Abstract
In this study, we aimed to evaluate the influence of diet-induced obesity on IL-6 deficiency-induced bone remodeling abnormality. Seven-week-old IL-6-/- mice and their wild type (WT) littermates were fed a standard diet (SD) or high-fat diet (HFD) for 25 weeks. Lipid formation and bone metabolism in mice tibiae were investigated by histochemical analysis. Both IL-6-/- and WT mice fed the HFD showed notable body weight gain, thickened cortical bones, and adipose accumulation in the bone marrow. Notably, the HFD normalized the bone phenotype of IL-6-/- mice to that of their WT counterpart, as characterized by a decrease in bone mass and the presence of an obliquely arranged, plate-like morphology in the trabecular bone. Alkaline phosphatase and osteocalcin expressions were attenuated in both genotypes after HFD feeding, especially for the IL-6-/- mice. Meanwhile, tartrate-resistant acid phosphatase staining was inhibited, osteoclast apoptosis rate down-regulated (revealed by TUNEL assay), and the proportion of cathepsin K (CK)-positive osteoclasts significantly increased in IL-6-/- mice on a HFD as compared with IL-6-/- mice on standard chow. Our results demonstrate that HFD-induced obesity reverses IL-6 deficiency-associated bone metabolic disorders by suppressing osteoblast activity, upregulating osteoclastic activity, and inhibiting osteoclast apoptosis.
Keywords: IL-6, obesity, bone metabolism, osteoblast, osteoclast
Introduction
Obesity and osteoporosis—commonly associated with a sedentary lifestyle and malnutrition—are increasingly gaining attention in modern society because both conditions have the potential to cause serious health-related consequences. Although usually considered separately, these two disorders seem to be closely related (Goulding et al. 2005). However, there is no consensus as to the relationship between obesity and osteoporosis, despite much related literature (Reid et al. 1994; Ionova-Martin et al. 2010). Obesity is traditionally considered to be favorable to bone formation, and thus protective against osteoporosis. Mechanical loading conferred by the increased body weight stimulates bone formation by increasing the proliferation and differentiation of local osteoblasts and osteocytes as well as decreasing apoptosis of osteoblastic lineage cells through the Wnt/β-catenin signaling pathway (Patsch et al. 2011). Clinical studies have shown that body weight or body mass index (BMI) is positively correlated with bone mineral density or bone mass, whereas low body weight or a low BMI is a risk factor for low bone mass and bone loss in humans (Felson et al. 1993; Ravn et al. 1999; Reid 2002).
Recent reports have demonstrated that high-fat diet-induced obesity is a cause of bone loss and may be regarded as a risk factor for osteoporosis (Jelcic 2010; Russell et al. 2010). A cross-sectional study of 200 young people aged 3 to 19 years showed that increased adiposity may be linked to an increased risk of bone fracture (Goulding et al. 2001). Another large cross-sectional study of 13,000 adult men and women also indicated a positive association between percentage body fat and osteopenia (Pollock et al. 2007). In animal studies, high-fat diet-induced obese mice demonstrated impaired bone microarchitecture and mechanical properties along with enhanced bone resorption and osteoclast formation (Halade et al. 2010; Halade et al. 2011; Patsch et al. 2011). Although several potential mechanisms involved in obesity-related stimulation of osteoclastogenesis have been proposed, including accumulated adipocyte-associated, low-grade inflammation (Halade et al. 2011) and changes in adipose tissue-derived hormones (Ducy et al. 2000; Wang et al. 2014), the precise mechanisms underlying the association between obesity and bone are still not well elucidated.
Interleukin-6 (IL-6) is a highly pleiotropic cytokine that affects numerous biological events, including bone remodeling (Kamimura et al. 2003). In bone, IL-6 is initially regarded as an osteoclastogenesis promoter because of its capacity to induce the differentiation of osteoclast precursor cells into mature and active osteoclasts both in vitro (Ishimi et al. 1990; Yokota et al. 2014) and in vivo (Binkley et al. 1994; Wong et al. 2006). Elevated IL-6-induced inflammation is also shown to be responsible for obesity-associated bone loss in mice (Halade et al. 2011). However, other groups have ascribed an inhibitory role to IL-6 in osteoclast formation and in bone resorption through a reduction in the expression of osteoclastic markers (TRAP, cathepsin K, calcitonin receptor) and the inhibition RANKL signaling pathways (Duplomb et al. 2008; Yoshitake et al. 2008). Meanwhile, the roles of IL-6 in osteoblastogenesis also remain controversial, with both positive and negative effects reported (Erices et al. 2002; Franchimont et al. 2005; Peruzzi et al. 2012; Kaneshiro et al. 2014). Therefore, to delineate whether IL-6 is anabolic or catabolic in bone metabolism, we previously performed histological analyses of tibiae using a middle-aged (32 weeks old) IL-6-/- murine model and demonstrated that IL-6-deficient middle-aged mice show increased bone formation and increased osteoclast number, with a significant reduction in osteoclastic bone resorption capacity and a high rate of apoptosis. Collectively, these changes caused an immature phenotype of the bone microstructure characterized by an increase in trabecular bone volume, suggestive of an inhibitory effect of IL-6 depletion on cell viability and bone resorptive ability of osteoclasts in mice (Liu et al. 2014).
Given the involvement of high-fat diet-induced obesity in bone metabolism, we postulated that a high-fat diet could alter, to some extent, the abnormalities associated with an IL-6 deficiency-induced bone phenotype. To confirm this speculation, we conducted a histological study on the tibiae of IL-6 knockout mice fed a high-fat diet or normal diet.
