Abstract
We targeted a habitat used differentially by deep-diving, air-breathing predators to empirically sample their prey's distributions off southern California. Fine-scale measurements of the spatial variability of potential prey animals from the surface to 1 200 m were obtained using conventional fisheries echosounders aboard a surface ship and uniquely integrated into a deep-diving autonomous vehicle. Significant spatial variability in the size, composition, total biomass, and spatial organization of biota was evident over all spatial scales examined and was consistent with the general distribution patterns of foraging Cuvier's beaked whales (Ziphius cavirostris) observed in separate studies. Striking differences found in prey characteristics between regions at depth, however, did not reflect differences observed in surface layers. These differences in deep pelagic structure horizontally and relative to surface structure, absent clear physical differences, change our long-held views of this habitat as uniform. The revelation that animals deep in the water column are so spatially heterogeneous at scales from 10 m to 50 km critically affects our understanding of the processes driving predator–prey interactions, energy transfer, biogeochemical cycling, and other ecological processes in the deep sea, and the connections between the productive surface mixed layer and the deep-water column.
Keywords: deep sea, acoustics, predator–prey, pelagic, heterogeneity
1. Introduction
The deep pelagic ocean is home to the ‘largest animal communities on the planet’ whether measured in terms of biomass, number of individuals, or areal extent [1]. Yet, the challenges of studying this immense, dark, hostile, and traditionally inaccessible zone mean many relevant aspects of its biology remain largely unexplored. Some basic biological dynamics appear to hold. For instance, the distribution of animals conforms to the boundaries of the principal oceanic water masses [2]. Additionally, some limited visual observations, primarily from remotely operated vehicles, have revealed that mid and deep waters have an incredibly diverse array of animals and particles that are quite active and highly spatially structured at small scales [3]. This complexity challenges the general assumption that at depth, conditions in the horizontal plane are spatially homogeneous and static over long time periods [4]. However, there are few direct data, primarily from cameras and nets, each with their own limitations with which to evaluate this contrast.
The analysis of predator stomach contents has contributed much to our knowledge of the distribution of deep sea species, particularly those that are rapid swimmers less likely to be captured in sample nets [5]. Marine mammals including toothed whales (odontocete cetaceans) and seals (phocid pinnipeds), have evolved remarkable physiological and behavioural characteristics to feed at depths of 1 000 m or deeper [6]. The repeated appearance of this physiologically challenging and energetically costly strategy in obligate air-breathers strongly suggests that there are valuable prey resources to be had in the deep sea. Despite the historical view that the deep ocean is horizontally homogeneous over large regions, deep-diving marine mammals show horizontal variation in foraging behaviour and habitat use at spatial scales from tens to hundreds of kilometres (e.g. [7–10]). These are precisely the scales over which direct measurements of cetacean and pinniped prey at depths of 1 000 m or more would fill key gaps in our understanding of the spatial structure of deep-sea biology. There are several significant challenges in obtaining such data. First, direct capture of the relatively large, fast swimming fish and squid requires extremely large nets and ships that can pull them, integrates the many kilometres over which the net is towed, and is difficult if not impossible to do quantitatively [11]. Imaging systems can provide insights at high resolution but only over relatively limited spatial extents [1]. Conventional ship-based, high-frequency active acoustic surveys to quantify the distribution and density of prey items using ships are effectively limited to approximately the upper 600 m of the water column. Recently, traditional fisheries acoustic mapping systems were integrated into an autonomous underwater vehicle (AUV) capable of diving up to 600 m and obtaining useful measurements of potential prey items for deep-diving marine mammals [12]. This integration of technologies opens new possibilities to use known variation in predator habitat use within a habitat to identify biologically significant regions in a seemingly featureless environment.
We sought to use this powerful new approach to remotely sense and quantify the distribution of potential marine mammal prey items in deep-water areas off southern California where several species of deep-diving marine mammals are known to occur and feed. We specifically focused on areas known to be important foraging habitats for the deepest diving marine mammals, the beaked whales. These include offshore areas around San Clemente Island where considerable research on the extreme deep-diving and geographical movement of Cuvier's beaked whales (Ziphius cavirostris) has been conducted due to the overlap between these animals and the US Navy's Southern California Anti-submarine Warfare Range (‘SOAR’). Local information on the behaviour, distribution, and individual behaviour of beaked whales was available from visual surveys and photo-identification [13], long-term satellite tag monitoring [14], as well as a broadly distributed array of monitoring hydrophones on the range tuned to detect foraging beaked whales in collaboration with the visual observation and tagging efforts and to provide long-term (monthly patterns over multiple years) acoustical monitoring of their distribution across a deep-water area of the SOAR range covering hundreds of square kilometres (D. Moretti, personal communication, 2012). This information was used to stratify our sampling within two defined sections of this area, allowing a priori predator distribution patterns indicating differential habitat use to identify regions of potentially contrasting prey characteristics. We combined acoustic and direct sampling measures of biotic resources at various depth in order to quantify the variability in deep-water resources over 10 m–50 km scales and to examine the connection between surface layers and these features.
