Abstract
Aim
Renal dysfunction is a common comorbidity in patients with heart failure and preserved ejection fraction (HFpEF). We sought to determine whether renal dysfunction was associated with measures of cardiovascular structure/function in patients with HFpEF.
Methods
We studied 217 participants from the PARAMOUNT study with HFpEF who had echocardiography and measures of kidney function. We evaluated the relationships between renal dysfunction [estimated glomerular filtration rate (eGFR) >30 and <60 mL/min/1.73 m2 and/or albuminuria] and cardiovascular structure/function.
Results
The mean age of the study population was 71 years, 55% were women, 94% hypertensive, and 40% diabetic. Impairment of at least one parameter of kidney function was present in 62% of patients (16% only albuminuria, 23% only low eGFR, 23% both). Renal dysfunction was associated with abnormal LV geometry (defined as concentric hypertrophy, or eccentric hypertrophy, or concentric remodelling) (adjusted P = 0.048), lower midwall fractional shortening (MWFS) (P = 0.009), and higher NT-proBNP (P = 0.006). Compared with patients without renal dysfunction, those with low eGFR and no albuminuria had a higher prevalence of abnormal LV geometry (P = 0.032) and lower MWFS (P < 0.01), as opposed to those with only albuminuria. Conversely, albuminuria alone was associated with greater LV dimensions (P < 0.05). Patients with combined renal impairment had mixed abnormalities (higher LV wall thicknesses, NT-proBNP; lower MWFS).
Conclusion
Renal dysfunction, as determined by both eGFR and albuminuria, is highly prevalent in HFpEF, and associated with cardiac remodelling and subtle systolic dysfunction. The observed differences in cardiac structure/function between each type of renal damage suggest that both parameters of kidney function might play a distinct role in HFpEF.
Keywords: Heart failure with preserved ejection fraction, Chronic kidney disease, Albuminuria, Glomerular filtration rate, Cardiovascular structure and function
Introduction
Renal dysfunction is one of the most common comorbidities in heart failure with preserved ejection fraction (HFpEF), with a prevalence of 30–60%1–3 which increases with ageing. It is defined by low-estimated glomerular filtration rate (eGFR) and/or high urinary albumin-to-creatinine ratio (UACR).4 Importantly, even mild impairment of renal function is associated with elevated cardiovascular (CV) risk.5,6 However, these two measures of renal dysfunction are only partly correlated and each provides prognostic information in heart failure patients.7,8
The prognostic relationship between renal dysfunction and CV outcomes may be mediated by abnormalities of cardiac structure and function. Cross-sectional findings from community cohorts and hypertensive populations demonstrate a high prevalence of left ventricular hypertrophy (LVH) and to lesser extent of diastolic dysfunction in patients with low eGFR or high UACR.9–13 Despite the frequency of renal dysfunction in HFpEF, there is a paucity of data regarding the relationships between kidney dysfunction and cardiac structure/function in this population.14,15 Furthermore, the association between the combined impairment of eGFR and UACR and CV remodelling has not been thoroughly investigated,16 specifically in HFpEF populations.
We analysed the associations between albuminuria and/or low eGFR and CV structure and function in subjects enrolled in the PARAMOUNT trial, a prospective study comparing the efficacy of therapy with an angiotensin receptor blocker (ARB) vs. an angiotensin receptor neprilysin inhibitor (ARNI) in patients with HFpEF.
Methods
Patient population
The design, baseline findings, and primary results of the Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) trial have been previously reported.17 Briefly, PARAMOUNT enrolled 301 patients with HFpEF, aged 40 years or older, with elevated NT-proBNP (>400 pg/mL) and a left ventricular ejection fraction (LVEF) ≥45%, while on active diuretic therapy. The main exclusion criteria included eGFR <30 mL/min/1.73 m2, non-cardiac dyspnoea, and significant valvular heart disease. The proportion of patients enrolled with atrial fibrillation was limited to ∼25% of the study population. In the present analysis, we included patients with analysable baseline echocardiographic data (n = 279) and with available eGFR and UACR data (n = 217, 72%).
Echocardiographic study
All echocardiograms were analysed in the Cardiovascular Imaging Core Laboratory at Brigham and Women's Hospital, Boston MA, USA. All measurements were made in triplicate in accordance with the recommendations of the American Society of Echocardiography (ASE).18 According to the echocardiographic protocol of the Cardiovascular Imaging Core Laboratory, both imaging depth and sector had to be optimized to maintain a frame rate of 50–80 frames per second. Left ventricular (LV) volumes were derived according to the modified biplane Simpson's method in the apical four-chamber and/or two-chamber views. The left ventricular mass (LVM) was calculated from LV linear dimensions and indexed to height2.7, according to ASE guidelines.18 Relative wall thickness (RWT) was calculated by the formula: [(2 × diastolic posterior wall thickness)/diastolic LV internal diameter]. Left ventricular hypertrophy was defined as LVM indexed to height2.7 >44 g/m2.7 in women and >48 g/m2.7 in men. Normal geometry was classified as RWT ≤0.42 and no LVH. Abnormal LV geometry was defined as either concentric remodelling (CR), or concentric hypertrophy (CH), or eccentric hypertrophy (EH). Concentric remodelling was defined as RWT >0.42 and no LVH; CH was defined as RWT >0.42 and LVH; and EH was defined as RWT ≤0.42 and LVH.18
Mitral flow velocities were assessed by positioning the sample volume of pulsed-wave Doppler at the tip of the mitral leaflets from the apical four-chamber view. Tissue Doppler E′ velocity was measured as the average of the values detected at the septal and lateral mitral annulus. Left atrial volume was assessed by the biplane area–length method from the apical two-chamber and four-chamber views at end-systole from the frame preceding mitral valve opening. Pulmonary artery systolic pressure (PASP) was estimated using Doppler tricuspid regurgitant velocity (V) as PASP = 4 (V2) + 10 mmHg.
