Abstract
αβ or γδ thymocytes whose T-cell receptors (TCRs) recognize endogenously expressed antigens (Ag) are autospecific and, thus, potentially self-reactive. In the thymus, such T cells are eliminated during T-cell development through a process known as negative selection. As a model of negative selection of γδ T cells, we have used G8 γδ–T cell transgenic mice, which express a γδ TCR that recognizes the nonpolymorphic MHC class I TLb molecule. Here, we demonstrate that negative selection of autospecific γδ T cells is almost complete in the adult thymus but is markedly attenuated in the neonatal thymus. A consequence of this attenuated negative selection is that potentially self-reactive γδ thymocytes are allowed to escape negative selection, undergo extrathymic differentiation, and find sanctuary in the intestinal epithelium. Interestingly, the ability of these potentially self-reactive γδ T cells to find sanctuary requires both the intestinal epithelial environment and the extrathymic presence of the self-Ag. The implications of these findings on the development and persistence of autoreactive T cells in autoimmune disease are discussed.
Introduction
Immature T cells that express an autospecific T-cell receptor (TCR) that recognizes an endogenously expressed antigen are eliminated in the thymus through the process of negative selection. This process has been shown in several systems to be an efficient method by which the body prevents potentially harmful autoreactive T cells from developing. During T-cell development, thymocytes that express a specific Vβ TCR that recognizes an endogenously expressed Mls superantigen are eliminated, and are thus virtually absent from the adult thymus (1, 2). Similarly, studies on transgenic (Tg) mice have shown that mature T cells that express a TCR that recognizes an endogenously expressed antigen (Ag) are also deleted (3–7).
Several lines of evidence suggest that, in contrast with the adult thymus, negative selection of autoreactive αβ and γδ T cells is either absent or markedly attenuated early in ontogeny. T cells that express Vβ TCRs that recognize an endogenously expressed Mls Ag are found in relatively large numbers in the neonatal thymus, but are virtually absent in the adult thymus (8–10). Similarly, in HY, αβ–T cell Tg mice, Teh et al. demonstrated that Tg αβ T cells that recognize the male HY antigen are found in almost equal numbers in the thymus of male and female mice at fetal day 16, but these cells begin to decrease markedly in the male thymus at or near the time of birth (11). Using G8 Tg γδ-TCR mice, Dent et al. demonstrated that Tg γδ T cells, which recognize an endogenously expressed TLb antigen (T10b or T22b), are found in the neonatal spleen, but are virtually absent in the adult spleen and thymus of TLb+ mice (12). Although their results suggest that negative selection of Tg γδ T cells is either absent or attenuated in the neonatal thymus, Dent et al. did not examine the neonatal thymus and therefore did not address the possibility that Tg γδ T cells found in the spleen of neonatal TLb+ mice were derived from an extrathymic pathway (13–16). Nevertheless, these results collectively suggest that the ability of the neonatal thymus to remove autospecific neonatal thymocytes is either absent or severely attenuated.
The fate of these autospecific neonatal thymocytes is an important issue that has not been addressed in the discussed neonatal studies. Presumably, a few cells escape out into the periphery and remain senescent at very low numbers, but most die from negative selection and possibly peripheral deletion. Therefore, it is interesting to note that like the neonatal thymus, the intestinal epithelial lymphocyte (IEL) population appears to be rich in T cells that bear autospecific TCR. The IEL population has been shown to be rich in αβ T cells, which express Vβ TCRs that recognize an endogenously expressed Mls Ag (17). Furthermore, studies on αβ– and γδ–T cell Tg mice, which express a TCR that recognizes endogenously expressed Ag, have shown that the IEL population is rich in T cells that express autospecific TCR (16, 18–20). However, how these IEL that express autospecific TCR escape negative selection is unclear. Present theory suggests that most IEL that express an autospecific TCR avoid thymic negative selection by developing by an extrathymic pathway (16, 17, 21). However, the relative contribution of the extrathymic pathway to the development of most IEL is controversial (22, 23). Nude mice that are congenitally athymic have virtually no TCR αβ IEL and have markedly reduced numbers of TCR γδ IEL compared with normal euthymic mice (22, 24). Furthermore, several studies have shown that many IEL, which were originally believed to develop solely through an extrathymic pathway, can also be derived from the fetal/neonatal thymus (25, 26).
In this study, we investigated whether there is a direct relationship between the absence or attenuation of negative selection in the neonatal thymus and the abundant number of IEL that express an autospecific TCR. Using G8 γδ Tg mice as a model of negative selection of γδ T cells, we demonstrate that negative selection is severely attenuated in the neonatal thymus so that up to 40% of day 2 neonatal thymocytes bear the autospecific Tg γδ TCR. Furthermore, a consequence of this attenuated negative selection is that potentially self-reactive Tg γδ neonatal thymocytes, which appear to be in the process of undergoing negative selection, are able to escape, differentiate extrathymically, and find sanctuary in intestinal epithelium, where they survive and incease in number. Lastly, we demonstrate that the ability of the intestinal epithelium to serve efficiently as a sanctuary for autospecific γδ T cells requires, paradoxically, the extrathymic presence of the self-Ag. The implications of these findings on the development of IEL and the development and persistence of autoreactive T cells found in autoimmune disease are discussed.
Methods
Mice.
Transgenic mice with or without the TLb Ag (Tgb/d or Tgd/d, respectively) were generated by breeding a single G8 Tgd/d founder male to either C57BL/6 (TLb+, H-2b+) or BALB/c (TLd+, H-2d+) females, respectively (3, 16, 27). Time of birth was considered day 0. All mice, including athymic mice bearing the TLb Ag (TLb+, C57BL/6 nu/nu) and those not bearing the TLb Ag (TLd, BALB/c nu/nu), were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). All mice were raised under specific pathogen–free conditions in the animal care facility at the La Jolla Institute of Allergy and Immunology.
Cell isolation, flow cytometry analysis, and cell sorting.
