Abstract
Background
Recent meta-analyses of resting-state networks in major depressive disorder (MDD) implicate network disruptions underlying cognitive and affective features of illness. Heterogeneity of findings to date may stem from the relative lack of data parsing clinical features of MDD such as phase of illness and the burden of multiple episodes.
Method
Resting-state functional magnetic resonance imaging data were collected from 17 active MDD and 34 remitted MDD patients, and 26 healthy controls (HCs) across two sites. Participants were medication-free and further subdivided into those with single v. multiple episodes to examine disease burden. Seed-based connectivity using the posterior cingulate cortex (PCC) seed to probe the default mode network as well as the amygdala and subgenual anterior cingulate cortex (sgACC) seeds to probe the salience network (SN) were conducted.
Results
Young adults with remitted MDD demonstrated hyperconnectivity of the left PCC to the left inferior frontal gyrus and of the left sgACC to the right ventromedial prefrontal cortex (PFC) and left hippocampus compared with HCs. Episode-independent effects were observed between the left PCC and the right dorsolateral PFC, as well as between the left amygdala and right insula and caudate, whereas the burden of multiple episodes was associated with hypoconnectivity of the left PCC to multiple cognitive control regions as well as hypoconnectivity of the amygdala to large portions of the SN.
Conclusions
This is the first study of a homogeneous sample of unmedicated young adults with a history of adolescent-onset MDD illustrating brain-based episodic features of illness.
Keywords: Amygdala, connectivity, depression, functional magnetic resonance imaging, illness course
Introduction
Emerging research has documented network abnormalities present during the resting state related to major depressive disorder (MDD). However, understanding the relevance and degree to which these network abnormalities relate to clinical features of illness and course of illness has only begun (Kerestes et al. 2012; Dichter et al. 2015). In particular, it is unclear whether relative hypo- and hyperconnectivity patterns within and between key networks are stable and trait-like or whether they stem directly from the acute disturbance of illness. Episodic, compensatory and burden features are likely to contribute to within-and between-study variability, obscuring key breakthroughs in understanding mechanisms of illness and prohibiting development of targeted treatments for MDD (Weisenbach et al. 2014). As studies of currently active illness dominate the literature, meta-analytic studies are likely to miss nuances discriminating episodic features of MDD (Pizzagalli, 2011).
One approach to begin to examine the distinctions inherent in a multiply determined, multiply defined illness such as MDD is to examine individuals as they pass through phases of illness including the acute disturbance of a depressive episode as well as remission and relapse. Examining these phasic patterns is also clinically relevant given an adequate understanding of risk for relapse in the remitted phase of MDD could help reduce public health burden via secondary prevention. The risk of repeated episodes increases as a function of previous episodes (Keller et al. 2007) and 70% of individuals in remission are at risk for future episodes. Adequate maintenance and novel interventions focused on secondary prevention have not been adequately explored to date.
Abnormalities in resting-state network connectivity have been consistently reported in MDD (Sundermann et al. 2014; Kaiser et al. 2015) and hyper-connectivity within the default mode network (DMN) may be the most commonly identified network abnormality in MDD (e.g. Sheline et al. 2009; Hamilton et al. 2015). The DMN was originally observed in the context of task-based studies to describe ‘task-negative’ regions that decrease in activation during performance of attention-demanding tasks and increase in activation during rest, mind-wandering or self-reflective thought (for a review, see Whitfield-Gabrieli & Ford, 2012). In contrast, a task-positive network includes regions that increase in activation during attention to demanding tasks (Fox et al. 2005). Task-positive and task-negative networks appear to act in opposition. For example, they have been shown to be anticorrelated during both cognitive tasks and during the resting state.
Two important, dissociable task-positive networks are the executive network (EN) and salience network (SN; Seeley et al. 2007). The SN supports emotion processing and autonomic regulation and incorporates regions such as the dorsal anterior cingulate cortex (ACC) and the orbital frontoinsula (Seeley et al. 2007). The SN overlaps with the affective network in regions such as the insula and regions of the SN are functionally connected with the cognitive control network (CCN) and DMN; thus, the SN is not strictly task-positive (Menon & Uddin, 2010). Dysfunction in the SN contributes to biases in emotion processing and autonomic regulation (Drevets et al. 2008; Briceño et al. 2013; for a review, see Price & Drevets, 2010). In contrast, the EN (also described as the CCN), modulates responses to stimuli that have already been identified as salient (Seeley et al. 2007; Menon, 2011). Taken together, aberrant network functioning may underlie and perpetuate observable clinical symptoms such as rumination (DMN), emotional dysregulation (CCN) and emotional reactivity (SN, e.g. Hamilton et al. 2012) exemplifying network models of psychopathology (Bressler & Menon, 2010; Menon, 2011).
Evidence that resting-state networks are disrupted in MDD has reinvigorated attempts to better parse clinical characteristics of illness as well as to understand putative markers of early treatment response and illness course. For example, a recent review of resting-state functional magnetic resonance imaging (fMRI) within the context of treatment response highlighted hyperconnectivity within the DMN and hypoconnectivity within the CCN in distinguishing individuals with treatment-resistant depression (de Kwaasteniet et al. 2015; Dichter et al. 2015). Treatment response, in contrast, appears to be associated with increased connectivity between frontal and limbic brain regions, possibly refiecting increased control over emotion processing and regulation (also see Crowther et al. 2015). An investigation of neural network markers of illness course and burden is both necessary and timely in advancing research on early identification, prospective clinical course prediction and novel treatment targets; however, limited work to date has exploited the opportunity to examine differences across phases of illness in unipolar depression. In bipolar illness, some evidence has begun to emerge as network connectivity captured during task-based fMRI has been used to demarcate manic from depressive episodes among individuals with bipolar disorder (e.g. Perlman et al. 2012). In sum, foundational knowledge of network disruptions may assist in the identification of novel treatment targets.
