Abstract
Frequency-dependent selection may drive adaptive diversification within species. It is yet unclear why the occurrence of alternative reproductive tactics (ARTs) is highly divergent between major animal taxa. Here we aim to clarify the environmental and social conditions favouring the evolution of intra-population variance of male reproductive phenotypes. Our results suggest that genetically determined ARTs that are fixed for life evolve when there is strong selection on body size due to size-dependent competitiveness, in combination with environmental factors reducing size benefits. The latter may result from growth costs or, more generally, from age-dependent but size-independent mortality causes. This generates disruptive selection on growth trajectories underlying tactic choice. In many parameter settings, the model also predicts ARTs to evolve that are flexible and responsive to current conditions. Interestingly, the conditions favouring the evolution of flexible tactics diverge considerably from those favouring genetic variability. Nevertheless, in a restricted but relevant parameter space, our model predicts the simultaneous emergence and maintenance of a mixture of multiple tactics, both genetically and conditionally determined. Important conditions for the emergence of ARTs include size variation of competitors, which is inherently greater in species with indeterminate growth than in taxa reproducing only after reaching their terminal body size. This is probably the reason why ARTs are more common in fishes than in other major taxa.
Keywords: ESS, evolutionary branching, genetic polymorphism, individual-based simulations, sexual selection, sperm competition
1. Introduction
During the past 150 years, substantial conceptual and empirical progress in evolutionary biology has enhanced our understanding of how and why the mean expression of phenotypic traits is changing within populations in response to natural and sexual selection [1]. More recently, important insights have also been made to explain within-population trait variation as a result of natural selection [2]. Such adaptive variation may emerge from selection for specialization in terms of, for instance, resource utilization [3], competitiveness [4] or ‘personality’ [5]. Prominent examples of such apparent intra-population trait variation come from the study of alternative reproductive tactics (ARTs) [6–10]. Nevertheless, the interplay between ecology and the emergence of intra-population trait variation still remains enigmatic [5,11].
In many animal species, individuals within one sex adopt different tactics in order to reproduce [7,8]. This may for instance be reflected in discrete distributions of behavioural, physiological and/or morphological characteristics [12]. ARTs may occur in both sexes. Yet, although female ARTs have evolved occasionally (see e.g. [13]), far more examples of ARTs have been described for males [12,14]. Typically, male ARTs conform to a ‘producer-scrounger’ type [11], with one ‘bourgeois’ tactic defending territories or nests in which females will reproduce, whereas ‘parasitic’ tactics are characterized by their intra-sexual exploitation of this reproductive resource [12]. This can be achieved, for instance, by sneaking into bourgeois males' territories to mate there with females. Obviously, these contrasting tactics will select for divergent phenotypes [15,16]. Dominant males will have a benefit from strong competitiveness or fighting prowess (generally referred to as resource holding potential), whereas sneaking males will often benefit from a cryptic appearance combined with swiftness and speed. ARTs thus represent a fascinating case of intra-population specialization manifested in many behavioural and life-history traits. Yet we have an imperfect understanding of the environmental conditions favouring the evolutionary emergence of such specialization.
To understand the evolution of ARTs, it is important to acknowledge that different kinds of ARTs can be distinguished, dependent on when and how tactic-specific phenotypic determination occurs. One important distinction is between ‘fixed’ and ‘flexible’ alternatives [16]. The key difference is that when males apply fixed tactics, the ‘decision’ of which tactic to use is made irreversibly at one stage (usually early) in their lifetime. This is exemplified by ARTs in many insects [10,17–19], where males either develop large weapons important in territorial fights or spare this investment [20]. Yet once they are sexually mature they cannot switch tactics. This is distinctly different from flexible systems in which, for instance, males use one tactic when being young or small, and eventually switch tactic use when they get older and larger (e.g. [6,21,22]). Regardless of whether tactics are fixed for life or flexible, there is usually genetic variance regarding the environmental thresholds at which individuals should express one or the other phenotype [11,23]. Rarely, ARTs are determined by simple genetic mechanisms (e.g. [24–26]), but the vast majority of ARTs are apparently conditionally determined and subject to significant external influences [7,8,10]. Possibly, the factors favouring a conditional expression of tactics, such as a high reliability of environmental or status cues [27,28] and low cost of plasticity [29], often occur in mating systems with ARTs [10].
Considerable research effort has been devoted to understand the stable coexistence of alternative reproductive behaviours in a population. The prime explanation for a polymorphism of ARTs is negative frequency-dependent selection [8,12,30]. This mechanism seems very plausible regarding the maintenance of producer-scrounger types of ARTs: if a bourgeois tactic becomes very common, it will increase the fitness of males using the parasitic tactic because there will be many males whose investment can be exploited, and competition from other parasitic males will be low. If reproductive parasites are very common, however, each parasitic male will have relatively low reproductive success due to intense competition from other parasitic males, and males pursuing the bourgeois tactic will be relatively better off. Nevertheless, few studies have actually demonstrated negative frequency-dependent selection acting on ARTs in natural populations [13,24,31].
Status-dependent selection may also generate scope for ARTs. For instance, males using a less beneficial tactic may apply a ‘best-of-a-bad-job’ strategy [17]. Here, phenotypic variation in ARTs can be explained if variation is maintained in some underlying state-character, such as condition, size or age. In that case, applying one tactic will be beneficial in certain conditions, whereas applying the other will be beneficial in other conditions [8,23]. The idea of a status-dependent switch-point at which males should use different tactics has generally found good empirical support in cases where tactics obviously result in unequal fitness benefits (e.g. [18]).
Interestingly, in some species males apply more than two different ARTs. These can either be determined by the same mechanism, for instance coexistence of distinct genotypes (e.g. [24,25]) or status-dependence including multiple states [19], or by a mixture of mechanisms. Multiple ARTs seem to occur especially in fish mating systems [16]. For instance, in the cichlid Lamprologus callipterus, three tactics co-occur: a parasitic tactic adopted by genetically distinct dwarf males [26], an opportunistic sneaker tactic applied mainly by small males before they eventually become territorial, and a bourgeois tactic of males building nests to attract mates [32]. Likewise, the ocellated wrasse Symphodus ocellatus may adopt three different tactics involving nest-building territory owners, collaborative satellite and purely parasitic sneaker males [21]. A similar phenomenon has been observed in the bluegill sunfish Lepomis macrochirus, in which males are either nest holders, sneakers or satellites, which mimic female behaviour [33]. The evolution and maintenance of such multiple ARTs is currently not well understood [34], possibly except in circumstances where these follow so-called rock–paper–scissors dynamics [24].