2. Material and methods
(a). Approach
In this study, we used a novel, autonomous echosounder system integrated into an advanced AUV (REMUS 600) capable of diving to 600 m [12] to obtain the first measurements of the distribution of potential prey items at relatively great depths (more than 1 000 m) where beaked whales feed. We had two specific goals with regard to the horizontal distribution of our sampling. The first was to investigate adjacent areas within the sonar training range (SOAR) for which extensive acoustic, visual, and tag-based measurements of feeding behaviour were available and suggested differential habitat usage and potential underlying differences in deep-water biology. The second was to obtain comparative measurements in a bathymetrically similar nearby area off the SOAR range which experiences far less sonar use and thus might provide an accessible alternate feeding area for animals moving off the range area during sonar disturbance. Acoustic prey data were collected in conjunction with net tows and physical habitat measures. A combination of ship-based and AUV-based measurements was used simultaneously to examine the relationship between shallow- and deep-water organisms, testing the hypothesis that differences in deep-water biomass reflect differences in upper water column biomass.
(b). Survey design
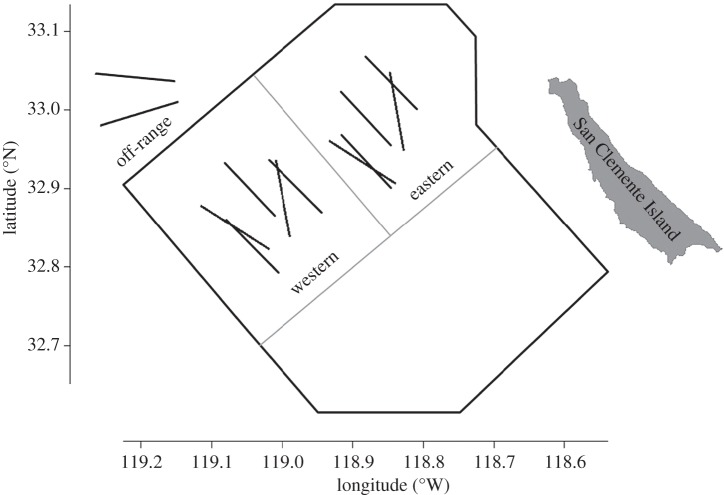
A key consideration for the active acoustic sampling design to measure the distribution and density of deep-water biota was the incorporation of what was known about the sub-mesoscale habitat use of deep foraging predators, particularly Cuvier's beaked whales, within the survey region. Recent progress has been made in understanding various life-history characteristics, including habitat utilization, through applications of medium-term tags (days to months) for tracking surface locations and some aspects of diving behaviour of Cuvier's beaked whales off California [13,14] and passive acoustic monitoring of their species-typical echolocation clicks (D. Moretti, personal communication, 2012). Over 20 satellite-linked tracking tags have been deployed on this species on the SOAR range. This underwater acoustic monitoring facility contains 172 bottom-mounted hydrophones covering nearly 1 800 km2 that are designed to track undersea vehicles but that have been used to monitor Cuvier's beaked whales and other species. Combined recent data from visual observations and encounters, tagging/tracking of individuals, and passive acoustic monitoring strongly suggests that this is an important beaked whale feeding area and that there may be preferential habitat use within it. Specifically, Cuvier's beaked whales detected on the SOAR range using these methods have historically been more commonly distributed in the western portions of the range relative to eastern areas. Based on these a priori observations of the distribution of deep-foraging predators, we constructed a blocked sampling design to investigate prey distribution in lower use (‘eastern’) and higher use (‘western’) zones of the SOAR range, as well as a bathymetrically similar ‘off-range’ zone immediately to the north (figure 1).
Figure 1.
The locations of our sampling transects, each 10 km in length, are shown relative to the US Navy's SOAR range, outlined in black, off southern California. A priori Ziphius habitat use zones are delineated by grey lines to show a ‘high use’ zone in the northwest quadrant of the range and ‘low use’ in the northeast quadrant. Zones to the north of the range are the closest similar habitats that might be used by animals if range activities displace them.
In each sampling zone, 10 km long transects were surveyed during daylight hours over 4 days in September 2013 using the R/V New Horizon and the specialized REMUS AUV. Transect locations within each sampling zone were chosen to sample discrete areas that represented the general bathymetry of the region and to effectively use limited available time with suitable weather conditions and access to the SOAR range. In each of the sampling zones on SOAR, five transects were conducted with measured mean bottom depths of 1 250, 1 310, 1 580, 1 600, and 1 650 m in the western zone and 1 300, 1 300, 1 580, 1 610 and 1 650 m in the eastern zone. Transects running parallel to the easternmost and westernmost boundaries of the range were sampled on 20 and 21 September with at least one transect in each zone sampled each day. Transects running obliquely relative to the boundaries of the range were sampled on 28 and 29 September. Transects from the two zones were 6.5 km apart at their closest approach. The off-range zone was only sampled with two transects, averaging 1 310 and 1 380 in measured bottom depth on 29 September. Each transect consisted of a single conductivity, temperature, depth (CTD) profile to a depth of 1 000 m near the beginning of the survey, ship-based acoustic measurements taken continuously at a vessel speed between 1.8 and 2.6 m s−1 offset slightly (approx. 100 m) from the AUV travelling along the same course at a speed of 1.8 m s−1, and a depth targeted oblique trawl conducted at 1.8 m s−1.
