Abstract
Deregulated expression of fibroblast growth factor receptors (FGFRs) and their ligands plays critical roles in tumorigenesis. The gene expression of an alternatively spliced isoforms of FGFR3, FGFR3IIIc, was analyzed by RT-PCR in samples from patients with esophageal carcinoma (EC), including esophageal squamous cell carcinoma (ESCC) and adenocarcinoma (EAC). The incidence of FGFR3IIIc was higher in EC [12/16 (75%); p=0.073] than in non-cancerous mucosa (NCM) [6/16 (38%)]. Indeed, an immunohistochemical analysis of early-stage ESCC showed that carcinoma cells expressing FGFR3IIIc stained positively with SCC-112, a tumor marker, and Ki67, a cell proliferation marker, suggesting that the expression of FGFR3IIIc promotes cell proliferation. We used EC-GI-10 cells endogenously expressing FGFR3IIIc as a model of ESCC to provide mechanistic insight into the role of FGFR3IIIc in ESCC. The knockdown of endogenous FGFR3 using siRNA treatment significantly abrogated cell proliferation and the overexpression of FGFR3IIIc in cells with enhanced cell proliferation. EC-GI-10 cells and ESCC from patients with EC showed endogenous expression of FGF2, a specific ligand for FGFR3IIIc, suggesting that the upregulated expression of FGFR3IIIc may create autocrine FGF signaling in ESCC. Taken together, FGFR3IIIc may have the potential to be an early-stage tumor marker and a molecular target for ESCC therapy.
Keywords: fibroblast growth factor receptor 3, esophageal cancer, cell proliferation, molecular target therapy, biological tumor marker
Introduction
The human genome contains 22 genes that code for fibroblast growth factors (FGFs). FGFs induce cell proliferation, differentiation and migration by binding to specific receptors (Basilico and Moscatelli 1992; Tanaka et al. 1992; Yamasaki et al. 1996). Fibroblast growth factor receptors (FGFRs) are encoded by four genes. The consensus structure of FGFRs consists of three Ig-like domains in the extracellular region, a single spanning transmembrane domain, and a split tyrosine kinase domain in the cytoplasmic region (Johnson and Williams 1993). When FGF binds to FGFRs, the tyrosine kinase domain in the cytoplasmic region of the receptors is activated and generates signals through, for example, the Ras-MAPK, PI3K-Akt and PLC-γ-PKC pathways to induce cell proliferation, differentiation, migration and oncogenesis (Powers et al. 2000). FGFR3 in particular is known to harbor oncogenic activity in several types of cancers. Malignant progression by the enhanced expression or variation in FGFR3 has been reported for bladder cancer, colon cancer and multiple myeloma (Gomez-Roman et al. 2005; Qing et al. 2009; Haugsten et al. 2010; Sonvilla et al. 2010; Wesche et al. 2011). In recent studies, cell proliferation in these cancers was shown to be suppressed by a neutralizing antibody against FGFR3 or a tyrosine kinase inhibitor of FGFR3, indicating that the activation of FGFR3 contributes to the malignant progression of cancers (Tomlinson et al. 2007; Qing et al. 2009; Miyake et al. 2010).
In Japan, approximately 90% of esophageal carcinoma (EC) is esophageal squamous cell carcinoma (ESCC) (Shigeoka and Shiozaki 2004), and the overall death rate in the general population for patients with EC has been reported to be 15.7 per 100,000 for men and a 2.6 per 100,000 for women. The prognosis of patients with EC is known to be poor, with the 5-year relative survival rates remaining at approximately 30%. This rate is lower than those for patients with other gastrointestinal cancers such as stomach, colon and rectal cancers (Satoh and Sakata 2009; Wakao et al. 2013).
Two alternatively spliced isoforms of FGFR3 possess an alternative sequence for the C-terminal half of the third Ig domain (IgIII), encoded by a separate 5’-exon IIIa and 3’-exon, either IIIb or IIIc, which determines FGF specificity. FGFR3IIIc has been shown to have a broader ligand spectrum (FGF1, 2, 4, 5, 6, 8, 9, 16, 17, 18, 19, 20, 21, and 23) than that of FGFR3IIIb (FGF1, 9, 16, and 20) (Ornitz et al. 1996; Kanai et al. 1997; Zhang et al. 2006)
The expression of FGFR isoforms is temporally and spatially regulated in embryos as well as in normal adult organs. The expression of FGFR3IIIb has been associated with an epithelial lineage, whereas FGFR3IIIc is predominantly expressed in non-epithelial cells and tissues (Murgue et al. 1994). Ligands are produced in either epithelial or mesenchymal tissues and generally activate receptors of the opposite tissue specificity. Pathological states can result from a breakdown in binding specificity, as is common in the types of cancers that display an overexpression of FGFs (Beenken and Mohammadi 2009). For example, in colorectal cancer, FGFR3IIIc expression is upregulated but FGFR3IIIb is downregulated with increasing tumor stage, resulting that a ratio of IIIc/IIIb expression that increases with increasing stage (Sonvilla et al. 2010). FGF18, a specific ligand for FGFR3IIIc, is also upregulated in colorectal cancer (Shimokawa et al. 2003), suggesting that FGFR3IIIc imparts changes such as cell proliferation and migration by mediating the effects of FGF18 effects in colorectal cancer (Sonvilla et al. 2010). In EC, a previous study demonstrated that the expression of FGF2, a specific ligand for FGFR3IIIc, is upregulated in the patients with poor prognosis (Barclay et al. 2005).
In the present study, we assessed the expression of FGFR3 isoforms in EC, including ESCC and the neighboring non-cancerous mucosa (NCM) by RT-PCR and immunohistochemical analysis, and found that cells from patients with early-stage EC express FGFR3IIIc. In an in vitro study, lentivirus-induced FGFR3IIIc overexpression in EC-GI-10 cells promoted the cell proliferative activity. These results suggest that FGFR3IIIc may have the potential to be an early-stage tumor marker and a molecular target for ESCC therapy.
Materials & Methods
Tissue Specimens
Fresh samples of EC and NCM for RT-PCR analysis were obtained from patients undergoing surgery for EC at Kitano Hospital in Osaka, Japan, after obtaining informed consent from each patient enrolled in the study (Table 1). Formalin-fixed, paraffin-embedded tissue sections of ESCC for immunohistochemical analysis were retrieved from the pathology files of Shinshu University Hospital in Matsumoto, Japan (Table 2). The study plan, including the use of human samples, was approved by the Ethics Committees of Kitano Hospital, Shinshu University School of Medicine, and Kyoto Sangyo University. Moreover, the study was conducted in full conformity with the current revision of the Declaration of Helsinki.
Table 1.