Isolation of IEL and cells from the thymus, spleen, and lymph node has been described previously (28). IEL described specifically as Tgb/d neonatal thymus–derived were isolated from C57BL/6 nude mice grafted 6 weeks previously with Tgb/d day 2 neonatal thymus (described below). Two- or 3-color flow cytometry analysis was performed with a FACScan flow cytometer from Becton Dickinson and Co. (Franklin Lakes, New Jersey, USA). The data were analyzed with the Macintosh CellQuest program. Cell sorting was performed with a FACStar cell sorter from Becton Dickinson and Co.
All antibodies were obtained from PharMingen (San Diego, California, USA) unless otherwise noted. Antibodies and reagents used were as follows: FITC-conjugated and nonconjugated anti-Vγ2 (UC3-10A6), FITC-conjugated and biotin-conjugated anti-TCRβ (H57-597), PE-conjugated anti–Thy 1.2, biotin-conjugated anti-CD8β (Caltag Laboratories Inc., Burlingame, California, USA), biotin-conjugated anti–H-2Kd, biotin-conjugated anti–H-2Kb, PE-conjugated anti-CD4, PE-conjugated anti-CD8α, PE-conjugated CD45RB, PE-conjugated anti-HSA, PE-conjugated B220, and streptavidin-PE (GIBCO BRL, Gaithersburg, Maryland, USA).
Neonatal thymectomy and thymus grafting of nude mice.
Neonatal thymectomy was performed on day 2 neonates as described previously (29, 30). Grafting of nude mice was performed by placing either 2 lobes of day 2 neonatal thymus or a portion of adult thymus (trimmed to be of similar size to a pair of day 2 neonatal thymuses) under the kidney capsule as described previously (31).
Proliferation assay.
Unless otherwise stated, responder cells (2 × 105) were cultured with either plate-bound mAbs (3 μg/mL) or stimulator cells (2 × 105 irradiated spleen cells) for 36 hours in 100 μL RPMI with 10% FCS, and then pulsed with 1 μCi of hydrogen-3 for 12 hours before harvest. Assays were performed at day 1, day 2, and day 3, in triplicate.
Cytotoxic assay.
The ability of IEL to induce the cytotoxic killing of human Jurkat T cells (primarily through a Fas-mediated mechanism) has been previously described (32). In brief, Jurkat target cells (106 cells/mL) were labeled with 5 mCi/mL [3H]thymidine for 2 hours. Unincorporated [3H]thymidine was removed by 2 washes with HBSS. IEL effector cells were cultured with labeled Jurkat target cells (2 × 104) at various effector target ratios in flat-bottomed, 96-well plates that had been previously coated with 3 μg/mL of anti-Vγ2 mAb. After 12 hours, cells were harvested using a Skatron cell harvester, and the quantity of [3H]thymidine-labeled unfragmented DNA was calculated as follows: % DNA fragmentation = 100[1 – (cpm experimental group/cpm control group)] ± SD. Assays were done in triplicate.
Measurement of IL-2 from Tgb/d neonatal thymus–derived Vγ 2 IEL.
IL-2 was quantified from the supernatant of 2 × 105 IEL cultured with stimulator cells (2 × 105 irradiated TLb+ spleen cells) for 48 hours in 200 μL of RPMI and 10% FCS media, using a murine IL-2 ELISA kit (Endogen Inc., Woburn, Massachusetts, USA), following the manufacturer’s instructions.
Results
γδ T cells expressing autospecific TCR are deleted in the adult thymus but are found abundantly in the IEL population.
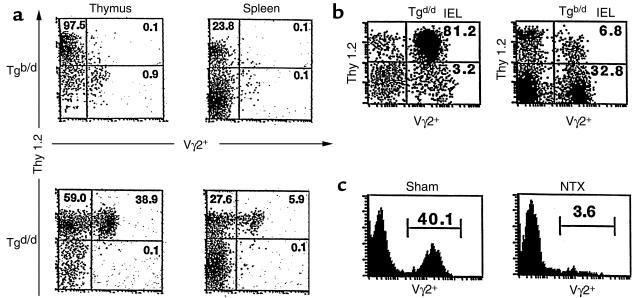
In G8 γδ–TCR Tg mice, Tg γδ T cells express the Vγ2+ TCR that recognizes the T10b or T22b gene product of the nonclassical MHC class I TL region (33, 34). Thus, in Tg mice with an H-2d+ background (Tgd/d), neither T10b nor T22b is present (TLb–), and thus Vγ2+ T cells are found in very high numbers in the thymus, spleen, and lymph node (Figure 1a and data not shown). However, in Tg mice with an H-2b+ background (Tgb/d), T10b or T22b is present (TLb+); in the adult mice, Vγ2+ T cells are deleted and thus virtually absent in the thymus, spleen, and lymph node (Figure 1a and data not shown). The absence of Vγ2+ T cells in the spleen and lymph node of Tgb/d mice reflects either efficient negative selection of Vγ2+ thymocytes, or (possibly) peripheral deletion of the few Vγ2+ thymocytes that escape negative selection in the Tgb/d thymus. In contrast, γδ T cells found in the IEL population appear to be an exception (27). The virtual absence of Vγ2+ T cells in the thymus and periphery of Tgb/d mice contrasts profoundly with the abundant number of Vγ2+ T cells found in the IEL population in both Tgd/d and Tgb/d mice (Figure 1b). To explain this paradox, present theory suggests that most Tgb/d Vγ2+ IEL are derived from an extrathymic pathway (16).
Figure 1.
(a) Flow cytometry (FCM) 2-color analysis of thymus and spleen from Tgb/d (top row) and Tgd/d (bottom row) mice. (b) FCM analysis of IEL from Tgd/d (left) and Tgb/d (right) mice. (c) FCM histogram analysis of IEL from 6-week-old Tgb/d mice that were sham operated (left) and thymectomized (NTX; right) on day 2 of life. Data shown represent 1 of 2 independent experiments.
The majority of Tgb/d Vγ2+ IEL are thymus dependent.
