Abstract
The 5-hydroxytryptamine-3 (5-HT3) receptor antagonists such as ondansetron have been used to prevent and treat nausea and vomiting for over 2 decades. This study was to determine whether ondansetron could serve as a perpetrator drug causing transporter-mediated drug-drug interactions in humans. Twelve unrelated male healthy Chinese volunteers were enrolled into a prospective, randomized, double-blind, crossover study to investigate the effects of ondansetron or placebo on the pharmacokinetics of and the response to metformin, a well-characterized substrate of organic cation transporters and multidrug and toxin extrusions (MATEs). Ondansetron treatment caused a statistically significantly higher Cmax of metformin compared with placebo (18.3 ± 5.05 versus 15.2 ± 3.23; P = 0.006) and apparently decreased the renal clearance of metformin by 37% as compared with placebo (P = 0.001). Interestingly, ondansetron treatment also statistically significantly improved glucose tolerance in subjects, as indicated by the smaller glucose area under the curve in the oral glucose tolerance test (10.4 ± 1.43) as compared with placebo (11.5 ± 2.29 mmol∙mg/l) (P = 0.020). It remains possible that ondansetron itself may affect glucose homeostasis in human subjects, but our clinical study, coupled with our previous findings in cells and in animal models, indicates that ondansetron can cause a drug-drug interaction via its potent inhibition of MATE transporters in humans.
Introduction
The properties of pharmacokinetics are crucial determinants of drug response. For a long time the study of pharmacokinetics has been largely focused on drug metabolizing enzymes (van Schaik, 2008; Hirota et al., 2013; Samer et al., 2013; Zanger and Schwab, 2013), but increasing evidence has clearly suggested the importance of membrane transporters in pharmacokinetics (Lu et al., 2010; Hua et al., 2012; Barton et al., 2013). For example, disposition of certain cationic drugs may be determined by their uptake via organic cation transporters (OCTs) from circulation to hepatocytes and/or renal tubular cells. More recently, the multidrug and toxin extrusion (MATE, SLC47A) proteins have been characterized as H+/organic cation antiporters mediating the excretion of cationic compounds into urine (Otsuka et al., 2005). OCTs and MATEs share a significant number of substrates and inhibitors (Nies et al., 2011). They work together to transcellularly translocate cationic drugs and serve as an essential system for drug renal elimination (Ayrton and Morgan, 2008; Wang et al., 2008; Matsushima et al., 2009).
Specifically, human OCT2 (hOCT2/SLC22A2) is highly expressed in the basolateral membrane of proximal tubules and mediates the uptake of cationic drugs into the kidney (Filipski et al., 2008), and hMATE1 (SLC47A1) and hMATE2-K (SLC47A2) are major transporters in the apical membrane (Masuda et al., 2006) that are responsible for the elimination of certain drugs from renal tubular cells into urine. The coordinated expression and activities of OCT2 and MATEs may be significant determinants of drug disposition, drug-drug interactions (DDIs), and drug response variability.
Our recent data have indicated that ondansetron, a 5-hydroxytryptamine-3 (5-HT3) receptor antagonist for the treatment of nausea and vomiting, as induced by chemotherapy, has a much more potent inhibition of MATEs than OCT2, based on the respective inhibitory constant (Ki) (MATE1, 0.035 µM; MATE2-K, 0.015 µM; OCT2, 3.85 µM; and OCT1 >100 µM), and it increases the systemic exposure and renal accumulation of metformin, a cationic probe drug, primarily via its inhibition on Mate1 in mice (Li et al., 2013). Furthermore, the inhibition of ondansetron on Mate1 could enhance mouse nephrotoxicity induced by cisplatin, a substrate of OCT2 and MATEs (Pimenov et al., 1990; Iwata et al., 2012; Yonezawa, 2012). These data suggest that inhibition of MATEs (human MATE1 and MATE2-K), but not OCT2, could serve as a rate-limiting mechanism underlying DDIs occurring in the kidney.
However, species differences in pharmacokinetics sometimes make extrapolating data from preclinical studies in animal models to human subjects difficult (Dresser et al., 2000). It has been reported, for example, that there are striking species differences in the interaction of erlotinib, ninotinib, and imatinib with mouse orthologs of Oct1, Oct3, and Mate1 in comparison with the respective human orthologs (Minematsu and Giacomini, 2011). It is thus necessary to conduct clinical studies to confirm whether ondansetron, the potent MATE inhibitor, can serve as a perpetrator drug causing transporter-mediated DDI in human subjects.
This study was designed to determine whether ondansetron affected the pharmacokinetics of and response to metformin that is not subject to metabolism but rather to a well-characterized substrate of human OCTs and MATEs. We report here, for the first time, that administration of ondansetron significantly altered metformin disposition and possibly its glucose-lowering effect in healthy Chinese volunteers.
Materials and Methods
Participants.
The clinical study protocol was approved by the research ethics committee of the Central South University, People’s Republic of China. A written and informed consent in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) were provided to all participants. Twelve unrelated male healthy volunteers from the mainland of the People’s Republic of China were enrolled into this prospective randomized, double-blind, crossover clinical study. The healthy status of participants was assessed via medical history, physical examination, and routine clinical laboratory tests. The men’s mean age was 22.1 ± 2.0 years old and mean body mass index was 22.4 ± 4.1 kg/m2. They were nonsmokers and had not taken any prescription drugs, traditional Chinese medicines, or herbal supplements for at least 2 weeks before our study. They also had no history of substance abuse or dependence.
Clinical Study Design.