We previously compared young adults in the remitted phase of MDD (rMDD) with healthy controls (HCs) and found hyperconnectivity of the DMN, as indexed by the posterior cingulate cortex (PCC) seed, and SN, as indexed by the subgenual ACC (sgACC) and amygdala seeds, with the CCN and that these differences were related to rumination and sustained attention (Jacobs et al. 2014). Thus, we sought to extend this novel work by comparing rMDD with active MDD (aMDD) to examine whether the observed network abnormalities were similar or distinct during different states of illness course (in v. out of episode). We also undertook a second analysis to investigate the influence of multiple episodes (i.e. illness burden). In order to extend upon our previous work we used the same PCC, sgACC and amygdala seeds as probes of the DMN and SN.
In sum, unanswered questions of clinical importance remain regarding the direction and extent of disrupted neural connectivity in MDD, particularly in relation to vulnerabilities that remain into remission and may point to pre-illness risk factors. We hypothesized that we would replicate our finding of increased connectivity within and between regions of the DMN (as indexed by seed-based connectivity of the PCC with the whole brain) and within and between the SN (as indexed by connectivity of the sgACC and amygdala seeds to the whole brain) among unmedicated young adults with rMDD compared with HCs. We hypothesized that these identified patterns in rMDD would differ from peers in the active state of illness and that hypoconnectivity would be observed among aMDD patients compared with both HCs and rMDD patients. Furthermore, we hypothesized that overall patterns of network connectivity would distinguish all MDD (both rMDD and aMDD) from HCs (episode-independent effect) as well as distinguish aMDD from rMDD (episode-dependent effect; Mayberg et al. 1999; Harrison et al. 2008). Last, as a secondary investigation into the nuances inherent to MDD, we investigated whether illness burden –defined as number of episodes – was associated with differential connectivity patterns.
Method
Participants
The current study was approved by the University of Michigan (UM) and the University of Illinois at Chicago (UIC) Institutional Review Boards. After a complete description of the study to participants, written informed consent was obtained. All participants completed structured diagnostic interviews (see online Supplementary material). Participants were considered rMDD if they previously met criteria for at least one major depressive episode (MDE) and scored below seven on the Hamilton Rating Scale for Depression (HAM-D; Hamilton, 1960). aMDD individuals were experiencing a current MDE and scored higher than 12 on the HAM-D. HCs did not meet current or past criteria for MDD or any other Axis I or II psychiatric disorder and had no first-degree relatives with a history of psychiatric illness. In addition, participants were required to be medication free for a minimum of 14 days prior to the scan (0 individuals were on fluoxetine, which has a longer half-life, and the majority had not received medication for over 3 months), and those with substance abuse or dependence within the past 6 months were excluded. An initial sample of 103 individuals in the 18–25 years age range was preprocessed and outliers were removed based on movement (see online Supplementary material). A final sample of 77 individuals with usable fMRI data included 17 aMDD, 34 rMDD and 26 HCs (n = 50 female, 65% female; demographic differences between the usable and full sample are reported in the online Supplementary material). These individuals were further subdivided based on level of burden, with 15 individuals reporting a single episode (three aMDD, 12 rMDD) and 29 individuals reporting multiple episodes (11 aMDD, 18 rMDD). Data on number of episodes were not available for seven individuals in the final sample.
fMRI acquisition
Both sites included an eyes-open resting-state scan acquired over 8 min. At UM (17 HC, 17 rMDD, 10 aMDD), scans were collected with a 3.0 T GE Signa scanner (USA) using T2*-weighted single shot reverse spiral sequence with the following parameters: 90 degree flip, field-of-view 20, matrix size = 64 × 64, slice thickness = 4 mm, 30 ms echo time, 29 slices. Scans at UIC (nine HC, 17 rMDD, seven aMDD) were collected with a 3.0 T GE Discovery scanner (USA) using parallel imaging with ASSET and T2* gradient-echo axial echo planar imaging (EPI) with the following parameters: 90 degree flip, field-of-view 22, matrix size = 64 × 64, slice thickness = 3 mm, 22.2 ms echo time, 44 slices, with a repetition time (TR) of 2000 ms with a total of 240 TRs collected. At both sites, high-resolution anatomic T1 scans were obtained for spatial normalization and motion was minimized with foam pads, a visual tracking line (UIC only) and/or cross (UIC and UM) on the display, and by conveying the importance of holding still to participants, with a TR of 2000 ms and 240 TRs collected.
fMRI preprocessing
Slice timing, realignment, co-registration, warping [DARTEL to Montreal Neurological Institute (MNI) template] and smoothing (5 mm full width at half maximum) were all completed with SPM8 batch scripts, including visual inspection after each step (see online Supplementary material).
