Abstract
Wild isolates of the nematode Caenorhabditis elegans perform social behaviours, namely clumping and bordering, to avoid hyperoxia under laboratory conditions. In contrast, the laboratory reference strain N2 has acquired a solitary behaviour in the laboratory, related to a gain-of-function variant in the neuropeptide Y-like receptor NPR-1. Here, we study the evolution and natural variation of clumping and bordering behaviours in Pristionchus pacificus nematodes in a natural context, using strains collected from 22 to 2400 metres above sea level on La Réunion Island. Through the analysis of 106 wild isolates, we show that the majority of strains display a solitary behaviour similar to C. elegans N2, whereas social behaviours are predominantly seen in strains that inhabit high-altitude locations. We show experimentally that P. pacificus social strains perform clumping and bordering to avoid hyperoxic conditions in the laboratory, suggesting that social strains may have adapted to or evolved a preference for the lower relative oxygen levels available at high altitude in nature. In contrast to C. elegans, clumping and bordering in P. pacificus do not correlate with locomotive behaviours in response to changes in oxygen conditions. Furthermore, QTL analysis indicates clumping and bordering to represent complex quantitative traits. Thus, clumping and bordering behaviours represent an example of phenotypic convergence with a different evolutionary history and distinct genetic control in both nematode species.
Keywords: Pristionchus pacificus, La Réunion Island, social behaviour, natural variation, oxygen, high-altitude hypoxia
1. Introduction
Recent years have seen a growing interest in phenotypic convergence and parallelism, the independent evolution of similar traits in different organisms or groups of organisms [1]. Several case studies in animals and plants allow inroads into the molecular basis of phenotypic convergence, thereby enhancing the understanding of how constraints shape evolutionary patterns. Nonetheless, it is important to distinguish between convergence and parallelism as the former describes a phenotypic pattern at any hierarchical level in closely or distantly related organisms, whereas the latter describes a shared molecular explanation mostly between closely related species [1]. As with other evolutionary patterns, the understanding of convergence and parallelism depends on case studies in organisms that ultimately allow molecular and mechanistic insights. Nematodes represent a powerful example for such case studies. In particular, the nematode Pristionchus pacificus has been established as a satellite model organism for molecular and mechanistic comparison with Caenorhabditis elegans [2]. Detailed comparisons of multiple developmental and physiological processes between these two nematodes provided important molecular insights. While previous studies did not focus on phenotypic convergence and rather aimed for an understanding of the evolution of developmental processes (evo-devo), they revealed the originally surprising, but common principle of developmental systems drift (DSD), the notion that developmental processes leading to similar and homologous morphological features are still specified by non-homologous molecular processes [3]. One key example of DSD is vulva development in C. elegans and P. pacificus, which results in a homologous organ system that is formed by homologous precursor cells but is regulated by distinct signalling pathways [4]. Here, we expand our comparative studies between P. pacificus and C. elegans to an obvious example of phenotypic convergence in association with social behaviour with the ultimate aim to understand its evolutionary history and genetic and physiological control.
Pristionchus pacificus and C. elegans are members of different nematode families and are separated for more than 200 myr [5], thus representing distantly related species. They share, by convergent evolution, an androdioecious mode of reproduction by self-fertilizing hermaphrodites and males [6]. In contrast, both species differ strongly in their ecology and population genetics [7,8]. While C. elegans is known to live freely in soil and composts [9], P. pacificus is found in soil and often in a necromenic association with scarab beetles. In this entomophilic association, the nematode stays in an arrested dauer stage as long as the beetle is alive and resumes development only after the death of the insect, feeding on growing microbes on the beetles' cadaver [8].
Pristionchus pacificus is unique in the genus Pristionchus in having a worldwide distribution that includes abundant populations on La Réunion Island [10]. La Réunion belongs to the Mascareignes Islands and represents the youngest (2–3 Ma) and steepest (up to 3070 metres above mean sea level, hereafter m.a.s.l.) island in the chain. The island harbours a complex suite of 19 habitat types or ‘ecozones’, including one of the most active volcanoes in the world [11]. Intense sampling across the island has resulted in the isolation of more than 600 P. pacificus strains to date and they are characterized by a very high genetic diversity [10]. Indeed, La Réunion strains nearly cover the complete worldwide genetic diversity of P. pacificus (according to mitochondrial and microsatellite genetic markers) because of multiple independent invasions of the nematode to the island with different beetle species at different time points in the island's history [10]. This genetic diversity, coupled with the large set of La Réunion ecozones, provides a powerful system for studying the evolution of ecologically relevant traits in wild populations undergoing divergence in response to the environment [7,12].
Examples of such traits are oxygen-induced behaviours, which have been described extensively in C. elegans. Many natural isolates show ‘clumping’ and ‘bordering’ behaviours when grown on standard nematode growth medium (NGM) agar plates seeded with Escherichia coli OP50 [13,14]. Clumping is the aggregation of C. elegans nematodes in feeding groups, whereas bordering indicates the preference of worms for the bacterial lawn border. Clumping and bordering are often described as ‘social feeding’ behaviours, because they are more pronounced in the presence of bacterial food [14]. Social behaviours have been suggested to serve hyperoxia avoidance (i.e. an excess supply of oxygen) because they are induced by the 21% oxygen concentration present in the laboratory and they result in the nematodes being exposed to lower oxygen concentrations. Specifically, the border of the bacterial lawn is thicker and consumes more oxygen [13], whereas the oxygen concentration in clumps decreases even further owing to consumption by the nematodes [15]. Because C. elegans lives in an oxygen-variable environment in nature [9], where oxygen levels can fluctuate from 21% to anaerobic levels [16,17], avoidance of 21% oxygen has been proposed to be beneficial in these environments in order to escape surface exposure and enable accumulation on bacterial food sources [18].
