Abstract
Drying of the tissue section, partial or total, during immunostaining negatively affects both the staining of tissue antigens and the ability to remove previously deposited antibody layers, particularly during sequential rounds of de-staining and re-staining for multiple antigens. The cause is a progressive loss of the protein-associated water up to the removal of the non-freezable water, a step which abolishes the immunoavailability of the epitope. In order to describe and prevent these adverse effects, we tested, among other substances, sugars, which are known to protect unicellular organisms from freezing and dehydration, and stabilize drugs and reagents in solid state form in medical devices. Disaccharides (lactose, sucrose) prevented the air drying-induced antigen masking and protected tissue-bound antigens and antibodies from air drying-induced damage. Complete removal of the bound antibody layers by chemical stripping was permitted if lactose was present during air drying. Lactose, sucrose and other disaccharides prevent air drying artifacts, allow homogeneous, consistent staining and the reuse of formalin-fixed, paraffin-embedded tissue sections for repeated immunostaining rounds by guaranteeing constant staining quality in suboptimal hydration conditions.
Keywords: sugars, disaccharide, lactose, sucrose, antigen masking, antibody, multiple immunostaining, elution, stripping, freeze-drying
Introduction
An undesired effect of routine processing (formalin fixation followed by dehydration and paraffin embedding; FFPE) is antigen masking (Shi et al. 1991; Shin et al. 1991), which results in partially reversible shielding of the existing tissue epitopes from detection by antibodies or probes. Dehydration and formalin fixation are the two main agents of masking. The conformational changes caused by the combined loss of water and the contact between active formalin-induced binding sites, results in an irreversible, high-energy misfolding of proteins (Fowler et al. 2007). This effect may take place on formalin-fixed frozen sections as well if allowed to dry before applying the primary antibody (Curran and Gregory 1978).
However, controlled freezing of aldehyde-fixed tissue at low temperature in the presence of sucrose and gum acacia (Holt et al. 1960) produces cryosections with a preserved antigenicity, even if dried and then stored for a long time (Griffith and Posthuma 2002; Ino 2003). The sucrose is required to allow cryosectioning and preservation of the ultrastructural details (Holt and Hicks 1961).
Drying causes undesired negative changes on native proteins (Carpenter and Crowe 1988). One example is proteins, such as antibodies, attached to a western blot membrane, which cannot be removed by applying a Laemmli SDS, 2-mercaptoethanol stripping buffer, unless a proteinaceous solution such as milk is allowed to wet the membrane before drying (Kaufmann et al. 1987).
In general, drying of a tissue section, complete or partial, during the process of immunohistochemical staining causes irreversible artifacts and a loss of the antigenicity, and this can affect the staining in an erratic and unpredictable fashion, introducing artifacts that can alter the reproducibility of an immunoassay or the diagnostic reliability of individual preparations (Bussolati and Leonardo 2008; Lin and Prichard 2011; Dabbs 2013; Taylor et al. 2013).
Routine immunohistochemistry entails a limited number of steps during which excess fluid is removed from the section in order to allow the deposition of an appropriately diluted reagent. These steps run the risk of unwanted drying of the section.
Sequential immunostaining of tissue sections often requires extensive manipulation involving manual or automated indirect immunohistochemistry or immunofluorescence, coverslipping, removal of the coverslip and of any alcohol-soluble dye present, removal of the bound antibodies and repeating a staining sequence (Gendusa et al. 2014). These protocols risk air drying of the sections. Also, the application of water-based mounting media containing variable amount of proteins, glycerol and between 50% and 70% water (Bancroft and Gamble 2008) can result in a loss of protein-bound water.
The observations described in this paper were first observed while developing a protocol for sequential, repeated staining of the same section (Gendusa et al. 2014). On rare occasions, we noticed two apparently unrelated phenomena: antigen re-masking and an inability to strip deposited antigen-antibody layers, even in the absence of frank drying of the section, prompting de novo repetition of the staining.
Suspecting that drying or simply reducing the amount of water in the tissue during sequential immunostaining may affect both the antigen availability and the ability to strip the section of bound antibodies, we investigated conditions that causes these phenomena and methods to prevent such an adverse effect. In this work, we describe the variables associated with this drying event and prevention of drying using sugars.
Materials & Methods
Tissues
FFPE fully anonymous human leftover material used (spleen, tonsil, lymph node, kidney) was exempt from the San Gerardo Institutional Review Board (IRB) approval as per Hospital regulations (ASG-DA-050 Donazione di materiale biologico a scopo di ricerca e/o sperimentazione, May 2012).
Three-µm sections were cut and placed on polylysine-coated glass slides, baked in a 60°C oven for 1 hr, deparaffinized in D-limonene and brought to water through a graded alcohol series until further use. Section thicknesses other than 3 µm or other clearing agents (e.g., xylene) were not investigated.
Chemicals
D-Lactose monohydrate (C12H22O11), sucrose (C12H22O11), glucose (C6H12O6) and galactose (C6H12O6) were purchased from A.C.E.F. s.p.a. (Fiorenzuola D’Arda, Italy). Fructose (C6H12O6), mannitol (C6H14O6), bovine gelatin, bovine serum albumin, glycin, sodium azide and urea were purchased from Sigma-Aldrich (Milan, Italy).
Antigen Retrieval
Sections hydrated in distilled water were inserted into radio-transparent slide holders (#S2029; Dako, Glostrup, Denmark). They were then transferred to an 800-ml glass container filled with retrieval solutions (10 mM EDTA in Tris-buffer pH 8) (Cattoretti et al. 1993) and irradiated continuously in a household microwave oven for 8 min or until vigorous boiling, followed by 20 min of intermittent electromagnetic radiation to maintain constant boiling.
The AR buffer was supplemented with 10% lactose or sucrose for experiments involving drying during retrieval.
Drying Protocols
Oven Drying
Antigen-retrieved (AR) slides, either unstained or labelled with the primary antibody, were wetted for 1 hr at room temperature in a humid chamber with Tris-buffered saline (TBS) or the desired solution of reagent-grade sugar, glycine, bovine serum albumin or gelatin in distilled water. For some experiments, the sugars were diluted in TBS.
Excess fluid was drained and the slides were air-dried in the vertical position for 1 hr at 60°C in a dry oven (Heraterm, Thermo Scientific; Langenselbold, Germany). The slides were re-hydrated in TBS before proceeding with the subsequent steps.
Freeze Drying
Dewaxed, AR-treated sections were frozen in liquid nitrogen, placed in a Christ Alpha 1-2 LD freeze dryer (Martin Christ Gefriertrocknungsanlagen GmbH; Germany), dried for 24 or 48 hr at 0.09 mb at -53°C.
Desiccator
Dewaxed, AR-treated sections were placed under vacuum (0.14 mb) in a plastic desiccator (Sigma-Aldrich), together with moisture absorbants in excess (silica with traces of cobalt chloride for dryness estimate) for 24 or 48 hr at room temperature.
Accidental Drying
The accidental drying of sections during staining was also reproduced by depositing a small amount of antibody dilution buffer (100–300 µl) onto the tissue section, gently tilting the slide at an approximate 15° angle so that only part of the section remained wet, allowing sufficient time for drying by visual inspection, washing the section and performing the immunostain. This procedure was designed in order to separate the effect of partial drying from the reduced time in which the antibody actually contacts the antigen.