Instead of assuming that Tgb/d Vγ2+ IEL are derived from an extrathymic pathway, we entertained the possibility that the development of Tgb/d Vγ2+ IEL was dependent on the thymus. Therefore, we thymectomized Tgb/d mice on neonatal day 2 and examined the IEL population after 6 weeks. Figure 1c demonstrates that neonatal thymectomy resulted in a nearly complete depletion of Vγ2+ IEL in adult Tgb/d mice, suggesting that development of a vast majority of Vγ2+ IEL in Tgb/d mice is dependent on the thymus. In addition, examination of the IEL phenotype of neonatal thymectomy Tgb/d mice revealed that there were also very few TCR αβ IEL (less than 5%, data not shown). Overall, these results are consistent with our previous observation that neonatal thymectomy results in the depletion of most TCR αβ IEL and TCR γδ IEL (22).
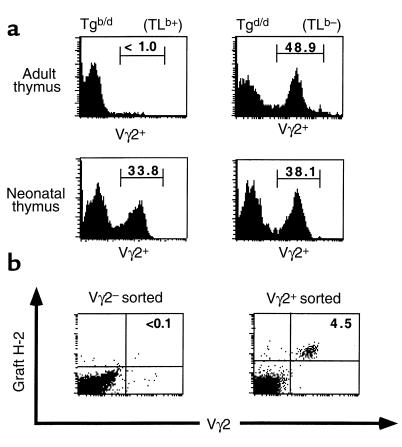
Vγ2+ thymocytes are increased in the neonatal Tgb/d thymus.
Neonatal thymectomy results suggest that most Tgb/d Vγ2+ IEL are derived from the thymus. However, it is unlikely that the adult Tgb/d thymus is a major source of Tgb/d Vγ2+ IEL, because Vγ2+ thymocytes are virtually absent in this organ (Figure 1a). Therefore we examined the Tgb/d neonatal thymus as a potential source of Tgb/d Vγ2+ IEL. Unlike Tgb/d adult thymocytes, a large percentage of Tgb/d neonatal thymocytes express the Vγ2+ TCR (Figure 2a). The percentage of Tgb/d Vγ2+ thymocytes varied considerably among the thymuses (ranging between 7% and 40%, data not shown), and persisted at increased levels up to 7 days of age. By 2 weeks of age, the percentage of Vγ2+ thymocytes became severely diminished and similar to the level found in adult Tgb/d thymus (data not shown).
Figure 2.
Negative selection is absent or attenuated in the neonatal thymus. (a) FCM histogram analysis for Vγ2 expression in adult (top row) and neonatal (day 2, bottom row) thymus from Tgb/d (left) and Tgd/d (right) mice. Data shown represent 1 of 3 independent experiments. (b) Tgb/d Vγ2+ but not Tgb/d Vγ2– neonatal thymocytes can give rise to Vγ2+ IEL. Flow cytometry–sorted Tgb/d Vγ2+ (3 × 105) and Vγ2– (106) neonatal thymocytes were injected into TLb+ athymic C57BL/6 mice. Six weeks later, IEL of injected athymic mice were examined for the presence of graft-derived (H-2d+) Tg Vγ2+ IEL. Data shown represent 1 of 3 independent experiments.
Tgb/d Vγ2+ neonatal thymocytes can give rise to Tgb/d Vγ2+ IEL.
Some compelling evidence suggests that the murine intestinal epithelium is a site for extrathymic T-cell development (13, 19, 21, 23, 35). Hence, Tgb/d Vγ2+ neonatal thymocytes may not migrate to the intestinal epithelium, but Tgb/d Vγ2– neonatal thymocytes could migrate to the intestinal epithelium and develop extrathymically into Tgb/d Vγ2+ IEL. To address this question, flow cytometry–purified Tgb/d Vγ2+ or Tgb/d Vγ2– thymocytes were injected into congenitally athymic TLb+ nude C57BL/6 hosts. Figure 2b demonstrates that Tgb/d Vγ2+, but not Tgb/d Vγ2– neonatal thymocytes, are capable of generating Vγ2+ IEL. This suggests that it is unlikely that Tgb/d Vγ2– neonatal thymocytes migrate to the intestinal epithelium and differentiate into Vγ2+ IEL. Furthermore, examination of lymph node and spleen of TLb+ nude mice injected with Tgb/d Vγ2+ neonatal thymocytes revealed no detectable presence of Tg Vγ2+ T cells (data not shown). This suggests that Tgb/d Vγ2+ neonatal thymocytes are predestined to either migrate to the intestinal epithelium or undergo peripheral deletion in the spleen and lymph node.
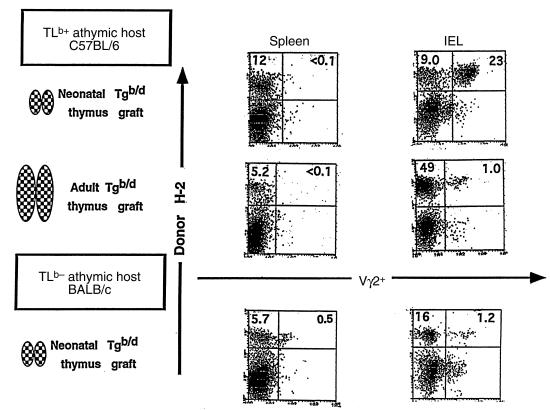
Efficient migration of Tgb/d Vγ2+ neonatal thymocytes to the intestinal epithelium requires the extrathymic presence of the TLb Ag.
