SUMMARY
The immunoglobulin heavy-chain (Igh) locus undergoes large-scale contraction in pro-B cells, which facilitates VH-DJH recombination by juxtaposing distal VH genes next to the DJH-rearranged gene segment in the 3′ proximal Igh domain. By using high-resolution mapping of long-range interactions, we demonstrate that local interaction domains established the three-dimensional structure of the extended Igh locus in lymphoid progenitors. In pro- B cells, these local domains engaged in long-range interactions across the Igh locus, which depend on the regulators Pax5, YY1, and CTCF. The large VH gene cluster underwent flexible long-range interactions with the more rigidly structured proximal domain, which probably ensures similar participation of all VH genes in VH-DJH recombination to generate a diverse antibody repertoire. These long-range interactions appear to be an intrinsic feature of the VH gene cluster, because they are still generated upon mutation of the Eµ enhancer, IGCR1 insulator, or 3′ regulatory region in the proximal Igh domain.
INTRODUCTION
B lymphocytes generate humoral immunity to foreign pathogens by creating a diverse antigen receptor repertoire through V(D)J recombination, which assembles the variable regions of immunoglobulin (Ig) genes from variable (V), diversity (D), and joining (J) gene segments during B cell development. The recombination of Ig genes is tightly controlled within the B lymphoid lineage: the Ig heavy-chain (Igh) locus undergoes rearrangements in early B cell development prior to Ig light-chain genes in pre-B cells. DH-JH rearrangements at the Igh locus are initiated in lymphoid progenitors followed by VH-DJH recombination in pro-B cells. The temporal order of V(D)J recombination is largely determined by the accessibility of the different Ig gene segments to the V(D)J recombinase, which is controlled by multiple epigenetic mechanisms (Jhunjhunwala et al., 2009; Perlot and Alt, 2008).
The Igh locus is composed of the 3′ proximal region of 266 kb length consisting of 16 DH, 4 JH, and 8 CH gene segments and of the distal VH gene cluster extending over a 2.44 Mb region, which contains 195 VH genes with the largest VH gene family consisting of 89 VHJ558 genes (Johnston et al., 2006). VH-DJH recombination at the Igh locus is regulated at several levels including the relocation of Igh alleles from peripheral to central nuclear positions (Fuxa et al., 2004; Kosak et al., 2002) and antisense transcription in the VHJ558 gene region (Bolland et al., 2004). Both Igh alleles also undergo homologous pairing in pro-B cells, which ensures that VH-DJH recombination simultaneously takes place on only one of the two Igh alleles (Hewitt et al., 2009).
The Igh locus furthermore contracts by looping in pro-B cells, which juxtaposes distal VH genes next to proximal DH segments to facilitate VH-DJH rearrangements (Fuxa et al., 2004; Jhunjhunwala et al., 2008; Kosak et al., 2002; Roldá n et al., 2005; Sayegh et al., 2005). Moreover, the Igh locus undergoes decontraction at the next developmental stage, which separates VH genes from the proximal Igh domain, thereby preventing VH-DJH rearrangement of the second, DJH-rearranged Igh allele in pre-B cells (Roldá n et al., 2005). The pro-B cell-specific contraction of the Igh locus depends on the B cell commitment factor Pax5 (Fuxa et al., 2004) and the ubiquitous transcriptional regulator YY1 (Liu et al., 2007). Long-range chromatin looping at complex loci is known to depend on the CCCTC-binding factor (CTCF) and its associated cohesin complex (Hadjur et al., 2009; Nativio et al., 2009; Splinter et al., 2006). Notably, multiple CTCF- and cohesin-binding sites are colocalized throughout the VH gene cluster (Degner et al., 2011; Ebert et al., 2011), and shRNA knockdown experiments implicated CTCF in the regulation of Igh locus contraction in pro-B cells (Degner et al., 2011).
Several cis-acting elements control the activity of the Igh locus. The intronic Eµ enhancer is essential for Igh recombination by regulating germline transcription and chromatin accessibility in the DH-JH region (Afshar et al., 2006; Chakraborty et al., 2009; Perlot et al., 2005). The Eµ enhancer was also implicated in Igh locus contraction by mediating loop formation with two VH gene regions (Guo et al., 2011a). The 3′ regulatory region (3′RR) downstream of the CH region consists of four DNase I hypersensitive sites (HS1–HS4), which function as potent enhancers in late B cell development (Vincent-Fabert et al., 2010). The downstream 3′CBE region also consists of four DNase I hypersensitive sites (HS5–HS7 and site “38’ [here referred to as HS8]) and may constitute the 3′ boundary of the Igh locus (Garrett et al., 2005), because it contains nine CTCF-binding elements (CBEs) that colocalize with cohesin-binding sites (Degner et al., 2011; Ebert et al., 2011). The intergenic control region 1 (IGCR1) with its two CBEs is located 2.1 kb upstream of the DHFL16.1 segment in the 100 kb region separating the DH and VH gene regions (Guo et al., 2011b). Specific mutation of these two CTCF-binding sites in IGCR1/CBE mutant mice revealed that they function as insulator elements to regulate ordered and lineage-specific V(D)J recombination at the Igh locus (Guo et al., 2011b). The large VH gene cluster contains 14 Pax5-activated intergenic repeat (PAIR) elements, which are interspersed together with the VH3609 genes in the distal VHJ558 gene region (Ebert et al., 2011). The PAIR elements, which bind Pax5, E2A, CTCF, and cohesin, give rise to long noncoding antisense transcripts only in pro-B cells, suggesting that they regulate distal VH-DJH recombination possibly by controlling Igh locus contraction (Ebert et al., 2011).
The 3D architecture of the Igh locus was so far studied at low spatial resolution in single cells by DNA fluorescent in situ hybridization (DNA-FISH) (Fuxa et al., 2004; Jhunjhunwala et al., 2008; Roldá n et al., 2005). Here, we have used 4C sequencing (4Cseq), which provides high-resolution analysis of chromatin loops at the cell population level and is based on the chromosome conformation capture-on-chip (4C) technique adapted for deep sequencing (van de Werken et al., 2012). 4C sequencing revealed that the Igh locus consists of local interaction domains in lymphoid progenitors. In committed pro-B cells, the local domains in the VH gene region participate in long-range interactions across the entire Igh locus by generating a continuum of flexible loops that probably contribute to the generation of a diverse immunoglobulin repertoire by providing a similar opportunity for each VH gene to contact the proximal DJH-rearranged gene segment prior to VH-DJH recombination.
RESULTS
High-Resolution Mapping of Long-Range Interactions in the Igh Locus by 4C Sequencing
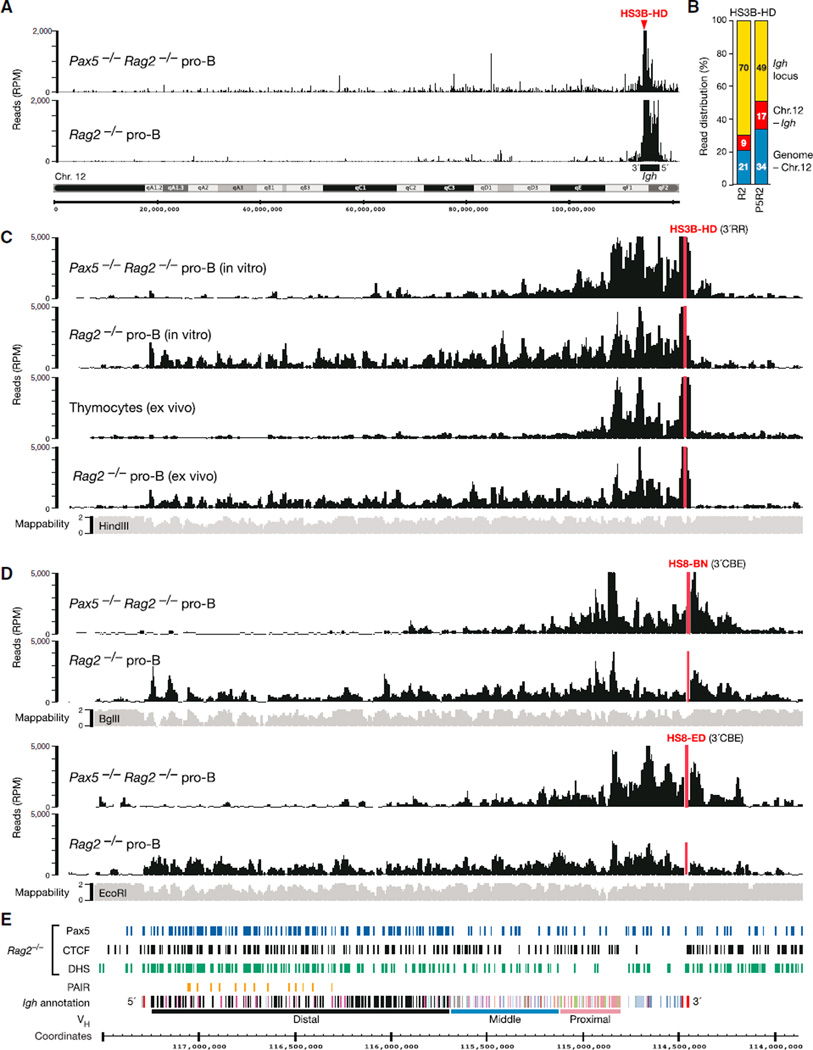
To determine the 3D architecture of the Igh locus, we used short-term cultured pro-B cells on a recombination-deficient Rag2 mutant background for 4C sequencing with 16 different viewpoints across the Igh locus, which are referred to by the closest annotated feature and the combination of primary (HindIII [H], EcoRI [E], or BglII [B]) and secondary (DpnII [D] or NlaIII [N]) restriction enzymes used for 4C template preparation (see Supplemental Experimental Procedures for a general description of the 4C-seq concept). We performed these 4Cseq experiments in the presence or absence of Igh locus contraction by analyzing Rag2−/− and Pax5−/−Rag2−/− pro-B cells, respectively (Fuxa et al., 2004). Figures 1A and 1B show a typical 4C-seq result obtained with the HS3B-HD viewpoint in the Igh 3′ regulatory region (3′RR). Most 4C-seq reads (70%) mapped to the contracted Igh locus in Pax5-expressing Rag2−/− pro-B cells, whereas fewer sequence reads (49%) with a narrower distribution were localized to the decontracted Igh locus in Pax5−/−Rag2−/− pro-B cells. 4C-seq experiments with other viewpoints revealed the same trend (Figure S1A available online). We therefore conclude that long-range interactions are largely confined to the Igh locus in pro-B cells undergoing locus contraction, whereas Igh sequences more readily interact with chromosomal sites throughout the genome in the absence of locus contraction.