The participants were randomly assigned to one of the two treatment groups. In group A, the men received oral doses of 8 mg of ondansetron (Qilu Pharmaceutical, Shandong, People’s Republic of China) once daily for 5 days. On day 6, after fasting overnight, a last oral dose of 8 mg of ondansetron was administered at 7 AM. A dose of 850 mg of metformin was administrated with 150 ml of water at 8 AM. Each man then drank 100 ml of water containing 75 g of glucose at 9 AM. Blood samples (5 ml) were taken before and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16, and 24 hours after the metformin intake. Standardized meals were provided 5 hours after the administration of metformin. All participants were asked to drink 250 ml of water every 4 hours to maintain urine flow and pH after the metformin dose.
Urine samples were collected during the following intervals: 0 to 2, 2 to 4, 4 to 8, 8 to 12, and 12 to 24 hours after metformin administration. The volume and pH of urine were measured for each interval, and 20 ml of the urine sample was kept at −20°C for analysis of metformin content. The plasma samples were centrifuged and separated in 30 minutes and were immediately stored at −80°C. From days 16 to 20, the men received placebo once daily for 5 days. On day 21, a last placebo treatment was administered at 7 AM; 850 mg of metformin was then administered, and the blood and urine samples were collected as previously described for days 6 to 9.
In group B, the men were treated with placebo first and later with ondansetron; the other procedures were the same as conducted for group A. All participants were randomized to either group A or group B.
Analytic Assays Determining Drug Concentrations.
Plasma and urinary concentrations of metformin were determined by a highly specific and sensitive method of high-pressure liquid chromatography with tandem mass spectrometry (Agilent 1260 HPLC/Agilent 6120 quadrupole MS; Agilent, Palo Alto, CA). The determination of plasma ondansetron concentration was performed on a Waters 2695 MICROMASS Quattro Micro API tandem quadrupole system (Waters Corporation, Milford, MA). The details of the methods are described in the (Supplemental Data).
Analysis of Blood Chemistries.
Venous blood samples were obtained before the last dose of ondansetron or placebo at days 6 and 21. The indicators of hepatic and renal functions, including creatinine, blood urea nitrogen, alanine aminotransferase, aspartate aminotransferase, and albumin, were measured by an automatic biochemistry analyzer (7600-020; Hitachi, Tokyo, Japan) in the First Affiliated Xiang-Ya Hospital, Central South University, People Republic of China.
Pharmacokinetic Analysis.
A noncompartmental method was applied to analyze the plasma drug concentrations (metformin and ondansetron), using WinNonlin (version 4; Pharsight Corporation, Mountain View, CA). In brief, the maximum plasma concentration (Cmax) and time to the maximum plasma concentration (Tmax) were directly calculated from the plasma concentration–time profile. All other parameters, including the elimination rate constant (ke), elimination half-life (T1/2), area under the concentration–time curve from 0 to 2 hours (AUC0–2 h) and the extrapolation to infinity (AUC0–∞), renal clearance (CLR), apparent oral clearance (CLoral), apparent oral volume of distribution (Voral), and the fraction of metformin excreted in the urine (fe,u 0–24 h), were calculated as described elsewhere (Shu et al., 2008).
Oral Glucose Tolerance Test (OGTT).
The response to metformin was assessed by a 2-hour oral glucose tolerant test (OGTT). In brief, 75 g of glucose was administrated to each man at 9 AM (0 hours), which was 1 hour after the intake of metformin at 8 AM on days 6 and 21. A series of blood samples were obtained from venous blood at 0, 0.5, 1, 1.5, and 2 hours for glucose measurement. Plasma was separated by centrifugation, and the plasma glucose level was analyzed by an automatic biochemistry analyzer in the First Affiliated Xiang-Ya Hospital of Central South University. The AUC of plasma glucose concentration–time plot was determined by linear trapezoidal method with extrapolation from 0 to 2 hours.
Statistical Analysis.
Statistical analyses were performed with SPSS (version 11.5 for Windows; SPSS Inc., Chicago, IL). Data are presented as the mean ± S.E. The test for normality was performed on all the pharmacokinetic parameters. Differences in the pharmacokinetic and OGTT parameters for the absence or presence of ondansetron were detected by univariate analysis of variance using individual code numbers, drugs (placebo or ondansetron), and groups (A or B) as between-subject factors. P < 0.05 was considered statistically significant.
Results
Effect of Ondansetron on Metformin Pharmacokinetics in Healthy Subjects.
We randomized healthy men into two groups for our two-phase crossover clinical study as described in Materials and Methods. An oral dose of 850 mg of metformin was administrated to each man after six doses of ondansetron or placebo treatment to determine the effect of ondansetron on metformin pharmacokinetics.