To explore the fate of Tgb/d Vγ2+ neonatal thymocytes in a model that mimics normal ontogeny as closely as possible, we grafted Tgb/d (H-2b+/d+) neonatal thymus into TLb+ (H-2b+) adult nude mice. In this model, cells derived from the thymus graft (donor) can be distinguished from cells derived from the nude hosts by a mAb that specifically recognizes the donor-specific class I H-2Kd molecule (shown as donor H-2+ in Figure 3). As shown in Figure 3 (upper row), grafting of the Tgb/d neonatal thymus into a TLb+ adult nude host readily generated graft-derived cells in both the spleen and IEL populations. Not surprisingly, the Tgb/d neonatal thymus failed to generate Vγ2+ T cells in the spleen of the nude TLb+ host. Presumably, this is either because Tgb/d Vγ2+ neonatal thymocytes cannot escape negative selection or they undergo deletion after escaping into the periphery. In contrast, the Tgb/d neonatal thymus was very efficient at generating Vγ2+ IEL when grafted into TLb+ nude hosts. Phenotypic studies of the Vγ2+ IEL generated by the Tgb/d neonatal thymus graft in TLb+ nude hosts suggests that they are mature (HSA–) and bear a phenotype that resembles the Vγ2+ IEL found normally in Tgb/d mice (CD8αd+, Thy 1–, and B220+), rather than the Vγ2+ IEL found in Tgd/d mice (CD8–, Thy 1+, B220–) (16 and data not shown). In addition, the functional properties of Tgb/d neonatal thymus graft–derived Vγ2+ IEL appear to be very similar to those reported for Vγ2+ IEL found in Tgb/d mice (27). Upon anti-Vγ2 TCR stimulation or culture with irradiated TLb+ spleen cells as stimulators, Tgb/d neonatal thymus graft–derived Vγ2+ IEL proliferated poorly and produced nearly undetectable levels of IL-2 (data not shown). Furthermore, unlike Vγ2+ IEL from Tgd/d mice, which readily mediated the cytotoxic killing of Jurkat T cells upon stimulation with anti-Vγ2 mAb, both Tgb/d neonatal thymus graft–derived Vγ2+ IEL and Vγ2+ IEL from Tgb/d mice exhibited very weak cytotoxicity against human Jurkat T cells (data not shown).
Figure 3.
Efficient homing of Tgb/d Vγ2+ neonatal thymocytes to the intestinal epithelium requires the extrathymic presence of the TLb Ag. FCM analysis of IEL and spleen cells from (top row) athymic C57BL/6 (self-Ag TLb+) mice and (bottom row) athymic BALB/c (TLd+) mice that were grafted 6 weeks previously with the Tgb/d (day 2) neonatal thymus. (middle row) FCM analysis of IEL and spleen cells from athymic C57BL/6 (self-Ag TLb+) mice that were grafted with the Tgb/d (6-week-old) adult thymus. Donor H-2+ cells represent cells derived from the neonatal thymus graft as determined by the appropriate anti–H-2K mAb (anti–H-2Kb or anti–H-2Kd). Data shown represent 1 of 3 independent experiments.
To determine whether the ability of Tgb/d Vγ2+ neonatal thymocytes to escape negative selection and migrate to the intestinal epithelium is a consequence of absent or attenuated negative selection during the neonatal period, nude TLb+ hosts were grafted with Tgb/d adult thymus, in which the negative selection of Tg Vγ2+ is almost complete (Figures 1 and 2). Figure 3 (middle row) demonstrates that the adult Tgb/d thymus generated graft-derived cells in both the IEL and spleen populations of nude TLb+ hosts, but relatively few were Vγ2+ T cells. Although it is possible that the adult Tg thymus simply could not generate Vγ2+ IEL, this is unlikely because grafting of Tgd/d adult thymus into TLd+ BALB/c nude mice readily generated Vγ2+ IEL (data not shown). Overall, these results suggest that it is the absence or attenuation of negative selection during neonatal development that allows Tgb/d neonatal thymus to generate Vγ2+ IEL in TLb+ nude hosts.
Next, we examined whether the extrathymic presence of the TLb Ag was required for the generation of Vγ2+ IEL in the nude host by grafting the Tgb/d neonatal thymus into TLd+ BALB/c nude mice, which do not bear the TLb Ag. In this model, cells derived from the thymus graft (donor) can be distinguished from cells derived from the nude host by a mAb that specifically recognizes the donor-specific class I H-2Kb molecule (shown as donor H-2+ in Figure 3). Although Tgb/d neonatal thymus readily generated T cells in the spleen and IEL populations of TLd+ hosts, only a relatively small percentage of those T cells was Vγ2+ (Figure 3, bottom row). Although the total percentage of Tgb/d neonatal thymus graft–derived Vγ2+ T cells found in the spleen and IEL of athymic TLd+ hosts was very small, it constituted almost 10% of the total number of Tgb/d neonatal thymus graft–derived T cells. This suggests that in the absence of the extrathymic TLb Ag, autospecific Tgb/d Vγ2+ neonatal thymocytes are capable of escaping negative selection and surviving in both the spleen and the intestinal epithelium at very low levels. However, as shown in Figure 3 (top row), when the Tgb/d neonatal thymus was grafted into TLb+ nude mice, the extrathymic presence of the TLb Ag appeared to induce peripheral deletion of Tgb/d Vγ2+ T cells in the spleen, and paradoxically, survival and increase in number of Tgb/d Vγ2+ T cells in the intestinal epithelium. Overall, these results suggest that the intestinal epithelium of TLb+ mice, but not TLd+ mice, can serve as a sanctuary for Tgb/d Vγ2+ neonatal thymocytes that have escaped negative selection and peripheral deletion.
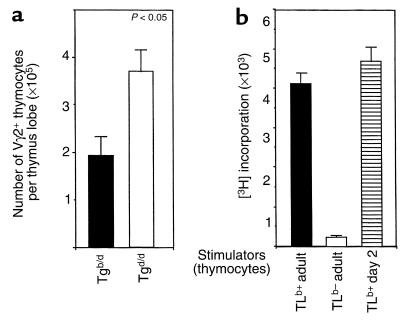
Negative selection of Vγ2+ thymocytes is present but attenuated in the neonatal Tgb/d thymus.
If neonatal Tgb/d thymus is a major source of Tgb/d Vγ2+ IEL, as suggested by our results, then an important question is whether negative selection of autospecific Tgb/d Vγ2+ T cells is attenuated or simply absent in the neonatal thymus. As shown in Figure 4a, the total number of Vγ2+ thymocytes in the Tgb/d neonatal thymus was reduced by 50% when compared with Tgd/d neonatal thymus, suggesting that some form of negative selection is present in the neonatal Tgb/d thymus. However, because there was a decrease of more than 95% in the total number of Vγ2+ thymocytes found in the adult Tgb/d thymus compared with adult Tgd/d thymus (data not shown), these results also suggest that negative selection of Vγ2+ thymocytes in the neonatal Tgb/d thymus is present, but attenuated.