Figure 1. High-Resolution Analysis of Long-Range Interactions within the Igh Locus.
(A) Interaction of the HS3B-HD viewpoint (red) with chromosome 12 sequences in cultured Pax5−/−Rag2−/− and Rag2−/− pro-B cells. The 20 kb running mean values of the 4C-seq reads were plotted as reads per million mapped sequence reads (RPMs). A black bar denotes the Igh locus.
(B) Relative distribution of the 4C-seq reads (HS3B-HD viewpoint) in the Igh locus, rest of chromosome 12 (Chr.12 – Igh), or entire genome minus chromosome 12 (Genome – Chr.12).
(C) Pax5-dependent long-range interactions of the HS3B region in ex vivo sorted and in vitro cultured cells of the indicated genotypes.
(D) 4C-seq patterns of the HS8-BN and HS8-ED viewpoints in cultured Pax5−/−Rag2−/− and Rag2−/− pro-B cells. A 20 kb running mean mappability track (gray) indicates to what degree (on a scale from 0 to 2) the end sequences of HindIII (C), BglII (D), and EcoRI (D) fragments can be unambiguously mapped to genomic Igh sequences. One representative 4C-seq experiment is shown for each viewpoint and cell type (C and D).
(E) Annotation of the C57BL/6 Igh locus. The distinct VH gene families (different colors) in the distal, middle, and proximal VH gene regions (Johnston et al., 2006) as well as the DH (gray) and CH (blue) elements and Eµ and 3′RR enhancers (red) in the 3′ proximal Igh domain are shown together with the PAIR elements (orange) and the mm9 genomic coordinates of mouse chromosome 12. The indicated DNase I hypersensitive (DHS) regions and CTCF- and Pax5-binding sites were determined in Rag2−/− pro-B cells by paired-end sequencing and peak calling (MACS; p value < 10−10) (Ebert et al., 2011; Revilla-I-Domingo et al., 2012).
See also Figure S1.
Within the Igh locus, sequences at the HS3B-HD viewpoint efficiently associated only with elements of the Igh 3′ region in the absence of Igh locus contraction in cultured Pax5−/− Rag2−/− pro-B cells and ex vivo isolated thymocytes (Figure 1C). In contrast, interactions with multiple sequences throughout the entire Igh locus were seen in both cultured and ex vivo isolated Rag2−/− pro-B cells, which validates the use of cultured pro-B cells for 4C-seq analysis (Figure 1C). Because the observed interaction patterns were highly reproducible in three independent 4C-seq experiments (Figure S1B), we subsequently show the average pattern of replica experiments, if available. Of note, the long-range interactions observed with the HS3B-HD viewpoint abruptly terminated at the 3′CBE region and the most 5′ VH gene (J558.89pg.195) of the Igh locus (Figure 1C).
The Igh locus contains many interspersed repeat sequences (Johnston et al., 2006), which prevent mapping of the corresponding 4C-seq reads to the Igh locus. Consequently, the end sequences of HindIII, BglII, and EcoRI fragments differ in their mappability along the Igh locus (Figures 1C and 1D, gray bars). We therefore used (in addition to HindIII), BglII and EcoRI for the preparation of 4C templates. The 4C-seq data obtained with the overlapping viewpoints HS8-BN and HS8-ED in the 3′CBE region demonstrated that their interactions in pro-B cells were also confined to the 3′ proximal region in the absence of Pax5 and extended along the entire Igh locus in the presence of Pax5 (Figures 1D and 1E), similar to the results obtained with the HS3B-HD viewpoint (Figure 1C). Together, these 4Cseq data demonstrate that the 3′RR and 3′CBE regions form multiple loops across the entire Igh locus in committed pro-B cells.
Loop Formation in the 3′ Proximal Igh Domain
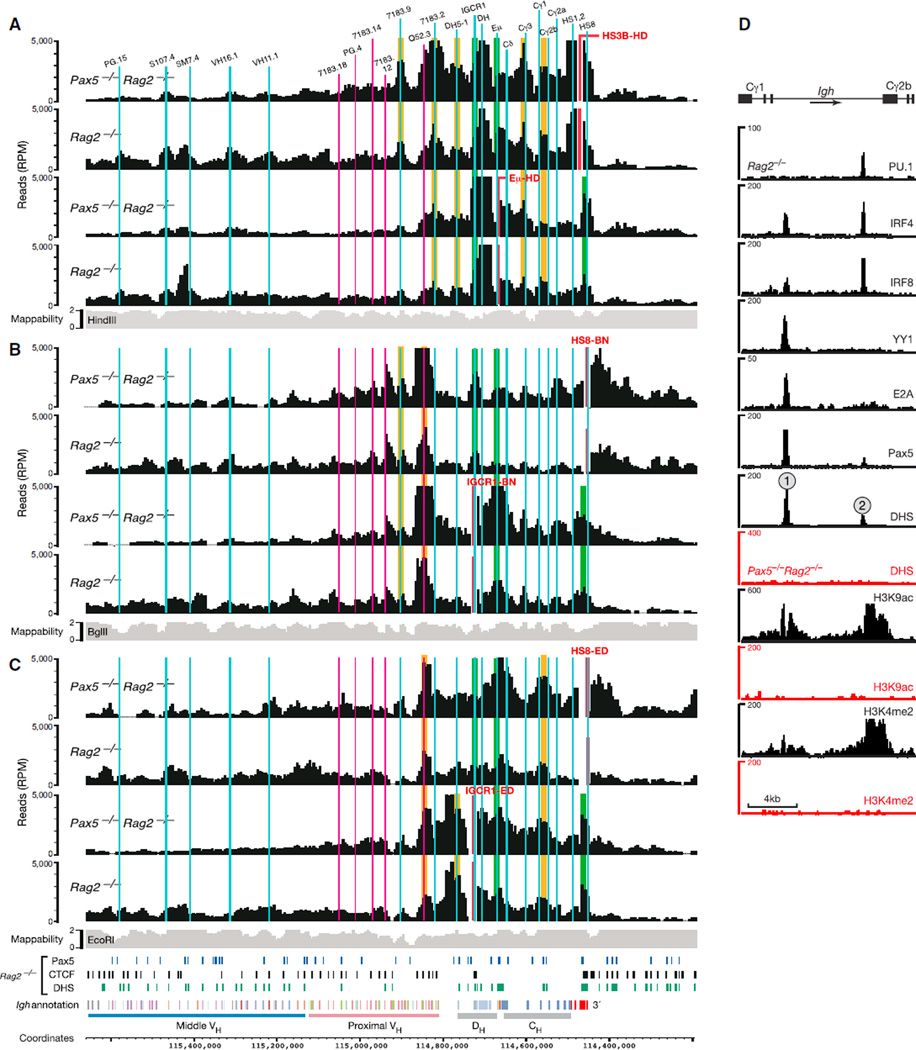
The proximal Igh region is known to form loops between the regulatory IGCR1, Eµ, 3′RR, and 3′CBE regions in pro-B cells (Degner et al., 2011; Guo et al., 2011a, 2011b). Our 4C-seq analyses with IGCR1, Eµ, HS3B (3′RR), and HS8 (3′CBE) viewpoints revealed that strong interactions in the proximal Igh region were restricted to a ~0.5 Mb long region in the absence of locus contraction in Pax5−/−Rag2−/− pro-B cells (Figure 2) and thymocytes (Figure S2A). Notably, the interaction patterns observed for each viewpoint were similar in Rag2−/− pro-B cells compared to Pax5−/−Rag2−/− pro-B cells (Figure 2) and thymocytes (Figure S2A), indicating that locus contraction does not influence loop formation in the proximal Igh domain.
Figure 2. Loop Formation in the 3′ Proximal Igh Region.
(A–C) 4C-seq interaction patterns revealed by the indicated viewpoints (red) in short-term cultured Pax5−/−Rag2−/− and Rag2−/− pro-B cells. Vertical lines indicate the positions of relevant VH, DH, and CH gene segments as well as IGCR1, Eµ, and DNase I hypersensitive sites constituting the 3′RR (HS1–HS4) and 3′CBE (HS5–HS8) regions. Previously known and newly identified interactions are highlighted in green and orange, respectively. See legend of Figure 1 for further explanations. The data in (A) are based on average values of three independent experiments, and the results shown in (B) and (C) are derived from one experiment.
(D) Characterization of the Cγ1-Cγ2b region. The sequences between the Cγ1 and Cγ2b gene segments were analyzed for binding of the indicated transcription factors and the presence of DHS sites and active histone modifications (H3K4me2 and H3K9ac) by deep sequencing of Pax5−/−Rag2−/− (red) and Rag2−/− (black) pro-B cells (Revilla-I-Domingo et al., 2012).
See also Figure S2.
