term airway diseases. At the same time, we also found that TF (tissue factor) was elevated in BALF of mice and NPA of children infected with RSV (data not shown), which might be related to the upregulation of PAR2. TF can simultaneously stimulate PAR1 and PAR2, and Sutherland and colleagues reported that TF promoted PAR2-mediated HSV1 infection (38). In this study, we showed that the increased level of MMP-12 induced by RSV infection was downregulated after PAR2 inhibition, which confirmed the report on other proteases except PAR1 involving MMP-12 (39). Moreover, PAR2 was suppressed in PCF-degenerated mice infected with RSV. We also found that the level of MMP-12 was increased after PAR2 agonist administration in PCF-degenerated mice infected with RSV (data not shown). The results implied that PCF-modulated MMP-12 production occurred at least in part via mediating PAR2. The ablation of sensory neurons (by capsaicin) markedly decreased the PAR2-mediated airway constriction and virtually abolished PAR2-mediated pulmonary inflammation and edema (19). In addition, Xu and Xu reported that CS stimulated PCFs to release SP and activate NK1R, promoting MMP-12 production (13). We also found an obvious decrease in levels of SP and NK1R in PCF-degenerated mice infected with RSV (data not shown), which might be further evidence of signaling for PCFs modulating MMP-12 production. This will require further investigation.
Taking these findings together, we have shown that PCFs are involved in long-term airway inflammation and AHR modulated at least in part by MMP-12 production, and the activation of PAR2 might be associated with PCF-modulated MMP-12 production. The regulation of PAR2 activation in PCFs is also an area for further detailed investigation. Our initial findings indicated that the inhibition of PCF activity would be targeted therapeutically for virus infection-induced long-term airway disorders.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81170010), the National Natural Science Foundation of China for Young Scholars (81301424), and the Foundation of NIH R01 (HL119683-01).
We thank Yong Lin for providing technology throughout the course of this work. We also thank the ABSL2 animal facility for housing and breeding mice at Lovelace Respiratory Research Institute.
We declare that we have no conflicts of interest regarding the publication of this paper.
REFERENCES
- 1.Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, Lyons K, Schweiger TL, Zheng J, Schechtman KB, Castro M, Bacharier LB. 2015. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 135:1171–1178. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J, Schechtman KB, Strunk RC, Castro M. 2012. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 130:91–100. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estripeaut D, Torres JP, Somers CS, Tagliabue C, Khokhar S, Bhoj VG, Grube SM, Wozniakowski A, Gomez AM, Ramilo O, Jafri HS, Mejias A. 2008. Respiratory syncytial virus persistence in the lungs correlates with airway hyperreactivity in the mouse model. J Infect Dis 198:1435–1443. doi: 10.1086/592714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikkel MB, Quint JK, Mallia P, Wedzicha JA, Johnston SL. 2008. Respiratory syncytial virus persistence in chronic obstructive pulmonary disease. Pediatr Infect Dis J 27:S63–S70. doi: 10.1097/INF.0b013e3181684d67. [DOI] [PubMed] [Google Scholar]
- 5.Mejias A, Chavez-Bueno S, Gomez AM, Somers C, Estripeaut D, Torres JP, Jafri HS, Ramilo O. 2008. Respiratory syncytial virus persistence: evidence in the mouse model. Pediatr Infect Dis J 27:S60–S62. doi: 10.1097/INF.0b013e3181684d52. [DOI] [PubMed] [Google Scholar]
- 6.Zang N, Li S, Li W, Xie X, Ren L, Long X, Xie J, Deng Y, Fu Z, Xu F, Liu E. 2015. Resveratrol suppresses persistent airway inflammation and hyperresponsivess might partially via nerve growth factor in respiratory syncytial virus-infected mice. Int Immunopharmacol 28:121–128. doi: 10.1016/j.intimp.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Coleridge JC, Coleridge HM. 1984. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99:1–110. [DOI] [PubMed] [Google Scholar]