Stage of 16 Esophageal Carcinoma Samples from Patients Whose Specimens Were Analyzed by RT-PCR.
| Variables | No. of Patients |
|
|---|---|---|
| n | % | |
| Gender | ||
| Male | 14§ | 87.5 |
| Female | 2 | 12.5 |
| Stage | ||
| I | 5 | 31.3 |
| II | 1 | 6.3 |
| III | 9§ | 56.2 |
| IV | 1 | 6.3 |
Two patients were diagnosed as having adenocarcinoma.
Table 2.
RT-PCR Analysis and Immunohistochemical Analysis of FGFR3IIIc Expression in Esophageal Squamous Cell Carcinoma.
| Analysis | Patient No. | FGFR3IIIc Expression (EC or ESCC/NCM or N) |
|---|---|---|
| RT-PCR analysis | E1 | -/+ |
| E2 | +/- | |
| E3 | +/+ | |
| E4 | +/- | |
| E5 | +/- | |
| E6 | +/- | |
| E7 | +/- | |
| E8 | +/- | |
| E9 | -/- | |
| E10 | +/+ | |
| E11 | -/- | |
| E12 | -/- | |
| E13 | +/+ | |
| E14 | +/+ | |
| E15 | +/+ | |
| E16 | +/- | |
| Immunohistochemical Analysis | A1 (Fig. 2) | +/- |
| A2 (Fig. 3A, 3B, 3C) | +/- | |
| A3 (Fig. 3D, 3E) | +/- | |
| A4 (Fig. 3F, 3G) | +/- | |
| A5 (data not shown) | +/- | |
| A6 (data not shown) | + § |
EC, Esophageal carcinoma; NCM, non-cancerous mucosa; ESCC, esophageal squamous cell carcinoma area; N, Normal esophageal epithelium area; +, positive expression or staining; -, negative expression or staining; § N did not appear in this patient sample.
RNA Extraction
Total RNA was extracted from tissue specimens using ISOGEN (Nippon Gene; Tokyo, Japan) according to the manufacturer’s protocol.
RT-PCR Analysis
First-strand cDNA was synthesized using the Superscript First-Strand Synthesis System for RT-PCR (#11904-018; Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. PCR for the expression of GAPDH, FGFRs and FGF2 in EC and neighboring NCM from patients and EC-GI-10 cells was performed using TaqDNA polymerase (Promega; Fitchburg, WI) or Gotaq® Green Master Mix (Promega). The primer sequences are listed in Table 3.
Table 3.
PCR Primer Sequences.
| Gene Name | Sequence | |
|---|---|---|
| FGFR1IIIb | forward | 5’-ACCTGACCACAGAATTGGAGGCTAC-3’ |
| reverse | 5’-ATTGAACAGGGTCAGCACCTCCGCATCCGAGCTATTAATTCCCGA-3’ | |
| FGFR1IIIc | forward | 5’-ACCTGACCACAGAATTGGAGGCTAC-3’ |
| reverse | 5’-ATGAACACCTCCATTTCCTTGTCGGTGGTATTAACTCCAGCAGT-3’ | |
| FGFR2IIIb | forward | 5’-AAGCTGGACTGCCTGCAAATGCCT-3’ |
| reverse | 5’-TCCGTCACATTGAACAGAGCC-3’ | |
| FGFR2IIIc | forward | 5’-AAGCTGGACTGCCTGCAAATGCCT-3’ |
| reverse | 5’-CTCAATCTCTTTGTCCGTGGTG-3’ | |
| FGFR3IIIb | forward | 5’-CTGTCGAGCCACCAATTTCATAGGC-3’ |
| reverse | 5’-GACAGGTCCTTGTCAGTGGCATC-3’ | |
| FGFR3IIIc | forward | 5’-CTTGCACAACGTCACCTTTGAG-3’ |
| reverse | 5’-GACAGGTCCTTGTCAGTGGCATC-3’ | |
| FGFR4 | forward | 5’-CCAACGCATGGAGAAGAAACTGCAT-3’ |
| reverse | 5’-TTCTCCCCATGGAAGGCCTGT-3’ | |
| FGF2 | forward | 5’-CTTCTTCCTGCGCATCCATCC-3’ |
| reverse | 5’-TCAGCTCTTAGCAGACATTGG-3’ | |
| GAPDH | forward | 5’-ACCACAGTCCATGCCATCAC-3’ |
| reverse | 5’-TCCACCACCCTGTTGCTGTA-3’ | |
Antigen Adsorption
A recombinant human FGFR3IIIc Fc chimera (#766-FR-050, lot.CWZ0713081; R&D Systems, Minneapolis, MN), which contains the extracellular region of FGFR3IIIc and Fc region of human IgG1, was incubated with anti-FGFR3IIIc antibody (cat#MAB7662, lot.GHK0411041; R&D Systems) in PBS containing 0.1% Tween-20, 3% BSA without γ-globulin (Wako; Kyoto, Japan), and 3% horse serum for 1 hr at room temperature. The mixture was then used for western blot analysis and immunohistochemistry as an antigen-adsorbed primary antibody. The concentration of the recombinant human FGFR3IIIc Fc chimera was 10-fold higher than that of the anti-FGFR3IIIc antibody.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections from ESCC patients were deparaffinized and rehydrated in graded alcohols. Tissue sections were incubated with 10 mM citrate buffer, pH 6.0, at 95°C for 20 min for epitope retrieval. The sections were blocked with PBS containing 0.1% Tween-20, 3% BSA without γ-globulin (Wako), and 3% horse serum for 30 min at room temperature to eliminate non-specific binding. In immunoperoxidase staining, the sections were treated with 3% hydrogen peroxide for 12 min at room temperature before blocking. The sections were incubated with a monoclonal anti-FGFR3IIIc antibody (#MAB7662, lot GHK0411041, R&D Systems; 1:25 dilution), rabbit anti-SCC-112 antibody (#A3000-088A, lot A300-088A-1, Bethyl Laboratories, Inc., Montgomery, TX; 1:100 dilution) or monoclonal anti-Ki67 antibody (#M7240, lot 00040373, Agilent Technologies, Santa Clara, CA; 1:100 dilution) at 4°C overnight. The sections were incubated with biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA; 1:50 dilution) or biotinylated anti-rabbit IgG (Invitrogen, Carlsbad, CA; 1:50 dilution) for 1 hr at room temperature. The sections were incubated with an Alexa Fluor 488-conjugated secondary antibody and Alexa Fluor 594-conjugated streptavidin (Life Technologies, Rockville, MD; 1:100 dilution) for 1 hr at room temperature. The nucleus was stained by 4’, 6-diamidino-2-phenylindole (DAPI; Invitrogen).
Regarding immunoperoxidase staining, sections were stained by using the Vectastain Elite ABC kit (#PK-6100; Vector Laboratories) and Peroxidase Substrate kit DAB (PK-4100; Vector Laboratories) according to the manufacturer’s protocol. Sections were counterstained using Mayer’s hematoxylin solution (Wako). Microphotographs were taken with an Eclipse E800 (Nikon; Tokyo, Japan) or a DM5500 Q (Leica Microsystems; Wetzlar, Germany).