(c). Oceanographic profiles
Near the first point of each transect, a profile of temperature, pressure, oxygen, fluorescence and beam transmission, and beam attenuation at 650 nm was collected using the ship's CTD to provide simple metrics of physical habitat differences among regions. Using custom routines, all CTD data were aligned to account for instrument lags, data were filtered, edited for loops, and low-pass filtered before calibrations were applied to convert data to appropriate measures. Each profile was examined qualitatively for differences. In addition, the depth of the thermocline was calculated by finding the largest point-to-point difference in 0.5 m averaged downcast values visually compared to profiles for validation. The average temperature, oxygen, fluorescence, beam transmission, and beam attenuation were calculated above the thermocline and over 900–1 000 m for each profile. These values were compared using an analysis of variance (ANOVA) to determine possible effects of zone. Post-hoc Dunnett's C tests were conducted to examine observed differences.
(d). Net trawls
Net tows were conducted with a 4 m2 mouth opening Isaacs–Kidd midwater trawl with a 3.2 mm mesh net towed at a speed of approximately 1.8 m s−1. To facilitate sampling, the net was equipped with a real-time pressure sensor (Simrad PI-32) that provides second-by-second depth information. The net was towed obliquely up from 500 to 250 m for a total duration of 20 min and then hauled back at a rate of 20 m of wire per minute. Deeper net tows were collected but not systematically and are not included here. The net, which is dark in colour, towed at relatively high speeds, and with minimal hardware to create a head wake effectively sampled organisms with body lengths between 1 and 35 cm. With the exception of gelatinous organisms which were classified and discarded, net samples were immediately preserved in 4% buffered formalin in seawater. In the laboratory, the total biomass of net contents was measured and individuals identified to species. Fish, shrimp, and krill length were also measured. A multiple analysis of variance (MANOVA) was used to assess the effect of sampling zone on measures of biomass and abundance of abundant taxonomic groups. Post-hoc Dunnett's C tests were conducted to examine observed differences.
(e). Active acoustic measurements
Active acoustic sampling was conducted from both a deep-water AUV and ship-based acoustic sampling to provide measures of animals throughout the water column. Ship-based echosounders included Simrad EK60 s a 38 kHz (12° split-beam), and 70, 120, and 200 kHz (7° split-beams) split-beam system with transducers deployed downward looking 2 m beneath the surface of the vessel. Each echosounder used a 512 µs long pulse at a rate of 1 Hz and a source level < 180 dB re 1 µPa (RMS).
The REMUS-600 AUV carried a custom echosounder payload described in Moline et al. [12]. Briefly, the REMUS-600 is a moderately sized AUV, weighing only 250 kg with dimensions of 3.25 × 0.3 m diameter. It has an operational depth of 600 m, with a range in our configuration of over 100 km and duration of approximately 20 h. Once beneath the surface, it is capable of level flight with no detectable tilt and roll and produces no bubbles. As part of the custom payload, the AUV houses downward looking, split-beam echosounders (Simrad EK60 s) at 38 and 120 kHz (7° beams) and a PC104 format computer that controls data acquisition. Each echosounder used a 512 µs long pulse at a rate of 1 Hz and a source level < 180 dB re 1 µPa (RMS). Data from these instruments had a lower noise floor than comparable systems on the ship, resulting in gains in operational sampling range of 30–40%, permitting single target and volume scattering data from both systems to be used at ranges between 50 and 650 m. As the AUV was flown at a consistent depth of 550 m for all surveys, this corresponds to 600 and 1 200 m water depth.
The ship-based echosounders were calibrated using a standard sphere method [15] at the beginning of the research cruise. To calibrate the echosounders inside the AUV, two approaches were employed as described by Moline et al. [12]. First, we employed a standard sphere method to calibrate the echosounders at the surface by tethering the AUV to the side of the research vessel and moving the sphere methodically below the transducers. To validate the AUV echosounder calibrations at depth, we conducted approximately 5 km long parallel surveys with the ship-based and AUV echosounders over areas with extensive scattering layers. The AUV was held at a constant depth of 50, 300, or 500 m for each survey to allow comparisons of volume scattering strength between the two platforms to be made over the diving depth range of the AUV and the volume scattering strength was compared statistically, revealing no effects of pressure on the resulting measures, described in detail by Moline et al. [12].
Acoustic scattering data from both platforms was processed using Echoview software. First, the seafloor and any noise artefacts were removed. Then a measure of integrated scattering (nautical area scattering coefficient, NASC in m2 nmi−2) was calculated over the entire length of each transect over a range of depth intervals using a −85 dB Sv integration threshold. To make different depth ranges comparable, each value was scaled to 100 m of vertical range. From the ship-based data, scattering was integrated from 5 to 600 m. Integration was conducted from 600 to 1 200 m in the AUV data. Using an ANOVA, integrated acoustic scattering was compared across sampling zones for the full water column, upper water column, lower water column, and 900–1 200 m.
Single targets, e.g. only one target per acoustic reverberation volume for each pulse [16], were extracted from both the 38 and 120 kHz data from both the ship (upper 600 m) and AUV (600–1 200 m). Detection criteria were based on those presented by Benoit-Bird et al. [17] for squid measured at similar ranges [18] which were empirically confirmed using a target strength threshold of −60 dB. Single targets could be localized with 10 cm of resolution in the horizontal plane and 10 cm in the vertical plane. For comparison, the number of targets detected was divided by the volume sampled by each beam to provide a measure of the numerical density of targets. This density was compared across sampling zones for the full water column, upper water column, lower water column, and 900–1 200 m, using an ANOVA.