- 8.Lee LY, Pisarri TE. 2001. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125:47–65. doi: 10.1016/S0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 9.Auais A, Adkins B, Napchan G, Piedimonte G. 2003. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol 285:L105–L113. doi: 10.1152/ajplung.00004.2003. [DOI] [PubMed] [Google Scholar]
- 10.Tan YR, Yang T, Liu SP, Xiang Y, Qu F, Liu HJ, Qin XQ. 2008. Pulmonary peptidergic innervation remodeling and development of airway hyperresponsiveness induced by RSV persistent infection. Peptides 29:47–56. doi: 10.1016/j.peptides.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Long X, Li S, Xie J, Li W, Zang N, Ren L, Deng Y, Xie X, Wang L, Fu Z, Liu E. 2015. MMP-12-mediated by SARM-TRIF signaling pathway contributes to IFN-gamma-independent airway inflammation and AHR post RSV infection in nude mice. Respir Res 16:11. doi: 10.1186/s12931-015-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Xu F, Wang R, Seagrave J, Lin Y, March TH. 2007. Cigarette smoke-induced hypercapnic emphysema in C3H mice is associated with increases of macrophage metalloelastase and substance P in the lungs. Exp Lung Res 33:197–215. doi: 10.1080/01902140701459514. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Xu F. 2010. Role of neurogenic substance P in overexpression of alveolar macrophages' neurokinin 1 receptor in mice exposed to cigarette smoke. Exp Lung Res 36:243–254. doi: 10.3109/01902140903398275. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Yang YM, Kim K, Shin DM, Yoon JH, Cho HJ, Choi JY. 2012. Protease-activated receptor 2 mediates mucus secretion in the airway submucosal gland. PLoS One 7:e43188. doi: 10.1371/journal.pone.0043188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jose RJ, Williams AE, Chambers RC. 2014. Proteinase-activated receptors in fibroproliferative lung disease. Thorax 69:190–192. doi: 10.1136/thoraxjnl-2013-204367. [DOI] [PubMed] [Google Scholar]
- 16.Antoniak S, Mackman N. 2014. Multiple roles of the coagulation protease cascade during virus infection. Blood 123:2605–2613. doi: 10.1182/blood-2013-09-526277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan RS, Stewart GA, Goldie RG, Henry PJ. 2004. Altered expression and in vivo lung function of protease-activated receptors during influenza A virus infection in mice. Am J Physiol Lung Cell Mol Physiol 286:L388–L398. doi: 10.1152/ajplung.00286.2003. [DOI] [PubMed] [Google Scholar]
- 18.Antoniak S, Owens AP III, Baunacke M, Williams JC, Lee RD, Weithauser A, Sheridan PA, Malz R, Luyendyk JP, Esserman DA, Trejo J, Kirchhofer D, Blaxall BC, Pawlinski R, Beck MA, Rauch U, Mackman N. 2013. PAR-1 contributes to the innate immune response during viral infection. J Clin Investig 123:1310–1322. doi: 10.1172/JCI66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. 2005. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol 175:2598–2605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- 20.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 21.Jancso G, Kiraly E, Jancso-Gabor A. 1977. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurons. Nature 270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- 22.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. 2000. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Li J, Wu Y, Wu J, Hotchandani R, Cunningham K, McFadyen I, Bard J, Morgan P, Schlerman F, Xu X, Tam S, Goldman SJ, Williams C, Sypek J, Mansour TS. 2009. A selective matrix metalloprotease 12 inhibitor for potential treatment of chronic obstructive pulmonary disease (COPD): discovery of (S)-2-(8-(methoxycarbonylamino)dibenzo[b,d]furan-3-sulfonamido)-3-methylbutanoic acid (MMP408). J Med Chem 52:1799–1802. doi: 10.1021/jm900093d. [DOI] [PubMed] [Google Scholar]