Left ventricular ejection fraction was calculated using the biplane Simpson's method from LV end-diastolic and end-systolic volume. Midwall fractional shortening (MWFS), a measure of LV systolic function that, for its nature, and in contrast with LVEF, is more reliable in case of abnormal LV geometry, was calculated according to the recommendations of the American Society of Echocardiography.18 Using a vendor-independent 2D speckle tracking software (2D Cardiac Performance Analysis version 4.5, TomTec Imaging Systems, Munich, Germany), we evaluated deformation parameters. Data were obtained using apical four-chamber and two-chamber views for longitudinal deformation. For myocardial deformation analysis, we excluded patients with non-DICOM images, with lack of a full cardiac cycle, >1 segment dropout or significant foreshortening of LV. In our study sample (217 patients), longitudinal strain was quantifiable in 168 participants.
End-systolic stiffness (Ees) was used as a measure of LV systolic stiffness: it was calculated with the modified single-beat method from SV, arm-cuff blood pressures, and pre-ejection and total systolic periods determined on pulsed-wave Doppler of aortic flow, LVEF, and an estimated normalized ventricular elastance at arterial end-diastole, as previously validated against invasive assessment.19 Arterial stiffness was characterized as effective arterial elastance (Ea).20 Arterial elastance was estimated as end-systolic pressure (ESP) divided by SV, determined echocardiographically using the Simpson's method of discs; LV ESP was calculated as 0.9 multiplied by arm-cuff SBP at the time of echocardiography. Ventricular–vascular coupling was assessed as the ratio Ea/Ees.
Blood/urine sampling procedures and definitions of renal parameters
Serum and spot urine samples were obtained at baseline. Glomerular filtration rate for this analysis was estimated using a recently validated formula that incorporates both creatinine and cystatin C values (CKD-EPI creatinine-cystatin C equation).9,21 Urinary albumin, and creatinine concentrations, detected in a spot urine sample, were used to calculate the UACR (mg/g).
Reduced eGFR was defined at a threshold of 60 mL/min/1.73 m2. High UACR was defined using gender-specific cut-off values for microalbuminuria (17 mg/g for men and 25 mg/g for women),22 since it has been suggested that women tend to have higher albumin urine excretion in physiological circumstances. As a sensitivity analysis, we also analysed UACR considering a fixed sex-independent cut-off of 30 mg/g. To assess the relationship between renal dysfunction and parameters of CV structure and function, patients were first stratified into two categories: subjects with reduced eGFR and/or high UACR were classified as renal dysfunction, while those with preserved eGFR and normal UACR were defined as no renal dysfunction. Secondly, we assessed the relationship between the type of renal function impairment and CV derangement creating four categories of kidney function: no renal dysfunction (reference), high UACR with preserved eGFR, normal UACR and low eGFR, both abnormal.
Statistical analysis
Continuous data are presented as mean and standard deviation or median and inter-quartile range. Categorical variables are shown as counts and percentages. Log transformations were applied to skewed variables (NT-proBNP, UACR, and cystatin C). Clinical characteristics and CV structure and function were compared between different groups of HFpEF patients, according to the type of kidney impairment, using two-sided t-tests, Wilcoxon rank sum tests, X2 tests, Fisher exact tests, or ANOVA as determined by variable type and distribution. Multivariable-adjusted linear and logistic regression models were used to study the association between echocardiographic parameters of cardiac structure and function (outcome variables) and renal function. Models were adjusted for age, sex, presence of diabetes, and covariates that differed significantly by renal function status on univariable analyses: previous admission for HF, anaemia, pulse pressure (PP). For ventricular and vascular stiffness analysis, each model was also adjusted for baseline heart rate, which has been shown to be related to these dependent variables, instead of PP (since blood pressure data are used to calculate stiffness parameters). All tests were two-sided and P-values of <0.05 were considered statistically significant. Statistical analyses were performed with STATA 12.0 (Stata Corp, College Station, TX, USA).
Results
Clinical characteristics
Out of 279 patients with analysable baseline echocardiographic data, eGFR was available in 256 and UACR in 238 patients, leading to a final sample size of 217 (72%) patients with available data on both parameters of renal function. The population was elderly (mean age 71), 55% women, 74% Caucasian, 40% with a history of diabetes, and the majority was hypertensive (94%). Impairment of at least one parameter of kidney function (albuminuria and/or low eGFR) was present in 62% of HFpEF patients: 16% had only albuminuria, 23% had only low eGFR, while 23% had both (Table 1). A similarly high prevalence of renal dysfunction (59%) was demonstrated applying the sex-independent cut-off for abnormal UACR (30 mg/g).
Table 1.
Baseline clinical/laboratory characteristics of the study population by renal dysfunction absence vs. presence
| No renal dysfunction, n = 82 (38%) | Renal dysfunction n = 135 (62%) | P-value | |
|---|---|---|---|
| eGFR (mL/min/1.73 m2) | 78 ± 13 | 55 ± 19 | <0.001 |
| UACR (mg/g) | 8.0 (4.4, 11.5) | 30.1 (11.5, 70.5) | <0.001 |
| Cystatin C (mg/L) | 1.0 (0.9, 1.1) | 1.4 (1.1, 1.5) | <0.001 |
| Age (years) | 69 ± 9 | 72 ± 9 | 0.037 |
| Women (%) | 49 (60) | 71 (53) | 0.30 |
| Body mass index (kg/m2) | 30.3 ± 5.8 | 30.8 ± 6.0 | 0.52 |
| Systolic BP (mmHg) | 135 ± 14 | 137 ± 15 | 0.25 |
| Diastolic BP (mmHg) | 79 ± 8 | 77 ± 11 | 0.14 |
| Pulse pressure (mmHg) | 56 ± 13 | 60 ± 14 | 0.022 |
| Heart rate (b.p.m.) | 68 ± 13 | 69 ± 13 | 0.95 |
| NYHA (%) | |||
| Class I | 1 (1) | 1 (1) | 0.90 |
| Class II | 66 (80) | 107 (79) | |
| Class III | 15 (18) | 27 (20) | |
| Prior HF hospitalization | 24 (29) | 69 (51) | 0.002 |
| HF aetiology (%) | |||
| Ischaemic | 29 (36) | 55 (41) | 0.75 |
| Hypertensive | 42 (52) | 66 (49) | |
| Other | 10 (12) | 14 (10) | |
| History of diabetes | 27 (33) | 60 (44) | 0.09 |
| Presence of atrial fibrillation | 23 (28) | 42 (31) | 0.63 |
| History of CHD | 33 (40) | 66 (49) | 0.22 |
| History of hypertension | 76 (93) | 128 (95) | 0.52 |
| Current smoker | 3 (4) | 9 (7) | 0.54 |
| Haemoglobin (g/dL) | 13.9 ± 1.3 | 13.3 ± 1.7 | 0.008 |
| Anaemia (%) | 10 (13) | 38 (29) | 0.005 |
| Baseline therapy (%) | |||
| ACE-I or ARBs | 78 (95) | 124 (92) | 0.36 |
| β-Blockers | 70 (85) | 111 (82) | 0.55 |
| Aldosterone antagonists | 14 (17) | 34 (25) | 0.16 |
Renal dysfunction defined as the presence of impairment of UACR and/or eGFR.
