Abstract
Bupropion is a widely used antidepressant and smoking cessation aid in addition to being one of two US Food and Drug Administration–recommended probe substrates for evaluation of cytochrome P450 2B6 activity. Racemic bupropion undergoes oxidative and reductive metabolism, producing a complex profile of pharmacologically active metabolites with relatively little known about the mechanisms underlying their elimination. A liquid chromatography-tandem mass spectrometry assay was developed to simultaneously separate and detect glucuronide metabolites of (R,R)- and (S,S)-hydroxybupropion, (R,R)- and (S,S)-hydrobupropion (threo) and (S,R)- and (R,S)-hydrobupropion (erythro), in human urine and liver subcellular fractions to begin exploring mechanisms underlying enantioselective metabolism and elimination of bupropion metabolites. Human liver microsomal data revealed marked glucuronidation stereoselectivity [Clint, 11.4 versus 4.3 µl/min per milligram for the formation of (R,R)- and (S,S)-hydroxybupropion glucuronide; and Clmax, 7.7 versus 1.1 µl/min per milligram for the formation of (R,R)- and (S,S)-hydrobupropion glucuronide], in concurrence with observed enantioselective urinary elimination of bupropion glucuronide conjugates. Approximately 10% of the administered bupropion dose was recovered in the urine as metabolites with glucuronide metabolites, accounting for approximately 40%, 15%, and 7% of the total excreted hydroxybupropion, erythro-hydrobupropion, and threo-hydrobupropion, respectively. Elimination pathways were further characterized using an expressed UDP-glucuronosyl transferase (UGT) panel with bupropion enantiomers (both individual and racemic) as substrates. UGT2B7 catalyzed the stereoselective formation of glucuronides of hydroxybupropion, (S,S)-hydrobupropion, (S,R)- and (R,S)-hydrobupropion; UGT1A9 catalyzed the formation of (R,R)-hydrobupropion glucuronide. These data systematically describe the metabolic pathways underlying bupropion metabolite disposition and significantly expand our knowledge of potential contributors to the interindividual and intraindividual variability in therapeutic and toxic effects of bupropion in humans.
Introduction
Bupropion [(±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl) amino]-1-propanone] is widely used for the treatment of depression, for seasonal affective disorder, as a smoking cessation aid (Dwoskin et al., 2006; Dhillon et al., 2008), and recently has been coformulated with naltrexone for weight management in obese patients (Yanovski and Yanovski, 2015); however, bupropion antidepressant (Thase et al., 2005) and smoking cessation (Hurt et al., 1997; Jorenby et al., 1999; Dale et al., 2001) treatment outcomes vary widely among patients. The use of immediate release bupropion is also associated with dose-dependent adverse effects, including seizures (Davidson, 1989).
The mechanisms contributing to the variable clinical response and adverse effects of bupropion are not fully known. Alterations in the metabolism of bupropion and its primary metabolites have been posited to contribute to this variability. Early animal studies suggest that bupropion is a more effective antidepressant in mice than in rats (Soroko et al., 1977; Ferris et al., 1983), and hydroxybupropion plasma exposure was much higher in mice than in rats (Welch et al., 1987). Thus, differences in the formation of potentially active metabolites may explain species differences in bupropion effect. In humans, bupropion undergoes extensive hepatic metabolism via oxidation of the t-butyl moiety to hydroxybupropion and reduction of the aminoketone group to two amino alcohols (erythro- and threo-hydrobupropion), with <1% of the administered dose excreted unchanged in urine (Findlay et al., 1981; Lai and Schroeder, 1983; Schroeder, 1983; Laizure et al., 1985; Posner et al., 1985; Welch et al., 1987). Several preclinical studies in addition to human data provide compelling evidence that bupropion metabolites are pharmacologically active (Perumal et al., 1986; Golden et al., 1988; Bondarev et al., 2003; Damaj et al., 2004; Silverstone et al., 2008; Zhu et al., 2012). Bupropion has also been reported as a clinically relevant inhibitor of CYP2D6, with in vitro evidence suggesting that bupropion metabolites (rather than the parent drug) mediated this interaction (Reese et al., 2008). Consideration of metabolite pharmacokinetics further reinforces their potential contribution to overall bupropion effect. Steady-state plasma exposures of hydroxybupropion and threo-hydrobupropion after bupropion administration are 17- and 7-fold higher, respectively, than the parent drug (Laizure et al., 1985; Posner et al., 1985; Benowitz et al., 2013), and metabolites accumulate substantially in plasma and cerebrospinal fluid during repeated administration. Therefore, it is important to understand the factors that contribute to differential tissue and plasma exposure of active bupropion metabolites.
Of the bupropion metabolites so far described, hydroxybupropion has been studied in detail. Bupropion has one chiral center and is administered clinically as a 50:50 racemic mixture. Hydroxylation of racemic bupropion to hydroxybupropion is exclusively catalyzed by cytochrome P450 2B6 (CYP2B6) (Faucette et al., 2000; Hesse et al., 2000). This reaction is one of two US Food and Drug Administration (FDA)-recommended in vitro and in vivo probes of CYP2B6 (FDA CDER, 2012). Elevated hydroxybupropion plasma exposure is associated with improved bupropion treatment outcomes in depression (Laib et al., 2014) and smoking cessation (Zhu et al., 2012); however, hydroxybupropion plasma exposure varied widely among patients and is only partially explained by CYP2B6 genetic variation (Benowitz et al., 2013). Bupropion hydroxylation creates an additional chiral center, and two diastereomers [(2R,3R)- and (2S,3S)-hydroxybupropion] have been quantified in human plasma (Suckow et al., 1997; Coles and Kharasch, 2007; Xu et al., 2007). Wheres plasma exposure of (2R,3R)-hydroxybupropion is >20-fold higher than (2S,3S)-hydroxybupropion (Kharasch et al., 2008), some pharmacologic activity of bupropion may reside in (2S,3S)-hydroxybupropion (Bondarev et al., 2003; Damaj et al., 2004; Hansard et al., 2011). The marked stereoselectivity in hydroxybupropion disposition observed in vivo is not fully explained by CYP2B6 as the rate of (S)-bupropion hydroxylation in expressed CYP2B6 and human liver microsomes is only 3- and 1.5-fold higher, respectively, than (R)-bupropion (Coles and Kharasch, 2008). Mechanisms other than CYP2B6-mediated formation (e.g., further elimination processes) may account for observed stereoselective disposition.
In vitro (Skarydova et al., 2014; Connarn et al., 2015) and in vivo (Benowitz et al., 2013) studies suggest that bupropion reduction to form two hydrobupropion metabolites is the major mechanism of bupropion clearance. Reduction of the keto group by 11β-hydroxysteroid dehydrogenase type 1 and other carbonyl reductases (Molnari and Myers, 2012; Meyer et al., 2013; Skarydova et al., 2014; Connarn et al., 2015) appears to favor formation of threo-hydrobupropion (Benowitz et al., 2013). Although this reduction creates an additional chiral center, potentially generating two distinct threo-hydrobupropion and two erythro-hydrobupropion diastereomers, data describing stereoselective elimination pathways and effect are lacking.
The main objective of the present study was to explore the mechanisms underlying enantioselective metabolism of bupropion metabolites by investigating the UGT-mediated metabolism of hydroxybupropion and threo- and erythro-hydrobupropion.
Materials and Methods
Chemicals.
All chemicals and solvents were high-performance liquid chromatogrphy grade or higher. Acetonitrile, methanol, and acetic acid were purchased from Fisher Scientific Company LLC (Hanover Park, IL). Laboratory water was prepared for liquid chromatography-tandem mass spectrometry (LC-MS/MS) applications using a Nanopure Infinity UV system (Barnsteas/Thermolyne, Dubuque, IA). UDPGA, alamethicin, Trizma base, and magnesium chloride (MgCl2) were purchased from Sigma-Aldrich (St. Louis, MO). Internal standard, nevirapine, was supplied through the National Institutes of Health AIDS Research and Reference Reagent Program (Germantown, MD). Bupropion, R-bupropion, S-bupropion, (2R,3R)-hydroxybupropion (R,R-hydroxybupropion), (2S,3S)-hydroxybupropion (S,S-hydroxybupropion), racemic erythro-hydrobupropion, racemic threo-hydrobupropion, (S,S)- and (R,R)-hydrobupropion β-d-glucuronides (threo-hydrobupropion glucuronides) and racemic erythro-hydrobupropion β-d-glucuronide (EGLUC1 and EGLUC2) were purchased from Toronto Research Chemicals (Toronto, ON).
Microsomal Preparations.
Mixed-gender pooled human liver microsomes (HLMs) (20 mg/ml) were purchased from Corning (Woburn, MA), and recombinant UGTs (rUGT) (UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2B4, UGT2B7, UGT2B10, UGT2B15, and UGT2B17) (5 mg/ml) were purchased from Discovery Labware, Inc. (Woburn, MA). All microsomal preparations were stored at −80°C until analysis.
LC-MS/MS Method Development.