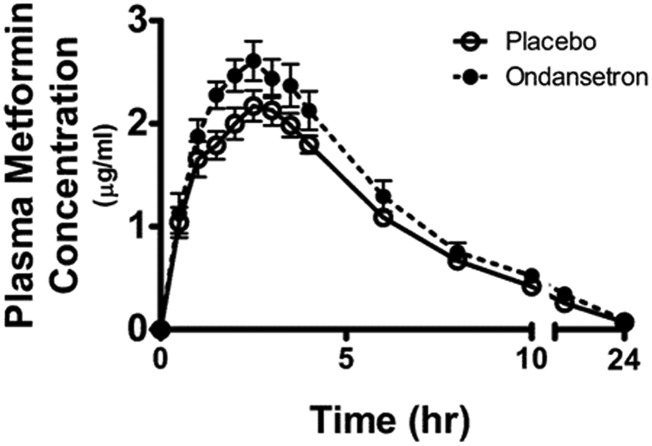
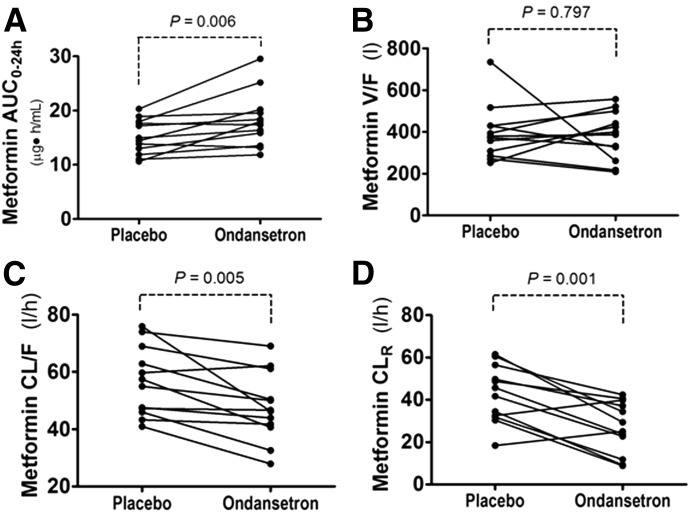
Overall, ondansetron treatment significantly affected the pharmacokinetics of metformin (Figs. 1 and 2; Table 1). The metformin concentration in plasma was higher in the subjects after ondansetron treatment than after placebo. The differences were statistically significant for plasma metformin levels at most time points (1, 1.5, 2.0, 2.5, 3.5, 4.0, 10, 12, and 24 hours) (P < 0.05, Fig. 1). Ondansetron treatment caused a statistically significantly higher Cmax compared with placebo (P = 0.014, Table 1). The AUC of metformin after ondansetron treatment was markedly greater than after placebo treatment (P = 0.006 for AUC0–24 h; P = 0.004 for AUC0–∞; Fig. 2A; Table 1). As expected, ondansetron administration led to a statistically significantly decreased apparent oral clearance (CL/F, 15.7% decrease, P = 0.005; Fig. 2C; Table 1) when compared with placebo treatment. The difference in apparent oral clearance between the placebo and ondansetron treatment was mainly due to an increased AUC0–24 h of metformin by ondansetron because the same men received the same dose of metformin with placebo or ondansetron in the crossover study (CL/F = dose/AUC0–24 h/weight).
Fig. 1.
The plasma concentration-time curves of metformin after oral administration in healthy men (n = 12) who received either ondansetron or placebo treatment. Ondansetron (8 mg) or placebo was administrated at 8 PM daily for 5 days, and the last dose was taken at 7 AM on the sixth day. Metformin (850 mg) was then administered at 8 AM. Blood samples for the pharmacokinetic analysis were drawn up to 24 hours after metformin administration. Data represent the mean ± S.E.
Fig. 2.
The effect of ondansetron on the pharmacokinetic parameters of oral metformin in healthy men. (A) AUC. (B) V/F (oral volume of distribution; volume of distribution divided by oral bioavailability). (C) CL/F (oral clearance; clearance divided by oral bioavailability). (D) CLR (renal clearance). Statistical difference between the two treatments is indicated by the P values as shown.
TABLE 1.
Metformin pharmacokinetic parameters from healthy individuals who were administered placebo or ondansetron
P < 0.05 was considered statistically significant.
| Parameters | Ondansetron |
P value | |
|---|---|---|---|
| Absence | Presence | ||
| AUC0–24 h (µg⋅h/ml) | 15.2 ± 3.23 | 18.3 ± 5.05 | 0.006 |
| AUC0–∞ (µg⋅h/ml) | 15.7 ± 3.25 | 19.0 ± 5.20 | 0.004 |
| Cmax (µg/ml) | 2.28 ± 0.52 | 2.75 ± 0.64 | 0.014 |
| Tmax (h) | 2.63 ± 0.64 | 2.25 ± 0.79 | 0.158 |
| T1/2 (h) | 4.8 ± 0.9 | 5.5 ± 0.9 | 0.124 |
| V/F (l) | 394 ± 132 | 382 ± 115 | 0.797 |
| CL/F (l/h) | 56.6 ± 12.0 | 47.7 ± 12.0 | 0.005 |
| CLR (l/h) | 42.3 ± 12.9 | 27.2 ± 11.7 | 0.001 |
| fe.u 0–24 h (%) | 75.0 ± 15.9 | 57.8 ± 24.6 | 0.014 |
CL/F, oral clearance (clearance over oral bioavailability); V/F, oral volume of distribution (volume of distribution divided by oral bioavailability).
The effect on metformin clearance by ondansetron was even more apparent when the renal clearance (CLR) was calculated (CLR = Cu × Vu/Cp; where Cu and Cp, are metformin concentration in urine or plasma, respectively, and Vu is the urine volume in the given unit of time). As compared with placebo treatment, ondansetron treatment caused a 37% decrease of CLR (P = 0.001; Fig. 2D; Table 1). Consistently, the individuals excreted less metformin in the urine and had higher plasma concentrations when they received ondansetron treatment than the placebo treatment.
The fraction of metformin eliminated into the urine (fe,u 0–24 h) was less after taking ondansetron compared with placebo (17.2% less; P = 0.014; Table 1). Metformin has been reported to be not metabolized in the liver, so only a small fraction is excreted into the bile (Ito et al., 2010; Shingaki et al., 2015). Consistently, the effect of ondansetron treatment on metformin clearance was mainly explained by its effect on renal clearance (Table 1). In comparing the ondansetron and placebo treatments, we found no differences in the oral volume of distribution (V/F; P = 0.797; Fig. 2B; Table 1) or the Tmax; other pharmacokinetic differences also were not found to be insignificant. Our results with humans are consistent with our previous findings in mice that ondansetron alters the pharmacokinetics of metformin (Li et al., 2013).