Cell Lines
The EC-GI-10 ESCC line, which was established from Japanese male (65-year-old ESCC patient), was obtained from the RIKEN cell bank (Tsukuba, Japan) and cultured in Dulbecco’s Modified Eagle’s Medium (Nissui; Tokyo, Japan) containing 10% fetal bovine serum (GE Healthcare; Little Chalfont, UK), 4.5 g/L glucose, 2 mM L-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin (Sato et al. 1987). The authenticity of the EC-GI-10 cells used in this study was validated by the STR-PCR method performed by the RIKEN cell bank, where the cells were tested for mycoplasma by DNA staining and PCR and validated as negative. We also sent the cells to the ICLAS Monitoring Center (Kawasaki, Japan) to test for mycoplasma infection by PCR and they were confirmed to be negative. HEK293T cells were purchased from ATCC (Manassas, VA) and cultured in DMEM supplemented with 10% FBS.
Lentivirus Construction and Induction
Human FGFR3IIIb and FGFR3IIIc cDNAs were amplified by PCR using FGFR3IIIb or FGFR3IIIc specific primers and subcloned into the pHAGE lentiviral backbone vector at NotI/BamHI sites. FGFR3 primer pairs were 5’-GGGGATCCGGCCCTTCACGTCCGCGAGCCCC-3’ and 5’-GGGCGGCCGCGCCGCCATGGGCGCCCCTGCCTGCGCC-3’. EGFP in the pHAGE lentiviral backbone vector was generously provided by Dr. A. Mammoto (Boston Children’s Hospital). Each FGFR3IIIb, FGFR3IIIc, and EGFP in the pHAGE lentiviral backbone vector was co-transfected with the helper plasmids (tat, rev, gag-pol and VSV-G) into HEK293 cells, as described previously (Mostoslavsky et al. 2005; Mammoto et al. 2007; Shimizu et al. 2008). Viral supernatants were assembled and concentrated at 38,000 × g for 1.5 hr at 4°C. The virus was infected with 10 μg/ml of polybrene (Millipore; Billerica, MA) to express FGFR3IIIb, FGFR3IIIc, or EGFP in EC-GI-10 cells or HEK293T cells.
Design of siRNAs
We designed the original siRNA sequences that targeted the 3’-UTR in human FGFR3 mRNA sequences (FGFR3 siRNA) to knock down the endogenous FGFR3 in EC-GI-10 cells, since the lentivirus vector encoding FGFR3IIIb or FGFR3IIIc does not include the 3’-UTR. The siRNA sequences were designed by the Whitehead siRNA Selection Web Server (Yuan et al. 2004). We validated that the siRNA knocked down the endogenous FGFR3 in EC-GI-10 cells, but not lentivirus expressed FGFR3IIIb or FGFR3IIIc (Fig. 4B). siRNAs were synthesized by Japan Bio Services Co. (Saitama, Japan). The FGFR3 siRNA pairs were 5’-GAUGCUGUGUAUAUGGUAUTT-3’ and 5’-AUACCAUAUACACAGCAUCTT-3’. The sicontrol pairs were 5’-UGGUUUACAUGUCGACUAATT-3’ and 5’-UUAGUCGACAUGUAAACCATT-3’.
Figure 4.
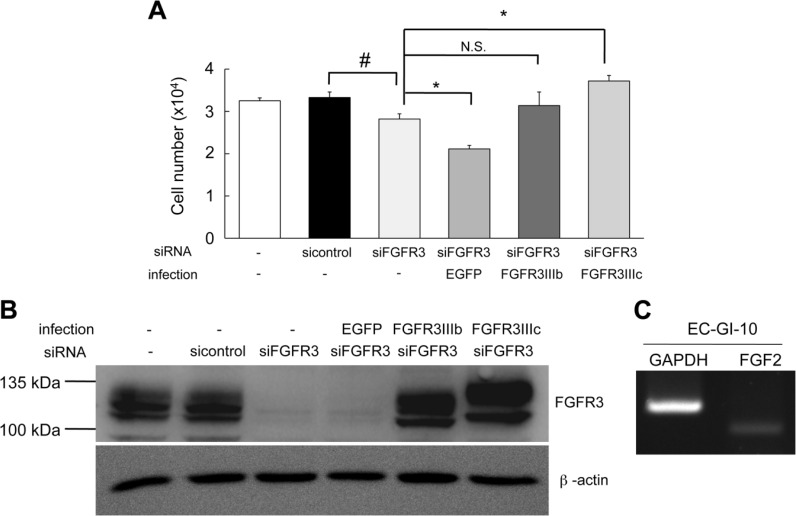
Enhanced expression of FGFR3IIIc accelerated cell proliferation. (A) Cell proliferation was significantly lower (by 15%) in EC-GI-10 cells treated with FGFR3 siRNA (siFGFR3) than in EC-GI-10 cells treated with sicontrol (sicontrol) after 5 days in culture. Cell proliferation in FGFR3IIIc-overexpressed siFGFR3-EC-GI-10 cells (FGFR3IIIc) was significantly higher than that in siFGFR3-EC-GI-10 cells (by 1.3-fold), whereas the overexpression of FGFR3IIIb (FGFR3IIIb) did not significantly affect cell proliferation rates. Cell proliferation was significantly lower (~25%) in EGFP-infected siFGFR3-EC-GI-10 cells (EGFP) than in siFGFR3-EC-GI-10 cells. The parental EC-GI-10 cells were not treated with siRNA (-) and not infected (-). Cell numbers are the mean ± S.E.M. The experiment was performed with n=6 samples, and repeated thrice. (#, p<0.05 versus the sicontrol cells and *, p<0.05 versus the siFGFR3 cells; t-test, N.S., not significant). (B) Western blotting shows FGFR3 expression levels of parental cells and cells treated with sicontrol, EGFP, siFGFR3, FGFR3IIIb, or FGFR3IIIc. β-actin was expressed equally among the cells. (C) RT-PCR shows that FGF2 was endogenously expressed in parental EC-GI-10 cells.
Cell Proliferation Assay
EC-GI-10 cells (3 × 105 cells/4 ml) were seeded into a 6-cm dish. Cells were then transfected with 3 nM of FGFR3 siRNA or control siRNA (sicontrol) by siLentFectTM Lipid (Bio-Rad; Hercules, CA) according to the manufacturer’s protocols. After 24 hr, the cells were then infected with a lentivirus encoding FGFR3IIIb, FGFR3IIIc or EGFP with 10 μg/ml of polybrene (Millipore). After 24 h, these cells were plated onto a well (6 x 103 cells/500 μl) in a 24-well plate with DMEM containing 1% fetal bovine serum, 4.5 g/L glucose, 2 mM L-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. After 5 days in culture, the cells were collected by trypsinization and the number of cells counted with a Coulter Counter ZM cell counter (Beckman Coulter Inc., Brea, CA).
