Abstract
Studies into the complex interaction between an organism and changes to its biotic and abiotic environment are fundamental to understanding what regulates biodiversity. These investigations occur at many phylogenetic, temporal and spatial scales and within a variety of biological and geological disciplines but often in relative isolation. This issue focuses on what can be achieved when ecological mechanisms are integrated into analyses of deep-time biodiversity patterns through the union of fossil and extant data and methods. We expand upon this perspective to argue that, given its direct relevance to the current biodiversity crisis, greater integration is needed across biodiversity research. We focus on the need to understand scaling effects, how lower-level ecological and evolutionary processes scale up and vice versa, and the importance of incorporating functional biology. Placing function at the core of biodiversity research is fundamental, as it establishes how an organism interacts with its abiotic and biotic environment and it is functional diversity that ultimately determines important ecosystem processes. To achieve full integration, concerted and ongoing efforts are needed to build a united and interactive community of biodiversity researchers, with education and interdisciplinary training at its heart.
Keywords: biodiversity, functional biology, scale-dependency, ecomorphology, macroevolution
1. Introduction
Biodiversity, defined broadly as the number and variety of organisms, is a complex property that emerges at many spatial, temporal and phylogenetic scales [1]. The drivers of diversification are therefore studied by researchers in a variety of biological and geological fields. The determinants of biodiversity are often split into biotic and abiotic factors. Abiotic factors include geochemical cycles, geological processes (e.g. volcanic eruptions, bolide impacts, continental drift, orogenesis, glaciations), sea-level changes, climate and weather, most of which are intricately linked. Similar cross-dependencies are also found within the set of biotic factors, which comprise organismal ecology and physiology, biotic interactions (e.g. predation, competition, mutualism) and community structure. Resource availability can be regarded as a composite trait driven by a combination of biotic and abiotic factors. The relative importance of biotic and abiotic factors in determining biodiversity has been disputed (e.g. [2–5]) but this division is almost certainly unproductive [6]. It is how each of these biotic and abiotic factors jointly interact to influence the rates of speciation/birth, extinction/death, phenotypic change and immigration/dispersal that ultimately drives diversification (figure 1). Understanding how taxa respond ecologically and evolutionarily to changes in their biotic and abiotic environment can play an important role in conservation (e.g. [7,8]). Thus a holistic understanding of the regulators of biodiversity across biological scales is crucial.
Figure 1.
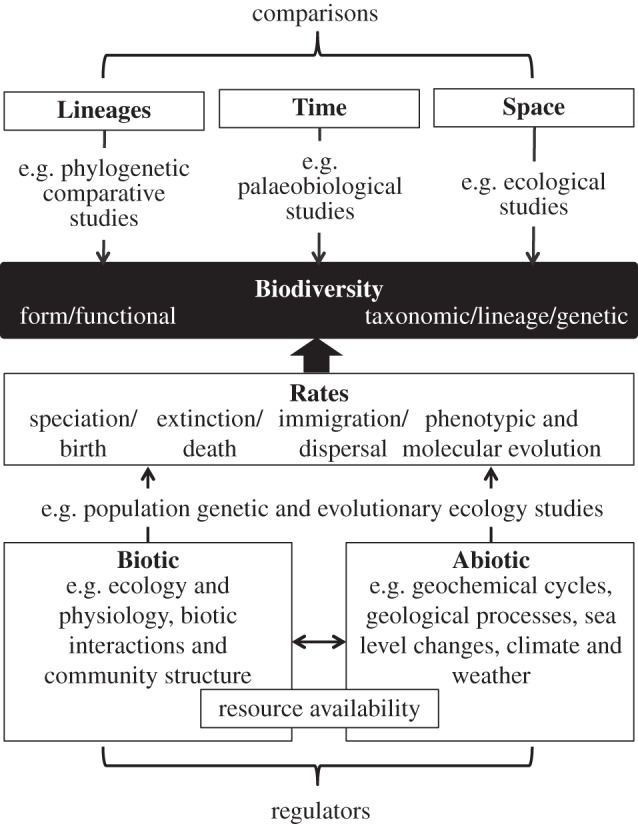
Integrative biodiversity. Biodiversity is a complex property that is regulated by a variety of biotic and abiotic factors. Such factors, e.g. ecology and physiology, biotic interactions, community structure (all biotic) but also geochemical cycles and processes, sea-level changes, climate and weather (all abiotic), control the processes that drive evolutionary rates and ultimately determine the evolution of biodiversity. The regulators of biodiversity can be studied by comparing diversity among lineages on a phylogeny, time periods, or areas across a variety of temporal, spatial and phylogenetic scales. We argue that a holistic approach is necessary, combining all of these aspects along with a renewed emphasis on functional biology and scaling effects.
In this article, we focus on the promising future for integrative biodiversity research. We start with a brief and somewhat biased history of the recent growth of synthetic biodiversity research as, similar to understanding the drivers of biodiversity, we first have to understand the past to forecast the future. In the context of biodiversity, understanding the past and present regulators will ultimately result in much refined predictions of future patterns, which is critical in the light of the current biodiversity crisis and its potential societal consequences [7,9–11]. We argue that, to improve synthesis and to increase the relevance of studies of regulators of biodiversity in deep time to conservation, we need (i) an increased focus on form and function as well as (ii) a better understanding of the dependency of the results on scale. A necessary requirement to achieve these changes is (iii) improved interdisciplinary integration, through concerted efforts to build a united and interactive community of biodiversity researchers.
We begin with a brief and biased history of integrative biodiversity research. For much of the twentieth and twenty-first centuries, distinct sub-disciplines of biology and geology have investigated the determinants of different aspects of biodiversity in relative isolation (figure 1). Palaeobiologists were arguably the first to quantitatively study the drivers of biodiversity (reviewed by Signor [12]) and continue to do so by estimating changes in taxon diversity and phenotypic disparity between distinct stratigraphic time-bins. Over the past 30 years, phylogenetic comparative biologists have also begun to address the determinants of large-scale diversity patterns, primarily by comparing the rates of speciation and phenotypic evolution between phylogenetic lineages (e.g. [13,14]). Although some phylogenetic macroevolutionary studies comprise reconstructions of the changes in diversity over time as provided by time-calibrated phylogenies (e.g. [15,16]), temporal inferences, especially in deep time, are limited without inclusion of fossil data. In contrast, ecologists have mainly focused on the regulators of spatial patterns of biodiversity. Macroecologists and biogeographers mainly compare species richness or functional diversity between regions at local to global scales [17], while community ecologists have concentrated on the drivers of differences in species diversity between communities, as measured by richness and the evenness of abundance (e.g. [18,19]). Finally, evolutionary ecologists and (population) geneticists have focused on the ecological and genetic mechanisms that lead to differentiation over short time periods (e.g. [20,21]). Even though we have gained tremendous insights into how lineages respond ecologically or evolutionarily to a change in their biotic and abiotic environment from such studies, the focus on a particular timescale or aspect of biodiversity has limited what can potentially be learnt about the complex interactions that regulate biodiversity.