The current frameworks modelling the occurrence of ARTs have been extremely useful to predict, for instance, the expected frequencies of different morphs, and the evolutionarily stable thresholds at which males should switch between tactics. Nevertheless, our understanding of the environmental conditions favouring the evolution of ARTs in the first place is much more limited. Indeed, theoretical attempts to model the coexistence [8,30,34] and genetic architecture [27,29] of ARTs typically assume that two distinct phenotypes already exist in a population. By contrast, the evolutionary emergence of distinct types from initially continuously distributed characters, which is a conceptually disparate question [2,35], has not been addressed by theoretical studies of ARTs, as these typically focus on the maintenance of already present variation. To illustrate this conceptual difference, the explanatory framework to understand the ecological conditions favouring the evolution of anisogamy [36] is distinctly different from sex allocation theory [37], which aims to understand sex-ratio variation and investment in male and female function.
Here, we take an original approach towards understanding the impact of environmental conditions on the emergence and maintenance of ARTs. Our aim is to model well-known and widespread general situations. We therefore develop a model reflecting a frequently observed mating pattern with competition for ‘territories’ limited in supply (see, e.g. [7,15]). We assume indeterminate body growth (as in most invertebrate and vertebrate lineages; see [38]), and that the ability to acquire and monopolize a territory will depend on male size (see [20,39]). Furthermore, investment in growth is an evolving strategy. Our assumptions are thus well anchored in natural systems and combine reality with generality. We use this model to unravel underlying evolutionary mechanisms, and specifically analyse under which ecological and competitive circumstances two (or eventually more) tactics spontaneously evolve from an initially monomorphic population. Furthermore, the model does not make inherent assumptions whether tactic choice is a fixed, irreversible lifetime decision or whether individuals are able to use tactics flexibly. Instead, in our model these attributes can evolve, also enabling conclusions about which underlying mechanism of tactic choice is likely to emerge.
2. Basic model assumptions
We used an individual-based simulation approach with reproduction occurring in discrete bouts at every time step. At each time step individuals compete for territories, which will be acquired by the most competitive individuals (see [4] for a similar modelling approach, [39] for empirical data). Males unable to compete for territories successfully may still attempt to reproduce, but all males with reproductive potential will bear the cost of sperm production, which is traded off against growth and somatic maintenance (see e.g. [40]). At each reproductive bout, a certain number of offspring are produced and the new population will be formed by these and the survivors from the previous reproductive bout. Survival probability is determined by individual mortality rate, which depends both on size and on the growth and sperm investment strategy of each individual (see below for specific assumptions and their justification). There are thus two strategies evolving in the population, a growth- and a sperm-investment strategy, both affecting reproductive success and mortality (see table 1 for an overview of fixed and evolving model parameters).
Table 1.
Definition of variables and parameters used in the model.
| parameter | description | evolving/fixed |
|---|---|---|
| growth | ||
| L | asymptotic body size | evolving |
| K | initial growth rate | evolving |
| α | basal Gompertz mortality rate | fixed |
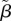 |
effect of growth on age-specific mortality rate | fixed |
| size-dependent mortality | ||
| μi | mortality rate of very large individuals | fixed |
| μσ, μ0 | size-dependent mortality change | fixed |
| premating intra-sexual selection | ||
| n | number of territories in population | fixed |
| ɛ | effect of body size on territory acquisition | fixed |
| σT | minimum body size required for territory holders | fixed |
| postmating intra-sexual selection | ||
| sa, sb, sc | size-dependent sperm investment strategy | evolving |
| r | loading factor (relative advantage of parasitic males) | fixed |
| γ, σp | size-dependent sneaking ability | fixed |
3. Growth and mortality
(a). Growth
Generally, body growth rate is fastest during early life stages and declines with increasing size and age [41]. This is captured by modelling growth according to the van Bertalanffy growth equation:
| 3.1 |
In this expression, L denotes asymptotic body size and K is a measure of relative growth rate. There are thus two evolving growth parameters comprising a growth strategy. This equation is apt to model indeterminate growth and has typically been applied to model fish growth, where it seems particularly suitable (cf. [41]). However, we note that the assumption remains adequate even for species with determinate growth, as long as not all characteristics of individual size or condition are fixed at the onset of sexual maturity (as typically in birds, mammals and insect income breeders). Assumptions and results from an extension of this indeterminate growth model implying a fixed body size at sexual maturation (as, for instance, in insect capital breeders) is presented in the electronic supplementary material.
(b). Growth costs—longevity and ageing
There are several lines of evidence demonstrating costs of an increased early-life growth rate [42,43]. In our model, the cost of growth is therefore manifested in reduced longevity. Specifically, we assume individuals with an initial faster growth rate will pay these costs later in life in terms of an increase in their age related mortality rate, thus in an increased rate of senescence. In our model, mortality rate conforms to the Gompertz model, 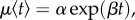 where t is age, and α and β are parameters that represent the baseline (age-independent) mortality and senescent component (age-dependent), respectively (see e.g. [44]). In the van Bertalanffy growth model, individuals have an initial (and maximal) growth rate of
where t is age, and α and β are parameters that represent the baseline (age-independent) mortality and senescent component (age-dependent), respectively (see e.g. [44]). In the van Bertalanffy growth model, individuals have an initial (and maximal) growth rate of 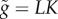 . We assume that the magnitude of this growth rate affects the rate at which they will later age, thus
. We assume that the magnitude of this growth rate affects the rate at which they will later age, thus 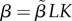 and
and 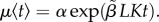 Together with the size-dependent mortality rate (see below), the assumptions conform largely to Siler's [45] competing hazard model.
Together with the size-dependent mortality rate (see below), the assumptions conform largely to Siler's [45] competing hazard model.
(c). Size-dependent mortality
Smaller individuals often have a higher mortality rate (μ) due to, for instance, predation (i.e. (dμ/dσ) < 0; e.g. [46–48]). This size-dependent mortality is modelled here as (from [49])
| 3.2 |
where μi is the mortality rate for very large individuals and μσ a parameter specifying how strongly mortality rate changes with size, while the parameter σ0 characterizes at which size the size-dependent component of mortality drops to e−1 = 0.368, relative to its value at size σ = 0.
