Abstract
Over 100 years have elapsed since the discovery of Chagas disease and there is still much to learn regarding pathogenesis and treatment. Although there are antiparasitic drugs available, such as benznidazole and nifurtimox, they are not totally reliable and often toxic. A recently released negative clinical trial with benznidazole in patients with chronic Chagas cardiomyopathy further reinforces the concerns regarding its effectiveness. New drugs and new delivery systems, including those based on nanotechnology, are being sought. Although vaccine development is still in its infancy, the reality of a therapeutic vaccine remains a challenge. New ECG methods may help to recognize patients prone to developing malignant ventricular arrhythmias. The management of heart failure, stroke and arrhythmias also remains a challenge. Although animal experiments have suggested that stem cell based therapy may be therapeutic in the management of heart failure in Chagas cardiomyopathy, clinical trials have not been promising.
Keywords: Arrhythmias, Chagas cardiomyopathy, Chagas disease, heart failure, myocardial fibrosis, nanotechnology, oxidative stress, stem cell therapy, vaccine development
1. General considerations: transmission, epidemiology, natural history and clinical manifestations
Chagas disease, also known as American trypanosomiasis, is a zoonotic disease caused by the infection with the protozoan Trypanosoma cruzi. This disease was initially described in 1909 by the Brazilian physician Carlos Chagas, who named the parasite in honor of his mentor, Oswaldo Cruz. Infection is endemic in Mexico, Central and South America, where it is a serious public health problem. It remains, even today, an important cause of morbidity and mortality in endemic areas of Latin America as well as among infected individuals that have immigrated to non-endemic areas of the world. In recent years, autochthonous cases of Chagas disease have been reported in the US, especially along the southern border with Mexico.[1,2] Due to increased immigration, individuals with Chagas disease have been identified in the US, Canada, Europe, Australia and Japan.[3–5]
Chagas disease is a neglected tropical disease. The World Health Organization recently estimated that it affects 5.7 million people and many millions more are at risk.[6] According to the Global Burden of Disease Study Group, between 1990 and 2013, the estimated annual global mortality from Chagas disease decreased from 12,699 to 10,611 cases a year, with a decrease of potential years of life lost from 343,000 to 245,000 years.[7]
Human infection is acquired through parasite-laden secretions from hematophagous triatomine insects in endemic areas.[1] In many endemic areas of South America, such as in Brazil, the vector has been virtually eliminated and can be considered residual.[8] The disease can also be transmitted through blood transfusions, laboratory accidents, organ donations and vertical transmission from mother-to-child, which are reported even in non-endemic areas.[9] Oral transmission is now recognized as a major mode of transmission that cause sporadic, small human outbreaks, especially in the Amazon region of Brazil.[10]
The clinical course of Chagas disease is divided into acute and chronic phases. The acute phase is usually mildly symptomatic and often misdiagnosed as a febrile illness of childhood. Severe acute infection occurs in approximately 1% of the patients and is manifested by fever, chills, rash, liver function abnormalities, acute myocarditis, pericardial effusion, acute heart failure and/or meningoencephalitis. Clinical manifestations of acute Chagas disease acquired by the oral route are often more severe. The incidence of clinically apparent acute infection has substantially decreased since the near interruption of transmission by vectors and via blood transfusions in most Latin American countries. Blood transfusion-associated acute Chagas disease is rare due to mandatory screening of blood donors in endemic countries. Organ donation may result in acute Chagas disease if the donor or recipient has not been appropriately screened. Acute exacerbation of infection may result in the setting of the administration of immunosuppressive drugs or as a result immunosuppressive diseases (i.e., HIV/AIDS).[4] During acute infection, trypomastigote forms of T. cruzi are readily observed in the bloodstream or detected by PCR.
The acute phase of the disease lasts for approximately 2–3 months, and gradually evolves into the indeterminate stage of chronic disease. In the indeterminate stage, patients have serological evidence of infection, but no clinical, radiologic, electrocardiographic or echocardiographic evidence of heart disease.[1,4] It has been demonstrated that patients in the indeterminate stage who were followed up for 5–10 years had an excellent prognosis, in that they evolved to clinical disease, mostly cardiac, at a rate of ~2% per year.[11] Overall, 20–40% of seropositive individuals develop clinically relevant Chagas heart disease. Digestive involvement, characterized by the presence of megacolon or megaesophagus, occurs in approximately 10% of the cases, but its prevalence appears to vary with the geographic origin of the patients and strain of parasite.[12,13]
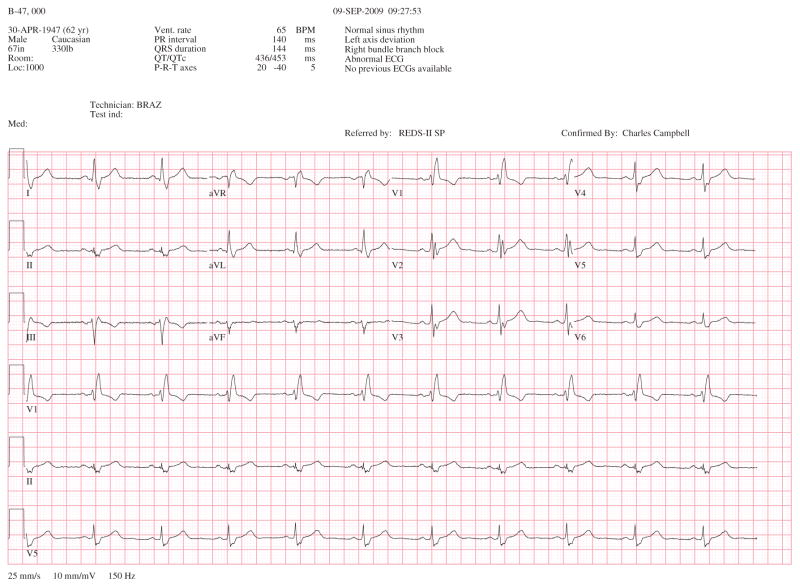
Chagas cardiomyopathy is the result of cardiac remodeling and the most important clinical manifestation of Chagas disease, because of its frequency, severity and impact on morbidity and mortality (Figure 1). The chronic phase lasts throughout the lifetime of the patient and results in a shortened survival rate. It is a complex and unpredictable disease that includes a wide spectrum of manifestations, ranging from minor myocardial involvement to patients with terminal cardiac failure, with a high incidence of arrhythmias.[1,4] The clinical presentation of Chagas disease varies according to the degree of myocardial damage. The mild cardiac form of heart disease can occur without left ventricular (LV) dysfunction and is frequently characterized by the presence of some abnormalities on ECG such as premature ventricular contractions and conduction system alterations, typically right-bundle branch block with or without left anterior hemiblock [14] (Figure 2). In these patients, echocardiographic abnormalities may be observed such as segmental LV wall motion abnormalities and diastolic dysfunction. As the disease progresses, LV systolic dysfunction, dilated cardiomyopathy, heart failure, arrhythmias, thromboembolic events and sudden death may occur.[1,4] Table 1 summarizes typical and nonspecific ECG abnormalities observed in Chagas cardiomyopathy, according to the Brazilian Expert Consensus in Chagas Disease.[15]
Figure 1. Heart of a patient who died of Chagas cardiomyopathy.
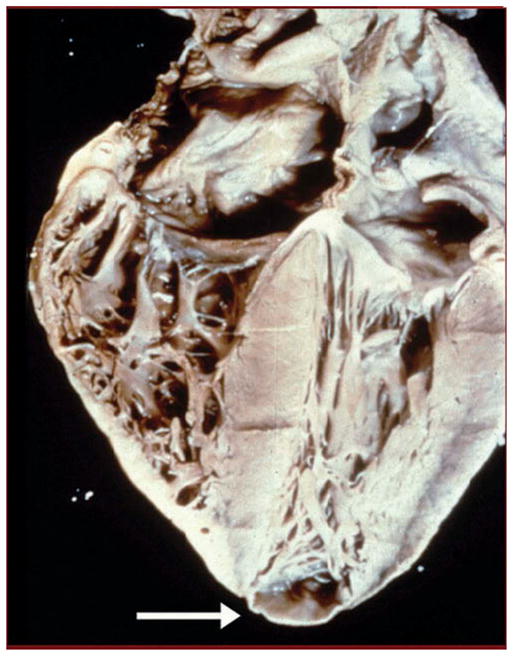
There is cardiac enlargement and a prominent apical aneurysm (arrow).
Courtesy of Armed Forces Institute of Pathology, United States.
Figure 2.
This is a 12-lead ECG of a patient with severe Chagas cardiomyopathy, showing right bundle branch block and left anterior hemiblock.
Table 1.
Typical and nonspecific ECG abnormalities in Chagas cardiomyopathy, according to the Brazilian Expert Consensus in Chagas Disease.
| Typical ECG changes for Chagas disease |
| – Right bundle branch block, associated or not with left anterior fascicular block |
| – Frequent ventricular premature beats (>1 by ECG), polymorphous or repetitive |
| – Nonsustained ventricular tachycardia |
| – Second and third degree atrioventricular block |
| – Sinus bradycardia with heart rate ≥40 bpm |
| – Sinus node dysfunction |
| – Left bundle branch block |
| – Atrial fibrillation |
| – Electrical inactive segment |
| – Primary alterations of ST-T wave |
| Nonspecific ECG changes observed in Chagas disease |
| – Sinus bradycardia with heart rate ≥40 bpm |
| – Low limb voltage |
| – Nonspecific ST-T changes |
| – Incomplete right bundle branch block |
| – Left anterior fascicular block |
| – Isolated ventricular premature beats |
| – First degree atrioventricular block |
The clinical course of Chagas cardiomyopathy is variable.[16] The prognosis of patients with Chagas cardiomyopathy appears to be worse compared with patients with idiopathic cardiomyopathy, even when adjusted for relevant risk factors.[17] Several factors related to the prognosis of Chagas disease are noted in longitudinal studies, but the value of some of these markers has yet to be validated.[16] The most consistent independent predictors of death are LV dysfunction, New York Heart Association (NYHA) functional class, and non-sustained ventricular tachycardia, which reflect a more advanced degree of myocardial damage.[18] Prediction scores of the risk of death were developed and may help to recognize high-risk Chagas disease patients.[16]
2. Inflammation, oxidative stress and fibrosis
Infection of the myocardium elicits an intense inflammatory response which may be viewed as a “double-edge sword.” Although necessary for the control of parasite proliferation, inflammation results in tissue damage leading to myocardial fibrosis and cardiac remodeling [19–22] (Figure 3). A detailed description of the immunology of Chagas disease is beyond the scope of this paper; however, the observed pro-inflammatory response includes, but is not limited to, secretion of Th1 cytokines and chemokines, eicosanoids, and endothelin-1 during T. cruzi infection. The cells that participate in innate and adaptive immune responses including dendritic cells, macrophages, CD4+ and CD8+ T cells, cytotoxic T cells and lytic antibody producing B cells are activated.[23,24] Activation of CD4+ and CD8+ T cells that synthesize Th1 cytokines generally correlates with myocardial damage, at least in experimental models, and may be modulated by IL-17 and T regulatory cells.[21,25,26] During acute infection, mononuclear cells produce high levels of IFN-γ,[27] TNF-α and IL-6.[28]
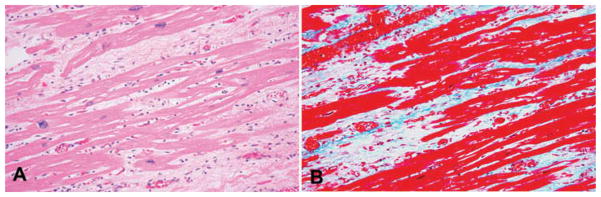
Figure 3. Histopathology of the heart from a patient who died while awaiting cardiac transplant.
(A) H&E showing bands of fibrous tissue. (B) Trichrome staining showing the bands of fibrous tissue.