The realization that a synthesis of disciplines, data and methods is required to capture the true mechanisms underlying biodiversity patterns is not new. For example, Doyle & Donoghue [22] demonstrated that fossil and extant data are both important for macroevolutionary studies at a time when arguments were being made that fossil data were now obsolete thanks to molecular phylogenetics. However, recent years have seen a surge in calls for integration, usually between pairs of sub-disciplines. There are a growing number of papers expounding the virtues of synthesizing fossil and phylogenetic perspectives on macroevolution (e.g. [10,23–28]) as well as ecology into macroevolutionary analyses [29]. Already much progress has been made on the integration of fossil data into phylogenetic analyses to provide a unified time-frame for diversification (e.g. [30,31]) and joint extinct and extant analyses of diversification and trait evolution are growing (e.g. [32,33]). The past decade or so has also witnessed the merging of two fields to develop the new discipline of phylogenetic community ecology [34–36] along with the emergence of conservation palaeobiology [37], the rise of palaeobiogeography ([38] and see [39]) and the recognition of the importance of biodiversity time series [9] and palaeo(macro)ecology. This issue builds upon these efforts, highlighting the importance of integrating the dynamic nature of environmental, biological and geological processes through time and thus necessitating the synthesis of many of these individual fields.
To realize perfect integration requires a complete record of the biotic and abiotic environment over time and space, throughout the Earth's history, and a global to local context is also required. However, reaching such idealistic levels of integration is hindered by the inherent complexity of combining large and very disparate datasets as well as the extreme difficulty of finding study systems where all the necessary data are available. Some clades such as mammals have well-understood phylogenies and quantifiable functional traits (e.g. [40]), yet their fossil record is not as detailed and rich as that of molluscs (e.g. [41]) or foraminiferans (e.g. [42]). Currently, some systems may be best suited to fine-scale analyses over short periods of time (e.g. [43]), whereas other systems enable the analysis of broad-scale patterns (e.g. [44]), but ideally they should be accessible by both palaeontologists and neontologists. However, the bottom line is that integrative approaches can provide insights that are unavailable to studies limited to the traditional discipline-specific approach, especially concerning the mechanisms that drive diversification and determine extinction risk. These approaches may combine data from different disciplines or may place data in a novel context by analysing them using approaches from other disciplines, but all transcend traditional disciplinary boundaries and sometimes scales to provide a fresh perspective on the regulators of biodiversity.
Much has already been gained from recent efforts that have achieved a partial integration of a subset of factors that affect the evolution of diversity. Box 1 provides a snapshot of current examples of integrative biodiversity research. We focus on case studies that are centred on palaeontological data, illustrating the potential power of the inclusion of the fossil record. The quality of the fossil record varies phylogenetically (some clades have higher fossilization potential), spatially (some environments have higher fossilization potential), as well as temporally (younger deposits tend to have a more complete record) [54], but carefully performed palaeontological research produces valuable historical datasets [1,11]. It is therefore unsurprising that combinations of palaeobiological and phylogenetic comparative data demonstrate that integration improves our ability to discern among processes that govern diversification (e.g. [42]). Similarly, the application of principles taken from biogeography and community ecology to fossil datasets helps us understand biotic interactions in the past and enables understanding of the evolution of current biodiversity over geological timescales (e.g. [41,44,49]).
Box 1. Examples of integrative biodiversity research.
The past decade has witnessed an increased motivation to study biodiversity in an integrative context. Examples could be drawn across different fields, but we want to highlight papers that focused on the importance of fossil data and the concept of deep time in understanding the regulators of biodiversity. These papers fall in a range of different topics and are briefly summarized below.
Fossils, phylogenies and diversification. Cenozoic macroperforate foraminifera are remarkable and may be one of the best study systems available at this time [45], with outstanding fossil record and reasonable understanding of their molecular diversity, allowing for an phylogenetically informed analysis. Importantly, several morphological features known to correlate with life history and ecology in extant groups (i.e. ecomorphological traits) are well preserved in the fossil record and thus allow for assessing the influence of ecological features on the rates of evolution. With this exceptional dataset, Ezard et al. [42] were able to demonstrate that the interplay between species' ecology and environmental change drove differential speciation and extinction over macoevolutionary timescales.
Fossils, climate and the latitudinal diversity gradient. In this issue, Fenton et al. [46] extend the work on the foraminifera system by considering the spatial distributions of taxa through time by joining data and techniques from palaeobiology, phylogenetic comparative biology and macroecology to study the latitudinal diversity gradient, the phenomenon of decreasing species richness with latitude. This study reveals that the modern foraminiferan diversity gradient was established by the end of the Eocene, likely as a result of a global cooling trend. In a broader context, this work and other studies (e.g. [41,44]) convincingly demonstrate the utility of testing environmental models developed for modern communities within fossil assemblages that experienced very different climatic parameters far beyond current boundaries. Studying the dynamic responses to such dramatically different climatic conditions should prove useful for better predictions of future ecosystem development.
Biotic interactions and diversification in the fossil record. Other recent integrative analyses include tackling the difficult question of identifying biotic interactions over deep time (e.g. [47,48]) and the ongoing synthesis of community ecology with palaeobiology [49]. The latter approach is potentially valuable for conservation applications as it focuses attention on species and their specific roles in their palaeocommunity, assessing their role in ecosystem functioning rather than the number of taxa. Reconstructions of palaeocommunities and food webs have a long tradition in palaeontology, and especially over recent years have taken a prominent role in determining extinction risks in biodiversity crises in earth history (e.g. [50–52]). Preserved ecologically relevant morphological traits make it possible to assign palaeofaunas into major trophic guilds and characterize the overall composition and structure of palaeocommunities [53]. If stratigraphic resolution and fossil record allow, one can reconstruct feasible food webs over geological time. A prime example is the analysis of the Permian–Triassic mass extinction event, suggesting that the stability of ecosystems largely depended on functional diversity and trophic interactions and not species richness per se [49]. This finding highlights the importance of identifying functional traits that help in interpreting biotic interactions.
Diversification at small spatial and temporal scales. While most recent examples of integrative fossil studies provide a global perspective of biodiversity over prolonged phases of geological time (10–100s of million years), this approach still has much to offer at smaller spatial and temporal scales. For example, Lake Malawi features a young radiation of gastropods [43], with four extant species that originated during the Holocene and display considerable molecular divergence. The fossil record of this species complex reveals that morphological stasis characterized a substantial part of the history of these diverging lineages, a surprising result that would likely have been overlooked if it were not for the analysis of palaeontological time series. Moreover, these young fossil records are particularly relevant to conservation [11].
The differences in spatial and temporal scale across disciplines are worth examining in more detail. As discussed by Ezard et al. [55], the impression that biotic drivers dominate over short timescales and abiotic over longer times [4] may have been driven by scaling issues. The identification of biological interactions and co-occurrence at the scale of the fossil record is difficult but not impossible to study [6]. On the other hand, the impact of abiotic factors may be obscured by lagged responses in the geologic record [56] or concealed by biotic interactions within ecological studies focused on a single time point [57]. Recent evidence supports the suspicion that biotic and abiotic factors working at different temporal scales may be a ‘false dichotomy’. Over macroevolutionary timescales, competition explains the speciation and extinction dynamics of ecologically similar marine brachiopods and clams better than any of the measured palaeoenvironmental proxies [58]. New developments in phylogenetic comparative methods similarly allow competition models to be tested among lineages [59,60]. However, at this macroscale, it is difficult to truly ascertain if the lineages did interact and enforce a selective impact upon one another. For example, did competition for meat between canid clades generate diversity-dependence [47]? An increased focus on functional biology can help to establish niche similarity between taxa but further investigation is needed into scaling-up the concept of competition, which occurs between individuals, to evolutionary interactions between species [3] and higher taxa.