Data are n (%), mean ± SD, median (IQR).
NYHA, New York Heart Association; BP, blood pressure; eGFR, estimated glomerular filtration rate. Albuminuria defined as UACR ≥17 in men and ≥25 in females. Anaemia defined as haemoglobin levels <12 g/dL in women and <13 g/dL in men.
Compared with subjects with preserved renal function, the presence of kidney dysfunction was associated with older age, whereas gender did not differ between the groups. Those with renal dysfunction were likely to have had a previous HF hospitalization; however, aetiology of HF and symptoms did not vary. While anaemia was more common and diabetes tended to be more prevalent in subjects with renal dysfunction, other traditional CV risk factors were similar between the groups. Patients in the two groups had similar therapy. Heart rate did not vary based on kidney function, whereas renal dysfunction was associated with higher PP. As seen in Table 3, diabetes was more common in the UACR alone group, as was a history of CAD. Conversely, older age, anaemia, and women sex were more prevalent in patients with low eGFR (alone or in combination with high UACR). Patients included in this analysis (n = 217) had higher BMI (30.6 ± 5.9 kg/m2 vs. 28.3 ± 5.1 kg/m2), SBP (137 ± 15 vs. 131 ± 17 mmHg), and beta-blocker therapy use (83% vs. 68%) compared with the ones not included, but were similar with respect to all other baseline characteristics.
Table 3.
Clinical characteristics and cardiovascular structure and function by estimated glomerular filtration rate and urinary albumin-to-creatinine ratio combined categories
| Reference: no renal dysfunction, n = 82 (38%) | High UACR/preserved eGFR, n = 35 (16%) | Normal UACR/low eGFR, n = 50 (23%) | High UACR/low eGFR, n = 50 (23%) | Unadjusted P-value | |
|---|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) | 78 ± 13 | 85 ± 36 | 46 ± 10 | 46 ± 8 | <0.001 |
| UACR (mg/g) | 8.0 (4.4, 11.5) | 42 (31, 111) | 8 (6, 13) | 67 (34, 119) | <0.001 |
| Clinical characteristics | |||||
| Age (years) | 69 ± 9 | 66 ± 11 | 73 ± 7 | 75 ± 8 | <0.001 |
| Women (%) | 49 (60) | 13 (37) | 38 (76) | 20 (40) | <0.001 |
| Body mass index (kg/m2) | 30.3 ± 5.8 | 31.2 ± 6.2 | 31.8 ± 6.8 | 29.5 ± 4.6 | 0.22 |
| Pulse pressure (mmHg) | 56 ± 13 | 62 ± 16 | 61 ± 15 | 59 ± 13 | 0.10 |
| Prior HF hospitalization (%) | 24 (29) | 14 (40) | 24 (48) | 31 (62) | 0.002 |
| History of diabetes (%) | 27 (33) | 24 (69) | 14 (28) | 22 (44) | 0.001 |
| History of CHD (%) | 33 (40) | 22 (63) | 20 (40) | 24 (48) | 0.12 |
| History of hypertension (%) | 76 (93) | 34 (97) | 48 (96) | 46 (92) | 0.77 |
| Anaemia | 10 (13) | 9 (26) | 14 (29) | 15 (31) | 0.033 |
| LV structure (%) | |||||
| LVEDV (mL) | 108 ± 23 | 131 ± 27 | 109 ± 35 | 117 ± 32 | 0.001 |
| LVESV (mL) | 46 ± 14 | 58 ± 18 | 45 ± 26 | 49 ± 17 | 0.008 |
| SWT (cm) | 0.90 ± 0.16 | 0.96 ± 0.11 | 1.02 ± 0.21 | 0.99 ± 0.20 | 0.001 |
| PWT (cm) | 0.82 ± 0.11 | 0.87 ± 0.12 | 0.90 ± 0.20 | 0.91 ± 0.20 | 0.007 |
| LVM (g) | 134 ± 33 | 161 ± 31 | 155 ± 49 | 159 ± 52 | 0.001 |
| LVMi (g/m2.7) | 36 ± 10 | 40 ± 10 | 41 ± 13 | 41 ± 12 | 0.010 |
| RWT (cm) | 0.36 ± 0.06 | 0.36 ± 0.06 | 0.40 ± 0.11 | 0.39 ± 0.10 | 0.011 |
| LV systolic function (%) | |||||
| LVEF | 58 ± 7 | 56 ± 8 | 60 ± 9 | 59 ± 6 | 0.10 |
| Cardiac output (L/min) | 4193 ± 1069 | 5106 ± 1402 | 4298 ± 1318 | 4544 ± 1195 | 0.002 |
| MWFS | 17.6 ± 2.0 | 17.4 ± 1.6 | 16.4 ± 2.4 | 16.8 ± 2.4 | 0.011 |
| GLS | −14.4 ± 3.4 | −14.6 ± 3.3 | −15.3 ± 3.3 | −15.1 ± 3.0 | 0.50 |
| LV diastolic function | |||||
| E/E′ | 14.2 ± 7.3 | 15.2 ± 6.9 | 15.0 ± 5.3 | 14.6 ± 6.5 | 0.44 |
| LAV (mL) | 62 ± 24 | 73 ± 28 | 68 ± 31 | 71 ± 26 | 0.14 |
| NT-proBNP (pg/mL) | 744 (321, 1174) | 741 (457, 1275) | 873 (572, 1508) | 1361 (828, 2293) | <0.001 |
| Measures of arterial–LV coupling | |||||
| Ea (mmHg/mL) | 2.05 ± 0.49 | 1.84 ± 0.50 | 2.06 ± 0.57 | 1.90 ± 0.55 | 0.10 |
| Ees (mmHg/mL) | 2.53 ± 0.99 | 2.20 ± 0.77 | 2.72 ± 1.04 | 2.40 ± 0.93 | 0.14 |
| Ea/Ees | 0.94 ± 0.61 | 0.94 ± 0.41 | 0.85 ± 0.41 | 0.88 ± 0.36 | 0.76 |
Data are mean ± SD, median (IQR).
LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; SWT, septal wall thickness; PWT, posterior wall thickness; LVM, left ventricular mass; LVMi, left ventricular mass indexed to height2.7; RWT, relative wall thickness; LVEF, left ventricular ejection fraction; MWFS, midwall fractional shortening; GLS, global longitudinal strain; E/E′, mitral inflow to mitral relaxation velocity ratio; LAV, left atrial volume; Ea, effective arterial elastance; Ees, left ventricular end-systolic elastance; Ea/Ees, ventricular–vascular coupling.
Cardiovascular structure and function according to kidney function
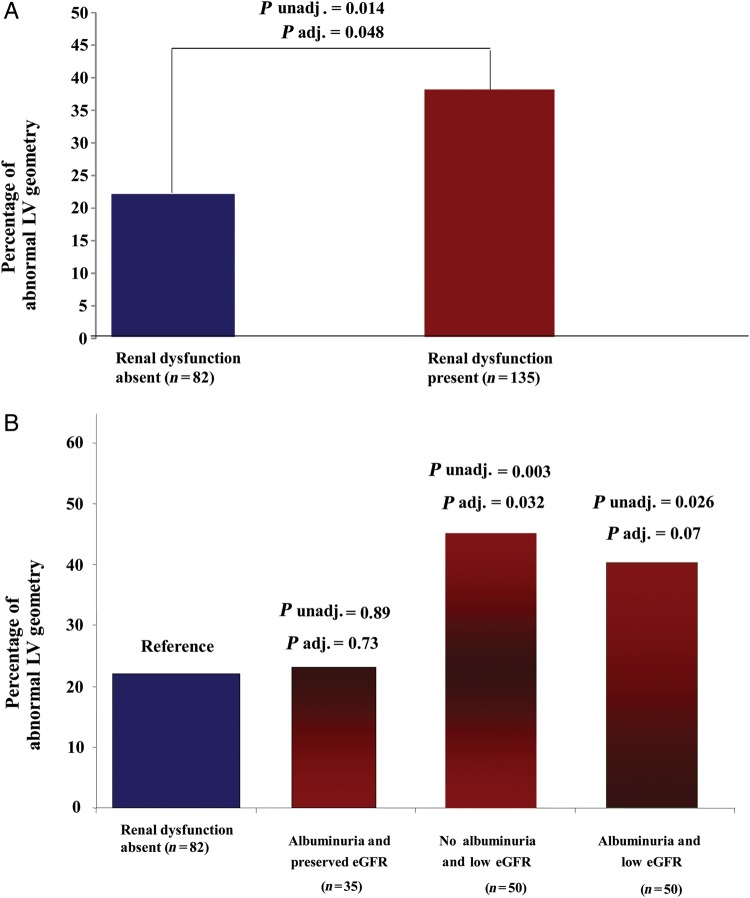
Differences in CV structure and function according to the presence or absence of renal dysfunction are shown in Table 2 and Figure 1. In both unadjusted and adjusted analyses, patients with renal dysfunction (eGFR >30 and <60 mL/min/1.73 m2 and/or high UACR), compared with subjects with preserved renal function, consistently showed greater LV wall thicknesses, LV mass, and a higher prevalence of abnormal LV geometry (either LV CH, or CR, or EH), combined with higher NT-proBNP, and lower MWFS. To account for the greater prevalence of HF hospitalizations (51 vs. 29%) and older age of patients with renal dysfunction compared with those without (72 vs. 69 years old), all our models were adjusted for these two covariates as well as others deemed to be clinically important (sex, PP, anaemia, history of diabetes). Renal dysfunction was associated with a higher prevalence of abnormal LV geometry (OR: 2.0, P = 0.048), with female sex the only other significant predictor of structural differences (OR: 2.4, P = 0.009). Conversely, ventricular-vascular systolic stiffness indexes and other parameters of LV systolic/diastolic function were similar between the two groups.
Table 2.