LC-MS/MS analytical method development to separate and detect bupropion, hydroxybupropion, and threo- and erythro-hydrobupropion was performed using an API 3200 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) equipped with an electrospray ionization (ESI) source and coupled to a high-performance liquid chromatography system consisting of two LC-20AD pumps, SIL-20AHT UFLC autosampler, DGU-20A3 degasser, and a CBM-20A system controller (Shimadzu, Columbia, MD). Data acquisition and processing were performed using Analyst software (version 1.5.1, AB SCIEX). Analyte concentrations were quantified using Analyst software by interpolation from matrix-matched calibration curves and quality controls with dynamic assay ranges of 0.1–1000 ng/ml for all analytes. The calibration standards and quality controls were judged for batch quality based on the 2013 FDA guidance for industry regarding bioanalytical method validation (FDA CDER, 2013). Chromatographic separation was achieved using a Luna C18-2 column (150 × 4.6 mm i.d.; 5-µm particle size; Phenomenex, Torrance, CA) and mobile phase consisting of methanol (mobile phase B) and water containing 0.1% acetic acid (mobile phase A) using the following gradient: initial conditions of 50% mobile phase B followed by a linear gradient to 90% mobile phase B between 0.01 and 16 minutes, then re-equilibrated to initial conditions between 16.01 minutes and 20 minutes using a total flow rate of 0.8 ml/min.
Initially, no glucuronide standards were commercially available, so urine collected from healthy volunteer(s) fter the administration of a single 100-mg oral tablet of racemic bupropion hydrochloride (Sandoz Inc., Princeton, NJ) was used for flow injection analysis (FIA) to obtain optimal instrument and compound-dependent parameters for the assay. Healthy volunteers were enrolled into a trial at the Indiana University School of Medicine Clinical Research Center. The Indiana University School of Medicine Institutional Review Board approved the study protocol, and all participants signed an approved, informed consent before enrollment. The trial is registered at the ClinicalTrials.gov (NCT02401256). The obtained urine sample was centrifuged at 12,000 rpm for 10 minutes before injection of resulting supernatant. Initial parameters for FIA were set according to parent drug FIA results. All data were collected in positive ion mode. As some authentic standards [(S,S)- and (R,R)-hydrobupropion (threo-hydrobupropion) β-d-glucuronides and racemic erythro-hydrobupropion β-d-glucuronide] were later commercially available, FIA was repeated for these glucuronides fter acquisition. Matrix-matched calibration curves were generated to directly quantify the glucuronide metabolites of threo- and erythro-hydrobupropion using the available glucuronide standards with a dynamic assay range of 0.5-1000 ng/ml. (R,R)- and (S,S)-hydroxybupropion glucuronides were quantified using the standard curves of threo-hydrobupropion glucuronides with the same isomeric configuration as glucuronide standards were not commercially available for these metabolites. The possibility that ionization efficiency and other system parameters may yield differing LC-MS/MS response between these structurally related metabolites could not be excluded. As such, the reported nmol formation and excretion rates of (R,R)- and (S,S)-hydroxybupropion glucuronides could be viewed more appropriately as nmol equivalents relative to threo-hydrobupropion glucuronides with the same isomeric configuration.
Urine Sample Analysis.
Urine samples were collected during four intervals (0–12, 12–24, 24–36, and 36–48 hours) over a 48-hour period after bupropion administration to healthy volunteers (n = 10). An aliquot of each urine sample (50 μl) was diluted with water-methanol (1:1) to 100 µl, 25 µl of 250 ng/ml nevirapine was added as internal standard, the sample was centrifuged (12,000 rpm × 10 minutes), and the resulting supernatant (10μl) was injected into the LC-MS/MS system.
Optimization of Microsomal Incubation Conditions.
Pilot incubation experiments were performed using HLMs to identify potential glucuronide metabolites of hydroxybupropion and to optimize incubation and LC-MS/MS analysis conditions. Racemic hydroxybupropion was dissolved and serially diluted in methanol to the required concentration. Methanol was removed by drying in a speed vacuum before reconstituting with HLMs. HLMs were diluted with Tris HCl buffer (pH 7.4, 100 mM) containing MgCl2 (5 mM) and activated by addition of alamethicin (50 µg/mg protein) on ice for 15 minutes with gentle agitation every 5 minutes. For the pilot studies, hydroxybupropion (100 µM) and 25 µl of activated HLMs (desired concentrations) were diluted in 125 µl Tris HCl buffer and preincubated for 5 minutes at 37°C. The reaction was initiated by adding 100 µl of UDPGA (5 mM dissolved in buffer) to yield a final reaction volume of 250 µl. The reaction was terminated by the addition of 100 µl of acetonitrile at predetermined time intervals. Nevirapine (25 µl of 500 ng/ml in methanol) was added as an internal standard to the incubation samples before analysis. The samples were vortexed vigorously for 20 seconds and left on ice for 15 minutes. Samples were then evaporated for 10 minutes, followed by centrifugation at 12,000 rpm for 5 minutes in an Eppendorf model 5415C centrifuge (Brinkman Instruments, Westbury, NY). The resulting supernatant (10 µl) was injected onto the LC-MS/MS system. Chromatographic separation of the bupropion glucuronide metabolites was achieved on a Luna C18-2 column (150 × 4.6 mm i.d.; 5-µm particle size; Phenomenex, Torrance, CA) using a mobile phase consisting of methanol (mobile phase B) and water containing 0.1% acetic acid (mobile phase A) using the following gradient: initial conditions of 2% mobile phase B followed by a linear gradient to 90% mobile phase B between 0.01 and 10 minutes and then reequilibrated to initial conditions between 10.01 and 14 minutes using a total flow rate of 0.8 ml/min.
Using the incubation and LC-MS/MS assay conditions already described, linearity in the formation rate of the observed hydroxybupropion glucuronides was established with regard to microsomal protein concentration and incubation time. Hydroxybupropion (100 μM) was incubated in HLMs (0.0–1.0 mg protein/ml) in the presence of the UDPGA (5 mM) at 37°C across a range of incubation times (0–90 minutes). Optimal incubation conditions were determined to be 60 minutes of incubation time using 1 mg/ml microsomal protein.
Kinetic Analysis.
An abbreviated substrate concentration range was used to obtain initial kinetic parameter estimates using Phoenix WinNonlin (Pharsight Corp., Cary, NC) with simulations to guide final concentration selection. Rates of hydroxybupropion, threo-hydrobupropion, and erythro-hydrobupropion glucuronide formation were determined by incubating a range of substrate concentrations (25–1000 μM) encompassing the predicted Km or S50. Incubation conditions were optimized for linearity with respect to time and protein concentration for each substrate as described here (data not shown).
Reaction Phenotyping Using a Recombinant UGT Enzyme Panel.
Hydroxybupropion, (R,R) and (S,S)-hydroxyburpopion, threo-hydrobupropion, or erythro-hydrobupropion (50 µM) were incubated with HLMs (1 mg/ml) or each individual recombinant UGT enzyme (0.5 mg/ml, UGT 1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, 2B17, and vehicle/vector control). Reactions were terminated at 60 minutes by the addition of acetonitrile and analyzed via LC-MS/MS as described herein.
Data Analysis.
Urinary excretion kinetics were analyzed both as absolute (ng) and molar (nmol) amounts (based on individual urine collection volumes) of each metabolite in relation to bupropion using Phoenix WinNonlin (version 6.4, Pharsight Corp.). Apparent in vitro enzyme kinetic parameters, maximum formation rate (Vmax), and substrate concentration resulting in 50% of Vmax (Km or S50), were obtained via nonlinear regression by fitting the single site Michaelis-Menten (V = Vmax × [S]/Km + [S]) or Hill (V = Vmax × [S]h/S50h + [S]h) equation (where h = Hill coeffficient) to substrate concentration versus apparent metabolite formation velocity data using Phoenix WinNonlin. Clint (Vmax/Km) or Clmax (Vmax × (h − 1)/Km + h(h − 1)1/h) (Houston and Kenworthy, 2000) was calculated for substrates described by the simple Michaelis-Menten or Hill equation, respectively. Unless noted, data are presented as mean of duplicate incubations, with error bars showing data variability for n = 2.
Results
LC-MS/MS Method Development.
The following were the optimized instrument parameters: interface temperature at 500°C, nebulizer gas (nitrogen) (GS1) 60.0 psi, heater gas (GS2) 55.0 psi, curtain gas (CUR) 25.0 psi, IonSpray Voltage 3500 V and collision activated dissociation 6.0 psi. The optimized compound-dependent MS/MS parameters are outlined in Table 1.
TABLE 1.