While there is still much to do to achieve full integration, it is becoming increasingly clear that the mechanisms underlying biodiversity patterns can only be recovered by combining multiple perspectives, be it at small or large temporal, spatial or phylogenetic scales. A central theme that emerges from our examples aside from scaling issues is the importance of studying the functional characteristics of species. We further develop these ideas in the next two sections. The first (i) proposes that trait-based approaches, guided by the principles of ecomorphology and functional biology, are key to the synthesis of biodiversity. Function provides a unifying theme common to all sub-disciplines that aim for an improved understanding of biodiversity regulators and is critical for conservation [61]. The second (ii) highlights the concern that biodiversity patterns and processes may be scale-dependent and suggests that greater efforts are necessary to elucidate the extent of this issue empirically and experimentally.
2. The future of integrative biodiversity research
(a). Integration of form and function
Functional biology, which we broadly define as any discipline connecting phenotype, its function and, importantly, its biological role, is important for a better understanding of the evolution of biodiversity in near and deep time. As functional biology establishes linkages between diversity, form, function, performance, fitness and major niche dimensions [62,63], it aids the identification of key determinants of organismal survival. In a general sense, a focus on the analysis of functional traits allows the assessment of structural, physiological, biochemical and behavioural capabilities of an organism to interact with its abiotic and biotic environment and thus predict how they may respond to changes [64]. The need to put function at the core of biodiversity research stems from the fact that it is functional diversity, not species, phylogenetic or morphological diversity, that determines important ecosystem processes [65,66]. It is therefore particularly important to understand how biotic and abiotic factors regulate functional diversity because it has conservation implications, including but not limited to: ecosystem stability [67], invasion tolerance [68] and ecosystem services [69].
When preparing macroevolutionary analyses, it is important to quantify traits with well-understood functional and ecological implications. Functional traits include ecological, morphological and physiological measurements, for example metabolic rate, burrowing behaviour, leaf nitrogen content, bark thickness, leaf size or dental and other feeding structures [61], as well as performance metrics such as maximum sustained swimming speed. Yet, many features used in biodiversity analyses may have no importance regarding organismal fitness, have at best a vague functional relevance, or are simply very hard to understand from a functional perspective. To make the identification of functional traits even harder, it is often the combination of many underlying traits that determines the exact properties of the organism, requiring detailed and careful analyses of sometimes very complex systems. The concept of many-to-one mapping of form to function [70] is important to consider in this context. It has been demonstrated that a large diversity of morphological structures can produce the same performance [71], i.e. it is often impossible to identify a single trait as a performance proxy. A good example for many-to-one mapping is the role of cranial musculoskeletal anatomy in suction feeding in aquatic vertebrates [72]. The suction forces acting on the prey in front of the predator's mouth are the biologically most relevant performance, and these can be modulated by many underlying components such as size and shape of the buccal cavity and mouth, the mechanical lever system involved in suction generation, and the size and speed of muscles that operate the levers [73].
Functional biology takes a central role in assessing how complex trait systems work, as its principles are firmly grounded in physical models of organismal form [74]. Functionally relevant traits may include laboratory or field-based measures of performance (e.g. acceleration capacity [75]) as well as continuously valued measures of anatomical structures such as linear, area and volumetric dimensions, or ratios of these (e.g. [72]). They may also represent discretely scored traits or result from an ordination of continuous and discrete traits (e.g. [76]). Regardless of the type of measurement or variable, it is however crucial to demonstrate its correlation with function, performance and ecology of the organism, which can be challenging, especially when using ordination techniques or geometric morphometrics as they often represent hard to interpret combinations of various traits. Prior to performing macroevolutionary trait analyses, one should understand how a structure functions, how well it performs and what its biological relevance is, otherwise the mechanistic underpinnings of resulting patterns are likely to remain blurry.
As becomes clear from the above paragraphs, functional biology is fundamentally important for linking organismal form and fitness. Hence, an increased appreciation of functional traits is central to the discussion of biodiversity, as it provides the opportunity to mechanistically link the ability of organisms to cope with challenges imposed by the environment and influence the functioning of their ecosystem. This link is crucial to better understand biodiversity dynamics in a temporally and spatially heterogeneous environment, and it is therefore fundamentally important that form and function as well as taxonomic diversity are fully integrated into the investigation of the regulators of diversity. The analysis of functional traits also makes it possible to study the specific effect of organisms on their ecosystem and evaluate the specific ecological role of organisms and their effect on species interactions. Analyses of functional diversity should enable us to understand how species compete for resources in a spatial and temporal context and how they alter the abiotic and biotic characteristics of the environment that they occupy [66]. We suggest that analyses attempting to infer these kinds of interactions without consideration of functional traits may be prone to yielding misleading results, as cause and effect of biodiversity regulators are not mechanistically linked.
Functional biology also improves the integration of palaeobiological evidence at larger evolutionary scales. Functional approaches are not restricted to extant species alone, as many traits with well-understood fitness and performance implications have an excellent fossil record. It is therefore often possible to analyse functional diversity across large temporal scales, integrating a palaeontological perspective with other approaches. Careful studies of ecomorphology, guided by physical models, principles of functional morphology and modern phylogenetic comparative methods, allow for the quantitative inference of diet (e.g. [53,77,78]), locomotor mode (e.g. [79]) and diel activity patterns (e.g. [80]), all of which are important to reconstruct the functional diversity of palaeocommunities. Detailed functional morphological analyses of extant species to firmly establish the linkages between form, function and ecology/behaviour are a prerequisite for thorough studies of functional biology in the fossil record.
Finally, it is also worth noting that functional biology is also relevant at much smaller evolutionary scales, when assessing genetic and phenotypic responses to changes in the biotic and abiotic environment [81]. An emerging theme over the past years has been to understand genome to phenome linkages, and ultimately this promises to be insightful when assessing the biological consequences of genetic diversity.
(b). Investigation of scale-dependency
Scale-dependency is a key challenge to developing a holistic understanding of the regulators of biodiversity. Different responses to changes in the biotic and abiotic environment may be identified depending on the scale of the study and whether biodiversity is measured phylogenetically (individuals to higher taxa), spatially (local or global) or temporally (seasons to millennia). Despite recognition across ecological and evolutionary disciplines of the importance of scale [1,82–85] and the interaction between time and space [57,86], the specific implications and extent of scaling issues are poorly understood [6]. Related to the issues of scale is the question of whether different species concepts identify inherently different units, for example morphospecies require consistent and persistent phenotypic differentiation while biospecies only require reproductive isolation. Ensuring that taxonomic units are congruent is particularly important when combining extinct and extant species' data, as genetic- and phylogenetic-based concepts are becoming increasingly popular in biology but they are difficult to apply to the fossil record. The successful integration of biodiversity research requires that we understand what generates mismatches between scales and the identification of appropriate units for space–time comparisons across scales. Evolutionary rates [87–89], which are central to biodiversity research (figure 1), may at first appear to be a good choice for seamlessly integrating diversity data across scales, as they explicitly account for time. However, rates themselves can also be highly scale-dependent, with the fastest rates occurring at the smallest scales ([90], but see [91]). We therefore urge greater research effort to be directed towards investigations of scale-dependence, with an ultimate goal of developing universal biodiversity indices.