For all targets identified at both frequencies (more than 85% of all single targets detected), the intensity of the echo at 120 kHz was subtracted from the 38 kHz intensity to provide information on target identity. The effect of sampling zone on the distribution of this measure was analysed using a Kruskal–Wallis non-parametric test. Targets were identified as consistent with squid, if their target strength at 38 kHz was 3.5–10 dB higher than their 120 kHz target strength (as in [17]) and fish if target strength values between the two frequencies were no more than 3 dB different. The distribution of the target strength values (typically interpreted as a size metric) of ‘squid’ and ‘fish’ targets between 900 and 1 200 m was analysed as a function of sampling zone using Kruskal–Wallis tests.
The heterogeneity of the distribution of targets consistent with squid or fish at the deepest range of our sampling (900–1 200 m) was analysed as a function of spatial scale. First, the variance in target density averaged over each transect was calculated between transects within each sampling zone. This served as a normalizer of variance at smaller scales. Each transect was then broken up into 10 segments, each 1 km long and the variance in average target density between each segment was divided by the between transect variance. This procedure was replicated at 100 and 10 m scales. ANOVA was used to examine the effects of scale and sampling zone on the measures of distributional heterogeneity for ‘squid’ targets.
3. Results
(a). Oceanographic profiles
Examination of profiles showed that physical measures of the habitat were remarkably similar across profiles with surface temperatures of approximately 20°C and a shallow thermocline between 25 and 30 m above water gradually cooling from 12.5°C to about 4°C at 1 000 m. Salinity in the surface mixed layer was consistently 33.7 before increasing sharply to 34.2 down to about 200 m then increasing to 34.5 at 1 000 m. On all casts, oxygen decreased slowly from a value near 6 ml l−1 at the surface to 0.23 ml l−1 at about 600 m, remaining low and stable in deeper water. While we found a significant effect of sampling zone on measures of the habitat (ANOVA F = 56.9, d.f. = 10,2, p < 0.01), post-hoc analysis revealed no significant differences in the measures of thermocline depth or average values of temperature, salinity, or oxygen in the surface mixed layer or at depth. In contrast to the physical and chemical variables, measures of fluorescence, beam transmission, and beam attenuation varied as a function of sampling zone in surface waters. In the eastern zone, fluorescence and beam attenuation in the surface mixed layer were significantly higher than in the off-range zone where it was significantly higher than the western zone while beam transmission showed the opposite pattern (p < 0.05 for all post-hoc comparisons). The western zone was 10–15% different from the eastern zone for each measure while the off-range zone was 5–10% different. Optical measurements were not significantly different at greater depths.
(b). Net trawls
There was a significant, overall effect of sampling zone on the various measures from net samples collected at water depths between 250 and 500 m (F = 40.5, d.f. = 10,2, p = 0.01). There were also significant effects on total biomass, and the abundance of gelatinous organisms, myctophids, all fish, squid, amphipods, and euphausiids (p < 0.05 for all comparisons) but no significant effects on the abundance of hatchetfish, gastropods, or shrimp (p > 0.3 for all comparisons). Post-hoc analyses revealed that for all measures where a significant difference was found, the eastern and off-range zones had higher measures of biomass and abundance with the exception of the abundance of euphausiids which was highest in the western zone. The total biomass, abundance of gelatinous organisms, and the abundance of squid was also significantly higher in the eastern relative to the off-range sampling zone.
(c). Active acoustic measurements
There was a significant effect of depth range (d.f. = 5,54, F = 28.7, p < 0.05) and sampling zone (d.f. = 2,54, F = 25.2, p < 0.02) as well as an interaction between these two variables (d.f. = 10,54, F = 59.2, p < 0.01) on 38 kHz acoustic scattering (figure 2). The number of individual, 38 kHz targets was significantly affected by depth range (d.f. = 5,54, F = 41.6, p < 0.02) and sampling zone (d.f. = 2,54, F = 98.2, p < 0.01) and there was a significant interaction term (d.f. = 10,54, F = 66.1, p < 0.01; figure 3).
Figure 2.
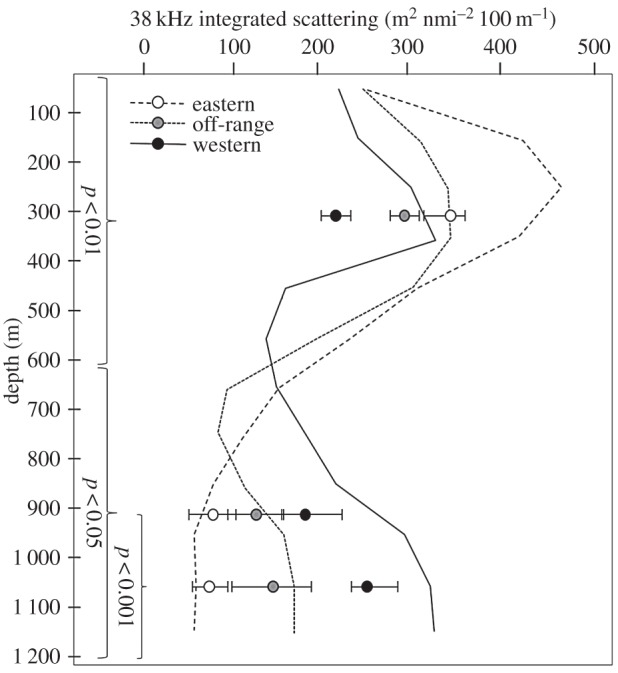
Acoustic scattering integrated over 100 m depth averaged over the transects in each zone is shown as a function of depth and sampling zone. In addition, integrations are shown for the upper 600 m of the water column, 600–1 200 m, and 900–1 200 m for each zone. Error bars show the total range of values for transects in that zone. Significance levels for post-hoc analysis of the effects of zone on extended integration depths are shown for each panel.