Compound dependent parameters optimized to hydroxybupropion glucuronide
| Analytes | Q1/Q3 | DP | EP | CEP | CE | CXP |
|---|---|---|---|---|---|---|
| m/z | v | v | v | v | v | |
| Erythro-hydrobupropion | 242.10/168.00 | 36 | 5.5 | 12 | 24 | 3 |
| EGLUC 1 | 418.00/168.00 | 45 | 8.5 | 30 | 35 | 5 |
| EGLUC 2 | 418.00/168.00 | 45 | 8.5 | 30 | 35 | 5 |
| Threo-hydrobupropion | 242.10/168.00 | 36 | 5.5 | 12 | 24 | 3 |
| (1R,2R)-hydrobupropion glucuronide | 418.00/168.00 | 45 | 8.5 | 30 | 35 | 5 |
| (1S,2S)-hydrobupropion glucuronide | 418.00/168.00 | 45 | 8.5 | 30 | 35 | 5 |
| hydroxybupropion | 256.00/238.00 | 30 | 5.5 | 25 | 24 | 4 |
| (S,S)-hydroxybupropion glucuronide | 432.00/184.00 | 56 | 8.5 | 16 | 24 | 3 |
| (R,R)-hydroxybupropion glucuronide | 432.00/184.00 | 56 | 8.5 | 16 | 24 | 3 |
CE, collision energy; CEP, cell entrance potential; CXP, collision cell exit potential; DP, declustering potential; ELGUC, erythro-hydrobupropion glucuronide; EP, entrance potential.
Chromatographic Separation, Detection, and Identification of Bupropion Metabolites.
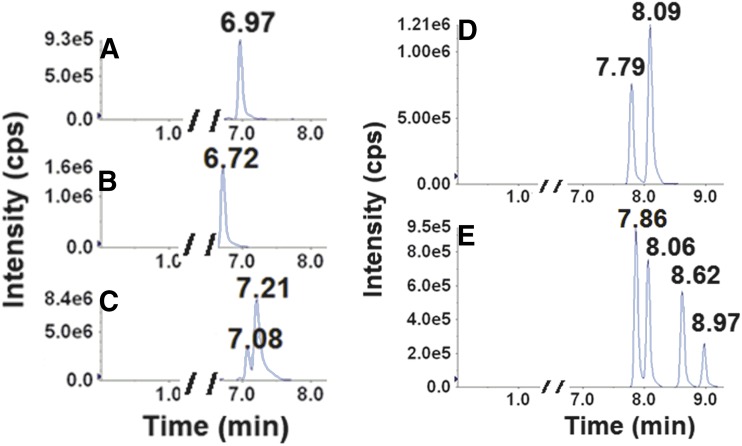
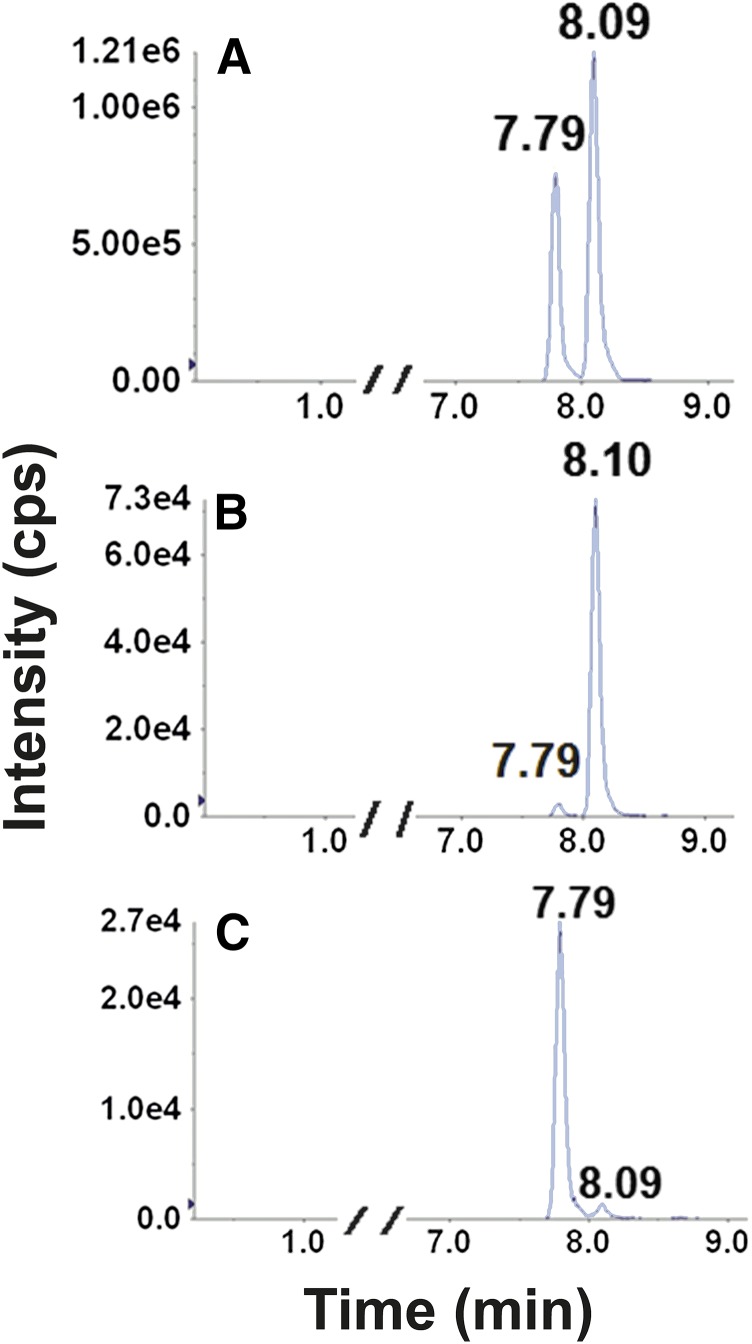
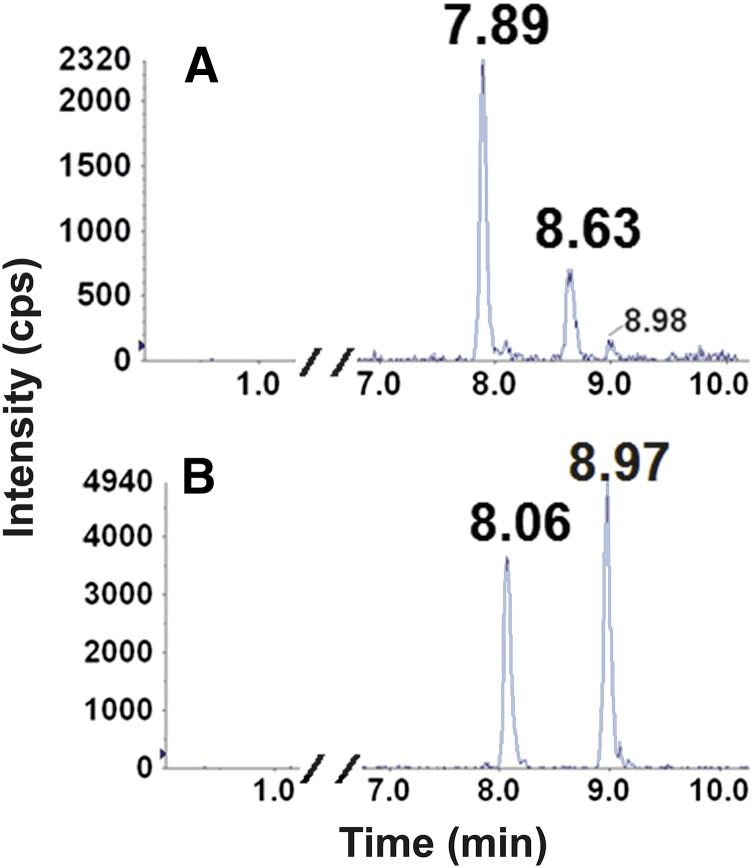
Retention times (RTs) and mass transitions of bupropion substrates and metabolites are outlined in Table 2. Baseline chromatographic separation of individual enantiomers of hydroxybupropion and threo- and erythro-hydrobupropion was not achieved using the outlined chromatographic conditions (Fig. 1, A–C). In contrast, baseline separation and detection of the enantiomeric bupropion glucuronide metabolites were achieved for the first time (Fig. 1, D and E). The glucuronides of hydroxybupropion (racemic or enantiomers) were not available commercially. Thus, identification of the two urinary glucuronides of hydroxybupropion were carried out by monitoring metabolites formed during incubation of racemic-, (S,S)-, and (R,R)-hydroxybupropion with HLMs supplemented with UDPGA. In microsomal incubations containing racemic hydroxybupropion, two glucuronide metabolites (Fig. 2A) that had the same retention times as those observed in urine (Fig. 1D) were generated. The glucuronide metabolite eluting first (RT 7.79 minutes) was exclusively formed in incubations using (S,S)-hydroxybupropion as the substrate (Fig. 2C); the later eluting glucuronide (RT 8.09 minutes) was exclusively formed in incubations utilizing (R,R)-hydroxybupropion as the substrate (Fig. 2B). Peaks corresponding to these glucuronide metabolites were not observed in the negative control incubations (no cofactor, no substrate, or no incubation). Although no hydroxybupropion glucuronides (racemic or enantiomers) were available to us commercially to confirm the specific identity of the metabolites, it is reasonable to suggest that (S,S)- and (R,R)-hydroxybupropion undergo UGT-mediated conjugation to form (S,S)- and (R,R)-hydroxybupropion glucuronides, respectively. Similarly, as shown in Fig. 3, two putative glucuronide peaks for each substrate were identified when racemic threo- (RTs 7.89, 8.63 minutes) (Fig. 3A) or erythro-hydrobupropion (RTs, 8.06 and 8.97 minutes) (Fig. 3B) was incubated with HLMs supplemented with UDPGA. Based on the RTs of authentic standards, the two glucuronide peaks formed from racemic threo-hydrobupropion were consistent with (1R,2R)-hydrobupropion glucuronide (RT, 7.89 minutes) and (1S,2S)-hydrobupropion glucuronide (RT, 8.63 minutes) (Fig. 3A). Our data from hydroxybupropion suggest that (1R,2R)-hydrobupropion and (1S,2S)-hydrobupropion undergo conjugation by UGTs to form (1R,2R)-hydrobupropion glucuronide (RT, 7.89 minutes) and (1S,2S)-hydrobupropion glucuronide (RT, 8,63 minutes), respectively. Confirmation of the identity of the two glucuronides formed from incubations of racemic erythro-hydrobupropion, erythro-hydrobupropion glucuronide (EGLUC) 1, and EGLUC2 remains to be determined as neither individual enantiomers of the potential substrates (erythro-hydrobupropion) nor enantiomers of the products (putative glucuronide metabolites) are commercially available; however, injection of an authentic glucuronide standard obtained from TRC (labeled: racemic erythro-hydro bupropion β-D-glucuronide) into the LC-MS/MS system yielded only a single peak that was consistent with the second glucuronide peak (RT, 8.97 minutes). This standard was confirmed to be a mislabeled single enantiomer of erythro-hydrobupropion β-d-glucuronide (data available from TRC), which suggests that the metabolite at RT, 8.97 minutes, is (1R, 2S)-hydrobupropion glucuronide (EGLUC2) and the glucuronide peak at RT, 8.06 minutes, is (1S, 2R)-hydrobupropion (EGLUC1).