Immunocytochemistry
HEK293T cells (3 × 105 cells/4 ml) were seeded into a 6-cm dish. After 24 hr, the cells were infected with a lentivirus encoding either FGFR3IIIb or FGFR3IIIc with 10 μg/ml of polybrene (Millipore). After a further 24 hr, these cells were lifted and plated into the wells (5 × 104 cells/100 μl) of a 96-well plate coated with 2.7 μg/ml of poly-D-lysine hydrobromide (Sigma-Aldrich; St. Louis, MO). EC-GI-10 cells were plated into a well (2 × 105 cells/2 ml) of a 6-well plate. After 24 hr, the cells were fixed with a 4% paraformaldehyde phosphate-buffered solution (Wako) for 20 min, and were blocked with PBS containing 0.1% Tween-20, 3% BSA without γ-globulin (Wako), and 3% horse serum for 30 min at room temperature. The cells were incubated with a monoclonal anti-FGFR3IIIc antibody (#MAB7662, lot. GHK0411041, R&D Systems; 1:50 dilution) or rabbit anti-SCC-112 antibody (#A3000-088A, lot. A300-088A-1, Bethyl Laboratories, Inc.; 1:100 dilution) at 4°C overnight. EC-GI-10 cells were treated with 0.2% Triton X-100 for 5 min at room temperature before blocking. The cells were incubated with an Alexa Fluor 594-conjugated secondary antibody (Life Technologies; 1:100 dilution) for 1 hr at room temperature. The nuclei were stained with DAPI (Invitrogen) in 0.2% Triton X-100. Microphotographs were taken with an ECLIPSE Ti-U (Nikon).
Western Blot Analysis
EC-GI-10 cells were lysed with a cold RIPA buffer containing 50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 100 mM NaCl and 1% protease inhibitor (Nacalai Tesque; Kyoto, Japan). The cell lysates were electrophoresed through 7.5% polyacrylamide gels and transferred to PVDF membranes (Immobilon-P; Millipore), and were analyzed by immunoblotting with the monoclonal anti-β-actin antibody (Sigma-Aldrich; 1:2000 dilution), anti-FGFR3 antibody (C-15) (Santa Cruz Biotechnology, Dallas, TX; 1:2000 dilution) and monoclonal anti-FGFR3IIIc antibody (cat#MAB7662, lot.GHK411041, R&D Systems; 1:500 dilution). After incubation with primary antibodies overnight at 4°C, the membrane was incubated with peroxidase-conjugated donkey anti-mouse IgG (#715-035-150, lot. 120343, Jackson ImmunoResearch, Suffolk, UK; 1:5000 dilution) or peroxidase-conjugated donkey anti-rabbit IgG (#711-035-152, lot. 117895, Jackson ImmunoResearch; 1:5000 dilution) for 1 hr at room temperature. The blots were treated with chemiluminescence substrate solution (#34080, Thermo Fisher Scientific; Waltham, MA) according to the manufacturer’s protocol and exposed to LAS-4000 mini (Fujifilm Co.; Tokyo, Japan) to reveal immunoreactive bands.
Statistical Analysis
Data are presented as the mean ± S.E.M. A one-way analysis of variance (ANOVA) followed by post-hoc testing was performed. Data were analyzed using Excel 2008 (Microsoft Corporation; Redmond, WA) with the t-test. A Fisher’s exact test was carried out using the JMP Statistical Discovery Software version 9.0 (SAS Institute Inc.; Cary, NC). Statistical significance was set at p<0.05.
Results
Enhanced Expression of FGFR3IIIc in Human EC
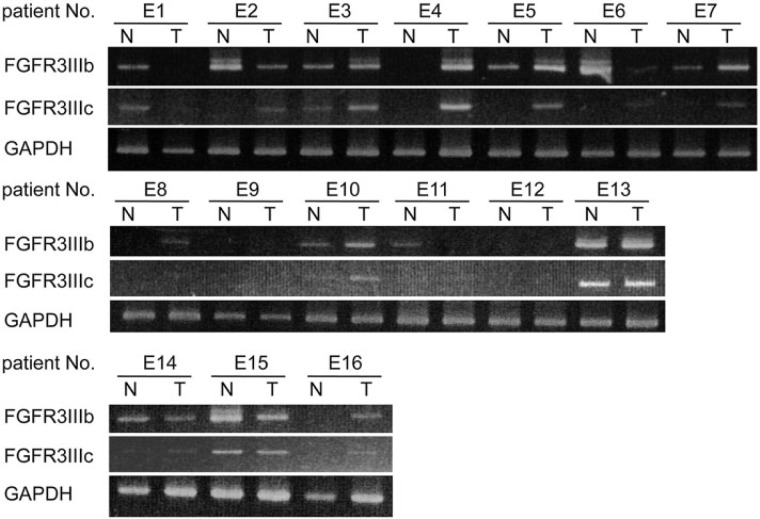
We obtained primary EC and neighboring NCM samples from patients (n=16) who had undergone curative resection. The 16 EC patients comprised 14 ESCC patients, including 1 patient who had been diagnosed with spindle cell carcinoma, and 2 adenocarcinoma (EAC) patients. We obtained ESCC specimens from all stages (Table 1). The gene expression of FGFR3IIIb and FGFR3IIIc was determined by RT-PCR. FGFR3IIIc gene expression was confirmed in 38% of all NCM (6/16) and 75% of all EC (12/16, p=0.073), with 11/14 (79%) and 1/2 (50%) for ESCC and EAC, respectively (Table 4). The incidence of FGFR3IIIc expression was 2-fold higher in EC than in NCM. However, no difference was observed in the incidence of FGFR3IIIb expression between EC (12/16, 75%; p=1.000) and NCM (11/16, 69%) regions (Table 4). Figure 1 shows the expression of FGFR3IIIb and FGFR3IIIc in 16 ESCC specimens (E1-E16). Although the expression of the other FGFRs (FGFR1, FGFR2, and FGFR4) was analyzed in both NCM and EC, no differences were noted in the incidence of expression between ESCC and NCM (Supplemental Fig. S1).
Table 4.
FGFR3IIIb and FGFR3IIIc Expression in Esophageal Carcinoma (EC) and Non-Cancerous Mucosa (NCM).
| FGFR3IIIb Expression |
FGFR3IIIc Expression |
|||
|---|---|---|---|---|
| NCM (N) | EC (T) | NCM (N) | EC (T) | |
| Stage of ESCC Patients (n=14) | ||||
| I | 5/5 (100%) | 3/5 (60%) | 4/5 (80%) | 3/5 (60%) |
| II | 1/1 (100%) | 1/1 (100%) | 0/1 (0%) | 1/1 (100%) |
| III | 4/7 (57%) | 6/7 (86%) | 1/7 (14%) | 6/7 (86%) |
| IV | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) |
| Stage of EAC Patients (n=2) | ||||
| III | 0/2 (0%) | 1/2 (50%) | 0/2 (0%) | 1/2 (50%) |
| Incidence of all patients (n=16) | 11/16 (69%) | 12/16 (75%) (p=1) |
6/16 (38%) | 12/16 (75%) (p=0.073) |
ESCC, Esophageal squamous cell carcinoma; EAC, Adenocarcinoma; T, tumor; N, non-tumor; p<0.05 versus the NCM; Fisher’s exact test.