Reproduced from Johndrow et al. (2014) [51] Microbes and Infection, with permission of Elsevier.
In chronic Chagas cardiomyopathy, T cells specific for T. cruzi as well as T cells that recognize cardiac-myosin are detected.[29,30] The cells infiltrating the myocardium of the patients likely express Th1 transcription factor (T-bet) and low levels of transcription factors related to Th2, Treg and Th17 cells subsets.[31] Other inflammatory mediators including IL-18, TNF-α, CCL21 and CCL2 [32,33] may be involved in induction of transforming growth factor-β (TGF-β), the development of cardiac fibrosis and cardiac myocyte hypertrophy.[21]
TGF-β is an important factor in the induction of inflammation and fibrosis. In different cell types, TGF-β contributes to tissue repair and remodeling [34] and in cell differentiation to myofibroblasts.[35] TGF-β also induces connective tissue growth factor and is critical for the preservation of the matrix by suppressing the activity of matrix metalloproteinases and inducing the production of protease inhibitors.[35] Moreover, TGF-β modulates the cardiac myocyte phenotype and triggers hypertrophic effects.[35,36] In the myocardium of Chagas disease patients, there are increased levels of TGF-β and other growth factors, such as granulocyte-macrophage-colony-stimulating factor and platelet-derived growth factor [37] that may mediate fibrosis. In a retrospective study of 54 Chagas disease patients, TGF-β1 was independently associated with all-cause mortality and hospitalization due to heart failure or cardiac arrhythmias.[38]
Recently, it was demonstrated that Galectin-3, a carbohydrate-binding protein, induced collagen deposition and cardiac fibrosis in T. cruzi infection.[39] Furthermore, it was suggested that Galectin-3 could contribute to the development of heart failure [36,40] and its inhibition results in reduction of cardiac fibrosis.[41] Deposits of type III and type IV collagens, laminin and fibronectin occur during subacute and chronic phases of T. cruzi infection.[42] Treatment with the anti-trypanosomal agents, benznidazole or MK-436 resulted in regression of the inflammatory and fibrotic lesions.[43] However, this effect may be parasite strain-dependent.[43]
Myocardial biopsies from patients with chronic Chagas cardiomyopathy have demonstrated that fibrosis is associated with activation of the Smad2 pathway, suggesting accentuated canonical TGF-β signaling. In murine Chagas disease, increased level of TGF-β in α2-macroglobulin knockout mice was associated with increased fibrosis. T. cruzi–infected mice treated with TGF-β inhibitors, SB-43154231 or GW78838848, showed a consistent reduction of heart fibrosis as determined by expression of fibronectin and type I collagen.[44] Others have shown that administration of ganglioside GM1 reduced arrhythmias in patients with Chagas disease [45] and fibrosis in T. cruzi–infected mice.[46] The antifibrotic effect was associated with a significant reduction in myocardial expression of TGF-β and in IFN-γ, TNF-α and CCL5/RANTES, all proinflammatory cytokines and chemokines. Thus, treatment with ganglioside GM1 may offer a potential therapy for modulating myocardial damage in chronic Chagas disease.
Endothelin-1 (ET-1) is a 21 amino acid peptide which has been implicated in the pathogenesis of cardiac fibrosis including that associated with Chagas disease. Mice treated with phosphoramidon, an inhibitor of endothelin converting enzyme, which mediates the synthesis of ET-1, resulted in a reduction in cardiac pathology including fibrosis when administered early in infection.[47] Additionally, T. cruzi infection of mice in which the ET-1 gene has been selectively knocked out resulted in a marked reduction in cardiac fibrosis.[48] In both of these models, cardiomyopathy was ameliorated and determined by cardiac imaging studies.
Verapamil is a calcium-channel blocker which has been demonstrated to reduce mortality and significantly reduce inflammation and fibrosis in infected mice treated early in infection.[49,50] This effect was not demonstrable when treatment was begun during the chronic stage. Thus, many of these experimental studies suggest that the reduction of cardiac fibrosis is dependent, in part, on the timing of therapy, mouse species and parasite strain.
The Garg laboratory has pioneered studies on the role of oxidative stress in chronic Chagas cardiomyopathy. T. cruzi–induced intracellular Ca+2 flux, required for parasite invasion,[52] elicits mitochondrial loss of membrane potential and an increase in the generation of mitochondrial reactive oxygen species (mtROS) contributing to oxidative adducts in cardiac myocytes.[53,54] The mitochondrial inefficiency in the setting of oxidative phosphorylation continues during the chronic disease phase.[55,56] Enhancing the host’s capacity to scavenge ROS by chemical antioxidant or by genetic overexpression of mitochondrial superoxide dismutase led to improved mitochondrial function that was associated with a significant control of oxidative and inflammatory stress and cardiac remodeling in T. cruzi–infected mice.[57,58] Chagas disease patients with clinically symptomatic disease exhibited higher levels of oxidative and inflammatory stress markers and mitochondrial dysfunction than was noted in clinically asymptomatic infected individuals.[55,59] It is not mechanistically proven what triggers mitochondrial defects and why it is not arrested to prevent continuous oxidative damage. Some evidence suggest that mtROS-induced DNA adducts result in poly(ADP-ribose) polymerase 1 (PARP1) activation in infected cardiac myocytes.[60] PARP1 is a DNA repair enzyme; however, its hyperactivation results in excessive utilization of NAD+ (substrate) and PARylation of mitochondrial proteins, thus, setting the stage for metabolic and oxidative imbalance in the heart. Other studies suggest that PARP1/PAR result in activation of the NF-κB pathway of proinflammatory cytokines (e.g., TNF-α, IL-1β) production in infected cardiac myocytes.[60,61] Thus, it is possible that targeting PARP1 will provide dual benefits in controlling Chagas disease inflammatory and oxidative stress, to be tested in future studies.
3. Vaccine development
Studies in animal models and humans have revealed the pathogenic mechanisms found during disease progression, and the features of protective immunity.[24] Parasite-specific CD4+ T cells have been suggested to assist in T. cruzi control through secretion of Th1 cytokines (e.g., IFN-γ, IL2), amplification of the phagocytic activity of macrophages, stimulation of B-cell proliferation, and antibody production and differentiation and activation of CD8+ T cells (reviewed in [24]). T. cruzi antigen-specific CD8+ T cells contribute to parasite control, either by cytolysis of the infected cells or by secretion of Th1 cytokines (IFN-γ) that induce trypanocidal activity (reviewed in [62]). Accordingly, several antigens (e.g., GP90, cruzipain, GP82, ASP2, GP56, Tc52, CRP, TSA1, Tc24) have been tested for their ability to elicit protective immunity to T. cruzi in experimental animals (reviewed in [63]). A majority of the candidate antigens were selected based upon their potential to be recognized by antibodies in infected mice and have proved to be efficacious as vaccine in providing some degree of protection from T. cruzi infection. In parallel, efforts to enhance the protective efficacy of subunit vaccines against T. cruzi have included testing the use of adjuvants, e.g., saponin, CpGODN, IL-12 and GMCSF cytokines,[23,64] attenuated strain of Salmonella [65] or adenovirus [66] for antigen delivery; and heterologous prime-boost protocols.[67]
To prevent investigator bias in vaccine design, the Garg group developed a computational/bioinformatic algorithm for screening the T. cruzi sequence database for the vaccine candidates.[68] These studies identified 11 potential candidates that were submitted to rigorous analysis for eliciting immunity against T. cruzi.[68,69] Eventually, these were narrowed down to TcG1, TcG2 and TcG4 as the candidates that exhibited relevant characteristics as vaccine candidates. These antigens were highly conserved in clinically relevant T. cruzi isolates, expressed (mRNA/protein) in trypomastigotes and amastigotes (mammalian stages) of T. cruzi, and released during parasite differentiation in host cell cytoplasm, a characteristic required for antigen presentation for T-cell activation.[68] These antigens showed antigen-specific antibody (IgG1, IgG2a and IgG2b) and/or CD8+ T-cell responses in T. cruzi-–infected mice and dogs; and were also recognized by antibody response in Chagas disease patients from distinct study sites (Argentina–Bolivia and Mexico–Guatemala), and expressed in diverse strains of the circulating parasites (Unpublished Data nj garg and [70]). Immunization with TcG1, TcG2 and TcG4 as a DNA vaccine provided T-cell immunity (TcG2 = TcG4 > TcG1) that was additive when the antigens were co-delivered and achieved a significant (but modest) control of challenge infection in mice and dogs.[68,69,71] The delivery of these antigens as heterologous prime/boost vaccine provided protective immunity consisting of parasite- and antigen-specific lytic antibodies and type 1 CD8+ cytotoxic T lymphocytes against challenge infection and chronic disease [72–74] that was significantly better than that observed with DNA-prime/DNA-boost vaccine. [68,69]
The enhanced efficacy of a heterologous prime/boost approach for vaccine delivery could be because delivery of antigens as DNA vaccines elicits robust T-cell responses, which are critical for the development of T-cell-dependent antibody responses, and DNA immunization is also highly effective in priming antigen-specific memory B cells.[75] Delivery of vaccine candidates as recombinant proteins is generally more effective at eliciting antibody responses and may directly stimulate antigen-specific memory B cells to differentiate into antibody-secreting cells, resulting in production of high-titer, antigen-specific antibodies.[76] In a recent study, it was demonstrated that TcG2 and TcG4 DNA prime/protein boost vaccine achieved long-term protection against T. cruzi infection and Chagas disease, suggesting the vaccine-induced effector T cells can be long-lived and provide protection from parasitic infection. This vaccine-induced immunity waned slightly after 6 months post booster immunization, but was still sufficient to provide twofold control of invading pathogens [77] that, according to mathematical modeling, is sufficient to break the parasite transmission cycle [78] and prevent disease progression.[79] Pertinent to the theme of this review, it is important to note that many of the studies discussed highlight the importance of a preventive or therapeutic vaccine to control T. cruzi infection by at least decreasing parasite burden, cardiac tissue inflammation and damage or increasing survival if not providing sterile immunity. This has recently been underscored in papers by Ma et al. [80] and Pereira et al. [81].
4. Stroke and atrial fibrillation
Chagas disease is a risk factor for stroke that may result in marked functional disability and death.[82–85] The incidence of thromboembolic events in patients with Chagas disease varies widely, depending on the population studied.[86] Necropsy studies assessing patients with advanced cardiomyopathy showed high rates of cerebral infarction,[87] and an increase in the prevalence of stroke is expected as a result of aging of the T. cruzi-infected population in Latin America.[88]
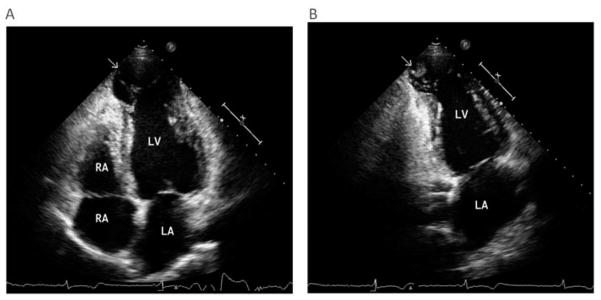
Cardioembolism is the main cause of stroke in patients with Chagas disease.[89] Several risk factors for stroke have been reported including heart failure, LV systolic dysfunction, left atrial enlargement, apical aneurysm, mural thrombus and arrhythmias.[82,84,85,89] However, stroke may occur in patients without clinical evidence of heart disease; a careful echocardiographic study may reveal mural thrombus and apical aneurysms (Figure 4A & B).[83,90] A cross-sectional study found that the classic vascular risk factors, including hypertension, diabetes mellitus and smoking, are less common in stroke patients with Chagas disease compared with those without.[91] Recurrence of stroke has been estimated to occur in 20% of patients with Chagas disease.[83]
Figure 4. Echocardiography of a patient with chronic chagas cardiomyopathy.