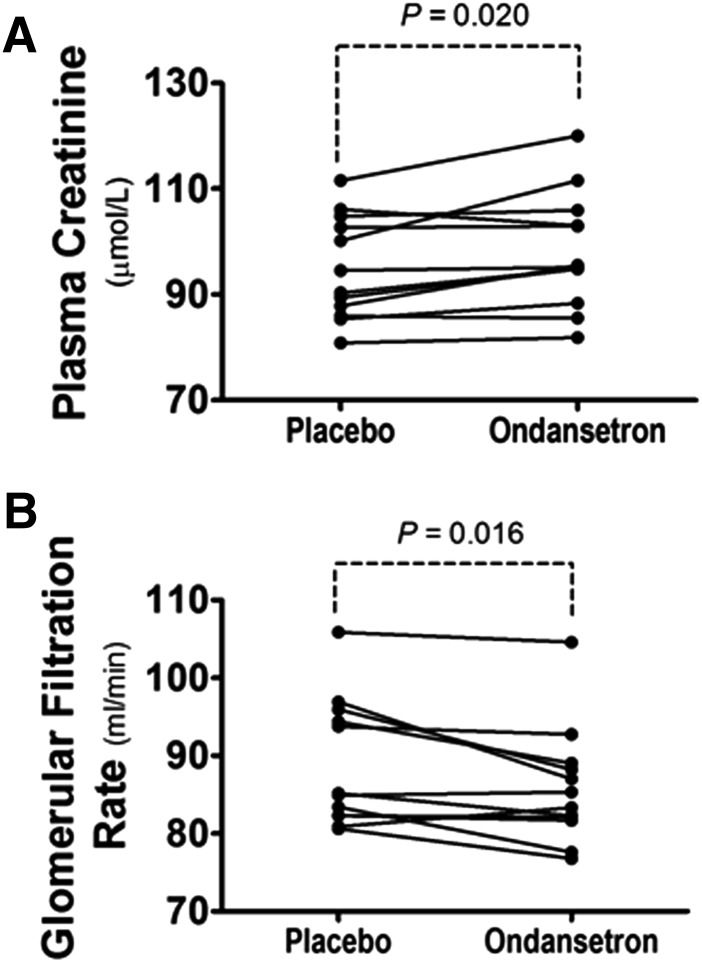
To collect further evidence of the effect of ondansetron treatment on MATE activities, we also investigated whether ondansetron treatment can alter the plasma level of creatinine, an endogenous substrate of MATE transporters that has been described elsewhere (Tanihara et al., 2009). We found that ondansetron treatment statistically increased the plasma level of creatinine in the healthy subjects as compared with placebo (98.3 ± 11.0 versus 95.0 ± 9.84 mmol/l; P = 0.020; Fig. 3A). The estimated glomerular filtration rate was calculated by the Cockcroft-Gault equation, Ccr = [(140 − Age) × Weight (kg)]/[0.818 × Scr (µmol/l)], where Ccr is the creatinine clearance and Scr is the serum creatinine. Consistently, ondansetron treatment caused a reduction in the apparent glomerular filtration rate in healthy men (P = 0.016; Fig. 3B).
Fig. 3.
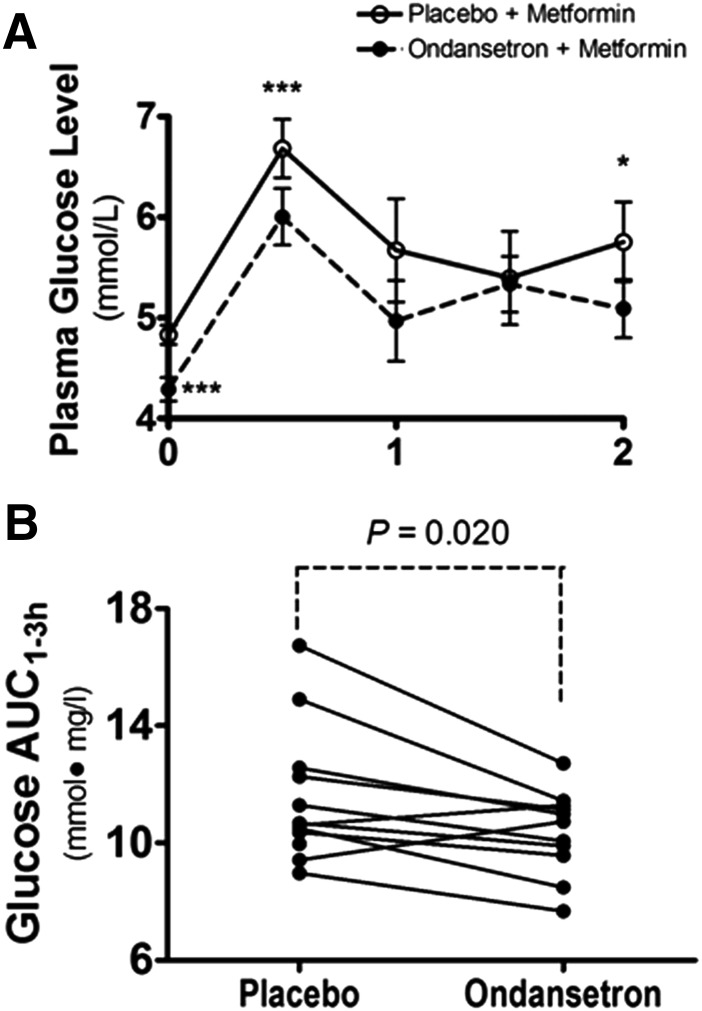
The effect of ondansetron on response to oral metformin in healthy men. (A) The 2-hour time course of plasma glucose concentrations for OGTT after metformin treatment in healthy men who received ondansetron or placebo treatment. Ondansetron (8 mg) or placebo was administrated at 8 PM daily for 5 days, and the last dose was taken at 7 AM in the sixth day. Metformin (850 mg) was then administered at 8 AM, and 75 g of glucose was given at 9 AM for a 2-hour OGTT. The data are expressed as mean ± S.E.M. *P < 0.05, ***P < 0.001 significant difference between the two treatments. (B) The glucose exposure with OGTT (AUC) after metformin administration in healthy men after ondansetron or placebo treatment. Ondansetron significantly decreased the AUC of OGTT in the healthy men as compared with placebo. *P ≤ 0.05, statistically different between the two treatments.