The concept of a nested hierarchy of biodiversity across scales is well established within ecology and conservation biology [83,92,93], and although hierarchical approaches have been implemented within (macro)evolutionary studies (e.g. [94]), we suggest that this perspective needs to be fully embraced. Our current lack of knowledge concerning the extent to which scale or different measures of diversity (lineage, spatial, temporal) influence the relative importance of various biotic and abiotic factors limits the predictive ability of macroevolutionary studies and thus also their application. Conservation studies and policies typically focus on the impact of biotic and abiotic factors such as climate change on much shorter timescales, i.e. decades or even shorter. This is juxtaposed by the fact that a deeper time perspective may be necessary if we wish to preserve the evolutionary and ecological processes that will generate diversity in the future as well as to establish ‘natural’ base lines [11]. We agree with Jablonski [6] that a multilevel approach is the way forward for empirical investigations into the drivers of biodiversity. Analyses using multiple scales of comparison and measures of diversity are necessary to elucidate the extent of scale-dependency and identify consistent relationships between the different biodiversity patterns across scales as well as between lineages, time and space.
Experimental studies will play a crucial role in establishing the link between the regulators of biodiversity at different scales and when compared between phylogenetic lineages, areas and times. Experimental studies provide the ability to directly manipulate biotic interactions and also measure selection as well as organismal or community responses to imposed abiotic or biotic changes. However, how such changes play out over large spatial, temporal or phylogenetic scales is difficult to ascertain. In contrast, macroscale evolutionary and ecological analyses are primarily limited to inferring ecological and evolutionary processes from the fitting of evolutionary models and correlative patterns. These comparative studies rely on multiple independent associations between the biotic or abiotic factor(s) and the biodiversity variable to ensure statistical validity of the relationship (e.g. [95]). Unfortunately, at these scales, patterns may be generated by the action of many processes [6] and similar biodiversity signatures can be generated by quite distinct processes [96]. Experimental evolution is therefore likely to become increasingly relevant to biodiversity studies [97]. Rapid generation times enable the investigation of short- and long-term responses enabling individual-through to community-level effects to be studied. Experimental evolution also allows for the evolution of biotic interactions, niches and the formation of communities to be studied in real-time (e.g. [98]), while changes in fitness can be measured directly by competing new strains against the ancestral strains. Any changes in organismal fitness can be related to specific changes in the organism's genome.
Despite the relative simplicity of experimental communities and the small spatial scales, these systems are already helping to disentangle the influence of evolutionary history, biotic interactions and abiotic changes, and to improve the link to conservation through direct manipulation. For example, although it is well established that as biodiversity increases so does productivity (see review by Tilman et al. [99]) and thus also ecosystem services [100], recent bacterial experiments have demonstrated that the strength of this biodiversity–ecosystem function relationship is modified by niche-width and evolutionary history [101]. Directly relevant to conservation was their finding that although on average the loss of specialists had a stronger effect on ecosystem functioning, the loss of a generalist species may actually have disproportionate effects when there is low functional redundancy [101].
Computer models and Artificial Life simulations have a similar potential to contribute to the integration of biodiversity research across scales (e.g. [102]) as they can follow the patterns from the scale of the individual through to clades and ecosystems. Although computer-evolved communities are built up from individuals following relatively simplistic rules, they are less constrained logistically and spatially than real experiments. Artificial life simulations thus have the potential to enable the investigation of the mechanisms generating spatial as well as temporal hierarchies of biodiversity.
To achieve ideal levels of integration across scales requires increased interactions among empiricists, theoreticians, experimental ecologists, microevolutionary scientists including experimental evolutionists and Artificial Life researchers. The increased involvement of evolutionary and ecological theoreticians may prove particularly important for synthesis. While there are many ecological and evolutionary models that predict specific biodiversity outcomes of individual changes in the biotic or abiotic environment on the small-scale, robust mathematical theory relating these to macroscale dynamics is missing [6].
(c). Improved interdisciplinary integration
The preceding sections have shown that integration is needed not only across the traditional disciplines concerned with biodiversity (palaeobiology, phylogenetic comparative biology, macroecology, biogeography, community ecology, evolutionary ecology and population genetics) but also with the emerging fields of Artificial Life and experimental evolution, especially when scale issues are considered. Calling for integrative research is easy but the implementation is difficult. Although undoubtedly important, there is a limit to what can be achieved by individual special issues or meetings bringing together researchers from disparate fields to talk to one another. We need ongoing and concerted efforts to build a united approach to biodiversity, and to create a research environment where it is easy and natural for researchers with different areas of expertise to interact. However, there are many cultural barriers to overcome when working across disciplinary boundaries [103,104]. These consist of practical issues, such as the use of discipline-specific jargon, which includes subtle differences in the understanding and implications of the same term, for example ‘adaptive radiation’ in the neontological and palaeontological literature [105,106]. There are also societal difficulties to bridge, such as the need to build a team of trusted individuals whose skills and knowledge you may struggle to evaluate, as well as the potential lack of credit for, and recognition of, the value of interdisciplinary work by disciplinary peers, granting agencies, university administrators and promotion committees [104]. Finally, the regular opportunity to interact is of paramount importance; currently researchers who focus on different aspects of diversity (e.g. genetics, evolution, ecology, conservation or palaeobiology) are often members of different research groups, work in different university departments and buildings, publish in different journals and go to discipline-specific annual conferences. The impact of these barriers is often underappreciated but a successful biodiversity synthesis will require a dramatic cultural shift.
The continuing investment in, and development of, interdisciplinary biodiversity centres will be critical to the future of integrated biodiversity research. The goal of such centres is to foster active interactions between biodiversity researchers within museums and across university campuses by providing a centralized and shared location, seminars and classes (e.g. Biodiversity and Climate Research Centre (Bik-F), University of Florida Biodiversity Initiative). While some with larger remit aim to develop international cross-disciplinary collaborations by running working groups (e.g. German Centre for Integrative Biodiversity Research—iDiv) or hosting guest researchers (e.g. Centre for Ecological and Evolutionary Synthesis at the University of Oslo), these centres also play an important role in the development of necessary cyber-infrastructure [107].
Biodiversity research will continue to be enhanced through sustained investment in big-data and data-sharing, as the complex and scale-dependent nature of ecological systems requires vast datasets and computational power to investigate [108,109]. Much has already been learnt through analysing data collated and shared by data-driven initiatives, which have been funded by national granting agencies as well as non-profit organizations. For example, existing projects provide researchers with worldwide access to: museum specimens (e.g. Integrated Digitized Biocollections—iDigBio), fossil diversity and biogeography (e.g. the Paleobiology Database, FAUNMAP), extant species biogeographic information (e.g. Global Biodiversity Information Facility—GBIF, Ocean Biogeographic Information System—OBIS, IUCN species distribution maps), morphological and genetic data (e.g. GenBank, MorphBank, Morphobank) and phylogenetic hypotheses (e.g. TreeBASE), as well as current, historical and palaeo-climate information (e.g. Climate Data Online from NOAA, Consortium for Spatial Information CGIAR-CSI, Neotoma palaeoenvironmental database).