To mimic accidental drying during AR, paired sections were processed with or without disaccharides in the AR buffer; one section was immediately extracted from the hot buffer and left to dry until the second, allowed to cool in the buffer, was ready to be processed further in a conventional fashion.
For experiments testing the effect of drying on bound antibodies, 10% lactose or sucrose was added to the antibodies, optimally diluted in TBS-bovine serum albumin BSA (Sigma-Aldrich), NaN3, incubated overnight on the AR tissue section, blotted and dried unwashed in an oven as above.
Long-term Drying
The protective effect of lactose on AR epitopes was tested on sections that were dewaxed, AR, immersed in 10% lactose, oven dried and then stored at room temperature in a slide box for 2, 4 or 6 months. Immediately after the preparation and at each interval, the sections were stained for Ki-67, bcl-2, CD14 and CD79a. Sections cut from the same tissue block and sections cut at the beginning of the experiment, but not processed, and stored in the same box were processed routinely in parallel and stained for the same antigens. Long-term, dry-stored, lactose-protected sections underwent a second AR step to test the effect of time-dependent masking.
Immunohistochemistry (IHC) Protocols
Sections were rinsed in TBS, pH 7.5, containing 0.01% Tween-20 (TBS-T) and blocked for non-specific binding with a 5% w/v defatted (<1.25% fat) powdered milk solution in TBS (Latte Scremato Spray, Reire s.r.l., Reggio Emilia, Italy) for 1 hr.
Endogenous enzymes were blocked either because of AR (phosphatases (Cattoretti et al. 1993)) or with a 5-min incubation in 1% NaN3 solution to which 3% H2O2 was added (peroxidases (Li et al. 1987)).
Subsequently, the slides were incubated with the appropriately diluted primary antibodies overnight in a humid chamber at room temperature (Kartell; Milan, Italy).
Primary antibodies were mouse monoclonal antibodies Ki-67/MIB1, BCL2, CD20, CD45, CD68/PGM1, vimentin-V9, rabbit CD3e, S-100, anti-IgM-FITC conjugated (Dako), CD79a/HM57 (Sigma-Aldrich), rabbit anti BCL6 (Santa Cruz Biotechnology; Dallas, TX), and rabbit monoclonal anti-Ki67/SP6, Pax5 (Thermo Scientific). Secondary antibodies were Envision+ Polymer anti-rabbit, anti-mouse (Dako), AP-conjugated Sheep anti-FITC F(ab) (Roche; Monza, Italy), AP-conjugated donkey anti-rabbit, and anti-mouse (Jackson ImmunoResearch; Suffolk, UK).
Primary and secondary antibodies were diluted in TBS containing 5% BSA. The sections were then washed four times for 15 min each in TBS-T and incubated for additional 30 min with the appropriate second or third immunoreagent as follows:
Peroxidase IHC
After the primary antibody incubation rinse, the slides were incubated for 30 min with a species-specific peroxidase-conjugated polymer (Dako). After the final rinse, the peroxidase was developed with a 3-3’-diaminobenzidine (DAB; Dako) or aminoethylcarbazole (AEC; Sigma-Aldrich) as chromogens.
Immunoalkaline IHC
After the primary antibody incubation rinse, the slides were incubated for 30 min with a species-specific, calf Alkaline Phosphatase (AP)-conjugated secondary antibodies for 30 min, rinsed for 60 min in 0.5 M NaCl prepared in 0.005 M TBS, and developed with the nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (NBT-BCIP) chromogen (Sigma-Aldrich) at 37°C in a humidity chamber with visual inspection between 20 min to 2 hr (minimum to maximum developing times in these experiments). The developing time was recorded for each experiment.
Counterstaining and Coverslipping
A light hematoxylin counterstain was applied when appropriate and the sections coverslipped with Eukitt mounting medium (Sigma-Aldrich) or Glycergel (Dako), with the latter used in the cases of alcohol soluble dyes such as AEC or NBT-BCIP.
In order to minimize the shearing force applied by the solidifying gelatine while mounting, the sections and the molten gelatin were placed on a heat plate with waterbath (VPPS 200050; Lanzoni S.r.l., Bologna, Italy).
Antibody Elution and Sequential Immunostaining
Elution with 2-Mercaptoethanol/SDS (2-ME/SDS) was done by mixing 20 ml 10% w/v SDS with 12.5 ml Tris HCl 0.5 M, pH 6.8, and 67.5 ml ultra-pure water, to which 0.8 ml 2-Mercaptoethanol (Sigma-Aldrich) was added under a hood, as published previously (Gendusa et al. 2014). Incubation in the buffer was done in a shaking waterbath (SW23, JULABO Italia SRL; Milan, Italy), pre-heated at 56°C for 30 min. Subsequently, the sections were washed for 1 hr in distilled water, with water changes every 15 min. Finally, the sections were rinsed in TBS-T for 5 min and the non-specific binding blocked with a 5% w/v defatted milk solution in TBS-T for 1 hr before a subsequent immunostaining round.
Virtual Whole-slide Imaging
Stained slide images were acquired with Aperio FL and an Aperio CS whole-slide scanners (Leica Microsystems, Milan, Italy), as published previously (Buscone et al. 2014). Individual single-stained images taken with light microscopy were acquired with the ImageScope software (Aperio), optimized for contrast with Adobe Photoshop CS3 (Adobe Systems Incorporated; San Jose, CA) and mounted with Adobe Illustrator.
Results
Effect of Water Removal on Antigen Detectability
A series of staining conditions are shown in Fig. 1. Dewaxed, AR sections in distilled water were transferred to TBS as a control or air-dried in an oven at 60°C for 1 hr, rehydrated and immunostained for nuclear (Ki-67), cytoplasmic (bcl2) or membrane antigens (CD3) (Figs. 1 and 2). Air-drying invariably reduced the immunostaining intensity in a non-uniform manner to near complete loss (see dry column in Fig. 1). The effect was observed also for CD79a, Pax5, S-100, anti IgM, CD20, CD68, vimentin and BCL6 (data not shown). Residual staining was sometimes seen at the borders, over section folds, or corresponding to crystal-like buffer salts dried deposits (Figs. 2 and 3).
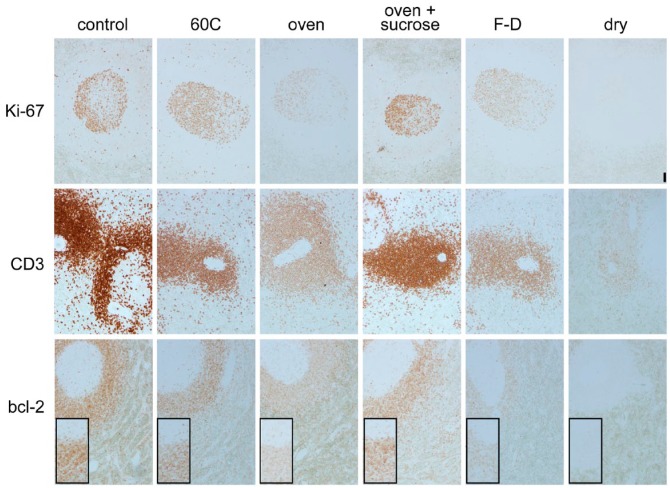
Figure 1.