Figure 4.
(a) Negative selection of Tgb/d Vγ2+ neonatal thymocytes is attenuated in day 2 neonatal thymus. The total number of Vγ2+ neonatal thymocytes is reduced by approximately 50% in Tgb/d neonatal thymus when compared with Tgd/d neonatal thymus. Data are shown as the mean of more than 20 neonatal thymus lobes examined separately for each group. (b) The TLb self-Ag is functionally present in the neonatal Tgb/d thymus. To test for the functional presence of the TLb Ag, 105 irradiated stimulator cells were cultured with adult Tgd/d thymocytes that were sorted to remove TCR β cells to avoid a nonspecific allogeneic response. Data shown represent 1 of 2 independent experiments.
TLb Ag is present in the Tgb/d neonatal thymus.
We next explored the question of whether the attenuated level of negative selection in the Tgb/d neonatal thymus may simply be the result of lower TLb Ag expression in the neonatal thymus. To test this hypothesis, we looked for the functional presence of the TLb Ag by testing the ability of irradiated Tgb/d neonatal thymocytes to induce the proliferation of Tgd/d Vγ2+ adult thymocytes, which should specifically recognize the TLb Ag. To avoid an allogeneic response, we sorted and removed TCR β thymocytes before culturing with irradiated Tgb/d neonatal thymocytes. Hence, any proliferative response should be the result of Tgd/d Vγ2+ thymocytes recognizing the TLb Ag. Figure 4b demonstrates that the TLb Ag is functionally present in day 2 neonatal Tgb/d thymus at levels equal to if not higher than those found in the adult Tgb/d thymus. As expected, no functional TLb Ag was detectable in the Tgd/d adult thymus. Overall, these results suggest that the attenuation of negative selection of Tgb/d Vγ2+ neonatal thymocytes is unlikely to be due to a lower level of TLb Ag expression in the Tgb/d neonatal thymus.
Phenotypic examination suggests that Tgb/d Vγ2+ neonatal thymocytes have encountered the TLb Ag at an immature stage in development.
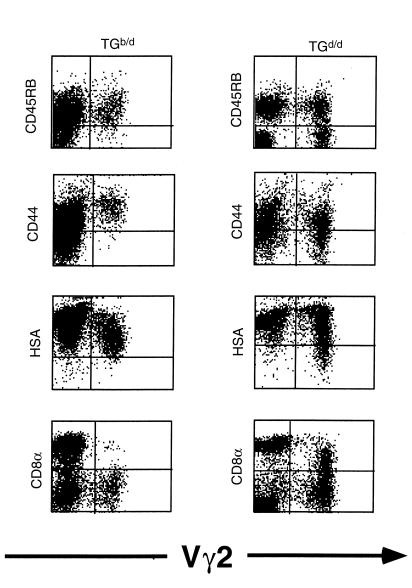
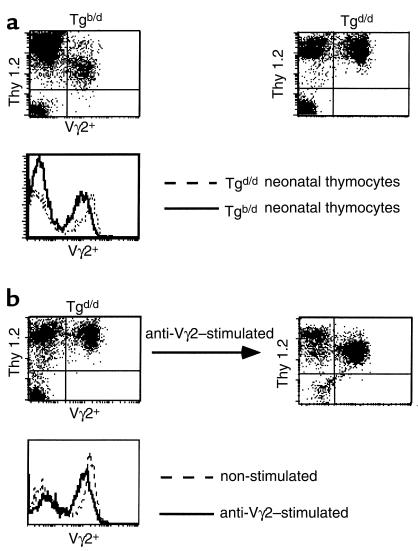
If negative selection of Tgb/d Vγ2+ neonatal thymocytes is partially functional and the TLb Ag is present in the neonatal thymus, we rationalized that there should be some evidence that Vγ2+ Tgb/d neonatal thymocytes have encountered the TLb self-Ag. Comparison of Tgb/d and Tgd/d Vγ2+ neonatal thymocytes for several phenotypic markers of differentiation and activation revealed that Tgd/d Vγ2+ neonatal thymocytes are comprised of a heterogeneous population expressing various levels of CD8α, CD45RB, CD44, and HSA. In contrast, Tgb/d Vγ2+ neonatal thymocytes are a relatively homogenous population and are uniformly CD8–, CD45RBhi, CD44hi, and HSAd+ (Figure 5). Because Tgb/d Vγ2+ neonatal thymocytes, but not Tgd/d Vγ2+ neonatal thymocytes, are virtually all positive for CD44 and HSA, which have been reported to be markers of activation and thymocyte immaturity (36–39), these results suggest that Tgb/d Vγ2+ neonatal thymocytes have been activated (presumably by the TLb Ag), but are also blocked at an immature stage of development. Furthermore, closer phenotypic examination revealed that Tgb/d Vγ2+ neonatal thymocytes express significantly lower levels of Thy 1 and Vγ2+ TCR than do naive Tgd/d Vγ2+ neonatal thymocytes (Figure 6a). Therefore, we tested the hypothesis that lower levels of Thy 1 and TCR expression are markers of TLb Ag recognition by examining naive Tgd/d Vγ2+ neonatal thymocytes before and after activation in vitro with either anti-Vγ2 mAb or irradiated TLb+ Tgb/d spleen cells. Figure 6b demonstrates that, consistent with our hypothesis, activation of Tgd/d Vγ2+ neonatal thymocytes resulted in lower levels of Thy 1 and Vγ2 TCR expression.
Figure 5.
FCM analysis of neonatal Tgb/d (left) and Tgd/d (right) thymocytes for markers of T-cell development and activation. Data shown represent 1 of 3 independent experiments.
Figure 6.
Phenotypic examination of Thy 1 and Vγ2+ TCR levels suggests that most Tgb/d Vγ2+ neonatal thymocytes have recognized the TLb Ag. (a) Tgb/d Vγ2+ neonatal thymocytes express lower levels of Thy 1 and Vγ2+ TCR than do Tgd/d Vγ2+ neonatal thymocytes. (b) Stimulation of Tgd/d neonatal thymocytes with anti-Vγ2 TCR mAb resulted in decreased levels of Thy 1 and Vγ2+ TCR. Data shown represent 1 of 2 separate experiments.