Effect of Ondansetron Treatment on Metformin Response in Healthy Subjects.
Although we mainly sought to determine the effect of ondansetron treatment on the pharmacokinetics of metformin, we also wondered whether metformin response in our healthy subjects could be altered by ondansetron treatment. Metformin is mainly used to lower blood glucose levels in diabetic patients. The effect of metformin on glucose levels is not obvious in nondiabetic subjects (Hermann, 1979). However, this effect can be observed in healthy subjects challenged with an oral dose of glucose, which increases the plasma glucose levels in an OGTT (Gyr et al., 1971). We compared the effects on OGTT outcome between ondansetron and placebo treatment in our healthy volunteers who also received metformin treatment 1 hour before OGTT.
We observed that ondansetron treatment caused significantly lower plasma glucose levels during the 2-hour OGTT compared with placebo (Fig. 4A). Consistently, the AUC after OGTT was significantly smaller for ondansetron treatment as compared with placebo (10.4 ± 1.43 versus 11.5 ± 2.29 mmol∙mg/l; P = 0.020; Fig. 4B). These data suggest that ondansetron treatment, in addition to its effect on pharmacokinetics, may correspondingly alter the pharmacodynamics of metformin in humans. However, it should be noted that the effect of ondansetron treatment alone on glucose tolerance remains unclear, and the improved OGTT may partially, if not entirely, result from the synergistic pharmacodynamics of the two drugs.
Fig. 4.
The effect of ondansetron treatment on the plasma concentration of creatinine. (A) Glomerular filtration rate (GFR) (estimated from creatinine clearance, Ccr) in healthy men. (B) Statistical difference between ondansetron or placebo treatment is indicated by the P value as shown.
Plasma Concentrations of Ondansetron in Healthy Subjects.
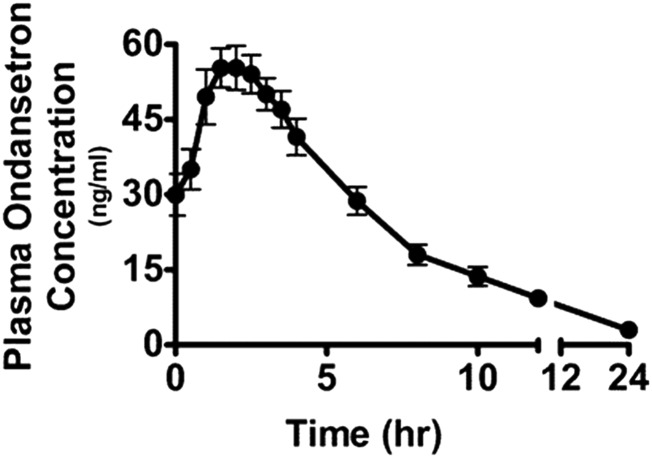
We analyzed the plasma concentrations of ondansetron in the men to further assess the interaction between ondansetron and metformin. After six doses of ondansetron as described in Materials and Methods, we determined the plasma concentrations of ondansetron before and after metformin treatment. The mean plasma concentration–time profile for all subjects is shown in Fig. 5, and we calculated the relevant pharmacokinetic parameters. The peak concentration of plasma ondansetron (55.3 ng/ml or 0.2 μmol/l) was observed to be higher than previously reported for a single dose (26.2 ng/ml, 0.09 μmol/l), and the Tmax was around 2 hours. Interestingly, we did not observe any correlation between the ondansetron concentrations and the increment of metformin or glucose AUC with ondansetron (data not shown).
Fig. 5.
The pharmacokinetic profile of ondansetron in healthy subjects. Healthy men (n = 12) received oral doses of 8 mg of ondansetron once daily for 5 days. On day 6, after the men had an overnight fast, a last oral dose of 8 mg ondansetron was administered at 7 AM. The plasma concentration-time curve of ondansetron is shown 1 hour after the last ondansetron dose (time 0) in the healthy men. Data represent mean ± S.E.
Discussion
The current study provides the first evidence to support that ondansetron has a significant effect on metformin pharmacokinetics and response in humans. Specifically, the pharmacokinetic properties of metformin, including AUC, Cmax, CLR, and fe,u 0–24 h, were significantly altered by ondansetron administration in healthy Chinese volunteers. Metformin is well characterized as a substrate of OCTs and MATEs, although ondansetron is an inhibitor of these transporters with a much more inhibitory potency for MATEs. In combination with our findings in cell and animal models (Li et al., 2013), our present study indicates that ondansetron can serve as a perpetrator drug in DDIs via its potent inhibition of MATEs.
The DDIs occurring in the liver, which have been studied for quite some time, are mainly attributed to alteration in the activities of drug-metabolizing enzymes and less to those of drug transporters. However, it has been recently recognized that the DDIs occurring in the kidney frequently involve the inhibition of transporters at the membrane of tubular cells (Morrissey et al., 2013). The transport system for organic cationic compounds in the tubular cells, consisting of the basolateral uptake transporters and the apical efflux transporters, is believed to be critical to the renal excretion of such compounds.
It has been long documented that inhibition of the uptake transporters such as OCT2 is the main mechanism underlying renal DDIs involving organic cations (Ciarimboli, 2011). However, recent findings concerning apical transporters and in vitro inhibition, put forward with the new concept about DDIs with cationic compounds, have challenged previous interpretations of the data (Koepsell et al., 2007; Nies et al., 2011). For example, cimetidine has been commonly employed in vitro to inhibit OCT activity and in vivo to study the renal secretion of organic cations in humans. However, Ito et al. (2012) demonstrated that cimetidine exhibits much stronger inhibition on MATEs than OCTs in vitro; further, they found that it increases the plasma creatinine level by decreasing the efflux of creatinine from the proximal tubules into the urine and not by preventing the creatinine uptake into the kidney. Thus, the inhibition of MATEs, but not OCTs, is a likely explanation for the DDIs with cimetidine in renal elimination (Ito et al., 2012).