TABLE 2.
RTs and mass transitions of bupropion metabolites
| Substrates/Metabolites | RT | Observed Mass Q1 | Fragment Quantifier Q3 |
|---|---|---|---|
| min | Da | Da | |
| Erythro-hydrobupropion | 6.92 | 242.10 | 168.00 |
| EGLUC 1 | 8.03 | 418.00 | 168.00 |
| EGLUC 2 | 8.93 | 418.00 | 168.00 |
| Threo-hydrobupropion | 7.05 | 242.10 | 168.00 |
| (1R,2R)-hydrobupropion glucuronide | 7.84 | 418.00 | 168.00 |
| (1S,2S)-hydrobupropion glucuronide | 8.59 | 418.00 | 168.00 |
| hydroxybupropion | 6.75 | 256.00 | 238.00 |
| (S,S)-hydroxybupropion glucuronide | 7.80 | 432.00 | 184.00 |
| (R,R)-hydroxybupropion glucuronide | 8.10 | 432.00 | 184.00 |
| (R,R)-hydroxybupropion | 6.59 | 256.00 | 131.00 |
| (S,S)-hydroxybupropion glucuronide | 7.78 | 432.00 | 184.00 |
| (R,R)-hydroxybupropion glucuronide | 8.08 | 432.00 | 184.00 |
| (S,S)-hydroxybupropion | 6.60 | 256.00 | 131.00 |
| (S,S)-hydroxybupropion glucuronide | 7.75 | 432.00 | 184.00 |
| (R,R)-hydroxybupropion glucuronide | 8.05 | 432.00 | 184.00 |
EGLUC, Erythro-hydrobupropion glucuronide.
Fig. 1.
Representative chromatographic traces for bupropion and its metabolites detected in urine collected from a healthy volunteer administered a single 100-mg oral dose of bupropion. Urine (200 µl) was centrifuged, and the resulting supernatant (50 µl) was injected into the LC-MS/MS system after filtration. Left: Traces of bupropion (A), racemic hydroxybupropion (B), and racemic erythro-/threo-hydrobupropion (C) at RTs of 6.97, 6.72, 7.08, 7.21 minutes, respectively, corresponding to Q1/Q3 m/z transitions of 240/184, 256/238, 242.1/168, and 242.1/168. Right: (D) Traces of (SS)-hydroxybupropion glucuronide (RT, 7.79 minutes) and (RR)-hydroxybupropion glucuronide (RT, 8.09 minutes) correspond to Q1/Q3 m/z transitions of 432/184 for both metabolites. Right: (E) Traces of (1R,2R)-hydrobupropion glucuronide (RT, 7.86 minute) and (1S,2S)-hydrobupropion glucuronide (RT, 8.62 minutes), and erythro-hydrobupropion glucuronide (EGLUC) 1 (RT, 8.06 minutes), and EGLUC 2 (RT, 8.97 minutes), corresponding to Q1/Q3 m/z transitions of 418/168.
Fig. 2.
Representative chromatographic traces for glucuronides of racemic hydroxybupropion (A), (RR)-hydroxybupropion (B), and (SS)-hydroxybupropion (C) in HLM incubates. Racemic hydroxy-, (S,S)-hydroxy-, or (R,R)-hydroxy-bupropion (10 µM) was incubated in duplicate for 60 minutes at 37°C with alamethicin (50 µg/mg protein final concentration) activated HLMs (1 mg/ml protein) and UDPGA (5 mM). Reactions were terminated by the addition of acetonitrile (100 µl). After the addition of the internal standard (nevirapine, 25 µl of 500 ng/ml in methanol), sample was vortex-mixed, centrifuged, and dried for 10 minutes, and the resulting supernatant (50µl) was injected into the LC-MS/MS system. (A) Traces of (S,S)-hydroxybupropion glucuronide (RT, 7.79 minute)s and (R,R)-hydroxybupropion glucuronide (RT, 8.09 minutes) obtained from incubation containing racemic hydroxybupropion. (B) Traces of (S,S)-hydroxybupropion glucuronide (RT, 7.79 minutes) and (R,R)-hydroxybupropion glucuronide (RT, 8.10 minutes) obtained from incubation containing (R,R)-hydroxybupropion. C) Traces of (S,S)-hydroxybupropion glucuronide (RT, 7.79 minutes) and (R,R)-hydroxybupropion glucuronide (RT, 8.09 minutes) obtained from incubation containing (S,S)-hydroxybupropion. All peaks correspond to Q1/Q3 m/z transitions of 432/184.
Fig. 3.
Representative chromatographic traces for glucuronides of threo- (A) and erythro-hydrobupropion (B) in HLM incubates. Erythro-hydrobupropion (25 µM) or threo-hydrobupropion (25 µM) was incubated for 60 minutes at 37°C with alamethicin (50 µg/mg protein final concentration) activated HLMs (1 mg/ml protein) and UDPGA (5 mM). Reaction was terminated by the addition of 100 µl of acetonitrile. After the addition of the internal standard (nevirapine, 25 µl of 500 ng/ml in methanol), sample was vortex-mixed, centrifuged and dried for 10 minutes, and the resulting supernatant (5 µl) was injected into LC-MS/MS system. (A) (1R,2R)-hydrobupropion glucuronide (RT, 7.86 minutes) and (1S,2S)-hydrobupropion glucuronide (RT, 8.62 minutes) obtained from incubations containing threo-hydrobupropion and (B) erythro-hydrobupropion glucuronide (EGLUC) 1 (RT, 8.06 minutes) and EGLUC 2 (RT, 8.97 minutes) obtained from incubation containing erythro-hydrobupropion; all peaks correspond to Q1/Q3 m/z transitions of 418/168.
Urinary Excretion of Bupropion Glucuronides.