Discussion
Using G8 γδ Tg mice, we have demonstrated that the negative selection of γδ T cells bearing an autospecific TCR is markedly attenuated in the neonatal thymus. A consequence of this attenuated negative selection is that potentially self-reactive thymocytes are allowed to escape negative selection, and migrate to the intestinal epithelium where they survive and increase in number. Although several studies have shown that negative selection is either absent or attenuated in the neonatal thymus, our study is the first to demonstrate that the fate of autospecific neonatal thymocytes can be other than death by negative selection. What makes our results intriguing is that we have also demonstrated that despite our autospecific neonatal γδ thymocytes appearing to be in the process of undergoing negative selection, the intestinal epithelium somehow serves as a sanctuary for these autospecific neonatal thymocytes. Lessons learned from studying this pathway are relevant to our understanding of how autoreactive T cells are allowed to develop and persist in autoimmune disease. It is worth noting that most studies that have addressed the fate of autospecific T cells have done so by transferring naive autospecific T cells into a host that expresses an Ag that is recognized by the transferred autospecific T cells. These studies, however, may not provide accurate models for studying the fate of autoreactive T cells in autoimmune disease, because they assume that autoreactive T cells are naive and have never encountered the self-Ag in development (i.e., negative selection). Our study addresses this issue and specifically examines the fate of autospecific T cells that have truly escaped functional negative selection.
From our model, 3 criteria appear to be required for autospecific thymocytes to escape negative selection and find sanctuary. The first criterion is that negative selection must be attenuated so that thymocytes expressing an autospecific TCR are allowed to escape negative selection. In our model, the neonatal period provides a brief window of time during which autospecific thymocytes can escape negative selection. It would be interesting to speculate whether stress or infection (which are both associated with autoimmune disease) can induce a similar phenomenon. The second criterion is that the self-Ag must be present extrathymically. Grafting of the Tgb/d neonatal thymus generated a large number of Vγ2+ T cells in the intestinal epithelium of only Ag+ TLb+ hosts, but not in Ag– TLd+ hosts (Figure 3). This criterion is puzzling, because our results suggest that Tgb/d Vγ2+ neonatal thymocytes have already encountered the TLb Ag and are rendered unresponsive to TCR stimulation (data not shown). The third criterion appears to be the environment. In our model, grafting of the Tgb/d neonatal thymus into TLb+ null hosts generated a large population of Vγ2+ T cells only in the intestinal epithelium and not elsewhere (Figure 3 and data not shown). This suggests that the intestinal epithelium provides a unique environment that allows the survival and expansion of autospecific neonatal thymocytes. The nature of this environment, however, is not presently known.
We also attempted to determine whether encountering the TLb Ag intrathymically early in ontogeny gives Vγ2+ neonatal thymocytes a selective advantage in their ability to migrate to the intestinal epithelium of TLb+ nude mice. Unfortunately, neither injection of Tgd/d Vγ2+ thymocytes nor grafting of the Tgd/d neonatal thymus into TLb+ nude mice generated any graft-derived cells in the IEL, spleen, or lymph node populations of the nude host (data not shown). This is probably because host natural killer cells reject thymus graft–derived T cells in a fully allogeneic model. On the other hand, grafting of Tgd/d neonatal thymus into a syngeneic TLd+ nude host readily generated Tg Vγ2+ IEL (data not shown). This suggests that, at the very least, encountering the TLb self-Ag in the neonatal thymus is not necessary for Tg Vγ2+ neonatal thymocytes to migrate to the intestinal epithelium.
An important question is whether our observations are also applicable to autospecific neonatal αβ thymocytes. Studies on αβ T cells, which recognize an endogenously expressed Mls Ag, and Tg αβ T cells, which recognize the male HY Ag, have shown that autospecific αβ T cells are found in relatively large numbers in both the neonatal thymus and IEL populations (8–11, 17, 20). These findings suggest that reminiscent of our observations regarding autospecific neonatal γδ thymocytes, the intestinal epithelium can also serve as a sanctuary for autospecific neonatal αβ thymocytes. However, whether these thymocytes can migrate to the intestinal epithelium and find sanctuary has not been directly demonstrated and is presently under investigation.