Cardiovascular structure and function by renal dysfunction absence vs. presence
| No renal dysfunction, n = 82 (38%) | Renal dysfunction, n = 135 (62%) | P-value (unadjusted) | P-value (adjusted)a | |
|---|---|---|---|---|
| LV structure | ||||
| LVEDV (mL) | 108 ± 23 | 118 ± 33 | 0.025 | 0.16 |
| LVESV (mL) | 46 ± 14 | 50 ± 21 | 0.19 | 0.70 |
| SWT (cm) | 0.90 ± 0.16 | 0.99 ± 0.19 | <0.001 | 0.001 |
| PWT (cm) | 0.82 ± 0.11 | 0.90 ± 0.18 | <0.001 | 0.016 |
| LVM (g) | 134 ± 33 | 158 ± 46 | <0.001 | 0.002 |
| LVMi (g/m2.7) | 36 ± 10 | 41 ± 12 | 0.001 | 0.012 |
| RWT (cm) | 0.36 ± 0.06 | 0.39 ± 0.10 | 0.018 | 0.056 |
| LV systolic function (%) | ||||
| LVEF | 58 ± 6 | 59 ± 8 | 0.44 | 0.18 |
| Cardiac output (L/min) | 4193 ± 1069 | 4601 ± 1327 | 0.020 | 0.022 |
| MWFS | 17.6 ± 2.0 | 16.8 ± 2.2 | 0.008 | 0.009 |
| GLS | −14.4 ± 3.8 | −15.1 ± 3.2 | 0.18 | 0.33 |
| LV diastolic function | ||||
| E/E′ | 14.2 ± 7.3 | 14.9 ± 6.1 | 0.49 | 0.25 |
| LAV (mL) | 62 ± 24 | 70 ± 28 | 0.031 | 0.09 |
| NT-proBNP (pg/mL) | 744 (321, 1174) | 967 (601, 1834) | <0.001 | 0.006 |
| Measures of arterial–LV coupling | ||||
| Ea (mmHg/mL) | 2.05 ± 0.49 | 1.94 ± 0.55 | 0.14 | 0.15b |
| Ees (mmHg/mL) | 2.53 ± 0.99 | 2.49 ± 0.96 | 0.75 | 0.15b |
| Ea/Ees | 0.94 ± 0.61 | 0.88 ± 0.39 | 0.45 | 0.93b |
Data are mean ± SD, median (IQR).
LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; SWT, septal wall thickness; PWT, posterior wall thickness; LVM, left ventricular mass; LVMi, left ventricular mass indexed to height;2.7 RWT, relative wall thickness; LVEF, left ventricular ejection fraction; MWFS, midwall fractional shortening; GLS, global longitudinal strain; E/E′, mitral inflow to mitral relaxation velocity ratio; LAV, left atrial volume; Ea, effective arterial elastance; Ees, left ventricular end-systolic elastance; Ea/Ees, ventricular–vascular coupling.
aAdjusted for age, sex, PP, previous admission for HF, history of diabetes, anaemia.
bAdjusted for age, sex, heart rate, previous admission for HF, history of diabetes, anaemia.
Figure 1.
Prevalence of abnormal left ventricular geometry according to binary categories of kidney function (presence vs. absence of renal dysfunction based on both estimated glomerular filtration rate and urinary albumin-to-creatinine ratio values) (A), or to four categories of renal function (urinary albumin-to-creatinine ratio normal and estimated glomerular filtration rate preserved, either presence of albuminuria alone or low-estimated glomerular filtration rate alone, both impaired) (B). Abnormal left ventricular geometry defined as either concentric remodelling, or concentric hypertrophy, or eccentric hypertrophy. P-values from multiple logistic regression, using abnormal left ventricular geometry as outcome, adjusting for age, sex, history of diabetes, anaemia, previous admission for heart failure, pulse pressure.
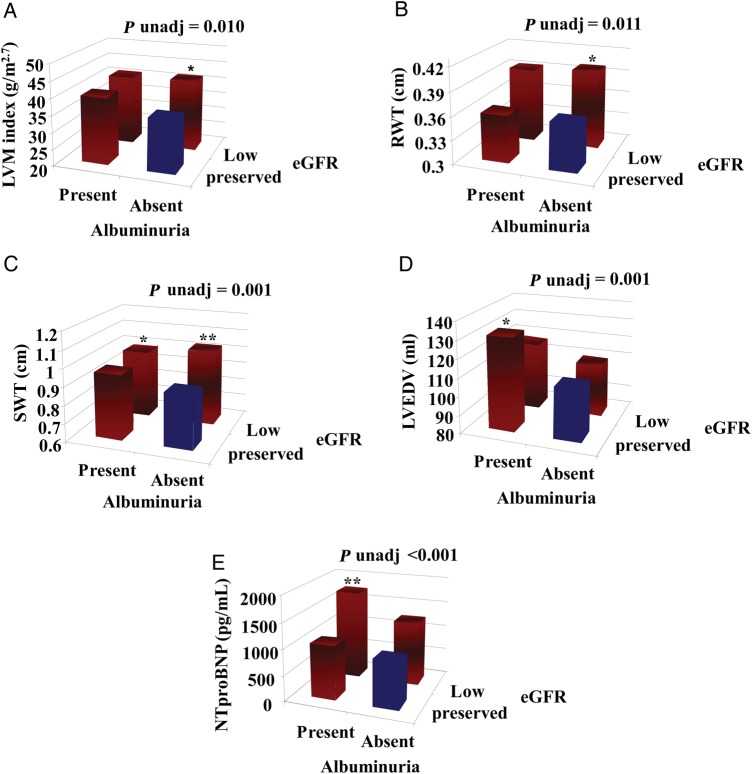
Considering four categories of renal function based on eGFR and UACR values, patients with reduced eGFR (>30 and <60 mL/min/1.73 m2) and no albuminuria had significantly greater LV wall thicknesses, LVM, and a higher prevalence of abnormal LV geometry compared with patients without renal dysfunction (Tables 3 and 4, Figures 1, 2, and 3). Furthermore, patients with reduced eGFR alone had lower MWFS. In addition to the significant association between low eGFR and MWFS, we also found that low eGFR was significantly associated with increased RWT, which was subsequently associated with reduced MWFS when added to the fully adjusted model (P < 0.001). Our data suggest the possibility that approximately half the effect of low eGFR on MWFS is mediated through the effect of low eGFR on RWT, while the other half occurs independently of RWT (beta = −1.22 without adjustment for RWT; beta = −0.52 after adjustment). Analogous results were observed according to the presence vs. absence of renal dysfunction.
Table 4.