In contrast to wild isolates, the laboratory reference strain N2 has acquired a solitary behaviour in the laboratory, related to a polymorphism in the neuropeptide Y-like receptor encoded by the npr-1 gene [14]. Specifically, the npr-1 variant found in the N2 strain causes a gain-of-function phenotype that creates a hyperactive neural circuit [14,19], which alters a wide variety of behaviours and traits including the loss of aggregation and locomotive behaviours [20]. In addition, neuronal sensitivity to oxygen depends on the globin-encoding gbl-5 gene. Animals of the strain CB4856 from Hawaii are sensitive to small shifts from 20% to 21% and respond by increasing the rate of reorientation movements (omega turns) [21], whereas animals of the N2 strain respond only to larger changes in oxygen concentrations [15]. Similar to npr-1, the glb-5 N2 variant has arisen under laboratory conditions and has been retained through clonal propagation [21].
In P. pacificus, the reference strain RS2333, as well as the majority of original wild isolates, shows a solitary behaviour in the laboratory similar to C. elegans N2 [6]. In addition, solitary behaviours are observed in all 28 Pristionchus species currently in culture [8]. In contrast, first observations of some La Réunion-derived P. pacificus strains indicated the presence of social strains in this species. Given this strong difference in the overall solitary versus social feeding pattern between P. pacificus and C. elegans, we systematically explore whether the natural variation in solitary versus social behaviours in P. pacificus populations on La Reunion Island represents an example of phenotypic convergence. We also investigate if such behavioural differences between P. pacificus strains are oxygen-induced. In general, P. pacificus nematodes should be exposed to more stable atmospheric oxygen levels as both microenvironments in which P. pacificus is found—the decaying beetle carcass and La Réunion volcanic soils—are characterized by good aeration. Thus, in comparison with compost heaps where C. elegans is found, P. pacificus might be adapted to a more oxygen-constant lifestyle, close to atmospheric oxygen levels. Consequently, one would predict that P. pacificus should not avoid high levels of oxygen, displaying solitary behaviour in the laboratory.
One important factor influencing the amount of oxygen that animals experience in nature is altitude-associated hypobaric hypoxia, meaning the decrease of atmospheric oxygen partial pressure (pO2) with increased altitude. While the atmospheric concentration of oxygen is constant at any altitude (21%), the pO2 is reduced from 21 to 16 kPa when rising from sea level to 2100 m.a.s.l., with a consequent 20% reduction of oxygen available for respiration [22]. In consequence, P. pacificus populations inhabiting high-altitude environments may have adapted to or developed a preference for lower oxygen levels, and they might avoid hyperoxic conditions in the laboratory by social behaviour, similarly to wild C. elegans isolates. Interestingly, one of the four well-established P. pacificus lineages is exclusively found in high altitudes on La Réunion [10]. In general, there are four mitochondrial and microsatellite lineages (clades; referred to as ‘A’, ‘B’, ‘C’ and ‘D’) of P. pacificus on La Réunion Island [10]. Clade B is endemic to high-altitude locations (2100–2400 m.a.s.l.) and is the only clade found in association with the stag beetle Amneidus godefroyi [10]. Amneidus godefroyi is a monotypic beetle endemic to La Réunion [23]. It lives under volcanic rocks, possesses short wings and is unable to disperse by flying. The inability of A. godefroyi to disperse across long distances has resulted in the isolation of P. pacificus clade B strains, which show no admixture with other clades [10]. Modelling studies and divergence dating support the idea that all lineages, including clade B, evolved prior to the colonization of La Réunion Island [12]. However, strains genetically similar to clade B have never been observed outside La Réunion, whereas clades A and D are known from other parts of the world [10]. While the location of origin of P. pacificus clade B outside of La Réunion Island remains unknown, it is likely that this clade has co-evolved with A. godefroyi for a substantial period of the island's history. The high-altitude habitat of A. godefroyi and P. pacificus clade B is characterized by an atmospheric pO2of around 16.4 kPa [22], whereas the exact pO2 level in the beetle microhabitat is not known.
Here, we provide a systematic analysis of social versus solitary feeding behaviour of 106 P. pacificus wild isolates from La Réunion. Unlike in C. elegans, we find predominantly solitary strains. However, we also observe intriguing evidence for a subset of social strains, most of which are of lineage B ancestry, inhabiting high-altitude locations. We show that clumping and bordering behaviour among P. pacificus social strains are regulated by oxygen, suggesting that these strains may have adapted to the hypobaric hypoxia associated with high altitude or may have evolved a preference for low oxygen levels. We contrast our results in P. pacificus with those in C. elegans, providing an example of phenotypic convergence in oxygen-induced social behaviours. Interestingly, we find that both species have different evolutionary histories for clumping and bordering behaviours, which is characterized by at least partially distinct genetic control. Finally, we compare locomotive omega-turn rates in response to shifts in oxygen levels between social and solitary P. pacificus strains, observing strikingly different patterns from those reported in C. elegans.
2. Methods
(a). Strains
In total, 106 strains isolated from different beetle species on La Réunion Island, and the laboratory reference strain RS2333, were used in this study (electronic supplementary material, table S1). Strains were maintained at 20°C using standard methods, unless otherwise indicated [24].
