Abstract
Sperm competition, a prevalent evolutionary process in which the spermatozoa of two or more males compete for the fertilization of the same ovum, leads to morphological and physiological adaptations, including increases in energetic metabolism that may serve to propel sperm faster but that may have negative effects on DNA integrity. Sperm DNA damage is associated with reduced rates of fertilization, embryo and fetal loss, offspring mortality, and mutations leading to genetic disease. We tested whether high levels of sperm competition affect sperm DNA integrity. We evaluated sperm DNA integrity in 18 species of rodents that differ in their levels of sperm competition using the sperm chromatin structure assay. DNA integrity was assessed upon sperm collection, in response to incubation under capacitating or non-capacitating conditions, and after exposure to physical and chemical stressors. Sperm DNA was very resistant to physical and chemical stressors, whereas incubation in non-capacitating and capacitating conditions resulted in only a small increase in sperm DNA damage. Importantly, levels of sperm competition were positively associated with sperm DNA fragmentation across rodent species. This is the first evidence showing that high levels of sperm competition lead to an important cost in the form of increased sperm DNA damage.
Keywords: sperm competition, rodents, sperm DNA fragmentation, capacitation, oxidative stress, sperm chromatin structure assay
1. Introduction
Sperm competition occurs when females mate with more than one male and the spermatozoa of those males compete for the fertilization of the same ovum [1,2]. Sperm competition is a prevalent phenomenon and its occurrence leads to several evolutionary adaptations at both the morphological and physiological levels [2,3]. High levels of sperm competition are associated with an increase in the production, storage and allocation of spermatozoa, as well as with enhanced sperm function [3–5]. In rodents, high levels of sperm competition lead to a higher proportion of spermatozoa that are morphologically normal, motile and capable of reaching and fertilizing the ovum [6,7], as well as in modifications in sperm dimensions that may result in improvements in sperm movement [8,9]. In many taxa, sperm swimming velocity, an important feature of sperm function, is also higher in those species that experience high levels of sperm competition [9–12].
Sperm DNA integrity is vital for the transmission of paternal genetic material. The occurrence of DNA damage in sperm cells is associated with reduced rates of fertilization, abortion and developmental problems with adverse effects for offspring [13–17]. The origins of this sperm DNA damage are varied, including strand breaks that originate during the chromatin remodelling during spermiogenesis and DNA fragmentation induced by oxidative stress [18,19]. Sperm cells are especially sensitive to DNA damage because of the absence of DNA repair mechanisms; the process of abortive apoptosis, in which sperm cells are only able to undergo a restricted apoptotic process that leads to DNA fragmentation but renders these cells still capable of fertilization; and the low levels of ROS-scavenging enzymes due to the limited volume of the cytosolic space in sperm cells [14,18].
Given that high levels of sperm competition are associated with a general enhancement of sperm function, two general scenarios may be possible. On the one hand, under high sperm competition conditions, species may evolve spermatozoa and accessory gland fluids that manage to prevent sperm DNA damage, and therefore it could be predicted that in species with high levels of sperm competition, spermatozoa will have (i) lower levels of baseline DNA damage and (ii) higher resistance to stressors that can impact DNA integrity. Such reduced baseline levels and higher protection against DNA damage can be attained, for example, by increasing antioxidant measures in the male reproductive tract, seminal fluid and spermatozoa themselves. On the other hand, the increased metabolism needed to fuel higher sperm swimming velocity in species that experience high levels of sperm competition [20] could lead to a marked rise in the production of reactive oxygen species (ROS) within sperm cells, resulting in a net loss in DNA integrity, despite countermeasures evolved to prevent or repair such damage. In addition, the faster rates of spermatogenesis in species with high levels of sperm competition [3] may result in less controlled DNA condensation and packaging of DNA leading to higher incidences of DNA fragmentation. In any of the latter two cases, an increase in DNA damage should be regarded as a cost accrued by species with high levels of sperm competition.