Education will be key to overcoming cultural barriers [110,111] and making an integrative approach to biodiversity mainstream. Training in interdisciplinarity can help to overcome disciplinary constraints and to foster creativity, as well as promote commitment to interdisciplinary work [112]. Students at all career stages need to be trained to think and work broadly and collaboratively. Therefore, greater institutional investment in interdisciplinary biodiversity degree programmes and (post)graduate groups, along with the further development of individual courses and workshops, will help to ensure the future of integrated biodiversity research. Inspiration can perhaps be drawn from the few very successful Integrative Biology groups in the US, which promote interdisciplinary research at all levels of biological organization through clustering together, either as a department (e.g. UC Berkeley) or a graduate programme (e.g. University of Chicago), a broad range of biologists including palaeontologists whose research complements one another. Additional endeavours that will enhance integration include the development of enduring forums for the discussion and publication of research on every aspect of biodiversity, whether applied or theoretical, ecological or evolutionary, and spanning ecological and palaeontological timescales as existing journals and conferences tend to be limited in scope or are one-off ventures.
3. Conclusion
Biodiversity research in deep time can become increasingly rigorous and relevant to conservation by shifting our focus from taxon counts to function and investigating scale-dependency. Biological diversity is a multi-scale concept across time and space [1] but we still lack robust theoretical and empirical evidence describing spatial and temporal interactions and how lower-level processes scale-up. To quantify the extent of scaling effects and determine the linkages between scales requires interdisciplinary collaboration, while experimental evolution and Artificial Life potentially present exciting opportunities to directly measure biodiversity across temporal and spatial scales. This work will help us to understand how current biodiversity loss and changing abiotic and biotic environments may also affect the processes that will generate diversity in the future. Across scales, functional biology mechanistically links the ability of an organism to respond to changing biotic and abiotic environments, which combined with a better understanding of biases in the fossil record can provide the clearest evidence for biotic interactions over deep time. By measuring functional diversity, we are also directly quantifying the determinants of ecosystem processes so crucial for conservation. A promising future for biodiversity research lies in the integration of deep- and near-time approaches through broader interdisciplinary collaboration and the movement away from descriptive to more predictive approaches.
Acknowledgements
We thank Tom Ezard, Mike Benton and Tiago Quental for inviting us to provide this perspective, as well as Graham Slater and Sarah Gilman for helpful discussion and comments. We also thank Danielle Silvestro and an anonymous reviewer for helpful comments. This work took inspiration from discussions held at the National Evolutionary Synthesis Center (NESCent: NSF #EF-0905606) during our catalysis meeting (‘Integrating approaches to macroevolution: combining fossils and phylogenies', held at the National Evolutionary Synthesis Center, Durham NC, 11–13 April 2013) and methods workshop (‘Integrating approaches to macroevolution: combining fossils and phylogenies', held at the National Evolutionary Synthesis Center, Durham NC, 22–29 July 2014).
Authors' contributions
S.A.P. and L.S. contributed equally to the development and writing of this paper.
Competing interests
We declare no competing interests.
Funding
Additional support for this work was provided by NSF DEB-1256894 to S.A.P.
References
- 1.Badgley C. 2003. The multiple scales of biodiversity. Paleobiology 29, 11–13. () [DOI] [Google Scholar]
- 2.Barnosky AD. 2001. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. J. Vertebrate Paleontol. 21, 172–185. ( 10.1671/0272-4634(2001)021%5B0172:DTEOTR%5D2.0.CO;2) [DOI] [Google Scholar]
- 3.Vermeij GJ. 1994. The evolutionary interaction among species: selection, escalation, and coevolution. Annu. Rev. Ecol. Syst. 25, 219–236. ( 10.1146/annurev.es.25.110194.001251) [DOI] [Google Scholar]
- 4.Benton MJ. 2009. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323, 728–732. ( 10.1126/science.1157719) [DOI] [PubMed] [Google Scholar]
- 5.Erwin DH. 2012. Novelties that change carrying capacity. Exp. Zool. B Mol. Dev. Evol. 318, 460–465. ( 10.1002/jez.b.21429) [DOI] [PubMed] [Google Scholar]
- 6.Jablonski D. 2008. Biotic interactions and macroevolution: extensions and mismatches across scales and levels. Evolution 62, 715–739. ( 10.1111/j.1558-5646.2008.00317.x) [DOI] [PubMed] [Google Scholar]
- 7.Dietl GP, Flessa KW. 2011. Conservation paleobiology: putting the dead to work. Trends Ecol. Evol. 26, 30–37. ( 10.1016/j.tree.2010.09.010) [DOI] [PubMed] [Google Scholar]
- 8.Orzechowski EA, et al. 2015. Marine extinction risk shaped by trait–environment interactions over 500 million years. Glob. Change Biol. 21, 3595–3607. ( 10.1111/gcb.12963) [DOI] [PubMed] [Google Scholar]
- 9.Dornelas M, et al. 2013. Quantifying temporal change in biodiversity: challenges and opportunities. Proc. R. Soc. B 280, 20121931 ( 10.1098/rspb.2012.1931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz SA, Schnitzler J, Eronen JT, Hof C, Böhning-Gaese K, Graham CH. 2013. Diversity in time and space: wanted dead and alive. Trends Ecol. Evol. 28, 509–516. ( 10.1016/j.tree.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 11.Kidwell SM. 2015. Biology in the Anthropocene: challenges and insights from young fossil records. Proc. Natl Acad. Sci. USA 112, 4922–4929. ( 10.1073/pnas.1403660112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Signor PW. 1990. The geologic history of diversity. Annu. Rev. Ecol. Syst. 21, 509–539. ( 10.1146/annurev.es.21.110190.002453) [DOI] [Google Scholar]
- 13.Slowinski JB, Guyer C. 1993. Testing whether certain traits have caused amplified diversification: an improved method based on a model of random speciation and extinction. Am. Nat. 142, 1019–1024. ( 10.1086/285586) [DOI] [PubMed] [Google Scholar]
- 14.Collar DC, Near TJ, Wainwright PC. 2005. Comparative analysis of morphological diversity: does disparity accumulate at the same rate in two lineages of centrarchid fishes? Evolution 59, 1783–1794. ( 10.1111/j.0014-3820.2005.tb01826.x) [DOI] [PubMed] [Google Scholar]