Materials & Methods
Animals and Diets
The study was approved by the Institutional Animal Care and Use Committee of Shandong University and Hokkaido University. Seven-week-old, male, IL-6-/- mice and their wild type (WT) littermates (The Jackson Laboratory; Bar Harbor, ME) were used in these experiments. IL-6-/- mice were backcrossed onto the C57BL/6 background for 10 generations and then intercrossed to produce homozygotes. During the initial 7 days of acclimatization, all mice received a standard diet (SD), the mice were divided into four groups (n=8 each). WT mice that remained on the SD served as the control group (WT & SD group), while other WT mice were switched to high-fat diet (HFD) (WT & HFD group) for 25 weeks. Both diets were purchased from Oriental Yeast Co. Ltd. (Tokyo, Japan). The IL-6-/- mice were also randomly divided into two groups: mice in one group were received the SD (IL-6-/- & SD group) and those in the other group were fed a HFD (IL-6-/- & HFD group) for the same period. All mice were maintained in a temperature-controlled room at 25°C under a 12-hr light/dark diurnal cycle, with free access to food and water throughout the experiment. Body weight was recorded weekly. After 25 weeks’ breeding, control and diet-induced obese mice were subjected to transcardiac perfusion with a fixative of 4% paraformaldehyde in a 0.1 M phosphate buffer (pH 7.4). Tibiae were removed and immersed in the same fixative for additional 12 hr. Following fixation, samples were decalcified with 10% EDTA-2Na solution for 3 weeks at 4°C. After that, the specimens were dehydrated through an ascending ethanol series and then embedded in paraffin using standard procedures. For observing general morphology, 5-μm-thick paraffin sections were prepared, and dewaxed sections were stained with hematoxylin and eosin (H&E).
H&E Staining and Bone Histomorphormetry
H&E staining was performed to investigate the morphology of the metaphyses in mice among the four groups. Stained sections were observed and digital images were taken with a light microscope (Olympus BX-53; Tokyo, Japan). Image-Pro Plus software (version 6.2; Media Cybernetics, Rockville, MD) was used to measure bone histomorphometric parameters, including trabecular bone volume (BV/TV, bone volume/tissue volume × 100%), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp). Eight slices for each sample were used for quantitative histomorphometric analysis to obtain the mean value.
Visible adipocytes (>30 μm in diameter) were also counted within the trabecular region (i.e., from the growth plate to 2 mm distally) using a light microscope (Olympus BX-53).
Immunohistochemistry for Alkaline Phosphatase, Osteocalcin, Cathepsin K and Tartrate-resistant Acid Phosphatase Staining
Paraffin sections were dewaxed in xylene and rehydrated in an ethanol series for immunohistochemical staining for tissue nonspecific alkaline phosphatase (ALP), osteocalcin (OCN), and cathepsin K (CK). Endogenous peroxidases were blocked by incubating sections in 0.3% hydrogen peroxide for 30 min at room temperature. Then, dewaxed paraffin sections were pre-incubated with 1% bovine serum albumin in phosphate-buffered saline (BSA-PBS) for 20 min to reduce non-specific binding. Sections were incubated with (1) rabbit anti-serum against rat tissue-nonspecific ALP at a dilution of 1:200, (2) goat anti-rat OCN antibody at a dilution of 1:150, or (3) goat anti-rat CK antibody at a dilution of 1:100, all diluted in BSA-PBS at room temperature for 2 hr. After rinsing with PBS, the sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Chemicon International Inc.; Temecula, CA) at a dilution of 1:100 for 1 hr at room temperature. All immunoreactions were visualized with diaminobenzidine (DAB) staining. Tartrate-resistant acid phosphatase (TRAP) staining for osteoclast visualization was performed as previously reported (Liu et al. 2014). Staining results were observed by light microscopy and all sections were slightly counterstained with methyl green. The mean optical density of ALP and OCN were measured in three randomly selected, non-overlapping microscopic fields from each section using Image-Pro Plus 6.2 software. Area of interest (AOI) was manually selected in a histogram-based manner.
Cell Apoptosis Assay
Cell apoptosis was determined using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) method using the TACS 2TdT-Blue Label In Situ Apoptosis Detection Kit (Trevigen Inc.; Gaithersburg, MD). Dewaxed sections were incubated with 1% proteinase K (Trevigen Inc.) diluted 1:200 at 37°C for 15 min, followed by inhibition of the endogenous peroxidases at room temperature for 5 min. After treatment with TdT enzyme at a dilution of 1:50 at 37°C for 1 hr, sections were incubated with HRP-conjugated streptavidin at room temperature for 15 min. The reaction was made visible with the blue label solution provided in the kit. Apoptotic osteoclasts were identified as being both TUNEL and TRAP positive in the same field of view on serial sections.
Statistical Analysis
Bone structure parameters and integrated optical density of ALP, OCN and CK as well as the number of osteoclasts/field were assessed with Image-Pro Plus software. All values are presented as the mean ± standard deviation. One-way ANOVA was used for multiple groups’ comparison, and the mean value of each group was compared using the Student-Newman-Keuls (SNK) test. p<0.05 was considered to be statistical significant.
Results
Changes in Body Weight and Effect of High-fat Diet on Bone Marrow Adiposity
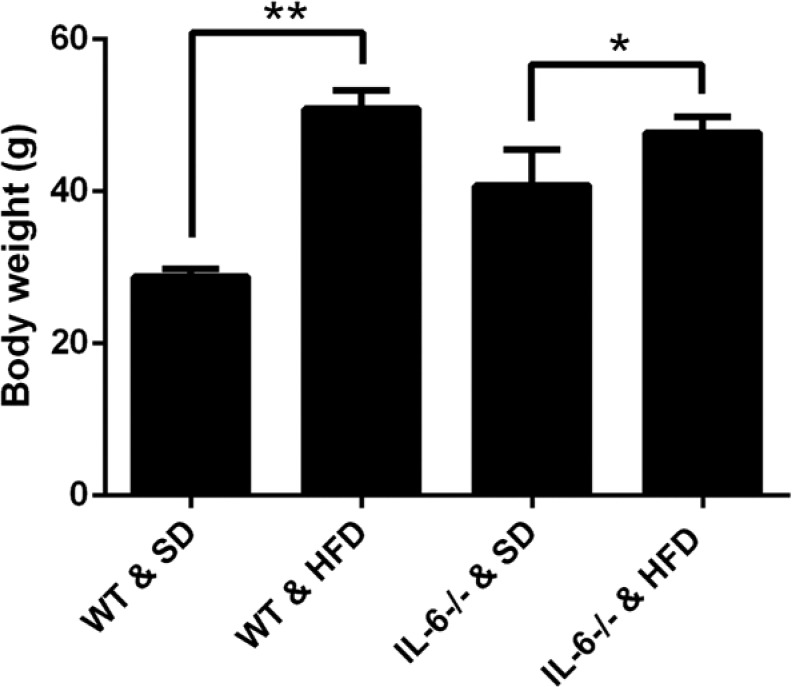
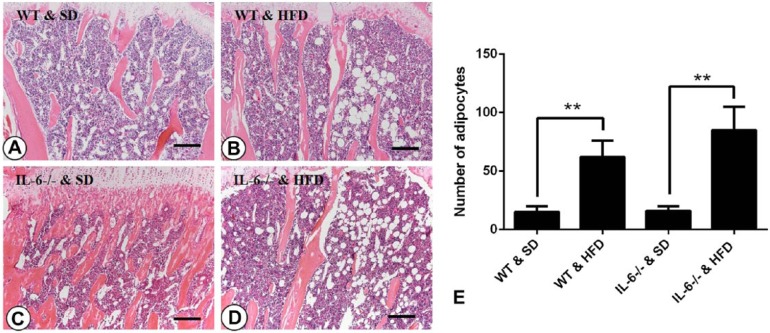
In this study, we found that 25 weeks of feeding on a HFD significantly increased the body weights of mice as compared with those fed a SD, especially between the WT groups (28.75 ± 1.06 g vs 50.86 ± 2.42 g for WT mice fed with SD and HFD, p<0.001; 40.76 ± 4.75 g vs 47.72 ± 2.08 g for IL-6-/- mice fed with SD and HFD, p<0.05, respectively; Fig. 1). To confirm the effect of a HFD on adipogenesis within the bone marrow microenvironment, bone marrow adiposity was evaluated via H&E staining. On the SD, mice showed no significant differences in the number or size of adipocytes between IL-6-/- and WT mice (Fig. 2A, 2C and 2E). On the HFD, the number and size of adipocytes were significantly increased as compared with the respective control mice fed a SD (Fig. 2B, 2D and 2E). This indicates that the HFD enhances adipogenesis within the bone marrow microenvironment.