Effect of water removal on antigenicity. Spleen sections were antigen-retrieved (AR) and then immunostained for Ki-67, CD3 and bcl-2 in control conditions (control), after 1 hr at 60°C in a waterbath (60°C), after 1 hr at 60°C in a dry oven (oven), same as previous but soaked in 10% sucrose in water before oven-drying (oven + sucrose), after freeze-drying for 48 hr (F-D), or after drying in a desiccator (dry). The inset shows higher magnification detail of the lymphoid cells. Original magnification 10×; Scale, 100 µm. No nuclear counterstain.
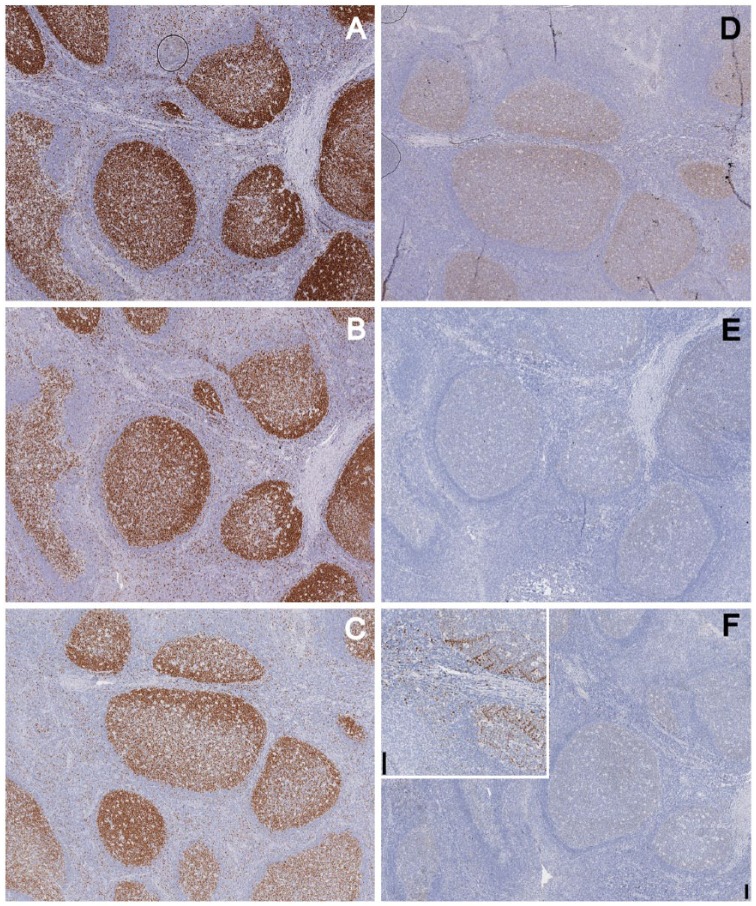
Figure 2.
Effect of air drying and proteic solutions on staining. Tonsil tissue was immunostained for Ki-67 without (A) or after air-drying (B–D). Before air-drying, the sections were immersed in buffer (B), or in a solution of 5% defatted milk (C) or glycine (D). Note faint residual staining in the germinal center of the air-dried section. Occasionally, with this pre-treatment, an uneven residual staining was noted on section folds or thicker portions (D). Original magnification 10×; Scale, 100 µm.
Figure 3.
Protective effect of lactose on air-dried sections. Serial tonsil sections were immunostained for Ki-67 after antigen retrieval (AR; A) and after post-AR air-drying (B–F). Before air-drying, the sections were immersed in buffer containing varying concentrations of lactose: 10% (B), 5% (C), 1% (D), 0.2% (E) or no lactose (F) (inset: crystal-like staining). Note the direct relationship between lactose concentration and staining. Original magnification 2×; Scale, 100 µm. Light hematoxylin nuclear counterstain.
The effect was not dependent on temperature, as maintaining the section at a fixed 60°C in distilled water for the same amount of time did not changed the immunoreactivity (Fig. 1). Air drying differs from freeze drying because, in the latter, a small amount of non-freezable water, about 5% of the total water, remains bound to the protein (Crowe et al. 1990), which, in our case, represents the antigen-retrieved epitope. Freeze drying for 48 hr reduced the immunoreactivity, but did not abolish it, whereas full dehydration by desiccation did (Fig. 1). Freeze drying for a shorter time (24 hr) produced an immunostain close, if not identical, to the control (data not shown).
Variations in the amount of residual staining in similarly treated sections stained with different antibodies were observed.
In order to embed the fixed tissue in paraffin, water must be replaced first with a water-miscible solvent (e.g., ethanol) and subsequently with xylene and paraffin; this process has been often referred to as “dehydration”. Replacing the water content of the antigen-retrieved sections with 100% ethanol or methanol through a graded series of progressive aqueous dilutions, or directly from the aqueous buffer into acetone did not reduced the immunostaining to a noticeable extent after gradually bringing the sections back to buffer (data not shown).
Thus, a reduction in the amount of water in an AR section by air drying does affect its immunostainability, depending on the amount of water removed.
Oven drying at 60°C for 1 hr is an effective method to remove most of the water from a tissue section and was used as the reference method for drying for the other experiments in this paper.
Once fully dehydrated, a second AR treatment, identical to first one, could only partially restore the immunostainability of the sections (Fig. 4).
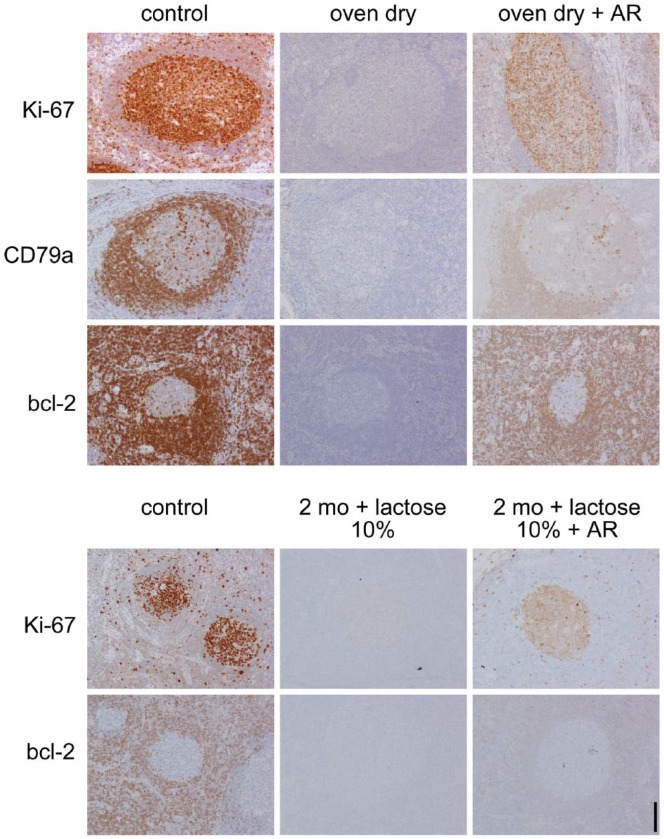
Figure 4.