4. Competition for territories
We assume that reproduction takes place within a restricted number of locations (n). Usually, larger and heavier males will be superior competitors [39] and thus better able to acquire these territories. Nevertheless, larger males will not always outcompete smaller males, yet the probability that they will do so is likely to increase with increasing differences in body size (see e.g. [50]). An intuitive way to model this is to assume that body size (σ) reflects true competitiveness, whereas realized competitiveness (c) may deviate somewhat from this in any given situation. Specifically,
| 4.1 |
where ζ is a random number drawn from a normal distribution with mean 0 and standard deviation ɛ. The parameter ɛ thus determines how strongly territory acquisition is dependent on body size. Small values of ɛ signify that males will always win against infinitesimally smaller individuals, whereas large values indicate that territory acquisition will be a random event and thus independent of body size (see [4] for a similar approach).
In many cases, males must have a certain minimum size to effectively defend territories. This is the case, for instance, when territories are partly built or constructed by males. In the cichlid fish, Lamprologus callipterus, for instance, males collect snail shells as female egg-laying substrate, and only males larger than 9 cm standard length are able to lift and swim with these shells [51]. Such an effect can additionally be incorporated in the model by assuming that competition for territories only occurs between males with a body size (σ) larger than a certain threshold size σT.
5. Competition for fertilizations
(a). Access to territories
Individuals not acquiring a territory may use an alternative tactic and attempt to sneak fertilizations in territories of dominant males. Their success in doing so is often dependent on body size [52–56]. Our approach is related to most empirical (i.e. statistical) approaches for analysing changes in the probability of an outcome (logistic regression). In principle, we assume that the log-odds that a sneaking male will successfully enter a dominant male's territory and steal fertilizations there changes linearly with size:
| 5.1 |
Thus, the parameter σp specifies at which size the log-odds of success is zero, hence parasitic males have a 50% chance to succeed. The parameter γ, on the other hand, determines how strongly parasitic ability decreases with increasing size (or increases if γ < 0). These assumptions translate into a logistic equation describing the probability of sneaking success as a function of body size
| 5.2 |
For most simulations, we will assume that sneaking success increases with decreasing size (i.e. γ > 0). There are several lines of evidence indicating that this is most often the case, for instance due to inconspicuousness and increased manoeuvrability [52–54].
(b). Cost of sperm investment
Fertilization success in sperm competition is often determined by males' sperm investment (see e.g. [57]), and larger males usually have larger testes [58]. In our model, sperm investment is an evolving size-dependent strategy. Specifically, we model sperm investment in terms of the associated costs males pay for sperm production. We further assume that there is a trade-off between sperm production and investment in maintenance (see e.g. [40]) so that an increase of sperm production is expressed as an increase in mortality rate. Thus, investment cost is modelled as mortality rate due to cost of sperm production conditioned on male size:
| 5.3 |
The evolving parameters sa, sb and sc define the form of the size-dependent sperm investment reaction norm. The quadratic term allows that distinct tactics potentially can evolve. Without it, the assumptions would force sperm investment to change linearly with size, yet we are interested also in nonlinear scenarios where, for instance, only small and large males invest in sperm. Explicitly assuming that the cost of sperm production is related to body size, the investment cost associated with actual sperm production is as follows:
| 5.4 |
(c). Sperm raffle and roles
Fertilization success may not only be influenced by male sperm reserves, but also by male roles [59]. Owing to their distinct roles, parasitic males may have a fertilization advantage or disadvantage relative to territorial males (e.g. [26,60]). This may depend, for instance, on a difference in ejaculate timing, or tactic-specific differences in spatial proximity to the females/eggs (see e.g. [54]). In concordance with the well-established loaded raffle principle [59], we let the loading factor r determine parasitic male advantage (disadvantage if r < 1) in relation to the territorial male according to
| 5.5 |
where vp denotes the fertilization of a parasitic male having sperm reserves equalling sp in competition with a territorial male investing st (and possibly n other parasitic males investing sp,i). Assumptions on asymmetric sperm competition success can easily be relaxed by assuming r = 1, signifying a fair raffle.
6. Individual-based simulations
(a). General simulation structure
In the individual-based simulations, we assume haploid genetics with 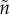 offspring sired in each territory at each time step. Mutations take place during the formation of new individuals at a rate of 0.001 and a mutation step that is normally distributed around 0.05. Following mutation and recombination, it is determined (depending on size- and age-dependent mortality
offspring sired in each territory at each time step. Mutations take place during the formation of new individuals at a rate of 0.001 and a mutation step that is normally distributed around 0.05. Following mutation and recombination, it is determined (depending on size- and age-dependent mortality 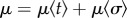 ) which individuals are likely to survive until the next reproductive bout. At each time step, reproduction takes place as described above (territory acquisition, potential sneaking and sperm competition, fertilization). Simulations were generally performed for 10 000 reproductive bouts, as this was generally sufficient for populations to stabilize at an evolutionary equilibrium.
) which individuals are likely to survive until the next reproductive bout. At each time step, reproduction takes place as described above (territory acquisition, potential sneaking and sperm competition, fertilization). Simulations were generally performed for 10 000 reproductive bouts, as this was generally sufficient for populations to stabilize at an evolutionary equilibrium.
(b). Identification of the evolution of alternative reproductive tactics
Potential genetically distinct tactics were identified in a two-step process. A genetic polymorphism would be manifested in a bimodal distribution of the evolving growth and sperm investment parameters. We therefore routinely performed a statistical analysis on the distribution of these parameters in the final populations, using the test.equality function in the R package mixtools [61]. This tests the hypothesis that a distribution is homogeneous against the alternative that it consists of a mixture of distributions, yet the procedure is sensitive to cryptically overlapping distributions. This does not necessarily reflect a polymorphism but may, for instance, reflect the spread of an advantageous genotype in a population, which is not entirely at its evolutionary equilibrium. The positives of this first scan were therefore subsequently manually inspected, and we regarded a genetic polymorphism to have established only if the distributions were truly non-overlapping. Furthermore, we performed four simulations with different starting conditions for each unique parameter combination, and more than half of the simulations (i.e. at least three out of four) had to be positives to be regarded as a genetic polymorphism. The repeatability of simulation outcomes was very high, so usually either none or all four simulations resulted in a polymorphism.