Figure 1.
Body weight alterations of WT and IL-6-/- mice fed a standard diet (SD) or high-fat diet (HFD). Comparing within the same genotypes, the HFD significantly increased the body weight of WT and IL-6-/- mice, especially those in the WT groups, as compared with their respective counterparts fed a SD. Data are the mean ± SD, *p<0.05, **p<0.001, comparing the same genotype.
Figure 2.
Histological images of the bone marrow and quantification of adipocyte numbers. Few adipocytes were observed in the (A) WT & SD (standard diet) and (C) IL-6-/- & SD groups. When fed a high-fat diet (HFD), both WT mice (B) and IL-6-/- mice (D) displayed a significant increase in the number of adipocytes. (E) Statistical analysis of the number of adipocytes in each group. Data are the mean ± SD, **p<0.001, compared within the same genotype. Scale, 50 μm.
Histological Manifestation in the Tibiae and Statistical Analysis
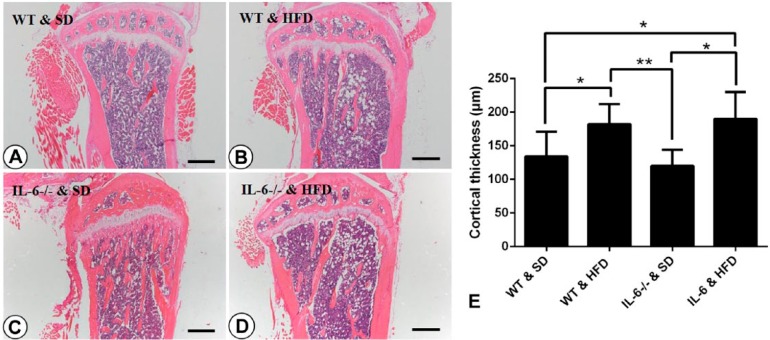
Histological tibial changes showed that 32-week-old WT mice fed a SD had sparse and dispersed, island-shaped, trabecular bone, whereas the age-matched HFD-fed WT mice showed thickened cortical bone and obliquely arranged plate-like trabecular in the same areas (Fig. 3A, 3B, 3E). Compared with their WT counterparts, tibiae from IL-6-/- & SD mice displayed significantly increased trabecular bone volume fraction (p<0.001, Tb.BV/TV), trabecular number (p<0.001, Tb.N), trabecular thickness (p<0.05, Tb.Th) and trabecular separation (p<0.001, Tb.Sp) in the metaphysis, whereas HFD feeding dramatically reduced the aforesaid bone histomorphometric parameters and transformed the abnormal bone morphology of IL-6-/- & SD mice to resemble that of WT & HFD mice (Fig. 3C, 3D; Table 1). However, there were no significant differences in the morphology or architecture of the bone metaphysis between WT and IL-6-/- mice on a HFD (Fig. 3B, 3D).
Figure 3.
Histological appearance of mesial tibiae and statistical analysis for the thickness of cortical bone. H&E staining for mesial tibiae of (A) WT & SD, (B) WT & HFD, (C) IL-6-/- & SD and (D) IL-6-/- & HFD groups. Trabecular bone in WT & SD mice (A) was sparse and irregularly arranged, whereas dramatically increased trabecular bone was observed in IL-6-/- & SD mice (C). Feeding on the HFD caused a marked increase in cortical bone thickness and transformed the trabecular bone to an obliquely arranged, plate-like morphology in both WT mice (B) and IL-6-/- mice (D). (E) Statistical analysis of cortical bone thickness for the mice in each group. Data are the mean ± SD; *p<0.05, **p<0.001, compared with other groups. SD, standard diet; HFD, high-fat diet. Scale, 125 μm.
Table 1.
Histomorphometric Parameters of Tibiae after High-Fat Diet Feeding.
| Treatment Groups |
||||
|---|---|---|---|---|
| Parameters | WT & SD | WT & HFD | IL-6-/- & SD | IL-6-/- & HFD |
| Tb.BV/TV (%) | 15.3 ± 4.2 | 19.5 ± 6.1* | 36.7 ± 8.7## | 21.4 ± 5.7** |
| Tb.N (mm-1) | 2.03 ± 0.51 | 2.23 ± 0.68 | 4.15 ± 1.34## | 2.14 ± 0.46** |
| Tb.Th (μm) | 23.6 ± 3.2 | 24.8 ± 4.9 | 32.2 ± 5.8# | 26.4 ± 5.5* |
| Tb.Sp (μm) | 321 ± 49 | 298 ± 37 | 182 ± 31## | 302 ± 53** |
Data are the mean ± SD (n=8 for each group); Abbreviations: Tb, trabecular; BV, bone volume; TV, total volume; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; WT, wild type; IL-6-/-, IL-6 knockout; SD, standard diet; HFD, high-fat diet. *p<0.05, **p<0.001, comparing the same genotype; #p<0.05, ##p<0.001, comparing the same diet.