Previously, we and others have found that ondansetron is an inhibitor of OCT1 (Tzvetkov et al., 2012), OCT2, MATE1, and MATE2-K in cell cultures (Kido et al., 2011; Li et al., 2013). However, ondansetron has a larger inhibitory potency against MATEs as compared with OCTs (Tzvetkov et al., 2012; Li et al., 2013). It has been reported that the peak plasma concentrations of ondansetron in patients range from 0.10 to 0.42 µM from the dose of 8 mg to 32 mg daily (Stout et al., 2010). A Ki value of at least more than one-tenth of Cmax has been considered for the drug to cause a clinically relevant interaction with another drug that is a substrate of the same transporter (Fenner et al., 2009).
In our present clinical study, we analyzed the pharmacokinetics of metformin with and without ondansetron treatment. As expected, ondansetron significantly enhanced the plasma concentrations of metformin. The clearance of metformin was also significantly reduced by ondansetron treatment. In particular, ondansetron treatment decreased the excretion of metformin from the kidney into the urine, as indicated by a lower fe,u 0–24 h. As ondansetron did not inhibit OCT2 very well at the clinical plasma concentrations achieved in this study (Cmax 0.2 µM), estimated from the inhibitory constant (Ki) by ondansetron (see the Introduction), it might not affect the uptake of metformin into the kidney. Approximately 75% of ondansetron is bound to plasma proteins. Even the concentrations of unbound ondansetron were sufficient to inhibit the apical MATEs and may thus reduce the secretion of metformin from the kidney into the urine, causing the increased accumulation of metformin in the kidney and consequently the enhanced plasma metformin levels.
The renal OCT system has endogenous substrates in addition to xenobiotic ones. Creatinine, a breakdown product of creatinine phosphate in the muscle, has been reported to be a weak substrate of OCT2 and MATEs (Urakami et al., 2004; Tanihara et al., 2009). Creatinine uptake is stimulated by the expression of hOCT2, rOCT1, rOCT2, hMATE1, hMATE2-K, and rMATE1 in human embryonic kidney 293 cells. Nakamura et al. (2010) have reported that in Mate1 knockout mice the blood level of creatinine is significantly elevated as compared with wild-type mice (Nakamura et al., 2010). In our present study, ondansetron significantly increased the plasma level of creatinine and accordingly decreased the creatinine clearance in healthy Chinese men, which is very likely due to the inhibition of MATE transporters by ondansetron. Ondansetron administration thus likely results in DDIs such as that with metformin and endogenous substrates in renal elimination via potent inhibition of MATEs.
The 5-HT3 receptor antagonists such as ondansetron have long been used clinically for the prevention and treatment of nausea and vomiting, particularly in patients receiving highly emetogenic chemotherapeutic regimens (Gustavson et al., 2003). Metformin, a biguanide, is widely used for the treatment of hyperglycemia in patients with type 2 diabetes mellitus. The drug is also taken by women with gestational diabetes who may experience nausea and vomiting (Siu et al., 2002). Moreover, increased understanding of the molecular mechanisms of metformin action has led to clinical trials of the drug for the prevention and treatment of various cancers (Beck and Scheen, 2013; Malek et al., 2013; Leone et al., 2014). Therefore, it is clinically possible that a 5-HT3 antagonist and metformin may be prescribed to a patient together. We have found that 5-HT3 antagonists as a drug class were potent MATE inhibitors in cellular studies (data not published). In our present study, the 5-HT3 antagonist ondansetron caused increased exposure and decreased clearance of metformin in human subjects. Consistently, we observed an enhanced response to glucose challenge by the combination treatment of ondansetron and metformin as compared with that of placebo and metformin.
One previous study showed that ondansetron could significantly suppress rimonabant-induced increases in blood glucose, particularly at 40 minutes and 120 minutes in OGTT in mice (Mandhane et al., 2012). However, it is not clear whether ondansetron alone can affect the plasma glucose levels in Mandhane et al's (2012) study. Another study showed that pretreatment with ondansetron was able to reduce the hyperglycemic response induced by central administration of quipazine; however, ondansetron injection alone into the third ventricle had no effect on plasma glucose concentrations in rodents (Carvalho et al., 2005). There have been no reports showing any effect of ondansetron on blood glucose levels in humans.
Although it may seem unlikely, we believe it is still possible that ondansetron alone may improve glucose tolerance in humans. The mechanism underlying the pharmacodynamic interaction between ondansetron and metformin remains to be elucidated. The effects of ondansetron on the pharmacokinetics and possibly pharmacodynamics of metformin should be taken into account when the two drugs are prescribed together. More importantly, considering the wide clinical application of the antiemetic ondansetron and other structurally similar 5-HT3 receptor antagonists, including tropiseton, palonasetron, alosetron, granisetron, azasetron, and dolasetron, more clinical research is needed to determine how important and extensive the DDIs mediated by MATE transporters are.
As an example, the coadministration of 5-HT3 receptor antagonists such as ondansetron and platinum compounds such as cisplatin is clinically common. Cisplatin, a substrate of OCT2 and MATEs (Ciarimboli, 2012), has been widely used to treat malignant solid tumors. However, severe side effects, particularly nephrotoxicity, hinder the clinical application of cisplatin in higher doses (Arany and Safirstein, 2003; Sastry and Kellie, 2005). Ondansetron can be used to prevent and treat cisplatin-induced nausea and vomiting. In our previous animal study, however, the treatment with ondansetron significantly enhanced the accumulation of cisplatin in the kidney; via potent inhibition of MATE function, ondansetron further deteriorated the renal damage induced by cisplatin. Our current findings suggest that ondansetron may significantly inhibit the activity of MATEs in vivo in humans as well. It is therefore likely that the risk of nephrotoxicity in human patients could be heightened when cisplatin is coadministrated with the antiemetic ondansetron. Further clinical assessment of the effect by ondansteron on the renal safety profile of cisplatin is warranted.