Cross-correlation analysis
Time series was detrended and mean centered. Physiological correction was performed by regressing out mean signal from white matter and cerebral spinal fluid (Behzadi et al. 2007). Motion parameters and deviations in pitch, roll and yaw were regressed out within first-level models (Jo et al. 2013). Global signal was not regressed due to collinearity violations with gray matter signal, problematic misestimates of and introductions of anticorrelations (Fox et al. 2009), and effect on distance–micromovement relationships (Jo et al. 2013). Finally time-series were band-pass filtered over 0.01–0.10 Hz. Seeds were derived based on previous literature examining resting-state connectivity of the PCC to examine the DMN (Bluhm et al. 2011; Alexopoulos et al. 2012) as well as the amygdala (McCabe & Mishor, 2011; Pannekoek et al. 2013) and sgACC (Margulies et al. 2007; Kelly et al. 2009) to probe the SN. The following coordinates were used: PCC (DMN, −5, −50, 36), amygdala (SN, −23, −5, −19), sgACC (SN, −4, 21, −8), with left coordinate seeds. Two SN seeds were used in light of prior work (Jacobs et al. 2014) suggesting that these two seeds do not capture entirely overlapping networks among healthy individuals when using seed-based strategies.
Correlation coefficients were calculated between mean time course for seed regions and all other voxels of the brain, resulting in three-dimensional correlation coefficient images (r images), transformed to Z scores using a Fisher transformation and compared in SPM8. Whole-brain correction was achieved at p < 0.05 by conducting 1000 Monte Carlo simulations in AlphaSim to determine a joint threshold of height and extent (p < 0.005, cluster extent of 440 mm3).
Statistical analyses
Two random-effects multivariate analyses of covariance (MANCOVAs) were computed. The first examined disease and episodic (episode-independent effects) effects by comparing aMDD, rMDD and HCs, covarying for sex and site. The second random-effects MANCOVA addressed disease burden by comparing HCs, individuals with a single MDE and those with multiple MDEs, from both the active and remitted groups, covarying for HAM-D score, sex and site. We report and display findings meeting group-level F test thresholds and interpret regions of main group effects through examination of post-hoc tests. None of these results was influenced by site as reported in the online Supplementary material. We also tested whether any identified group differences were related to age of participant or to clinical features including level of depression and anxiety using the HAM-D and Hamilton Rating Scale for Anxiety.
Results
Participant demographics and clinical characteristics
Table 1 details the current sample. Individuals in the aMDD group reported higher HAM-D scores [F = 291.11, degrees of freedom (df) = 2, p < 0.01] and a greater number of MDEs (F = 16.36, df = 2, p < 0.01) than individuals in the rMDD group and HCs. In addition, more individuals in the aMDD group had a history of co-morbid anxiety disorders (F = 25.55, df = 2, p < 0.01), endorsed using psychiatric medications in the past (χ2 = 7.25, df = 1, p = 0.03) and were approximately 1 year older (F = 4.18, df = 2, p = 0.02) than aMDD and HCs. Individuals with multiple MDEs (hereafter ME MDD, mean = 13.82, S.D. = 3.48) compared with a single MDE (hereafter SE MDD, mean = 18.20, S.D. = 2.70; F = 17.92, df = 1, p < 0.01) reported an earlier age of illness onset but did not differ from the single episode group on any other clinical or demographic feature including family history (all p > 0.05).
Table 1.
Demographic and clinic characteristics
| HC (n = 26) | rMDD (n = 34) | aMDD (n = 17) | |
|---|---|---|---|
| Mean age*, years (S.D.) | 21.15 (1.49) | 21.06 (1.54) | 22.35 (1.80) |
| Females, n (%) | 14 (54) | 25 (74) | 11 (65) |
| Caucasian, n (%) | 19 (76) | 25 (74) | 6 (67) |
| Mean education, years (S.D.) | 14.84 (1.14) | 14.41 (1.39) | 14.69 (1.40) |
| Mean IQ estimate (S.D.) | 106.9 (9.7) | 108.4 (9.8) | 110.5 (9.3) |
| Mean HAM-D* (S.D.) | 0.35 (1.0) | 2.35 (2.82) | 18.65 (3.67) |
| History of co-morbid substance, n (%) | 2 (8) | 11 (32) | 3 (18) |
| History of co-morbid anxiety*, n (%) | 2 (8) | 12 (35) | 15 (88) |
| Past psychiatric medication*, n (%) | 0 | 21 (62) | 6 (36) |
| Mean number of MDEs* (S.D.) | N.A. | 1.82 (1.21) | 7.20 (8.88) |
| Mean age of first onset, years (S.D.) | N.A. | 15.83 (3.09) | 14.94 (4.02) |
| Site, n | |||
| University of Michigan | 17 | 17 | 10 |
| University of Illinois at Chicago | 9 | 17 | 7 |
HC, Healthy controls; rMDD, remitted major depressive disorder; aMDD, active major depressive disorder; S.D., standard deviation; IQ, intelligence quotient; HAM-D, Hamilton Rating Scale for Depression; MDEs, major depressive episodes; N.A., not applicable;
p < 0.05.
All figures display results meeting F test thresholds only, with post-hoc contrasts indicated in Tables for only those regions surviving whole-brain correction with F test significance.