- 15.Harmon LJ, Schulte JAI, Larson A, Losos JB. 2003. Tempo and mode of evolutionary radiation in Iguanian lizards. Science 301, 961–964. ( 10.1126/science.1084786) [DOI] [PubMed] [Google Scholar]
- 16.Price SA, Schmitz L, Oufiero C, Eytan RI, Dornburg A, Smith WL, Friedman M, Near TJ, Wainwright PC. 2014. Two waves of colonization straddling the K−Pg boundary formed the modern reef fish fauna. Proc. R. Soc. B 281, 20140321 ( 10.1098/rspb.2014.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid WV. 1998. Biodiversity hotspots. Trends Ecol. Evol. 13, 275–280. ( 10.1016/S0169-5347(98)01363-9) [DOI] [PubMed] [Google Scholar]
- 18.Ricklefs RE, Schluter D. 1993. Species diversity in ecological communities: historical and geographical perspectives. Chicago, IL: University of Chicago Press. [Google Scholar]
- 19.Ricklefs RE. 1987. Community diversity: relative roles of local and regional processes. Science 235, 167–171. ( 10.1126/science.235.4785.167) [DOI] [PubMed] [Google Scholar]
- 20.Bolnick DI, Doebeli M. 2003. Sexual dimorphism and adaptive speciation: two sides of the same ecological coin. Evolution 57, 2433–2449. ( 10.1111/j.0014-3820.2003.tb01489.x) [DOI] [PubMed] [Google Scholar]
- 21.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 22.Doyle JA, Donoghue MJ. 1987. The importance of fossils in elucidating seed plant phylogeny and macroevolution. Rev. Palaeobot. Palynol. 50, 63–95. ( 10.1016/0034-6667(87)90040-6) [DOI] [Google Scholar]
- 23.Benton MJ, Emerson BC. 2007. How did life become so diverse? The dynamics of diversification according to the fossil record and molecular phylogenetics. Paleontology 50, 23–40. ( 10.1111/j.1475-4983.2006.00612.x) [DOI] [Google Scholar]
- 24.Benton MJ. 2015. Exploring macroevolution using modern and fossil data. Proc. R. Soc. B 282, 20150569 ( 10.1098/rspb.2015.0569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jablonski D, Shubin NH. 2015. The future of the fossil record: paleontology in the 21st century. Proc. Natl Acad. Sci. USA 112, 4852–4858. ( 10.1073/pnas.1505146112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Losos JB. 2011. Seeing the forest for the trees: the limitations of phylogenies in comparative biology. Am. Nat. 177, 709–727. ( 10.1086/660020) [DOI] [PubMed] [Google Scholar]
- 27.Slater GJ, Harmon LJ. 2013. Unifying fossils and phylogenies for comparative analyses of diversification and trait evolution. Methods Ecol. Evol. 4, 699–702. ( 10.1111/2041-210X.12091) [DOI] [Google Scholar]
- 28.Tarver JE, Donoghue P. 2011. The trouble with topology: phylogenies without fossils provide a revisionist perspective of evolutionary history in topological analyses of diversity. Syst. Biol. 60, 700–712. ( 10.1093/sysbio/syr018) [DOI] [PubMed] [Google Scholar]
- 29.McInnes L, et al. 2011. Integrating ecology into macroevolutionary research. Biol. Lett. 7, 644–646. ( 10.1098/rsbl.2011.0358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronquist F, Klopfstein S, Vilhelmsen L, Schulmeister S, Murray DL, Rasnitsyn AP. 2012. A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Syst. Biol. 61, 973–999. ( 10.1093/sysbio/sys058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heath TA, Huelsenbeck JP, Stadler T. 2014. The fossilized birth–death process for coherent calibration of divergence-time estimates. Proc. Natl Acad. Sci. USA 11, E2957–E2966. ( 10.1073/pnas.1319091111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slater GJ, Harmon LJ, Alfaro ME. 2012. Integrating fossils with molecular phylogenies improves inference of trait evolution. Evolution 66, 3931–3944. ( 10.1111/j.1558-5646.2012.01723.x) [DOI] [PubMed] [Google Scholar]
- 33.Betancur R, Ortí G, Pyron RA. 2015. Fossil-based comparative analyses reveal ancient marine ancestry erased by extinction in ray-finned fishes. Ecol. Lett. 18, 441–450. ( 10.1111/ele.12423) [DOI] [PubMed] [Google Scholar]
- 34.Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW. 2009. The merging of community ecology and phylogenetic biology. Ecol. Lett. 12, 6930715 ( 10.1111/j.1461-0248.2009.01314.x) [DOI] [PubMed] [Google Scholar]
- 35.Johnson MT, Stinchcombe JR. 2007. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol. Evol. 22, 250–257. ( 10.1016/j.tree.2007.01.014) [DOI] [PubMed] [Google Scholar]
- 36.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505. ( 10.1146/annurev.ecolsys.33.010802.150448) [DOI] [Google Scholar]
- 37.Dietl GP, Kidwell SM, Brenner M, Burney DA, Flessa KW, Jackson ST, Koch PL. 2015. Conservation paleobiology: leveraging knowledge of the past to inform conservation and restoration. Annu. Rev. Earth Planet. Sci. 43, 79–103. ( 10.1146/annurev-earth-040610-133349) [DOI] [Google Scholar]
- 38.Lieberman BS. 2003. Paleobiogeography: the relevance of fossils to biogeography. Annu. Rev. Ecol. Evol. Syst. 34, 51–69. ( 10.1146/annurev.ecolsys.34.121101.153549) [DOI] [Google Scholar]
- 39.Silvestro D, Zizka A, Bacon CD, Cascales-Miñana B, Salamin N, Antonelli A. 2016. Fossil biogeography: a new model to infer dispersal, extinction and sampling from palaeontological data. Phil. Trans. R. Soc. B 371, 20150225 ( 10.1098/rstb.2015.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slater GJ. 2015. Iterative adaptive radiations of fossil canids show no evidence for diversity-dependent trait evolution. Proc. Natl Acad. Sci. USA 112, 4897–4902. ( 10.1073/pnas.1403666111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang S, Roy K, Valentine JW, Jablonski D. 2015. Convergence, divergence, and parallelism in marine biodiversity trends: integrating present-day and fossil data. Proc. Natl Acad. Sci. USA 112, 4903–4908. ( 10.1073/pnas.1412219112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ezard TH, Aze T, Pearson PN, Purvis A. 2011. Interplay between changing climate and species’ ecology drives macroevolutionary dynamics. Science 332, 349–351. ( 10.1126/science.1203060) [DOI] [PubMed] [Google Scholar]
- 43.Van Bocxlaer B, Hunt G. 2013. Morphological stasis in an ongoing gastropod radiation from Lake Malawi. Proc. Natl Acad. Sci. USA 110, 13 892–13 897. ( 10.1073/pnas.1308588110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolland J, Condamine FL, Beeravolu CR, Jiguet F, Morlon H. 2015. Dispersal is a major driver of the latitudinal diversity gradient of Carnivora. Glob. Ecol. Biogeogr. 24, 1059–1071. ( 10.1111/geb.12354) [DOI] [Google Scholar]
- 45.Ezard TH, Thomas GH, Purvis A. 2013. Inclusion of a near-complete fossil record reveals speciation-related molecular evolution. Methods Ecol. Evol. 4, 745–753. ( 10.1111/2041-210X.12089) [DOI] [Google Scholar]