One cannot use the genotypes to identify any conditionally expressed flexible tactics. Instead, we identified the occurrence of successful sneaking attempts of sexually mature males (i.e. s > 0), which were of the same genotype as territory holders, yet did not secure a territory, as a diagnostic of an evolving conditional alternative tactic. If this frequency on average exceeded 1% in the last hundred generations of each simulation, we considered it to be an alternative tactic. This criterion level is high enough to disregard non-adaptive mutant tactics inevitably present in the population, and sufficiently low to avoid inadvertent exclusion of biologically significant alternative tactics occurring at low frequencies.
Table 1 reveals that the model parameter space is relatively large, and that we had to trade off range for resolution. For each set of simulations, we therefore divided the values of two focal parameters in a 10 × 10 grid to perform a first round of simulations. To increase our resolution specifically in parts with an ambiguous outcome, we further divided those sections where the outcome in neighbouring sections were qualitatively different in a smaller 3 × 3 grid to perform a second round of simulations. Here we present results from 9728 simulations in total, using 2432 different parameter combinations, where we focused on the effect of territory competition, growth costs, sperm competition, size-dependent mortality and sneaking ability on the evolution of ARTs.
7. Results
(a). The evolution of genetically fixed alternative reproductive tactics
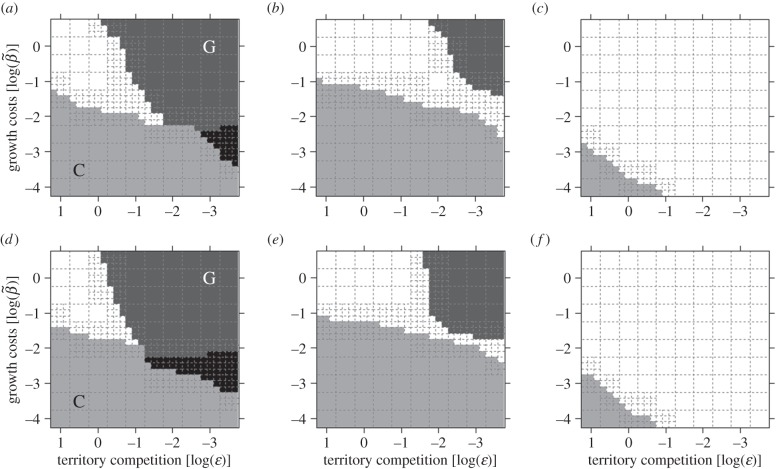
In our model, the parameter ɛ determines how strongly male resource holding potential is influenced by body size (i.e. how intensively selection on body size will be influenced by territorial competition). Generally, this parameter strongly affects the evolution of ARTs based on a genetic polymorphism (figures 1 and 2). This is especially true in combination with high growth costs (figure 1), which are reflected by reduced longevity. In these situations, there are two contrasting factors affecting selection on growth rate in opposite directions: strong competition for territories, which selects for increased growth, and growth-inflicted mortality costs, which reduce growth benefits. Furthermore, the evolution of a genetic polymorphism is facilitated by a fair raffle of sperm (figure 1). When parasitic males have a strong fertilization advantage, ARTs evolve under more restricted conditions only (figure 1b,e), and a territorial male advantage in sperm competition completely eliminated the potential for genetic ARTs in the investigated parameter space.
Figure 1.
Evolutionary emergence of a genetic polymorphism (G, dark grey areas), conditionally flexible tactics (C, light grey areas) and a mixture of three ARTs (black areas) in our simulations depending on varying parameter values of growth costs (y-axis), selection intensity on body size due to territorial competition (x-axis; decreasing values reflect strong effect of body size on territory acquisition) and sperm raffle mechanism: (a) fair raffle (r = 1), (b) parasitic advantage (r = 10), (c) bourgeois advantage (r = 0.1). Panels (d–f) correspond to panels (a–c), respectively, except for the additional assumption that males need a certain minimum size (σT = 25) in order to establish and defend a territory. The centre of each dashed-line quadrat signifies a parameter value combination that was repeated four times. Other parameter values were σT = 0, n = 500, α = 10−6, μi = 10−4, μ0 = 10, σp = −1, μσ = −2, γ = 1.5.
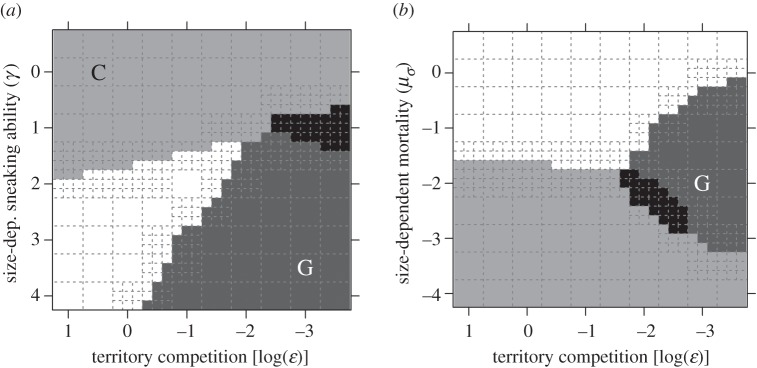
Figure 2.
Evolutionary emergence of a genetic polymorphism (G, dark grey areas), conditionally flexible tactics (C, light grey areas) and a mixture of three ARTs (black areas) in our simulations depending on varying parameter values of selection intensity on body size due to territorial competition (decreasing values reflect strong effect of body size on territory acquisition) and (a) size-dependent sneaking ability and (b) size-dependent mortality. In (a) negative values of log(γ) indicate lower sneaking ability for large individuals. In (b) negative values of log(μσ) indicate lower mortality for large individuals. The centre of each dashed-line quadrat signifies a parameter value combination that was repeated four times. Other parameter values were: r = 1, σT = 0, n = 500, α = 10−6, μi = 10−4, μ0 = 10, σp = −1, 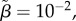 μσ = −2(a), γ = 1.5.
μσ = −2(a), γ = 1.5.
In addition, we found that assuming a minimum size for males in order to use a territory slightly increased the potential for a genetic polymorphism to evolve, yet the effect was rather marginal (cf. figure 1a–c and d–f). Two other environmental factors affecting selection on male body size strongly affected whether genetically distinct ARTs evolved or not, namely size effects on sneaking ability and size-dependent mortality rate (figure 2). The likelihood of the emergence of a genetic polymorphism is greater if sneaking ability decreases strongly with increasing size (figure 2a). The pattern for size-dependent mortality rate was more complex. Genetic polymorphisms were absent both when size-dependent mortality rate was very strong and when very weak, suggesting that it only evolves at intermediate levels (figure 2b).