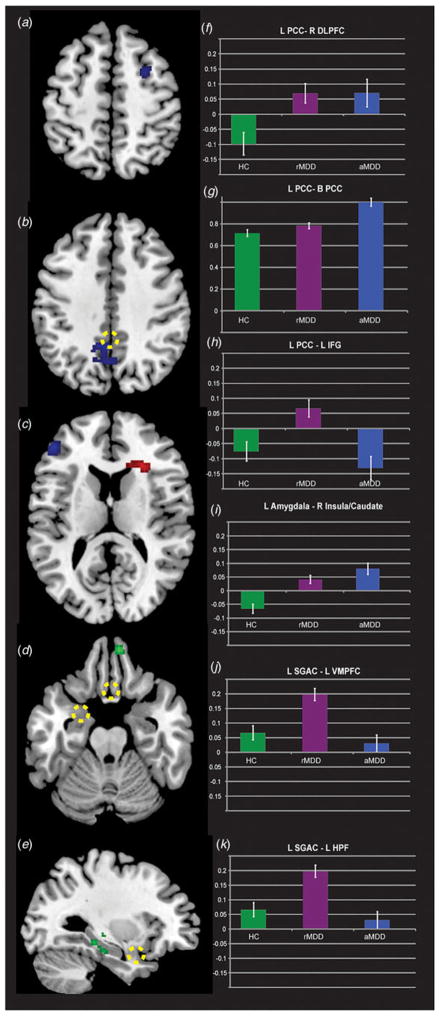
Episode-dependent and -independent connectivity of the PCC
Fig. 1 illustrates PCC seed-based connectivity differences for the main effect of group (F tests, blue). F test group differences were observed in three regions: the left inferior frontal gyrus (IFG), right middle frontal gyrus (MFG) and adjacent areas to the seed within the left PCC. In contrast, participants with aMDD demonstrated weakened connectivity to the left IFG compared with rMDD, suggestive of an episode-dependent feature of illness. In addition, participants with aMDD demonstrated amplified connectivity within the DMN (i.e. to adjacent portions of the PCC) compared with rMDD, also suggestive of an episode-dependent feature of illness. Table 2 details significant group differences and the direction of post-hoc effects indicating clinical correlations. In contrast, all MDD demonstrated amplified connectivity of the left PCC to the right MFG compared with HCs, suggestive of an episode-independent feature of illness.
Fig. 1.
Differential connectivity between active and remitted major depressive disorder (aMDD and rMDD, respectively) compared with healthy controls (HC). Left (L) posterior cingulate cortex (PCC) seed probing default mode network connectivity highlighting differences between groups at the F test level (blue, panels a, b and c on the left and corresponding bar graphs on the right in panels f, g and h). Differences in amygdala connectivity based upon episodic state are illustrated at the F test level (panel c with extracted values in panel i). Subgenual anterior cingulate (SGAC) connectivity differences are also illustrated at the F test level (green, panels d and e, with corresponding extracted values in panels j and k). Dashed yellow circles illustrate the locations of the PCC, amygdala and SGAC seeds. Views are axial for panels a to d and left sagittal for panel e. R, Right; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; VMPFC, ventromedial prefrontal cortex; HPF, hippocampal formation. Values are means, with standard errors represented by vertical bars.
Table 2.
Group differences between active and remitted MDD compared with healthy controls using the PCC, amygdala and sgACC seeds
| MNI coordinates
|
|||||||
|---|---|---|---|---|---|---|---|
| Contrast/lobe | BA | x | y | z | Z | Cluster size, mm3 | Post-hoc comparison |
| Left PCC, F test group | |||||||
| Frontal | |||||||
| Inferior frontal | 46 | −46 | 42 | 12 | 4.20 | 592 | rMDD > aMDDa,b* |
| Middle frontal | 10 | 28 | 6 | 54 | 3.22 | 472 | All MDD > HC |
| Limbic | |||||||
| Posterior cingulate | 31/7 | −6 | −50 | 36 | 4.34 | 2672 | aMDD > rMDDa* |
| Left amygdala, F test group | |||||||
| Subcortical | |||||||
| Claustrum/insula | 13 | 26 | 28 | 16 | 4.11 | 856 | All MDD > HC |
| Left sgACC, F test group | |||||||
| Frontal | |||||||
| Orbital frontal | 11 | 8 | 52 | −24 | 3.97 | 536 | rMDD > aMDDa* |
| Temporal | |||||||
| Hippocampus | −32 | −24 | −4 | 3.47 | 528 | rMDD > aMDDa* | |
MDD, Major depressive disorder; PCC, posterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; BA, Brodmann’s area; MNI, Montreal Neurological Institute; rMDD, remitted MDD; aMDD, active MDD; HC, healthy controls.
p < 0.05, significantly correlated (within all MDD) with a the Hamilton Depression Scale and b age of first episode. No clusters are significantly correlated with the Hamilton Anxiety Scale.
Episode-dependent connectivity of the amygdala
All MDD demonstrated amplified connectivity of the left amygdala with the right anterior insula, caudate and claustrum compared with HCs, indicative of state-independent features of illness (Fig. 1, in red).
Episode-dependent connectivity of the sgACC
Individuals with rMDD exhibited amplified connectivity of the left sgACC to the right orbitofrontal cortex (OFC) and left hippocampus compared with aMDD, suggesting that weakened connectivity among aMDD may be an episode-dependent feature of illness.