(A) Two-dimensional echocardiography at the apical four-chamber view of the left ventricle demonstrating a dilated apical aneurysm. There is a distinct limit between the normal contractile myocardium and the dyskinetic apex (arrow) with bulging motion during systole. (B) Two-chamber apical view showing an apical thrombus (arrow). There is a mass attached to the left ventricular apical aneurysm with a margin distinct from the underlying wall, which is dyskinetic.
LA: Left atrium, LV: Left ventricle; RA Right atrium; RV Right ventricle.
Atrial fibrillation often indicates advanced myocardial damage and is a predictor of mortality in patients with Chagas cardiomyopathy.[4,92,93] In addition, atrial fibrillation is an important risk factor for stroke, independent of the LV function.[82,94] Reports from a prospective hospital-based case–control study revealed that approximately 15% of patients with Chagas disease with stroke who were consecutively admitted to the hospital had atrial fibrillation. [82,85] Furthermore, a population-based cohort study showed that the presence of atrial fibrillation was a predictor of the risk of mortality from stroke in T. cruzi–infected elderly patients.[94] The risk of stroke varies widely among patients with atrial fibrillation, depending on other clinical features. Increased left atrial volume has been shown to be associated with an increased risk for stroke, independent of atrial fibrillation, age and other clinical risk factors for cerebrovascular disease.[95]
Studies assessing the source of embolism in Chagas disease found that the presence of left atrial thrombi was not associated with ischemic cerebral events.[83,90] However, low left atrial appendage flow velocity has been demonstrated to be an important predictor of embolic risk in atrial fibrillation, regardless of the presence of thrombus in the left atrium.[96] The relation of LV dysfunction and stroke appears to be partially mediated by stasis of flow in the left atrial appendage.[97] Indeed, cerebral infarction in Chagas disease occurs more commonly in the advanced chronic heart disease than in asymptomatic patients in the indeterminate stage of chronic disease.[84,85,87,94]
Although cardioembolism is the main cause of ischemic stroke related to Chagas disease, cryptogenic and small vessel strokes can also occur, especially in patients with mild chronic heart disease.[82] The increased proportion of patients with Chagas disease who are smokers, and have diabetes, obesity, and sedentary lifestyles may increase the risk of stroke.[82,98]
5. Electrocardiology of ventricular arrhythmias
Chagas cardiomyopathy is also an arrhythmogenic condition and sudden death may be the first manifestation of the disease.[99] Chagas disease may present with ventricular premature beats, short runs of non-sustained ventricular tachycardia (VT) or with malignant VT.[100] This is most likely due to areas of necrosis and fibrosis resulting from myocardial inflammation. This is also associated with microvascular lesions or autonomic changes that regulate blood perfusion of the injured myocardium. The damage to the intercellular junctions provokes changes in electrical potential and impair conduction of the stimulus between cells, causing electrical uncoupling, slow conduction of stimuli and unidirectional block.[100] This process, associated with the fibrotic areas, forms the reentrant circuit that causes ventricular arrhythmias.
The recognition of fibrosis, either by cardiac magnetic resonance imaging (CMRI) or eletrocardiological methods, allows the recognition of patients prone to develop arrhythmic complications.[101–103] CMRI, using the delayed enhancement technique, can be useful to select patients with global or regional ventricular dysfunction, with high degree of fibrosis and at higher risk for clinical VT.[103] The presence of zones of slow conduction in the myocardium may be recognized by the presence of prolonged filtered QRS duration obtained by signal averaged ECG, which was shown to be an independent predictor of death in Chagas disease patients.[101] Since both CMRI and signal-averaged ECG are not readily available, an alternative simple, noninvasive method is a 12-lead ECG QRS scoring system, which quantifies myocardial fibrosis, correlates with the scar, evaluated by CMRI, and with LV dysfunction and with malignant VT.[102]
The role of autonomic nervous system dysfunction in the mechanisms of ventricular arrhythmias and sudden death in Chagas cardiomyopathy has been the subject of investigations for decades,[104,105] since the classical description by Koeberle of the “cardiopatia parasympathicopriva”.[106]
Chagas disease patients may have reduced vagal modulation over the sinus node, causing reduced heart rate response to physiological and pharmacological stimulus,[107–109] as well as impaired heart rate variability, measured using time and frequency domain and nonlinear methods.[110,111] These abnormalities occur early in the course of the disease, even before the development of overt heart disease or LV dysfunction, generally with mild abnormalities,[109,111] suggesting an underlying disease-specific mechanism. Vagal dysfunction appears to be more prominent in patients with LV dysfunction [112] and can be detected even in older patients with Chagas disease, but under 70 years of age.[113] Cardiac sympathetic denervation has also been detected in some patients by iodine-123 (I-123) meta-iodobenzylguanidine scintigraphic studies,[114,115] and can occur early in the course of the disease.[115]
Although it was demonstrated that autonomic dysfunction is an early and typical finding in the natural history of human Chagas disease,[105,111] the association between impaired autonomic cardiac modulation and sudden death remains elusive. Small studies have shown an association between sympathetic innervation defects (as detected by iodine-123 meta-iodobenzylguanidine scintigraphic studies) and sustained VT. [114] In addition, autonomic-driven abnormal heart rate dynamics have been shown to precede VT in Chagas disease patients.[116] No major cohort study demonstrated a prognostic value of abnormal autonomic tests or reduced heart rate variability and the clinical value of these methods is still controversial.
More robust data exist linking the presence of cardiac repolarization abnormalities and the risk of death in Chagas cardiomyopathy. Abnormal QT interval or increased QTd dispersion,[117] as well as T-wave axis deviation [118] or primary T-wave abnormalities in the 12-lead ECG,[93] were associated with an increased risk of death in cohort studies. Other methods of evaluation of repolarization variability, as T-wave amplitude variability [119] and T-wave spatial heterogeneity,[120] were independently associated with higher risk in a relatively small cohort. Microvolt T-wave alternans, evaluated by a commercial method and useful in other conditions, is abnormal in Chagas cardiomyopathy patients and may be helpful in the risk stratification of Chagas disease patients.[121]
6. Heart failure: advances in pharmacological treatment
The treatment modalities employed in the management of heart failure in patients with Chagas cardiomyopathy are similar to that employed in the management of heart failure of other etiologies.[1] Therapy relies on the inhibition of renin-angiotensin-aldosterone and sympathetic systems, including a combination of three types of drugs: diuretics, beta blockers and angiotensin-converting enzyme (ACE) inhibitors.[1] ACE inhibitors remain the first-line treatment for inhibition of the renin-angiotensin system in heart failure in the setting of Chagas disease, but angiotensin receptor blockers are reasonable alternatives in patients who cannot tolerate the side effects of ACE inhibitors. ACE inhibitors have been shown to improve cardiac function from Chagas heart disease. Although studies were not powered to evaluate mortality.[122,123]
Beta blockers are another option in the management of Chagas heart disease. They improve clinical status and may reduce mortality.[122,124,125] However, previous studies demonstrated that the frequency of beta blocker use was lower in patients with Chagas disease when compared with other etiologies of heart failure.[17,124,125] This is likely due to concerns related to the possible risk of bradycardia and hypotension. A recommended strategy in the management of heart failure in the setting of Chagas disease is to give ACE inhibitors and diuretics at first to compensate for congestive symptoms. After the improvement of clinical status, beta blockers can be safely given at targeted doses, if necessary reducing ACE inhibitors doses.[122] Aldosterone receptor antagonists seem to be safe in Chagas cardiomyopathy and should be added in patients who are already on ACE inhibitors (or angiotensin receptor blockers) and beta blockers in symptomatic patients.[1]
Digoxin has long been used in the treatment of patients with heart failure secondary to Chagas cardiomyopathy. This drug may be added to the initial regimen in patients with severe symptoms who have not yet responded symptomatically to standard therapy.[1] Patients should not be given digoxin if they have significant sinus or atrioventricular blockade unless it has been addressed with the insertion of a permanent pacemaker. The drug should be used cautiously in patients taking other drugs that can depress sinus or atrioventricular nodal function or affect digoxin levels, especially amiodarone or beta blockers. Although treatment with digoxin had no effect on mortality in non-Chagas heart failure, the use of this drug can improve symptoms and exercise tolerance in patients with Chagas cardiomyopathy.[1,126]
There are insufficient data to make broad recommendations regarding anticoagulation in patients with Chagas cardiomyopathy. In general, anticoagulation is recommended for patients with Chagas disease who have permanent/paroxysmal atrial fibrillation, previous thromboembolic events, or the presence of a cardiac thrombus detected by echocardiography.[127] Sousa et al., prospectively studying 1043 patients with chronic Chagas disease,[128] developed a risk score for ischemic attacks. LV dysfunction was given 2 points and apical aneurism, abnormalities of the ST-T segment and age >48 years, 1 point each. Anticoagulation with warfarin was indicated in patients with 4–5 points (4.4%/year risk of stroke versus 2.0%/year risk of severe bleeding). The role of new anticoagulants and anti-platelet drugs in the prevention of thromboembolic events has yet to be determined in the setting of Chagas disease.
Heart transplantation can be used for selected patients with refractory end-stage heart failure [127] and may be the only option for some refractory patients.[129] This approach has been successful at some centers. However, the problems with this approach include high cost, donor availability and accessibility especially in rural areas. Additionally, there is the risk of re-activation of the infection as a result of the administration of immunosuppressive drugs.[130] Recurrent life-threatening ventricular arrhythmia that is refractory to all currently available treatments is a less common indication for cardiac transplantation in patients with Chagas cardiomyopathy. Monitoring of the reactivation of infection with T. cruzi after heart transplantation should be performed routinely.[127] Biventricular pacing could be an effective alternative for selected patients,[131,132] but should be restricted to patients who presented with spontaneous or pacemaker-induced left bundle branch block, since those with right-bundle branch block do not benefit with the implantation of the pacemaker.[133] Multidisciplinary approach of heart failure patients, including cardiac rehabilitation and pharmaceutical care, may be helpful in improving symptoms and quality of life in Chagas disease patients.[134,135]
7. Heart failure: stem cell therapy
As stem cell therapy has been used increasingly often for other cardiomyopathies (for review see [136,137]), a number of studies have now applied variations of this strategy in rodent models of chronic Chagas cardiomyopathy and a small number of clinical trials have begun. One strategy for therapy is to use the entire population of bone marrow mononuclear cells (BMMCs), which contains hematopoietic progenitors and also mesenchymal stem cells (MSCs), which are capable of differentiating into multiple cell types including cardiac myocytes and endothelial cells.[138]
An initial report, in a mouse model of Chagas cardiomyopathy,[139] demonstrated that intravenous BMMC injection significantly reduced cardiac inflammation and fibrosis for up to 6 months after treatment. This study also used enhanced green fluorescent protein-tagged donor cells to show that at least some BMMCs had migrated to and were retained within the heart. In a follow-up study using cardiac MRI, tail vein injection of BMMCs was shown to prevent and even reverse the right ventricular (RV) dilatation induced by both acute (1 month) and chronic (up to 6 months) T. cruzi-infection.[140] Subsequently, it was reported that fibrosis and inflammation were reversed in chronically infected mouse heart 2 months after mononuclear cell injection.[141] Moreover, microarray analysis in this study revealed that the extensive gene expression changes previously identified in chronic Chagas cardiomyopathy [142] were largely (about 85%) restored to control values by cell therapy. Because the proportion of MSCs in the BMMC population is very low (<0.02%; [143]) and these cells are both anti-inflammatory and capable of differentiating into myocytes, purified MSCs have been evaluated for efficacy in cell therapy.