(b). Behavioural assays
The assay for quantification of bordering and clumping behaviours in C. elegans [14] was modified for P. pacificus as follows: 6 cm NGM plates containing 2.1% agar were seeded 2 days before the assay with 50 ml of E. coli OP50 in LB medium, resulting in a circular lawn of about 10 mm in diameter with a border width of approximately 1 mm. Sixty well-fed young adult worms from uncrowded plates were transferred by pipetting to the assay plate, and located outside the OP50 lawn. The assay plates were incubated in darkness for 3 h at 20°C (the standard culture temperature at the laboratory), to allow the nematodes to freely distribute across the bacterial lawn. After 3 h, both bordering and clumping behaviours were measured simultaneously as described [14]. Three or four replicates per strain were performed for each assay.
The regulation of bordering and clumping behaviours by oxygen was analysed by performing the above assay in a custom-fabricated Plexiglas chamber [25]. In our assays, the air pressure remained always constant when oxygen concentration was manipulated. We decided to use particular oxygen levels and temperatures in our experiments similar to those used previously in related experiments in C. elegans. This allows for a maximal comparison of the dataset. However, it is important to note that while the atmospheric pO2 at high-altitude locations is known (table 1), the exact pO2 level in the beetle carcass microenvironment is currently unknown and might change over time. Therefore, we focus in many experiments on a comparison between 21% and 16% oxygen, which represent conditions found in the natural habitats (sea level versus 2100 m.a.s.l.). For some experiments, however, we reduced oxygen levels further to 10% and 4% to compare with previous C. elegans studies, although it is unlikely that these conditions mimic those found in nature.
Table 1.
Pristionchus pacificus populations from La Réunion Island analysed in this study.
| location | location code | altitude (m) | lineage | number of strainsa | pO2b | %O2c |
|---|---|---|---|---|---|---|
| Saint Benoit | SB | 22 | A | 10 | 20.9 | 100 |
| Grand Etang | GE | 527 | C/D | 8/10 | 19.7 | 94 |
| Colorado | CO | 697 | C | 10 | 19.2 | 92 |
| Plaines des Lianes | PL | 774 | D | 9 | 19.1 | 92 |
| Sans Souci | SS | 1353 | C | 9 | 17.8 | 86 |
| Trois Bassin | TB | 1378 | C | 10 | 17.8 | 85 |
| Plaines de Cafres | PC | 1578 | C | 10 | 17.4 | 84 |
| Neu du Boeuf | NB | 2152 | B | 10 | 16.4 | 78 |
| Coteau Kerveguen | CK | 2158 | B | 10 | 16.4 | 78 |
| Le Cratere Commerson | CC | 2327 | B | 10 | 16.2 | 77 |
aA population is defined by location and lineage. The number of individuals per population varies between 8 and 10. The altitude and the nematode lineage isolated from each location are shown. For a more detailed list of P. pacificus strains used in this study, see electronic supplementary material, table S1.
bAtmospheric oxygen partial pressure given in kPa (see Methods).
cAmbient oxygen level calculated as a percentage of sea-level value. Note that at 2150 m.a.s.l., the oxygen available for respiration is 22% lower than at sea level.
Assay plates were prepared similarly to the clumping/bordering assay but using 10 cm NGM agar plates. The nematodes were allowed to distribute freely on the bacterial lawn for 1 h under a gas mixture of 21% oxygen and 79% nitrogen. The gas mixture was delivered at a constant flow rate of 50 ml min−1 during all assays via a static gas mixer connected to mass flow controllers (Vögtling Instruments) operated by LabView software (National Instruments). Images were captured every 10 min with a digital camera (JAI BM-500, Stemmer Imaging) in order to quantify bordering and clumping behaviours. This assay was performed for three clade B strains (RSB001, RSC011 and RSA076) and RS2333, with three replicates completed per strain. For each time point, the three measurements were pooled for statistical analysis (see electronic supplementary material, Methods). The bordering and clumping assay for RSB001 under fixed oxygen concentrations was performed similarly to previous assays, but the animals were exposed for 3 h to a constant gas flow delivering a particular oxygen concentration (see electronic supplementary material, figure S3). Then, the number of bordering and clumping animals was measured as before. Three replicates were performed for each oxygen concentration.
Oxygen-evoked turning responses were monitored as described previously [25–27]. Briefly, NGM plates were seeded with OP50 bacteria (grown overnight). Twenty young adult animals were confined to a 28 × 28 mm region using Whatman filter paper. Plates were covered with the aerotaxis chamber and gas flow was delivered at 50 ml min−1. Oxygen concentration was changed every 6 min as described in electronic supplementary material, figure S4. Animals were recorded at 3 frames s−1 with a digital camera (JAI BM-500, Stemmer Imaging) and the StreamPix 5 software (NorPix), and automatically tracked using a customized image-processing and analysis script written in Matlab software (MathWorks, Natick, MA). Twenty replicates per assay were performed. Ω-turn rate values were calculated in 15 s windows. The means of Ω-turns during the 3 min before and after the oxygen shift were calculated for each replicate.
(c). Quantitative trait locus mapping analysis
To investigate the genetic complexity of the clumping/bordering trait, recombinant inbred lines (RILs) were produced by mating the RSB001 and RS2333 strains as previously described [28]. Ninety-four of these RILs were phenotyped and genotyped by mRNA sequencing. Association between phenotypes and genotypes was calculated by performing a Wilcoxon rank-sum test (see electronic supplementary material, Methods for detailed information).