In order to test these two opposing predictions relating levels of sperm competition, sperm structure and function, and sperm DNA damage, we evaluated sperm DNA integrity in three series of experiments using the sperm chromatin structure assay (SCSA). The SCSA is an optimal technique to quantify populations of DNA-fragmented spermatozoa in rodents [21] and other mammals [22], including humans [23]. First, we performed a comparative analysis to assess baseline levels of DNA fragmentation among 18 species of rodents that differ in their levels of sperm competition. In addition, we used SCSA data to determine if the proportion of spermatozoa in immature stages (see ‘DNA fragmentation assessment’ below) is higher in species with high levels of sperm competition, which could be related to faster spermatogenesis in these species [3]. We also assessed the possible changes in DNA integrity after a long-term incubation that mimics the period of time and conditions of sperm survival in the female tract before undergoing changes required for fertilization. As already mentioned, spermatozoa are more motile and swim faster in species with high levels of sperm competition, possibly leading to an increased production of ROS. We thus hypothesized that increases in sperm DNA damage driven by incubation will be higher as the level of sperm competition increases.
Second, we assessed whether capacitation of spermatozoa, as influenced by different levels of sperm competition, has any impact on sperm DNA damage. Capacitation is a physiological switching-on that occurs in the oviduct and is essential for mammalian spermatozoa. Molecular and cellular changes taking place during capacitation render spermatozoa capable of interacting with the ovum and surrounding coats, and undergoing the acrosome reaction in response to ovum signals [24,25]. Completion of capacitation is accompanied by the development of hyperactivated motility, which is thought to facilitate sperm transport and ovum penetration during the final stages of fertilization [25]. Hyperactivated motility, because of its presumed increase in energetic demands, could lead to an enhanced production of ROS and thus increased sperm DNA damage. To test this prediction, we incubated sperm cells from three species of mice from the genus Mus that differ in their levels of sperm competition [6] under non-capacitating and capacitating conditions, and quantified sperm DNA integrity.
Third, we reasoned that spermatozoa from species with varying levels of sperm competition may respond differently to a series of external stressors. To assess the effect of different stressors, we exposed sperm cells from the same three Mus species to physical (freeze–thawing plus vortexing) and chemical (DNase-I and H2O2) stressors to determine if any increase in DNA damage promoted by such stressors is influenced by differing levels of sperm competition. DNase-I is an endonuclease that cleaves DNA phosphodiester bonds and thus induces DNA strand breaks. Exposing spermatozoa in vitro to DNase-I is an indirect approach to determine if species differ in their degree of sperm DNA compaction. During sperm maturation, the chromatin becomes extremely condensed when histones are replaced by protamines [26]. The inter- and intra-molecular disulfide bonds between the protamine molecules confer chromatin compaction and stabilization [27], and thus higher protection for the nuclear DNA from damaging factors, including ROS. In fact, deficient protamination of sperm chromatin results in DNA damage and infertility [28]. Given that such DNA compaction may differ between species, the spermatozoa of different species may endure different levels of DNA damage under the same conditions [29,30]. Since the action of DNase-I depends on its access to DNA, a higher induction of DNA strand breaks by DNase-I in some species would give a measure of impaired compaction in those species. H2O2 is normally used as an oxidative stressor due to its membrane permeability and readiness to form the highly reactive hydroxyl radical that predominantly causes single-strand breaks and oxidative base damage [31]. By exposing sperm cells to different levels of H2O2 and assessing the ensuing DNA damage, we were able to determine if the three Mus species have different sensitivity to oxidative stress [14], and, if so, whether such species differences are related to their differing levels of sperm competition.
2. Material and methods
(a). Animals
We studied adult males (when ages were known, we used males that were four to six months of age) from a total of 18 species. Males from Mus musculus (n = 16), M. pahari (n = 4), M. spretus (n = 16) and M. spicilegus (n = 15) came from wild-derived colonies, which had been kept in captivity at the Museo Nacional de Ciencias Naturales for only a few generations. Males from M. castaneus (n = 8), M. caroli (n = 10), M. domesticus (n = 6) and M. macedonicus (n = 7) were purchased from the Laboratory of Genome and Populations of the University of Montpellier II, France. Males from Cricetulus griseus (n = 5), Lemniscomys barbarus (n = 9), Mastomys natalensis (n = 6), Mesocricetus auratus (n = 6), M. minutoides (n = 8), Micromys minutus (n = 5), Phodopus campbelli (n = 5), P. roborovskii (n = 5) and P. sungorus (n = 7) were purchased from local vendors. Males from Apodemus sylvaticus (n = 13) were trapped in Palencia, Spain, during the reproductive season, taken to the laboratory, and housed in individual cages for 10–15 days before sample collection (to minimize perceived risk of sperm competition by males). All males were maintained under standard conditions (14 h light–10 h darkness, 22–24°C, 55–60% relative humidity); with food (rodent chow, Harlan Laboratories; seeds and fresh apple) and water provided ad libitum.