Rescue of antigenicity after drying with or without lactose. Top: Tonsil sections, antigen-retrieved (AR), were immunostained for Ki-67, CD79a and bcl-2 in control conditions (control), after 1 hr at 60°C in a dry oven (oven dry), and after a second AR process performed after being oven-dried, before immunostaining (oven dry + AR). Bottom: Tonsil sections were immunostained for Ki-67 and bcl-2 with a routine protocol: freshly after being cut (control); after dewaxing, AR, soaking in lactose, oven-drying, and storage at room temperature for 2 months (2 mo + lactose 10%); or treated as before, but subjected to AR again before immunostaining (2 mo + lactose 10% + AR). Original magnification 10×; Scale, 100 µm. Light nuclear counterstain.
Disaccharides Protect from Air Drying-induced Antigen Masking
Immersing the sections before air drying in a 5% solutions of BSA, gelatin or glycin had no protective effect (Fig. 2 and data not shown). Urea 6 M caused tissue distortion because of crystal formation (data not shown).
Defatted milk (5% in TBS) provided a partial protection (Fig. 2). Because defatted milk is approximately 50% carbohydrates, the remaining being proteins (casein, lactalbumin), we tested the milk sugar lactose at 10% concentration and we found it to be 100% effective in protecting the antigen from the detrimental effect of air drying. Lower concentrations (5%, 1%, 0.2%) were progressively less protective (Figs. 1 and 3).
We also tested monosaccharides: 5% galactose, glucose, fructose or mannose did not protect the section from showing an air drying-dependent reduction in immunostainability (data not shown).
We noted in these and in all of the other experiments that, in the presence of any dilution of the disaccharide, the immunostaining was evenly distributed over the section, without staining artifacts at the border. Diluting the saccharides in TBS instead of distilled water did not change this protective effect (data not shown).
The protective effect of the pre-incubation with the disaccharide was lost upon removal of the sugar: lactose-protected, air-dried, rehydrated sections went unstained if air-dried again without the sugar (data not shown).
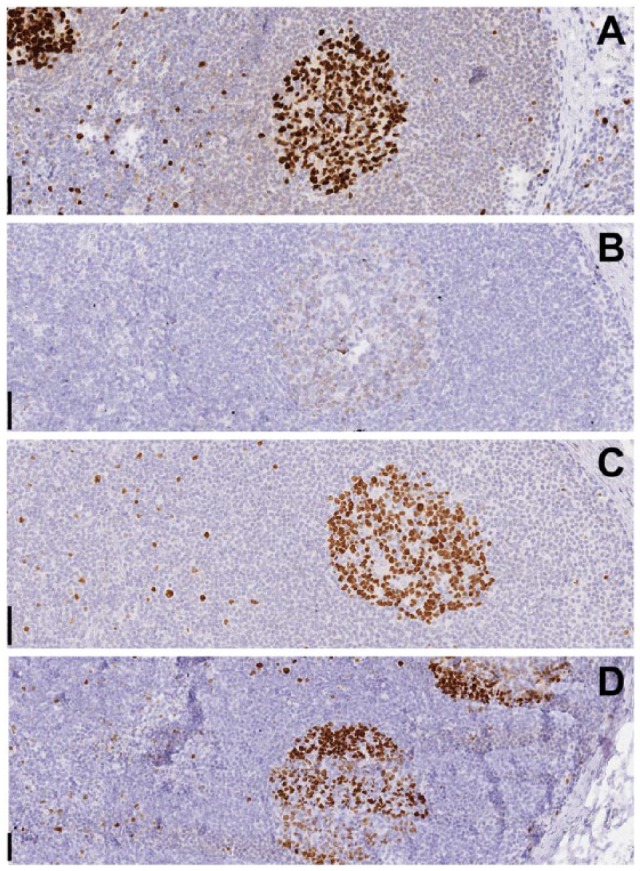
Lactose or sucrose 10% solutions were effective in preventing re-masking caused by accidental drying during the AR procedure (Shi et al. 1991): extracting the sections at mid-boiling from an AR solution resulted in an absence of staining, whereas the presence of sucrose or lactose in the AR buffer completely reversed the negative effect (Fig. 5).
Figure 5.
Protective effect of lactose and sucrose on accidental drying during antigen retrieval (AR). A formalin-fixed, paraffin-embedded (FFPE) lymph node is stained for the proliferation nuclear marker Ki-67 with a routine established method after heat-mediated AR in 0.1 M EDTA buffer, pH 8 (A, B), the same buffer containing 10% lactose (C, D), or 10% sucrose (E, F). Sections (B), (D) and (F) on the right have been extracted from buffer while still boiling and left to dry before proceeding with immunostaining. (A), (C) and (E) were allowed to cool in the buffer before proceeding with the immunostaining. Note that the presence of lactose or sucrose in the AR buffer prevents the drying-induced absence of immunostaining observed in (B). Original magnification 1×; Scale, 1 mm. Insets, magnification 10×; Scale, 100 µm. Light hematoxylin nuclear counterstain.
Lactose and sucrose were effective in less-stringent drying conditions, as might be seen in accidental loss of the horizontal position during incubation or by dropping one glass slide end in the incubation chamber. A loss of staining was evident on the high end of a non-horizontally placed slide when a disaccharide was not present. However, the presence of the sugar allowed even staining on the dried section end (Fig. 6). Again, no artifactual staining was noticed at the border of the tissue section.
Figure 6.
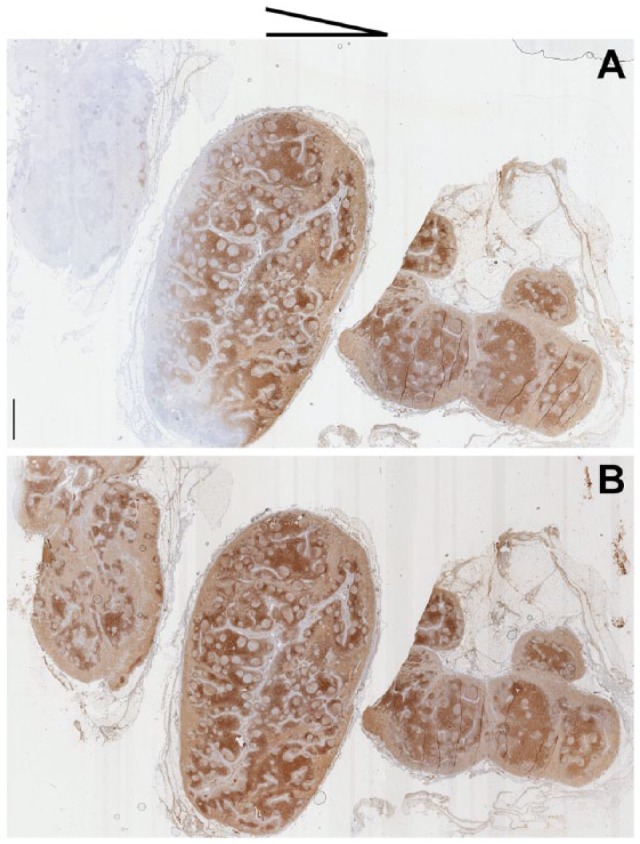
Lactose prevents staining artifacts due to partial drying of sections during staining. Two lymph node serial sections were immunostained for CD3 after both were tilted with an angle of about 15° (see scheme on top for direction of tilting). (A) Section incubated with regular antibody diluent; (B) section incubated with antibody diluent plus 10% lactose. Note the loss of staining on the anti-gravitational end of the section in (B). Original magnification. Whole slide image 1×; Scale, 1 mm. Light hematoxylin nuclear counterstain.