Our 4C-seq analyses confirmed the existence of the previously identified chromatin loops between the IGCR1, Eµ, 3′RR (HS3B), and 3′CBE (HS8) regions (Figure 2, highlighted in green). Our 4C-seq analyses detected two new contact sites in the Igh constant gene region (Figure 2, highlighted in orange). A region close to the Cγ3 gene segment was contacted by the viewpoints HS3B-HD and Eµ-HD (Figure 2A). The viewpoints IGCR1-ED, Eµ-HD, HS3B-HD, and HS8-ED all interacted with a region located between the Cγ1 and Cγ2b gene segments (Figures 2A and 2C), which contained two DNase I hypersensitive (DHS) regions (referred to as Cγ1-2b DHS sites 1 and 2) in Rag2−/− pro-B cells (Figure 2D). Pax5 not only bound to both Cγ1-2b DHS sites together with other transcription factors but also was required for the formation of these DHS sites and their active chromatin state (H3K4me2, H3K9ac; Figure 2D).
Notably, four interaction sites were identified upstream of the IGCR1 region in the absence of Igh locus contraction. The viewpoints HS3B-HD, Eµ-HD, and IGCR1-ED strongly associated with sequences close to the DH5-1 pseudogene, which is located in the 100 kb region separating the DH and VH gene regions (Figures 2A and 2C). The proximal VH7183.2.3 gene region (also known as VH81X) was efficiently contacted by the viewpoints HS3B-HD and Eµ-HD, the upstream VHQ52.3.8 gene sequences by the four viewpoints IGCR1-ED, IGCR1-BN, HS8-ED, and HS8-BN, and the VH7183.9.15 gene region by the viewpoints HS3B-HD and HS8-BN (Figures 2A–2C). Not all interaction sites were equally well detected by the two overlapping viewpoints in the IGCR1 (ED and BN) or HS8 (ED and BN) region. The observed differences may be explained by the different mappabilities of the EcoRI and BglII end sequences (Figures 2B and 2C), the presence of unique features in the nonoverlapping part of these viewpoints (Figure S3), or possible PCR biases. Despite these potential limitations, our systematic 4C-seq analysis detected new chromatin loops within the proximal Igh domain in pro-B cells. Hence, more chromatin loops than previously anticipated contribute to the 3D architecture of the proximal Igh region in the absence of locus contraction.
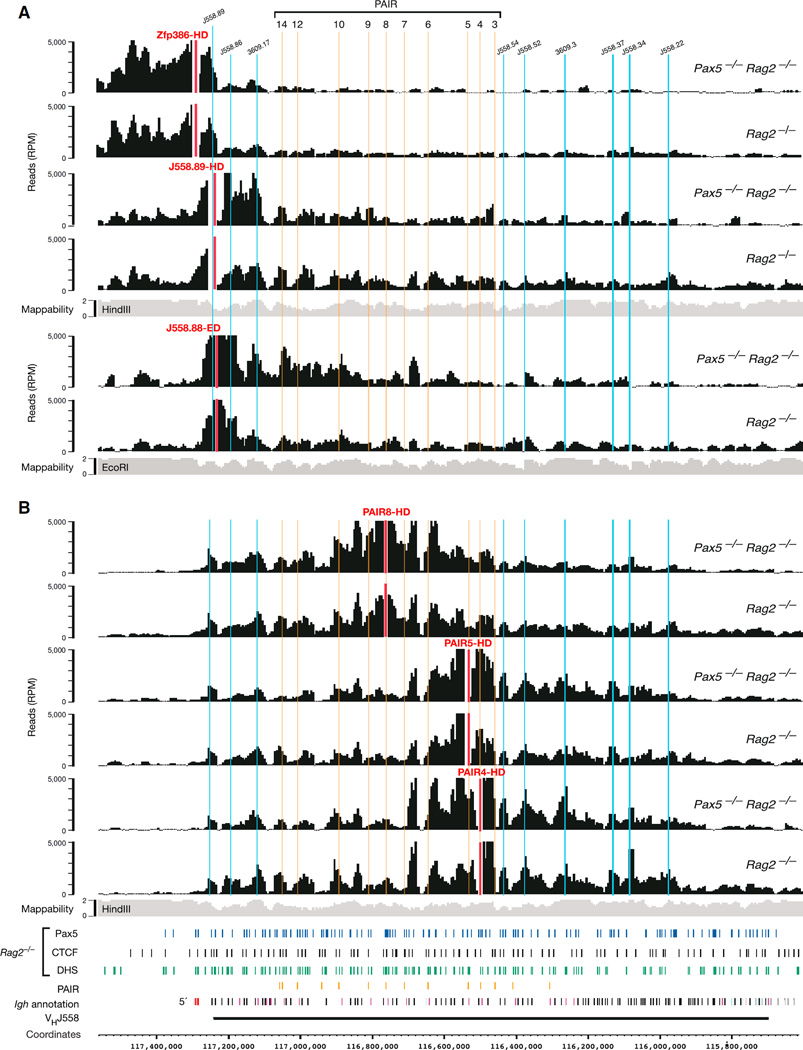
Folding Pattern of the Distal VH Gene Region
We next investigated the 3D conformation at the Igh 5′ end by using viewpoints corresponding to the last two VHJ558 and upstream Zfp386 genes (Figure S3). The viewpoint Zfp386-HD almost exclusively interacted with sequences upstream of the last VHJ558.89pg.195 gene in both Pax5−/−Rag2−/− and Rag2−/− pro-B cells (Figure 3A). In contrast, the J558.89-HD viewpoint efficiently interacted with upstream and downstream sequences in both pro-B cell types (Figure 3A) similar to the situation observed with the two HS8 viewpoints at the Igh 3′ end (Figures 2B and 2C). The J558.88-ED viewpoint predominantly contacted sequences in the distal VH gene cluster, although it is separated by only 6.3 kb from the upstream J558.89-HD viewpoint. Hence, these data indicate that the 5′ and 3′ boundaries of the Igh locus are located at or near the VHJ558.89pg.195 gene and HS8 site, respectively.
Figure 3. Local Interactions within the Distal VH Gene Cluster in the Absence of Locus Contraction.
(A) 4C-seq interaction profiles at the Igh 5′ end in cultured Pax5−/−Rag2−/− and Rag2−/− pro-B cells. Vertical lines indicate the positions of PAIR elements (yellow) and selected VH genes (blue).
(B) 4C-seq interaction patterns detected with the indicated PAIR viewpoints (red) in the distal VH gene region. See legend of Figure 1 for further explanations. One 4C-seq experiment was performed with viewpoints Zfp386-HD and J558.88-ED, and the data of all other viewpoints are based on average values of three experiments.
See also Figure S3.
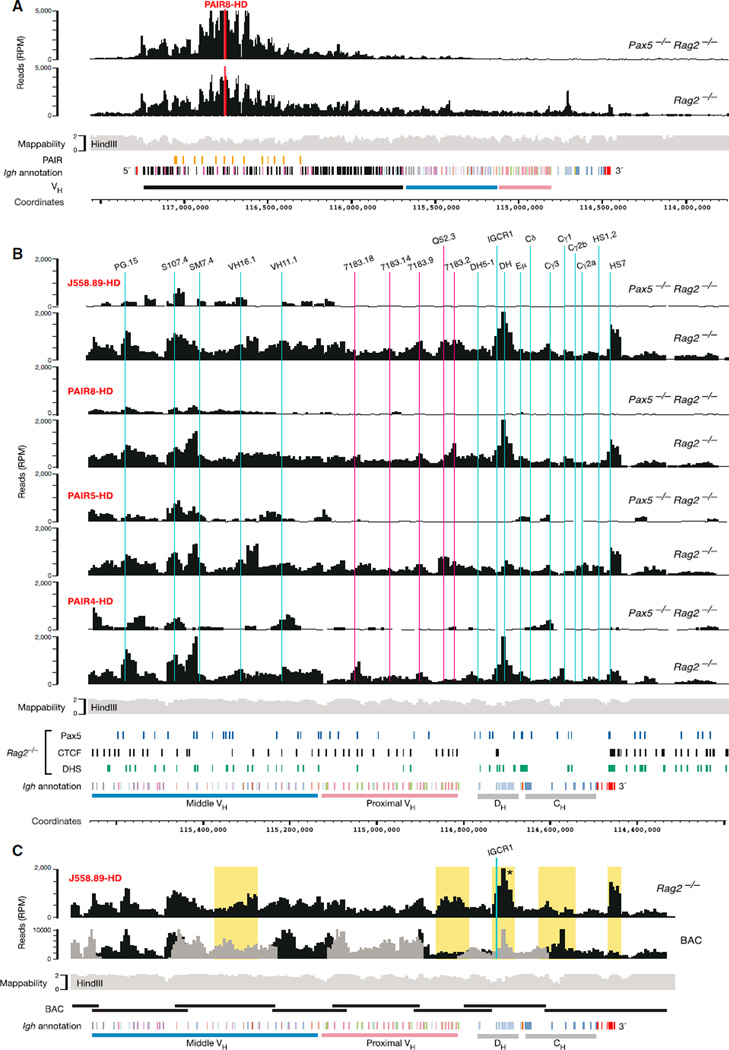
Three viewpoints in the PAIR region (PAIR4,5,8-HD) revealed an interaction pattern in the distal VH gene region that was unique in two aspects. First, the interactions detected with all three viewpoints extended from the Igh 5′ boundary over a 1.3 Mb region spanning almost the entire VHJ558 gene cluster in Pax5−/− Rag2−/− and Rag2−/− pro-B cells (Figure 3B) and is thus 2.5 times larger than the 0.5 Mb domain in the 3′ proximal Igh region in the absence of locus contraction. Second, the 4C-seq profiles of both pro-B cell types were similar for not just one, but for all three PAIR viewpoints (Figure 3B) as well as for the J558.70-HD viewpoint (Figure S2B). In contrast, the J606.5-HD viewpoint near the 3′ end of the distal VH gene cluster was part of a different local interaction region that minimally overlapped with the PAIR element-containing interaction domain in Pax5−/−Rag2−/− pro-B cells (Figure S2B). These data therefore identified a large interaction domain, which spans most of the distal VHJ558 gene cluster in the absence of locus contraction.