3. Results
(a). Pristionchus pacificus shows natural variation in clumping and bordering behaviours
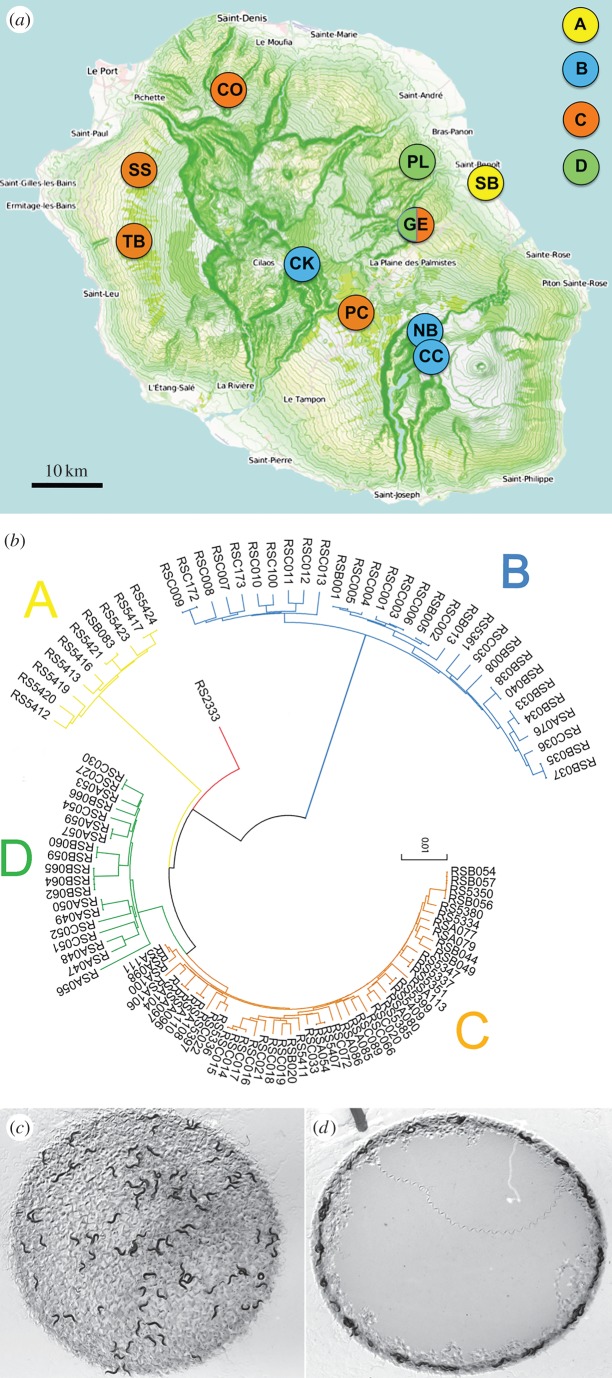
In C. elegans, all but a single strain display social behaviours under laboratory conditions, possibly as a result of a 21% oxygen avoidance behaviour acquired in their natural habitats. To analyse natural variation in clumping and bordering behaviours in P. pacificus, we phenotyped 106 strains isolated from 11 populations on La Réunion Island (figure 1a and table 1; electronic supplementary material, S1) collected from 22 to 240 m.a.s.l. This set of strains includes representatives of all four clades of P. pacificus that inhabit different ecozones on the island (figure 1b). In addition, we analysed RS2333 from California, which serves as a laboratory reference strain similar to C. elegans N2.
Figure 1.
(a) Map of La Réunion Island showing the locations included in this study and the nematode lineages [10] isolated from them (modified from openstreetmap.org, © OpenStreetMap contributors). (b) Unrooted neighbour-joining phylogenetic tree for La Réunion P. pacificus strains analysed in this study and the reference strain, RS2333. Colour code refers to the lineage of the strains: yellow, clade A; red, RS2333 (clade A); blue, clade B; orange, clade C; green, clade D. (c) Clumping/bordering assay for the P. pacificus RS2333 strain, which shows a solitary behaviour under laboratory conditions, characterized by low levels of clumping and bordering. (d) Clumping/bordering assay for the P. pacificus RSB001 strain, isolated at 2327 m.a.s.l., which shows strong clumping and bordering behaviours under laboratory conditions.
Overall, the percentage of bordering animals among La Réunion strains followed a continuous distribution from 40% to 100%, whereas the percentage of animals in clumps followed a continuous distribution from 0% to 50% (electronic supplementary material, figure S1). The distinction between social and solitary strains is therefore not as clear-cut in P. pacificus. In contrast to C. elegans, we predominantly found evidence for strains showing low and medium clumping/bordering levels in our dataset, including RS2333 (figure 1c). However, we also found a subset of social strains that perform high levels of clumping and bordering, most of which are of lineage B ancestry, such as RSB001 (figure 1d).
(b). Natural variation in clumping and bordering in Pristionchus pacificus is associated with three highly correlated factors: altitude, lineage and beetle host
To characterize the bordering and clumping behaviours further, we first tested whether the two behaviours were themselves associated across the whole dataset. In addition, we tested for correlations independent of genetic lineage by analysing clade C individually, because it represents the most abundant clade in our dataset. The Pearson product–moment correlation coefficient for bordering versus clumping was r = 0.83 (p < 0.001) for the whole sample set and r = 0.72 (p < 0.001) among clade C, indicating a strong positive correlation between the two behaviours that is independent of genetic lineage.
We next wanted to examine the various factors that may be contributing to the natural variation in bordering and clumping in P. pacificus. In particular, we wanted to test whether these behaviours were significantly associated with altitude, given our hypothesis that atmospheric pO2 may be driving this natural variation. Indeed, we performed analysis of variance for the whole dataset and found that altitude does significantly partition the variance in clumping and bordering among strains (F9,96 = 28.22; p < 0.001 and F9,96 = 38.41; p < 0.001, for bordering and clumping, respectively; electronic supplementary material, figure S2 and table S2a). Pairwise comparisons with Tukey's post hoc analysis revealed that these differences were due to significantly higher clumping/bordering between altitude locations above 2100 and below 1600 m.a.s.l. (electronic supplementary material, table S2b). Taken together, these results indicate that the substantial natural variation in clumping and bordering observed among wild isolates of P. pacificus is associated with altitude in the tested strains. However, in practice, high-altitude strains are exclusively correlated with clade B ancestry (r = 1.000, p < 0.001), and the beetle host A. godefroyi (r = 0.931, p < 0.001). Therefore, the contribution of these three individual factors cannot be disentangled, and thus represent a complex phenomenon, the evolutionary meaning of which will be discussed below.