We have shown that Tgb/d Vγ2+ neonatal thymocytes can give rise to Tgb/d Vγ2+ IEL. Interestingly, the phenotypes of these 2 lineage-related populations are markedly different. For example, most Tgb/d Vγ2+ neonatal thymocytes are HSA+, B220–, CD8– , Thy 1lo, whereas most Tgb/d Vγ2+ IEL are HSA–, B220+, CD8+, Thy 1– (Figure 5 and data not shown). This suggests that several additional developmental steps are required before Tgb/d Vγ2+ neonatal thymocytes can develop into Tgb/d Vγ2+ IEL. Whether these steps take place intrathymically or extrathymically (presumably at the intestinal epithelium) is unclear. The absence of a discrete population of Tgb/d Vγ2+ neonatal thymocytes that phenotypically resemble Tgb/d IEL suggests that despite their thymic origin, Tgb/d Vγ2+ neonatal thymocytes differentiate further extrathymically before becoming Tgb/d Vγ2+ IEL. However, we can not rule out the possibility that a very small portion of Tgb/d Vγ2+ neonatal thymocytes differentiates intrathymically before leaving the thymus and migrating to the intestinal epithelium. Although this hypothesis is speculative, it may explain why the injection of Tgb/d neonatal thymocytes was significantly less efficient at generating Tgb/d Vγ2+ IEL in the TLb+ nude host than was grafting of the neonatal Tgb/d thymus (compare Figure 2b and Figure 3, top row).Our results with neonatal thymectomy and thymus grafting strongly suggest that most (if not all) Tgb/d Vγ2+ IEL are derived from the thymus. Thymectomy on day 2 almost completely eliminated Tgb/d Vγ2+ IEL (Figure 1c), and grafting of the neonatal Tgb/d thymus into a TLb+ host generated very large numbers of Tgb/d Vγ2+ IEL (Figure 3, top row). At first sight, our results appear to directly conflict with the study by Barrett et al. (16), who used the adult thymectomy radiation bone marrow chimera (ATXBM) as a model for extrathymic T-cell development and concluded that Tgb/d Vγ2+ IEL are derived from an extrathymic pathway. Although we are willing to concede that some Tgb/d Vγ2+ IEL can develop through such a pathway, several lines of reasoning suggest that the results of the ATXBM model probably exaggerate the significance of the this pathway. First, the radiation bone marrow chimera model only demonstrates that some Tgb/d Vγ2+ IEL can develop through an extrathymic pathway. Second, the ATXBM model is not natural and does not duplicate normal ontogeny as well as the thymus graft and neonatal thymectomy experiments do. Lastly, radiation probably alters the intestinal epithelium so that it induces the appearance of T cells, which normally would not be there. To prove this, we injected Tgb/d spleen cells (which have virtually no Vγ2+ T cells) into a TLb+ RAG null host and obtained virtually no Tg Vγ2+ IEL, as expected. However, when we irradiated the TLb+ RAG null mice with 400 rads before injection of Tgb/d spleen cells, there was an almost 30-fold increase in Tg Vγ2+ IEL in the recipient TLb+ RAG null host (Lin et al., unpublished observations). Our conclusion is supported by the study of Rocha et al., who demonstrated that reconstitution of thymectomized, nonirradiated RAG null mice with bone marrow cells generated very few γδ IEL (40). Collectively, these results suggest that most of the γδ IEL seen in the ATXBM model probably would not appear under normal nonirradiated conditions, and that most γδ IEL (including Tgb/d Vγ2+ IEL) are derived from the thymus.
If Tgb/d Vγ2+ neonatal thymocytes can escape negative selection and efficiently migrate to and increase in number at the intestinal epithelium, as suggested by our data, it is unclear what purpose this would serve in developing a functional immune repertoire. Because our data suggest that most Tgb/d Vγ2+ IEL are derived from the neonatal thymus, it is not surprising that the phenotype and the functional properties of Tgb/d neonatal thymus–derived Vγ2± IEL are essentially the same as those of Tgb/d Vγ2+ IEL. The latter have been extensively studied by Barrett et al. (16, 27), who demonstrated that Tgb/d Vγ2+ IEL have acquired tolerance. Although the development of tolerance may protect the intestinal epithelium from being harmed by potentially autoreactive T cells, it is not clear what functional purpose would be served by expanding a large population of both tolerant and potentially autoreactive T cells. One possibility is that this pathway may be an efficient way of generating IEL that produce TH2-type cytokines rather than the TH1-type cytokines, which may be more harmful to the intestinal epithelium in an immune reaction. Using 2C Tg mice that express an αβ TCR that recognizes an endogenous peptide Ag presented by the MHC class I H-2Ld, Guehler et al. (41) demonstrated that Tg IEL from Ag+ mice proliferated poorly and produced very little IL-2 or IFN-γ, yet also demonstrated by RT-PCR that Tg IEL from Ag+ mice were more likely than Tg IEL from their experimental Ag– mice to produce TH2-type cytokine IL-4. Whether this phenomenon occurs for αβ IEL in other Tg mice or for our Tg Vγ2+ IEL has yet to be shown. Finally, our results may have some relevance to human γδ IEL. Groh et al. recently demonstrated that some human γδ IEL are capable of recognizing stress-induced self MHC class I–related molecules (42). Their results suggest that a similar pathway of development, which we have described here, may also occur for human γδ IEL.
Acknowledgments
The authors would like to thank M. Kronnenberg and M. Huflejt for careful reading of the manuscript, M. Reaves for her secretarial assistance, and W.A. Olsen for his support. This research was supported by K08 Award DK-02445-01 to T. Lin and grant R01 GM-52735 (to D.R. Green) from the National Institutes of Health.
References
- 1.Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatability complex. Nature. 1988;332:35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 2.Acha-Orbea H, Palmer E. Mls: a retrovirus exploits the immune system. Immunol Today. 1991;12:356–361. doi: 10.1016/0167-5699(91)90066-3. [DOI] [PubMed] [Google Scholar]
- 3.Dent AL, et al. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 4.Teh HS, Kishi H, Scott B, von Boehmer H. Deletion of autospecific T cells in T cell receptor transgenic mice spare cells with normal TCR levels and low levels of CD8 molecules. J Exp Med. 1989;169:795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisielow P, Bluethmann H, Startz UD, Steinmetz M, von Boehmer H. Tolerance in T cell receptor transgenic mice involves deletion of non-mature CD4+CD8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 6.Sha WC, et al. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 7.Sha WC, et al. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 8.Speiser DE, et al. Neonatal tolerance to Mls-1a determinants: deletion or anergy of Vβ6+ T lymphocytes depending upon MHC compatibility of neonatally injected cells. Int Immunol. 1991;3:127–134. doi: 10.1093/intimm/3.2.127. [DOI] [PubMed] [Google Scholar]