Flexible Long-Range Interactions across the VH Gene Cluster Mediate Locus Contraction
As expected, the Eµ-HD and HS3B-HD viewpoints of the proximal Igh domain specifically interacted with the distal VH gene region only upon locus contraction in Rag2−/− pro-B cells, but not in Pax5−/−Rag2−/− pro-B cells (Figure 4A). Notably, the long-distance interaction patterns of these proximal viewpoints were highly similar not only between each other (Figure 4A) but also in comparison with the local interaction patterns of the three PAIR (4,5,8-HD) viewpoints (Figure 3B). Likewise, the long-distance interaction profiles of Rag2−/− pro-B cells were also comparable between the proximal HS8-BN and IGCR1-BN or HS8-ED and IGCR1-ED viewpoints (Figures 4B and 4C). However, the 4C-seq profiles of Rag2−/− pro-B cells differed in the distal VH gene region between the three pairs of proximal region viewpoints similar to the observed changes in the mappability of the end sequences between the HindIII, BglII, and EcoRI sites that were used for 4C template preparation (Figures 4A–4C). One possible explanation for the above results could be that the observed peaks of the 4C-seq profiles identify interacting sequences at the base of chromatin loops. Alternatively, the 4C-seq patterns may result from a continuum of flexible long-range loops in the distal VH gene region, particularly in view of the fact that each 4C-seq experiment was performed with approximately 1 million pro-B cells, thus revealing an average of all interactions in the entire cell population (Figure S4A).
Figure 4. Flexible Long-Range Interactions along the Distal VH Gene Region in the Presence of Locus Contraction.
(A–C) Long-range interactions of the indicated proximal viewpoints (red) with the distal VH gene region in cultured Pax5−/−Rag2−/− and Rag2−/− pro-B cells. See legend of Figure 1 for further explanations. The data of the HS3B-HD and Eµ-HD viewpoints (A) are average values of three experiments, and all other viewpoints (B, C) were analyzed once.
(D) Comparison of the 4C-seq patterns obtained with random BAC libraries and Rag2−/− pro-B cells (analyzed from HS3B-HD). The sequencing pattern of each BAC (with its size indicated below) was normalized by setting the highest peak to an RPM value of 10,000 (Figure S4B) prior to merging the patterns of the two BAC libraries (indicated in black and gray).
Abbreviation: Del., internal BAC deletion. See also Figure S4.
To test these hypotheses, we generated two random 4C-seq libraries with purified DNA of nonoverlapping C57BL/6 BAC clones that covered the entire mouse Igh locus (Figures S4B–S4D). To this end, HindIII-digested BAC DNA was ligated at high concentration to favor random ligation, and the randomly ligated HindIII fragments were subsequently processed for deep sequencing in the same way as the 4C samples of pro-B cells (see Supplemental Experimental Procedures). In addition to the random assortment of HindIII sites, the sequencing of random BAC libraries also controlled for two potential artifacts, because it was subjected to the same mappability issues and potential PCR biases as the 4C-seq analysis of pro-B cells. As shown in Figure 4D, the sequence pattern of the BAC libraries was similar to the Rag2−/− pro-B cell interaction profile in the distal VH gene region. We conclude therefore that the VH gene region is characterized by a continuum of flexible long-range interactions.
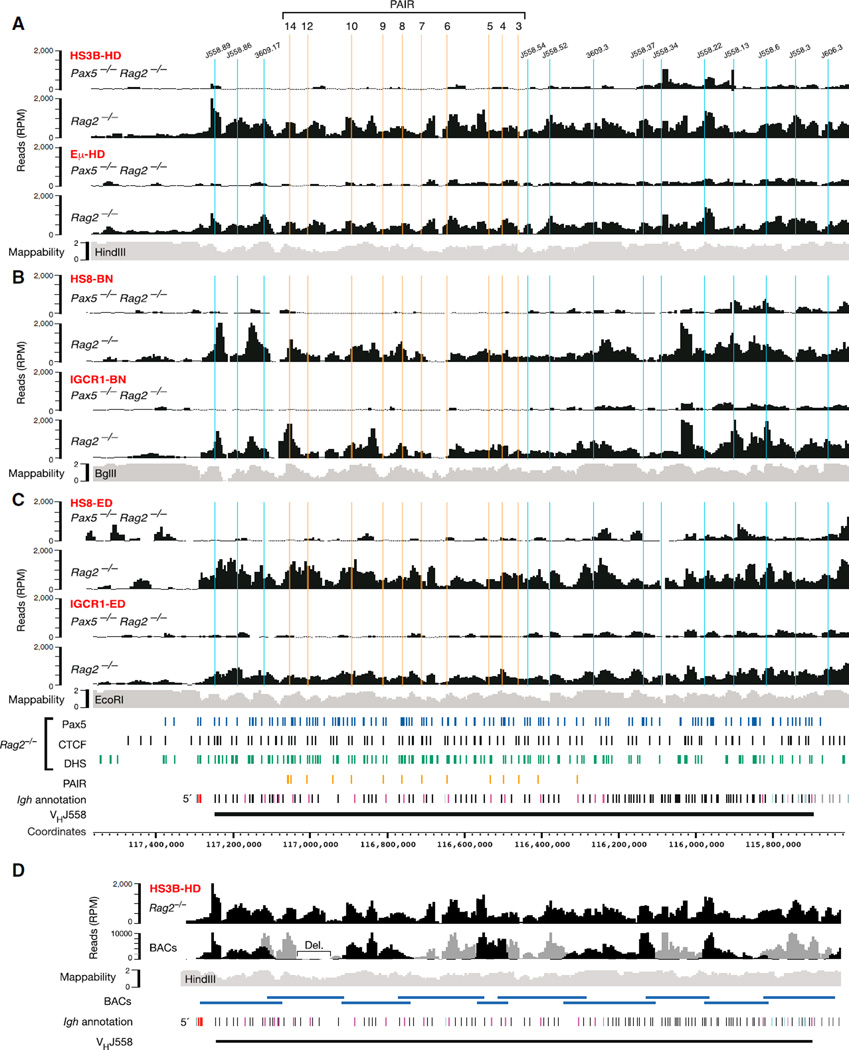
Locus Contraction Promotes Interaction of Distal VH Genes with Proximal CBE Regions
As shown by previous DNA-FISH analyses, the distal VH gene region interacts with the proximal Igh domain only upon locus contraction in Rag2−/− pro-B cells. Here we revealed the details of these interactions by 4C-seq analysis by using distal J558 (89,70-HD) and PAIR (4,5,8-HD) viewpoints (Figures 5A, 5B, and S2B). The long-range interactions of these distal viewpoints with the proximal Igh domain were significantly different (Figure 5B) from the local interaction patterns observed with proximal viewpoints (Figure 2). In particular, the J558.89-HD and three PAIR (4,5,8-HD) viewpoints showed only minimal interactions with the Eµ enhancer and elements in the CH region (Figure 5B), which were, however, strongly contacted by proximal viewpoints. Instead, the distal viewpoints efficiently interacted with the IGCR1/DH and 3′CBE (HS7 and HS8) regions (Figure 5B), which are the only elements containing CTCF-binding sites in the proximal Igh domain (Degner et al., 2011; Ebert et al., 2011). These data suggest that locus contraction brings the 5′ and 3′ Igh boundaries into close proximity and may mediate long-range interactions between distal and proximal Igh sequences through CTCF-binding sites in the proximal Igh domain. Moreover, analysis of the Igh BAC libraries with the J558.89-HD viewpoint indicated that the random sequencing pattern of the BACs and the 4C-seq interaction profile of Rag2−/− pro-B cells were largely similar in the proximal and middle VH gene regions but significantly differed in the Igh 3′ proximal domain (Figures 5C and S4E). These results therefore indicate that the proximal Igh domain assumes a more rigid 3D architecture compared to the flexible loops observed in the VH gene region.
Figure 5. Long-Range Interactions of the Distal VH Gene Region with the Proximal Igh Domain.
(A) 4C-seq interaction pattern of PAIR8 with sequences across the Igh locus in cultured Pax5−/−Rag2−/− and Rag2−/− pro-B cells.
(B) Long-range interactions of the indicated distal viewpoints (red) with sequences of the Igh 3′ region in the indicated cultured pro-B cell types. See legends of Figures 1 and 2 for further explanations. The data of all viewpoints in (A) and (B) are average values of three experiments except for the PAIR4-HD analysis of Pax5−/−Rag2−/− pro-B cells (average of two experiments).
(C) Comparison of the 4C-seq patterns between random BAC libraries and Rag2−/− pro-B cells (analyzed from J558.89-HD). See legend of Figure 4D for normalization of the BAC sequencing pattern. Yellow shading denotes regions with a 4C-seq pattern that differs between the random BAC libraries and Rag2−/− pro-B cells. The specificity of the 4C-seq interactions in the IGCR1/DH region (asterisk) of Rag2−/− pro-B cells compared to the random BAC library was confirmed by 3C-qPCR (Figure S4E).