Figure 1.
Gene expression of FGFR3 isoforms in esophageal carcinoma (EC) and non-cancerous mucosa (NCM). Total RNA was extracted from the specimens of EC patients. The gene expression of FGFR3 in 16 patients (E1 to E16) was analyzed by RT-PCR. FGFR3IIIc expression was clearly detected in the EC (T; tumor) of 12 patients (E2, E3, E4, E5, E6, E7, E8, E10, E13, E14, E15, and E16) and in the NCM (N; non-tumor) of 6 patients (E1, E3, E10, E13, E14, and E15). FGFR3IIIb expression was clearly detected in the EC (T) of 12 patients and in the NCM (N) of 11 patients. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
FGFR3IIIc Has the Potential to be a New Biomarker of ESCC from the Early Stage
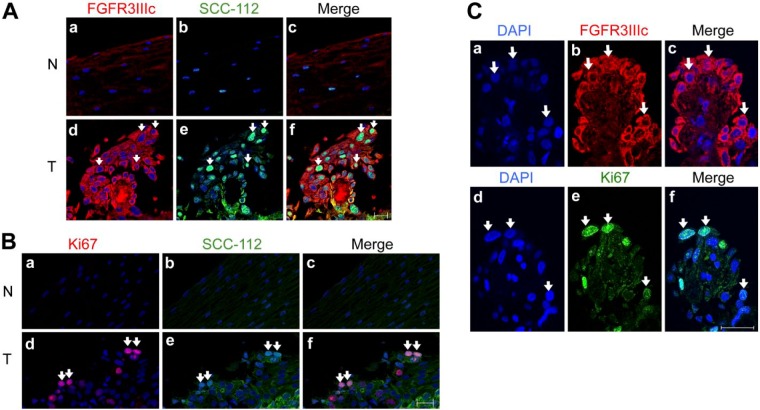
FGFR3IIIc was expressed in the early stage of ESCC (Table 4). An immunohistochemical analysis of ESCC from patients diagnosed with TNM classification stage 0 showed that the cells expressing SCC-112, a tumor marker, were also positively stained for FGFR3IIIc (Zheng et al. 2008) (Fig. 2A, parts d, e, f: white arrows). Neither SCC-112- nor FGFR3IIIc-positive cells were detected in normal esophageal epithelium cells (Fig. 2A, parts a, b, c). Most EC cells (SCC-112 positive) were also positive for Ki-67, a proliferating cell marker (Fig. 2B, parts d, e, f: white arrows). FGFR3IIIc-expressing cells also expressed Ki-67 in serial sections of the specimens (Fig. 2C, parts c, f: white arrows). These results indicate that the expression of FGFR3IIIc was enhanced in EC cells but not in the normal mucosa or in other types of cells, and that FGFR3IIIc may contribute to signal-enhanced cell proliferation.
Figure 2.
Enhanced expression of FGFR3IIIc was associated with proliferating esophageal carcinoma (EC) cells. (A) Immunofluorescence staining of FGFR3IIIc (a, d) and SCC-112 (b, e) in non-cancerous mucosa (NCM; N, non-tumor) and esophageal squamous cell carcinoma (ESCC) (T, tumor), which was diagnosed as stage 0. In confocal microscopic images, strong staining of FGFR3IIIc (red) was observed in EC cells (d) but not in normal esophageal epithelium cells (a). FGFR3IIIc-positive cells in ESCC were consistent with SCC-112-positive cells (green) (d, e, f: white arrows). Nuclei were stained with DAPI (blue). (B) Immunofluorescence staining of Ki-67 (red; a, d) and SCC-112 (green; b, e) in NCM (N) and ESCC (T). The strong staining of Ki-67 was observed in EC cells, and Ki67-positive cells were consistent with SCC-112-positive cells in ESCC (d, e, f: white arrows). On the other hand, staining of Ki-67 and SCC-112 were not observed in NCM (a, b, c). (C) Immunofluorescence staining of DAPI (a, d), FGFR3IIIc (b), Ki-67 (e) and merged images (c, f) in ESCC samples. The expression of FGFR3IIIc was detected in the same cells, which also expressed Ki-67 in consecutive sections (c, f; white arrows). Scale, 20 μm.
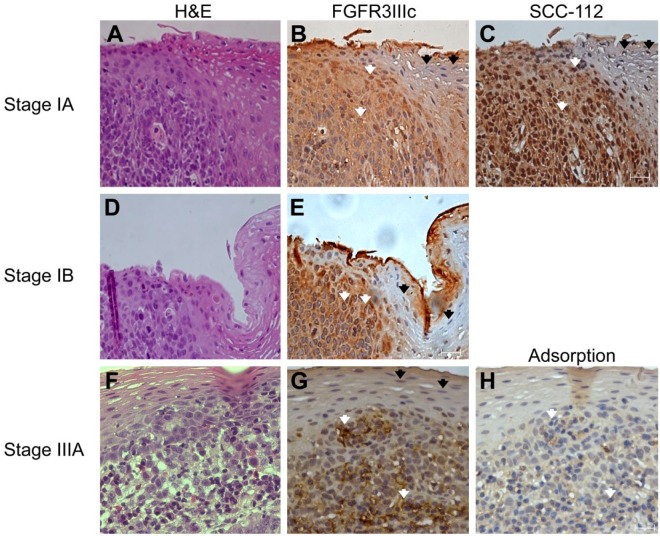
We further analyzed the expression of FGFR3IIIc in esophageal tissues from other patients diagnosed with TNM classification stage IA that contained both EC cells and normal esophageal epithelium cells (Fig. 3A–3C). Strong staining for FGFR3IIIc was observed in EC cells (Fig. 3B: white arrows). Cells positively stained for FGFR3IIIc were distinguishable from negatively stained cells by a clear border, indicating that normal esophageal epithelium cells do not express FGFR3IIIc (Fig. 3B: black arrows). The FGFR3IIIc-positive area was consistent with the same area of strong staining for SCC-112 in the nuclei on consecutive sections (Fig. 3C: white arrows), but was not observed in SCC112-negative areas (Fig. 3C: black arrows). The strong staining of FGFR3IIIc in the EC area (Fig. 3E and G: white arrows) was also observed in tissue samples from different patients who were diagnosed with TNM classification stage IB (Fig. 3D and 3E) and stage IIIA (Fig. 3F and 3G), but was absent in normal esophageal epithelium areas (Fig. 3E and 3G: black arrows). Staining of FGFR3IIIc in the EC area was specific, because FGFR3IIIc did not stain with the anti-FGFR3IIIc antibody after pre-adsorption with recombinant human FGFR3IIIc Fc chimera (Fig. 3H; Supplemental Fig. S4A and S4B).