Connectivity differences based upon number of episodes (burden)
Burden effects using the PCC seed
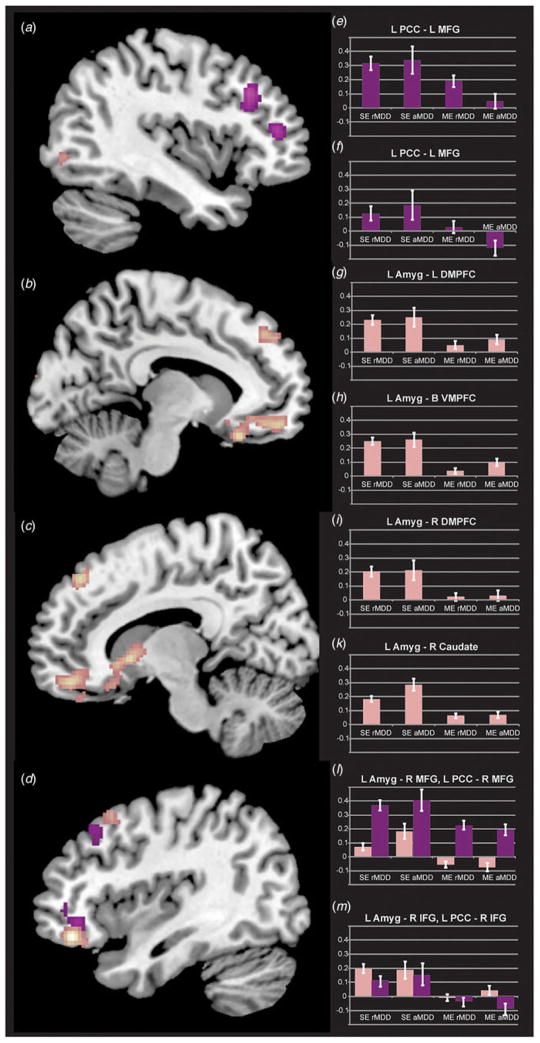
SE MDD exhibited a general pattern of weaker negative connectivity of the left PCC with a number of CCN regions including the bilateral IFG and MFG as well as the right inferior parietal lobule, relative to both HCs and ME MDDs (Fig. 2). Fig. 2 also illustrates increased positive connectivity in SE MDD of the left PCC seed to the left MFG, relative to the HC and ME MDD groups. Table 3 details all significant group differences. All single v. multiple differences remained significant after covarying for age of first MDE.
Fig. 2.
Differential connectivity between single-episode (SE) and multiple-episode (ME) major depressive disorder (MDD). Panels illustrate effects of burden in single episode v. multiple episode, further subdivided by in v. out of episode status (the latter provided to clarify that episode-linked and burden-linked effects are distinct). Views are sagittal for panels a and b (left) to g and h (right), and bar graphs generally align with the cluster from which they were extracted. Panel a (purple) indicates regions of decreased left (L) posterior cingulate cortex (PCC) connectivity in ME MDD relative to SE MDD in the left middle frontal gyrus (MFG; also panels c and d). Decreased connectivity of the left amygdala (Amyg) in ME MDD was present and illustrated in the bilateral dorsomedial and ventromedial portions of the prefrontal cortex (DMPFC and VMPFC, respectively; panels b and g, pink, also panels e, f and i), and right caudate (panel g, also panel k). Panel h demonstrates decreased connectivity in ME MDD for the left PCC (purple) and left amygdala (pink) to closely linked middle frontal (MFG) and inferior frontal gyrus (IFG) regions, with these extracted data illustrated in panels l and m. rMDD, Remitted MDD; aMDD, active MDD; R, right; B, bilateral. Values are means, with standard errors represented by vertical bars.
Table 3.
Group differences between single and multiple major depressive episodes compared with healthy controls using the PCC, amygdala and sgACC as seeds
| MNI coordinates
|
|||||||
|---|---|---|---|---|---|---|---|
| Contrast/lobe | BA | x | y | z | Z | Cluster size, mm3 | Post-hoc comparison |
| Left PCC, F test group | |||||||
| Frontal | |||||||
| Middle frontal | 9 | −40 | 26 | 34 | 4.08 | 1440 | Single > HC, multiplea,b |
| Middle frontal | 9/46 | 28 | 24 | 36 | 3.33 | 1272 | Single > HC, multiple |
| Inferior frontal | 46 | −44 | 42 | 12 | 3.9 | 936 | Single > HCa,b |
| Inferior frontal | 47/11 | 34 | 42 | 4 | 3.37 | 648 | Single > HC, multipleb |
| Parietal | |||||||
| Inferior parietal | 22 | 52 | −54 | 22 | 2.83 | 440 | Single > multiple |
| Left amygdala, F test group | |||||||
| Frontal | |||||||
| Medial frontal | 11 | 8 | 50 | −16 | 4.13 | 7048 | Single > HC, multipleb |
| Middle frontal | 11 | 38 | 40 | −14 | 4.72 | 1392 | Single > multipleb |
| Middle frontal | 6 | 42 | 18 | 52 | 3.54 | 1368 | Single > multipleb |
| Superior frontal | 8 | 12 | 40 | 48 | 3.85 | 1024 | Single > multiple |
| Superior frontal | 8 | −8 | 50 | 40 | 3.57 | 752 | Single > multiple |
| Limbic | |||||||
| Uncus/amygdala | 34 | 20 | −4 | −22 | 3.66 | 552 | Single, multiple > HC |
| Temporal | |||||||
| Inferior temporal | 20 | 68 | −26 | −16 | 4 | 616 | Single > multiple |
| Occipital | |||||||
| Cuneus | 18 | −14 | −98 | 18 | 3.48 | 608 | HC > single, multiple |
| Inferior occipital | 19 | −38 | −78 | −2 | 3.42 | 504 | HC > multipleb |
| Subcortical | |||||||
| Caudate head | 8 | 12 | −2 | 3.86 | 1600 | HC > multipleb | |
| Left sgACC, F test group | |||||||
| Frontal | |||||||
| Superior frontal | 10 | 20 | 64 | 20 | 3.61 | 1072 | HC < multiple |
| Inferior frontal | 44/47 | 38 | 20 | 22 | 3.79 | 712 | Single > multipleb |
| Occipital | |||||||
| Middle occipital | 18 | −12 | −98 | 22 | 3.98 | 528 | HC, multiple > singlea |
PCC, Posterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; BA, Brodmann’s area; MNI, Montreal Neurological Institute; HC, healthy controls.
p < 0.05, within all major depressive disorder, significantly correlated with a the Hamilton Depression Scale and b age of first episode. No clusters are significantly correlated with the Hamilton Anxiety Scale.