(b). Sperm suspension preparation
Males were sacrificed by cervical dislocation and weighed. Testes were removed and weighed. Mature spermatozoa were collected from the caudae epididymides and vasa deferentia. In several species, DNA fragmentation has been shown not to differ between the spermatozoa obtained from the cauda and ejaculated spermatozoa [32]. We placed the tissue in a Petri dish containing medium prewarmed to 37°C, made several cuts and allowed spermatozoa to swim out for a period of 10 min. We used two types of media depending on the experiment, one being a non-capacitating medium (Hepes-buffered modified Tyrode's medium, mT-H) and the other a capacitating medium (mT-HB, containing 15 mM NaHCO3, equilibrated with 5% CO2) [33]. mT-H supports sperm survival but not the development of changes required for fertilization (i.e. ‘capacitation’, the physiological switching on of spermatozoa required for sperm–oocyte interactions), whereas mT-HB supports capacitation and the ensuing hyperactivation [33]. After 10 min of incubation for swim-out, the sperm suspension (approx. 10–20 × 106 spermatozoa ml−1) was transferred to a prewarmed Eppendorf tube. Each sperm suspension was maintained at 37°C until processing.
(c). Comparative analyses
We performed two sets of comparative analyses. In the first set of analyses, we quantified the baseline levels of sperm DNA fragmentation in 18 rodent species (see sample sizes in table 1). We collected a subsample of sperm suspension from each male immediately after sperm swim-out (10 min after reproductive tract was excised). We snap-froze each sperm sample in liquid nitrogen and stored frozen samples at −80°C until SCSA analysis. In the second set of analyses, we quantified the effect of incubation for 3 h on sperm DNA integrity. For a subset of males used in the first set of analyses (see sample sizes for each species in the electronic supplementary material, table S1), we incubated sperm suspensions at 37°C in mT-H medium under air for 3 h as described previously [34]. We then snap-froze each sperm sample in liquid nitrogen and stored the samples at −80°C until SCSA analysis.
Table 1.
Relationship between levels of sperm competition and SCSA values. Values represent the mean ± s.e.m. RTS, relative testes size; tDFI, total DNA fragmentation index; HDS, high DNA stainability.
| species | n | body mass (g) | testes mass (g) | RTS | tDFI | HDS |
|---|---|---|---|---|---|---|
| Apodemus sylvaticus | 13 | 29.85 ± 0.69 | 0.96 ± 0.02 | 2.27 ± 0.05 | 19.11 ± 2.06 | 39.34 ± 2.95 |
| Cricetulus griseus | 5 | 33.72 ± 0.38 | 1.78 ± 0.04 | 3.83 ± 0.11 | 27.65 ± 2.58 | 15.03 ± 2.36 |
| Lemniscomys barbarus | 9 | 42.45 ± 1.36 | 0.7 ± 0.06 | 1.25 ± 0.1 | 14.27 ± 2.49 | 13.51 ± 3.77 |
| Mastomys natalensis | 6 | 80.76 ± 5.52 | 0.92 ± 0.06 | 1.02 ± 0.06 | 27.66 ± 3.16 | 37.27 ± 3.66 |
| Mesocricetus auratus | 6 | 111.02 ± 8.59 | 3.32 ± 0.23 | 2.88 ± 0.11 | 19 ± 4.26 | 17.78 ± 1.69 |
| Micromys minutus | 5 | 8.09 ± 0.43 | 0.12 ± 0.01 | 0.77 ± 0.03 | 3.42 ± 1.02 | 2.21 ± 0.53 |
| Mus caroli | 10 | 18.11 ± 0.39 | 0.15 ± 0.01 | 0.5 ± 0.02 | 4.29 ± 0.59 | 10.8 ± 1.67 |