In conclusion, in healthy men, the pharmacokinetics of metformin are significantly affected by ondansetron treatment, likely via potent inhibition of renal MATE function. Ondansetron treatment consistently enhances the glucose-lowering effect by metformin. The inhibition of transporters such as MATEs by ondansetron for cationic compounds, in particular those primarily eliminated from the kidney, is likely to be clinically significant and warrants further study.
Supplementary Material
Abbreviations
- AUC
area under the concentration–time curve
- AUC0–2 h
AUC from 0 to 2 hours
- AUC0–∞
AUC extrapolated from 0 to infinity
- CLoral
apparent oral clearance
- CLR
renal clearance
- DDI
drug-drug interaction
- fe,u 0–24 h
fraction excreted in the urine over 24 hours
- 5-HT3
5-hydroxytryptamine-3
- Ki
inhibitory constant
- MATE
multidrug and toxin extrusion
- OCT
organic cation transporter
- OGTT
oral glucose tolerance test
- Tmax
time to the maximum plasma concentration
- Voral
apparent oral volume of distribution
Authorship Contributions
Participated in research design: Shu, Polli, Zhou, Li.
Conducted experiments: Yang, Guo, Zhang.
Performed data analysis: Li, Yang, Guo, Shu.
Wrote or contributed to the writing of the manuscript: Li, Shu.
Footnotes
This work was support in part by the National Natural Science Foundation of the People’s Republic of China [Grants 81373477 and 81570533] (to Q.L.), the National Institutes of Health National Institute of General Medical Sciences [Grant R01GM099742], and the U.S. Food and Drug Administration [Grant U01FD004320] (to Y.S.).
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Arany I, Safirstein RL. (2003) Cisplatin nephrotoxicity. Semin Nephrol 23:460–464. [DOI] [PubMed] [Google Scholar]
- Ayrton A, Morgan P. (2008) Role of transport proteins in drug discovery and development: a pharmaceutical perspective. Xenobiotica 38:676–708. [DOI] [PubMed] [Google Scholar]
- Barton HA, Lai Y, Goosen TC, Jones HM, El-Kattan AF, Gosset JR, Lin J, Varma MV. (2013) Model-based approaches to predict drug-drug interactions associated with hepatic uptake transporters: preclinical, clinical and beyond. Expert Opin Drug Metab Toxicol 9:459–472. [DOI] [PubMed] [Google Scholar]
- Beck E, Scheen AJ. (2013) [Metformin, an antidiabetic molecule with anti-cancer properties]. Rev Med Liege 68:444–449. [PubMed] [Google Scholar]
- Carvalho F, Barros D, Silva J, Rezende E, Soares M, Fregoneze J, de Castro-E-Silva E. (2005) Hyperglycemia induced by pharmacological activation of central serotonergic pathways depends on the functional integrity of brain CRH system and 5-HT3 receptors. Horm Metab Res 37:482–488. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G. (2011) Role of organic cation transporters in drug-induced toxicity. Expert Opin Drug Metab Toxicol 7:159–174. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G. (2012) Membrane transporters as mediators of cisplatin effects and side effects. Scientifica (Cairo) 2012:473829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser MJ, Gray AT, Giacomini KM. (2000) Kinetic and selectivity differences between rodent, rabbit, and human organic cation transporters (OCT1). J Pharmacol Exp Ther 292:1146–1152. [PubMed] [Google Scholar]
- Fenner KS, Troutman MD, Kempshall S, Cook JA, Ware JA, Smith DA, Lee CA. (2009) Drug-drug interactions mediated through P-glycoprotein: clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug. Clin Pharmacol Ther 85:173–181. [DOI] [PubMed] [Google Scholar]
- Filipski KK, Loos WJ, Verweij J, Sparreboom A. (2008) Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res 14:3875–3880. [DOI] [PubMed] [Google Scholar]
- Gustavson SM, Chu CA, Nishizawa M, Farmer B, Neal D, Yang Y, Vaughan S, Donahue EP, Flakoll P, Cherrington AD. (2003) Glucagon’s actions are modified by the combination of epinephrine and gluconeogenic precursor infusion. Am J Physiol Endocrinol Metab 285:E534–E544. [DOI] [PubMed] [Google Scholar]
- Gyr N, Berger W, Fridrich R, Denes A, Stalder GA. (1971) [Effect of dimethylbiguanide on stomach emptying and on oral glucose tolerance]. Schweiz Med Wochenschr 101:1876–1879. [PubMed] [Google Scholar]
- Hermann LS. (1979) Metformin: a review of its pharmacological properties and therapeutic use. Diabete Metab 5:233–245. [PubMed] [Google Scholar]
- Hirota T, Eguchi S, Ieiri I. (2013) Impact of genetic polymorphisms in CYP2C9 and CYP2C19 on the pharmacokinetics of clinically used drugs. Drug Metab Pharmacokinet 28:28–37. [DOI] [PubMed] [Google Scholar]
- Hua WJ, Hua WX, Fang HJ. (2012) The role of OATP1B1 and BCRP in pharmacokinetics and DDI of novel statins. Cardiovasc Ther 30:e234–e241. [DOI] [PubMed] [Google Scholar]
- Ito S, Kusuhara H, Kuroiwa Y, Wu C, Moriyama Y, Inoue K, Kondo T, Yuasa H, Nakayama H, Horita S, et al. (2010) Potent and specific inhibition of mMate1-mediated efflux of type I organic cations in the liver and kidney by pyrimethamine. J Pharmacol Exp Ther 333:341–350. [DOI] [PubMed] [Google Scholar]
- Ito S, Kusuhara H, Yokochi M, Toyoshima J, Inoue K, Yuasa H, Sugiyama Y. (2012) Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther 340:393–403. [DOI] [PubMed] [Google Scholar]
- Iwata K, Aizawa K, Kamitsu S, Jingami S, Fukunaga E, Yoshida M, Yoshimura M, Hamada A, Saito H. (2012) Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin Exp Nephrol 16:843–851. [DOI] [PubMed] [Google Scholar]