Effects of High-fat Diet on Osteogenesis
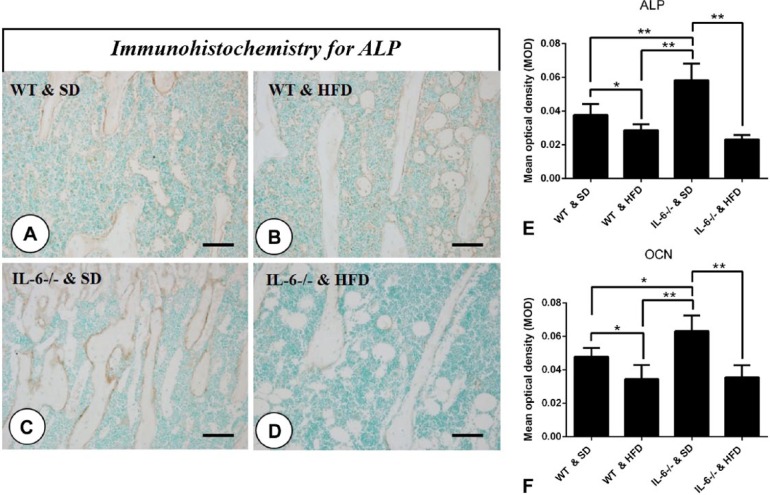
In WT & HFD mice, ALP expression at the trabecula surface of the tibiae was slightly decreased as compared with that measured in the SD-fed littermates (Fig. 4A, 4B and 4E). In contrast, ALP expression in IL-6-/- & SD mice was significantly enhanced as compared with that in the other groups, with HFD feeding remarkably attenuating the degree of ALP staining in IL-6-/- mice to the level of that seen in the WT & HFD mice (Fig. 4C, 4D and 4E). OCN, another critical osteogenic marker expressed in the bone matrix, was similarly reduced by feeding on the HFD, especially for mice in the IL-6-/- & HFD group (OCN immunostaining not shown; statistical analysis for mean optical density is shown in Fig. 4F).
Figure 4.
Effect of the high-fat diet (HFD) on the expression of osteogenic markers. Immunohistochemistry for alkaline phosphatase (ALP) (A–D) in mesial tibiae of each group. The expression of ALP was dramatically increased in IL-6-/- & SD (standard diet) group (C), whereas IL-6-/- mice fed a HFD showed significantly inhibited expression of ALP (D). Additionally, HFD feeding slightly reduced the expression of ALP in WT mice (B) as compared with their SD-fed littermates (A). (E) Statistical analysis of ALP immunostaining intensity. (F) Osteocalcin (OCN) exhibited a similar expression profile relative to ALP (OCN immunohistochemistry not shown) Data are the mean ± SD, *p<0.05, **p<0.001, compared with the other groups. Scale, 25 μm.
Effects of High-fat Diet on Osteoclastogenesis and Cathepsin K Expression
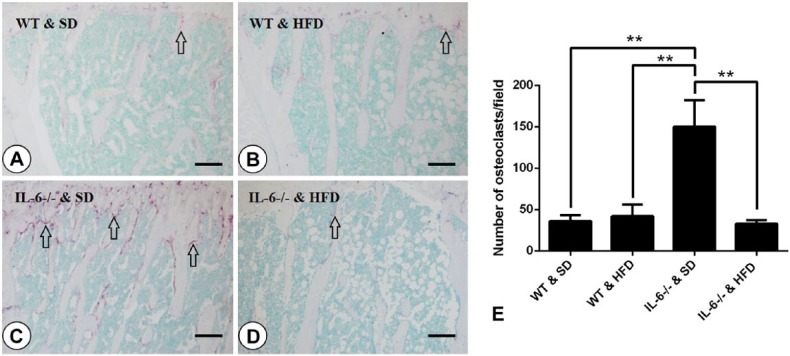
Osteoblast-mediated bone formation and osteoclast-mediated bone resorption are functionally interrelated in bone remodeling. To gain insight into the changes to osteoclastogenesis after HFD feeding, we used TRAP staining to visualize osteoclasts in the metaphyses of tibiae and score the number of TRAP-positive multinucleated cells (MNCs). We found that WT mice fed a HFD showed a similar number of TRAP-positive osteoclasts as mice on a standard diet (WT & SD group; Fig. 5A, 5B), indicating that the HFD exerted no significant effect on osteoclastogenesis in WT mice. Intriguingly, the number of osteoclasts per field for mice in the IL-6-/- & SD group was 3-fold higher than that seen for mice in the WT & HFD group (42 ± 8 vs 148 ± 36 for WT & SD and IL-6-/- & SD mice, respectively; Fig. 5A, 5C and 5E), and this increase was abrogated after feeding on the HFD (Fig. 5C, 5D and 5E).
Figure 5.
Effect of a high-fat diet (HFD) on osteoclastogenesis. Tartrate-resistant acid phosphatase (TRAP) staining of mesial tibiae (A–D) and osteoclast quantification (E) were performed. Arrows indicate TRAP-positive multinucleated cells (purple), identified as osteoclasts. The TRAP-positive osteoclasts were scarce in the WT & SD (standard diet) group (A) and in the WT & HFD group (B). IL-6-/- & SD mice showed dramatically increased numbers of osteoclasts (C) and this was reduced to the level of that seen in the WT groups when the mice were fed a HFD (D). (E) Statistical analysis of the number of osteoclasts per field from each group. Data are the mean ± SD; **p<0.001 compared among the groups. Scale, 50 μm.