Jasmin and colleagues [144,145] demonstrated that tail vein injected MSCs were as effective as BMMCs in reducing RV dilation and restoring expression patterns of selected genes. Tracking of nanoparticle-labeled cells revealed significant homing to the heart at early times after injection in infected mice, although improvement of cardiac function was attributed to paracrine action because newly differentiated labeled myocytes were not found in the heart. The success achieved with BMMC and MSC therapy in rodent model of Chagas cardiomyopathy led to clinical trials evaluating safety and efficacy of BMMC cell therapy in these patients.[144,146,147] Both trials involved patients with heart failure due to chronic Chagas cardiomyopathy, with severely compromised left ventricular ejection fraction (LVEF) and NYHA class III or IV. Bone marrow was aspirated and BMMCs enriched through gradient centrifugation were injected in coronary arteries. After 25 days, patients received G-CSF injections for 5 days (based on studies demonstrating that this factor decreased fibrosis and inflammation: [148]). Results of the initial trial were demonstrated safety of the procedure and small but significant increase in LVEF and 6-min walking.
Efficacy of BMMCs in patients with chronic Chagas cardiomyopathy was tested in a more extensive phase 2/3 study,[147] with data from 183 patients analyzed. The results showed that both groups, BMMCs and placebo, improved LVEF at 6 and 12 months follow-up, but there was no significant difference between the two groups. This was also observed in secondary endpoints such as the 6-min walking distance, and NYHA class. It was concluded that the procedure was safe but that injection of autologous BMMCs in patients with chronic Chagas cardiomyopathy did not provide additional benefit when compared to standard drug therapy.
Substantial progress has thus been made toward realizing therapeutic efficacy for chronic Chagas cardiomyopathy in rodent models and is beginning to clarify mechanisms underlying efficacy. However, significant therapeutic benefit of stem cell therapy has not been demonstrated in the few clinical trials that have been undertaken testing cell-based approaches in Chagas disease patients. In order to optimize translation of the success in small animal studies to clinical practice, several new strategies could be applied.
Intermediate-sized models, such as dogs, should be used to confirm rodent results whenever possible.[146]
Novel stem cell types could be used in the studies. Most studies of cell therapy in chronic Chagas cardiomyopathy have used BMMCs or MSCs, but there are multiple other sources of MSCs, including adipose tissue, dental pulp, umbilical cord and other tissues. It is notable that early studies by Guarita-Souza et al. [149] injected a mixture of skeletal myoblasts and mesenchymal bone marrow–derived cells that had been co-cultured for 2 weeks into the LV of chronically infected rats. At 1 month after injection, end-systolic and diastolic volumes were decreased and ejection fraction was increased. This study raises the possibility that cells with greater differentiation potential might show promise. One new avenue worth pursuing is reprogramming to reverse fate of differentiated blood cells or fibroblasts by a cocktail of only a few transcription factors,[150] thereby providing cells that might replace damaged myocytes.
Manipulations of treatments used to vary such parameters as route of delivery and co-therapy with adjunctive treatment. In small animal model studies, stem cells have been delivered through injection into tail vein, local injection into the myocardium, and even intraperitoneally. In patient trials, the route of delivery is generally though catheterization for local placement within the cardiac circulation.
Severity of disease at time of treatment and possible presence of comorbidities should be carefully evaluated in human patients who qualify for the trials on the basis of disease severity.
8. Parasite persistence and specific treatment
The role of the persistence of a low-grade T. cruzi infection in the development of chronic heart disease had been a matter of controversy for decades.[151–154] The presence of both tissue parasitism and parasitemia in chronic Chagas disease has been established and supported by the transmission of the disease by chronically infected persons by blood and placental route, [155,156] as well as the reactivation of the disease due to immunosuppression after organ transplantation, HIV infection or autoimmune diseases.[157] There is substantial evidence that parasite persistence is important in the progression of chronic Chagas disease. In a landmark study, Zhang and Tarleton [158] showed, in a murine model, that parasite persistence correlates with the presence of disease in the heart and the clearance of parasites from tissues was associated with amelioration of inflammation. Subsequently, Schijman et al. demonstrated that the extent of inflammation, fibrosis and severity of the disease was associated with persistence of parasite DNA in cardiac lesions obtained from Chagas disease patients.[159] These findings were confirmed by Benvenuti et al. [160], who analyzed heart biopsies from 29 individuals for T. cruzi DNA and found 25 positive by PCR, as well as a significant positive association between myocardial parasite persistence and high-grade myocarditis. In an article from the NIH-sponsored REDS II cohort, Sabino et al. [161] studied 499 T. cruzi seropositive blood donors and 101 patients with clinically diagnosed Chagas cardiomyopathy. Rates of PCR detection of T. cruzi DNA in blood were significantly higher in those diagnosed with Chagas cardiomyopathy (75.2%) compared with seropositive donors without cardiomyopathy (51.3%). Presence of parasitemia significantly correlated with known markers of disease progression, such as QRS prolongation, reduced LV ejection fraction and higher levels of troponin and NTpro-BNP.[161]
The importance of the parasite persistence in the development of Chagas cardiomyopathy is also supported by a large non-randomized clinical trial reported by Viotti et al. [162], showing that benznidazole treatment, compared with no treatment, was associated with reduced progression of Chagas disease and increased negative seroconversion for patients with chronic disease.
The results from various studies suggested that specific antiparasitic therapy could potentially halt the progression of the disease. [162,163] In fact, most experts recommend the anti-trypanosomal treatment for most of patients with chronic T. cruzi infection, with or without cardiomyopathy, especially in those below 50 years of age.[152,163–165] However, there are many concerns related to the toxicity of the currently available drugs, the absence of evidence of benefit in those older than 50 years of age and the absence of a randomized clinical trial that documented the beneficial effect of the specific treatment.[127,166] There are only two drugs currently available for the treatment of Chagas disease, nifurtimox and benznidazole, both associated with significant toxicity, especially in adults. A recent meta-analysis on trypanocidal drugs for chronic asymptomatic Chagas disease included 13 studies involving 4229 participants and showed that, although trypanocidal drugs reduce parasite-related outcomes, the effect on clinical outcomes remains elusive.[167] It was expected that the results of a multicenter, randomized, double-blinded, placebo-controlled trial would demonstrate a beneficial effect of benznidazole on cardiac outcomes in patients with chronic infection (BENEFIT study).[168] However, the recently published results of that study revealed that although there was a significant reduction in parasitemia, benznidazole therapy did not halt the progression of cardiac disease.[169]
Treatment of Chagas disease is mandatory for all acute infections, caused by vector transmission, oral transmission, congenital infection, laboratory accidents or organ transplantation associated with reactivation due to immunosuppression and for children with chronic infection up to 12 or up 18 years of age.[127]
Posaconazole, a triazole antifungal drug with high trypanocidal activity, was a promising antiparasitic drug, but a randomized clinical trial recently published showed that most patients treated with posaconazole recur with detectable parasitemia during a follow-up period of 10 months.[170] Another randomized clinical trial evaluating the association of posaconazole and benznidazole, the STOP-CHAGAS trial (NCT01377480), is currently underway. The antiparasitic effect of the anti-arrhythmic drug amiodarone, demonstrated in experimental studies, was not confirmed in the clinical setting.[171]
9. Other developments in drug therapy
Considering the uncertainties regarding the effectiveness and the toxicity of currently available drugs, there is a renewed interest in the discovery and development of new drugs for treatment of chronic Chagas disease.[172,173] A major goal of drug therapy is the delivery of drug not only to the bloodstream but also to the intracellular site and nanotechnology offers the possibility of enhancing the drug delivery to intracellular sites. There are several drug strategies that show promise with respect to limiting the severe vascular/cardiac consequences of chronic inflammatory response to the chronic infection. These drug strategies are based on the reported efficacy of nitric oxide (NO) and its related bioactive forms,[174–176] curcumin [177] and selenium. [178,179] Two of these (NO and curcumin) also hold promise with respect to limiting infectivity. A major limitation in translating the promising preclinical studies into actual therapies that can be deployed and administered in a cost- and time-effective manner is the issue of drug delivery with respect to enhancing bioavailability, rate and duration of delivery, mode of delivery and site-specific targeting to tissues.
Nanoparticle platforms have been developed that show promise with respect to overcoming many of the drug delivery limitations. One such platform is based on a hybrid composition in which the high porosity of a silane-derived hydrogel is “plugged” using chitosan to form a glass-like hydrogen bonding network.[180] NO and N2O3, a potent nitrosating agent, can be generated from nitrite within this glassy matrix. The release of NO or any other incorporated therapeutics does not occur when the nanoparticles are either dry or in a nonaqueous medium. The introduction of water triggers the release. Release rates can be easily controlled by tuning the composition of the nanoparticle. These nanoparticles can deliver therapeutic payloads through topical,[181] mucosal, intravenous [182] and intraperitoneal administration routes.[182] Mucosal delivery of myristic acid containing NO releasing nanoparticles produces sustained systemic NO-related vascular consequences seemingly comparable to the sustained response to NO administered intravenously (IV) releasing nanoparticles (unpublished results).
The NO releasing nanoparticles have been shown to be highly effective as a very broad spectrum antimicrobial agent. [133,183–185] Additionally, and most significantly, is that these nanoparticles, when administered IV in animal models, have been shown to be anti-inflammatory [182] with respect to vascular dysfunction and the inflammatory cascades induced by several triggers including acellular hemoglobin, acute hemorrhage,[186] and lipopolysaccharide induced endotoxemia. These biocompatible nontoxic NO-releasing nanoparticles were shown to normalize the cytokine profiles, restore/increase tissue perfusion, deactivate platelets, inhibit leukocyte activation and normalize shock-activated macrophage populations (unpublished results). Similar anti-inflammatory properties were observed for IV infused nanoparticles containing a curcumin–selenium complex (unpublished results) and for curcumin-loaded nanoparticles applied topically to mouse knees in an induced osteoarthritis model (unpublished results). The relevant finding with respect to Chagas disease is that the nanoparticles traversed the skin and are then localized in fat pads where drug is stored and slowly released. The implication is that these nanoparticles can be used topically to create a large reservoir of curcumin (or curcumin–selenium complex) in fatty tissues that will provide sustained therapeutic levels not achievable by oral administration.
A newly developed platform incorporates paramagnetic nanocrystalline centers (derived from gadolinium oxide) into the hybrid hydrogel nanoparticles described above. These paramagnetic nanoparticles can be rapidly and efficiently localized subsequent to intravenous administration using a powerful external magnetic field placed at the target site. This platform would allow for targeted delivery of high levels of NO, nitrosothiols, curcumin, curcumin–selenium to the heart. Similarly, this paramagnetic platform could deliver siRNAs designed to repair or rebuild damaged myocardium. Nanoparticle technology will very likely open up new therapeutic options for the treatment of many aspects of Chagas disease.
Expert commentary
Chagas disease is an important cause of heart disease not only in endemic areas of Latin America but also among those that have emigrated from endemic areas to non-endemic areas of North America, and Europe where physicians are now encountering what is for them a “new disease.” Chagas disease persists as a disease related to poverty, both in endemic and non-endemic countries, affecting people who came from rural areas with poor housing conditions. The access of those millions of infected persons to health care and drugs remains one the most difficult challenges of this neglected disease.
Chagas disease is a complex disease with a very long natural history and a wide spectrum of clinical manifestations, ranging from asymptomatic patients without apparent cardiomyopathy to severe cardiac and digestive manifestations. Although several aspects of the disease had been elucidated in the last decades, important questions remain unsolved. While several studies have documented mechanisms involved in the development of inflammation and fibrosis, as well as the role of the oxidative stress, the translational aspects of these studies have not been fully achieved. A better understanding of the role of parasite persistence in the severity of the manifestations of the chronically infected patients is an important area of research for future studies.
There only two antiparasitic drugs commercially available for clinical use that have problems with both efficacy and toxicity. The first phase of the BENEFIT trial which aimed to evaluate the effectiveness of benznidazole in chronic Chagas disease, has finished the required follow-up period and the results have been disappointing. Thus, new and safer effective drugs are needed. It is possible that new strategies can be developed from innovative approaches, as effective vaccines or the use of nanotechnology for targeted delivery of antiparasitic drugs.