- 46.Fenton IS, Pearson PN, Dunkley Jones T, Farnsworth A, Lunt DJ, Markwick P, Purvis A. 2016. The impact of Cenozoic cooling on assemblage diversity in planktonic foraminifera. Phil. Trans. R. Soc. B 371, 20150224 ( 10.1098/rstb.2015.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silvestro D, Antonelli A, Salamin N, Quental TB. 2015. The role of clade competition in the diversification of North American canids. Proc. Natl Acad. Sci. USA 112, 8684–8689. ( 10.1073/pnas.1502803112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyman T, Linder HP, Pena C, Malm T, Wahlberg N. 2012. Climate-driven diversity dynamics in plants and plant-feeding insects. Ecol. Lett. 15, 889–898. ( 10.1111/j.1461-0248.2012.01782.x) [DOI] [PubMed] [Google Scholar]
- 49.Roopnarine PD, Angielczyk KD. 2015. Community stability and selective extinction during the Permian–Triassic mass extinction. Science 350, 90–93. ( 10.1126/science.aab1371) [DOI] [PubMed] [Google Scholar]
- 50.Dunne JA, Williams RJ, Martinez ND, Wood RA, Erwin DH. 2008. Compilation and network analyses of Cambrian food webs. PLoS Biol. 6, 0693–0708. ( 10.1371/journal.pbio.0060102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Froebisch N, Froebisch J, Sander PM, Schmitz L, Rieppel O. 2013. A macropredatory ichthyosaur from the Middle Triassic and the origin of modern trophic networks. Proc. Natl Acad. Sci. USA 110, 1393–1397. ( 10.1073/pnas.1216750110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott RW. 1978. Approaches to trophic analyses of paleocommunities. Lethaia 11, 1–14. ( 10.1111/j.1502-3931.1978.tb01210.x) [DOI] [Google Scholar]
- 53.Van Valkenburgh B. 1988. Trophic diversity in past and present guilds of large predatory mammals. Paleobiology 14, 155–173. [Google Scholar]
- 54.Kidwell SM, Holland SM. 2002. The quality of the fossil record: implications for evolutionary analyses. Annu. Rev. Ecol. Syst. 33, 561–588. ( 10.1146/annurev.ecolsys.33.030602.152151) [DOI] [Google Scholar]
- 55.Ezard THG, Quental TB, Benton MJ. 2016. The challenges to inferring the regulators of biodiversity in deep time. Phil. Trans. R. Soc. B 371, 20150216 ( 10.1098/rstb.2015.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Blasio FV, Liow LH, Schweder T, De Blasio BF. 2015. A model for global diversity in response to temperature change over geological time scales, with reference to planktic organisms. J. Theor. Biol. 365, 445–456. ( 10.1016/j.jtbi.2014.10.031) [DOI] [PubMed] [Google Scholar]
- 57.Wiens JA. 1989. Scaling in ecology. Funct. Ecol. 3, 385–397. ( 10.2307/2389612) [DOI] [Google Scholar]
- 58.Liow LH, Reitan T, Harnik PG. 2015. Ecological interactions on macroevolutionary time scales: clams and brachiopods are more than ships that pass in the night. Ecol. Lett. 18, 1030–1039. ( 10.1111/ele.12485) [DOI] [PubMed] [Google Scholar]
- 59.Drury J, Clavel J, Morlon H. 2015. Estimating the effect of competition on trait evolution using maximum likelihood inference. bioRxiv . See 10.1101/023473. [DOI]
- 60.Nuismer SL, Harmon LJ. 2015. Predicting rates of interspecific interaction from phylogenetic trees. Ecol. Lett. 18, 17–27. ( 10.1111/ele.12384) [DOI] [PubMed] [Google Scholar]
- 61.Diaz S, Purvis A, Cornelissen JHC, Mace GM, Donoghue MJ, Ewers RM, Jordano P, Pearse WD. 2013. Functional traits, the phylogeny of function, and ecosytem service vulnerability. Ecol. Evol. 3, 2958–2975. ( 10.1002/ece3.601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wainwright PC. 1996. Ecological explanation through functional morphology: the feeding biology of sunfishes. Ecology 77, 1336–1343. ( 10.2307/2265531) [DOI] [Google Scholar]
- 63.Arnold SJ. 1983. Morphology, performance and fitness. Am. Zool. 23, 347–361. ( 10.1093/icb/23.2.347) [DOI] [Google Scholar]
- 64.Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882–892. ( 10.1111/j.0030-1299.2007.15559.x) [DOI] [Google Scholar]
- 65.Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E. 1997. The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302. ( 10.1126/science.277.5330.1300) [DOI] [Google Scholar]
- 66.Diaz S, Cabido M. 2001. Vive la difference: plant functional diversity matters to ecoystem processes. Trends Ecol. Evol. 16, 646–655. ( 10.1016/S0169-5347(01)02283-2) [DOI] [Google Scholar]
- 67.McCann KS. 2000. The diversity–stability debate. Nature 405, 228–233. ( 10.1038/35012234) [DOI] [PubMed] [Google Scholar]
- 68.Pokorny ML, Sheley RL, Zabinski CA, Engel RE, Svejcar TJ, Borkowski JJ. 2005. Plant functional group diversity as a mechanism for invasion resistance. Restor. Ecol. 13, 448–459. ( 10.1111/j.1526-100X.2005.00056.x) [DOI] [Google Scholar]
- 69.Díaz S, Lavorel S, de Bello F, Quétier F, Grigulis K, Robson TM. 2007. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl Acad. Sci. USA 104, 20 684–20 689. ( 10.1073/pnas.0704716104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wainwright PC, Alfaro ME, Bolnick DI, Hulsey CD. 2005. Many-to-one mapping of form to function: a general principle in organismal design? Integr. Compar. Biol. 45, 256–262. ( 10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]
- 71.Alfaro ME, Bolnick DI, Wainwright PC. 2005. Evolutionary consequences of many-to-one mapping of jaw morphology to mechanics in labrid fishes. Am. Nat. 165, E140–E154. ( 10.1086/429564) [DOI] [PubMed] [Google Scholar]
- 72.Wainwright PC, Carroll AM, Collar DC, Day SW, Higham TE, Holzman RA. 2007. Suction feeding mechanics, performance, and diversity in fishes. Integr. Compar. Biol. 47, 96–106. ( 10.1093/icb/icm032) [DOI] [PubMed] [Google Scholar]
- 73.Carroll AM, Wainwright PC, Huskey SH, Collar DC, Turingan RG. 2004. Morphology predicts suction feeding performance in centrarchid fishes. J. Exp. Biol. 207, 3873–3881. ( 10.1242/jeb.01227) [DOI] [PubMed] [Google Scholar]
- 74.Wainwright PC. 1991. Ecomorphology: experimental functional anatomy for ecological problems. Am. Zool. 31, 680–693. ( 10.1093/icb/31.4.680) [DOI] [Google Scholar]
- 75.Vanhooydonck B, Herrel A, Damme RV, Irschick DJ. 2006. The quick and the fast: the evolution of acceleration capacity in Anolis lizards. Evolution 60, 2137–2147. ( 10.1111/j.0014-3820.2006.tb01851.x) [DOI] [PubMed] [Google Scholar]
- 76.Anderson PS, Friedman M, Brazeau MD, Rayfield EJ. 2011. Initial radiation of jaws demonstrated stability despite faunal and environmental change. Nature 476, 206–209. ( 10.1038/nature10207) [DOI] [PubMed] [Google Scholar]
- 77.Kelley NP, Motani R. 2015. Trophic convergence drives morphological convergence in marine tetrapods. Biol. Lett. 11, 20140709 ( 10.1098/rsbl.2014.0709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gill PG, Purnell MA, Crumpton N, Brown KR, Gostling NJ, Stampanoni M, Rayfield EJ. 2014. Dietary specializations and diversity in feeding ecology of the earliest stem mammals. Nature 512, 303–305. ( 10.1038/nature13622) [DOI] [PubMed] [Google Scholar]