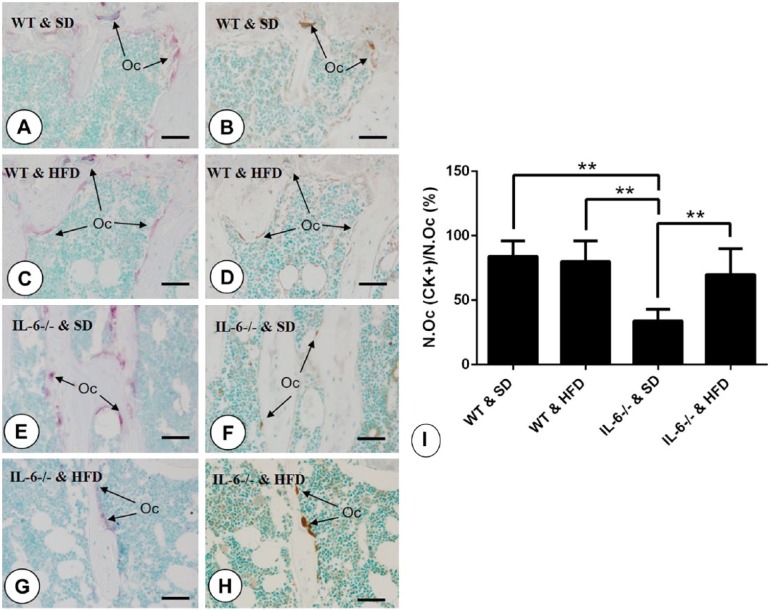
Cathepsin K is one of the most important enzymes secreted by osteoclasts for the degradation of bone matrix. In this study, CK expression was used as an indicator of osteoclast activity. In WT mice, the number of CK-positive osteoclasts was comparable with that of TRAP-positive cells (Fig. 6A–6D, 6I). But, when compared with the IL-6-/- & SD group, the percentage of CK-positive osteoclasts as a function of the total number of TRAP-positive cells dramatically dropped (Fig. 6E, 6F and 6I), indicating that IL-6 knockout suppressed the activity of osteoclasts. However, feeding on the HFD in IL-6-/- mice changed this discrepancy in the proportions of CK-positive and TRAP-positive cells to approximate that of WT mice (Fig. 6G, 6H and 6I).
Figure 6.
Immunolocalization of cathepsin K (CK) and statistical analysis. Arrows indicate TRAP-positive (purple) and CK-positive (brown) osteoclasts in serial sections. (A, C, E, G) The distribution of TRAP-positive osteoclasts located at the border of trabecular bone in (A) WT & SD, (C) WT & HFD, (E) IL-6-/- & SD, and (G) IL-6-/- & HFD groups. (B, D, F, H) Staining of CK at the same areas among (B) WT & SD, (D) WT & HFD, (F) IL-6-/- & SD, (H) IL-6-/- & HFD groups. (I) Statistical analysis of CK-positive osteoclasts account for total TRAP-positive osteoclasts (No.Oc (CK+)/No.Oc). Data are the mean ± SD; **p<0.001, compared among the groups. SD, standard diet; HFD, high-fat diet. Scale, 10 μm.
Effects of High-fat Diet on Apoptosis of Osteoclasts
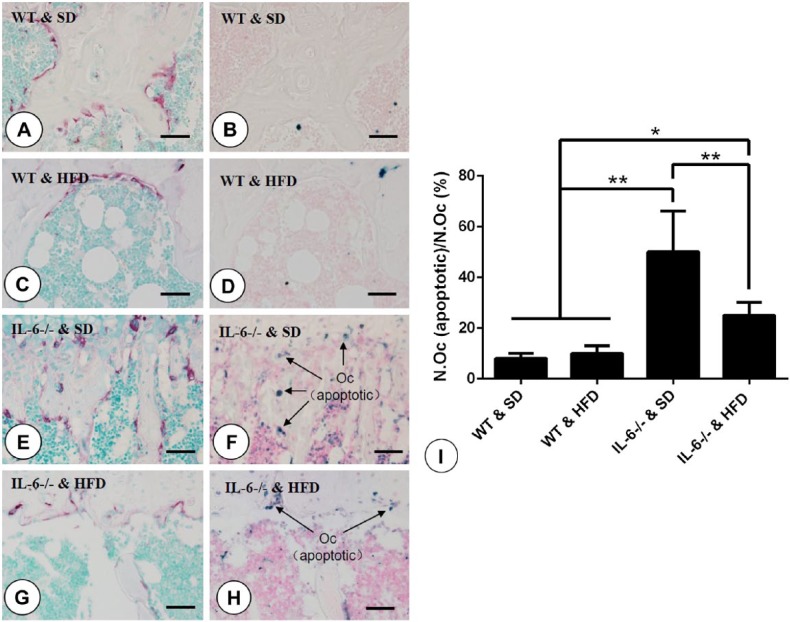
Our previous study showed that IL-6 depletion promoted osteoclastogenesis with a concomitant increase in apoptosis of osteoclasts in mouse tibiae, and we believed that this could be partly explained by the increase in osteoclast cell number and asymmetric bone resorption activity (Liu et al. 2014). In the present study, our results showed that there was almost no or only a small amount of visible apoptotic bodies in the micro-vision field in WT mice fed either a SD or HFD (Fig. 7A–7D). In contrast, in IL-6-/- & SD mice, the number of apoptotic bodies significantly increased as compared with that in the WT groups, especially at sites where TRAP-positive multinucleated cells were located, as observed in serial slices (Fig. 7E, 7F and 7I). Feeding with a HFD significantly reduced this apoptosis in bone marrow cells, including the number of TRAP-positive multinucleated osteoclasts, in IL-6-/- mice (Fig. 7G, 7H and 7I).
Figure 7.
TUNEL assay of osteoclasts in tibial sections and statistical analysis. (A, C, E, G) Distribution of TRAP-positive multinucleated cells located at the border of trabecular bone in mice from (A) WT & SD, (C) WT & HFD, (E) IL-6-/- & SD, (G) IL-6-/- & HFD groups. (B, D, F, H) TUNEL assay indicating apoptotic bodies (blue) within the same areas for (B) WT & SD, (D) WT & HFD, (F) IL-6-/- & SD, (H) IL-6-/- & HFD groups. Arrows indicate the apoptotic osteoclasts identified when comparing serial sections. (I) Statistical analysis of the number of osteoclasts (apoptotic) as a function of the total number of osteoclasts. Data are the mean ± SD; **p<0.001 compared among the groups. SD, standard diet; HFD, high-fat diet. Scale, 10 μm.