(b). The evolution of conditionally flexible alternative reproductive tactics
The circumstances favouring the evolution of flexible ARTs were generally different from those favouring genetically determined ARTs fixed for life (cf. figures 1 and 2). Large costs of growing, which facilitate the evolution of genetic ARTs, severely limit the evolution of flexible tactics. Furthermore, competition-related selection on body size, which favours the evolution of genetically determined ARTs, reduces the potential for flexible tactics to emerge, albeit less strongly than large costs of growing (figure 1). The expression of conditionally flexible tactics was also favoured under conditions where selection on growth and body size were relaxed. In particular, weak size-dependent sneaking ability (figure 2a) and mortality (figure 2b) promote the occurrence of a flexible tactic.
(c). The evolution of multiple tactics
Certain parameter combinations favoured the evolution of multiple ARTs, so that three male types coexisted within the population, two parasitic types developing from separate life-history trajectories and a bourgeois male type. As the conditions favouring genetically fixed versus conditionally flexible tactics were generally distinctly different, and coexistence of similar phenotypes may be further constrained by competitive exclusion, the areas of overlap were rather restricted (figures 1 and 2). Nevertheless, in those cases where multiple ARTs evolved, the population always consisted of one bourgeois and two parasitic tactics, of which one was conditional and one was genetically determined.
(d). Relative sperm investment
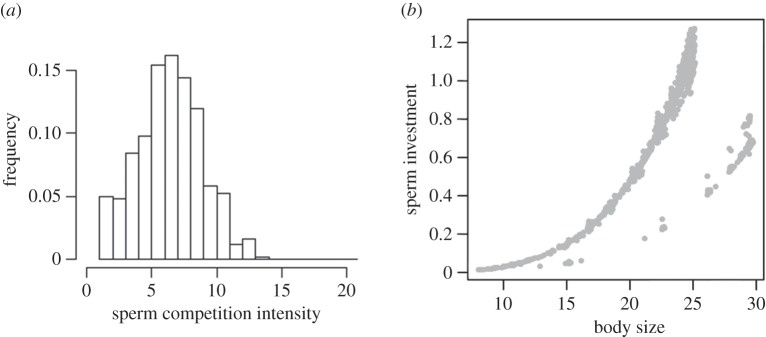
In all simulations, the relative sperm investment evolved to a higher level for the tactics applying parasitic reproduction. This was true irrespective of whether there was an asymmetry in sperm competition risk between the two tactic types (cf. figure 3).
Figure 3.
Exemplary results from a simulation resulting in a genetic polymorphism with large bourgeois individuals (occurring at low frequency) and smaller parasites (occurring at high frequency. (a) The frequency distribution of the number of successful sneaking attempts in each territory (=sperm competition intensity), whereas (b) is the size-sperm investment relationship for the two morphs (represented here by dots for each individual in the population). Parameter values: r = 1, σT = 0, n = 500, α = 10−6, μi = 10−4, μ0 = 10, σp = −1, μσ = −2, γ = 1.5, ɛ = 10−3.
8. Discussion
This study attempts to unravel the ecological and social circumstances in which ARTs evolve from an initially homogeneous population. We used very general assumptions; thus our model inferences are not limited to restricted conditions, specified, for instance, by the mode of fertilization (internal or external). Also, our assumption of indeterminate growth is not necessarily restrictive. As long as some characteristic affecting male tactic-specific reproductive success (e.g. body condition) can increase after sexual maturation, our results will still pertain even if adult body size is fixed. By means of an individual-based simulation model, we capture many of the typical features seen in natural systems. In some situations, populations remained homogeneous with respect to mating tactics, whereas in others genetically fixed ARTs, size-dependent conditional ARTs or a mixture of both evolved.
Our simulations revealed that the selection regimes facilitating the evolution of genetically fixed and conditionally flexible tactics are notably different. Genetically distinct tactics evolve especially when selection on growth and body size is acting in opposite directions. For instance, our simulations showed that a combination of severe growth costs and strong selection on body size due to competition for territories increases the likelihood of genetically distinct ARTs. Here, male–male competition will select for fast growth and large body size, as only the largest males will be able to exploit their reproductive potential. Yet, simultaneously, growth costs will be large. Growing slightly slower will relax the costs of growing, but this will be extremely unfavourable in terms of territory acquisition. Remaining much smaller, however, will strongly reduce growth costs and thereby create a different mating niche to males, which then can parasitize the reproductive effort of other males. Hence, intermediate phenotypes will be selected against resulting in disruptive selection acting on male growth trajectories. Crucially, as the major element favouring small body size is to avoid growth costs, the determination of the growth trajectory must be made early in life, thus selecting for genetically fixed tactics.
The evolution of conditionally flexible tactics, on the other hand, seems not to be facilitated by disruptive selection. The probable reason for this is that, ultimately, smaller males applying a sneaker tactic will later grow to become large. Thus, applying a different tactic will not release males from the costs and constraints of growing large. Instead, a conditionally flexible tactic can only be advantageous when the cost of applying it does not severely compromise reproductive potential later in life. A conditional parasitic tactic will impose additional costs due to, for instance, sperm investment. A male applying a sneaker tactic can thus either maintain a high growth rate at a cost caused by a reproductive handicap or maintain energy expenditure by enhancing early reproduction at the cost of reduced growth. This line of reasoning also applies to insects, for instance, despite their fixed body size, if success as a bourgeois male depends on some other body characteristic (such as body condition) that changes in the adult stage, and as long as applying a parasitic tactic withdraws resources from developing this trait. Our simulations reveal that a conditionally flexible tactic is likely to evolve only when the additional cost to growth is small (figure 1), the cost of reduced growth in terms of size-dependent mortality is small (figure 2b) and/or when the benefit of a conditional tactic is high, due to high sneaking success even when males are large (figure 2a).