| Mus castaneus | 8 | 18.82 ± 0.34 | 0.08 ± 0.01 | 0.26 ± 0.02 | 5.41 ± 0.68 | 8.23 ± 2.19 |
| Mus domesticus | 6 | 19.7 ± 0.6 | 0.15 ± 0.01 | 0.49 ± 0.02 | 4.97 ± 1.04 | 12.22 ± 2.82 |
| Mus macedonicus | 7 | 20.1 ± 0.64 | 0.3 ± 0.01 | 0.95 ± 0.03 | 3.92 ± 1.29 | 8.74 ± 0.81 |
| Mus minutoides | 8 | 5.84 ± 0.48 | 0.11 ± 0.01 | 0.89 ± 0.06 | 7.18 ± 0.2 | 3.51 ± 0.28 |
| Mus musculus | 16 | 22. 86 ± 1.29 | 0.14 ± 0.01 | 0.42 ± 0.02 | 3.55 ± 1.09 | 9.35 ± 0.67 |
| Mus pahari | 4 | 33.15 ± 0.61 | 0.13 ± 0 | 0.28 ± 0.01 | 4.49 ± 0.53 | 9.04 ± 1.82 |
| Mus spicilegus | 15 | 17.85 ± 0.56 | 0.45 ± 0.01 | 1.58 ± 0.03 | 3.42 ± 0.59 | 10.28 ± 0.76 |
| Mus spretus | 16 | 17.85 ± 0.76 | 0.28 ± 0.01 | 1 ± 0.04 | 2.97 ± 0.25 | 5.73 ± 0.72 |
| Phodopus campbelli | 5 | 51.31 ± 3.46 | 2.09 ± 0.12 | 3.25 ± 0.13 | 15.21 ± 1.18 | 9.82 ± 2.44 |
| Phodopus roborovskii | 5 | 24.64 ± 1.06 | 0.93 ± 0.09 | 2.53 ± 0.21 | 17.2 ± 3.17 | 5.05 ± 0.98 |
| Phodopus sungorus | 7 | 43.08 ± 1.87 | 0.9 ± 0.05 | 1.6 ± 0.08 | 23.45 ± 2.7 | 12.18 ± 2.14 |
(d). Effect of capacitation
We used males (n = 5 per species) from three species of Mus that differ greatly in their levels of sperm competition: M. musculus, M. spretus and M. spicilegus. These three species have been characterized as a good model for studies on sperm competition in rodents, representing low, intermediate and high levels of sperm competition, respectively [6]. For each male, we incubated spermatozoa from one cauda epididymis under non-capacitating conditions (medium mT-H, under air, at 37°C) and spermatozoa from the other cauda under capacitating conditions (medium mT-HB, under 5% CO2/air, at 37°C). In both cases, spermatozoa were incubated for 90 min. Sperm samples were taken at time 0 and at 90 min. For each male, we thus collected four different samples: spermatozoa incubated under non-capacitating conditions at time 0 and after 90 min, and spermatozoa incubated under capacitating conditions at time 0 and after 90 min. All samples were snap-frozen in liquid nitrogen and then stored at −80°C until SCSA analysis.
(e). Effects of physical and chemical stress
We examined the effect of stressors on spermatozoa from the three mouse species with different sperm competition levels, as above, namely M. musculus, M. spretus and M. spicilegus (n = 5 per species). For each male, we collected spermatozoa from both caudae in mT-H medium under air, allowing sperm to swim out for 10 min. We divided the resulting sperm suspension in 12 aliquots. One aliquot was directly snap-frozen in liquid nitrogen without any treatment (control). Another aliquot was snap-frozen in liquid nitrogen, thawed at room temperature, vortexed during 10 s and snap-frozen again (vortex treatment). The remaining ten samples were exposed to different concentrations of DNase-I and H2O2, in line with the concentrations used for laboratory mouse spermatozoa in a previous study [35]. The use of these concentrations of DNase-I and H2O2 provided the opportunity to directly compare our results with those of a previous comparative study on mammals [35].