Normal Long-Range Interactions in the Absence of Individual 3′ Regulatory Elements
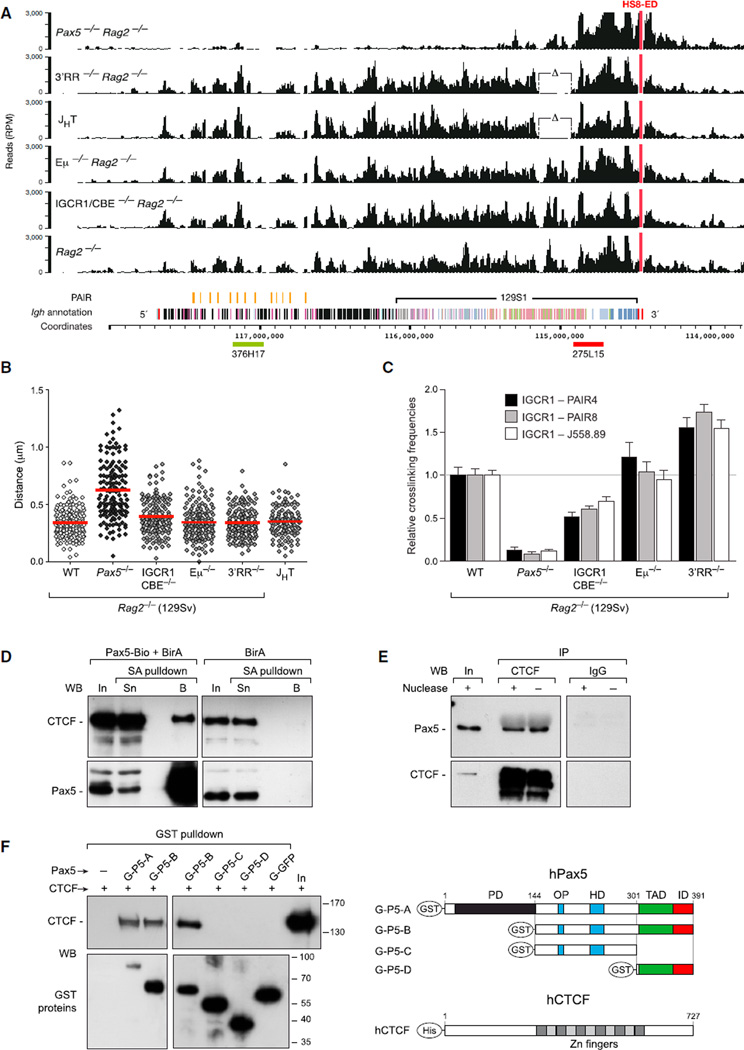
To investigate the influence of regulatory elements in the 3′ proximal domain on long-range Igh interactions, we examined the dual CBE mutation of the IGCR1 element (Guo et al., 2011b), the core Eµ enhancer deletion (Perlot et al., 2005), a deletion of the entire 3′RR (Vincent-Fabert et al., 2010), and the JHT mutation (eliminating the DHQ52, JH and Eµ elements; Gu et al., 1993) in 129Sv pro-B cells (for further explanation see Supplemental Experimental Procedures). As shown in Figures 6A and S5A, 4C-seq analyses with proximal (HS8-ED) and distal (J558.89-HD) viewpoints revealed similar long-range interaction patterns across the entire VH gene region in homozygous JHT pro-B cells as well as in Rag2−/− pro-B cells containing one of the 3′RR, Eµ, or IGCR1/CBE mutations compared to control Rag2−/− pro-B cells. We conclude therefore that individual mutation of either the IGCR1, Eµ, or 3′RR region had no effect on longrange interactions across the Igh locus in Rag2−/− pro-B cells.
Figure 6. Role of CTCF and 3′ Regulatory Elements in Long-Range Igh Interactions.
(A) 4C-seq analysis of cultured 129Sv pro-B cells of the indicated genotypes with the HS8-ED viewpoint (red). The homozygous JHT deletion eliminates the DHQ52, JH, and Eµ elements (Gu et al., 1993). The 129Sv sequence reads were mapped to genomic C57BL/6 sequences except for the available 129S1 Igh sequences (see Supplemental Experimental Procedures). A 120 kb deletion (Δ) was present in the E14 (129Ola) ESCs used to generate the JHT and 3′RR mutant alleles. 4C-seq experiments were performed once with Rag2−/− 3′RR (–/–) mutant, Rag2−/− Eµ (–/–) mutant, and JHT-deficient pro-B cells and twice with 129Sv Rag2−/−, Pax5−/−Rag2−/−, and Rag2−/− IGCR1/CBE (–/–) mutant pro-B cells.
(B) Two-color 3D DNA-FISH analysis with Igh BAC probes (indicated in A). Dot plots show the distances measured between the two DNA signals of individual Igh alleles together with the average distance determined for each genotype.
(C) 3C-qPCR analysis of the IGCR1 interaction with distal PAIR4, PAIR8, and VHJ558.89 sequences in cultured 129Sv pro-B cells of the indicated genotypes. A total of 12 3C templates of each genotype were used to determine the relative crosslinking frequency (see Supplemental Experimental Procedures), which was set to 1 for Rag2−/− pro-B cells and is shown with the standard error of the mean.
(D) Coprecipitation of CTCF with Pax5-Bio by streptavidin (SA) pull-down of nuclear extracts prepared from Abl-MLV-transformed pro-B cells of the Pax5Bio/Bio (Pax5-Bio) or control Rosa26BirA/BirA (BirA) genotype. The input (In; 1/100), supernatant (Sn; 1/100), and streptavidin-bound (B) precipitate were analyzed by immunoblotting (WB) with CTCF and Pax5 antibodies.
(E) Coimmunoprecipitation of Pax5 from a nuclear extract of Rag2−/− pro-B cells with CTCF antibodies followed by immunoblotting with a biotinylated rat anti-Pax5 mAb (detected with streptavidin-coupled horse radish peroxidase). Input (In; 1/100) and rabbit IgG were used as controls. Where indicated, DNA was digested with the endonuclease Benzonase during nuclear extract preparation.
(F) Direct Pax5-CTCF protein interaction. The hexahistidine-tagged CTCF and GST-Pax5 proteins (schematically depicted to the right) were affinity purified from baculovirus-infected Sf9 cells (Figure S5B) and used for in vitro binding and GST pull-down assays followed by immunoblotting of the precipitates with CTCF and GST antibodies (see Supplemental Experimental Procedures). A protein size marker (in kilodaltons) and input (In; 1/20) are shown. Empty glutathione-sepharose beads (−) and GST-GFP were used as negative controls. Abbreviations are as follows: G, GST; P5, Pax5; PD, paired domain; OP, octapeptide; HD, partial homeodomain; TAD, transactivation domain; ID, inhibitory domain.
See also Figure S5.
To confirm these findings with an independent method, we analyzed the same double mutant pro-B cells by 3D DNA-FISH with BAC probes (Figure 6A) from the 5′ and 3′ end of the VH gene cluster. As shown in Figure 6B, the distribution and average value of the measured distances between the two DNA signals were similar in homozygous JHT pro-B cells and Rag2−/− pro-B cells containing one of the 3′RR, Eµ, or IGCR1/CBE mutations compared to control Rag2−/− pro-B cells. In contrast, the distance between the probe signals was much greater for the decontracted Igh locus in Pax5−/−Rag2−/− pro-B cells, as published (Fuxa et al., 2004). In summary, the results of both 4C-seq and 3D DNA-FISH analyses demonstrate that the long-range interactions mediating Igh locus contraction in pro-B cells do not depend on functional IGCR1, Eµ, or 3′RR elements.
CTCF-Binding Sites in the IGCR1 Region Contribute to Long-Range Looping
Although the IGCR1/CBE mutation had no global effect on long-distance interactions in the Igh locus, we next investigated whether the two CTCF-binding sites (CBEs) in the IGCR1 region contribute to the formation of long-range loops with distal Igh sequences. To this end, we performed 3C-qPCR experiments with mutant and control Rag2−/− pro-B cells by using primers located in HindIII fragments containing VHJ558.89, PAIR8, PAIR4, and IGCR1 sequences (Figure 6C). Whereas the relative crosslinking frequency was high for the interaction between all three distal Igh elements and the IGCR1 region in Rag2−/− Eµ mutant and Rag2−/− 3′RR mutant pro-B cells, it was low in the locus contraction-deficient Pax5−/−Rag2−/− pro-B cells and was reduced by about 50% in Rag2−/− IGCR1/CBE mutant pro-B cells compared to Rag2−/− pro-B cells (Figure 6C). We conclude therefore that functional CBEs in the IGCR1 element are required for efficient loop formation with sequences in the distal VH gene cluster.
Because CTCF and Pax5 are required for long-range interactions and bind to multiple sites in the Igh locus (Ebert et al., 2011), we hypothesized that both transcription factors may mediate looping by binding to each other. To test this idea, we took advantage of Pax5Bio/Bio pro-B cells expressing a Pax5 protein with a C-terminal biotin acceptor sequence that is efficiently biotinylated in vivo by coexpression of the E. coli biotin ligase BirA (McManus et al., 2011). Streptavidin pull-down experiments with nuclear extracts of Pax5Bio/Bio pro-B cells or BirA-expressing Pax5+/+ pro-B cells revealed that CTCF specifically coprecipitated with the biotinylated Pax5-Bio protein in contrast to the control BirA extract (Figure 6D). In the reverse order, Pax5 was coimmunoprecipitated with anti-CTCF but not with unspecific IgG antibodies from nuclear extracts of Rag2−/− pro-B cells even after nuclease-mediated DNA digestion (Figure 6E). We next performed in vitro binding and GST pull-down assays with affinity-purified human CTCF and GST-Pax5 fusion proteins, which were expressed in baculovirus-infected Sf9 insect cells (Figure S5B). These in vitro binding experiments revealed that CTCF could directly interact with full-length Pax5 (G-P5-A) and a Pax5 mutant lacking the paired domain (G-P5-B), but not with polypeptides containing only central (G-P5-C) or C-terminal (G-P5-D) Pax5 sequences (Figure 6F). Similar results were obtained with GST pull-down assays with bacterially expressed GST-Pax5 fusion proteins (Figure S5C). These in vitro binding data are consistent with the possibility that Pax5 and CTCF may directly interact with each other in pro-B cells. As shown by ChIP-seq analysis of Pax5−/−Rag2−/− and Rag2−/− pro-B cells (Figure S5D), the CTCF-binding patterns at the Igh locus were highly similar in the absence and presence of Pax5. Hence, CTCF binds to its sites in the Igh locus independently of its interaction with Pax5.