Discussion
Our previous study demonstrated that middle-aged IL-6-/- mice showed abnormal bone microstructure in their tibiae, including delayed development of the tibial metaphysis and the presence of a more abundant, thicker but immature trabecular bone, which was perhaps caused by a down-regulation in osteoclast activity and an increase in their rate of apoptosis (Liu et al. 2014). In the present study, HFD-induced obesity was established in IL-6-/- mice to investigate the effect of long-term consumption of a HFD on IL-6 deficiency-induced abnormal bone microstructure. In addition to the markedly increased body weight gain, feeding on a HFD normalized the bone phenotype of IL-6-/- mice to that of the WT control, as characterized by a thickened cortical bone and an obliquely arranged plate-like trabecular bone. Specifically, the significantly attenuated bone resorption activity and survival capacity of osteoclasts in the IL-6-/- mice were restored by feeding on a HFD, which may account for the higher extent to which the abnormal bone microstructure was restored.
HFD is found to be positively correlated with an increase in body mass and fat accumulation (Ghibaudi et al. 2002; Hariri and Thibault 2010). As expected, mice on the HFD for 25 weeks showed significantly higher body weights and bone marrow adiposity than mice fed a standard chow. Although recent reports have shown that excessive fat mass is associated with decreased bone mineral density and may be a risk factor for osteoporosis (Halade et al. 2011; Fehrendt et al. 2014), obesity is also thought to be beneficial to bone and prevent bone loss and osteoporosis (Douchi et al. 1997; Kopelman 2000), with the mechanical loading conferred by this increased body weight thought to stimulate bone formation by decreasing apoptosis and increasing the proliferation and differentiation of osteoblasts and osteocytes (Ehrlich and Lanyon 2002) through Wnt/β-catenin signaling (Sawakami et al. 2006; Bonewald and Johnson 2008). In the present study, tibial histological sections from HFD mice showed obliquely arranged plate-like trabeculae, as well as increased cortical thickness and bone mass, as demonstrated by the increased BV/TV ratio; these findings are in agreement with the profitable effect of body weight gain on bone mass and this change may be associated with increased gravity induced-adaptation of bone microstructure. It is suggested that conflicting results in reports that examine the relationship between HFD induced-obesity and bone mass may be attributable to differences in the ages of animals examined. Animal studies as well as clinical observations have revealed that obesity in younger animals, including adolescents and children, is associated with reduced bone quality and increased bone fracture incidence (Taylor et al. 2006; Ionova-Martin et al. 2010), whereas obesity in adults or aged animals is postulated to cause an increase in bone size and a reduction in fracture incidence (Albala et al. 1996; Nguyen et al. 2005; Reid 2008). We note that the mice in this study were middle-aged by the completion of the study, and this may explain why we observed an increase in bone mass rather than bone loss after HFD feeding. Moreover, the fatty acid profiles of the different chows might affect the outcome of the HFD-induced changes in bone metabolism (Watkins et al. 2000; Weiss et al. 2005); indeed, HFDs from different manufacturers are composed of varied components, especially the ratio of (n-6)/(n-3) fatty acids, and this may result in distinct bone phenotypes among the various studies.
In our previous study, IL-6 deficient mice presented with significantly enhanced osteoblastogenesis and bone formation, as IL-6 inhibits osteoblast differentiation (Franchimont et al. 2005) and has been shown to decrease the expression of differentiation markers in osteoblasts (Yang et al. 2007). In the present study, immunohistochemical staining for markers of osteogenesis showed that the expressions of two critical osteoblast differentiation markers, ALP and OCN, were attenuated after HFD feeding as compared with littermates fed a SD. This was particularly the case for IL-6-/- groups, in which the staining intensity for both osteogenic markers was reduced to that of the WT & SD group, indicating an inhibition of bone formation by HFD-induced obesity. It is well known that adipocytes and osteoblasts are derived from a common multipotent mesenchymal stem cell and that obesity may increase adipogenesis while decreasing osteoblastogenesis (Rosen and Bouxsein 2006). However, our results demonstrated that bone mass was increased in WT mice fed a HFD, and this result did not reflect the impaired osteogenic ability that is caused by exaggerated adipogenesis. This discrepancy could be explained by the fact that increased body weight associated with obesity may counteract the detrimental effects of obesity on bone metabolism, as body weight or body mass index (BMI) is reported to be positively correlated with bone mass (Felson et al. 1993; Reid 2002) and low body weight or BMI may be a risk factor for low bone mass in humans (Ravn et al. 1999).
Regarding the osteoclastogenesis, TRAP staining showed no significant change in the number of osteoclasts in the WT groups, which differs from the results of several other studies that fat deposition in the bone marrow microenvironment stimulates osteoclast formation and bone resorption (Cao et al. 2010; Halade et al. 2011). This inconsistency may be caused by differences in the study animals in terms of age, dietary fat levels, and time or duration of HFD feeding. Interestingly, the elevated osteoclastogenesis for mice in the IL-6-/- & SD group (as evidenced by the increased number of TRAP-positive osteoclasts) was significantly reduced after a HFD. This phenomenon may be attributable to the inhibited osteoblastogenesis of IL-6-/- mice by the HFD, which attenuates osteoclastogenesis via the RANKL/RANK/OPG pathway (Lacey et al. 1998; Yasuda et al. 1998).