In the clinical setting, there are many challenges related to the paucity of specific studies addressing questions about the specificity of the care of patients with Chagas cardiomyopathy. Further research is needed on the use of diagnostic methods and for guiding treatment of the various clinical syndromes seen with Chagas disease. Promising new treatments, such as stem cell therapy, have not fulfilled our expectations, and research to understand why stem therapy results have been different in mice and humans should lead to improved stem cell therapeutics for this infection.
Five-year view
Since research on Chagas disease has now intensified, we expect to see, in the next 5-year span, more data on the diagnostic and prognostic values of several clinical tests, especially of ECG-derived and blood biomarkers. The initial results of the BENEFIT trial while disappointing may open the possibility of new clinical trials, perhaps employing a combination of drugs.
The role of mitochondrial oxidative stress and inflammation in pathogen control versus causing host injury can only be delineated with future longitudinal studies in humans, or large animal models, and will guide the promise of antioxidant and anti-inflammatory therapies in controlling progressive Chagas cardiomyopathy.
There remains a dearth of data on the immune response in human Chagas disease, and these data are needed for further development and testing of prophylactic and therapeutic efficacy of currently developed vaccine formulations as well as guide the development of next generation vaccines.
The promising results of mononuclear and mesenchymal stem cells in the rodent model of Chagas cardiomyopathy, in our opinion, warrant continued optimization in this model. Such studies should focus on selection of most effective and clinically applicable stem cell type, development of adjunctive therapy to improve cardiac recovery and determination of mechanisms responsible for functional improvement. Other large animal models will likely be useful for fine-tuning the translational studies to the clinic.
Key issues.
Chagas disease remains an important cause of morbidity and mortality in endemic areas of Latin America and among infected individuals that have immigrated to non-endemic areas of the world.
Myocardial fibrosis is a major pathophysiological process in Chagas cardiomyopathy. While the mechanistic basis is not entirely known, induction of TGF-β, Galectin-3, ET-1 and other proinflammatory cytokines and chemokines appears to be important.
Clinical recognition of fibrosis, either by cardiac magnetic resonance imaging or eletrocardiological methods, may allow the recognition of patients prone to develop arrhythmic complications.
Clinical management of stroke, atrial fibrillation and heart failure is still challenging and clinical studies in Chagas cardiomyopathy are needed.
Current research in vaccine development for prevention and/or therapy is only in its infancy but there is hope that this can be achieved.
Although stem-cell-based therapy showed promising results in animal experiments, clinical trials did not demonstrate clinical benefit in patients.
Specific antiparasitic therapy could potentially halt the progression of the disease; however, the toxicity and limited effectiveness of current available drugs, benznidazole and nifurtimox, are obstacles to their widespread use.
There is a renewed interest in the discovery and development of new drugs and methods for treatment of chronic Chagas disease; nanoparticle platforms have been developed that show promise with respect to improving drug delivery with respect to enhancing bioavailability, rate and duration of delivery, mode of delivery, and site-specific targeting to tissues.
Footnotes
Financial & competing interests disclosure
This study was supported by grants from the National Institutes of Health, grant numbers: HL094802, HL088230, AI054578 and AI107227 (to NJ Garg), HL110900 (to JM Friedman), GM104547 (to JN Lora) and AI098461 (to AL Ribeiro); HL122866 (to J Nagajyothi); and grants from the Brazilian National Research Council (CNPq - Bolsa de Produtividade em Pesquisa) to AL Ribeiro, FS Machado and MC Nunes; and grants from the Foundation for Research Support of Minas Gerais (FAPEMIG – Pesquisador Mineiro) to AL Ribeiro, FS Machado and MC Nunes. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Ribeiro AL, Nunes MP, Teixeira MM, et al. Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol. 2012;9(10):576–589. doi: 10.1038/nrcardio.2012.109. [DOI] [PubMed] [Google Scholar]
- 2.Garcia MN, Aguilar D, Gorchakov R, et al. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg. 2015;92(2):325–330. doi: 10.4269/ajtmh.14-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Molina JA, Perez AM, Norman FF, et al. Old and new challenges in Chagas disease. Lancet Infect Dis. doi: 10.1016/S1473-3099(15)00243-1. In press. [DOI] [PubMed] [Google Scholar]
- 4.Nunes MC, Dones W, Morillo CA, et al. Chagas disease: an overview of clinical and epidemiological aspects. J Am Coll Cardiol. 2013;62(9):767–776. doi: 10.1016/j.jacc.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS Negl Trop Dis. 2011;5(4):e1136. doi: 10.1371/journal.pntd.0001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90(6):33–44. [PubMed] [Google Scholar]
- 7.GBD 2013 Mortality and Cause of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silveira AC, Dias JC. The control of vectorial transmission. Rev Soc Bras Med Trop. 2011;44(Suppl 2):52–63. doi: 10.1590/s0037-86822011000800009. [DOI] [PubMed] [Google Scholar]
- 9.Bern C, Kjos S, Yabsley MJ, et al. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011;24(4):655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shikanai-Yasuda MA, Carvalho NB. Oral transmission of Chagas disease. Clin Infect Dis. 2012;54(6):845–852. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- 11.Sabino EC, Ribeiro AL, Salemi VM, et al. Ten-year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi-seropositive former blood donors. Circulation. 2013;127(10):1105–1115. doi: 10.1161/CIRCULATIONAHA.112.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Ayala A, Perez-Molina JA, Norman F, et al. Gastro-intestinal Chagas disease in migrants to Spain: prevalence and methods for early diagnosis. Ann Trop Med Parasitol. 2011;105(1):25–29. doi: 10.1179/136485910X12851868780423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souza DH, Vaz MD, Fonseca CR, et al. Current epidemiological profile of Chagasic megaesophagus in Central Brazil. Rev Soc Bras Med Trop. 2013;46(3):316–321. doi: 10.1590/0037-8682-0065-2013. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro AL, Sabino EC, Marcolino MS, et al. Electrocardiographic abnormalities in Trypanosoma cruzi seropositive and seronegative former blood donors. PLoS Negl Trop Dis. 2013;7(2):e2078. doi: 10.1371/journal.pntd.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Secretaria de Vigilância em Saúde do Ministério da Saúde. Consenso Brasileiro em Doença de Chagas. Rev Soc Bras Med Trop. 2005;38(Suppl 3):7–29. [PubMed] [Google Scholar]
- 16.Nunes MC, Carmo AA, Rocha MO, et al. Mortality prediction in Chagas heart disease. Expert Rev Cardiovasc Ther. 2012;10(9):1173–1184. doi: 10.1586/erc.12.111. [DOI] [PubMed] [Google Scholar]
- 17.Nunes MD, Barbosa MM, Ribeiro AL, et al. Predictors of mortality in patients with dilated cardiomyopathy: relevance of Chagas disease as an etiological factor. Rev Esp Cardiol. 2010;63(7):788–797. doi: 10.1016/s1885-5857(10)70163-8. [DOI] [PubMed] [Google Scholar]
- 18.Rassi A, Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115(9):1101–1108. doi: 10.1161/CIRCULATIONAHA.106.627265. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira MM, Gazzinelli RT, Silva JS. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18(6):262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- 20.Marino AP, da Silva A, dos SP, et al. Regulated on activation, normal T cell expressed and secreted (RANTES) antagonist (Met-RANTES) controls the early phase of Trypanosoma cruzi-elicited myocarditis. Circulation. 2004;110(11):1443–1449. doi: 10.1161/01.CIR.0000141561.15939.EC. [DOI] [PubMed] [Google Scholar]
- 21.Cunha-Neto E, Chevillard C. Chagas disease cardiomyopathy: immunopathology and genetics. Mediators Inflamm. 2014;2014:683230. doi: 10.1155/2014/683230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi ML, De Morais CF, Pereira Barreto AC, et al. The role of active myocarditis in the development of heart failure in chronic Chagas’ disease: a study based on endomyocardial biopsies. Clin Cardiol. 1987;10(11):665–670. doi: 10.1002/clc.4960101113. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Chagoyan JC, Gupta S, Garg NJ. Vaccine development against Trypanosoma cruzi and Chagas disease. Adv Parasitol. 2011;75:121–146. doi: 10.1016/B978-0-12-385863-4.00006-X. [DOI] [PubMed] [Google Scholar]
- 24.Machado FS, Dutra WO, Esper L, et al. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol. 2012;34(6):753–770. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariano FS, Gutierrez FR, Pavanelli WR, et al. The involvement of CD4 +CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 2008;10(7):825–833. doi: 10.1016/j.micinf.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.da Matta Guedes PM, Gutierrez FR, Maia FL, et al. IL-17 produced during Trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis. PLoS Negl Trop Dis. 2010;4(2):e604. doi: 10.1371/journal.pntd.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samudio M, Montenegro-James S, de Cabral M, et al. Differential expression of systemic cytokine profiles in Chagas’ disease is associated with endemicity of Trypanosoma cruzi infections. Acta Trop. 1998;69(2):89–97. doi: 10.1016/s0001-706x(97)00118-6. [DOI] [PubMed] [Google Scholar]
- 28.Moretti E, Basso B, Cervetta L, et al. Patterns of cytokines and soluble cellular receptors in the sera of children with acute Chagas’ disease. Clin Diagn Lab Immunol. 2002;9(6):1324–1327. doi: 10.1128/CDLI.9.6.1324-1327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonseca SG, Moins-Teisserenc H, Clave E, et al. Identification of multiple HLA-A*0201-restricted cruzipain and FL-160 CD8+ epitopes recognized by T cells from chronically Trypanosoma cruzi-infected patients. Microbes Infect. 2005;7(4):688–697. doi: 10.1016/j.micinf.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Cunha-Neto E, Kalil J. Autoimmunity in Chagas’ heart disease. Sao Paulo Med J. 1995;113(2):757–766. doi: 10.1590/s1516-31801995000200005. [DOI] [PubMed] [Google Scholar]
- 31.Nogueira LG, Santos RH, Ianni BM, et al. Myocardial chemokine expression and intensity of myocarditis in Chagas cardiomyopathy are controlled by polymorphisms in CXCL9 and CXCL10. PLoS Negl Trop Dis. 2012;6(10):e1867. doi: 10.1371/journal.pntd.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araujo-Jorge TC, Waghabi MC, Hasslocher-Moreno AM, et al. Implication of transforming growth factor-beta1 in Chagas disease myocardiopathy. J Infect Dis. 2002;186(12):1823–1828. doi: 10.1086/345882. [DOI] [PubMed] [Google Scholar]
- 33.Cunha-Neto E, Dzau VJ, Allen PD, et al. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas’ disease cardiomyopathy. Am J Pathol. 2005;167(2):305–313. doi: 10.1016/S0002-9440(10)62976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29(5):196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araujo-Jorge TC, Waghabi MC, Bailly S, et al. The TGF-beta pathway as an emerging target for Chagas disease therapy. Clin Pharmacol Ther. 2012;95(2):613–621. doi: 10.1038/clpt.2012.102. [DOI] [PubMed] [Google Scholar]