Burden effects using the amygdala seed
Individuals with SE and ME MDD demonstrated relatively weakened connectivity of the left amygdala to the left cuneus compared with HCs, an effect independent of episode. Those with ME MDD exhibited weakened connectivity of the amygdala to the left inferior occipital lobe and right caudate head relative to HCs. Individuals with a SE MDD exhibited amplified connectivity of the amygdala with multiple frontal regions relative to those who had experienced multiple MDEs, especially in the medial frontal cortex, extending into orbital and subgenual cingulate regions, potentially reflecting the burden of multiple episodes (Fig. 2). In Table 3, connectivity values for half of the 10 seeds from the left amygdala to regions of relative hypoconnectivity were positively correlated with age of first MDE onset among individuals with multiple MDEs. All SE v. ME MDD differences remained after covarying for age for first MDE.
Burden effects using the sgACC seed
HCs and ME MDD exhibited greater connectivity of the left sgACC to the left middle occipital gyrus when compared with individuals with a SE MDD (Fig. 2). Individuals with ME MDD demonstrated increased connectivity to the right superior frontal gyrus (SFG) compared with HCs. Individuals with a SE MDD demonstrated amplified connectivity to the right IFG compared with ME MDD. The superior and inferior frontal results remained significant after covarying for age of first MDE, whereas the middle occipital finding was no longer significant.
Discussion
The diagnostic category of MDD remains a heterogeneous phenotype divided into poorly refined and largely overlapping subsets. The present study provides insight into how features of illness such as number of MDEs (burden) and phase of illness (episode-dependent v. independent) can be used to understand differences in resting-state data among young, unmedicated adults. Notable differences in connectivity were observed based upon in v. out of episode status. Further dissociations between individuals with varying longitudinal burden were observed when using a seed-based approach to probe the DMN and SN. Finally, effects in the SN and DMN with the CCN were observed in all MDD (episode-independent) relative to HCs. Specifically, all MDD demonstrated increased connectivity of the left amygdala with the right anterior insula, implicating an episode-independent abnormality within the SN. All MDD also demonstrated increased connectivity of the PCC to the right MFG, indicating that some DMN abnormalities are independent of acute MDE.
Episode-dependent and episode-independent differences in resting-state connectivity
Young adults in the remitted state demonstrated hyperconnectivity of the PCC with regions of the CCN, whereas individuals within an active episode demonstrated hyperconnectivity of the PCC with additional regions of the DMN and hypoconnectivity of the PCC with frontal regions of the CCN, consistent with the broader literature. Thus, using the PCC seed results in divergent patterns within the DMN and between the DMN and CCN for the remitted v. active phases of illness suggesting they may be episode-dependent.
An additional notable episode-dependent effect of illness includes weakened connectivity of the sgACC to the OFC among participants with aMDD relative to rMDD. The left IFG appears to play an important role in the emotion regulation difficulties observed during acute illness including affective perception (Briceño et al. 2013). Individuals within an acute episode also exhibited enhanced connectivity within the DMN as well as from the amygdala to regions including the ipsilateral globus pallidus and putamen – also only in relation to the rMDD group. The globus pallidus and putamen are typically not described in the resting-state literature, although a recent study investigating basal ganglia connectivity in adolescent MDD found increased connectivity between striatal regions and portions of the CCN, DMN and SN (Gabbay et al. 2013). The observed episode-dependent effects suggest that more nuanced ascertainment criteria for studies of MDD may lead to greater homogeneity of results as well as unveil how patterns fluctuate across different phases of illness.
These specific findings extend and contextualize previous literature documenting abnormalities in connectivity between the DMN and CCN in MDD (Sheline et al. 2010; Kaiser et al. 2015) and suggest for the first time in a single study that hyperconnectivity of the DMN and SN with the CCN may be episode-independent. We specifically found that these abnormalities pertain to rMDD compared with both aMDD. This evidence indicates episodic features of illness (present only in one state, yet still different from HCs). It may also be an episode-independent feature predictive of disease course or perhaps even resilience. The SFG and MFG may represent an extension of the CCN that serves to down-regulate the DMN and SN among those who have recovered from MDD, perhaps representing an early compensatory response. Hyperconnectivity of frontal regions to the DMN in remitted individuals may be representative of increased cognitive control regulating the default mode, allowing these individuals to experience control over depressive symptoms such as rumination and negative internalizing states, allowing them to stay well over longer periods of time.