For the DNase-I treatment, five sperm aliquots in mT-H were centrifuged at 1677g (5000 r.p.m. in a MiniSpin Plus, Eppendorf Ibérica, Madrid, Spain) for 5 min. The resulting sperm pellet was resuspended in 100 µl of permeabilizing solution (0.1% sodium citrate and 0.1% Triton X-100, both from Sigma, Madrid, Spain, in mT-H) and incubated for 2 min at 37°C. This permeabilizing solution allows DNase-I to cross the sperm membrane. After centrifugation at 1677g for 5 min and removal of the supernatant, we added 500 µl of the corresponding DNase-I concentration (0, 1, 10, 100 or 1000 U ml−1 DNase-I from bovine pancreas expressed in Pichia pastoris; Roche Diagnostics, Mannheim, Germany). After each centrifugation, the sperm pellet was resuspended by lightly flicking the end of the tube. After 30 min of incubation at 37°C, we centrifuged each sample twice at 1677g for 5 min, each time removing the supernatant and adding new mT-H. Samples were then snap-frozen in liquid nitrogen.
For the H2O2 treatment, we first centrifuged five sperm aliquots in mT-H at 1677g for 5 min, then removed the supernatant and added 500 µl of the corresponding H2O2 solution (0, 0.1, 1, 10 or 100 mM H2O2 in mT-H) made from a commercial solution (35%, w/w; Sigma). After 1 h of incubation at 37°C, we centrifuged each sample twice at 1677g for 5 min, each time removing the supernatant and adding new mT-H. Samples were then snap-frozen in liquid nitrogen until DNA fragmentation analysis by means of SCSA.
(f). DNA fragmentation assessment
Chromatin stability was assessed by using the SCSA [23]. The SCSA is based on the susceptibility of the sperm DNA to acid-induced denaturation in situ and on the metachromatic properties of the stain acridine orange, which fluoresces green when associated with the intact double-stranded DNA helix, but red when associated with single-strand denaturated DNA and RNA [22,36]. SCSA has been previously used in rodent spermatozoa to determine DNA fragmentation with good results [21].
Samples were thawed on crushed ice, diluted with TNE buffer (0.15 M NaCl, 0.01 M Tris–HCl, 1 mM EDTA; pH 7.4) at a final sperm concentration of 2 × 106 cells ml−1, and 200 µl of sperm suspension were placed in a cytometry tube. Immediately, 400 µl of an acid-detergent solution (0.08 N HCl, 0.15 M NaCl, 0.1% Triton X-100; pH 1.4) were added to the tube. After exactly 30 s, 1.2 ml of acridine orange staining solution (0.037 M citric acid, 0.126 M Na2HPO4, 0.0011 M disodium EDTA, 0.15 M NaCl; pH 6.0, 4°C) containing 6 mg ml−1 electrophoretically purified acridine orange were added. Stained samples were analysed 3 min later by flow cytometry. Acridine orange was excited by using an argon laser providing 488 nm light. A total of 5000 events were accumulated for each sample. We expressed the extent of DNA denaturation in terms of DNA fragmentation index (DFI), which is the ratio of red to total (red plus green) fluorescence intensity (i.e. the level of denatured DNA over the total DNA) [22]. The DFI value was calculated for each sperm cell in a sample, and the resulting DFI frequency profile was obtained. Total DNA fragmentation index (tDFI) was defined as the percentage of spermatozoa with a DFI value over 25. High DNA stainability (HDS), which offers a measure of the percentage of immature sperm cells, was defined as the percentage of spermatozoa with green fluorescence higher than channel 600 (of 1024 channels).
(g). Statistical analyses
All statistical analyses were conducted using R v. 3.1.0 [37]. Normality was checked with the Shapiro–Wilk normality test. If normality was not met, we used a logarithmic transformation. Average values are reported as mean ± s.e.m. Significance level (α) was set at 0.05 for all the tests.