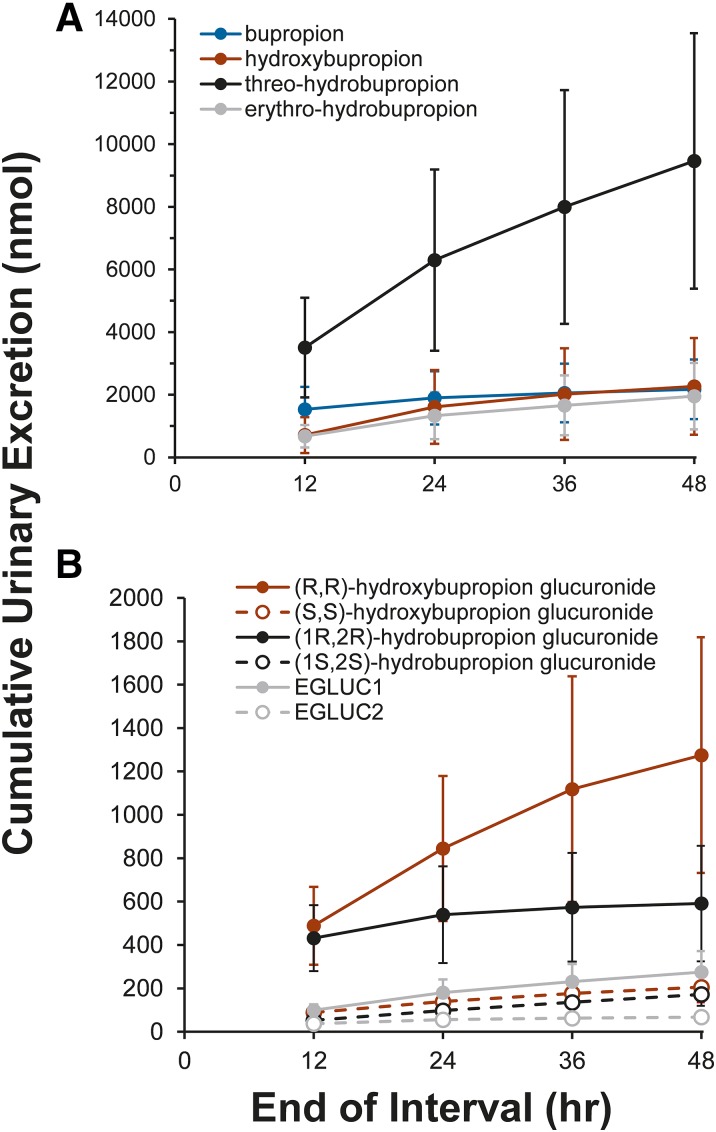
Less than 1% of the bupropion dose was excreted unchanged in the urine (Table 3). Approximately 10% of the administered bupropion dose was recovered in the urine as bupropion and metabolites by 48 hours. Threo-hydrobupropion was the predominant unconjugated bupropion metabolite detected in urine, followed by erythro-hydrobupropion and hydroxybupropion (Fig. 4A; Table 3). Threo-hydrobupropion accounted for approximately 50% of the total urinary bupropion metabolites. The predominant glucuronide metabolite excreted in the urine was (R,R)-hydroxybupropion glucuronide, followed (in order of magnitude) by (1R,2R)-hydrobupropion glucuronide, EGLUC1, (S,S)-hydroxybupropion glucuronide, (1S,2S)- hydrobupropion glucuronide, and EGLUC2 (Fig. 4B; Table 3). Glucuronide metabolites accounted for approximately 40%, 15%, and 7% of total excreted hydroxybupropion, erythro-hydrobupropion, and threo-hydrobupropion, respectively.
TABLE 3.
48-Hour bupropion and metabolite urinary excretion
Values denote means (n = 10 subjects) and 90% confidence intervals (CI).
| Analyte | 48-h Recovery, nmola Mean (90% CI) | % of Bupropion Dose Mean (90% CI) | ||
|---|---|---|---|---|
| Bupropion | 2172 | (1218–3125) | 0.60 | (0.3–0.86) |
| Hydroxybupropion | 2266 | (722–3811) | 0.67 | (0.21–1.12) |
| Threo-hydrobupropion | 9463 | (5384–13,542) | 5.24 | (2.98–7.51) |
| Erythro-hydrobupropion | 1953 | (894–3011) | 1.08 | (0.50–1.67) |
| (R,R)-hydroxybupropion glucuronide | 1275 | (732–1818) | 1.27 | (0.73–1.81) |
| (S,S)-hydroxybupropion glucuronide | 205 | (135–275) | 0.20 | (0.13–0.27) |
| EGLUC 1 | 275 | (179–372) | 0.26 | (0.17–0.36) |
| EGLUC 2 | 67 | (42–92) | 0.06 | (0.04–0.09) |
| (1R,2R)-hydrobupropion glucuronide | 591 | (325–857) | 0.57 | (0.31–0.82) |
| (1S,2S)-hydrobupropion glucuronide | 172 | (119–225) | 0.17 | (0.11–0.22) |
Erythro-hydrobupropion glucuronide (EGLUC).
(R,R)- and (S,S)-hydroxybupropion glucuronides represent nmol equivalents relative to threo-hydrobupropion glucuronides with the same isomeric configuration.
Fig. 4.
Urinary excretion kinetics of bupropion and metabolites. Urine was collected (0–48 hours) from healthy volunteers (n = 10) after oral administration of a single 100-mg dose of racemic bupropion. Cumulative urinary excretion was determined by LC-MS/MS monitoring of (A) bupropion (blue), hydroxybupropion (red), threo-hydrobupropion (black), erythro-hydrobupropion (gray), and (B) (R,R)-hydroxybupropion glucuronide (solid red line and symbols), (S,S)-hydroxybupropion glucuronide (dashed red line, open red symbols), (1R,2R)-hydrobupropion glucuronide (solid black line and symbols), (1S,2S)-hydrobupropion glucuronide (dashed black line, open black symbols), erythro-hydrobupropion glucuronide (EGLUC) 1 (solid gray line and symbols), and EGLUC2 (dashed gray line, open gray symbols) over time. Glucuronide 1 and glucuronide 2 were assigned based on RTs in Figs. 1–3 and are outlined in Table 2. Symbols and error bars denote the mean and 90% confidence interval of the observed cumulative urinary excretion (nmol) at the end of the collection interval, respectively.
Bupropion Metabolite Glucuronidation Kinetics.
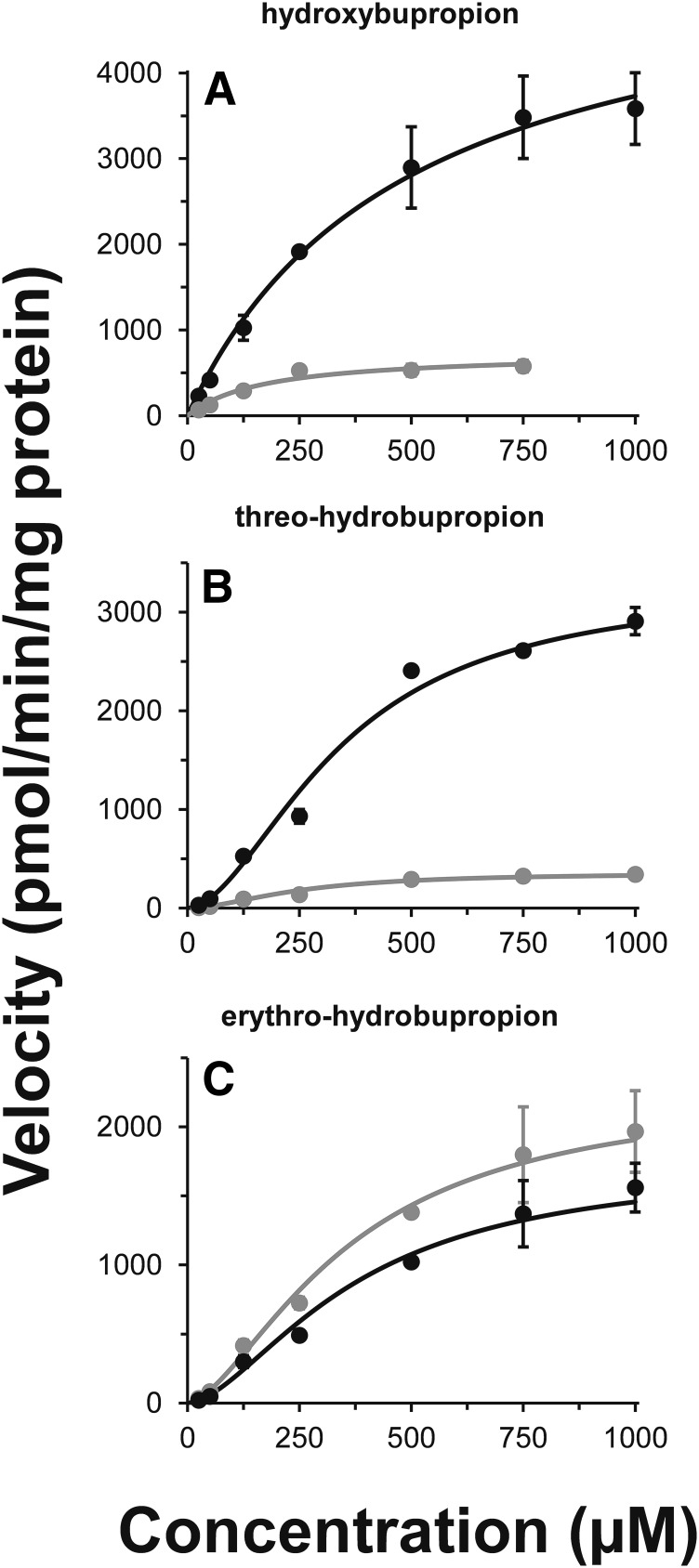
Glucuronide formation kinetics in HLMs demonstrated stereoselective glucuronidation of hydroxybupropion (Fig. 5A), threo-hydrobupropion (Fig. 5B), and erythro-hydrobupropion (Fig. 5C). Glucuronidation kinetics of hydroxybupropion is best described by the simple Michaelis-Menten equation, whereas threo- and erythro-hydrobupropion kinetics is best described by the Hill equation. Glucuronidation efficiency (Clint or Clmax) varied nearly 10-fold across the enantiomers evaluated. Kinetic parameters for glucuronide formation are displayed in Table 4.