Figure 3.
Expression of FGFR3IIIc was enhanced in esophageal carcinoma cells diagnosed as stage IA (A, B, and C), stage IB (D and E), stage IIIA (F, G and H), but not in normal esophageal epithelium cells. FGFR3IIIc was not stained by anti-FGFR3IIIc antibody after pre-adsorption with the recombinant human FGFR3IIIc Fc chimera (H). Hematoxylin and eosin staining (H&E stain, A, D, and F), FGFR3IIIc immunostaining (B, E, and G), and SCC-112 immunostaining (C). Strong staining for FGFR3IIIc was observed in esophageal carcinoma cells (B, E, and G: white arrows), and FGFR3IIIc expression was clearly restricted in the carcinoma area, with a clear border. Normal esophageal epithelium cells did not express FGFR3IIIc (B, E, and G: black arrows). FGFR3IIIc-positive cells were consistent with SCC-112-positive cells in the nuclei on consecutive sections (C: white arrows). Scale, 20 μm.
In all ESCC patients, the strong staining of FGFR3IIIc was observed only in EC cells (6/6, 100%) and not in normal esophageal epithelium cells (0/5, 0%) (Table 3).
These results indicate that FGFR3IIIc has the potential to be a new biomarker for ESCC at the early stage, because the expression of FGFR3IIIc is clearly restricted in EC cells at stage 0 (Fig. 2A, parts c, and f).
Enhanced Expression of FGFR3IIIc Accelerates Cell Proliferation by Endogenous FGF2
FGFR3IIIc was detected in cells that also expressed Ki-67 in serial sections (Fig. 2C, parts c, f). These results suggest that the enhanced expression of FGFR3IIIc accelerates cell proliferation in ESCC. To determine whether the expression of FGFR3IIIc contributed to cell proliferation in EC cells, we performed a cell proliferation assay using EC-GI-10 cells as a model of EC cells. EC-GI-10 cells are an ESCC line (Nagoya et al. 2013; Saito et al. 2015) that endogenously expresses FGFR3IIIc, FGFR3IIIb, and SCC-112, a tumor marker (Supplemental Fig. S2A and S2B). To evaluate whether the endogenous expression of FGFR3IIIb and FGFR3IIIc contribute to the proliferation of cells, we knocked down both isoforms using FGFR3 siRNA (siFGFR3), which targets the 3’-UTR of FGFR3 mRNA. Endogenous FGFR3 protein expression levels were lower in EC-GI-10 cells treated with siFGFR3 (siFGFR3-EC-GI-10 cells) than in cells treated with sicontrol (sicontrol-EC-GI-10 cells) (Fig. 4B). Cell proliferation was significantly reduced (by 15%) in siFGFR3-EC-GI-10 cells as compared with sicontrol-EC-GI-10 cells, indicating that endogenously expressed FGFR3 in EC-GI-10 cells enhanced cell proliferation. Next, we overexpressed FGFR3IIIc or FGFR3IIIb in siFGFR3-EC-GI-10 cells by lentiviral infection (FGFR3IIIc-overexpressed EC-GI-10 cells and FGFR3IIIb-overexpressed EC-GI-10 cells), and used siFGFR3-EC-GI-10 cells infected with EGFP-lentivirus as a control (EGFP-infected EC-GI-10 cells; Supplemental Fig. S3). Cell proliferation was significantly higher (by 1.3-fold) in FGFR3IIIc-overexpressed EC-GI-10 cells than in siFGFR3-EC-GI-10 cells (Fig. 4A). In contrast, cell proliferation was not enhanced in FGFR3IIIb-overexpressed EC-GI-10 cells over siFGFR3-EC-GI-10 cells (Fig. 4A). The cell proliferation in EGFP-infected EC-GI-10 cells was significantly lower (by 25%) than that in siFGFR3-EC-GI-10 cells, suggesting that the EGFP protein may reduce cell proliferation or be cytotoxic to the cells in this study (Liu et al. 1999). A western blot analysis revealed that the expression of FGFR3IIIc in FGFR3IIIc-overexpressed EC-GI-10 cells was similar to that of FGFR3IIIb in FGFR3IIIb-overexpressed EC-GI-10 cells (Fig. 4B). These results indicate that the strong expression of FGFR3IIIc in ESCC accelerated cell proliferation, whereas that of FGFR3IIIb did not.
FGF2 ligand specifically binds to FGFR3IIIc but not FGFR3IIIb (Chellaiah et al. 1994; Terada 2009). FGF2 in EC has been reported to be up-regulated and involved in the poor prognosis of patients (Barclay et al. 2005). An RT-PCR analysis was performed in order to confirm whether FGF2 was expressed in parental EC-GI-10 cells. FGF2 was detected in parental EC-GI-10 cells (Fig. 4C), suggesting that enhancements in cell proliferation by the expression of FGFR3IIIc were induced by endogenous FGF2 via an autocrine pathway.
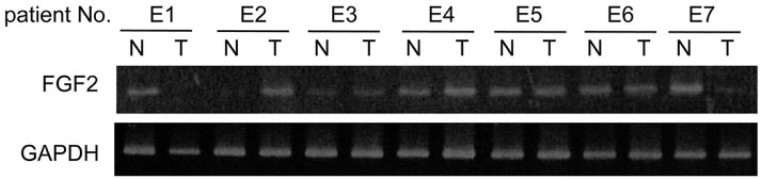
FGF2 Is Expressed in ESCC and NCM
An RT-PCR analysis was performed to determine whether the expression of FGF2 was higher in ESCC (T) than in NCM (N). No significant difference between was observed in the incidence of FGF2 expression between ESCC (6/7, 86%) and NCM (6/7, 86%) (Fig. 5), indicating that the expression of FGF2 did not change between NCM and ESCC.
Figure 5.
Gene expression of FGF2 in 7 patients (E1 to E7) by RT-PCR. No significant difference was observed in FGF2 expression between ESCC (T, 6/7, 86%) and NCM (N, 6/7, 86%). E1: Stage I, E2-E6: Stage III, E7: Stage II. T, tumor; N, non-tumor.
Discussion
Tumor markers, including the squamous cell carcinoma-related antigen (SCC), carcinoembryonic antigen (CEA), CA19-9, CYFRA, CA125, and mutated p53, are widely used to diagnose EC (Mealy et al. 1996; Song et al. 2009). A clinical study demonstrated that human EC markers had low sensitivity for cancer screening, and tumor marker levels of CEA, CA19-9, CA125, and SCC did not correlate with the stage of disease or short-term survival (Mealy et al. 1996).