Burden differences in resting-state connectivity
Connectivity from the amygdala seed resulted in discrimination between MDD groups with single v. multiple episodes, independent of episode status, which has not frequently been examined in the literature to date. Traditional sample sizes have not allowed for nuanced examinations of the effect of a first-onset v. multiple episodes, which may contribute to the relative dearth of reported amygdala-based connectivity findings in MDD (i.e. only four of 25 studies used an amygdala seed in MDD; Kaiser et al. 2015). Specifically, in the present study the amygdala was hyperconnected to the bilateral ventral medial PFC among individuals with SE MDD compared with ME MDD and HCs. The amygdala was also hyperconnected with other regions of the SN in SE v. ME MDD, which may implicate a temporal decoupling of the amygdala from SN regions after the burden of ME MDD (Lee et al. 2012). In contrast, this was not evident in the comparison between SE MDDs and HCs, offering further evidence that the recurrence of illness may lead to disruption of SN function through decoupling. To date, a few studies have implicated decreased network coupling in treatment-resistant depression (de Kwaasteniet et al. 2015; Dichter et al. 2015); however, our results suggest that this decoupling may occur much earlier in the course of recurrent MDD. Moreover, weakened connectivity between the left amygdala and right caudate among multiple MDEs compared with HCs indicates an effect of burden. The caudate is involved in reward and cognitive control and this hypoconnectivity among individuals with multiple episodes could relate to the anhedonic features often observed in treatment-resistant depression (Kerestes et al. 2012). Alternatively, the caudate also is known to perform subservient tasks within the CCN such as response inhibition (Aron et al. 2007; Langenecker et al. 2007). Collectively, these findings indicate that amygdala-based connectivity is worthy of examination among carefully selected depressed populations.
Interestingly, age of first onset was related to results deriving from the SE (typically hyper-) v. ME (typically hypo-) contrast. Upon closer examination, weakened connectivity was more readily observable among those with an earlier age of onset. It is possible that later onset of first MDE enables the CCN to develop more fully, and thus cross-network increases in connectivity are subsequently observed. It is also possible that early-onset MDE may relate to early trauma and increased number of episodes; some research examining early-life trauma suggests an adverse effect on development of the CCN (Rogosch et al. 1995; Majer et al. 2010; Spann et al. 2012) and may further affect relations of CCN to other networks. Furthermore, we do not know if there was enough time for later-onset MDD to develop multiple episodes to fully dissociate age of onset and number of episodes, or whether these differences may predate and predict the course of illness.
Limitations and future directions
We note several limitations of our study. First, the strict controls for movement resulted in a usable sample that was younger and less severe in symptomatology than the overall sample. These differences make the current results less generalizable. Second, the current data are cross-sectional and cannot discriminate between network abnormalities that render an individual vulnerable to the first onset of MDD, ME MDD, as opposed to a normal maturational process. To capture developmental trajectories that contribute to resiliency and risk in MDD, future longitudinal research could examine whether excessive coupling of intrinsic networks predicts first onsets of depression or relapse as adolescents transition into early adulthood. In addition, this study used retrospective interviews to capture illness and episode frequency, which may be biased by factors such as selective recall. Prospective studies of high-risk cohorts represent an important direction for future research. In addition, our stringent movement criteria resulted in the exclusion of a significant minority of the aMDD group which resulted in a modest group size; however, inclusion of these individuals would have resulted in different and potentially missed results (online Supplementary Fig. S1 illustrates those with movement obscure between-group effects). Our findings should be replicated with larger samples of individuals with aMDD. There were also differences in clinical demographics across sites. Furthermore, sex differences have been identified in previous research examining brain-based state and trait markers of illness (e.g. Versace et al. 2010). We controlled for sex but did not have adequate power to further analyse group × sex differences. Despite these limitations, we believe the current examination focused on a relatively early phase of illness provides a level of protection against potential confounds including complex treatment histories or neural scarring, making the current study innovative and important. Last, our examination of burden of illness (single v. multiple episodes) deserves further exploration and replication as there were very few aMDD with a single episode. However, examination of bar graphs suggests that the result of ME MDD was prominent in both aMDD and rMDD.
In conclusion, this is the first resting-state fMRI study illustrating features of both active (in-episode) and remitted (out-of-episode) MDD highlighting future directions that can better define risk for illness onset as well as early course markers. Furthermore, hypoconnectivity previously attributed to recurrent, treatment-resistant MDD (e.g. burden) may be present much earlier in illness. The current results address an understudied, yet important set of questions regarding the dissociation of episode-related, compensatory and early scar features of multiple episodes of illness. Ultimately, distinguishing between individuals who are at increased risk for multiple episodes may guide practice parameters for maintenance treatment and secondary prevention efforts.
Supplementary Material
Acknowledgments
Preparation of this manuscript was funded in support of R.H.J. by the UIC Center for Clinical and Translational Science UL1TR00050, NIH RO1 MH091811 (S.A.L.) and NIH RO1 MH101487 (S.A.L.). A.B., J.R.G. and L.M.J. were supported by NIH RO1 MH091811 (S.A.L.) and NIH RO1 MH101487 (S.A.L.). H.K. was funded by the Brain and Behavior Research Foundation. L.M.J. was funded by MH 101487 (S.A.L.). B.J.M. was supported by NIH K23 MH092648, and O.A. was supported by NIH R01 MH101487 (S.A.L.). M.P., M.S. and J.-K.Z. were funded through NIH R01 MH086858 and the Phil F. Jenkins Foundation. K.A.R. was supported by the National Center for Advancing Translational Sciences NIH 2KL2TR000434, NIH R01 MH091811 (S.A.L.), the Heinz C. Prechter Bipolar Research Fund at the University of Michigan Depression Center, and the Richard Tam Foundation. D.T.H. was supported by NIH K01 MH085035, and K.L.P. was funded through NIH NIMH R01 MH101497. The analytic scripts for the connectivity analysis were developed by R.C.W. with support from NIH/NINDS 1R01NS052514. S.A.L. was supported by NIH R01 MH091811, NIH R01 MH101487, and the Brain and Behavior Research Foundation. The authors would like to acknowledge the contributions of the Center for Magnetic Resonance Research 3T Program at UIC.