Fig. 5.
Kinetics for the formation of hydroxybupropion (A), threo-hydrobupropion (B), and erythro-hydrobupropion (C) glucuronides in HLMs. Racemic hydroxybupropion, threo-hydrobupropion, or erythro-hydrobupropion (25 µM–1000 µM) was incubated for 60 minutes at 37°C in duplicate with HLMs (1 mg/ml protein) and 5 mM UDPGA. The two putative glucuronide metabolites in each panel are consistent with (R,R)-hydroxybupropion glucuronide (black) and (S,S)-hydroxybupropion glucuronide (gray) (A), (1R,2R)-hydrobupropion glucuronide (black) and (1S,2S)- hydrobupropion glucuronide (gray) (B), erythro-hydrobupropion glucuronide (EGLUC) 1 (black) and EGLUC2 (gray) (C). Glucuronide 1 and glucuronide 2 were assigned based on RTs in Figs. 1–3 and re outlined in Table 2. Curves denote model-generated values obtained from nonlinear regression performed by fitting the Michaelis-Menten or Hill equation to substrate concentration versus apparent metabolite formation velocity using Phoenix WinNonlin (version 6.4). Symbols and error bars denote mean and S.D., respectively, of duplicate incubations.
TABLE 4.
Bupropion glucuronidation kineticsa
| Substrate | Km or S50 (µM) | Vmax | Clintd or Clmaxe |
|---|---|---|---|
| μM | pmol/min/mg | µl/min/mg | |
| (S,S)-hydroxybupropion glucuronidea | 172 ± 38.9 | 739 ± 59.6 | 4.31 |
| (R,R)-hydroxybupropion glucuronidea | 488 ± 98.3 | 5550 ± 507 | 11.4 |
| (1R,2R)-hydrobupropion glucuronideb | 343 ± 37.5 | 3290 ± 269 | 7.7 |
| (1S,2S)-hydrobupropion glucuronideb | 248 ± 27.1 | 358 ± 25.5 | 1.1 |
| EGLUC1c | 373 ± 63.0 | 1740 ± 212 | 3.1 |
| EGLUC2c | 360 ± 55.2 | 2280 ± 241 | 3.8 |
Values represent the parameter estimate ± S.E. obtained by fitting the Michaelis-Mentena (hydroxybupropion) or Hill.
Glucuronide 1 and glucuronide 2 denoted based on retention times in Figs. 1–3 and outlined in Table 2. Erythro-hydrobupropion glucuronide (EGLUC).
(threo- and erythro-hydrobupropion) equation to [substrate] versus metabolite formation velocity using Phoenix WinNonlin (version 6.4).
Intrinsic clearance (Clint), calculated as the ratio of Vmax to Km.
Maximal clearance (Clmax), calculated as the ratio of Vmax × (h − 1) to S50 + h(h − 1)1/h.
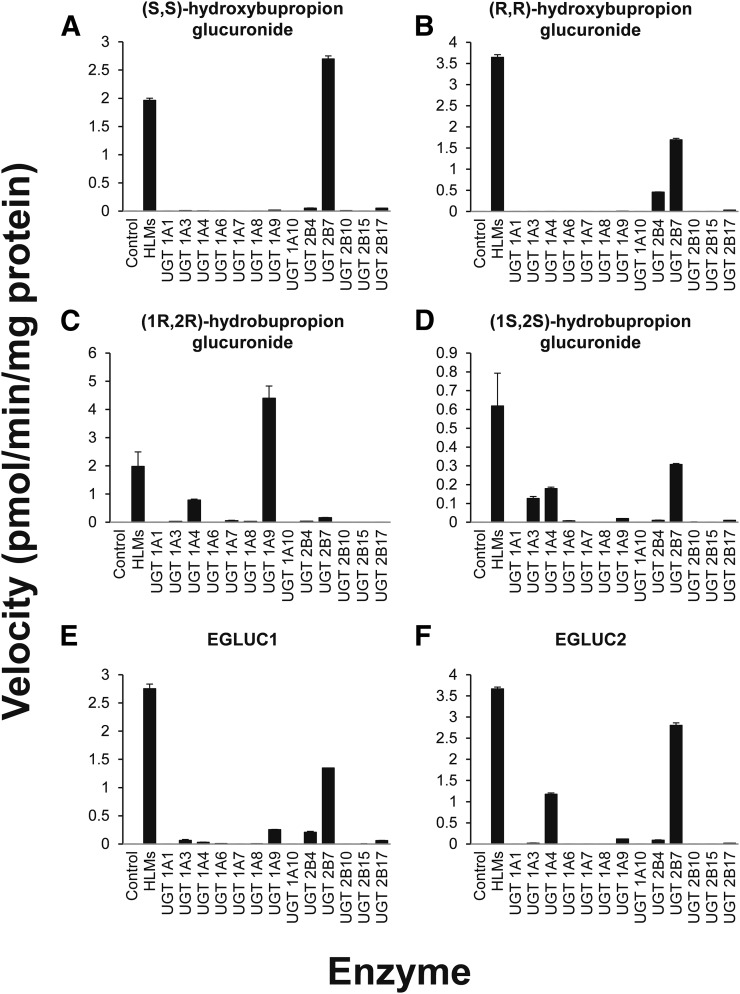
Isoform Selective Bupropion Glucuronidation.
UGT2B7 was identified as the most active isoform catalyzing in vitro hydroxybupropion glucuronidation, with UGT2B4 forming the glucuronides to a lesser extent. UGT2B7 and UGT2B4 catalyzed the glucuronidation of (R,R)-hydroxybupropion, whereas only UGT2B7 catalyzed glucuronidation of (S,S)-hydroxybupropion. Recombinant UGT1A4 and UGT1A9 catalyzed threo-hydrobupropion glucuronidation, wheres UGT2B7 was the most active isoform catalyzing erythro-hydrobupropion glucuronidation with UGT1A9 and UGT2B4 also forming glucuronides of erythro-hydrobupropion. These data expand our knowledge of the proposed human metabolic pathways involved in the stereoselective glucuronidation of bupropion and its metabolites (Fig. 6).
Fig. 6.
Bupropion glucuronide formation using HLMs and a panel of 13 rUGT isoforms. HLMs (1 mg/ml) or rUGT (0.5 mg/ml) were incubated with hydroxybupropion, (R,R)-hydroxybupropion (50 µM), (S,S)-hydroxybupropion (50 µM), threo-hydrobupropion (100 µM), or erythro-hydrobupropion (100 µM) and UDPGA (5 mM) (250 µl final reaction volume) at 37°C for 60 minutes. Velocities correspond to the formation of (S,S)-hydroxybupropoin glucuronide (A), (R,R)-hydroxybupropion glucuronide (B), (1R,2R)-hydrobupropion glucuronide (C), (1S,2S)-hydrobupropion glucuronide (D), erythro-hydrobupropion glucuronide 1 (EGLUC1) (E), or erythro-hydrobupropion glucuronide 2 (EGLUC2) (F). Glucuronide 1 and glucuronide 2 were assigned based on RT in Figs. 1–3 and are outlined in Table 2. Bars and error bars denote the mean and S.D., respectively, of duplicate incubations.
Discussion
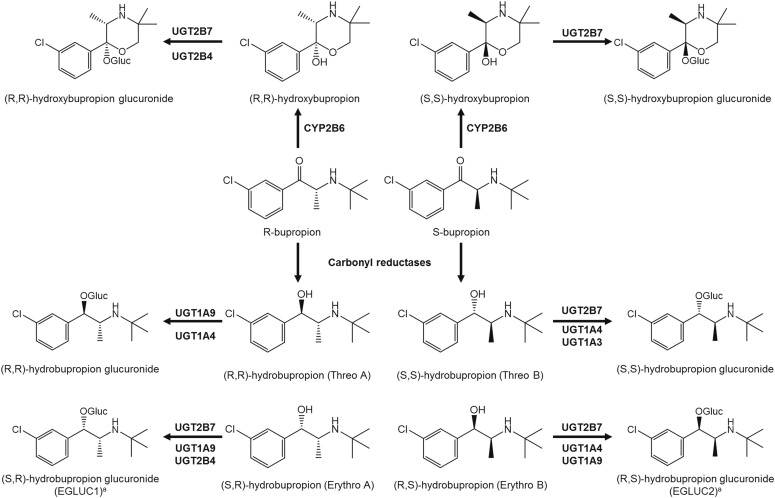
Racemic bupropion undergoes complex metabolism catalyzed by multiple enzymes to yield numerous metabolites in humans. These metabolites are important contributors to the therapeutic and toxic effects of the drug, but relatively little is known about the mechanisms governing their metabolism and elimination from the body. This report is the first to describe the stereoselective separation and detection of glucuronides of bupropion enantiomers excreted in the urine of healthy human volunteers, UGT-mediated elimination pathways using HLMs and bupropion enantiomers (both individual and racemic) as substrates to infer the glucuronide stereochemistry and in vitro to in vivo extrapolation, and the predominant UGT isoforms responsible for catalyzing glucuronidation of bupropion metabolites using a panel of recombinant UGTs. These data provide a deeper understanding of mechanisms underlying the disposition of pharmacologically active bupropion metabolites.