For the comparative analyses of DNA fragmentation, we performed regressions using phylogenetic generalized least-squares (PGLS) analyses to control for phylogenetic association [38]. All PGLS analyses were performed using the caper v. 0.5.2 package for R [39]. The PGLS analysis estimates a phylogenetic scaling parameter lambda (λ), which is then incorporated in the models to control for phylogenetic effects. The phylogenetic reconstruction used in the PGLS analyses is included in the electronic supplementary material, figure S1. We used tDFI or HDS as the dependent variable (given that HDS is a measure of the degree of sperm maturation, we only analysed HDS in the first series of analyses, but not in the subsequent analyses in which spermatozoa were subjected to various treatments). We used body mass and testes mass as predictors in the PGLS analyses. This provided a measure of the relationship between each dependent variable and relative testes mass (RTS). This latter variable has been shown to reflect sperm competition levels in rodents [40–42]. Given that body mass and testes mass are related to each other (i.e. they are non-orthogonal), a sequential (type I) sum of squares was used, adding the two predictors to the models in the following order: body mass, testes mass. For the graphical representation of RTS (figure 1), and for the calculation of RTS values in table 1, and only in these cases, RTS was calculated using Kenagy and Trombulak's rodent-specific regression equation: RTS = testes mass/0.031 × body mass0.77 [43].
Figure 1.
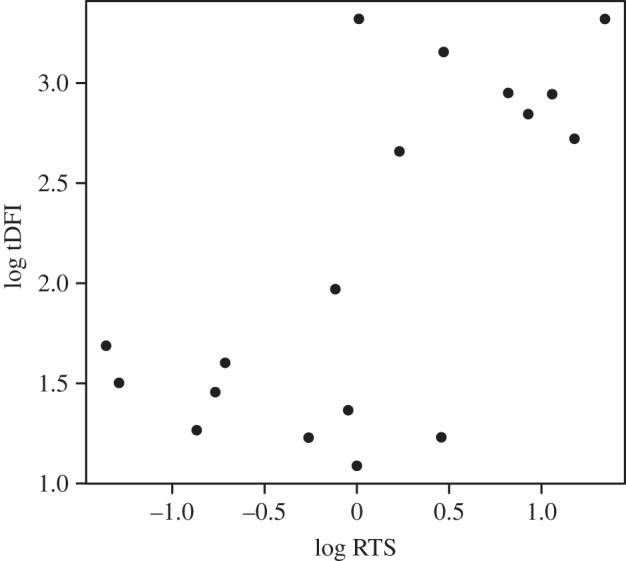
Relationship between relative testes size (RTS, sensu [43]) and total sperm DNA fragmentation index (tDFI). This representation does not include the phylogenetic corrections included in the statistical models.
For analyses comparing three Mus species, we performed two-way repeated measures ANOVAs. We used tDFI as the dependent variable, and species and treatment as factors (we also considered the interaction between species and treatment). The repeated measure was given by male identity.
3. Results
(a). Comparative analyses
The 18 species of rodents that we studied offer a large range of body masses (from 5.84 ± 0.48 g in M. minutoides to 111.02 ± 8.59 g in Me. auratus), testes masses (from 0.08 ± 0.01 g in M. castaneus to 3.32 ± 0.23 g in Me. auratus) and relative testes sizes (RTS; from 0.26 ± 0.02 in M. castaneus to 3.83 ± 0.11 in C. griseus). Values for body mass, testes mass, RTS, tDFI and HDS from these 18 species are summarized in table 1.
We did not predict a priori any relationship between body mass and either tDFI or HDS values. However, we found that larger species had higher tDFI values (PGLS: F1,15 = 23.2, p = 0.0002) and higher HDS values (PGLS: F1,15 = 8.12, p = 0.01).
We obtained baseline levels for tDFI and HDS from the 18 species. We found that higher RTS values were associated with higher tDFI values (PGLS: F1,15 = 6.8, p = 0.02; figure 1) but that RTS did not relate to HDS (PGLS: F1,15 = −0.15, p = 0.89; table 1).