Given that separate selection regimes facilitate the evolution of genetically fixed and conditionally flexible ARTs, the simultaneous evolution of three ARTs in a population was a relatively infrequent outcome of our simulations. Nevertheless, certain parameter combinations resulted in a mixture of three tactics in the final populations—a fixed-for-life parasitic tactic, a parasitic tactic applied by small males on a different life-history trajectory allowing males to adopt the bourgeois tactic later in life, and a bourgeois tactic. This possibly reflects its incidence in nature, where such a phenomenon does occur in a number of species (e.g. [21,26,33]). Indeed, the co-occurrence of fixed and flexible ARTs may be further constrained by the fact that coexisting flexible and genetic parasitic tactics will compete against each other, reducing the benefit of applying a parasitic tactic. In some parameter combinations, it is conspicuous that a genetic polymorphism did not evolve even though the pattern implied by simulations with adjacent parameter settings strongly suggests so. For instance, the combination of intense selection on body size due to territory competition and weak size-dependent mortality did not result in a genetic polymorphism, but in a conditionally determined sneaking tactic (bottom right of figure 2b). Whereas it is conceivable that a sneaker tactic is absent when mortality of small individuals is high (top right of figure 2b), it is not immediately apparent why the genetic polymorphism disappears when this assumption is strongly relaxed. Possibly, the benefit of remaining small will be strongly reduced because these conditions also favour the evolution of a flexible tactic, and this will considerably increase reproductive interference between the two sneaker tactics. We can examine this supposition by comparing the results with the complementary implementation of our model assuming a fixed size at sexual maturation (see electronic supplementary material, figure S1). Here, the expression of any size-dependent flexible parasitic tactic is prevented, as individuals will never change size when reproductively active. Indeed, a genetically determined fixed-for-life tactic evolves under a broader range of conditions in this situation. This possibly accounts for the observation that, overall, conditionally flexible ARTs seem to be considerably more frequent than genetically determined ARTs, and that multiple types relatively rarely co-occur [8]: the less constrained conditions leading to the evolution of a conditionally flexible tactic will frequently lead to the competitive exclusion of genetically determined ARTs.
In our simulations, the evolution of ARTs was especially favoured when there was a fair raffle of sperm compared with situations with a loaded raffle (figure 1). This was initially surprising, as one would naively expect a sperm competition advantage of parasitic males to favour the evolution of parasitic tactics. However, a strong parasitic sperm advantage completely degrades the benefit for males to grow large and defend a reproductive territory, which will act against the evolution of ARTs. Instead, males remain small and mutually steal fertilizations from each other. Thus, the mating pattern evolves towards group spawning. Also, the effect of assuming a minimum threshold size for territorial males did not correspond fully to our initial predictions. Intuitively, this should increase the potential for disruptive selection on male body size and thus the evolution of a polymorphism, as intermediately sized males should be disfavoured. However, in most situations where a genetic polymorphism did evolve, the strength of selection on male body size due to territory competition was intense, and most territorial males nonetheless outgrew this threshold size by far. Therefore, this additional disadvantage of intermediate size had only a marginal effect.
In all simulations, we found that males adopting parasitic tactics should invest relatively more in sperm competition than males applying a dominant bourgeois tactic. Partly this was expected, partly it was not. Often, sperm competition risk will be asymmetrically distributed across tactics: parasitic males will always face sperm competition, whereas bourgeois males will only occasionally face sperm competition. Theoretically, it has been shown that this asymmetry should select for different sperm allocation strategies, where sneaker males should invest more [59]. This theoretical prediction has generally been shown to hold (e.g. [32,58,62]). Nevertheless, here we found this pattern also when there was no asymmetry in sperm competition risk, hence when sperm competition levels were so high that bourgeois males also almost invariably face sperm competition (figure 3). Our interpretation is that the trade-off between growth and sperm investment will be stronger for bourgeois males, which will only be able to capitalize on the superior success of their tactic if they grow sufficiently large. Thus, the discrepancy in sperm investment usually observed across male tactics may be only partly due to the fact that sperm investment is more beneficial for parasitic males. The discrepancy is likely to be amplified by the circumstance that investing in sperm will be more costly in terms of reduced growth for bourgeois than for parasitic males. This result thus highlights the importance of separating the beneficial and costly aspects of trait development in order to fully understand phenotype evolution.
During the last decades, our understanding of adaptive diversification processes within populations has profoundly progressed (see e.g. [2]). The coexistence of ARTs is a prominent example of conspicuous within-population variability [7]. Nevertheless, understanding the population and environmental conditions promoting the evolution of ARTs has remained unclear. Theoretical studies of ARTs have focused on a disruptive selection scenario in which two distinct behaviour types were already present (see e.g. [8,23,30,34]). The question is then rather what favours a fixed genetic versus a conditional (yet irreversible) nature of strategies (e.g. [27,29]). In our approach, we do not inherently assume disruptive selection; instead, it results as an emergent property. We were interested in the environmental conditions generating selection on a monomorphic strategy, so that a different ‘mating niche’ may emerge. This therefore reflects an attempt to develop a general, ecologically explicit framework to understand the conditions favouring both the initial emergence as well as the maintenance of ARTs. We have outlined conditions under which genetically fixed versus conditionally flexible tactics are likely to evolve, and we have shown that these conditions are generally different. Nevertheless, we also identified the restricted conditions promoting the evolution of multiple tactics within populations that are controlled by different selection mechanisms. Our model framework facilitates the understanding of the occurrence of ARTs in natural populations by providing hypotheses that can be tested, for instance by using a comparative approach linking the occurrence of ARTs to specific ecological and social factors.
ARTs represent a distinct case of a broader class of within-population variation. Our model could therefore be used as a template and stimulate future approaches to better understand the emergence of plasticity and genetic polymorphism as a more general phenomenon, for instance, by illuminating the evolution of within-population behavioural variability exemplified by ‘personality’ trait variation (for review see [63]).
Supplementary Material
Data accessibility
All relevant data generated in this simulation study has been presented either in the article or in the electronic supplementary material.
Authors' contributions
M.T. conceived the study. L.E. and M.T. jointly designed the modelling framework. L.E. wrote the model, analysed the results and drafted the manuscript. L.E. and M.T. wrote the final version.
Competing interests
We have no competing interests.
Funding
Our research was funded by the Swiss National Science Foundation, grant no. 310030B_138660 to M.T.