YY1 Controls PAIR Function and Long-Range Interactions across the Igh Locus
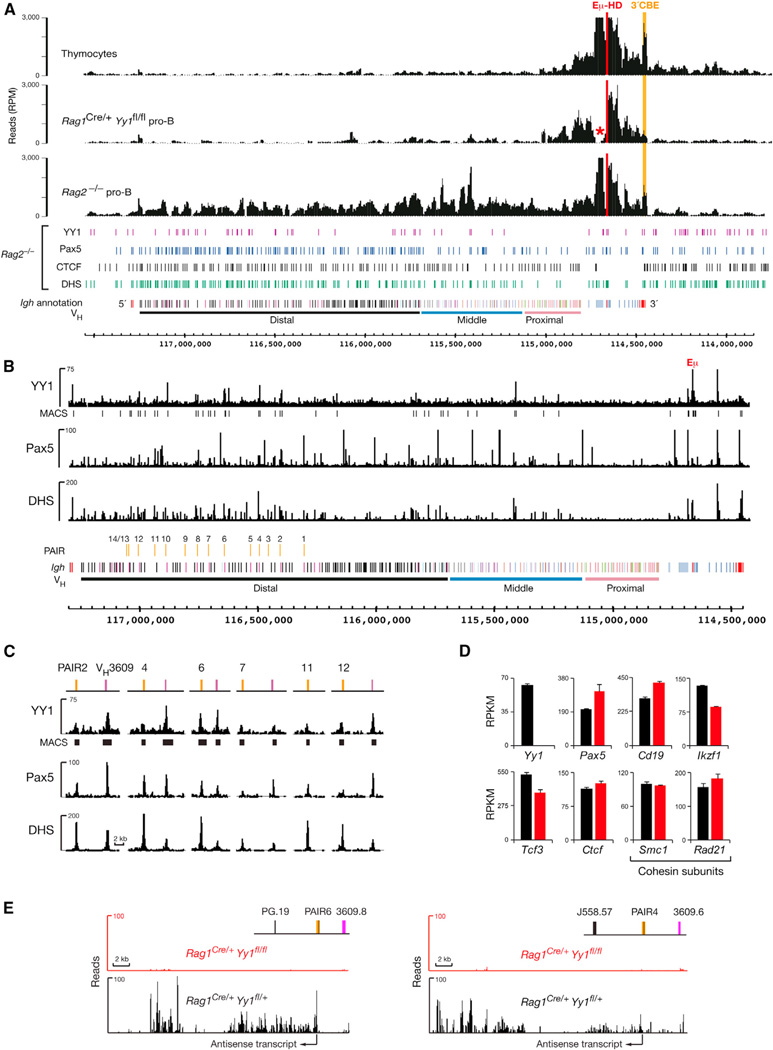
Given the involvement of the ubiquitous transcription factor YY1 in Igh locus contraction (Liu et al., 2007), we determined by 4Cseq analysis to what degree the conditional loss of YY1 affects long-range interactions in the Igh locus. Because B cell development was stringently arrested at the CD19+ pro-B cell stage in Rag1Cre/+Yy1fl/fl mice (Figure S6A), we used CD19 MACS sorting to isolate Yy1-deleted pro-B cells with high purity from the bone marrow of these mice (Figures S6B and S6C). 4C-seq analysis with the Eµ-HD and J606.5-HD viewpoints demonstrated that long-range interactions along the Igh locus could not be detected in YY1-deficient pro-B cells (Figures 7A and S6D), consistent with recent 3C-qPCR results indicating that YY1 is essential for looping of Eµ with PAIR4 and PAIR6 (Verma-Gaur et al., 2012). In contrast to Pax5−/− pro-B cells (Figure 2), local interactions in the proximal Igh domain were also altered in YY1-deficient pro-B cells, as shown by the loss of the Eµ-3′CBE interaction (Figure 7A). We therefore conclude that YY1 controls local as well as long-range interactions in the Igh locus.
Figure 7. YY1 Controls PAIR Function and Long-Range Interactions across the Igh Locus.
(A) 4C-seq analysis of ex vivo sorted Rag1Cre/+Yy1fl/fl pro-B cells (Figures S6B and S6C), control Rag2−/− pro-B cells, and thymocytes with the viewpoint Eµ-HD (red). The Eµ-3′CBE interaction is highlighted in orange. A red asterisk indicates the loss of DH sequences by DH-JH rearrangements in the recombination-proficient Rag1Cre/+Yy1fl/fl pro-B cells.
(B) Identification of YY1 peaks in the Igh locus by Bio-ChIP-sequencing of cultured pro-B cells from Yy1ihCd2/+Rosa26BirA/BirARag2−/− mice. Vertical bars below the Bio-ChIP-seq track identify significant YY1 peaks that were called by MACS with a p value of <10−10. For comparison, the Pax5-binding and DHS site patterns of Rag2−/− pro-B cells are shown (Revilla-I-Domingo et al., 2012).
(C) YY1 binding at PAIR elements and associated VH3609 genes.
(D and E) Analysis of YY1-dependent gene expression. The expression values (RPKM) of selected genes (D) and RNA-seq profiles of the antisense transcripts originating from PAIR4 and PAIR6 (E) are shown for Rag1Cre/+Yy1fl/fl (red) and control Rag1Cre/+Yy1fl/+ (black) pro-B cells together with the standard error of the mean (based on two RNA-seq experiments for each genotype).
See also Figure S6.
YY1 is known to bind to the Eµ enhancer (Park and Atchison, 1991), which is, however, dispensable for long-range Igh looping (Figures 6A and 6B). To search for YY1-binding sites involved in long-range interactions, we generated a Yy1ihCd2 allele expressing a YY1-Bio protein that can be biotinylated by the E. coli biotin ligase BirA (Figures S6E–S6H). B cell development was normal in Yy1ihCd2/+ mice (data not shown), so we used short-term cultured pro-B cells from Yy1ihCd2/+Rosa26BirA/BirARag2−/− mice for streptavidin-mediated chromatin precipitation coupled with deep sequencing (Bio-ChIP-seq). As shown in Figures 2D and 7B, two prominent YY1 peaks were identified by Bio-ChIP sequencing at the Eµ enhancer and Cγ1-2b DHS site 1 in the proximal Igh domain. YY1 peaks were unevenly distributed in the VH gene cluster with most peaks being present in the distal VH gene region (Figure 7B). Several of these YY1-binding sites were located together with Pax5 peaks at PAIR elements and their associated VH3609 genes (Figure 7C). We next examined by RNA sequencing whether YY1 controls the expression of known regulators of locus contraction. Pax5, Ikzf1 (Ikaros), Tcf3 (E2A), and Ctcf as well as Smc1 and Rad21 (encoding two cohesin subunits) were similarly expressed in sorted Rag1Cre/+Yy1fl/fl and control Rag1Cre/+Yy1fl/+ pro-B cells (Figure 7D), consistent with a direct role of YY1 in controlling Igh locus contraction. In contrast, the noncoding antisense transcripts originating from PAIR4 and PAIR6 were strongly reduced in the absence of YY1 (Figure 7E). Collectively, these data demonstrate that YY1 controls antisense transcription and long-range interactions by binding to multiple sites in the distal VH gene cluster.
DISCUSSION
VH-DJH recombination depends on long-range interactions between the VH gene cluster and proximal Igh domain containing the DJH-rearranged gene segment (Fuxa et al., 2004; Kosak et al., 2002). Here, we have investigated these interactions by high-resolution 4C sequencing, which revealed important aspects of Igh looping in lymphoid progenitors and committed pro-B cells.
In pro-B cells, all long-range Igh interactions were strictly confined to the Igh locus, which constitutes one large chromatin- folding unit. We identified the Igh 5′ and 3′ boundaries near the first 5′ VHJ558 gene and at the 3′CBE with its nine CTCF-cohesin-binding sites, because the distal J558.89 and proximal HS8 viewpoints efficiently interacted with both Igh and adjacent non-Igh sequences, as expected for boundary elements. We were, however, unable to further localize the 5′ boundary region because of the absence of conspicuous features such as a specific chromatin signature or clustering of CTCF-binding sites in the VHJ558.89 gene region (Ebert et al., 2011).
Distance measurements between 12 distinct Igh FISH probes combined with computer simulation predicted that the Igh locus is organized into compartments with clusters of loops separated by linkers (MLS model) in uncommitted pre-pro-B cells (Jhunjhunwala et al., 2008). Our 4C-seq analyses have provided experimental evidence for this notion, because the Igh locus in its “extended’ configuration consists of local interaction domains ranging from 0.5 Mb (3′ proximal region) to 1.3 Mb (5′ PAIR region) in uncommitted lymphoid progenitors and thymocytes. As shown by global Hi-C analyses, the entire mouse genome is organized into local chromatin interaction domains referred to as topological domains, which range from 0.5 to 3 Mb with a median size of 0.88 Mb (Dixon et al., 2012; Lin et al., 2012; Nora et al., 2012). The local interaction domains of the Igh locus also fit this size range, exhibit a similar 4C-seq pattern in both lymphoid progenitors and thymocytes, and thus probably correspond to topological domains, which are largely invariant between cell types (Dixon et al., 2012; Lin et al., 2012). However, the local interaction domains in the Igh locus partially overlap with each other and thus differ from topological domains, which are separated by stringent boundaries (Dixon et al., 2012; Nora et al., 2012). Notably, the local interactions in the VH gene cluster do not require Pax5 or PAIR activity for their formation in lymphoid progenitors and may thus reflect a default folding state of the Igh locus prior to full activation of the VH gene region in committed pro-B cells.