To determine the effect of incubation in non-capacitating medium (a condition that resembles that experienced by spermatozoa in the female reproductive tract), we assessed sperm tDFI and HDS after 3 h of incubation in 17 species (we were not able to obtain 3 h incubation samples from A. sylvaticus; tDFI and HDS values at 0 h and at 3 h of incubation are reported in the electronic supplementary material, table S1, although only tDFI data were analysed at 3 h, as explained above). Similar to the baseline results, after 3 h of incubation there was also a positive relationship between tDFI and RTS (PGLS: F1,14 = 5.55, p = 0.03). However, the increase in tDFI after 3 h of incubation was similar across species and thus was not related to RTS (PGLS: F1,14 = 1.12, p = 0.31; electronic supplementary material, table S1). Thus, the relationship between RTS and tDFI at 3 h was construed by the baseline differences between species and there were no differential effects of incubation time on changes in tDFI.
(b). Effect of capacitation
In the overall model, we found significant differences among the four treatments (control 0 min, control 90 min, capacitation 0 min and capacitation 90 min; F3,36 = 4.18, p = 0.01; figure 2). In subanalyses by species, there were no significant differences between treatments in M. musculus (F3,12 = 0.73, p = 0.55), whereas significant differences were detected in M. spretus (F3,12 = 5.7, p = 0.01) and M. spicilegus (F3,12 = 5.6, p = 0.01).
Figure 2.
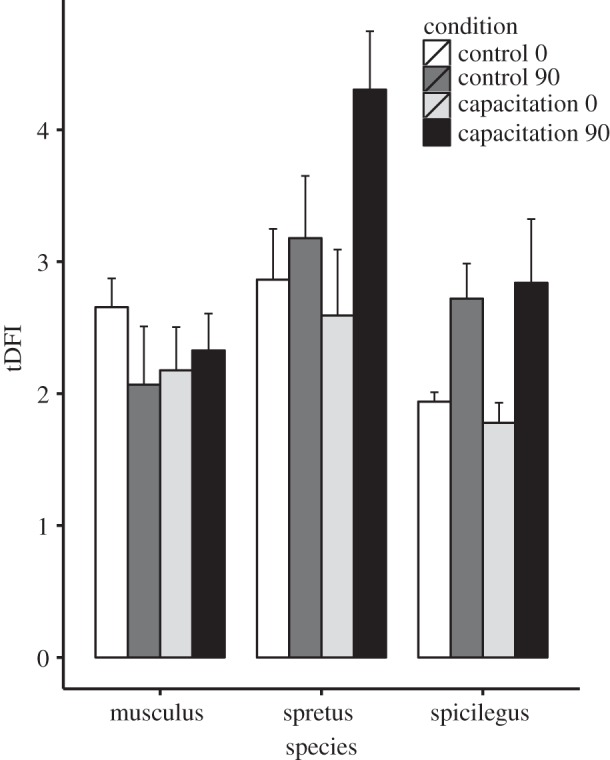
Effect of capacitation on sperm DNA fragmentation. Spermatozoa from M. musculus, M. spretus and M. spicilegus were incubated in non-capacitating (control 0 and control 90) or capacitating (capacitation 0 and capacitation 90) media during 10 + 0 min (control 0 and capacitation 0) or 10 + 90 min (control 90 and capacitation 90). tDFI, total sperm DNA fragmentation index. Mean and s.e.m. are presented (n = 5 for each species).
Values of tDFI were higher after 90 min of incubation under capacitating conditions in both M. spretus (capacitation 0 min versus capacitation 90 min: F1,4 = 13.4, p = 0.02) and M. spicilegus (F1,4 = 14.2, p = 0.02; figure 2). However, we found no difference between 90 min of incubation under non-capacitating and capacitating conditions (control 90 min versus capacitation 90 min: M. spretus: F1,4 = 3.57, p = 0.13; M. spicilegus: F1,4 = 0.003, p = 0.96).
(c). Effects of physical and chemical stressors
There was no increase in tDFI due to a vortex treatment (two-way repeated measures ANOVA: F1,12 = 0.1, p = 0.76; electronic supplementary material, figure S2a). Also, there were no differences in tDFI between the three species with this treatment (F2,12 = 0.54, p = 0.6).
There were no significant differences in tDFI values between treatments with various DNase-I concentrations (F4,48 = 2.23, p = 0.08) or among the three different species (F2,12 = 1.01, p = 0.4; electronic supplementary material, figure S2b).