References
- 1.Darwin C. 1859. On the origin of species by means of natural selection: or the preservation of favoured races in the struggle for life. London, UK: John Murray. [PMC free article] [PubMed] [Google Scholar]
- 2.Rueffler C, Van Dooren TJM, Leimar O, Abrams PA. 2006. Disruptive selection and then what? Trends Ecol. Evol. 21, 238–245. ( 10.1016/j.tree.2006.03.003) [DOI] [PubMed] [Google Scholar]
- 3.Dieckmann U, Doebeli M. 1999. On the origin of species by sympatric speciation. Nature 400, 354–357. ( 10.1038/22521) [DOI] [PubMed] [Google Scholar]
- 4.Baldauf SA, Engqvist L, Weissing FJ. 2014. Diversifying evolution of competitiveness. Nat. Commun. 5, 5233 ( 10.1038/ncomms6233) [DOI] [PubMed] [Google Scholar]
- 5.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. ( 10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 6.Taborsky M. 1998. Sperm competition in fish: ‘bourgeois’ males and parasitic spawning. Trends Ecol. Evol. 13, 222–227. ( 10.1016/S0169-5347(97)01318-9) [DOI] [PubMed] [Google Scholar]
- 7.Oliveira RF, Taborsky M, Brockmann HJ. 2008. Alternative reproductive tactics: an integrative approach. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Gross MR. 1996. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 11, 263 ( 10.1016/0169-5347(96)81050-0) [DOI] [PubMed] [Google Scholar]
- 9.Radwan J. 2009. Alternative mating tactics in Acarid mites. Adv. Study Behav. 39, 185–208. ( 10.1016/S0065-3454(09)39006-3) [DOI] [Google Scholar]
- 10.Buzatto BA, Tomkins JL, Simmons LW. 2014. Alternative phenotypes within mating systems. In The evolution of mating systems (eds Shuker DM, Simmons LW), pp. 106–128. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Taborsky M, Brockmann HJ. 2010. Alternative reproductive tactics and life history phenotypes. In Animal behaviour: evolution and mechanisms (ed. Kappeler P.), pp. 537–586. Berlin, Germany: Springer. [Google Scholar]
- 12.Taborsky M, Oliveira RF, Brockmann HJ. 2008. The evolution of alternative reproductive tactics: concepts and questions. In Alternative reproductive tactics: an integrative approach (eds Oliveira RF, Taborsky M, Brockmann HJ), pp. 1–21. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Svensson EI, Abbott J, Hardling R. 2005. Female polymorphism, frequency dependence, and rapid evolutionary dynamics in natural populations. Am. Nat. 165, 567–576. ( 10.1086/429278) [DOI] [PubMed] [Google Scholar]
- 14.Neff BD, Svensson EI. 2013. Polyandry and alternative mating tactics. Phil. Trans. R. Soc. B 368, 20120045 ( 10.1098/rstb.2012.0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Taborsky M. 2008. Alternative reproductive tactics in fish. In Alternative reproductive tactics: an integrative approach (eds Oliveira RF, Taborsky M, Brockmann HJ), pp. 251–299. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Eberhard WG. 1982. Beetle horn dimorphism: making the best of a bad lot. Am. Nat. 119, 420–426. ( 10.1086/283920) [DOI] [Google Scholar]
- 18.Tomkins JL, Brown GS. 2004. Population density drives the local evolution of a threshold dimorphism. Nature 431, 1099–1103. ( 10.1038/nature02918) [DOI] [PubMed] [Google Scholar]
- 19.Rowland JM, Emlen DJ. 2009. Two thresholds, three male forms result in facultative male trimorphism in beetles. Science 323, 773–776. ( 10.1126/science.1167345) [DOI] [PubMed] [Google Scholar]
- 20.Emlen DJ. 2008. The evolution of animal weapons. Ann. Rev. Ecol. Evol. Syst. 39, 387–413. ( 10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 21.Alonzo SH, Taborsky M, Wirtz P. 2000. Male alternative reproductive behaviours in a Mediterranean wrasse, Symphodus ocellatus: evidence from otoliths for multiple life-history pathways. Evol. Ecol. Res. 2, 997–1007. [Google Scholar]
- 22.Penn D, Brockmann HJ. 1995. Age-biased stranding and righting in male horseshoe crabs, Limulus polyphemus. Anim. Behav. 49, 1531–1539. ( 10.1016/0003-3472(95)90074-8) [DOI] [Google Scholar]
- 23.Tomkins JL, Hazel W. 2007. The status of the conditional evolutionarily stable strategy. Trends Ecol. Evol. 22, 522–528. ( 10.1016/j.tree.2007.09.002) [DOI] [PubMed] [Google Scholar]
- 24.Sinervo B, Lively CM. 1996. The rock-paper-scissors game and the evolution of alternative male strategies. Nature 380, 240–243. ( 10.1038/380240a0) [DOI] [Google Scholar]
- 25.Shuster SM, Wade MJ. 1991. Equal mating success among male reproductive strategies in a marine isopod. Nature 350, 608–610. ( 10.1038/350608a0) [DOI] [Google Scholar]
- 26.Wirtz Ocana S, Meidl P, Bonfils D, Taborsky M. 2014. Y-linked Mendelian inheritance of giant and dwarf male morphs in shell-brooding cichlids. Proc. R. Soc. B 281, 20140253 ( 10.1098/rspb.2014.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazel W, Smock R, Lively CM. 2004. The ecological genetics of conditional strategies. Am. Nat. 163, 888–900. ( 10.1086/386313) [DOI] [PubMed] [Google Scholar]
- 28.Lively CM. 1986. Canalization versus developmental conversion in a spatially variable environment. Am. Nat. 128, 561–572. ( 10.1086/284588) [DOI] [Google Scholar]
- 29.Plaistow SJ, Johnstone RA, Colegrave N, Spencer M. 2004. Evolution of alternative mating tactics: conditional versus mixed strategies. Behav. Ecol. 15, 534–542. ( 10.1093/beheco/arh029) [DOI] [Google Scholar]
- 30.Gadgil, M. 1972. Male dimorphism as a consequence of sexual selection. Am. Nat. 106, 574–580. ( 10.1086/282797) [DOI] [Google Scholar]
- 31.Gross MR. 1991. Evolution of alternative reproductive strategies: frequency-dependent sexual selection in male bluegill sunfish. Phil. Trans. R. Soc. B 332, 59–66. ( 10.1098/rstb.1991.0033) [DOI] [Google Scholar]
- 32.Schütz D, Pachler G, Ripmeester E, Goffinet O, Taborsky M. 2010. Reproductive investment of giants and dwarfs: specialized tactics in a cichlid fish with alternative male morphs. Funct. Ecol. 24, 131–140. ( 10.1111/j.1365-2435.2009.01605.x) [DOI] [Google Scholar]