Unadjusted and adjusted β-coefficients for parameters of cardiovascular structure and function significantly different at univariable analyses by estimated glomerular filtration rate and urinary albumin-to-creatinine ratio combined categories
| Normal UACR/preserved eGFR | High UACR /preserved eGFR | Normal UACR/low eGFR | High UACR/low eGFR | ||
|---|---|---|---|---|---|
| LVEDV (mL) | Reference | Unadjusted β-coefficient | 22.9** | 0.5 | 8.9 |
| Adjusted β-coefficient | 13.9* | 3.4 | 1.0 | ||
| LVESV (mL) | Reference | Unadjusted β-coefficient | 11.8** | −1.3 | 2.6 |
| Adjusted β-coefficient | 6.3 | 0.3 | −2.7 | ||
| SWT (cm) | Reference | Unadjusted β-coefficient | 0.07 | 0.13** | 0.09** |
| Adjusted β-coefficient | 0.06 | 0.13** | 0.08* | ||
| PWT (cm) | Reference | Unadjusted β-coefficient | 0.05 | 0.08** | 0.09** |
| Adjusted β-coefficient | 0.03 | 0.08* | 0.06* | ||
| LVM (g) | Reference | Unadjusted β-coefficient | 27** | 21** | 25** |
| Adjusted β-coefficient | 16 | 22** | 15 | ||
| LVMi (g/m2.7) | Reference | Unadjusted β-coefficient | 4.2** | 5.8** | 5.3** |
| Adjusted β-coefficient | 3.6 | 4.5* | 4.3 | ||
| RWT (cm) | Reference | Unadjusted β-coefficient | 0.000 | 0.041** | 0.035* |
| Adjusted β-coefficient | −0.001 | 0.036* | 0.033 | ||
| Cardiac output (L/min) | Reference | Unadjusted β-coefficient | 914** | 105 | 351 |
| Adjusted β-coefficient | 712** | 322 | 276 | ||
| MWFS (%) | Reference | Unadjusted β-coefficient | −0.25 | −1.20** | −0.82* |
| Adjusted β-coefficient | −0.19 | −1.22** | −0.98* | ||
| NT-proBNP (pg/mL) | Reference | Unadjusted β-coefficient | 148 | 341 | 840** |
| Adjusted β-coefficient | 48 | 287 | 741** |
LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; SWT, septal wall thickness; PWT, posterior wall thickness; LVM, left ventricular mass; LVMi, left ventricular mass indexed to height2.7; RWT, relative wall thickness; MWFS, midwall fractional shortening.
Adjusted for age, sex, PP, previous admission for HF, history of diabetes, anaemia.
*P < 0.05 for comparison vs. reference (both eGFR and UACR normal).
**P < 0.01 for comparison vs. reference (both eGFR and UACR normal).
Figure 2.
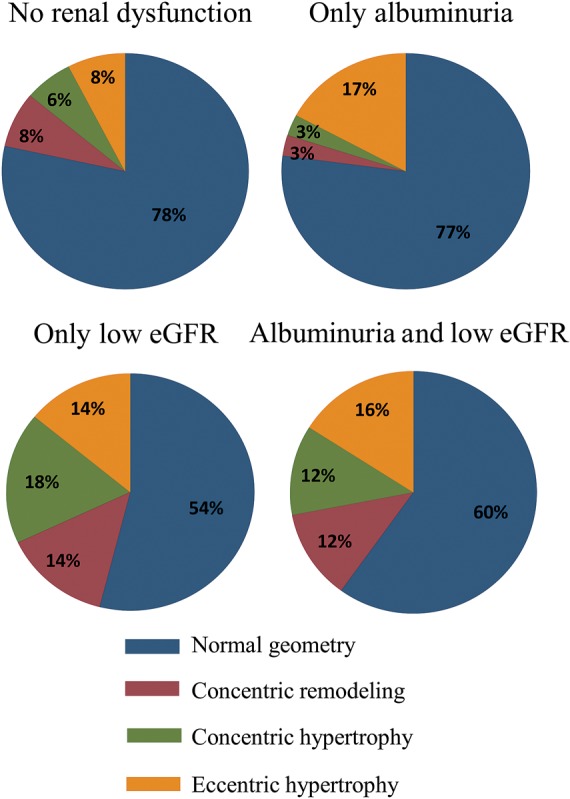
Prevalence of different types of abnormal left ventricular geometry according to the four categories of renal function.
Figure 3.
Cardiovascular structure and function according to estimated glomerular filtration rate and urinary albumin-to-creatinine ratio combined categories. Low-estimated glomerular filtration rate is between 30 and 60 mL/min/1.73 m2, preserved estimated glomerular filtration rate >60 mL/min/1.73 m2; albuminuria is defined using gender-specific cut-off values for microalbuminuria (17 mg/g for men and 25 mg/g for women). (A) Differences in left ventricular mass indexed to height2.7 . (B) Differences in relative wall thickness. (C) Differences in septal wall thickness. (D) Differences in left ventricular end-diastolic volume. (E) Differences in NT-proBNP. *Adjusted P < 0.05, **Adjusted P < 0.01 from multiple linear regression (adjusting for age, sex, pulse pressure, previous admission for heart failure, history of diabetes, anaemia).
Conversely, albuminuria with preserved eGFR was associated with larger ventricular size (higher LVEDV and LVESV), whereas LV mass, LV geometry, E/E′, and parameters of LV systolic function did not differ based on the UACR level in adjusted analysis (Table 4 and Figures 1–3). In univariable and multivariable analyses, patients with low eGFR and albuminuria had greater LV wall thicknesses, higher NT-proBNP, and lower MWFS compared with subjects with no renal dysfunction (Tables 3 and 4, Figure 3). The higher prevalence of abnormal LV geometry in patients with high UACR and low eGFR in univariable analysis did not reach statistical significance when accounting for covariates (Figure 1). Considering the different types of LV geometry according to the four categories of renal function (Figure 2), the presence of EH was twice as common in the albuminuria-only patients relative to the no renal dysfunction patients. Additionally, LV geometry patterns in patients with low eGFR alone vs. patients with combined renal impairment were quite similar. However, we were not powered to detect these types of interactions with the current sample size.
Across the four categories of kidney function there were no differences in filling pressures, LVEF, deformation parameters, and ventricular-vascular systolic stiffness indexes (Table 3). Cardiac structure/function results were comparable using the sex-independent cut-off for abnormal UACR (30 mg/g) (same level of significance displayed in Tables 2 and 3 for all the parameters studied).