(c). Clumping and bordering of clade B strains are highly regulated by oxygen
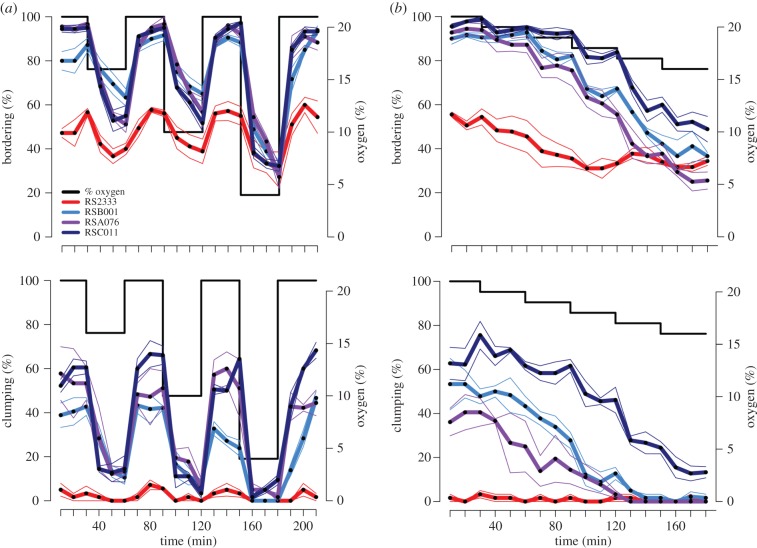
Clumping and bordering behaviours are regulated quantitatively by oxygen in the social C. elegans mutant npr-1 (ad609). A shift in oxygen concentration from 21% to 7% rapidly suppresses both behaviours, whereas smaller shifts from 21% to either 15% or 10% oxygen have a lesser effect. Similarly, shifting cultures back to 21% oxygen restores both behaviours. Likewise, oxygen influences the performance of clumping and bordering in the solitary N2 strain although in a much weaker manner [13]. To test if clumping and bordering in P. pacificus are also regulated by oxygen, we analysed both behaviours in specific assay systems using aerotaxis chambers (see Methods). Specifically, we performed shift and ramp assays using one strain from each clade B population (RSA076, RSB001 and RSC011) plus the reference strain RS2333.
In shift assays, we altered oxygen concentrations in 30 min intervals by shifting worms from 21% to 16%, 10% or 4%, respectively, returning to 21% in between (figure 2a; electronic supplementary material, table S3). We found that all strains reduced bordering in response to the shift from 21% oxygen to lower concentrations. The back-shift to 21% oxygen resulted in a complete return of bordering to the initial levels in all strains. However, this fluctuation was much stronger in clade B strains than in RS2333, resembling the pattern observed between social and solitary C. elegans strains [13]. For clumping, we observed a strong fluctuation in clade B strains in response to oxygen shifts, whereas RS2333 showed very low clumping under all conditions.
Figure 2.
Regulation of bordering and clumping behaviour by oxygen levels. (a) Comparison of the dynamic changes in bordering (top) and clumping (bottom) behaviours during oxygen shifts between clade B strains (RSB001, RSA076 and RSC011) and RS2333. Oxygen concentration changes every 30 min from 21 → 16 → 21 → 10 → 21 → 4 → 21%. (b) Comparison of the dynamic changes in bordering (top) and clumping (bottom) behaviours between clade B strains (RSB001, RSA076 and RSC011) and RS2333 during oxygen shifts from 21% to 16%, with a 1% decrement occurring every 30 min. In all graphs, black dots represent the mean and thin lines represent the standard error (s.e.m.).
In ramp assays, we decreased oxygen from 21% to 16% with a 1% decrement occurring every 30 min (figure 2b; electronic supplementary material, table S3). This reduction is equivalent to a 20% reduction in the amount of oxygen available for respiration when rising from sea level to 2100 m.a.s.l. (owing to the decrease from 21 to 16.4 kPa pO2; see table 1 and Methods). For both behaviours, results were generally similar to that seen in shift assays. Reduction in oxygen concentration from 21% to 16% led to a stronger decrease in bordering in clade B strains (from approx. 93% to approx. 39%) than in RS2333 (from approx. 53% to approx. 35%). Likewise, clumping was reduced strongly in clade B strains, being almost suppressed at 16% oxygen, whereas in RS2333 clumping was again very low under all conditions.
In order to estimate the relationship between oxygen and clumping/bordering behaviours for RSB001 as a clade B representative, independent values for bordering and clumping were obtained under different fixed oxygen concentrations (see Methods; electronic supplementary material, figure S3 and table S4). Regression analysis revealed that, in total, 94% (p < 0.001) of the variation in bordering and 88% (p = 0.004) of the variation in clumping could be explained by the given model (regression equations: bordering mean = −133.21 − 10.99x [O2]; clumping mean = −104.86 + 7.08x [O2]). Together, these experiments confirm that oxygen levels significantly regulate clumping and bordering behaviours in P. pacificus.