An evaluation of the osteoclastic ability of osteoclasts using CK expression showed that the discrepancy between increased osteoclast number and decreased osteoclastic ability for mice in the IL-6-/- & SD group was eliminated after they were fed a HFD. Meanwhile, the TUNEL assay showed that the IL-6 deficiency-induced increase in osteoclast apoptosis was also strongly inhibited by the HFD. Others have shown that a HFD-induced fat accumulation may affect the biological activity of osteoclasts through obesity-associated inflammation and adipocyte-derived hormones. Obesity is traditionally considered a state of low-grade inflammation associated with increased levels of pro-inflammatory cytokines, such as TNF-α, IL-1 and IL-6, which can exert both direct and indirect effects on bone metabolism (Shoelson et al. 2007; Halade et al. 2011). These cytokines have been shown to increase osteoclastogenesis (Khosla 2001), activate osteoclastic ability, and inhibit osteoclast apoptosis (Mundy 2007; Sutherland et al. 2009). Despite the absence of IL-6, the IL-6-/- mice showed a relatively normal bone architecture and osteoclastogenesis as well as restored osteoclast survival when fed a HFD, which raises the possibility that the up-regulation of other inflammatory cytokines (TNF-α, monocyte chemotactic protein-1, IL-1, among others) may compensate the loss of IL-6 in these mice, as these cytokines have been shown to have intricate and overlapping functions with IL-6. Another potential mechanism of note to account for this fat–osteoclast interaction is the production of hormones and adipokines, such as leptin and adiponectin, by adipose tissue, which may influence bone mineral density (BMD) (Reid 2002). Leptin may inhibit bone formation in trabecular bone through the sympathetic nervous system (Takeda et al. 2002). Cao et al. (2010) found that serum leptin levels of mice fed a HFD increased by more than 4-fold, which may inhibit bone formation, as reported in genetic- and diet-induced mouse models of obesity (Fujita et al. 2012). Adiponectin—a member of the adipokine family and possessing anti-inflammation properties—is thought to increase bone mass by suppressing osteoclastogenesis and activating osteoblastogenesis (Williams et al. 2009). Plasma adiponectin levels have been reported to decrease in obese mice as compared with that in non-obese mice (Bullen et al. 2007). Therefore, the aforementioned fat tissue-derived adipokines may be implicated in HFD-induced bone microstructure normalization in IL-6-/- mice.
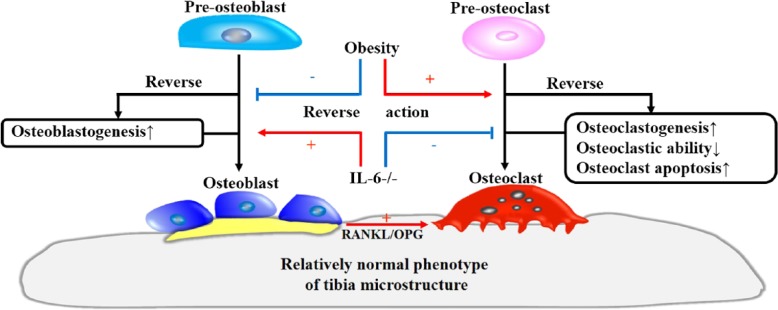
In conclusion, our results show that IL-6 deficiency-induced bone microstructure abnormalities are partially reversed by the long-term consumption of a HFD. This rehabilitation of bone metabolism may be achieved by means of HFD-induced suppression of osteoblastogenesis, an upregulation of osteoclastic activity, as well as an inhibition of osteoclast apoptosis (Fig. 8). Further research is required to focus on the mechanism of synaptic effect of IL-6 deficiency combined with a HFD on altered bone metabolism.
Figure 8.
Schematic model showing the roles of HFD-induced obesity and IL-6 knockout in mouse bone remodeling. IL-6 deficiency enhances bone formation by promoting osteoblastogenesis and suppressing bone resorption through an inhibition of osteoclastic ability and viability. In this process, the increase in osteoclastogenesis associated with enhanced osteoblastogenesis through the RANKL/OPG pathway cannot compensate for the reduced bone resorption that results from the decreased osteoclast activity; this, together, leads to the bone abnormalities observed in IL-6-/- mice. HFD-induced obesity restores the changes that occur in response to IL-6 deficiency through an inhibition of osteoblastogenesis and an increase in osteoclast action, such as osteoclastic resorptive ability and improved cell viability. These changes counteract the effects of IL-6 deficiency and thereby lead to the appearance of a relatively normal phenotype in tibial microstructure in IL-6-/- mice.
Footnotes
Author Contribution Statement: WF and BL for acquisition of data and drafting the article; YM and KO for animals and antibody support; TH for sample processing; JC and DL for revising article; WW and XH for statistical analysis; NA for conception; ML for design and manuscript review.
Competing Interests: The authors declared no potential competing interests with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported by the National Nature Science Foundation of China (grant No. 81271965; 81470719; 81311140173) and Specialized Research Fund for the Doctoral Program of Higher Education (grant No. 20120131110073) to Li M.
References
- Albala C, Yanez M, Devoto E, Sostin C, Zeballos L, Santos JL. (1996). Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord 20:1027-1032. [PubMed] [Google Scholar]
- Binkley NC, Sun WH, Checovich MM, Roecker EB, Kimmel DB, Ershler WB. (1994). Effects of recombinant human interleukin-6 administration on bone in rhesus monkeys. Lymphokine Cytokine Res 13:221-226. [PubMed] [Google Scholar]
- Bonewald LF, Johnson ML. (2008). Osteocytes, mechanosensing and Wnt signaling. Bone 42:606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen JW, Jr., Bluher S, Kelesidis T, Mantzoros CS. (2007). Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 292:E1079-1086. [DOI] [PubMed] [Google Scholar]
- Cao JJ, Sun L, Gao H. (2010). Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci 1192:292-297. [DOI] [PubMed] [Google Scholar]
- Douchi T, Oki T, Nakamura S, Ijuin H, Yamamoto S, Nagata Y. (1997). The effect of body composition on bone density in pre- and postmenopausal women. Maturitas 27:55-60. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. (2000). Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197-207. [DOI] [PubMed] [Google Scholar]
- Duplomb L, Baud’huin M, Charrier C, Berreur M, Trichet V, Blanchard F, Heymann D. (2008). Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology 149:3688-3697. [DOI] [PubMed] [Google Scholar]
- Ehrlich PJ, Lanyon LE. (2002). Mechanical strain and bone cell function: a review. Osteoporos Int 13:688-700. [DOI] [PubMed] [Google Scholar]
- Erices A, Conget P, Rojas C, Minguell JJ. (2002). Gp130 activation by soluble interleukin-6 receptor/interleukin-6 enhances osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Exp Cell Res 280:24-32. [DOI] [PubMed] [Google Scholar]
- Fehrendt H, Linn T, Hartmann S, Szalay G, Heiss C, Schnettler R, Lips KS. (2014). Negative influence of a long-term high-fat diet on murine bone architecture. Int J Endocrinol 2014:318924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Zhang Y, Hannan MT, Anderson JJ. (1993). Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 8:567-573. [DOI] [PubMed] [Google Scholar]