Figure 3.
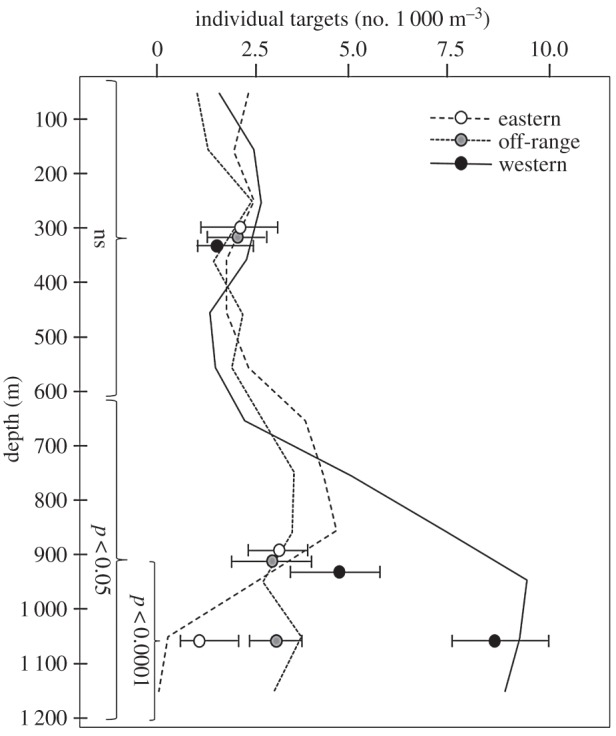
The density of individual targets as a function of depth range and sampling zone is shown over 100 m depth bins. Values are shown averaged over the upper 600 m of the water column, 600–1 200 m, and 900–1 200 m for each zone. Error bars show the total range of values for transects in that zone. Significance levels for post-hoc analysis of the effects of sampling zone are shown for each panel.
The distribution of target frequency response varied significantly among sampling zones (d.f. = 2, χ2 = 44.9, p < 0.01), as shown in figure 4 where the expected frequency response for fish and squid is highlighted. The fraction of targets consistent with squid was approximately 30% in the eastern zone, 42% in the off-range zone, and 53% in the western zone.
Figure 4.
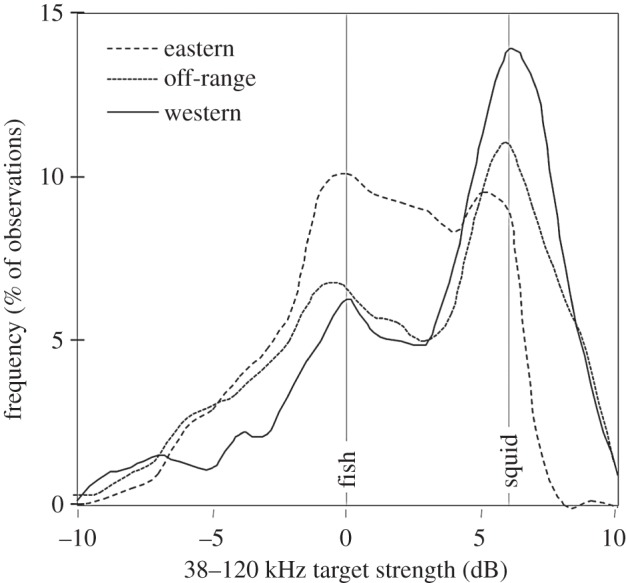
Smoothed histograms of the frequency response of individual targets detected between 900 and 1 200 m as a function of sampling zone. The expected frequency response for fish (0 dB) and squid (6 dB) are indicated by grey lines. The y-axis is the per cent of observations within each zone. This allows the relative proportion of different scatters to be observed across zones.
The distribution of target strength of fish targets did not vary significantly as a function of sampling zone (d.f. = 2, χ2 = 2.1, p = 0.32). The distribution of 38 kHz target strength for squid targets did vary significantly as a function of sampling zone (figure 5; d.f. = 2, χ2 = 34.5, p < 0.01). To aid interpretation of these results, the mode target strength was converted to an estimate of squid mantle length using relationships established for other squid [17], resulting in an estimated mode length of 16 cm in the eastern zone and 22 cm in the off-range and western zones. While the lack of length-target strength relationships available for deep-dwelling squid limits the interpretation of these as absolute values, relative differences are likely accurate given the consistency of the slope of this relationship across taxa [19].
Figure 5.