- 36.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51(4):600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reis MM, Higuchi ML, Aiello VD, et al. Growth factors in the myocardium of patients with chronic Chagasic cardiomyopathy. Rev Soc Bras Med Trop. 2000;33(6):509–518. doi: 10.1590/s0037-86822000000600001. [DOI] [PubMed] [Google Scholar]
- 38.Saraiva RM, Waghabi MC, Vilela MF, et al. Predictive value of transforming growth factor-beta1in Chagas disease: towards a biomarker surrogate of clinical outcome. Trans R Soc Trop Med Hyg. 2013;107(8):518–525. doi: 10.1093/trstmh/trt050. [DOI] [PubMed] [Google Scholar]
- 39.Pineda MA, Cuervo H, Fresno M, et al. Lack of galectin-3 prevents cardiac fibrosis and effective immune responses in a murine model of Trypanosoma cruzi infection. J Infect Dis. 2015;212(7):1160–1171. doi: 10.1093/infdis/jiv185. [DOI] [PubMed] [Google Scholar]
- 40.de Boer RA, Voors AA, Muntendam P, et al. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11(9):811–817. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 41.Yu L, Ruifrok WP, Meissner M, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6(1):107–117. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 42.Andrade SG, Grimaud JA, Stocker-Guerret S. Sequential changes of the connective matrix components of the myocardium (fibronectin and laminin) and evolution of cardiac fibrosis in mice infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1989;40(3):252–260. doi: 10.4269/ajtmh.1989.40.252. [DOI] [PubMed] [Google Scholar]
- 43.Andrade SG, Stocker-Guerret S, Pimentel AS, et al. Reversibility of cardiac fibrosis in mice chronically infected with Trypanosoma cruzi, under specific chemotherapy. Mem Inst Oswaldo Cruz. 1991;86(2):187–200. doi: 10.1590/s0074-02761991000200008. [DOI] [PubMed] [Google Scholar]
- 44.de Oliveira FL, Araujo-Jorge TC, de Souza EM, et al. Oral administration of GW788388, an inhibitor of transforming growth factor beta signaling, prevents heart fibrosis in Chagas disease. PLoS Negl Trop Dis. 2012;6(6):e1696. doi: 10.1371/journal.pntd.0001696. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Iosa D, Massari DC, Dorsey FC. Chagas’ cardioneuropathy: effect of ganglioside treatment in chronic dysautonomic patients: a randomized, double-blind, parallel, placebo-controlled study. Am Heart J. 1991;122(3 Pt 1):775–785. doi: 10.1016/0002-8703(91)90525-m. [DOI] [PubMed] [Google Scholar]
- 46.Cutrullis RA, Poklepovich TJ, Postan M, et al. Immunomodulatory and anti-fibrotic effects of ganglioside therapy on the cardiac chronic form of experimental Trypanosoma cruzi infection. Int Immunopharmacol. 2011;11(8):1024–1031. doi: 10.1016/j.intimp.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Jelicks LA, Chandra M, Shirani J, et al. Cardioprotective effects of phosphoramidon on myocardial structure and function in murine Chagas’ disease. Int J Parasitol. 2002;32(12):1497–1506. doi: 10.1016/s0020-7519(02)00136-4. [DOI] [PubMed] [Google Scholar]
- 48.Tanowitz HB, Huang H, Jelicks LA, et al. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect Immun. 2005;73(4):2496–2503. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Souza AP, Tanowitz HB, Chandra M, et al. Effects of early and late verapamil administration on the development of cardiomyopathy in experimental chronic Trypanosoma cruzi (Brazil strain) infection. Parasitol Res. 2004;92(6):496–501. doi: 10.1007/s00436-004-1080-1. [DOI] [PubMed] [Google Scholar]
- 50.Morris SA, Weiss LM, Factor S, et al. Verapamil ameliorates clinical, pathologic and biochemical manifestations of experimental chagasic cardiomyopathy in mice. J Am Coll Cardiol. 1989;14(3):782–789. doi: 10.1016/0735-1097(89)90126-5. [DOI] [PubMed] [Google Scholar]
- 51.Johndrow C, Nelson R, Tanowitz H, et al. Trypanosoma cruzi infection results in an increase in intracellular cholesterol. Microbes Infect. 2014;16(4):337–344. doi: 10.1016/j.micinf.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burleigh BA, Woolsey AM. Cell signalling and Trypanosoma cruzi invasion. Cell Microbiol. 2002;4(11):701–711. doi: 10.1046/j.1462-5822.2002.00226.x. [DOI] [PubMed] [Google Scholar]
- 53.Gupta S, Bhatia V, Wen JJ, et al. Trypanosoma cruzi infection disturbs mitochondrial membrane potential and ROS production rate in cardiomyocytes. Free Radic Biol Med. 2009;47(10):1414–1421. doi: 10.1016/j.freeradbiomed.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen JJ, Garg NJ. Mitochondrial generation of reactive oxygen species is enhanced at the Q(o) site of the complex III in the myocardium of Trypanosoma cruzi-infected mice: beneficial effects of an antioxidant. J Bioenerg Biomembr. 2008;40(6):587–598. doi: 10.1007/s10863-008-9184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen JJ, Yachelini PC, Sembaj A, et al. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med. 2006;41(2):270–276. doi: 10.1016/j.freeradbiomed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Wen JJ, Dhiman M, Whorton EB, et al. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 2008;10(10–11):1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhiman M, Wan X, Popov VL, et al. MnSODtg mice control myocardial inflammatory and oxidative stress and remodeling responses elicited in chronic Chagas disease. J Am Heart Assoc. 2013;2(5):e000302. doi: 10.1161/JAHA.113.000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen JJ, Bhatia V, Popov VL, et al. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas’ disease. Am J Pathol. 2006;169(6):1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhiman M, Estrada-Franco JG, Pando JM, et al. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas’ disease. Clin Vaccine Immunol. 2009;16(5):660–666. doi: 10.1128/CVI.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ba X, Gupta S, Davidson M, et al. Trypanosoma cruzi induces the reactive oxygen species-PARP-1-RelA pathway for up-regulation of cytokine expression in cardiomyocytes. J Biol Chem. 2010;285(15):11596–11606. doi: 10.1074/jbc.M109.076984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ba X, Garg NJ. Signaling mechanism of poly(ADP-ribose) polymerase-1 (PARP-1) in inflammatory diseases. Am J Pathol. 2011;178(3):946–955. doi: 10.1016/j.ajpath.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarleton RL. CD8+ T cells in Trypanosoma cruzi infection. Semin Immunopathol. 2015;37(3):233–238. doi: 10.1007/s00281-015-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garg N, Bhatia V. Current status and future prospects for a vaccine against American trypanosomiasis. Expert Rev Vaccines. 2005;4(6):867–880. doi: 10.1586/14760584.4.6.867. [DOI] [PubMed] [Google Scholar]
- 64.Garg N, Tarleton RL. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect Immun. 2002;70(10):5547–5555. doi: 10.1128/IAI.70.10.5547-5555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cazorla SI, Becker PD, Frank FM, et al. Oral vaccination with Salmonella as cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi. Infect Immun. 2007;76(1):324–333. doi: 10.1128/IAI.01163-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyahira Y, Takashima Y, Kobayashi S, et al. Immune responses against a single CD8+-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect Immun. 2005;73(11):7356–7365. doi: 10.1128/IAI.73.11.7356-7365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Alencar BC, Persechini PM, Haolla FA, et al. Perforin and Interferon-γ expression are required for CD4+ and CD8+ T cell-dependent protective immunity against a human parasite (Trypanosoma cruzi) elicited by a heterologous plasmid DNA prime-recombinant adenovirus 5 boost vaccination. Infect Immun. 2009;77(10):4383–4395. doi: 10.1128/IAI.01459-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhatia V, Sinha M, Luxon B, et al. Utility of the Trypanosoma cruzi sequence database for identification of potential vaccine candidates by in silico and in vitro screening. Infect Immun. 2004;72(11):6245–6254. doi: 10.1128/IAI.72.11.6245-6254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhatia V, Garg NJ. Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice. Clin Vaccine Immunol. 2008;15(8):1158–1164. doi: 10.1128/CVI.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta S, Wan X, Zago MP, et al. Antigenicity and diagnostic potential of vaccine candidates in human Chagas disease. PLoS Negl Trop Dis. 2013;7(1):e2018. doi: 10.1371/journal.pntd.0002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aparicio-Burgos JE, Ochoa-Garcia L, Zepeda-Escobar JA, et al. Testing the efficacy of a multi-component DNA-prime/DNA-boost vaccine against Trypanosoma cruzi infection in dogs. PLoS Negl Trop Dis. 2011;5(5):e1050. doi: 10.1371/journal.pntd.0001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta S, Garg NJ. Prophylactic efficacy of TcVac2 against Trypanosoma cruzi in mice. PLoS Negl Trop Dis. 2010;4(8):e797. doi: 10.1371/journal.pntd.0000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta S, Garg NJ. Delivery of antigenic candidates by a DNA/MVA heterologous approach elicits effector CD8(+)T cell mediated immunity against Trypanosoma cruzi. Vaccine. 2012;30(50):7179–7186. doi: 10.1016/j.vaccine.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta S, Garg NJ. TcVac3 induced control of Trypanosoma cruzi infection and chronic myocarditis in mice. PLoS One. 2013;8(3):e59434. doi: 10.1371/journal.pone.0059434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. 2005;175(2):633–639. doi: 10.4049/jimmunol.175.2.633. [DOI] [PubMed] [Google Scholar]
- 76.Bryan MA, Norris KA. Genetic immunization converts the trypanosoma cruzi B-Cell mitogen proline racemase to an effective immunogen. Infect Immun. 2010;78(2):810–822. doi: 10.1128/IAI.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S, Garg NJ. A two-component DNA-prime/protein-boost vaccination strategy for eliciting long-term, protective T cell immunity against Trypanosoma cruzi. PLoS Pathog. 2015;11(5):e1004828. doi: 10.1371/journal.ppat.1004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen JE, Gurtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293(5530):694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 79.Lee BY, Bacon KM, Connor DL, et al. The potential economic value of a Trypanosoma cruzi (Chagas disease) vaccine in Latin America. PLoS Negl Trop Dis. 2010;4(12):e916. doi: 10.1371/journal.pntd.0000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma Y, Weiss LM, Huang H. Inducible suicide vector systems for Trypanosoma cruzi. Microbes Infect. 2015;17(6):440–450. doi: 10.1016/j.micinf.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Pereira IR, Vilar-Pereira G, Marques V, et al. A human type 5 adenovirus-based Trypanosoma cruzi therapeutic vaccine re-programs immune response and reverses chronic cardiomyopathy. PLoS Pathog. 2015;11(1):e1004594. doi: 10.1371/journal.ppat.1004594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carod-Artal FJ, Vargas AP, Horan TA, et al. Chagasic cardiomyopathy is independently associated with ischemic stroke in Chagas disease. Stroke. 2005;36(5):965–970. doi: 10.1161/01.STR.0000163104.92943.50. [DOI] [PubMed] [Google Scholar]
- 83.Carod-Artal FJ, Gascon J. Chagas disease and stroke. Lancet Neurol. 2010;9(5):533–542. doi: 10.1016/S1474-4422(10)70042-9. [DOI] [PubMed] [Google Scholar]
- 84.Nunes MC, Barbosa MM, Ribeiro AL, et al. Ischemic cerebrovascular events in patients with Chagas cardiomyopathy: a prospective follow-up study. J Neurol Sci. 2009;278(1–2):96–101. doi: 10.1016/j.jns.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 85.Paixao LC, Ribeiro AL, Valacio RA, et al. Chagas disease: independent risk factor for stroke. Stroke. 2009;40(12):3691–3694. doi: 10.1161/STROKEAHA.109.560854. [DOI] [PubMed] [Google Scholar]