- Kido Y, Matsson P, Giacomini KM. (2011) Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem 54:4548–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. (2007) Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24:1227–1251. [DOI] [PubMed] [Google Scholar]
- Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. (2014) New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res 159:355–376. [DOI] [PubMed] [Google Scholar]
- Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, Polli JE, Shu Y. (2013) Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol 273:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Liao M, Cohen L, Xia CQ. (2010) Emerging in vitro tools to evaluate cytochrome P450 and transporter-mediated drug-drug interactions. Curr Drug Discov Technol 7:199–222. [DOI] [PubMed] [Google Scholar]
- Malek M, Aghili R, Emami Z, Khamseh ME. (2013) Risk of cancer in diabetes: the effect of metformin. ISRN Endocrinol 2013:636927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandhane S, Nayak P, Soni D, Jain S, Ashton JC, Rajamannar T. (2012) Induction of glucose intolerance by acute administration of rimonabant. Pharmacology 89:339–347. [DOI] [PubMed] [Google Scholar]
- Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, Ogawa O, Inui K. (2006) Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol 17:2127–2135. [DOI] [PubMed] [Google Scholar]
- Matsushima S, Maeda K, Inoue K, Ohta KY, Yuasa H, Kondo T, Nakayama H, Horita S, Kusuhara H, Sugiyama Y. (2009) The inhibition of human multidrug and toxin extrusion 1 is involved in the drug-drug interaction caused by cimetidine. Drug Metab Dispos 37:555–559. [DOI] [PubMed] [Google Scholar]
- Minematsu T, Giacomini KM. (2011) Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol Cancer Ther 10:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. (2013) Renal transporters in drug development. Annu Rev Pharmacol Toxicol 53:503–529. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K. (2010) Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol 80(11):1762–1767. [DOI] [PubMed] [Google Scholar]
- Nies AT, Koepsell H, Damme K, Schwab M. (2011) Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol 201:105–167. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. (2005) A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci USA 102:17923–17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenov AA, Rakhmilevich AL, Deev VV, Kirillova IV, Migdal TL, Andronova TM, Fuks BB. (1990) [Activation of cellular immunity in mice under normal conditions and in tumor growth during treatment with glucosaminyl muramyl dipeptide]. Vopr Med Khim 36:58–60. [PubMed] [Google Scholar]
- Samer CF, Lorenzini KI, Rollason V, Daali Y, Desmeules JA. (2013) Applications of CYP450 testing in the clinical setting. Mol Diagn Ther 17:165–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry J, Kellie SJ. (2005) Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr Hematol Oncol 22:441–445. [DOI] [PubMed] [Google Scholar]
- Shingaki T, Hume WE, Takashima T, Katayama Y, Okauchi T, Hayashinaka E, Wada Y, Cui Y, Kusuhara H, Sugiyama Y, Watanabe Y. (2015) Quantitative evaluation of mMate1 function based on minimally invasive measurement of tissue concentration using PET with [(11)C]metformin in mouse. Pharm Res 32:2538–2547. [DOI] [PubMed] [Google Scholar]
- Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, Sheardown SA, Yue L, Burchard EG, Brett CM, et al. (2008) Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 83:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu SS, Yip SK, Cheung CW, Lau TK. (2002) Treatment of intractable hyperemesis gravidarum by ondansetron. Eur J Obstet Gynecol Reprod Biol 105:73–74. [DOI] [PubMed] [Google Scholar]
- Stout PR, Bynum ND, Lewallen CM, Mitchell JM, Baylor MR, Ropero-Miller JD. (2010) A comparison of the validity of gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry analysis of urine samples II: amphetamine, methamphetamine, (±)-3,4-methylenedioxyamphetamine, (±)-3,4-methylenedioxymethamphetamine, (±)-3,4-methylenedioxyethylamphetamine, phencyclidine, and (±)-11-nor-9-carboxy-Δ⁹-tetrahydrocannabinol. J Anal Toxicol 34:430–443. [DOI] [PubMed] [Google Scholar]
- Tanihara Y, Masuda S, Katsura T, Inui K. (2009) Protective effect of concomitant administration of imatinib on cisplatin-induced nephrotoxicity focusing on renal organic cation transporter OCT2. Biochem Pharmacol 78:1263–1271. [DOI] [PubMed] [Google Scholar]
- Tzvetkov MV, Saadatmand AR, Bokelmann K, Meineke I, Kaiser R, Brockmöller J. (2012) Effects of OCT1 polymorphisms on the cellular uptake, plasma concentrations and efficacy of the 5-HT(3) antagonists tropisetron and ondansetron. Pharmacogenomics J 12:22–29. [DOI] [PubMed] [Google Scholar]
- Urakami Y, Kimura N, Okuda M, Inui K. (2004) Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm Res 21:976–981. [DOI] [PubMed] [Google Scholar]
- van Schaik RH. (2008) CYP450 pharmacogenetics for personalizing cancer therapy. Drug Resist Updat 11:77–98. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Yin OQ, Tomlinson B, Chow MS. (2008) OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics 18:637–645. [DOI] [PubMed] [Google Scholar]
- Yonezawa A. (2012) [Platinum agent-induced nephrotoxicity via organic cation transport system]. Yakugaku Zasshi 132:1281–1285. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Schwab M. (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.