- 9.Schneider R, et al. Fate of potentially self-reactive T cells in neonatal mice: analysis of Vβ6+ T cells in Mlsa mice. Thymus. 1989;13:35–43. [PubMed] [Google Scholar]
- 10.Schneider R, et al. Postnatal disappearance of self-reactive (Vβ6) cells from the thymus of Mlsa mice. J Exp Med. 1989;169:2149–2154. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teh HS, et al. Early deletion and late positive selection of T cells expressing a male-specific receptor in T-cell receptor transgenic mice. Dev Immunol. 1990;1:1–10. doi: 10.1155/1990/18208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dent AL, Matis LA, Bluestone JA, Hedrick SM. Evidence for programmed cell death of self-reactive gamma delta T cell receptor-positive thymocytes. Eur J Immunol. 1993;23:2482–2487. doi: 10.1002/eji.1830231016. [DOI] [PubMed] [Google Scholar]
- 13.Bandeira A, et al. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-antigen receptor γδ. Proc Natl Acad Sci USA. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperling A, et al. Selective expansion of Vgamma2-Vdelta7 TCR gamma delta cells in C57BL/6 mice is postnatal and extrathymic. J Immunol. 1997;159:86–91. [PubMed] [Google Scholar]
- 15.Yoshikai Y, Reis MD, Mak TW. Athymic mice express a high level of functional gamma-chain but greatly reduced levels of alpha- and beta-chain T-cell receptor messages. Nature. 1986;324:482–485. doi: 10.1038/324482a0. [DOI] [PubMed] [Google Scholar]
- 16.Barrett TA, et al. Mechanism of self-tolerance of γ/δ T cells in epithelial tissue. J Exp Med. 1992;175:65–70. doi: 10.1084/jem.175.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha B, Vassalli P, Guy-Grand D. The Vβ repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor αβ+ lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991;173:483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha B, von Boehmer H, Guy-Grand D. Selection of intraepithelial lymphocytes with CD8 alpha/alpha co-receptors by self antigen in the murine gut. Proc Natl Acad Sci USA. 1992;89:5336–5340. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poussier P, Teh HS, Julius M. Thymus-independent positive and negative selection of T cells expressing a major histocompatibility complex class I restricted transgenic T cell receptor αβ in the intestinal epithelium. J Exp Med. 1993;178:1947–1957. doi: 10.1084/jem.178.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz D, et al. An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes. J Exp Med. 1998;188:255–265. doi: 10.1084/jem.188.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha B, Vassalli P, Guy-Grand D. The extrathymic T cell development pathway. Immunol Today. 1992;13:449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- 22.Lin T, Matsuzaki G, Kenai H, Nomoto K. Extrathymic and thymic origin of IEL: are most IEL in euthymic mice derived from the thymus? Immunol Cell Biol. 1995;73:469–473. doi: 10.1038/icb.1995.73. [DOI] [PubMed] [Google Scholar]
- 23.Lefrancois L, Puddington L. Extrathymic intestinal T-cell development: virtual reality. Immunol Today. 1995;16:16–21. doi: 10.1016/0167-5699(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 24.Matsuzaki G, Lin T, Nomoto K. Differentiation and function of intestinal intraepithelial lymphocytes. Int Rev Immunol. 1994;11:47–53. doi: 10.3109/08830189409061716. [DOI] [PubMed] [Google Scholar]
- 25.Lin T, Matsuzaki G, Kishihara K, Mak TW, Nomoto K. Characteristics of fetal thymus-derived T cell receptor γδ intestinal intraepithelial lymphocytes. Eur J Immunol. 1994;24:1792–1798. doi: 10.1002/eji.1830240811. [DOI] [PubMed] [Google Scholar]
- 26.Lefrancois L, Olsen S. A novel pathway of thymus-directed T lymphocyte maturation. J Immunol. 1994;157:987–995. [PubMed] [Google Scholar]
- 27.Barrett TA, Tatsumi Y, Bluestone JA. Tolerance of T cell receptor γδ cells in the intestine. J Exp Med. 1993;177:1755–1762. doi: 10.1084/jem.177.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin T, et al. CD3–CD8+ intestinal intraepithelial lymphocytes (IEL) and the extrathymic development of IEL. Eur J Immunol. 1994;24:1080–1087. doi: 10.1002/eji.1830240511. [DOI] [PubMed] [Google Scholar]
- 29.Sjodin K, Dalmasso AP, Smith JM, Martinez C. Thymectomy in newborn and adult mice. Transplantation. 1966;1:521–525. doi: 10.1097/00007890-196301040-00011. [DOI] [PubMed] [Google Scholar]
- 30.Lin T, Matsuzaki G, Kenai H, Nakamura T, Nomoto K. Thymus influences the development of extrathymically derived intestinal intraepithelial lymphocytes early in ontogeny. Eur J Immunol. 1993;23:1968–1974. doi: 10.1002/eji.1830230836. [DOI] [PubMed] [Google Scholar]
- 31.Lin T, Matsuzaki G, Kenai H, Nomoto K. Progenies of fetal thymocytes are the major source of CD4–CD8+ αα intestinal intraepithelial lymphocytes early in ontogeny. Eur J Immunol. 1994;31:1785–1791. doi: 10.1002/eji.1830240810. [DOI] [PubMed] [Google Scholar]
- 32.Lin T, et al. Fas-ligand-mediated killing by intestinal intraepithelial lymphocytes. J Clin Invest. 1998;101:570–577. doi: 10.1172/JCI896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schild H, et al. The nature of MHC recognition by γ/δ T cells. Cell. 1994;76:29–36. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 34.Weintraub BC, Jackson MR, Hedrick SM. γδ T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J Immunol. 1994;153:3051–3058. [PubMed] [Google Scholar]
- 35.Guy-Grand D, et al. Different expression of the recombinant activating gene RAG-1 in various populations of thymocytes, peripheral T cells and gut thymus-independent intraepithelial lymphocytes suggest two pathways of T cell receptor rearrangement. Eur J Immunol. 1992;22:505–510. doi: 10.1002/eji.1830220232. [DOI] [PubMed] [Google Scholar]
- 36.Budd RC, et al. Distinction of virgin and memory T lymphocytes. J Immunol. 1987;138:3120–3124. [PubMed] [Google Scholar]
- 37.Wells FB, et al. Phenotypic and functional analysis of positive selection in the γδ T cell lineage. J Exp Med. 1993;177:1061–1070. doi: 10.1084/jem.177.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweighoffer E, Fowlkes BJ. Positive selection is not required for thymic maturation of transgenic γδ T cells. J Exp Med. 1996;183:2033–2041. doi: 10.1084/jem.183.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;35:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 40.Rocha B, Vassalli P, Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J Exp Med. 1994;180:681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guehler SR, Bluestone JA, Barrett TA. Immune deviation of 2C transgenic intraepithelial lymphocytes in antigen-bearing hosts. J Exp Med. 1996;184:493–503. doi: 10.1084/jem.184.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gamma-delta T cells. Science. 1998;13:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]