In contrast, the proximal Igh domain is already active under the control of the Eµ enhancer in lymphoid progenitors (Afshar et al., 2006; Perlot et al., 2005). The local 3D architecture of the proximal domain consists of loops between the IGCR1, Eµ, 3′RR, and 3′CBE regions (Degner et al., 2011; Guo et al., 2011a, 2011b). Our systematic 4C-seq analysis identified additional interactions of these regulatory regions with elements in the constant gene region and the first proximal VH genes in thymocytes, lymphoid progenitors, and committed pro-B cells. One of these interaction sites is located between the Cγ1 and Cγ2b gene segments in a region containing two DHS sites that bind Pax5 and other transcription factors in pro-B cells. The two Cγ1-2b DHS sites may function as Pax5-dependent enhancers regulating V(D)J or class switch recombination. The incorporation of proximal VH genes into the local 3D architecture of the proximal Igh domain nicely explains why only the first proximal VH genes undergo VH-DJH recombination in Pax5-deficient progenitors (Hesslein et al., 2003; Roldá n et al., 2005). Surprisingly, the most proximal VH genes also interact with the proximal Igh domain in thymocytes, although they never undergo VH-DJH recombination during T cell development, unless the CBEs of the IGCR1 insulator are inactivated (Guo et al., 2011b). We conclude therefore that a functional IGCR1 element can prevent VH-DJH recombination even if VH genes participate in interactions with the proximal Igh domain in thymocytes. Moreover, the function of the IGCR1 insulator seems to be neutralized already in the absence of locus contraction in Pax5-deficient progenitors.
Igh locus contraction was extensively studied at the single-cell level by low-resolution DNA-FISH analysis. Recent 3C experiments, which provide high-resolution analysis of individual loops in a population of a million pro-B cells, have so far identified only four focal long-distance interactions between the Eµ enhancer and the VH7183.18, VHJ558.1, PAIR4, and PAIR6 regions (Guo et al., 2011a; Verma-Gaur et al., 2012). In contrast, our systematic 4C-seq analyses based on 16 different viewpoints have revealed a continuum of flexible long-range interactions across the entire VH gene cluster in committed pro-B cells. This conclusion is based on the following evidence. First, long-range interactions were observed only in committed pro-B cells but not in Pax5- or YY1-deficient pro-B cells, indicating that these interactions were not caused by a 4C sequencing artifact. Second, 4C templates prepared with the same primary restriction enzyme (HindIII, EcoRI, or BglII) gave rise to similar long-range interaction patterns in the VH gene region when analyzed from different proximal viewpoints. Third, the interaction profiles were distinct between 4C-seq experiments performed with different restriction enzymes that differ in the mappability of their end sequences along the VH gene cluster. Finally, the long-range interaction patterns in the VH gene region of committed pro-B cells were similar to the sequencing profiles obtained with random Igh BAC libraries, thus supporting the idea that the sum of all flexible loops in a population of a million pro-B cells results in a continuum of long-range interactions. In contrast, the 3′ proximal Igh domain forms a more rigid 3D architecture, as shown by the fact that long-range loops from the distal VH gene region primarily interacted with the IGCR1/DH and 3′CBE regions in a manner that differed from the sequencing pattern of random Igh BAC libraries. We therefore hypothesize that the flexible long-range loops provide, at the pro-B cell population level, a similar probability for each VH gene to be juxtaposed next to the proximal DJH-rearranged gene segment to undergo VH-DJH recombination. By promoting equal participation of all VH genes in VHDJH recombination, flexible long-range loops contribute to the generation of a diverse antibody repertoire, which is essential for an effective immune response to foreign pathogens.
The question thus arises what sequence features may determine the structure of flexible loops in the VH gene cluster. Unfortunately, it is the flexible nature of the long-range loops itself that prevents the identification of interacting elements, which may be present at regular intervals similar to the dense distribution of CTCF- and Pax5-binding sites along the VH gene cluster (Ebert et al., 2011; Revilla-I-Domingo et al., 2012). Importantly, the local interaction domains in Pax5-deficient progenitors and the corresponding long-range loops in committed pro-B cells revealed similar 4C-seq patterns in the distal VH gene region. Hence, the flexible loops along the VH gene cluster may be a characteristic feature of the local interaction domains, which coalesce into a large interaction territory upon locus contraction by a so-far-unknown mechanism.
As shown by 4C sequencing, long-range interactions across the VH gene cluster were absent in pro-B cells lacking Pax5 or YY1, consistent with previous DNA-FISH analyses (Fuxa et al., 2004; Liu et al., 2007). In the absence of Pax5, local Igh domains were normally formed in lymphoid progenitors, as shown by the fact that Pax5 expression is activated only later in committed pro-B cells. Local Igh domains were also generated in the absence of the ubiquitous regulator YY1, although specific local interactions, such as the Eµ-3′CBE loop, required YY1 function. Bio-ChIP sequencing identified YY1-binding sites in the 3′ proximal domain and middle VH gene region. However, the majority of YY1-binding sites were found in the distal VHJ558 region, where YY1 peaks colocalized together with Pax5-binding sites at six PAIR elements (2, 4, 6, 7, 11, and 12) and their associated VH3609 genes. Consistent with this finding, noncoding antisense transcripts originating from PAIR4 and PAIR6 were lost in YY1-deficient pro-B cells (Verma-Gaur et al., 2012; this study) similar to Pax5 mutant pro-B cells (Ebert et al., 2011). Hence, Pax5 and YY1 both control the activity of PAIR elements and mediate long-range interactions across the VH gene cluster, thus supporting a role for PAIR elements in regulating Igh locus contraction.
The PAIR elements 4, 6, and 11 exhibit the highest transcriptional activity within the VH gene cluster and have therefore been postulated to induce long-range looping by relocating the PAIR-containing VH gene sequences to transcription factories in the nucleus where they interact with the proximal Igh domain, which is transcriptionally activated by the Eµ enhancer (Verma-Gaur et al., 2012). This hypothesis predicts that the PAIR elements with the highest transcriptional activity should constitute interaction hotspots in the distal VH gene region. In marked contrast, our 4C-seq experiments indicate that all VH gene sequences contact the proximal Igh domain with similar probability. Alternatively, the regularly spaced CTCF-cohesin-binding sites in the VH gene region may be involved in the formation of flexible loops within local interaction domains in lymphoid progenitors. Once expressed in committed pro-B cells, Pax5 may then provide another layer of interactions between the many Pax5-binding sites and CTCF-cohesin-binding elements in the Igh locus, thus promoting long-range interactions among local interaction domains. In support of this hypothesis, we have shown that Pax5 and CTCF interact with each other in pro-B cells and in vitro binding assays. Moreover, long-range interactions between IGCR1 and distal VH gene sequences depend on functional CTCF-binding sites in the IGCR1 region. Finally, long-range loops from the VH gene region primarily contact the IGCR1 and 3′CBE regions, which are the only elements containing CTCF-binding sites in the proximal Igh domain.
The chromatin accessibility and transcriptional activation of the proximal Igh domain is stringently controlled by the Eµ enhancer (Afshar et al., 2006; Chakraborty et al., 2009; Perlot et al., 2005). The VH-DJH recombination block, which is observed in Eµ mutant pro-B cells, is probably a secondary effect of the strongly reduced number of DJH-rearranged Igh alleles (Afshar et al., 2006; Perlot et al., 2005). Notably, the recombination of proximal and distal VH genes is equally affected by deletion of the Eµ enhancer (Perlot et al., 2005), implying that the VH-DJH recombination phenotype is not caused by the loss of Igh locus contraction. Moreover, germline transcription of proximal and distal VH genes proceeds normally in Eµ mutant pro-B cells, indicating that the activity of the Eµ enhancer is restricted to the proximal Igh domain (Afshar et al., 2006; Perlot et al., 2005). A recent report has, however, implicated the Eµ enhancer in the control of Igh locus contraction and thus VH-DJH recombination (Guo et al., 2011a). This conclusion was primarily based on 3D DNA-FISH analysis of the Igh contraction state in Rag2−/− Eµ mutant and JHT-deficient pro-B cells compared to control Rag2−/− pro-B cells (Guo et al., 2011a). By performing 4C-seq and 3D DNA-FISH experiments with the same pro-B cell types, we could, however, not confirm a role for the Eµ enhancer in the regulation of Igh locus contraction by these two independent methods. Similar to the Eµ enhancer, our 4C-seq and 3D DNAFISH analyses did not provide evidence for a role of the IGCR1 and 3′RR elements in Igh long-range looping, indicating that individual mutations of these three regulatory elements in the proximal Igh domain do not affect locus contraction. These data are compatible with the testable hypothesis that the VH gene region and proximal domain of the Igh locus have separable functions. A major and intrinsic function of the VH gene cluster seems to be the formation of long-range loops to present VH genes to the proximal domain, which functions as the business end of the Igh locus where V(D)J recombination takes place.
EXPERIMENTAL PROCEDURES
Detailed methods can be found in the Supplemental Information available online. All animal experiments were carried out according to valid project licenses, which were approved and regularly controlled by the Austrian Veterinary Authorities.
4C Sequencing
4C templates were prepared from pro-B cells and thymocytes, as described (Simonis et al., 2007). For each viewpoint, 16 individual PCR amplifications with 35 cycles were performed with ~150 ng of 4C template with specific primers that were extended with Illumina sequencing adaptors (Table S1). The pooled PCR products were selected for a fragment size less than 1 kb on agarose gels prior to single-end sequencing with the HiSeq 2000 system resulting in a read length of 100 nucleotides.
Random 4C-Seq BAC Library
For template generation, equimolar DNA mixtures of C57BL/6 BAC clones covering the mouse Igh locus (Figure S4B) were digested with HindIII and ligated a high DNA concentration followed by Sau3AI digestion, ligation at low DNA concentration, and PCR amplification with viewpoint-specific primers.