Introduction of a hydroxy functional group occurs during both the oxidative and reductive metabolism of bupropion to yield hydroxybupropion as well as erythro- and threo-hydrobupropion. These metabolites would be expected to undergo further metabolism via conjugation reactions (glucuronidation and/or sulfation) to enhance hydrophilicity and promote renal elimination. Indeed, glucuronide metabolites of hydroxybupropion and threo- and erythro-hydrobupropion have been detected in human urine after therapeutic doses of racemic bupropion (Welch et al., 1987; Petsalo et al., 2007; Benowitz et al., 2013). The estimated amount of free and total bupropion metabolites excreted in the urine from healthy volunteers administered bupropion to steady-state indicates that glucuronide metabolites account for approximately 75%, 25%, and 10% of total excreted hydroxybupropion, erythro-hydrobupropion, and threo-hydrobupropion, respectively (Benowitz et al., 2013). These percentages mirror the observed rank order in our study but differ slightly in absolute amount; however, the previously reported indirect method (subtractive using deconjugation by β-glucuronidase) (Benowitz et al., 2013) does not specifically provide information on glucuronide and sulfate conjugates, as nonselective cleavage of sulfate conjugates (qualitatively identified in urine of healthy volunteers administered bupropion) (Petsalo et al., 2007) can occur during extended enzymatic incubations, and only the sum of racemic mixtures were reported. This important difference in analytical methods likely explains the discrepancy between our work and previous reports. Nonetheless, these findings provide further confirmation that bupropion primary metabolites undergo metabolism via conjugation before urinary excretion.
Herein is the first report describing stereoselective glucuronidation of bupropion metabolites. We have directly identified two glucuronides each of hydroxybupropion, erythro-hydrobupropion, and threo-hydrobupropion in the urine of healthy volunteers administered a single 100-mg oral dose of bupropion. A previous study reported two glucuronide metabolites of hydroxybupropion and four glucuronides of threo-/erythro-hydrobupropion mixtures in human urine (Petsalo et al., 2007), which could suggest stereoselective glucuronidation; however, this study did not elucidate isomeric structures nor identify specific enantiomeric substrates. In addition, no quantitative information was provided to describe glucuronide disposition. Our data provide for the first time structural description of these glucuronides and, using in vitro experiments, show enantiomers from which these glucuronide metabolites originate. Of note, these glucuronides were chromatographically separated using achiral column chemistry, whereas enantiomers of their proximate substrates are not (present data and Petsalo et al., 2007), suggesting substantially unique configuration of the glucuronide metabolites compared with their corresponding substrates. After a single oral dose of racemic bupropion, the glucuronides of hydroxybupropion were the predominant conjugates detected in urine, in agreement with a previous report conducted at steady state (Benowitz et al., 2013). Of the glucuronides detected in urine, (R,R)-hydroxybupropion was the most prevalent species, providing further evidence for enantioselective urinary elimination of bupropion metabolites and conjugates. We confirm that threo-hydrobupropion is the primary unconjugated urinary bupropion metabolite. Overall, direct description of stereoselective urinary excretion of bupropion and metabolites at 48 hours agreed well with a previous (indirect, nonstereoselective) report of urinary recovery at 24 hours (Benowitz et al., 2013) and with findings after administration of C14-labeled bupropion (Johnston et al., 2002).
The enzymes responsible for the oxidative and reductive metabolism of bupropion to form hydroxybupropion, erythro-hydrobupropion, and threo-hydrobupropion have been investigated extensively (Faucette et al., 2000, 2001; Hesse et al., 2000; Bondarev et al., 2003; Damaj et al., 2004; Molnari and Myers, 2012; Zhu et al., 2012; Meyer et al., 2013; Skarydova et al., 2014). Metabolite exposure is presumably dependent on metabolic pathways leading to their formation and elimination; however, the enzymes responsible for glucuronidation and subsequent renal elimination of the pharmacologically important bupropion metabolites have not been fully elucidated. Here we quantitatively describe the stereoselective hepatic glucuronidation of bupropion. The most efficiently formed bupropion glucuronide in vitro, (R,R)-hydroxybupropion glucuronide was also the predominant urinary conjugate observed in vivo. Similarly, the next most efficiently formed glucuronide in vitro, threo-hydrobupropion glucuronide 1, was also the second most abundant glucuronide excreted in the urine. Interestingly, erythro-hydrobupropion glucuronide formation demonstrated similar in vitro efficiency but nearly 4-fold differences in mean urinary excretion, suggesting that additional mechanisms may underlie differential urinary excretion of these metabolites.
Characterization of the UGTs responsible for the conjugation of active bupropion metabolites is essential to understanding factors that may influence the interindividual and intraindividual variability in bupropion metabolite exposure, including the evaluation of potential drug-drug interactions and pharmacogenetic implications. UGT2B7 and UGT1A9, both being prominently expressed in the liver (Margaillan et al., 2015b), are likely the dominant isoforms responsible for hepatic glucuronidation of bupropion metabolites. These isoforms may also play an important role in the extrahepatic glucuronidation of bupropion, particularly in the kidney where UGT1A9 is the predominant isoform expressed (Margaillan et al., 2015a) and in the gut where UGT2B7 and UGT1A9 are present (Harbourt et al., 2012; Fallon et al., 2013). Further exploration of extrahepatic bupropion metabolism could more comprehensively describe bupropion metabolite disposition and facilitate quantitative in vitro-in vivo extrapolation of bupropion metabolite kinetics (Gill et al., 2012, 2013).
In summary, racemic bupropion undergoes complex metabolism catalyzed by multiple enzymes to yield numerous metabolites in humans. These metabolites are important contributors to the therapeutic and toxic effects of the drug. Despite their pharmacologic importance, relatively little has been known about the mechanisms governing their metabolism and elimination from the body. This is the first LC-MS/MS assay that allowed simultaneous separation and quantification of enantiomers of bupropion glucuronide metabolites in human urine and liver subcellular fractions. We elucidated for the first time isomeric structures of the glucuronides and identified specific enantiomeric substrates (Fig. 7). Furthermore, we provided quantitative information regarding the amount of each enantiomer excreted in urine and show enantioselective urinary elimination of bupropion glucuronide conjugates that is in concurrence with observed in vitro glucuronidation efficiency. Our data suggest that plasma exposure of active bupropion metabolites may be influenced by differences not only in their formation but also in their elimination via UGTs, notably UGT2B7 and UGT1A9. These novel findings significantly expand our knowledge of the metabolic pathways underlying bupropion disposition in humans (Fig. 7) and should form the basis for future studies designed to link bupropion metabolism and its clinical effect.
Fig. 7.
Proposed human metabolic pathways involved in the stereoselective oxidation, reduction, and glucuronidation of bupropion and its metabolites. aConfirmation of the identity of the two glucuronides formed from incubations of racemic erythro-hydrobupropion, erythro-hydrobupropion glucuronide (EGLUC) 1 and EGLUC2, remains to be definitively determined as neither individual enantiomers of the potential substrates (erythro-hydrobupropion) nor unambiguously identified enantiomers of the products (putative glucuronide metabolites) are commercially available.
Abbreviations
- FDA
US Food and Drug Administration
- FIA
flow injection analysis
- HLM
human liver microsome
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- P450
cytochrome P450
- RT
retention time
- rUGT
recombinant UGT
- UDPGA
UDP-glucuronic acid
- UGT
UDP-glucuronosyl transferase
Author Contributions
Participated in research design: Lu, Gufford, Desta.
Conducted experiments: Lu, Metzger.
Contributed new reagents or analytical tools: Lu, Jones.
Performed data analysis: Gufford, Desta.
Wrote or contributed to writing of the manuscript: Gufford, Lu, Jones, Desta.
Footnotes
This work was supported by the National Institutes of Health (NIH) National Institute of General Medical Sciences [Grant R01 GM078501]. B.T.G. is supported by the NIH General Medical Sciences [Grant T32 GM008425].