The preservation of full antigenicity obtained by the addition of disaccharide to buffers upon drying prompted the question of whether a dried, lactose-protected, AR section could withstand storage as compared to stored waxed sections. To this end, AR sections soaked in 10% lactose and oven-dried were compared with stored routine paraffin sections affixed to slides as well as freshly cut new sections from the block, at the beginning of the experiment and at 2-month intervals (Fig. 4), stained for Ki-67, bcl-2, CD14 and CD79a. Despite the presence of lactose, the dried sections lost the immunoreactivity upon storage. A second round of AR only partially restored the immunoreactivity on these stored sections (Fig. 4). However, further testing is required to determine if disaccharides can affect the long-term preservation of antigenicity or stability after sectioning.
Disaccharides Stabilize Primary Antibodies and Protect from Air Drying-induced Degradation
The removal of free water from the tissue induces conformational changes in the proteins, which may result in precipitation of antigens that could prevent them from being removed by extensive washing. Some of these changes are desirable, such as in the case of tissue components of a frozen section (Curran and Gregory 1980; Bancroft and Gamble 2008); others, less so (e.g., when a previously deposited antibody layer needs to be removed (Kaufmann et al. 1987) for multiplexing).
In order to test the effect of air drying on a bound antibody layer, tonsil sections were stained with a FITC-conjugated anti-IgM polyclonal antibody, washed, dried with or without the presence of saccharides and then counterstained.
Because the effect of drying in the presence or absence of modifiers may involve masking of the proteinaceous epitopes or removal of the bound immunoglobulin altogether, both resulting in a negative stain, we detected the bound Igs in parallel via a non-proteinaceous, small (354 Da), linear epitope, FITC, located on the same anti-IgM antibody.
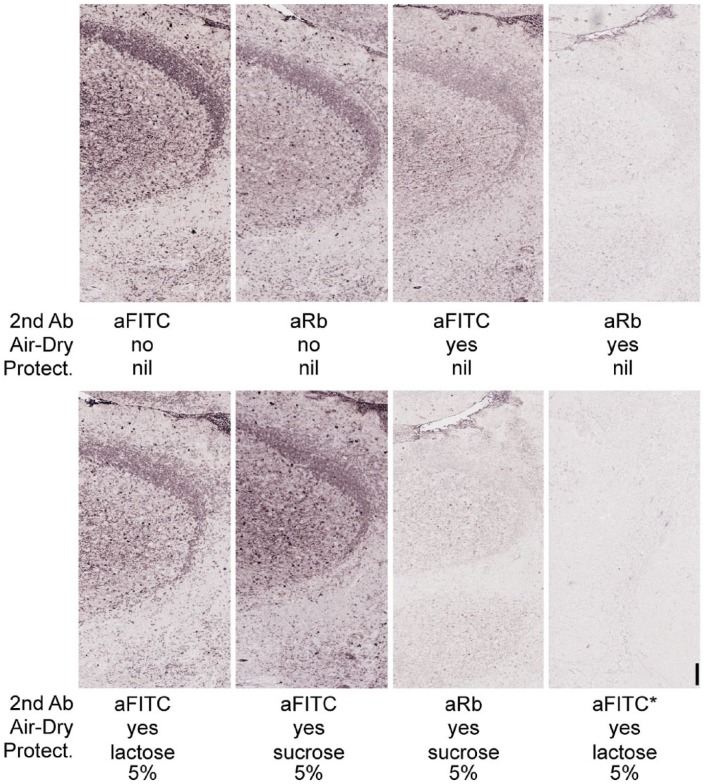
Air drying reduced the availability of the rabbit anti-IgM antibody for a secondary anti-rabbit antibody, as shown by the disproportionate reduction in staining as compared with the anti-FITC stained, air-dried companion slide (Fig. 7). Lactose and sucrose rescued the anti-rabbit staining after drying to a level close to the non-dried control (Fig. 7), and this was better shown by the anti-FITC staining than the anti-immunoglobulin staining.
Figure 7.
Protective effect of saccharides on bound primary antibodies. Serial tonsil sections were labelled with a FITC-conjugated anti-human IgM primary antibody and immunostained with an anti-FITC (top left) or anti-rabbit (top mid-left) secondary antibody. Air-drying in TBS after the primary antibody markedly reduced the rabbit antibody antigenicity for the anti-rabbit secondary (top right), less so for the anti-FITC antibody (top mid-right), whereas most of the antibody was still bound after rehydration. The presence of 5% lactose (bottom left) or 5% sucrose (bottom mid-left) in the buffer before drying improved the preservation of the FITC epitope, less so of the protein epitope (bottom mid-right). Air drying with or without sugars allowed 2-ME/SDS removal of the bound primary antibody (bottom right). Asterisk (*) refers to sections that were stripped before applying the anti-FITC AP-conjugated antibody. Original magnification 4×; Scale, 50 µm. No nuclear counterstain.
Monosaccharides such as glucose, but not galactose, protected to some extent the bound immunoglobulins (data not shown).
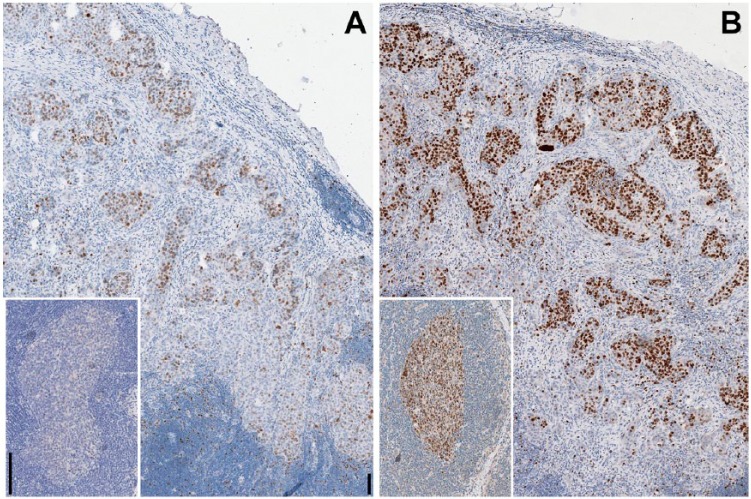
Oven air drying a section that had been incubated with a primary antibody (Ki-67, BCL6, CD45) and without washing the excess reagent, resulted in major reduction of its interaction with a secondary antibody, unless 10% lactose was present during the primary antibody incubation stage and during the drying stage; in this latter case, the expected staining distribution and strength were obtained (Fig. 8). Of note, no background or non-specific staining was produced in either condition.