(d). Oxygen-induced locomotive behaviour of social and solitary Pristionchus pacificus strains differs from Caenorhabditis elegans
In addition to clumping and bordering, oxygen also influences locomotive behaviours in C. elegans, like the Ω-turn rate. Increased Ω-turn rates while crawling are interpreted as responses to aversive stimuli, whereas decreases correspond to attractive stimuli [29]. Wild isolates, like CB4856 from Hawaii, respond to the aversive stimuli induced by shifts in oxygen concentration from 20% to 21% with increased rates of Ω-turns, whereas the solitary N2 strain does not modify its Ω-turn rate in response to these shifts [21,25]. To explore whether a similar pattern exists in P. pacificus, we analysed the Ω-turn rate of RSB001 and RS2333 under varying oxygen levels (see Methods). First, we compared locomotive behaviours in a 21–20–21% oxygen shift assay (electronic supplementary material, figure S4a and table S5). Surprisingly, we observed an opposite pattern in P. pacificus compared with C. elegans, where only social strains respond. Specifically, the solitary strain RS2333 reduced its Ω-turn rate when oxygen was shifted from 21% to 20%, and increased it again with the back-shift to 21%. This result is contrary to our expectation that the solitary strain RS2333 should not perceive the increase from 20% to 21% in the oxygen concentration as an aversive stimulus. In contrast, RSB001 maintained a constant Ω-turn rate during oxygen shifts, which is consistent with our previous observation that bordering and clumping in RSB001 remained constant when oxygen levels were reduced from 21% to 20% (figure 2b; electronic supplementary material, figure S3 and table S4).
Next, we analysed the ability of the two strains to respond to 21–16–21% oxygen shifts by modifying its Ω-turn rate, because 16% oxygen mimics the oxygen levels at the high-altitude clade B locations. Indeed, RSB001 and RS2333 reduced Ω-turn rates when oxygen levels were shifted from 21% to 16% (electronic supplementary material, figure S4b and table S5). Interestingly, after a back-shift from 16% to 21% oxygen, both strains increased the Ω-turn rate to levels that were more than double those observed at the initial 21% baseline. However, after a few minutes at 21%, the Ω-turn rate of both strains gradually declined towards baseline levels. Thus, both strains respond to oxygen shifts with changes in their locomotion patterns. These results indicate that the regulation of both oxygen-induced behaviours (Ω-turn and clumping/bordering) is uncoupled in P. pacificus and they suggest both a strain- and species-specific behaviour in response to changes of environmental oxygen. In C. elegans, gbl-5 was identified as a key regulator of oxygen sensitivity [21], and future studies in P. pacificus will reveal the genetic composition of oxygen sensitivity in this nematode. However, a full understanding of locomotive behaviours in P. pacificus and C. elegans requires a precise knowledge of the exact oxygen levels in the microhabitats of both organisms, an endeavour that is complicated by the miniature size of nematodes.
(e). Pristionchus pacificus clumping and bordering are complex quantitative traits independent of npr-1
To provide a first estimate of the genetic complexity of P. pacificus social behaviours, we performed a cross between males of the social strain RSB001 and hermaphrodites of the solitary strain RS2333. F1 hybrid animals displayed intermediate bordering behaviour, whereas clumping behaviour was similar to RS2333 (electronic supplementary material, figure S5a). Phenotypic analysis of clumping/bordering of 94 RILs showed that both behaviours were continuously distributed among strains, spanning the full range of values between parental strains (electronic supplementary material, figure S5b). These findings provide first evidence that clumping and bordering behaviours in P. pacificus represent complex traits. More specifically, the association between genotypes and phenotypes by a Wilcoxon rank-sum test revealed several peaks related to quantitative trait loci (QTL), which further suggests a polygenic control of both phenotypes. The strongest association is seen on chromosome IV with several QTL peaks that were not fully separated because of the small number of recombination events. In addition, chromosomes I and X also show QTLs although of lower effect (electronic supplementary material, figure S6). While the insufficient number of recombination events among the 94 RILs did not permit the identification of individual candidate genes, it allows the conclusion that P. pacificus social behaviours are regulated by complex quantitative traits. Furthermore, these data indicate that a substantially larger number of RILs will be necessary to clone individual QTLs, an endeavour that was very successful for other variable traits in P. pacificus strains [30,31].
In C. elegans, a sequence polymorphism in npr-1 underlies the behavioural difference between social and solitary strains [14]. In P. pacificus, the 1 : 1 orthologue of npr-1 is situated on Contig127-SNAP2012.14 on the X chromosome (electronic supplementary material, figure S7). Surprisingly, detailed analysis of the X chromosome QTL peak mentioned above did not provide evidence for a strong contribution of Ppa-npr-1 to the bordering and clumping phenotypes, because the most significant associated SNPs on the X chromosome are not found in the proximity of Ppa-npr-1. However, a smaller contribution of Ppa-npr-1 cannot be ruled out and awaits more detailed QTL analysis. Furthermore, sequence analysis indicates that both RS2333 and RSB001 possess a phenylalanine at npr-1 position 237, corresponding to the same variant present in social C. elegans strains (electronic supplementary material, figure S8). Taken together, our initial analysis of the genetic regulation suggests that P. pacificus clumping and bordering represent complex quantitative traits with at least partially different regulatory mechanisms.
4. Discussion
Here, we study the evolution and natural variation of clumping and bordering behaviours in P. pacificus nematodes using wild populations living at a variety of altitudes and heterogeneous environments on La Réunion Island. We find substantial natural variation in these behaviours among strains. In general, the amount and type of natural variation observed in P. pacificus contrasts with that found in C. elegans, where only a single laboratory strain has been shown to display solitary behaviour, whereas all wild isolates are social. Overall, P. pacificus strains fall into two categories: strains from locations below 1600 m.a.s.l. are predominantly solitary, whereas strains from locations above 2100 m.a.s.l. exclusively display social behaviour, specifically bordering and clumping. These findings suggest different evolutionary histories of social behaviour for these two nematode species, and thus social behaviours as observed in P. pacificus and C. elegans represent an example of phenotypic convergence.