References
- Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P., III (2013) Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics 23:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarev ML, Bondareva TS, Young R, Glennon RA. (2003) Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol 474:85–93. [DOI] [PubMed] [Google Scholar]
- Coles R, Kharasch ED. (2007) Stereoselective analysis of bupropion and hydroxybupropion in human plasma and urine by LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 857:67–75. [DOI] [PubMed] [Google Scholar]
- Coles R, Kharasch ED. (2008) Stereoselective metabolism of bupropion by cytochrome P4502B6 (CYP2B6) and human liver microsomes. Pharm Res 25:1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connarn JN, Zhang X, Babiskin A, Sun D. (2015) Metabolism of bupropion by carbonyl reductases in liver and intestine. Drug Metab Dispos 43:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LC, Glover ED, Sachs DP, Schroeder DR, Offord KP, Croghan IT, Hurt RD. (2001) Bupropion for smoking cessation : predictors of successful outcome. Chest 119:1357–1364. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. (2004) Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol 66:675–682. [DOI] [PubMed] [Google Scholar]
- Davidson J. (1989) Seizures and bupropion: a review. J Clin Psychiatry 50:256–261. [PubMed] [Google Scholar]
- Dhillon S, Yang LP, Curran MP. (2008) Bupropion: a review of its use in the management of major depressive disorder. Drugs 68:653–689. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. (2006) Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 12:178–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JK, Neubert H, Goosen TC, Smith PC. (2013) Targeted precise quantification of 12 human recombinant uridine-diphosphate glucuronosyl transferase 1A and 2B isoforms using nano-ultra-high-performance liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Drug Metab Dispos 41:2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28:1222–1230. [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Shord SS, Lecluyse EL, Lindley CM. (2001) Evaluation of the contribution of cytochrome P450 3A4 to human liver microsomal bupropion hydroxylation. Drug Metab Dispos 29:1123–1129. [PubMed] [Google Scholar]
- Ferris RM, Cooper BR, Maxwell RA. (1983) Studies of bupropion’s mechanism of antidepressant activity. J Clin Psychiatry 44:74–78. [PubMed] [Google Scholar]
- Findlay JW, Van Wyck Fleet J, Smith PG, Butz RF, Hinton ML, Blum MR, Schroeder DH. (1981) Pharmacokinetics of bupropion, a novel antidepressant agent, following oral administration to healthy subjects. Eur J Clin Pharmacol 21:127–135. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration Center for Drug Evaluation and Research (FDA CDER) (2012) Drug interaction studies: study design, data analysis, implications for dosing, and labeling recommendations (draft guidance).
- Food and Drug Administration Center for Drug Evaluation and Research (FDA CDER) (2013) Bioanalytical Method Validation (draft guidance).
- Gill KL, Gertz M, Houston JB, Galetin A. (2013) Application of a physiologically based pharmacokinetic model to assess propofol hepatic and renal glucuronidation in isolation: utility of in vitro and in vivo data. Drug Metab Dispos 41:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KL, Houston JB, Galetin A. (2012) Characterization of in vitro glucuronidation clearance of a range of drugs in human kidney microsomes: comparison with liver and intestinal glucuronidation and impact of albumin. Drug Metab Dispos 40:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden RN, De Vane CL, Laizure SC, Rudorfer MV, Sherer MA, Potter WZ. (1988) Bupropion in depression: II. The role of metabolites in clinical outcome. Arch Gen Psychiatry 45:145–149. [DOI] [PubMed] [Google Scholar]
- Hansard MJ, Jackson MJ, Smith LA, Rose S, Jenner P. (2011) A major metabolite of bupropion reverses motor deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated common marmosets. Behav Pharmacol 22:269–274. [DOI] [PubMed] [Google Scholar]
- Harbourt DE, Fallon JK, Ito S, Baba T, Ritter JK, Glish GL, Smith PC. (2012) Quantification of human uridine-diphosphate glucuronosyl transferase 1A isoforms in liver, intestine, and kidney using nanobore liquid chromatography-tandem mass spectrometry. Anal Chem 84:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. (2000) CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos 28:1176–1183. [PubMed] [Google Scholar]
- Houston JB, Kenworthy KE. (2000) In vitro-in vivo scaling of CYP kinetic data not consistent with the classical Michaelis-Menten model. Drug Metab Dispos 28:246–254. [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, et al. (1997) A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 337:1195–1202. [DOI] [PubMed] [Google Scholar]
- Johnston AJ, Ascher J, Leadbetter R, Schmith VD, Patel DK, Durcan M, Bentley B. (2002) Pharmacokinetic optimisation of sustained-release bupropion for smoking cessation. Drugs 62 (Suppl 2):11–24. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, et al. (1999) A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 340:685–691. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Mitchell D, Coles R. (2008) Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J Clin Pharmacol 48:464–474. [DOI] [PubMed] [Google Scholar]
- Lai AA, Schroeder DH. (1983) Clinical pharmacokinetics of bupropion: a review. J Clin Psychiatry 44:82–84. [PubMed] [Google Scholar]
- Laib AK, Brünen S, Pfeifer P, Vincent P, Hiemke C. (2014) Serum concentrations of hydroxybupropion for dose optimization of depressed patients treated with bupropion. Ther Drug Monit 36:473–479. [DOI] [PubMed] [Google Scholar]
- Laizure SC, DeVane CL, Stewart JT, Dommisse CS, Lai AA. (1985) Pharmacokinetics of bupropion and its major basic metabolites in normal subjects after a single dose. Clin Pharmacol Ther 38:586–589. [DOI] [PubMed] [Google Scholar]
- Margaillan G, Rouleau M, Fallon JK, Caron P, Villeneuve L, Turcotte V, Smith PC, Joy MS, Guillemette C. (2015a) Quantitative profiling of human renal UDP-glucuronosyltransferases and glucuronidation activity: a comparison of normal and tumoral kidney tissues. Drug Metab Dispos 43:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaillan G, Rouleau M, Klein K, Fallon JK, Caron P, Villeneuve L, Smith PC, Zanger UM, Guillemette C. (2015b) Multiplexed targeted quantitative proteomics predicts hepatic glucuronidation potential. Drug Metab Dispos 43:1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Vuorinen A, Zielinska AE, Strajhar P, Lavery GG, Schuster D, Odermatt A. (2013) Formation of threohydrobupropion from bupropion is dependent on 11β-hydroxysteroid dehydrogenase 1. Drug Metab Dispos 41:1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnari JC, Myers AL. (2012) Carbonyl reduction of bupropion in human liver. Xenobiotica 42:550–561. [DOI] [PubMed] [Google Scholar]
- Perumal AS, Smith TM, Suckow RF, Cooper TB. (1986) Effect of plasma from patients containing bupropion and its metabolites on the uptake of norepinephrine. Neuropharmacology 25:199–202. [DOI] [PubMed] [Google Scholar]
- Petsalo A, Turpeinen M, Tolonen A. (2007) Identification of bupropion urinary metabolites by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 21:2547–2554. [DOI] [PubMed] [Google Scholar]
- Posner J, Bye A, Dean K, Peck AW, Whiteman PD. (1985) The disposition of bupropion and its metabolites in healthy male volunteers after single and multiple doses. Eur J Clin Pharmacol 29:97–103. [DOI] [PubMed] [Google Scholar]
- Reese MJ, Wurm RM, Muir KT, Generaux GT, St John-Williams L, McConn DJ. (2008) An in vitro mechanistic study to elucidate the desipramine/bupropion clinical drug-drug interaction. Drug Metab Dispos 36:1198–1201. [DOI] [PubMed] [Google Scholar]
- Schroeder DH. (1983) Metabolism and kinetics of bupropion. J Clin Psychiatry 44:79–81. [PubMed] [Google Scholar]
- Silverstone PH, Williams R, McMahon L, Fleming R, Fogarty S. (2008) Convulsive liability of bupropion hydrochloride metabolites in Swiss albino mice. Ann Gen Psychiatry 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarydova L, Tomanova R, Havlikova L, Stambergova H, Solich P, Wsol V. (2014) Deeper insight into the reducing biotransformation of bupropion in the human liver. Drug Metab Pharmacokinet 29:177–184. [DOI] [PubMed] [Google Scholar]
- Soroko FE, Mehta NB, Maxwell RA, Ferris RM, Schroeder DH. (1977) Bupropion hydrochloride ((+/-) alpha-t-butylamino-3-chloropropiophenone HCl): a novel antidepressant agent. J Pharm Pharmacol 29:767–770. [DOI] [PubMed] [Google Scholar]
- Suckow RF, Zhang MF, Cooper TB. (1997) Enantiomeric determination of the phenylmorpholinol metabolite of bupropion in human plasma using coupled achiral-chiral liquid chromatography. Biomed Chromatogr 11:174–179. [DOI] [PubMed] [Google Scholar]
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, VanMeter S, Harriett AE, Wang Y. (2005) Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry 66:974–981. [DOI] [PubMed] [Google Scholar]
- Welch RM, Lai AA, Schroeder DH. (1987) Pharmacological significance of the species differences in bupropion metabolism. Xenobiotica 17:287–298. [DOI] [PubMed] [Google Scholar]
- Xu H, Loboz KK, Gross AS, McLachlan AJ. (2007) Stereoselective analysis of hydroxybupropion and application to drug interaction studies. Chirality 19:163–170. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ, Yanovski JA. (2015) Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA 313:1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Cox LS, Nollen N, Faseru B, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. (2012) CYP2B6 and bupropion’s smoking-cessation pharmacology: the role of hydroxybupropion. Clin Pharmacol Ther 92:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]