Figure 8.
Lactose prevents the adverse effect of drying on sections during staining. A lymph nodal breast cancer metastasis and a lymph node (inset) were incubated with the primary antibody (Ki-67/MIB 1 and BCL6, respectively) and dried unwashed, before counterstaining. The staining is reduced and abolished in (A); however, the presence of 10% lactose in (B) showed the staining to be unaffected by the drying. Original magnification 10×; Scale, 100 µm. Light hematoxylin nuclear counterstain.
Lactose Maintains Bound Antibodies in a Post-drying Erasable Conformation
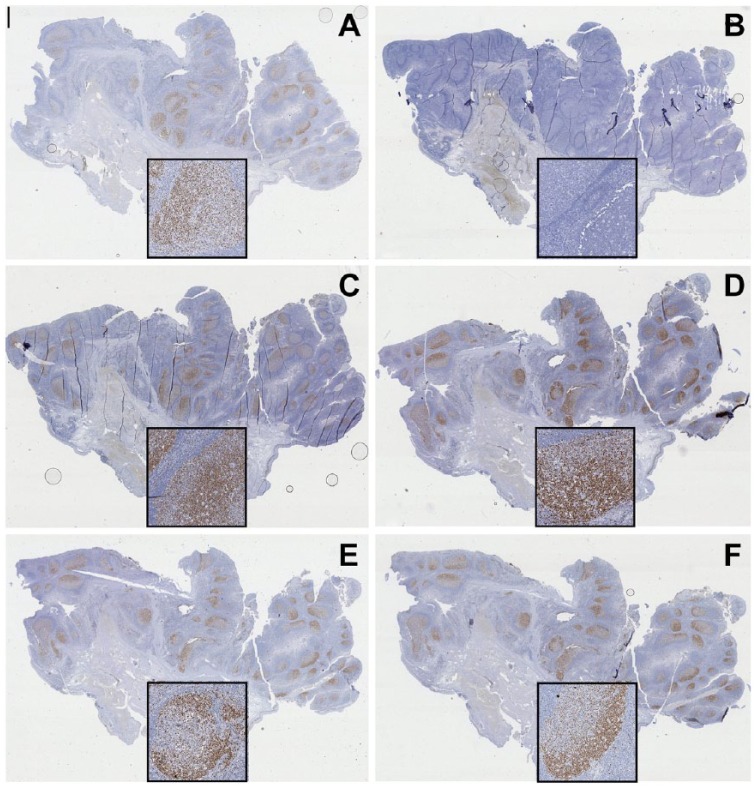
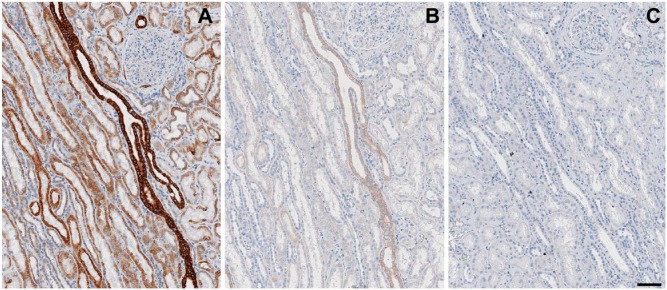
The air drying-induced stabilization effect on bound antibodies produced an inefficient removal by stripping (Kaufmann et al. 1987). We noticed a similar erratic effect on sections, which were variable and inconsistent depending on the type and density of the tissue antigen involved, the antibody layers deposited on the section, and poorly reproducible variations in tissue drying during staining and coverslipping. In order to address these issues, experiments were set up where single or multiple IHC layers, deposited on the sections, were thoroughly air-dried. Primary antibodies stained by indirect immunohistochemistry, such as anti-IgM, were completely erasable, despite the air-drying (Fig. 6). Anti-keratin, CD34 and anti-IgM antibodies, stained in double indirect immunohistochemistry (Cattoretti et al. 2006) and air-dried, left antibody residues after stripping, unless 10% lactose was present while drying (Fig. 9).
Figure 9.
Protective effect of lactose on the ability to erase bound antibody layers. A kidney section was immunostained for keratins (AE-1 + AE-3) with a mouse antibody (overnight), goat anti-mouse-FITC, mouse antibody, goat anti-mouse-FITC antibody sequence followed by anti-FITC HRP conjugate and DAB development (A). Before the anti-FITC conjugate, the sections were air dried in TBS (B) or TBS with 10% lactose (C), rehydrated, and stripped with 2-ME/SDS. Note the residual keratin staining in (B). Original magnification, 8×; Scale, 100 µm. Light hematoxylin nuclear counterstain.
Discussion
We have shown that the availability of an epitope in an AR FFPE section depends on a minimal amount of protein-associated water, which can be molecularly defined as non-freezable water. The definition of non-freezable water in this context is borrowed from cryoprotection studies, in which it has been shown that a very small amount of protein-associated water cannot readily been frozen but is responsible for maintaining the physical and enzymatic properties of proteins. Drying would remove this residual water and destroy the properties of the protein (Crowe et al. 1990). Removal of non-freezable water, about 5% of the total protein-associated water, abolishes the immunoreactivity. This is because the removal of water may affect the three-dimensional space distribution of the protein (Crowe et al. 1990) and thus the availability of the epitope, either directly affecting the antigen or any neighboring proteins that may condition the epitope availability. Removal of non-freezable water appears to occur during extensive drying, either intentional or accidental.
We also found that disaccharides, but not monosaccharides or proteins, protect the antigen from loss of this non-freezable water.
Sugars act thermodynamically and chemically as “water substitutes” (Crowe et al. 1990; Liao et al. 2002) when non-freezable, protein-associated water is removed, typically during air drying as compared with freeze drying (Carpenter and Crowe 1989; Carpenter et al. 1993; Wettlaufer and Leopold 1994). Hydrogen bonding between the -OH groups on the disaccharide and the polar groups on the protein preserve the conformation of the latter (Crowe et al. 1990; Liao et al. 2002). The sugar must be present at a defined concentration at the beginning of the dehydration process (Carpenter and Crowe 1989). Depending on the protein tested, the assay, and the method by which the water is removed, any of sucrose, trehalose, lactose or glucose can be effective, some more so than others (Carpenter et al. 1987; Carpenter et al. 1993; Liao et al. 2002; Prestrelski et al. 1995); below a certain threshold of water loss, the solute requirement for stabilization becomes very specific (Crowe et al. 1990).
The protective property of sugars against drying is remarkable but not entirely unexpected. Sugars are known for their protective effect against freezing and dehydration for a number of small organisms (Hubálek 2003). The protective effect of disaccharides is also well known in industry, as documented by numerous patents (Cook et al. 2010; Decker 1980; Drave and Whiteley 1986; Mayeresse 2012; Wettlaufer and Leopold 1994) and publications (Andya et al. 1999; Carpenter and Crowe 1989; Carpenter and Crowe 1988; Carpenter et al. 1993; Crowe et al. 1990; Dráber et al. 1995; Liao et al. 2002; Prestrelski et al. 1995; Whittier 1925).