It is important to note that the phenotypic convergence of social behaviours as seen in both nematodes is the result of not only independent evolutionary histories, but also different evolutionary trajectories. In particular, phenotypic frequencies indicate that the most parsimonious explanation for the observed phenomena is that social behaviour is basal in C. elegans. This is most likely to reflect an adaptation towards 21% oxygen avoidance in their natural habitats (soil, compost and rotten fruits), with the solitary behaviour only resulting under artificial conditions in one strain [21]. In contrast, solitary behaviour in P. pacificus is more widespread and possibly basal, which could be related to the ecological association of this nematode with beetles. While the exact oxygen level on beetle carcasses is unknown and might fluctuate, we speculate that the total spectrum of oxygen levels P. pacificus is exposed to is more stable and regular than those for C. elegans. Therefore, the solitary behaviour as found in most P. pacificus wild isolates might well represent an evolutionary adaptation. We speculate that the observed bordering and clumping behaviours of the strains isolated from high-altitude locations might indicate an adaptation to high-altitude-associated hypobaric hypoxia found in the wild. Because pO2 decreases with increased altitude, hypobaric hypoxia is one of the main environmental stresses associated with high-altitude environments. Although social P. pacificus strains should, in principle, be susceptible to evolve similarly to the N2 strain under laboratory conditions, the number of generations required for that to take place is difficult to predict. In this context, it is also important to note that the ‘evolution of the N2 strain’ was largely facilitated by artificial selection of those worm individuals that did not show clumping and bordering behaviour. In our propagation of worms, we usually prevent single worm propagation, and thereby actively prevent this type of artificial selection.
Although local adaptation presents an intriguing hypothesis for the variation in underlying social behaviours in P. pacificus, other explanations could also account for our results. In particular, alongside altitude, the second factor common among social strains of P. pacificus is genetic lineage, and with it the specific beetle host. While all strains belonging to clade B are social, most of the strains belonging to other clades are solitary. However, all clade B strains are also from high altitude and the same host beetle, indicating that the contribution of altitude, beetle host and phylogenetic lineage cannot easily be disentangled. Therefore, clumping and bordering may not represent a sole adaptation to oxygen, but might also be due to the distinct evolutionary history of the clade B lineage and its beetle host. In particular, distinct phenotypic variation in clade B may be a consequence of genetic drift in isolation after this clade colonized the island, or it may represent evolutionary ‘baggage’ carried over from an earlier adaptation in this lineage that is unrelated to altitude. Modelling studies estimate that colonization of La Réunion by the clade B lineage occurred around 158 000 generations ago [12], but unfortunately the origin of either the source population or the beetle host is currently unknown. The clade B host beetle, A. godefroyi, represents a monotypic genus, indicating that its evolution and history will remain difficult to determine. However, given that this beetle is also only found at high altitudes, it is likely that the beetle and nematode clade have co-evolved for a substantial period of the island's history, and perhaps together adapted to high-altitude habitats.
In conclusion, our analysis of the natural variation in social behaviours in P. pacificus provides evidence for phenotypic convergence of these behaviours in P. pacificus and C. elegans nematodes, related to different evolutionary histories in both nematode species. However, this convergence may not necessarily represent an example of parallelism, as the genetic architecture of the traits in P. pacificus appears much more complex than in C. elegans. A full molecular understanding of the genetic regulation of social feeding behaviours awaits future analysis in both nematodes, in particular a detailed molecular analysis of the associated QTLs in P. pacificus as introduced in this study.
Supplementary Material
Acknowledgements
We thank our La Réunion colleagues for logistic support during fieldwork, particularly Jacques Rochat (La Réunion Insectarium), and staff of La Réunion Parc National. We also thank Cameron Weadick for revision and discussion on the manuscript. Finally, we thank two anonymous reviewers for comments and suggestions on an earlier version of this manuscript.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
E.M. and R.J.S. designed research; E.M. performed research; E.M., A.M., C.R. and M.Z. analysed data; and E.M., A.M., M.Z., C.R. and R.J.S. wrote the paper.
Competing interests
We have no competing interests.
Funding
This work was supported by the Max Planck Society.