- Franchimont N, Wertz S, Malaise M. (2005). Interleukin-6: An osteotropic factor influencing bone formation? Bone 37:601-606. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Watanabe K, Maki K. (2012). Serum leptin levels negatively correlate with trabecular bone mineral density in high-fat diet-induced obesity mice. J Musculoskelet Neuronal Interact 12:84-94. [PubMed] [Google Scholar]
- Ghibaudi L, Cook J, Farley C, van Heek M, Hwa JJ. (2002). Fat intake affects adiposity, comorbidity factors, and energy metabolism of sprague-dawley rats. Obes Res 10:956-963. [DOI] [PubMed] [Google Scholar]
- Goulding A, Grant AM, Williams SM. (2005). Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res 20:2090-2096. [DOI] [PubMed] [Google Scholar]
- Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. (2001). Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr 139:509-515. [DOI] [PubMed] [Google Scholar]
- Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. (2011). Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol 46:43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Rahman MM, Williams PJ, Fernandes G. (2010). High-fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem 21:1162-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri N, Thibault L. (2010). High-fat diet-induced obesity in animal models. Nutr Res Rev 23:270-299. [DOI] [PubMed] [Google Scholar]
- Ionova-Martin SS, Do SH, Barth HD, Szadkowska M, Porter AE, Ager JW, 3rd, Ager JW, Jr., Alliston T, Vaisse C, Ritchie RO. (2010). Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity. Bone 46:217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T, et al. (1990). IL-6 is produced by osteoblasts and induces bone resorption. J Immunol 145:3297-3303. [PubMed] [Google Scholar]
- Jelcic J. (2010). [Influence of obesity on fracture risk in osteoporosis]. Lijec Vjesn 132:298-302. [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. (2003). IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol 149:1-38. [DOI] [PubMed] [Google Scholar]
- Kaneshiro S, Ebina K, Shi K, Higuchi C, Hirao M, Okamoto M, Koizumi K, Morimoto T, Yoshikawa H, Hashimoto J. (2014). IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab 32:378-392. [DOI] [PubMed] [Google Scholar]
- Khosla S. (2001). Minireview: the OPG/RANKL/RANK system. Endocrinology 142:5050-5055. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. (2000). Obesity as a medical problem. Nature 404:635-643. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. (1998). Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165-176. [DOI] [PubMed] [Google Scholar]
- Liu H, Feng W, Yimin, Cui J, Lv S, Hasegawa T, Sun B, Li J, Oda K, Amizuka N, Li M. (2014). Histological Evidence of Increased Osteoclast Cell Number and Asymmetric Bone Resorption Activity in the Tibiae of Interleukin-6-Deficient Mice. J Histochem Cytochem 62:556-564. [DOI] [PubMed] [Google Scholar]
- Mundy GR. (2007). Osteoporosis and inflammation. Nutr Rev 65:S147-151. [DOI] [PubMed] [Google Scholar]
- Nguyen ND, Pongchaiyakul C, Center JR, Eisman JA, Nguyen TV. (2005). Abdominal fat and hip fracture risk in the elderly: the Dubbo Osteoporosis Epidemiology Study. BMC Musculoskelet Disord 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch JM, Kiefer FW, Varga P, Pail P, Rauner M, Stupphann D, Resch H, Moser D, Zysset PK, Stulnig TM, Pietschmann P. (2011). Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism 60:243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi B, Cappariello A, Del Fattore A, Rucci N, De Benedetti F, Teti A. (2012). c-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP5 signalling. Nat Commun 3:630. [DOI] [PubMed] [Google Scholar]
- Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. (2007). Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr 86:1530-1538. [DOI] [PubMed] [Google Scholar]
- Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. (1999). Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res 14:1622-1627. [DOI] [PubMed] [Google Scholar]
- Reid IR. (2002). Relationships among body mass, its components, and bone. Bone 31:547-555. [DOI] [PubMed] [Google Scholar]
- Reid IR. (2008). Relationships between fat and bone. Osteoporos Int 19:595-606. [DOI] [PubMed] [Google Scholar]
- Reid IR, Evans MC, Ames RW. (1994). Volumetric bone density of the lumbar spine is related to fat mass but not lean mass in normal postmenopausal women. Osteoporos Int 4:362-367. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML. (2006). Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35-43. [DOI] [PubMed] [Google Scholar]
- Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. (2010). Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab 95:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. (2006). The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281:23698-23711. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A. (2007). Obesity, inflammation, and insulin resistance. Gastroenterology 132:2169-2180. [DOI] [PubMed] [Google Scholar]
- Sutherland KA, Rogers HL, Tosh D, Rogers MJ. (2009). RANKL increases the level of Mcl-1 in osteoclasts and reduces bisphosphonate-induced osteoclast apoptosis in vitro. Arthritis Res Ther 11:R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. (2002). Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305-317. [DOI] [PubMed] [Google Scholar]
- Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC, Brady S, Reynolds JC, Calis KA, Yanovski JA. (2006). Orthopedic complications of overweight in children and adolescents. Pediatrics 117:2167-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QP, Li XP, Wang M, Zhao LL, Li H, Xie H, Lu ZY. (2014). Adiponectin exerts its negative effect on bone metabolism via OPG/RANKL pathway: an in vivo study. Endocrine 47:845-853. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Li Y, Allen KG, Hoffmann WE, Seifert MF. (2000). Dietary ratio of (n-6)/(n-3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J Nutr 130:2274-2284. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Barrett-Connor E, von Muhlen D. (2005). Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am J Clin Nutr 81:934-938. [DOI] [PubMed] [Google Scholar]
- Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, Reid IR, Cornish J. (2009). In vitro and in vivo effects of adiponectin on bone. Endocrinology 150:3603-3610. [DOI] [PubMed] [Google Scholar]
- Wong PK, Quinn JM, Sims NA, van Nieuwenhuijze A, Campbell IK, Wicks IP. (2006). Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum 54:158-168. [DOI] [PubMed] [Google Scholar]
- Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko Camacho N, Bostrom MP. (2007). Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 41:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. (1998). Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota K, Sato K, Miyazaki T, Kitaura H, Kayama H, Miyoshi F, Araki Y, Akiyama Y, Takeda K, Mimura T. (2014). Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol 66:121-129. [DOI] [PubMed] [Google Scholar]
- Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. (2008). Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem 283:11535-11540. [DOI] [PubMed] [Google Scholar]