3C-qPCR Analysis
3C templates of pro-B cells were prepared and subjected to TaqMan PCR analysis with primers listed in Table S2, as described (Hagége et al., 2007).
Bio-ChIP-Seq Analysis of YY1 Binding
Chromatin was prepared from short-term cultured pro-B cells of Yy1ihCd2/+ Rosa26BirA/BirARag2−/− mice followed by streptavidin pull-down, as described (Ebert et al., 2011). The precipitated DNA were submitted to paired-end sequencing with the Illumina GAIIx system resulting in a read length of 76 nucleotides. YY1 peaks were called with MACS (version 1.3.6.1) and filtered for p values of <10−10.
Nuclear Extract Preparation and Coprecipitation Analysis
Pax5-Bio was precipitated from nuclear extracts of Abl-MLV-transformed Pax5Bio/Bio and Rosa26BirA/BirA pro-B cells by streptavidin pull-down, and CTCF was precipitated from nuclear extracts of cultured Rag2−/− pro-B cells with a CTCF mAb (clone 48, BD), as described (McManus et al., 2011).
Supplementary Material
Acknowledgments
We thank A. Sommer and P. Stolt-Berger at the Campus Science Support Facilities for deep sequencing and protein expression, E. Axelsson for RNA-seq analysis, W. van Ijcken and F. Grosveld for advice on 4C sequencing, J. Skok for help with DNA-FISH analysis, and J.-M. Peters for advice on CTCF purification. This research was supported by Boehringer-Ingelheim, the Vienna Science and Technology Fund (WWTF), an ERC Advanced Grant (291740- LymphoControl) from the European Community’s Seventh Framework Program, the Austrian GEN-AU initiative (financed by the Bundesminsterium für Bildung und Wissenschaft), and an EMBO fellowship (to T.S.).
Footnotes
ACCESSION NUMBERS
The 4C-seq and ChIP-seq data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSE43008.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, six figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2013.08.011.
REFERENCES
- Afshar R, Pierce S, Bolland DJ, Corcoran A, Oltz EM. Regulation of IgH gene assembly: role of the intronic enhancer and 5’DQ52 region in targeting DHJH recombination. J. Immunol. 2006;176:2439–2447. doi: 10.4049/jimmunol.176.4.2439. [DOI] [PubMed] [Google Scholar]
- Bolland DJ, Wood AL, Johnston CM, Bunting SF, Morgan G, Chakalova L, Fraser PJ, Corcoran AE. Antisense intergenic transcription in V(D)J recombination. Nat. Immunol. 2004;5:630–637. doi: 10.1038/ni1068. [DOI] [PubMed] [Google Scholar]
- Chakraborty T, Perlot T, Subrahmanyam R, Jani A, Goff PH, Zhang Y, Ivanova I, Alt FW, Sen R. A 220-nucleotide deletion of the intronic enhancer reveals an epigenetic hierarchy in immunoglobulin heavy chain locus activation. J. Exp. Med. 2009;206:1019–1027. doi: 10.1084/jem.20081621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner SC, Verma-Gaur J, Wong TP, Bossen C, Iverson GM, Torkamani A, Vettermann C, Lin YC, Ju Z, Schulz D, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc. Natl. Acad. Sci. USA. 2011;108:9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A, McManus S, Tagoh H, Medvedovic J, Salvagiotto G, Novatchkova M, Tamir I, Sommer A, Jaritz M, Busslinger M. The distal VH gene cluster of the Igh locus contains distinct regulatory elements with Pax5 transcription factor-dependent activity in pro-B cells. Immunity. 2011;34:175–187. doi: 10.1016/j.immuni.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Fuxa M, Skok J, Souabni A, Salvagiotto G, Roldán E, Busslinger M. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett FE, Emelyanov AV, Sepulveda MA, Flanagan P, Volpi S, Li F, Loukinov D, Eckhardt LA, Lobanenkov VV, Birshtein BK. Chromatin architecture near a potential 3′ end of the igh locus involves modular regulation of histone modifications during B-Cell development and in vivo occupancy at CTCF sites. Mol. Cell. Biol. 2005;25:1511–1525. doi: 10.1128/MCB.25.4.1511-1525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Guo C, Gerasimova T, Hao H, Ivanova I, Chakraborty T, Selimyan R, Oltz EM, Sen R. Two forms of loops generate the chromatin conformation of the immunoglobulin heavy-chain gene locus. Cell. 2011a;147:332–343. doi: 10.1016/j.cell.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Yoon HS, Franklin A, Jain S, Ebert A, Cheng HL, Hansen E, Despo O, Bossen C, Vettermann C, et al. CTCF-binding elements mediate control of V(D)J recombination. Nature. 2011b;477:424–430. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, Fisher AG, Merkenschlager M. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–413. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagège H, Klous P, Braem C, Splinter E, Dekker J, Cathala G, de Laat W, Forné T. Quantitative analysis of chromosome conformation capture assays (3C-qPCR) Nat. Protoc. 2007;2:1722–1733. doi: 10.1038/nprot.2007.243. [DOI] [PubMed] [Google Scholar]
- Hesslein DGT, Pflugh DL, Chowdhury D, Bothwell ALM, Sen R, Schatz DG. Pax5 is required for recombination of transcribed, acetylated, 5′ IgH V gene segments. Genes Dev. 2003;17:37–42. doi: 10.1101/gad.1031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SL, Yin B, Ji Y, Chaumeil J, Marszalek K, Tenthorey J, Salvagiotto G, Steinel N, Ramsey LB, Ghysdael J, et al. RAG-1 and ATM coordinate monoallelic recombination and nuclear positioning of immunoglobulin loci. Nat. Immunol. 2009;10:655–664. doi: 10.1038/ni.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhunjhunwala S, van Zelm MC, Peak MM, Cutchin S, Riblet R, van Dongen JJM, Grosveld FG, Knoch TA, Murre C. The 3D structure of the immunoglobulin heavy-chain locus: implications for long-range genomic interactions. Cell. 2008;133:265–279. doi: 10.1016/j.cell.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhunjhunwala S, van Zelm MC, Peak MM, Murre C. Chromatin architecture and the generation of antigen receptor diversity. Cell. 2009;138:435–448. doi: 10.1016/j.cell.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CM, Wood AL, Bolland DJ, Corcoran AE. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J. Immunol. 2006;176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG, Singh H. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- Lin YC, Benner C, Mansson R, Heinz S, Miyazaki K, Miyazaki M, Chandra V, Bossen C, Glass CK, Murre C. Global changes in the nuclear positioning of genes and intra- and interdomain genomic interactions that orchestrate B cell fate. Nat. Immunol. 2012;13:1196–1204. doi: 10.1038/ni.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K, Shi Y. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21:1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus S, Ebert A, Salvagiotto G, Medvedovic J, Sun Q, Tamir I, Jaritz M, Tagoh H, Busslinger M. The transcription factor Pax5 regulates its target genes by recruiting chromatin-modifying proteins in committed B cells. EMBO J. 2011;30:2388–2404. doi: 10.1038/emboj.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nativio R, Wendt KS, Ito Y, Huddleston JE, Uribe-Lewis S, Woodfine K, Krueger C, Reik W, Peters J-M, Murrell A. Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus. PLoS Genet. 2009;5:e1000739. doi: 10.1371/journal.pgen.1000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Atchison ML. Isolation of a candidate repressor/activator, NF-E1 (YY-1, δ), that binds to the immunoglobulin κ 3′ enhancer and the immunoglobulin heavy-chain µ E1 site. Proc. Natl. Acad. Sci. USA. 1991;88:9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlot T, Alt FW. Cis-regulatory elements and epigenetic changes control genomic rearrangements of the IgH locus. Adv. Immunol. 2008;99:1–32. doi: 10.1016/S0065-2776(08)00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlot T, Alt FW, Bassing CH, Suh H, Pinaud E. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc. Natl. Acad. Sci. USA. 2005;102:14362–14367. doi: 10.1073/pnas.0507090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla-I-Domingo R, Bilic I, Vilagos B, Tagoh H, Ebert A, Tamir IM, Smeenk L, Trupke J, Sommer A, Jaritz M, Busslinger M. The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis. EMBO J. 2012;31:3130–3146. doi: 10.1038/emboj.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán E, Fuxa M, Chong W, Martinez D, Novatchkova M, Busslinger M, Skok JA. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat. Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh CE, Jhunjhunwala S, Riblet R, Murre C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 2005;19:322–327. doi: 10.1101/gad.1254305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonis M, Kooren J, de Laat W. An evaluation of 3C-based methods to capture DNA interactions. Nat. Methods. 2007;4:895–901. doi: 10.1038/nmeth1114. [DOI] [PubMed] [Google Scholar]
- Splinter E, Heath H, Kooren J, Palstra R-J, Klous P, Grosveld F, Galjart N, de Laat W. CTCF mediates long-range chromatin looping and local histone modification in the β-globin locus. Genes Dev. 2006;20:2349–2354. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Werken HJ, de Vree PJ, Splinter E, Holwerda SJ, Klous P, de Wit E, de Laat W. 4C technology: protocols and data analysis. Methods Enzymol. 2012;513:89–112. doi: 10.1016/B978-0-12-391938-0.00004-5. [DOI] [PubMed] [Google Scholar]
- Verma-Gaur J, Torkamani A, Schaffer L, Head SR, Schork NJ, Feeney AJ. Noncoding transcription within the Igh distal V(H) region at PAIR elements affects the 3D structure of the Igh locus in pro-B cells. Proc. Natl. Acad. Sci. USA. 2012;109:17004–17009. doi: 10.1073/pnas.1208398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent-Fabert C, Fiancette R, Pinaud E, Truffinet V, Cogné N, Cogné M, Denizot Y. Genomic deletion of the whole IgH 3′ regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood. 2010;116:1895–1898. doi: 10.1182/blood-2010-01-264689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.