- 86.Pittella JE. Central nervous system involvement in Chagas disease: a hundred-year-old history. Trans R Soc Trop Med Hyg. 2009;103(10):973–978. doi: 10.1016/j.trstmh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 87.Aras R, da Matta JA, Mota G, et al. Cerebral infarction in autopsies of chagasic patients with heart failure. Arq Bras Cardiol. 2003;81(4):411–413. doi: 10.1590/s0066-782x2003001200008. [DOI] [PubMed] [Google Scholar]
- 88.Carod-Artal FJ. Policy implications of the changing epidemiology of chagas disease and stroke. Stroke. 2013;44(8):2356–2360. doi: 10.1161/STROKEAHA.113.000738. [DOI] [PubMed] [Google Scholar]
- 89.Dias JO, Junior, da Costa Rocha MO, de Souza AC, et al. Assessment of the source of ischemic cerebrovascular events in patients with Chagas disease. Int J Cardiol. 2014;176(3):1352–1354. doi: 10.1016/j.ijcard.2014.07.266. [DOI] [PubMed] [Google Scholar]
- 90.Nunes MC, Barbosa MM, Rocha MO. Peculiar aspects of cardiogenic embolism in patients with Chagas’ cardiomyopathy: a transthoracic and transesophageal echocardiographic study. J Am Soc Echocardiogr. 2005;18(7):761–767. doi: 10.1016/j.echo.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 91.Carod-Artal FJ, Vargas AP, Melo M, et al. American trypanosomiasis (Chagas’ disease): an unrecognised cause of stroke. J Neurol Neurosurg Psychiatry. 2003;74(4):516–518. doi: 10.1136/jnnp.74.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Espinosa RA, Pericchi LR, Carrasco HA, et al. Prognostic indicators of chronic chagasic cardiopathy. Int J Cardiol. 1991;30(2):195–202. doi: 10.1016/0167-5273(91)90095-7. [DOI] [PubMed] [Google Scholar]
- 93.Ribeiro AL, Marcolino MS, Prineas RJ, et al. Electrocardiographic abnormalities in elderly Chagas disease patients: 10-year follow-up of the Bambui Cohort Study of Aging. J Am Heart Assoc. 2014;3(1):e000632. doi: 10.1161/JAHA.113.000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lima-Costa MF, Matos DL, Ribeiro AL. Chagas disease predicts 10-year stroke mortality in community-dwelling elderly: the Bambui cohort study of aging. Stroke. 2010;41(11):2477–2482. doi: 10.1161/STROKEAHA.110.588061. [DOI] [PubMed] [Google Scholar]
- 95.Benjamin EJ, D’Agostino RB, Belanger AJ, et al. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92(4):835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 96.Ohara K, Hirai T, Fukuda N, et al. Relation of left atrial blood stasis to clinical risk factors in atrial fibrillation. Int J Cardiol. 2009;132(2):210–215. doi: 10.1016/j.ijcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 97.Nunes MC, Barbosa MM, Rocha ES, et al. Function of the left atrium in Chagas’ cardiomyopathy. Arq Bras Cardiol. 2005;84(6):452–456. doi: 10.1590/s0066-782x2005000600004. [DOI] [PubMed] [Google Scholar]
- 98.Carod-Artal FJ. Stroke: a neglected complication of American trypanosomiasis (Chagas’ disease) Trans R Soc Trop Med Hyg. 2007;101(11):1075–1080. doi: 10.1016/j.trstmh.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 99.Sternick EB, Martinelli M, Sampaio R, et al. Sudden cardiac death in patients with chagas heart disease and preserved left ventricular function. J Cardiovasc Electrophysiol. 2006;17(1):113–116. doi: 10.1111/j.1540-8167.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- 100.Barbosa MP, Carmo AA, Rocha MO, et al. Ventricular arrhythmias in Chagas disease. Rev Soc Bras Med Trop. 2015;48(1):4–10. doi: 10.1590/0037-8682-0003-2014. [DOI] [PubMed] [Google Scholar]
- 101.Ribeiro AL, Cavalvanti PS, Lombardi F, et al. Prognostic value of signal-averaged electrocardiogram in chagas disease. J Cardiovasc Electrophysiol. 2008;19(5):502–509. doi: 10.1111/j.1540-8167.2007.01088.x. [DOI] [PubMed] [Google Scholar]
- 102.Strauss DG, Cardoso S, Lima JA, et al. ECG scar quantification correlates with cardiac magnetic resonance scar size and prognostic factors in Chagas’ disease. Heart. 2011;97(5):357–361. doi: 10.1136/hrt.2010.210047. [DOI] [PubMed] [Google Scholar]
- 103.Mello RP, Szarf G, Schvartzman PR, et al. Delayed enhancement cardiac magnetic resonance Imaging can identify the risk for ventricular tachycardia in chronic Chagas’ heart disease. Arq Bras Cardiol. 2012;98(5):421–430. doi: 10.1590/s0066-782x2012005000031. [DOI] [PubMed] [Google Scholar]
- 104.Marin-Neto JA, Cunha-Neto E, Maciel BC, et al. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115(9):1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 105.Junqueira LF., Jr Insights into the clinical and functional significance of cardiac autonomic dysfunction in Chagas disease. Rev Soc Bras Med Trop. 2012;45(2):243–252. doi: 10.1590/s0037-86822012000200020. [DOI] [PubMed] [Google Scholar]
- 106.Koeberle F. Cardiopathia parasympathicopriva. Münch Med Wochenschr. 1959;101:1308–1310. [PubMed] [Google Scholar]
- 107.Amorim DS, Godoy RA, Manco JC, et al. Effects of acute elevation in blood pressure and of atropine on heart rate in Chagas’ disease. A Preliminary Report. Circulation. 1968;38(2):289–294. doi: 10.1161/01.cir.38.2.289. [DOI] [PubMed] [Google Scholar]
- 108.Marin-Neto JA, Maciel BC, Gallo JL, et al. Effect of parasympathetic impairment on the haemodynamic response to handgrip in Chagas’s heart disease. Br Heart J. 1986;55(2):204–210. doi: 10.1136/hrt.55.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villar JC, Leon H, Morillo CA. Cardiovascular autonomic function testing in asymptomatic T. cruzi carriers: a sensitive method to identify subclinical Chagas’ disease. Int J Cardiol. 2004;93(2–3):189–195. doi: 10.1016/j.ijcard.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 110.Guzzetti S, Iosa D, Pecis M, et al. Impaired heart rate variability in patients with chronic Chagas’ disease. Am Heart J. 1991;121(6 Pt 1):1727–1734. doi: 10.1016/0002-8703(91)90019-e. [DOI] [PubMed] [Google Scholar]
- 111.Ribeiro AL, Moraes RS, Ribeiro JP, et al. Parasympathetic dysautonomia precedes left ventricular systolic dysfunction in Chagas disease. Am Heart J. 2001;141(2):260–265. doi: 10.1067/mhj.2001.111406. [DOI] [PubMed] [Google Scholar]
- 112.Raadschilders L, Rocha MO, Sousa L, et al. Is autonomic function associated with left ventricular systolic function in Chagas heart disease patients undergoing treatment for heart failure? Rev Soc Bras Med Trop. 2014;47(2):239–242. doi: 10.1590/0037-8682-0013-2013. [DOI] [PubMed] [Google Scholar]
- 113.Ribeiro AL, Cassini P, Peixoto SV, et al. Vagal impairment in elderly Chagas disease patients: a population-based study (The Bambui Study) Int J Cardiol. 2011;147(3):359–365. doi: 10.1016/j.ijcard.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 114.Miranda CH, Figueiredo AB, Maciel BC, et al. Sustained ventricular tachycardia is associated with regional myocardial sympathetic denervation assessed with 123I-metaiodobenzyl-guanidine in chronic Chagas cardiomyopathy. J Nucl Med. 2011;52(4):504–510. doi: 10.2967/jnumed.110.082032. [DOI] [PubMed] [Google Scholar]
- 115.Landesmann MC, da Fonseca LM, de BP, et al. Iodine-123 metaiodobenzyl-guanidine cardiac imaging as a method to detect early sympathetic neuronal dysfunction in chagasic patients with normal or borderline electrocardiogram and preserved ventricular function. Clin Nucl Med. 2011;36(9):757–761. doi: 10.1097/RLU.0b013e31821772a9. [DOI] [PubMed] [Google Scholar]
- 116.Diaz JO, Makikallio TH, Huikuri HV, et al. Heart rate dynamics before the spontaneous onset of ventricular tachyarrhythmias in Chagas’ heart disease. Am J Cardiol. 2001;87(9):1123–1125. A10. doi: 10.1016/s0002-9149(01)01477-1. [DOI] [PubMed] [Google Scholar]
- 117.Salles G, Xavier S, Sousa A, et al. Prognostic value of QT interval parameters for mortality risk stratification in Chagas’ disease: results of a long-term follow-up study. Circulation. 2003;108(3):305–312. doi: 10.1161/01.CIR.0000079174.13444.9C. [DOI] [PubMed] [Google Scholar]
- 118.Salles GF, Xavier SS, Sousa AS, et al. T-wave axis deviation as an independent predictor of mortality in chronic Chagas’ disease. Am J Cardiol. 2004;93(9):1136–1140. doi: 10.1016/j.amjcard.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 119.Ribeiro AL, Rocha MO, Terranova P, et al. T-wave amplitude variability and the risk of death in Chagas disease. J Cardiovasc Electrophysiol. 2011;22(7):799–805. doi: 10.1111/j.1540-8167.2010.02000.x. [DOI] [PubMed] [Google Scholar]
- 120.Sassi R, Rivolta MW, Mainardi LT, et al. Spatial repolarization heterogeneity and survival in chagas disease. Methods Inf Med. 2014;53(4):464–468. doi: 10.3414/ME14-01-0002. [DOI] [PubMed] [Google Scholar]
- 121.Raadschilders L, Barbosa MP, Carmo AA, et al. Microvolt T-wave alternans in Chagas disease. Int J Cardiol. 2015;187:7–8. doi: 10.1016/j.ijcard.2015.03.253. [DOI] [PubMed] [Google Scholar]
- 122.Botoni FA, Poole-Wilson PA, Ribeiro AL, et al. A randomized trial of carvedilol after renin-angiotensin system inhibition in chronic Chagas cardiomyopathy. Am Heart J. 2007;153(4):544–548. doi: 10.1016/j.ahj.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 123.Roberti RR, Martinez EE, Andrade JL, et al. Chagas cardiomyopathy and captopril. Eur Heart J. 1992;13(7):966–970. doi: 10.1093/oxfordjournals.eurheartj.a060301. [DOI] [PubMed] [Google Scholar]
- 124.Issa VS, Amaral AF, Cruz FD, et al. Beta-blocker therapy and mortality of patients with Chagas cardiomyopathy: a subanalysis of the REMADHE prospective trial. Circ Heart Fail. 2010;3(1):82–88. doi: 10.1161/CIRCHEARTFAILURE.109.882035. [DOI] [PubMed] [Google Scholar]
- 125.Bestetti RB, Otaviano AP, Cardinalli-Neto A, et al. Effects of B-Blockers on outcome of patients with Chagas’ cardiomyopathy with chronic heart failure. Int J Cardiol. 2011;151(2):205–208. doi: 10.1016/j.ijcard.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 126.Muratore CA, Baranchuk A. Current and emerging therapeutic options for the treatment of chronic chagasic cardiomyopathy. Vasc Health Risk Manag. 2010;6:593–601. doi: 10.2147/vhrm.s8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andrade JP, Marin Neto JA, Paola AA, et al. I Latin American Guidelines for the diagnosis and treatment of Chagas’ heart disease: executive summary. Arq Bras Cardiol. 2011;96(6):434–442. doi: 10.1590/s0066-782x2011000600002. [DOI] [PubMed] [Google Scholar]
- 128.de Sousa AS, Xavier SS, de Freitas GR, et al. Prevention strategies of cardioembolic ischemic stroke in Chagas’ disease. Arq Bras Cardiol. 2008;91(5):306–310. doi: 10.1590/s0066-782x2008001700004. [DOI] [PubMed] [Google Scholar]
- 129.Fiorelli AI, Santos RH, Oliveira JL, Jr, et al. Heart transplantation in 107 cases of Chagas’ disease. Transplant Proc. 2011;43(1):220–224. doi: 10.1016/j.transproceed.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 130.Kransdorf EP, Zakowski PC, Kobashigawa JA. Chagas disease in solid organ and heart transplantation. Curr Opin Infect Dis. 2014;27(5):418–424. doi: 10.1097/QCO.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 131.Bestetti RB. Cardiac resynchronization therapy for patients with chronic systolic heart failure secondary to Chagas cardiomyopathy in the 21st century. Rev Bras Cir Cardiovasc. 2014;29(1):IV–VI. doi: 10.5935/1678-9741.20140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Araujo EF, Chamlian EG, Peroni AP, et al. Cardiac resynchronization therapy in patients with chronic Chagas cardiomyopathy: long-term follow up. Rev Bras Cir Cardiovasc. 2014;29(1):31–36. doi: 10.5935/1678-9741.20140008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cunnington C, Kwok CS, Satchithananda DK, et al. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non-left bundle branch block QRS morphology: meta-analysis of randomised controlled trials. Heart. 2015;101(18):1456–1462. doi: 10.1136/heartjnl-2014-306811. [DOI] [PubMed] [Google Scholar]