Sugars and sucrose in particular have been previously used in histology. The original papers describing the use of sucrose in cryosectioning mention its effect in preserving tissue enzymes, besides an excellent morphology (Holt et al. 1960). Later studies noted that tissues fixed in aldehyde in the presence of variable amounts of sucrose could be cryosectioned and the sections dried without a loss of antigenicity (Griffith and Posthuma 2002; Ino 2003); this is at odds with the common experience of drying a fixed section without any protection. However, at the beginning of the “Antigen Retrieval years”, the use of formalin-sucrose (Curran and Gregory 1978) all but disappeared in the routine practice, except for experimental laboratory ultracryotomy use and some histochemical reactions (Baker’s fixative).
In 1987, Kaufmann et al. described a protective effect of skimmed milk on antibodies bound to a western blot membrane, which would allow the membrane to be successfully stripped and re-probed. They did not assess the independent effects of milk protein and lactose separately, but this may have been the chemistry behind their observations.
We have now extended the demonstration of the stabilizing properties of sugars to tissue antigens in histology preparations. In particular, disaccharides protect FFPE tissue antigens that are re-exposed after AR, maintaining their immunoreactive conformation, despite the loss of water. The effect is concentration-dependent, but is not related to the reducing power of lactose, as monosaccharides, which are also reducing sugars, have no or little such effect. On native, unfixed globular proteins, such as immunoglobulins, lactose and sucrose partially prevent drying-induced conformational changes, which reduces the accessibility to a secondary anti-Ig antibody. Small, linear epitopes like FITC are less affected by air drying.
The effect is also transient, as removal of the sugar or prolonged storage at room temperature (data not shown) re-masks the epitope.
Surprisingly, lactose was unable to protect dried epitopes over time. AR could not or only partially rescue the lost immunogenicity on stored, lactose-protected or freshly dried sections. One explanation may be that, during storage, the antigens are progressively degraded, as previously reported (Xie et al. 2011). Alternatively, re-masking occurs over time even in the presence of water substitutes. However, the fact that AR could not rescue the immunoreactivity of dried sections suggests that additional masking mechanisms take place in these sections and the second time they are less amenable to be removal by AR.
We have been unable to choose sucrose over lactose or other disaccharides as the preferred agent; lactose, a reducing sugar, is the least soluble disaccharide in alcohol (Whittier 1925) and is less soluble in water than sucrose. In addition, it undergoes crystallization upon drying, which reduces its protective effect on proteins. It may also interact with lysine residues and cause glycation (Andya et al. 1999) with time. For these reasons, we favor sucrose. However, we could not demonstrate any residual protection after lactose was washed away from the tissue. In our hands, lactose is a reproducible, cheap and effective disaccharide, and performs as well as sucrose.
We have found that dehydration does not change the ability of an antibody to bind the antigen, because, as shown upon rehydration, most of the antibody remains in situ, where it bound in the first place. This is despite the drying-induced modifications to the antibody itself, as demonstrated by the reduced immunoavailability for an anti-Ig secondary antibody. This may be because the energy of the paratope–epitope bond prevents the desiccation-associated modification of the Ig antigen binding site, or because the paratope is intrinsically resistant to the drying-induced modifications. Ad hoc experiments are needed to address this question. In any case, disaccharides are known to protect the whole Ig, even for labile isotypes (Dráber et al. 1995), including the paratope.
Accidental dehydration during an immunostaining procedure is considered in the literature a mere nuisance, not worth more than a few lines in which the suggestion is to avoid it (Bussolati and Leonardo 2008; Dabbs 2013; Lin and Prichard 2011; Taylor et al. 2013). Furthermore, the concept is not further elaborated or dissected. However, we hypothesized that partial dehydration, undetectable by the naked eye, unavoidable during repeated, sequential immunostaining and slide coverslipping, was causing two major adverse effects: re-masking of antigens, with loss of stainability, and irreversible precipitation of antibody layers in situ, causing an inability to chemically destroy and strip them from the section. By devising reproducible experiments addressing all the conditions leading to complete and partial drying of the tissue sections, we found that disaccharides (lactose, sucrose) protect the epitopes in the sections and on native proteins (immunoglobulins) from all the negative effects of air drying during each step of the immunostaining process.
As a side observation, we must note that, in our conditions, the effect of drying of the section is a blank, negative slide and not any background or non-specific staining, contrary to what has been published so far (Bussolati and Leonardo 2008; Dabbs 2013; Lin and Prichard 2011; Taylor et al. 2013). This may have to do with different protocols used in the staining process and antibody or chromogen trapping in dried sections.
Incomplete drying and the interplay with the masking ability of FFPE tissue are variables difficult to control in daily practice. However, incorporating a 10% disaccharide throughout all the steps of the IHC process and particularly during critical steps of the immunohistochemical procedure (AR, washing steps, antibody incubation, for example) may avoid the adverse effects of uncontrolled, unavoidable or unwanted air drying. Further work is necessary to determine the value of the addition of disaccharides at various steps in the processing, but this data provides a basis for its use to protect from antigen loss during accidental tissue drying.
Acknowledgments
We wish to thank Ms. Lorella Riva, Sara Malachina, Loredana Tusa, Antonella Musarò, Gloria Arrigoni for technical help, Dr. Franco Ferrario for continuous support, Dr. Dario Cerri (Central Pharmacy Services, San Gerardo) for providing sugar samples, Reire s.r.l. (Reggio Emilia, Italy) for providing powdered milk samples, Carlo Sbona, Francesca Venerucci and Ilaria Priori (Bio-Optica, Milan, Italy) for expert advice on formalin chemistry, Professors Francesco Nicotra, Laura Francesca Cipolla, Francesca Raimondo, Laura Russo, Department of Biotechnology and Biosciences, Chemistry, UNIMIB for advice, discussions and the use of the lyophilization facilities, Prof. David L Rimm, Yale University School of Medicine, USA, for suggestions and discussion.
Footnotes
Author Contributions: GC and GB designed the experiments; GB, CRS, RG performed immunohistochemical tests and histopathology preparations; GC, SR and GB scored the immunohistochemistry preparations; GC and SR wrote the manuscript. All authors have read and approved the final manuscript.
Competing Interests: The authors declared no potential competing interests with respect to the research, authorship, and/or publication of this article. Giorgio Cattoretti as the sole inventor filed an US PTO Provisional Patent application N. 62062750, “Method to protect tissue sections on glass slides from dehydration-induced damage.” on October 10, 2014.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Rossella Gendusa and Carla Rossana Scalia’s salary are paid by a GlaxoSmithKline clinical research with the Azienda Ospedaliera San Gerardo (HGS1006-C1121) and by the Fondazione per la Ricerca Scientifica Termale (FoRST), IV call grants (Project ‘‘Lymphopoiesis In Secondary Lymphoid Tissue’’). The Aperio Scanscope was provided through a grant from the Regione Lombardia (Call for Independent Research, DDG 6716 del 1/7/2009). This project has been supported by Departmental University of Milano-Bicocca and Hospital funds.