- 33.Gross MR, Charnov EL. 1980. Alternative male life histories in bluegill sunfish. Proc. Natl Acad. Sci. USA 77, 6937–6940. ( 10.1073/pnas.77.11.6937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alonzo SH, Calsbeek R. 2010. The unstable dynamics of multiple alternative reproductive tactics. J. Evol. Biol. 23, 2614–2624. ( 10.1111/j.1420-9101.2010.02130.x) [DOI] [PubMed] [Google Scholar]
- 35.Chevin LM, Lande R. 2013. Evolution of discrete phenotypes from continuous norms of reaction. Am. Nat. 182, 13–27. ( 10.1086/670613) [DOI] [PubMed] [Google Scholar]
- 36.Randerson JP, Hurst LD. 2001. The uncertain evolution of the sexes. Trends Ecol. Evol. 16, 571–579. ( 10.1016/S0169-5347(01)02270-4) [DOI] [Google Scholar]
- 37.Charnov EL. 1982. The theory of sex allocation. Princeton, NJ: Prineton University Press. [Google Scholar]
- 38.Sebens KP. 1987. The ecology of indeterminate growth in animals. Ann. Rev. Ecol. Syst. 18, 371–407. ( 10.1146/annurev.es.18.110187.002103) [DOI] [Google Scholar]
- 39.Arnott G, Elwood RW. 2009. Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004. ( 10.1016/j.anbehav.2009.02.010) [DOI] [Google Scholar]
- 40.van Voorhies WA. 1992. Production of sperm reduces nematode life-span. Nature 360, 456–458. ( 10.1038/360456a0) [DOI] [PubMed] [Google Scholar]
- 41.von Bertalanffy L. 1957. Quantitative laws in metabolism and growth. Q. Rev. Biol. 32, 217–231. ( 10.1086/401873) [DOI] [PubMed] [Google Scholar]
- 42.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 43.Dmitriew CM. 2011. The evolution of growth trajectories: what limits growth rate? Biol. Rev. 86, 97–116. ( 10.1111/j.1469-185X.2010.00136.x) [DOI] [PubMed] [Google Scholar]
- 44.Pletcher SD. 1999. Model fitting and hypothesis testing for age-specific mortality data. J. Evol. Biol. 12, 430–439. ( 10.1046/j.1420-9101.1999.00058.x) [DOI] [Google Scholar]
- 45.Siler W. 1979. Competing-risk model for animal mortality. Ecology 60, 750–757. ( 10.2307/1936612) [DOI] [Google Scholar]
- 46.Sogard SM. 1997. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull. Mar. Sci. 60, 1129–1157. [Google Scholar]
- 47.Remmel T, Davison J, Tammaru T. 2011. Quantifying predation on folivorous insect larvae: the perspective of life-history evolution. Biol. J. Linn. Soc. 104, 1–18. ( 10.1111/j.1095-8312.2011.01721.x) [DOI] [Google Scholar]
- 48.Werner EE. 1986. Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am. Nat. 128, 319–341. ( 10.1086/284565) [DOI] [Google Scholar]
- 49.Taborsky B, Dieckmann U, Heino M. 2003. Unexpected discontinuities in life-history evolution under size-dependent mortality. Proc. R. Soc. B 270, 713–721. ( 10.1098/rspb.2002.2255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enquist M, Leimar O, Ljungberg T, Mallner Y, Segerdahl N. 1990. A test of the sequential assessment game: fighting in the cichlid fish Nannacara anomala. Anim. Behav. 40, 1–14. ( 10.1016/S0003-3472(05)80660-8) [DOI] [Google Scholar]
- 51.Schütz D, Taborsky M. 2005. The influence of sexual selection and ecological constraints on an extreme sexual size dimorphism in a cichlid. Anim. Behav. 70, 539–549. ( 10.1016/j.anbehav.2004.11.010) [DOI] [Google Scholar]
- 52.Ota K, Kohda M, Sato T. 2010. Why are reproductively parasitic fish males so small? Influence of tactic-specific selection. Naturwissenschaften 97, 1113–1116. ( 10.1007/s00114-010-0725-4) [DOI] [PubMed] [Google Scholar]
- 53.Moya-Larano J, El-Sayyid MET, Fox CW. 2007. Smaller beetles are better scramble competitors at cooler temperatures. Biol. Lett. 3, 475–478. ( 10.1098/rsbl.2007.0300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoltz JA, Neff BD. 2006. Male size and mating tactic influence proximity to females during sperm competition in bluegill sunfish. Behav. Ecol. Sociobiol. 59, 811–818. ( 10.1007/s00265-005-0127-3) [DOI] [Google Scholar]
- 55.Brockmann HJ. 2008. Alternative reproductive tactics in insects. In Alternative reproductive tactics: an integrative approach (eds Oliveira RF, Taborsky M, Brockmann HJ), pp. 177–223. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 56.Buzatto BA, Roberts JD, Simmons LW. 2015. Sperm competition and the evolution of precopulatory weapons: increasing male density promotes sperm competition and reduces selection on arm strength in a chorusing frog. Evolution 69, 2613–2624. ( 10.1111/evo.12766) [DOI] [PubMed] [Google Scholar]
- 57.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1111/j.1469-185x.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 58.Tomkins JL, Simmons LW. 2002. Measuring relative investment: a case study of testes investment in species with alternative male reproductive tactics. Anim. Behav. 63, 1009–1016. ( 10.1006/anbe.2001.1994) [DOI] [Google Scholar]
- 59.Parker GA. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection. (eds Birkhead TR, Møller AP), pp. 3–54. San Diego, CA: Academic Press. [Google Scholar]
- 60.Fu P, Neff BD, Gross MR. 2001. Tactic-specific success in sperm competition. Proc. R. Soc. Lond. B 268, 1105–1112. ( 10.1098/rspb.2001.1625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benaglia T, Chauveau D, Hunter DR, Young D. 2009. mixtools: an R package for analyzing finite mixture models. J. Stat. Softw. 36, 1–29. [Google Scholar]
- 62.Simmons LW, Emlen DJ, Tomkins JL. 2007. Sperm competition games between sneaks and guards: a comparative analysis using dimorphic male beetles. Evolution 61, 2684–2692. ( 10.1111/j.1558-5646.2007.00243.x) [DOI] [PubMed] [Google Scholar]
- 63.Dingemanse NJ, Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958. ( 10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data generated in this simulation study has been presented either in the article or in the electronic supplementary material.