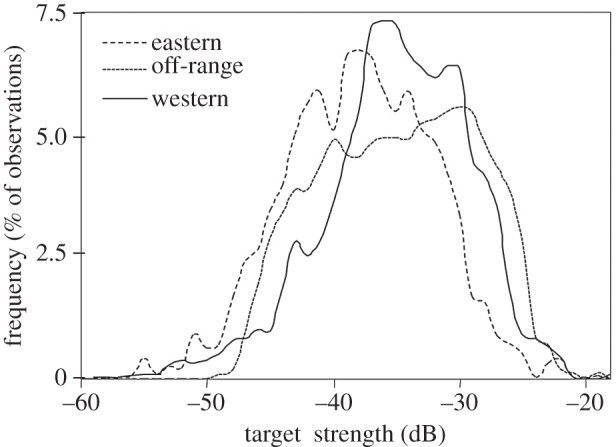
Smoothed histograms of the target strength of targets consistent with squid for each of the three habitat use categories. The y-axis is the frequency measured as the per cent of observations within each category.
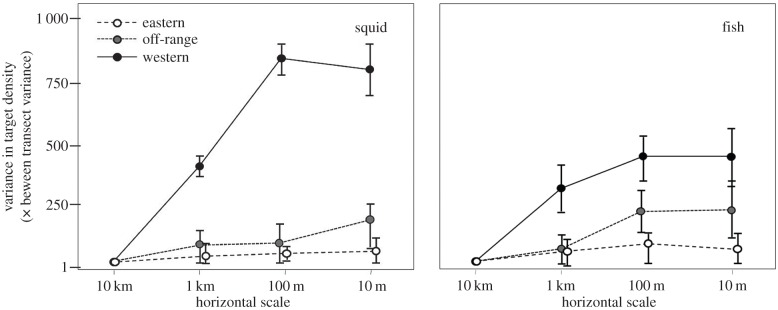
The spatial distribution of likely squid and fish was examined by looking at the variance in target density at a variety of scales within each transect (figure 6). This provides a description of the patchiness, or conversely, the evenness, of these resources. There were significant effect of scale (d.f. = 2,29, squid F = 66.7, p < 0.01; fish F = 21.8, p < 0.05) and sampling zone (d.f. = 2,29; squid F = 85.3, p < 0.01; fish F = 39.3, p < 0.05) as well as an interaction effect (d.f. = 4,29, squid F = 78.3, p < 0.01; fish F = 41.6, p < 0.5) on the variance in squid and fish density.
Figure 6.
To quantify the spatial distribution or patchiness of potential Ziphius prey over a range of scales, variance in the density of targets consistent with squid among segments of each transect was scaled by the between transect variance within each habitat use category as transects were split into smaller and smaller sections. Shown here are the data consistent with squid with error bars showing the variance in this measure between transects. Note that data from the eastern and off-range sampling zones are offset slightly for visual clarity however transect segments were not different in size.
4. Discussion
It is a long-held view that at depth in the ocean, conditions in the horizontal plane, both physical and biological, are spatially homogeneous and temporally static [4]. This view has been challenged by in situ imaging from tethered vehicles that has revealed unexpected spatial structure, ecological organization, and activity in the deep ocean [3]. However, the challenges in studying this harsh, inaccessible habitat have left gaps between studies at biogeographic scales and those of individual associations studied with imaging. Long-term sampling of Ziphius has revealed that they show significant variation in their use of habitat while foraging at scales of 10–50 km. These observations suggest structure in the resources used by these predators at these scales, something we sought to confirm using deep-water acoustic sampling facilitated by the application of a newly available platform for these sensors, a deep-diving AUV. These differences were reflected in measures of total biomass between zones at the depths that overlap with foraging effort by beaked whales (900–1 200 m).
The variability in the distribution of deep-sea biotic resources we measured also occurred at finer scales than the zones sampled. By examining the variance in the density of squid and fish along a transect with decreasing scale, significant heterogeneity at scales of 10–100 m was identified in the western zone. This density variance was consistent across all transects in the western zone, indicating that the animals we observed are clustered at scales of 10–100 m. In the eastern zone, only slight increases in variance were observed, as expected by chance within a small sample size. However, this increase is similar in scale to the increases in variance among segments, indicating that squid in this zone are relatively uniformly distributed with some random fluctuations. The reasons for the small-scale patches of squid in the western zone (and their absence in the eastern zone) are unclear as our measurements of deep-water habitat characteristics are not resolved to this scale. Variability at these intermediate scales has previously been extremely difficult to quantify in the deep ocean. Midwater trawls integrate samples in the horizontal plane so their resolution is seldom less than a kilometre while the imaging systems have small sampling volumes and are not typically moved over ranges large enough to detect patches at this scale as well as the gaps between them. The presence of biological heterogeneity at scales less than 100 m even over the short assessment time possible within this study contributes to the increasing evidence that our view of the deep ocean as homogeneous and static is vastly oversimplified. These observations of resource structure at scales of 100 m also provide a framework for how we study and interpret the behaviour of deep-diving predators.
Figure 2 provides a proxy for the total biomass of animals in various segments in the water column, combining acoustic data from the ship and the AUV. Integrating over the water column to a depth of 1 200 m obscures the differences observed between sampling zones. The effects of sampling zone are opposite in surface and deep waters with the patterns in the deep amplified below 900 m. This highlights the need to measure the biota at depth rather than using easier to obtain proxies from surface waters as these do not necessarily reflect the processes in deep water. These observations demonstrate that for deep-diving predators, surface-based sampling to predict prey resources may not only lead to different conclusions than direct sampling of the deep water, it may lead to completely inverse conclusions.