References
- 1.Rosenblum EB, Parent CE, Brandt EE. 2014. The molecular basis of phenotypic convergence. Annu. Rev. Ecol. Evol. Syst. 45, 203–226. ( 10.1146/annurev-ecolsys-120213-091851) [DOI] [Google Scholar]
- 2.Sommer RJ (ed.). 2015. Pristionchus pacificus—a nematode model for comparative and evolutionary biology. Leiden, The Netherlands: Brill. [Google Scholar]
- 3.True JR, Haag ES. 2001. Developmental system drift and flexibility in evolutionary trajectories. Evol. Dev. 3, 109–119. ( 10.1046/j.1525-142x.2001.003002109.x) [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Sommer RJ. 2011. Antagonism of LIN-17/Frizzled and LIN-18/Ryk in nematode vulva induction reveals evolutionary alterations in core developmental pathways. PLoS Biol. 9, e1001110 ( 10.1371/journal.pbio.1001110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diederich C, et al. 2008. The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat. Genet. 40, 1193–1198. ( 10.1038/ng.227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer RJ, Carta LK, Kim S-Y, Sternberg PW. 1996. Morphological, genetic and molecular description of Pristionchus pacificus sp. n. (Nematoda: Neodiplogastridae). Fund. Appl. Nematol. 19, 511–522. [Google Scholar]
- 7.Sommer RJ, McGaughran A.. 2013. The nematode Pristionchus pacificus as a model system for integrative studies in evolutionary biology. Mol. Ecol. 22, 2380–2393. ( 10.1111/mec.12286) [DOI] [PubMed] [Google Scholar]
- 8.Ragsdale EJ, Kanzaki N, Herrmann M. 2015. Taxonomy and natural history: the genus Pristionchus. In Pristionchus pacificus—a nematode model for comparative and evolutionary biology (ed. Sommer RJ.), pp. 77–120. Leiden, The Netherlands: Brill. [Google Scholar]
- 9.Frèzal L, Félix M-A. 2015. The natural history of model organisms: C. elegans outside the Petri dish. eLife 4, e05849 ( 10.7554/eLife.05849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan K, McGaughran A, Villate L, Herrmann M, Witte H, Bertelmes G, Sommer RJ. 2012. Multi-locus analysis of Pristionchus pacificus on La Réunion Island reveals an evolutionary history shaped by multiple introductions, constrained dispersal events, and rare out-crossing. Mol. Ecol. 21, 250–266. ( 10.1111/j.1365-294X.2011.05382.x) [DOI] [PubMed] [Google Scholar]
- 11.Strasberg D, Rouget M, Richardson DM, Baret S, Dupont J, Cowling RM. 2005. An assessment of habitat diversity and transformation on La Réunion Island (Mascarene Islands, Indian Ocean) as a basis for identifying broad-scale conservation priorities. Biodivers. Conserv. 14, 3015–3032. ( 10.1007/s10531-004-0258-2) [DOI] [Google Scholar]
- 12.McGaughran A, Morgan K, Sommer RJ. 2013. Unravelling the evolutionary history of the nematode Pristionchus pacificus: from lineage diversification to island colonization. Ecol. Evol. 3, 667–675. ( 10.1002/ece3.495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. 2004. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322. ( 10.1038/nature02714) [DOI] [PubMed] [Google Scholar]
- 14.de Bono M, Bargmann CI. 1998. Natural variation in a neuropeptide Y receptor homolog modifies social behaviour and food response in C. elegans. Cell 94, 679–689. ( 10.1016/S0092-8674(00)81609-8) [DOI] [PubMed] [Google Scholar]
- 15.Rogers C, Persson A, Cheung B, de Bono M.. 2006. Behavioural motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr. Biol. 16, 649–659. ( 10.1016/j.cub.2006.03.023) [DOI] [PubMed] [Google Scholar]
- 16.Tan KH. 2011. Principles of soil chemistry, 4th edn New York, NY: CRC Press. [Google Scholar]
- 17.Wang W, Wang X, Liu J, Ishii M, Igarashi Y, Cui Z. 2007. Effect of oxygen concentration on the composting process and maturity. Compost. Sci. Util. 15, 184–190. ( 10.1080/1065657X.2007.10702331) [DOI] [Google Scholar]
- 18.Persson A, Gross E, Laurent P, Busch KE, Bretes H, de Bono M.. 2009. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature 458, 1030–1033. ( 10.1038/nature07820) [DOI] [PubMed] [Google Scholar]
- 19.Macosko EZ et al. 2009. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458, 1171–1175. ( 10.1038/nature07886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen EC, Bloom JS, Gerke JP, Kruglyak L. 2014. A variant in the neuropeptide receptor npr-1 is a major determinant of Caenorhabditis elegans growth and physiology. PLoS Genet. 10, e1004156 ( 10.1371/journal.pgen.1004156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath PT et al. 2009. Quantitative mapping of a digenic behavioural trait implicates globin variation in C. elegans sensory behaviours. Neuron 61, 692–699. ( 10.1016/j.neuron.2009.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beall CM. 2007. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl Acad. Sci. USA 104, 8655–8660. ( 10.1073/pnas.0701985104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coquerel C. 1866. Faune de Bourbon (Ile de la Réunion). Coléoptères. Paris: Annales de la Société entomologique de France, pp. 326–327. [Google Scholar]
- 24.Pires da Silva A. 2013. Pristionchus pacificus protocols. WormBook. See http://www.wormbook.org/chapters/www_ppageneticprotocols.2/ppagenticprotocols.html. ( 10.1895/wormbook.1.114.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmer M, et al. 2009. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron 61, 865–879. ( 10.1016/j.neuron.2009.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. 2007. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70. ( 10.1038/nature06292) [DOI] [PubMed] [Google Scholar]
- 27.Ramot D, Johnson BE, Berry TL Jr, Carnell L, Goodman MB. 2008. The parallel worm tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS ONE 3, e2208 ( 10.1371/journal.pone.0002208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witte H, Moreno E, Rödelsperger C, Kim J, Kim JS, Streit A, Sommer RJ. 2014. Gene inactivation using the CRISPR/Cas9 system in the nematode Pristionchus pacificus. Dev. Genes Evol. 225, 55–62. ( 10.1007/s00427-014-0486-8) [DOI] [PubMed] [Google Scholar]
- 29.Ha H, et al. 2010. Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron 68, 1173–1186. ( 10.1016/j.neuron.2010.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer MG, Rödelsperger C, Witte H, Riebesell M, Sommer RJ. 2015. An orphan gene regulates intraspecific competition in nematodes by copy number variation. PLoS Genet. 11, e1005146 ( 10.1371/journal.pgen.1005146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kienle S, Sommer RJ. 2013. Cryptic variability in vulva development by cis-regulatory evolution of a HAIRY binding site. Nat. Commun. 4, 1714 ( 10.1038/ncomms2711) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.