- 79.Hutchinson JR, Gatesy SM. 2006. Dinosaur locomotion: beyond the bones. Nature 440, 292–294. ( 10.1038/440292a) [DOI] [PubMed] [Google Scholar]
- 80.Schmitz L, Motani R. 2011. Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science 332, 705–708. ( 10.1126/science.1200043) [DOI] [PubMed] [Google Scholar]
- 81.Nevo E. 2001. Evolution of genome–phenome diversity under environmental stress. Proc. Natl Acad. Sci. USA 98, 6233–6240. ( 10.1073/pnas.101109298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jablonski D. 2000. Micro- and macroevolution: scale and hierarchy in evolutionary biology and paleobiology. Paleobiology 26, 15–52. ( 10.1666/0094-8373(2000)26%5B15:MAMSAH%5D2.0.CO;2) [DOI] [Google Scholar]
- 83.Levin SA. 1992. The problem of pattern and scale in ecology. Ecol. Soc. 73, 1943–1967. ( 10.2307/1941447) [DOI] [Google Scholar]
- 84.Rahbek C. 2005. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 8, 224–239. ( 10.1111/j.1461-0248.2004.00701.x) [DOI] [Google Scholar]
- 85.Swenson NG, Enquist BJ, Pither J, Thompson J, Zimmerman JK. 2006. The problem and promise of scale dependency in community phylogenetics. Ecology 87, 2418–2424. ( 10.1890/0012-9658(2006)87%5B2418:TPAPOS%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 86.Dayton PK, Tegner MJ. 1984. The importance of scale in community ecology: a kelp forest example with terrestrial analogs. In A new ecology. Novel approaches to interactive systems (eds Price P, Slobodchikoff CN, Gaud WS), pp. 457–481. New York, NY: John Wiley & Sons. [Google Scholar]
- 87.Ackerly D. 2009. Conservatism and diversification of plant functional traits: evolutionary rates versus phylogenetic signal. Proc. Natl Acad. Sci. USA 106, 19 699–19 706. ( 10.1073/pnas.0901635106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haldane JBS. 1949. Suggestions as to quantitative measurement of rates of evolution. Evolution 3, 51–56. ( 10.2307/2405451) [DOI] [PubMed] [Google Scholar]
- 89.Gingerich PD. 1993. Quantification and comparison of evolutionary rates. Am. J. Sci. 293, 453–478. ( 10.2475/ajs.293.A.453) [DOI] [Google Scholar]
- 90.Gingerich PD. 2009. Rates of evolution. Annu. Rev. Ecol. Evol. Syst. 40, 657–675. ( 10.1146/annurev.ecolsys.39.110707.173457) [DOI] [Google Scholar]
- 91.Hunt G. 2012. Measuring rates of phenotypic evolution and the inseparability of tempo and mode. Paleobiology 38, 351–373. ( 10.1666/11047.1) [DOI] [Google Scholar]
- 92.Allen TFH, Starr TB. 1982. Hierarchy: perspectives for ecological complexity. Chicago, IL: University of Chicago Press. [Google Scholar]
- 93.Noss RF. 1990. Indicators for monitoring biodiversity: a hierarchical approach. Conserv. Biol. 4, 355–364. ( 10.1111/j.1523-1739.1990.tb00309.x) [DOI] [Google Scholar]
- 94.Jablonski D, Hunt G. 2006. Larval ecology, geographic range, and species survivorship in Cretaceous mollusks: organismic versus species-level explanations. Am. Nat. 168, 556–564. ( 10.1086/507994) [DOI] [PubMed] [Google Scholar]
- 95.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 96.Moen D, Morlon H. 2014. Why does diversification slow down? Trends Ecol. Evol. 29, 190–197. ( 10.1016/j.tree.2014.01.010) [DOI] [PubMed] [Google Scholar]
- 97.Kassen R. 2014. Experimental evolution and the nature of biodiversity. Greenwood Village, CO: Roberts and Company. [Google Scholar]
- 98.Bono LM, Gensel CL, Pfennig DW, Burch CL. 2012. Competition and the origins of novelty: experimental evolution of niche-width expansion in a virus. Biol. Lett. 9, 20120616 ( 10.1098/rsbl.2012.0616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471 ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 100.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 101.Gravel D, Bell T, Barbera C, Bouvier T, Pommier T, Venail P, Mouquet N. 2011. Experimental niche evolution alters the strength of the diversity–productivity relationship. Nature 469, 89–92. ( 10.1038/nature09592) [DOI] [PubMed] [Google Scholar]
- 102.Kim JT. 1997. Distance distribution complexity: a measure for the structured diversity of evolving populations. In Artificial Life V Proc. of the Fifth Int. Workshop on the Synthesis and Simulation of Living Systems, Nara, Japan, 16–18 May 1996 (eds CG Langton, K Shimohara). Cambridge, MA: The MIT Press.
- 103.Golde CM, Gallagher HA. 1999. The challenges of conducting interdisciplinary research in traditional doctoral programs. Ecosystems 2, 281–285. ( 10.1007/s100219900076) [DOI] [Google Scholar]
- 104.Pellmar TC, Eisenberg L. 2000. Barriers to interdisciplinary research and training. In Bridging disciplines in the brain, behavioral, and clinical sciences. Institute of Medicine (US) Committee on Building Bridges in the Brain, Behavioral, and Clinical Sciences (eds Pellmar TC, Eisenberg L), ch. 3 Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 105.Benson RB, Campione NE, Carrano MT, Mannion PD, Sullivan C, Upchurch P, Evans DC. 2014. Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 12, e1001896 ( 10.1371/journal.pbio.1001853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moen D, Morlon H. 2014. From dinosaurs to modern bird diversity: extending the time scale of adaptive radiation. PLoS Biol. 12, e1001854 ( 10.1371/journal.pbio.1001854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodrigo A, et al. 2013. Science incubators: synthesis centers and their role in the research ecosystem. PLoS Biol. 11, e1001468 ( 10.1371/journal.pbio.1001468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelling S, Hochachka WM, Fink D, Riedewald M, Caruana R, Ballard G, Hooker G. 2009. Data-intensive science: a new paradigm for biodiversity studies. BioScience 59, 613–620. ( 10.1525/bio.2009.59.7.12) [DOI] [Google Scholar]
- 109.Hampton SE, Strasser CA, Tewksbury JJ, Gram WK, Budden AE, Batcheller AL, Duke CS, Porter JH. 2013. Big data and the future of ecology. Front. Ecol. Environ. 11, 156–162. ( 10.1890/120103) [DOI] [Google Scholar]
- 110.Carpenter SR, et al. 2009. Accelerate synthesis in ecology and environmental sciences. BioScience 59, 699–701. ( 10.1525/bio.2009.59.8.11) [DOI] [Google Scholar]
- 111.Sidlauskas B, et al. 2010. Linking big: the continuing promise of evolutionary synthesis. Evolution 64, 871–880. ( 10.1111/j.1558-5646.2009.00892.x) [DOI] [PubMed] [Google Scholar]
- 112.Morse WC, Nielsen-Pincus M, Force J, Wulfhorst J. 2007. Bridges and barriers to developing and conducting interdisciplinary graduate-student team research. Ecol. Soc. 12, 1–14. [Google Scholar]