The contrast in sampling zone differences between the upper and lower water column is interesting in light of the view that deep-water biomass reflects surface productivity [4]. This may be generally true at biogeographic spatial scales or long time scales. However, the striking contrasts among zones with a 3.5-fold decrease in biota between the upper and lower halves of the water column in areas less preferable to beaked whales and no change in a proxy of biomass across this depth transition in a preferred habitat indicates different processes and connections between the surface and deep ocean over relatively short horizontal distances during this study. Measures of the physical properties of the water column did not vary across zones either at the surface or at depth, however measures of the surface biota showed remarkable differences that may be indicative of the processes at work. First, the upper water column had higher levels of fluorescence, higher scattering, and lower clarity in eastern relative to western zones. These eastern zones had abundant gelatinous organisms including many large medusa that were completely absent from samples in the western region. Interestingly, the eastern zone also had higher numbers of myctophids, all fish, squid, amphipods, and euphausiids than the western zone, reflecting in more detail the differences in total biomass observed in the acoustic measurements. Across taxa and size spectra from phytoplankton to zooplankton to micronketon, the surface waters of the eastern habitat had much higher standing stocks than the western habitat.
Predictions that the deep ocean reflects the biomass in surface layers suggest that there should be a logarithmic decline of food energy with increasing depth [4]. Across all of our observations, integrated acoustic scattering was within the same order of magnitude across all depth intervals. The largest difference between the upper and lower half of the water column was at most fourfold. In fact, within the western habitat, there was no difference in the acoustic scattering over the upper and lower water column. These measures of acoustic scattering do not reflect the total food energy in the water, however the similarity of their values is interesting, particularly in light of the relatively large size and thus high energy content of the biota encompassed in these measurements. During our study, the deep waters in this region appear to have high standing stocks of animals relative to those predicted by energy in the surface waters, raising questions about the transfer of energy through the water column that would need to be addressed by longer term observations and measures of production rather than standing stock.
5. Conclusion
Studying biology below the ocean's surface presents formidable challenges requiring the development of specialized tools. At great depths in the sea, a ‘limited number of samplers has been employed, each of which offers only a limited window on reality’ [20]. Here, we present the first results from a new approach to sampling biota between 600 and 1 200 m using conventional acoustic methods uniquely integrated within a deep-diving autonomous vehicle. This tool allowed us to resolve the locations of individual animals at depth, classify them based on their acoustic frequency response, estimate their relative size, and examine their distribution with high spatial resolution in a rapid, fine-scale, non-extractive manner. We used this tool to examine the spatial variability of relatively large animals capable of rapid swimming that could serve as prey for beaked whales over horizontal spatial scales ranging from 10 m to 50 km. This range of spatial scales fills a major gap between existing approaches examining biology at these depths and matches closely with the habitat selection and prey selection scales of foraging beaked whales.
Significant spatial variability in the biota was evident over all spatial scales examined by using the historic habitat use patterns of Ziphius to provide an ecological basis for our sampling design. Notably, we found differences between the eastern and western sides of the Navy's SOAR range. These approximately 25 km across zones were internally consistent with respect to the acoustic scattering, number of individual targets, percentage of squid, squid size distribution, and heterogeneity of targets within foraging depths for Ziphius but transects in different zones that were separated by just 6.5 km were significantly different with higher scattering and more, larger squid clustered at 10–100 m scales rather than randomly distributed on the western side of the range used more frequently by Ziphius. This kind of striking structure in the deep pelagic absent clear physical differences changes our view of this habitat as uniform. The high levels of acoustic scattering—similar in magnitude to those measured at the surface—also contradict the expectation that energy decreases exponentially with depth.
Using deep-diving, air-breathing predators to tell us about habitats we cannot access ourselves has revealed much about the organisms living deep in the ocean, providing an important link between our world and these vast habitats. Combining a new, deep-diving acoustic platform with the approach of letting predators lead us to important areas provided new insights into the spatial scales of heterogeneity of biotic resources at these inhospitable depths. Our results have important implications for deep-diving predators as well as the management of human interactions with these species. The revelation that large animals deep in the water column that serve as prey for beaked whales are so spatially heterogeneous at scales from 10 m to 50 km critically affects our understanding of the processes driving predator–prey interactions, energy transfer, biogeochemical cycling, and other ecological processes at depth in the ocean, the connections between the productive surface mixed layer and the deep-water column, and the scales at which we must approach these important questions.
Acknowledgements
Field efforts were supported by Chad Waluk, David O'Gorman, Ian Robbins, David Cade, Megan Cimino, Danielle Haulsee, Marnie Jo Zirbel, John Calambokidas, Ari Friedlander, and the Captain and crew of the R/V New Horizon. David Moretti, Greg Schorr, and Erin Falcone provided guidance on the experimental design. We gratefully acknowledge the encouragement and logistical support of program manager John Hall.
Data accessibility
C.T.D. data are archived as part of the Rolling Deck Repository. Processed acoustic data will be archived in Dryad. doi:10.5061/dryad.g8g20.
Author contributions
K.J.B.-B. participated in the study design, the collection of field data, interpretation of results, and led the acoustical and statistical analysis, and the drafting of the manuscript. B.L.S. led collaborations with beaked whale studies and participated in study design, the collection of field data, interpretation of results, and drafting of the manuscript. M.A.M. led the AUV operations and participated in the study design, the collection of field data, interpretation of results, and drafting of the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Funding was provided by the Strategic Environmental Research and Development Program (SERDP).