References
- Andya JD, Maa YF, Costantino HR, Nguyen PA, Dasovich N, Sweeney TD, Hsu CC, Shire SJ. (1999). The effect of formulation excipients on protein stability and aerosol performance of spray-dried powders of a recombinant humanized anti-IgE monoclonal antibody. Pharm Res 16:350-358. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Gamble M. (2008). Theory and Practice of Histological Techniques, Philidelphia: Churchill Livingstone/Elsevier. [Google Scholar]
- Buscone S, Argentieri MC, Pilla D, Cattoretti G. (2014). Whole-slide, quadruple immunofluorescence labeling of routinely processed paraffin sections. Appl Immunohistochem Mol Morphol 22:e1-7. [DOI] [PubMed] [Google Scholar]
- Bussolati G, Leonardo E. (2008). Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J Clin Pathol 61:1184-1192. [DOI] [PubMed] [Google Scholar]
- Carpenter JF, Crowe JH. (1989). An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 28:3916-3922. [DOI] [PubMed] [Google Scholar]
- Carpenter JF, Crowe JH. (1988). Modes of stabilization of a protein by organic solutes during desiccation. Cryobiology 25:459-470. [Google Scholar]
- Carpenter JF, Martin B, Crowe LM, Crowe JH. (1987). Stabilization of phosphofructokinase during air-drying with sugars and sugar/transition metal mixtures. Cryobiology 24:455-464. [DOI] [PubMed] [Google Scholar]
- Carpenter JF, Prestrelski SJ, Arakawa T. (1993). Separation of freezing- and drying-induced denaturation of lyophilized proteins using stress-specific stabilization. I. Enzyme activity and calorimetric studies. Arch Biochem Biophys 303:456-464. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C, Key G, D’Amato L, Sabattini E, Feudale E, et al. (1993). Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol 171:83-98. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Shaknovich R, Smith PM, Jack HM, Murty VV, Alobeid B. (2006). Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J Immunol 177:6930-6939. [DOI] [PubMed] [Google Scholar]
- Cook ME, Yang M, Etzel MR. (2010). Mixing protein with a saccharide compound in a liquid suspension and then drying to produce a solid that contains the protein and the saccharide. US Patent 7,750,117. [Google Scholar]
- Crowe JH, Carpenter JF, Crowe LM, Anchordoguy TJ. (1990). Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology 27:219-231. [Google Scholar]
- Curran RC, Gregory J. (1980). Effects of fixation and processing on immunohistochemical demonstration of immunoglobulin in paraffin sections of tonsil and bone marrow. J Clin Pathol 33:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran RC, Gregory J. (1978). Demonstration of immunoglobulin in cryostat and paraffin sections of human tonsil by immunofluorescence and immunoperoxidase techniques. Effects of processing on immunohistochemical performance of tissues and on the use of proteolytic enzymes to unmask antigens in sections. J Clin Pathol 31:974-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs DJ. (2013). Diagnostic Immunohistochemistry, Philidelphia: Elsevier Health Sciences. [Google Scholar]
- Decker RH. (1980). Sugar coated reagents for solid phase immunoassay. CA Patent 1,086,647. [Google Scholar]
- Dráber P, Dráberová E, Nováková M. (1995). Stability of monoclonal IgM antibodies freeze-dried in the presence of trehalose. J Immunol Methods 181:37-43. [DOI] [PubMed] [Google Scholar]
- Drave RAL, Whiteley SC. (1986). Preparation of reagents. EP Patent App. EP19,860,300,169 [Google Scholar]
- Fowler CB, Cunningham RE, O’Leary TJ, Mason JT. (2007). ‘Tissue surrogates’ as a model for archival formalin-fixed paraffin-embedded tissues. Lab Invest 87:836-846. [DOI] [PubMed] [Google Scholar]
- Gendusa R, Scalia CR, Buscone S, Cattoretti G. (2014). Elution of High Affinity (>10-9 KD) Antibodies from Tissue Sections: Clues to the Molecular Mechanism and Use in Sequential Immunostaining. J Histochem Cytochem 62:519-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JM, Posthuma G. (2002). A reliable and convenient method to store ultrathin thawed cryosections prior to immunolabeling. J Histochem Cytochem 50:57-62. [DOI] [PubMed] [Google Scholar]
- Holt Sj, Hicks Rm. (1961). Studies on formalin fixation for electron microscopy and cytochemical staining purposes. J Biophys Biochem Cytol 11:31-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt Sj, Hobbiger Ee, Pawan Gl. (1960). Preservation of integrity of rat tissues for cytochemical staining purposes. J Biophys Biochem Cytol 7:383-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek Z. (2003). Protectants used in the cryopreservation of microorganisms. Cryobiology 46:205-229. [DOI] [PubMed] [Google Scholar]
- Ino H. (2003). Antigen retrieval by heating en bloc for pre-fixed frozen material. J Histochem Cytochem 51:995-1003. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Ewing CM, Shaper JH. (1987). The erasable Western blot. Anal Biochem 161:89-95. [DOI] [PubMed] [Google Scholar]
- Li C, Ziesmer SC, Lazcano-Villareal O. (1987). Use of azide and hydrogen peroxide as an inhibitor for endogenous peroxidase in the immunoperoxidase method. J Histochem Cytochem 35:1457-1460. [DOI] [PubMed] [Google Scholar]
- Liao Y-H, Brown MB, Nazir T, Quader A, Martin GP. (2002). Effects of sucrose and trehalose on the preservation of the native structure of spray-dried lysozyme. Pharm Res 19:1847-1853. [DOI] [PubMed] [Google Scholar]
- Lin F, Prichard J. (2011). Handbook of Practical Immunohistochemistry: Frequently Asked Questions, New York: Springer. [Google Scholar]
- Mayeresse Y. (2012). Drying process for preserving an active agent as a highly viscous liquid. US Patent 8,173,411. [Google Scholar]
- Prestrelski SJ, Pikal KA, Arakawa T. (1995). Optimization of lyophilization conditions for recombinant human interleukin-2 by dried-state conformational analysis using Fourier-transform infrared spectroscopy. Pharm Res 12:1250-1259. [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL. (1991). Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741-748. [DOI] [PubMed] [Google Scholar]
- Shin RW, Iwaki T, Kitamoto T, Tateishi J. (1991). Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer’s disease brain tissues. Lab Invest 64:693-702. [PubMed] [Google Scholar]
- Taylor CR, Colley EC, Stead RH, Shi S-R, Nielsen S, L J, Nielsen M, Mànsson S, Rudbeck L, Petersen K, Pedersen HC, Schmidt J, Pace GE, Rasmussen OF, MacDonald D, Jørgensen JT, Saxena R, Badve S, Mollerup J, Wendelboe HG, Lykke A. (2013) ‘Immunohistochemical Staining Methods’, in Taylor CR, Rudbeck L. (eds.). IHC Guidebook, 6th edition, Denmark: Dako Denmark A/S, An Agilent Technologies Company. [Google Scholar]
- Wettlaufer SH, Leopold AC. (1994). Method of protecting biological materials from destructive reactions in the dry state. US Patent 5,290,765. [Google Scholar]
- Whittier EO. (1925). Lactose. A Review. Chem Rev 2:85-125. [Google Scholar]
- Xie R, Chung J-Y, Ylaya K, Williams RL, Guerrero N, Nakatsuka N, Badie C, Hewitt SM. (2011). Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem 59:356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]