Discussion
Among HFpEF patients enrolled in the PARAMOUNT trial, we found that renal dysfunction, defined as low eGFR and/or high UACR, was highly prevalent, and associated with cardiac remodelling and subtle systolic dysfunction. Compared with patients with preserved renal function, those with low eGFR and no albuminuria had a higher prevalence of abnormal LV geometry (either CH, or CR, or EH) and lower MWFS, as opposed to patients with albuminuria and preserved eGFR. Conversely, albuminuria alone was characterized by greater LV dimensions. The combination of low eGFR and albuminuria was associated with the presence of mixed structural and functional abnormalities, as shown by higher LV wall thickness, NT-proBNP, and lower MWFS. Our findings suggest that both eGFR and albuminuria might play a distinct role in HFpEF.
Based upon sex-specific cut-off value for UACR and CKD-EPI equation calculation formula for eGFR, this analysis allowed us to estimate the relevance of renal dysfunction, assessed by both domains, in HFpEF: similarly to a recent Japanese study3 our data demonstrated in a predominantly Caucasian population a high prevalence of renal dysfunction (∼60%) using both UACR and eGFR compared with previous studies using only eGFR (30–45%),1,2,23 further acknowledging the importance of this comorbidity in HFpEF. Of note, according to the PARAMOUNT entry criteria, patients with eGFR <30 mL/min/1.73 m2 were not included into the trial; likely, the prevalence of renal dysfunction in HFpEF may even be higher, taking into account also patients with severely reduced eGFR.
Additionally, to our knowledge for the first time, we were able to explore the presence of possible different associations between high UACR alone or low eGFR alone with abnormal cardiac structure and function in HFpEF, adjusting our models for the same covariates. PARAMOUNT data are concordant with previous studies in subjects with and without HF highlighting the bidirectional link between low eGFR and LV remodelling.10,13,14,24,25 Furthermore, compared with patients with preserved renal function those with reduced eGFR were further characterized by lower MWFS, despite similar LVEF and deformation parameters. These results suggest that MWFS may be an index of LV systolic function more reliable than ejection fraction, as previously demonstrated in case of abnormal LV geometry.26,27 Of note, MWFS might be less sensitive to noise than deformation analysis, in particular for images acquired at the FPS range normally encountered in clinical practice. Therefore, MWFS might be particularly useful for the detection of subtle systolic dysfunction in the setting of HFpEF.28
Conversely, while prior data in populations without HFpEF demonstrated an association between UACR and LVH,11,12,29,30 we showed in HFpEF patients that high UACR alone was associated with larger LV volumes, despite similar NT-proBNP31 and degree of abnormal LV geometry (either CR, CH, or EH) compared with patients without renal dysfunction. The different LV structure findings compared with prior studies may be partly due to the populations studied, namely patients with and without HFpEF, to the categorization of renal function applied in our study based on both eGFR and UACR values, and to the inclusion criteria used in PARAMOUNT. However, we have hypothesized a possible association between these structural abnormalities and albuminuria induced endothelial dysfunction, microvascular dysfunction, and ischaemia, in agreement with the newly proposed pathogenetic role for endothelial microvascular dysfunction for HFpEF development and with the already recognized many possible contributory mechanisms to HFpEF, beyond LVH.32,33 Eventually, also hyper activation of the renin–angiotensin system may be involved, with greater production of aldosterone and volume overload. The higher cardiac output encountered in albuminuria alone patients compared with the ones without renal dysfunction may support this theory, while previous studies gave contradictory results.31,34,35
Finally, Ea did not vary according to different categories of kidney function. These data are discordant with some previous studies,11,36 but are consistent with findings reported in a community-based study of HFpEF patients.15 Since Ea does not take into account factors that can contribute to increased load, such as pulse wave velocity and early reflected wave, it is possible that the use of parameters more reflective of central haemodynamics, such as aortic characteristic impedance (Zc), may lead to different results.37
Of note, recent evidence suggests that renal dysfunction might in part cause HFpEF.38 Although the present study does not provide causal evidence, PARAMOUNT data support the use of both eGFR and proteinuria to adequately evaluate kidney function and cardiac structure and function in HF patients.4,39 Furthermore, these data may potentially help to identify subgroups of HFpEF patients to target future clinical trials. Other possible future directions of research in this field may include the addiction of MRI results and/or of novel marker of renal damage, such as neutrophil gelatinase-associated lipocalin.34 Finally, these data suggest that interactions between the heart and kidney are complex, still incompletely understood, and merit prospective studies to gain more mechanistic insights.
Limitations of this analysis should be noted. First, the cross-sectional nature of the data precludes conclusions about causality. Second, our results may not be generalizable to HFpEF populations in the community, given the inclusion and exclusion criteria of the PARAMOUNT trial, thus requiring systematic and prospective evaluation. In particular, our results are mainly applicable to Caucasian race (∼75% of our study sample) and only to patients without severe impairment of eGFR, because these subjects were excluded from enrolment. Third, small sample sizes may have contributed to limited power to detect significant differences in our analyses and may limit the generalization of our findings. Furthermore, it would have been of interest to assess and control for inflammation in the study population, given the association of inflammation with both HFpEF and renal damage. Unfortunately, this was not feasible since there were no available data on inflammation parameters such as C-reactive protein. Finally, it was not possible to calculate the stress corrected MWFS, a more robust parameter of systolic function compared with MWFS, because the end-systolic posterior wall thickness was not assessed in the PARAMOUNT database.
In conclusion, renal dysfunction, as determined by both eGFR and UACR, is highly prevalent in HFpEF, and associated with cardiac remodelling and subtle systolic dysfunction. The observed differences in cardiac structure and function between each type of renal damage suggest that both parameters of kidney function might play a distinct role in HFpEF, although prospective validation is warranted.
Funding
This study was funded by Novartis Pharmaceuticals, East Hanover, NJ, USA.
Conflict of interest: M.R.Z., B.P., A.A.V., J.J.M., M.P., A.M.S., and S.D.S. have received research support and have consulted for Novartis. T.B. and M.L. are employees of Novartis. M.G., M.S., D.K.G., D.M.C., E.K.K., B.C., and A.B.S.S. declare that they have no conflict of interest.