- 134.Lima MM, Rocha MO, Nunes MC, et al. A randomized trial of the effects of exercise training in Chagas cardiomyopathy. Eur J Heart Fail. 2010;12(8):866–873. doi: 10.1093/eurjhf/hfq123. [DOI] [PubMed] [Google Scholar]
- 135.Sperandio da Silva GM, Chambela MC, Sousa AS, et al. Impact of pharmaceutical care on the quality of life of patients with Chagas disease and heart failure: randomized clinical trial. Trials. 2012;13:244. doi: 10.1186/1745-6215-13-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leite CF, Almeida TR, Lopes CS, et al. Multipotent stem cells of the heart-do they have therapeutic promise? Front Physiol. 2015;6:123. doi: 10.3389/fphys.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liebson PR. Stem-cell angiogenesis and regeneration of the heart: review of a saga of 2 decades. Clin Cardiol. 2015;38(5):309–316. doi: 10.1002/clc.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leblond AL, O’Sullivan J, Caplice N. Bone marrow mononuclear stem cells: potential in the treatment of myocardial infarction. Stem Cells Cloning. 2009;2:11–19. doi: 10.2147/sccaa.s6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Soares MB, Lima RS, Rocha LL, et al. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am J Pathol. 2004;164(2):441–447. doi: 10.1016/s0002-9440(10)63134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goldenberg RC, Jelicks LA, Fortes FS, et al. Bone marrow cell therapy ameliorates and reverses chagasic cardiomyopathy in a mouse model. J Infect Dis. 2008;197(4):544–547. doi: 10.1086/526793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Soares MB, Lima RS, Souza BS, et al. Reversion of gene expression alterations in hearts of mice with chronic chagasic cardiomyopathy after transplantation of bone marrow cells. Cell Cycle. 2011;10(9):1448–1455. doi: 10.4161/cc.10.9.15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soares MB, de Lima RS, Rocha LL, et al. Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J Infect Dis. 2010;202(3):416–426. doi: 10.1086/653481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, et al. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant Proc. 2013;45(1):434–439. doi: 10.1016/j.transproceed.2012.05.091. [DOI] [PubMed] [Google Scholar]
- 144.Jasmin, Jelicks LA, Koba W, et al. Mesenchymal bone marrow cell therapy in a mouse model of chagas disease. Where do the cells go? PLoS Negl Trop Dis. 2012;6(12):e1971. doi: 10.1371/journal.pntd.0001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jasmin J, Jelicks LA, Tanowitz HB, et al. Molecular imaging, biodistribution and efficacy of mesenchymal bone marrow cell therapy in a mouse model of Chagas disease. Microbes Infect. 2014;16(11):923–935. doi: 10.1016/j.micinf.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Carvalho AC, Carvalho AB. Stem cell-based therapies in chagasic cardiomyopathy. Biomed Res Int. 2015;2015:436314. doi: 10.1155/2015/436314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ribeiro dos SR, Rassi S, Feitosa G, et al. Cell therapy in Chagas cardiomyopathy (Chagas arm of the multicenter randomized trial of cell therapy in cardiopathies study): a multicenter randomized trial. Circulation. 2012;125(20):2454–2461. doi: 10.1161/CIRCULATIONAHA.111.067785. [DOI] [PubMed] [Google Scholar]
- 148.Macambira SG, Vasconcelos JF, Costa CR, et al. Granulocyte colony-stimulating factor treatment in chronic Chagas disease: preservation and improvement of cardiac structure and function. FASEB J. 2009;23(11):3843–3850. doi: 10.1096/fj.09-137869. [DOI] [PubMed] [Google Scholar]
- 149.Guarita-Souza LC, Carvalho KA, Woitowicz V, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation. 2006;114(1 Suppl):I120–I124. doi: 10.1161/CIRCULATIONAHA.105.000646. [DOI] [PubMed] [Google Scholar]
- 150.Shi Y, Desponts C, Do JT, et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3(5):568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 151.Cunha-Neto E, Teixeira PC, Nogueira LG, et al. Autoimmunity. Adv Parasitol. 2011;76:129–152. doi: 10.1016/B978-0-12-385895-5.00006-2. [DOI] [PubMed] [Google Scholar]
- 152.Machado FS, Tyler KM, Brant F, et al. Pathogenesis of Chagas disease: time to move on. Front Biosci (Elite Ed) 2012;4:1743–1758. doi: 10.2741/495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Teixeira AR, Hecht MM, Guimaro MC, et al. Pathogenesis of Chagas’ disease: parasite persistence and autoimmunity. Clin Microbiol Rev. 2011;24(3):592–630. doi: 10.1128/CMR.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bonney KM, Engman DM. Autoimmune pathogenesis of Chagas heart disease: looking back, looking ahead. Am J Pathol. 2015;185(6):1537–1547. doi: 10.1016/j.ajpath.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Coura JR. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions: a comprehensive review. Mem Inst Oswaldo Cruz. 2015;110(3):277–282. doi: 10.1590/0074-0276140362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rendell VR, Gilman RH, Valencia E, et al. Trypanosoma cruzi-infected pregnant women without vector exposure have higher parasitemia levels: implications for congenital transmission risk. PLoS One. 2015;10(3):e0119527. doi: 10.1371/journal.pone.0119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lattes R, Lasala MB. Chagas disease in the immunosuppressed patient. Clin Microbiol Infect. 2014;20(4):300–309. doi: 10.1111/1469-0691.12585. [DOI] [PubMed] [Google Scholar]
- 158.Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. J Infect Dis. 1999;180(2):480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- 159.Schijman AG, Vigliano CA, Viotti RJ, et al. Trypanosoma cruzi DNA in cardiac lesions of Argentinean patients with end-stage chronic chagas heart disease. Am J Trop Med Hyg. 2004;70(2):210–220. [PubMed] [Google Scholar]
- 160.Benvenuti LA, Roggerio A, Freitas HF, et al. Chronic American trypanosomiasis: parasite persistence in endomyocardial biopsies is associated with high-grade myocarditis. Ann Trop Med Parasitol. 2008;102(6):481–487. doi: 10.1179/136485908X311740. [DOI] [PubMed] [Google Scholar]
- 161.Sabino EC, Ribeiro AL, Lee TH, et al. Detection of Trypanosoma cruzi DNA in blood by PCR is associated with Chagas cardiomyopathy and disease severity. Eur J Heart Fail. 2015;17(4):416–423. doi: 10.1002/ejhf.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144(10):724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 163.Viotti R, Alarcon de NB, Araujo-Jorge T, et al. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother. 2014;58(2):635–639. doi: 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bern C. Chagas’ disease. N Engl J Med. 2015;373(5):456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 165.Sosa-Estani S, Segura EL. Integrated control of Chagas disease for its elimination as public health problem: a review. Mem Inst Oswaldo Cruz. 2015;110(3):289–298. doi: 10.1590/0074-02760140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Perez-Molina JA, Perez-Ayala A, Moreno S, et al. Use of benznidazole to treat chronic Chagas’ disease: a systematic review with a meta-analysis. J Antimicrob Chemother. 2009;64(6):1139–1147. doi: 10.1093/jac/dkp357. [DOI] [PubMed] [Google Scholar]
- 167.Villar JC, Perez JG, Cortes OL, et al. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev. 2014;5:CD003463. doi: 10.1002/14651858.CD003463.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marin-Neto JA, Rassi A, Jr, Avezum A, Jr, et al. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz. 2009;104(4):319–324. doi: 10.1590/s0074-02762009000900042. [DOI] [PubMed] [Google Scholar]
- 169.Morillo CA, Marin-Neto JA, Avezum A, et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med. 2015;373(14):1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 170.Molina I, Prat J, Salvador F, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370(20):1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 171.Carmo AA, Rocha MO, Silva JL, et al. Amiodarone and Trypanosoma cruzi parasitemia in patients with Chagas disease. Int J Cardiol. 2015;189:182–184. doi: 10.1016/j.ijcard.2015.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Alonso-Padilla J, Rodriguez A. High throughput screening for anti-Trypanosoma cruzi drug discovery. PLoS Negl Trop Dis. 2014;8(12):e3259. doi: 10.1371/journal.pntd.0003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lepesheva GI. Design or screening of drugs for the treatment of Chagas disease: what shows the most promise? Expert Opin Drug Discov. 2013;8(12):1479–1489. doi: 10.1517/17460441.2013.845554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guedes PM, Oliveira FS, Gutierrez FR, et al. Nitric oxide donor trans-[RuCl([15]aneN)NO] as a possible therapeutic approach for Chagas’ disease. Br J Pharmacol. 2010;160(2):270–282. doi: 10.1111/j.1476-5381.2009.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Machado FS, Tanowitz HB, Teixeira MM. New drugs for neglected infectious diseases: Chagas’ disease. Br J Pharmacol. 2010;160(2):258–259. doi: 10.1111/j.1476-5381.2010.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sesti-Costa R, Carneiro ZA, Silva MC, et al. Ruthenium complex with benznidazole and nitric oxide as a new candidate for the treatment of chagas disease. PLoS Negl Trop Dis. 2014;8(10):e3207. doi: 10.1371/journal.pntd.0003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nagajyothi F, Zhao D, Weiss LM, et al. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol Res. 2012;110(6):2491–2499. doi: 10.1007/s00436-011-2790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alvarenga Americano do Brasil PE, Pereira de SA, Hasslocher-Moreno AM, et al. Selenium Treatment and Chagasic Cardiopathy (STCC): study protocol for a double-blind randomized controlled trial. Trials. 2014;15:388. doi: 10.1186/1745-6215-15-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jelicks LA, de Souza AP, Araujo-Jorge TC, et al. Would selenium supplementation aid in therapy for Chagas disease? Trends Parasitol. 2011;27(3):102–105. doi: 10.1016/j.pt.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Friedman AJ, Han G, Navati MS, et al. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide. 2008;19(1):12–20. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 181.Tar M, Cabrales P, Navati M, et al. Topically applied NO-releasing nanoparticles can increase intracorporal pressure and elicit spontaneous erections in a rat model of radical prostatectomy. J Sex Med. 2014;11(12):2903–2914. doi: 10.1111/jsm.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cabrales P, Han G, Roche C, et al. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010;49(4):530–538. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Friedman A, Blecher K, Sanchez D, et al. Susceptibility of Gram-positive and -negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence. 2011;2(3):217–221. doi: 10.4161/viru.2.3.16161. [DOI] [PubMed] [Google Scholar]
- 184.Schairer DO, Chouake JS, Nosanchuk JD, et al. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence. 2012;3(3):271–279. doi: 10.4161/viru.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Macherla C, Sanchez DA, Ahmadi MS, et al. Nitric oxide releasing nanoparticles for treatment of Candida albicans burn infections. Front Microbiol. 2012;3:193. doi: 10.3389/fmicb.2012.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nachuraju P, Friedman AJ, Friedman JM, et al. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation. 2011;82(5):607–613. doi: 10.1016/j.resuscitation.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]