The constitutive activation of FGFR3 has been shown to drive malignant progression in multiple myeloma, colorectal cancer, and bladder cancer (Gomez-Roman et al. 2005; Qing et al. 2009; Sonvilla et al. 2010). A blockade of activated FGFR3, using highly specific antibody-targeting of FGFR3, reduced tumor growth in a t(4;14)-positive multiple myeloma mouse model (Qing et al. 2009). An antibody against FGFR3 or the use of PD173074, a selective tyrosine kinase inhibitor of FGFR3, suppressed cell proliferation in human bladder carcinoma and tumor growth in a mouse xenograft model (Gomez-Roman et al. 2005; Qing et al. 2009; Miyake et al. 2010). The overexpression of FGFR3IIIc induced cell proliferation in colon carcinoma cell lines and tumor growth in a mouse xenograft model (Sonvilla et al. 2010). Thus, these findings demonstrate that FGFR3 is tumorigenic.
Although several studies have shown FGFR1 and FGFR2 amplification in ESCC patients (Ishizuka et al. 2002; Kato et al. 2013), the upregulation of FGFR3 expression in EC has not yet been examined. We herein demonstrate that the expression of FGFR3IIIc was higher in EC regions than in NCM regions. In contrast, the expression of FGFR3IIIb was higher than that of FGFR3IIIc in NCM regions but remained unchanged in EC. In this study, due to the limited number of the patients who underwent curative resection, it was difficult to adjust for known prognostic factors such as age, sex, T and N stages for multivariable and univariable analyses of survival outcome (i.e., overall survival or progression-free survival) based on the expression levels of FGFR3IIIc.
An immunohistochemical analysis of ESCC from patients diagnosed as stage 0—defined as high-grade dysplasia by the TNM classification—showed that FGFR3IIIc is restricted to EC cells, indicating that, during the early stages of carcinogenesis, FGFR3IIIc could confer some benefit to enhance the proliferative activities for the progression of malignancy. Indeed, in this study, FGFR3IIIc was detected in EC cells, which also expressed Ki-67. Thus, FGFR3IIIc may be an early-stage tumor marker because the expression of FGFR3IIIc was clearly restricted to stage 0 EC cells. Certain molecular mechanisms associated with carcinogenesis in the esophageal epithelium may upregulate the expression of FGFR3IIIc, but not that of FGFR3IIIb.
SCC-112 has been reported to promote cell proliferation and contributes to tumorigenesis by interacting with p63, a transcription factor (Fessing et al. 2011) that affects the survival and proliferative capacity of squamous epithelia and promotes cell cycling (Barbieri et al. 2003; Zheng et al. 2008). We used SCC-112 as a tumor marker in our experiments, since SCC-112 expression was detected in EC but not in the normal counterpart (Zheng et al. 2008). In this study, FGFR3IIIc was detected in EC cells that also expressed SCC-112. However, we have yet to establish a functional relationship between FGFR3IIIc and SCC-112.
In the in vitro study, our results showed that the overexpression of FGFR3IIIc, but not that of FGFR3IIIb, enhanced cell proliferation in EC-GI-10 cells. Conversely, the knockdown of endogenous FGFR3 by siRNA reduced cell proliferation beyond that observed in cells treated with sicontrol. FGF2 was endogenously expressed in EC-GI-10 cells, suggesting that the enhanced expression of FGFR3IIIc was beneficial for cancer cell proliferation due to FGF2 binding via an autocrine pathway. In patients with ESCC, FGF2 was expressed in both NCM and ESCC regions, suggesting that the upregulated expression of FGFR3IIIc could trigger carcinogenesis of EC and malignant progression.
In conclusion, FGFR3IIIc has the potential to be not only an early-stage tumor marker but also a molecular target for ESCC therapy. However, the number of EC samples examined here was limited, and further studies are required to validate this conclusion.
Supplementary Material
Acknowledgments
We greatly thank Ayumi Yoshida, Shoko Matsushima, Hirotsugu Asano, Honami Sowa, Motoki Terada, Kensuke Sakurai, Shinichi Abe and Chikara Ohnishi for experimental support and Professor Naoki Itano for valuable discussion.
Footnotes
Author Contributions: NU, AS, MK, YI, SU, JN, and MS contributed to the conception and design the study. NU and AS performed the RT-PCR, immunohistochemistry, western blot analysis and cell proliferation assay. NU, AS and MS drafted the manuscript. All authors analyzed the data and have read and approved the final manuscript.
Competing Interests: The authors declare no competing or financial interests.
Funding: This work was partly supported by the Foundation for the Scientific Research Center in Kyoto Sangyo University from the Ministry of Education, Science, and Culture of Japan to NU, AS, and MKS.
References
- Barbieri CE, Barton CE, Pietenpol JA. (2003). Delta Np63 alpha expression is regulated by the phosphoinositide 3-kinase pathway. J Biol Chem 278:51408-51414. [DOI] [PubMed] [Google Scholar]
- Barclay C, Li AW, Geldenhuys L, Baguma-Nibasheka M, Porter GA, Veugelers PJ, Murphy PR, Casson AG. (2005). Basic fibroblast growth factor (FGF-2) overexpression is a risk factor for esophageal cancer recurrence and reduced survival, which is ameliorated by coexpression of the FGF-2 antisense gene. Clin Cancer Res 11:7683-7691. [DOI] [PubMed] [Google Scholar]
- Basilico C, Moscatelli D. (1992). The FGF family of growth factors and oncogenes. Adv Cancer Res 59:115-165. [DOI] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. (2009). The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8:235-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah AT, McEwen DG, Werner S, Xu J, Ornitz DM. (1994). Fibroblast growth factor receptor (FGFR) 3. Alternative splicing in immunoglobulin-like domain III creates a receptor highly specific for acidic FGF/FGF-1. J Biol Chem 269:11620-11627. [PubMed] [Google Scholar]
- Fessing MY, Mardaryev AN, Gdula MR, Sharov AA, Sharova TY, Rapisarda V, Gordon KB, Smorodchenko AD, Poterlowicz K, Ferone G, Kohwi Y, Missero C, Kohwi-Shigematsu T, Botchkarev VA. (2011). p63 regulates Satb1 to control tissue-specific chromatin remodeling during development of the epidermis. J Cell Biol 194:825-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roman JJ, Saenz P, Molina M, Cuevas Gonzalez J, Escuredo K, Santa Cruz S, Junquera C, Simon L, Martinez A, Gutierrez Banos JL, Lopez-Brea M, Esparza C, Val-Bernal JF. (2005). Fibroblast growth factor receptor 3 is overexpressed in urinary tract carcinomas and modulates the neoplastic cell growth. Clin Cancer Res 11:459-465. [PubMed] [Google Scholar]
- Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. (2010). Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res 8:1439-1452. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Tanabe C, Sakamoto H, Aoyagi K, Maekawa M, Matsukura N, Tokunaga A, Tajiri T, Yoshida T, Terada M, Sasaki H. (2002). Gene amplification profiling of esophageal squamous cell carcinomas by DNA array CGH. Biochem Biophys Res Commun 296:152-155. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Williams LT. (1993). Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res 60:1-41. [DOI] [PubMed] [Google Scholar]
- Kanai M, Goke M, Tsunekawa S, Podolsky DK. (1997). Signal transduction pathway of human fibroblast growth factor receptor 3. Identification of a novel 66-kDa phosphoprotein. J Biol Chem 272:6621-6628. [DOI] [PubMed] [Google Scholar]
- Kato H, Arao T, Matsumoto K, Fujita Y, Kimura H, Hayashi H, Nishiki K, Iwama M, Shiraishi O, Yasuda A, Shinkai M, Imano M, Imamoto H, Yasuda T, Okuno K, Shiozaki H, Nishio K. (2013). Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. Int J Oncol 42:1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ. (1999). Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun 260:712-717. [DOI] [PubMed] [Google Scholar]
- Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. (2007). Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem 282:23910-23918. [DOI] [PubMed] [Google Scholar]
- Mealy K, Feely J, Reid I, McSweeney J, Walsh T, Hennessy TP. (1996). Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 22:505-507. [DOI] [PubMed] [Google Scholar]
- Miyake M, Ishii M, Koyama N, Kawashima K, Kodama T, Anai S, Fujimoto K, Hirao Y, Sugano K. (2010). 1-tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido[2,3 -d]pyrimidin-7-yl]-urea (PD173074), a selective tyrosine kinase inhibitor of fibroblast growth factor receptor-3 (FGFR3), inhibits cell proliferation of bladder cancer carrying the FGFR3 gene mutation along with up-regulation of p27/Kip1 and G1/G0 arrest. J Pharmacol Exp Ther 332:795-802. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. (2005). Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther 11:932-940. [DOI] [PubMed] [Google Scholar]
- Murgue B, Tsunekawa S, Rosenberg I, deBeaumont M, Podolsky DK. (1994). Identification of a novel variant form of fibroblast growth factor receptor 3 (FGFR3 IIIb) in human colonic epithelium. Cancer Res 54:5206-5211. [PubMed] [Google Scholar]
- Nagoya H, Futagami S, Shimpuku M, Tatsuguchi A, Wakabayashi T, Yamawaki H, Kodaka Y, Kawagoe T, Watarai Y, Makino H, Miyashita M, Tsuchiya S, Crowe SE, Sakamoto C. (2013). Apurinic/apyrimidinic endonuclease-1 is associated with angiogenesis and VEGF production via upregulation of COX-2 expression in esophageal cancer tissues. Am J Physiol Gastrointest Liver Physiol 306:G183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. (1996). Receptor specificity of the fibroblast growth factor family. J Biol Chem 271:15292-15297. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. (2000). Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7:165-197. [DOI] [PubMed] [Google Scholar]
- Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K, Ross S, Stinson S, Dornan D, French D, Wang QR, Stephan JP, Wu Y, Wiesmann C, Ashkenazi A. (2009). Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest 119:1216-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Morishima K, Ui T, Hoshino H, Matsubara D, Ishikawa S, Aburatani H, Fukayama M, Hosoya Y, Sata N, Lefor AK, Yasuda Y, Niki T. (2015). The role of HGF/MET and FGF/FGFR in fibroblast-derived growth stimulation and lapatinib-resistance of esophageal squamous cell carcinoma. BMC Cancer 15:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kasono K, Ohba Y, Yashiro T, Fujii Y, Yoshida MA, Tsushima T, Shizume K. (1987). Establishment of a parathyroid hormone-like factor-producing esophageal carcinoma cell line (EC-GI). Jpn J Cancer Res 78:1044-1048. [PubMed] [Google Scholar]
- Satoh T, Sakata Y. (2009). On the path to standardizing esophageal cancer treatment in Japan. Gastrointest Cancer Res 3:77-79. [PMC free article] [PubMed] [Google Scholar]
- Shigeoka H, Shiozaki H. (2004). Surgery for resectable esophageal cancer in Japan. Ann Thorac Cardiovasc Surg 10:69-70. [PubMed] [Google Scholar]
- Shimizu A, Mammoto A, Italiano JE, Jr., Pravda E, Dudley AC, Ingber DE, Klagsbrun M. (2008). ABL2/ARG tyrosine kinase mediates SEMA3F-induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J Biol Chem 283:27230-27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa T, Furukawa Y, Sakai M, Li M, Miwa N, Lin YM, Nakamura Y. (2003). Involvement of the FGF18 gene in colorectal carcinogenesis, as a novel downstream target of the beta-catenin/T-cell factor complex. Cancer Res 63:6116-6120. [PubMed] [Google Scholar]
- Song K, Udagawa H, Norio A, Kei M. (2009). Treatment process and tumor marker of esophageal cancer. Esophagus 6:137-142. [Google Scholar]
- Sonvilla G, Allerstorfer S, Heinzle C, Stattner S, Karner J, Klimpfinger M, Wrba F, Fischer H, Gauglhofer C, Spiegl-Kreinecker S, Grasl-Kraupp B, Holzmann K, Grusch M, Berger W, Marian B. (2010). Fibroblast growth factor receptor 3-IIIc mediates colorectal cancer growth and migration. Br J Cancer 102:1145-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Miyamoto K, Minamino N, Takeda M, Sato B, Matsuo H, Matsumoto K. (1992). Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc Natl Acad Sci U S A 89:8928-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M, Ohnishi C, Ueno N, Shimizu A, Kanai M, Seo M. (2009). Enhanced expression of Fibroblast Growth Factor Receptor 3 in human skin cancer cells. Open Circulation Vascular J 2:30-36. [Google Scholar]
- Tomlinson DC, Hurst CD, Knowles MA. (2007). Knockdown by shRNA identifies S249C mutant FGFR3 as a potential therapeutic target in bladder cancer. Oncogene 26:5889-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao F, Nishimoto H, Katanoda K, et al. (eds.) (2013). Cancer statistics in Japan ‘13. Tokyo: Foundation for Promotion of Cancer Research (online resource). pp107. http://ganjoho.jp/en/professional/statistics/brochure/2013_en.html.
- Wesche J, Haglund K, Haugsten EM. (2011). Fibroblast growth factors and their receptors in cancer. Biochem J 437:199-213. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Miyake A, Tagashira S, Itoh N. (1996). Structure and expression of the rat mRNA encoding a novel member of the fibroblast growth factor family. J Biol Chem 271:15918-15921. [DOI] [PubMed] [Google Scholar]
- Yuan B, Latek R, Hossbach M, Tuschl T, Lewitter F. (2004). siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res 32:W130-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. (2006). Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 281:15694-15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng MZ, Zheng LM, Zeng YX. (2008). SCC-112 gene is involved in tumor progression and promotes the cell proliferation in G2/M phase. J Cancer Res Clin Oncol 134:453-462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.