- 24.Zang N, Xie X, Deng Y, Wu S, Wang L, Peng C, Li S, Ni K, Luo Y, Liu E. 2011. Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J Virol 85:13061–13068. doi: 10.1128/JVI.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flandre TD, Leroy PL, Desmecht DJ. 2003. Effect of somatic growth, strain, and sex on double-chamber plethysmographic respiratory function values in healthy mice. J Appl Physiol 94:1129–1136. doi: 10.1152/japplphysiol.00561.2002. [DOI] [PubMed] [Google Scholar]
- 26.Li XQ, Fu ZF, Alvarez R, Henderson C, Tripp RA. 2006. Respiratory syncytial virus (RSV) infects neuronal cells and processes that innervate the lung by a process involving RSV G protein. J Virol 80:537–540. doi: 10.1128/JVI.80.1.537-540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan Y, Yang T, Liu S, Liu H, Xiang Y, Qu F, Li H, Qin X. 2008. Infection with respiratory syncytial virus alters peptidergic innervation in the lower airways of guinea-pigs. Exp Physiol 93:1284–1291. doi: 10.1113/expphysiol.2008.043521. [DOI] [PubMed] [Google Scholar]
- 28.McGowan SE. 2007. Vitamin A deficiency increases airway resistance following C-fiber stimulation. Respir Physiol Neurobiol 157:281–289. doi: 10.1016/j.resp.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes D Jr, Collins PB, Khosravi M, Lin RL, Lee LY. 2012. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med 185:1190–1196. doi: 10.1164/rccm.201201-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schelegle ES, Walby WF. 2012. Vagal afferents contribute to exacerbated airway responses following ozone and allergen challenge. Respir Physiol Neurobiol 181:277–285. doi: 10.1016/j.resp.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu CC, Lin RL, Lin YS, Lee LY. 2013. Bronchoconstriction induced by increasing airway temperature in ovalbumin-sensitized rats: role of tachykinins. J Appl Physiol 115:688–696. doi: 10.1152/japplphysiol.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birrell MA, Bonvini SJ, Dubuis E, Maher SA, Wortley MA, Grace MS, Raemdonck K, Adcock JJ, Belvisi MG. 2014. Tiotropium modulates transient receptor potential V1 (TRPV1) in airway sensory nerves: a beneficial off-target effect? J Allergy Clin Immunol 133:679–687. doi: 10.1016/j.jaci.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubuis E, Wortley MA, Grace MS, Maher SA, Adcock JJ, Birrell MA, Belvisi MG. 2014. Theophylline inhibits the cough reflex through a novel mechanism of action. J Allergy Clin Immunol 133:1588–1598. doi: 10.1016/j.jaci.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. 2014. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A 111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhuri R, McSharry C, Brady J, Donnelly I, Grierson C, McGuinness S, Jolly L, Weir CJ, Messow CM, Spears M, Miele G, Nocka K, Crowther D, Thompson J, Brannigan M, Lafferty J, Sproule M, Macnee W, Connell M, Murchison JT, Shepherd MC, Feuerstein G, Miller DK, Thomson NC. 2012. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: relationship to disease severity. J Allergy Clin Immunol 129:655–663 e658. doi: 10.1016/j.jaci.2011.12.996. [DOI] [PubMed] [Google Scholar]
- 36.Churg A, Zhou S, Wright JL. 2012. Series “matrix metalloproteinases in lung health and disease”: matrix metalloproteinases in COPD. Eur Respir J 39:197–209. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 37.Foronjy RF, Taggart CC, Dabo AJ, Weldon S, Cummins N, Geraghty P. 2015. Type-I interferons induce lung protease responses following respiratory syncytial virus infection via RIG-I-like receptors. Mucosal Immunol 8:161–175. doi: 10.1038/mi.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutherland MR, Ruf W, Pryzdial EL. 2012. Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease-activated receptor 2 cofactors that enhance infection. Blood 119:3638–3645. doi: 10.1182/blood-2011-08-376814. [DOI] [PubMed] [Google Scholar]
- 39.Raza SL, Nehring LC, Shapiro SD, Cornelius LA. 2000. Proteinase-activated receptor-1 regulation of macrophage elastase (MMP-12) secretion by serine proteinases. J Biol Chem 275:41243–41250. doi: 10.1074/jbc.M005788200. [DOI] [PubMed] [Google Scholar]