References
- 1.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE I-PRESERVE Investigators. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 3.Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, Shimokawa H CHART-2 Investigators. Urinary albumin excretion in heart failure with preserved ejection fraction: an interim analysis of the CHART 2 study. Eur J Heart Fail. 2012;14:367–376. doi: 10.1093/eurjhf/hfs001. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, Lin J, Solomon CG, Jablonski KA, Rice MM, Steffes M, Domanski M, Hsia J, Gersh BJ, Arnold JM, Rouleau J, Braunwald E, Pfeffer MA. Prevention of Events With ACE Inhibition (PEACE) Investigators. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation. 2007;116:2687–2693. [Google Scholar]
- 6.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs. younger individuals: The HUNT II Study. Arch Intern Med. 2007;167:2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 7.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 8.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 9.Ix JH, Shlipak MG, Chertow GM, Ali S, Schiller NB, Whooley MA. Cystatin C, left ventricular hypertrophy, and diastolic dysfunction: data from the Heart and Soul Study. J Card Fail. 2006;12:601–607. doi: 10.1016/j.cardfail.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG Chronic Renal Insufficiency Cohort (CRIC) Study Group. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23:1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AM, Lam CS, Cheng S, Verma A, Desai AS, Rocha RA, Hilkert R, Izzo J, Oparil S, Pitt B, Thomas JD, Zile MR, Aurigemma GP, Solomon SD. The relationship between renal impairment and left ventricular structure, function, and ventricular-arterial interaction in hypertension. J Hypertens. 2011;29:1829–1836. doi: 10.1097/HJH.0b013e32834a4d38. [DOI] [PubMed] [Google Scholar]
- 12.Lieb W, Mayer B, Stritzke J, Doering A, Hense HW, Loewel H, Erdmann J, Schunkert H. Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: the MONICA/KORA Augsburg Echocardiographic Substudy. Nephrol Dial Transplant. 2006;21:2780–2787. doi: 10.1093/ndt/gfl364. [DOI] [PubMed] [Google Scholar]
- 13.Verma A, Anavekar NS, Meris A, Thune JJ, Arnold JM, Ghali JK, Velazquez EJ, McMurray JJ, Pfeffer MA, Solomon SD. The relationship between renal function and cardiac structure, function, and prognosis after myocardial infarction: the VALIANT Echo Study. J Am Coll Cardiol. 2007;50:1238–1245. doi: 10.1016/j.jacc.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Afshinnia F, Spitalewitz S, Chou SY, Gunsburg DZ, Chadow HL. Left ventricular geometry and renal function in hypertensive patients with diastolic heart failure. Am J Kidney Dis. 2007;49:227–236. doi: 10.1053/j.ajkd.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5:710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leoncini G, Viazzi F, Conti N, Baratto E, Tomolillo C, Bezante GP, Deferrari G, Pontremoli R. Renal and cardiac abnormalities in primary hypertension. J Hypertens. 2009;27:1064–1073. doi: 10.1097/HJH.0b013e3283281213. [DOI] [PubMed] [Google Scholar]
- 17.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 20.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Metra M, Cotter G, Gheorghiade M, Dei Cas L, Voors AA. The role of the kidney in heart failure. Eur Heart J. 2012;33:2135–2142. doi: 10.1093/eurheartj/ehs205. review). [DOI] [PubMed] [Google Scholar]
- 24.Pateinakis P, Papagianni A. Cardiorenal syndrome type 4-cardiovascular disease in patients with chronic kidney disease: epidemiology, pathogenesis, and management. Int J Nephrol. 2011;2011:938651. doi: 10.4061/2011/938651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 26.de Simone G, Devereux RB. Rationale of echocardiographic assessment of left ventricular wall stress and midwall mechanics in hypertensive heart disease. Eur J Echocardiogr. 2002;3:192–198. doi: 10.1053/euje.2002.0163. review) [DOI] [PubMed] [Google Scholar]
- 27.De Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MH, Laragh JH. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23:1444–1451. doi: 10.1016/0735-1097(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer H, Jacobs DR, Jr, Bild D, Post W, Saad MF, Detrano R, Tracy R, Cooper R, Liu K. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 30.Wachtell K, Palmieri V, Olsen MH, Bella JN, Aalto T, Dahlöf B, Gerdts E, Wright JT, Jr, Papademetriou V, Mogensen CE, Borch-Johnsen K, Ibsen H, Devereux RB. Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Losartan Intervention for Endpoint Reduction. Am Heart J. 2002;143:319–326. doi: 10.1067/mhj.2002.119895. [DOI] [PubMed] [Google Scholar]
- 31.van de Wal RM, Asselbergs FW, Plokker HW, Smilde TD, Lok D, van Veldhuisen DJ, van Gilst WH, Voors AA. High prevalence of microalbuminuria in chronic heart failure patients. J Card Fail. 2005;11:602–606. doi: 10.1016/j.cardfail.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Paulus WJ, Tschöpe C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 33.Shah AM, Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2012;9:555–556. doi: 10.1038/nrcardio.2012.123. [DOI] [PubMed] [Google Scholar]
- 34.Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol. 2008;295:F1589–F1600. doi: 10.1152/ajprenal.00142.2008. [DOI] [PubMed] [Google Scholar]
- 35.Danziger J. Importance of low-grade albuminuria. Mayo Clin Proc. 2008;83:806–812. doi: 10.4065/83.7.806. [DOI] [PubMed] [Google Scholar]
- 36.Edwards NC, Ferro CJ, Townend JN, Steeds RP. Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: a pattern resembling heart failure with preserved ejection fraction. Heart. 2008;94:1038–1043. doi: 10.1136/hrt.2007.137539. [DOI] [PubMed] [Google Scholar]
- 37.Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009;15:658–664. doi: 10.1016/j.cardfail.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–1431. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 39.Blecker S, Matsushita K, Köttgen A, Loehr LR, Bertoni AG, Boulware LE, Coresh J. High-normal albuminuria and risk of heart failure in the community. Am J Kidney Dis. 2011;58:47–55. doi: 10.1053/j.ajkd.2011.02.391. [DOI] [PMC free article] [PubMed] [Google Scholar]