Footnotes
Preliminary data were presented in part at the 53rd annual meeting of the American College of Neuropsychopharmacology in Phoenix, Arizona, 7–11 December 2014.
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291715002615
Declaration of Interest
None.
References
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. Journal of Neuroscience. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Clark CR, McFarlane AC, Moores KA, Shaw ME, Lanius RA. Default network connectivity during a working memory task. Human Brain Mapping. 2011;32:1029–1035. doi: 10.1002/hbm.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Briceño EM, Weisenbach SL, Rapport LJ, Hazlett KE, Bieliauskas LA, Haase BD, Ransom MT, Brinkman ML, Pecina M, Schteingart DE, Starkman MN, Giordani B, Welsh RC, Noll DC, Zubieta J-K, Langenecker SA. Shifted inferior frontal laterality in women with major depressive disorder is related to emotion-processing deficits. Psychological Medicine. 2013;43:1433–1445. doi: 10.1017/S0033291712002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther A, Smoski MJ, Minkel J, Moore T, Gibbs D, Petty C, Bizzell J, Schiller CE, Sideris J, Carl H, Dichter GS. Resting-state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacology. 2015;40:1659–1673. doi: 10.1038/npp.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kwaasteniet BP, Rive MM, Ruhé HG, Schene AH, Veltman DJ, Fellinger L, van Wingen GA, Denys D. Decreased resting-state connectivity between neurocognitive networks in treatment resistant depression. Frontiers in Psychiatry. 2015;6:28. doi: 10.3389/fpsyt.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. Journal of Affective Disorders. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Qingyang L, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP. Striatum-based circuitry of adolescent depression and anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:628–641. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical science. Biological Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yücel M. Modulation of brain resting-state networks by sad mood induction. PLoS ONE. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Jenkins LM, Gabriel LB, Barba A, Ryan KA, Weisenbach SL, Verges A, Baker AM, Peters AT, Crane NA, Gotlib IH, Zubieta J-K, Phan KL, Langenecker SA. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLOS ONE. 2014;9:e104366. doi: 10.1371/journal.pone.0104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, Saad ZS. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state fMRI. Journal of Applied Mathematics. 2013;2013:10. doi: 10.1155/2013/935154. 1155/2013/935154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, Nemeroff CB, Friedman ES, Gelenberg AJ, Kocsis JH, Dunner DL, Hirschfeld RM, Rothschild AJ, Ferguson JM, Schatzberg AF, Zajecka JM, Pedersen RD, Yan B, Ahmed S, Musgnung J, Ninan PT. The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) Study: outcomes from the 2-year and combined maintenance phases. Journal of Clinical Psychiatry. 2007;68:1246–1256. doi: 10.4088/jcp.v68n0812. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Bhagwagar Z, Nathan PJ, Meda SA, Ladouceur CD, Maloney K, Matuskey D, Ruf B, Saricicek A, Wang F, Pearlson GD, Phillips ML, Blumberg HP. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Research. 2012;202:30–37. doi: 10.1016/j.pscychresns.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Briceño EM, Hamid NM, Nielson KA. An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: a study in the basal ganglia. Brain Research. 2007;1135:58–68. doi: 10.1016/j.brainres.2006.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RSC, Hermens DF, Porter MA, Redoblado-Hodge A. A meta-analysis of cognitive deficits in first-episode major depressive disorder. Journal of Affective Disorders. 2012;140:113–124. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Majer M, Nater UM, Lin JM, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurology. 2010;10:61. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. NeuroImage. 2011;57:1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJA, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SARB, van der Wee NJA. Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. Journal of Affective Disorders. 2013;145:29–35. doi: 10.1016/j.jad.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Almeida JRC, Kronhaus DM, Versace A, LaBarbara EJ, Klein CR, Phillips ML. Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar Disorders. 2012;14:162–174. doi: 10.1111/j.1399-5618.2012.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D, Aber JL. The role of child maltreatment in early deviations in cognitive and affective processing abilities and later peer relationship problems. Development and Psychopathology. 1995;7:591–609. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences USA. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann MN, Mayes LC, Kalmar JH, Guiney J, Womer FY, Pittman B, Mazure CM, Sinha R, Blumberg HP. Childhood abuse and neglect and cognitive flexibility in adolescents. Child Neuropsychology. 2012;18:182–189. doi: 10.1080/09297049.2011.595400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann B, Beverborg MOL, Pfieiderer B. Toward literature-based feature selection for diagnostic classification: a meta-analysis of resting-state fMRI in depression. Frontiers in Human Neuroscience. 2014;8:692. doi: 10.3389/fnhum.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Ladouceur CD, Romero S, Birmaher B, Axelson DA, Kupfer DJ, Phillips ML. Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1249–1259. doi: 10.1016/j.jaac.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenbach SL, Kassel MT, Rao J, Weldon AL, Avery ET, Briceño EM, Ajilore O, Mann M, Kales HC, Welsh RC, Zubieta J-K, Langenecker SA. Differential prefrontal and subcortical circuitry engagement during encoding of semantically related words in patients with late-life depression. International Journal of Geriatric Psychiatry. 2014;29:1104–1115. doi: 10.1002/gps.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.