References
- 1.Robison BH. 2004. Deep pelagic biology. J. Exp. Mar. Biol. Ecol. 300, 253–272. ( 10.1016/j.jembe.2004.01.012) [DOI] [Google Scholar]
- 2.Backus R, Craddock J, Haedrich R, Robison B. 1977. Atlantic mesopelagic zoogeography. Fish. Western North Atlantic 7, 266–287. [Google Scholar]
- 3.Robison BH. 1995. Light in the ocean's midwaters. Sci. Am. 273, 60 ( 10.1038/scientificamerican0795-60) [DOI] [Google Scholar]
- 4.Haedrich R. 1996. Deep-water fishes: evolution and adaptation in the earth's largest living spaces. J. Fish Biol. 49, 40–53. ( 10.1111/j.1095-8649.1996.tb06066.x) [DOI] [Google Scholar]
- 5.Clarke MR. 1996. The role of cephalopods in the world's oceans: an introduction. Phil. Trans. R. Soc. Lond. B 351, 979–983. ( 10.1098/rstb.1996.0088) [DOI] [Google Scholar]
- 6.Martin AR, Reeves RR. 2009. Diversity and zoogeography. In Marine mammal biology—an evolutionary approach (ed. Hoelzel AR.), pp. 1–37. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 7.Watwood SL, Miller PJO, Johnson MP, Madsen PT, Tyack PL. 2006. Deep-diving foraging behaviour of sperm whales (Physeter macrocephalus). J. Anim. Ecol. 75, 814–825. ( 10.1111/j.1365-2656.2006.01101.x) [DOI] [PubMed] [Google Scholar]
- 8.Hooker SK, Baird RW. 1999. Deep-diving behaviour of the northern bottlenose whale, Hyperoodon ampullatus (Cetacea: Ziphiidae). Proc. R. Soc. Lond. B 266, 671–676. ( 10.1098/rspb.1999.0688) [DOI] [Google Scholar]
- 9.McConnell B, Chambers C, Fedak M. 1992. Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarct. Sci. 4, 393–398. ( 10.1017/S0954102092000580) [DOI] [Google Scholar]
- 10.Robinson PW, et al. 2012. Foraging behavior and success of a mesopelagic predator in the northeast Pacific Ocean: insights from a data-rich species, the northern elephant seal. PLoS ONE 7, e36728 ( 10.1371/journal.pone.0036728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wormuth JH, Roper CF. 1983. Quantitative sampling of oceanic cephalopods by nets: problems and recommendations. Biol. Oceanogr. 2, 357–377. [Google Scholar]
- 12.Moline MA, Benoit-Bird KJ, O'Gorman D, Robbins IC. 2015. Integration of scientific echosounders with an adaptable autonomous platform to extend our understanding of animals from the surface to the bathypelagic. J. Atmos. Oceanic Technol. 32, 2173–2186. ( 10.1175/JTECH-D-15-0035.1) [DOI] [Google Scholar]
- 13.Schorr GS, Falcone EA, Moretti DJ, Andrews RD. 2014. First long-term behavioral records from Cuvier's beaked whales (Ziphius cavirostris) reveal record-breaking dives. PLoS ONE 9, e92633 ( 10.1371/journal.pone.0092633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falcone EA, et al. 2009. Sighting characteristics and photo-identification of Cuvier's beaked whales (Ziphius cavirostris) near San Clemente Island, California: a key area for beaked whales and the military? Mar. Biol. 156, 2631–2640. ( 10.1007/s00227-009-1289-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foote KG, Vestnes G, Maclennan DN, Simmonds EJ. 1987. Calibration of acoustic instruments for fish density estimation: a practical guide. ICES Coop. Res. Rep. 144, 57. [Google Scholar]
- 16.Sawada K, Furusawa M, Williamson NJ. 1993. Conditions for the precise measurement of fish target strength in situ. Fish Sci. 20, 15–21. ( 10.3135/jmasj.20.73) [DOI] [Google Scholar]
- 17.Benoit-Bird KJ, Gilly WF, Au WWL, Mate BR. 2008. Controlled and in situ target strengths of the jumbo squid Dosidicus gigas and identification of potential acoustic scattering sources. J. Acoust. Soc. Am. 123, 1318–1328. ( 10.1121/1.2832327) [DOI] [PubMed] [Google Scholar]
- 18.Benoit-Bird KJ, Gilly WF. 2012. Coordinated nocturnal behavior of foraging jumbo squid Dosidicus gigas. Mar. Ecol. Prog. Ser. 455, 211–228. ( 10.3354/meps09664) [DOI] [Google Scholar]
- 19.McClatchie S, Macaulay G, Coombs RF. 2003. A requiem for the use of 20logLength for acoustic target strength with special reference to deep-sea fishes. ICES J. Mar. Sci. 60, 419–428. ( 10.1016/S1054-3139(03)00004-3) [DOI] [Google Scholar]
- 20.Merrett N, Gordon J, Stehmann M, Haedrich R. 1991. Deep demersal fish assemblage structure in the Porcupine Seabight (eastern North Atlantic): slope sampling by three different trawls compared. J. Mar. Biol. Assoc. UK 71, 329–358. ( 10.1017/S0025315400051638) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
C.T.D. data are archived as part of the Rolling Deck Repository. Processed acoustic data will be archived in Dryad. doi:10.5061/dryad.g8g20.