Similarly, there were not significant differences in tDFI depending on H2O2 concentrations (F4,48 = 0.67, p = 0.62) or among the three different species (F2,12 = 3.8, p = 0.06; electronic supplementary material, figure S2c).
4. Discussion
We found a positive relationship between levels of sperm competition and sperm DNA fragmentation across rodent species. This is the first time that an increase in sperm competition levels has been found to be associated with a functional cost involving sperm DNA integrity. This is a very important cost if the spermatozoa that reach the fertilization site contain damaged DNA, because spermatozoa exhibiting a significant degree of DNA fragmentation are still capable of fertilization [44]. It has to be considered that the increased numbers of spermatozoa with damaged DNA in species with high levels of sperm competition may not be part of the small sperm subpopulation that reaches the site of fertilization if any mechanism is in place in the female tract that aims towards selection against damaged sperm. This hypothesis could be tested in the future by quantitating sperm DNA integrity in sperm subpopulations to assess if the increased sperm DNA damage in species with high levels of sperm competition is spread across the whole sperm population or, instead, a particular subpopulation is affected. If the latter, it may be that high levels of sperm competition lead to an increased intra-male variation in sperm subpopulations, with subpopulations in the first sections of the female reproductive tract having higher levels of sperm DNA damage compared with sperm subpopulations reaching the last sections of the female reproductive tract. Another untested hypothesis is that the repair of DNA fragmentation that happens within the fertilized oocyte [45] may be more efficient or increased in species with high levels of sperm competition, leading to a coevolutionary process that may compensate for high levels of sperm DNA damage in such species.
The higher prevalence of sperm DNA fragmentation in species with high levels of sperm competition could be due to higher levels of oxidative stress in these species. Recently, it has been shown that the increase in sperm swimming velocity observed in rodent species that experience high levels of sperm competition is driven to a great extent by a rise in the content of sperm ATP [20,34,46]. That is, in species with high levels of sperm competition there seem to be metabolic changes and an increase in metabolism that allow sperm cells to swim faster [47]. This increased metabolism may lead to a rise in the production of ROS, which can damage not only lipids and proteins but also the DNA in sperm cells. In species with high levels of sperm competition there is a decrease in the percentage of polyunsatured fatty acids in the sperm membrane, which confers lipid protection against ROS [48,49]. According to our results, analogous measures (e.g. changes in protamination or compaction) appear not be in place in these species to counteract the damaging effects of ROS on sperm DNA. However, differences in the type and extent of protamination in relation to levels of sperm competition deserve future examination [50].
High DNA stainability (HDS) was higher in large-bodied species but was not associated with different levels of sperm competition. Absolute body size was also positively associated with higher levels of sperm DNA fragmentation (i.e. higher tDFI values). The significant relationship between body size and sperm DNA fragmentation across rodent species could be related to species differences in mass-specific metabolic rate [3], but further research is needed to clarify the meaning of such association.
High levels of sperm competition lead to faster spermatogenesis and an increase in sperm production [3]. Such enhanced sperm production does not seem to result in a higher proportion of immature spermatozoa (as measured by HDS) but could be partly responsible for the observed increase in sperm DNA fragmentation. Indeed, our incubation experiment indicates that species differences with regard to sperm DNA fragmentation may take place not after but before ejaculation (i.e. during spermatogenesis, sperm transport in the epididymis and storage in the cauda epididymis). We observed that after 3 h of incubation, there was a slight increase in sperm DNA damage relative to baseline levels, but such increase was similar across species and thus was not influenced by differing levels of sperm competition. Likewise, in many species as diverse as human, deer, boar and rhinoceros, a period of incubation that reflects the time that spermatozoa will remain in the female reproductive tract before capacitation does not result in any substantial increase in sperm DNA damage [51–55].
The process of sperm capacitation, including acquisition of fast motility (hyperactivation) by spermatozoa, also led to a small increase in sperm DNA fragmentation. Interestingly, such increase in sperm DNA fragmentation after incubation in capacitating medium occurred in the two species with higher levels of sperm competition (i.e. M. spretus and M. spicilegus) but not in the species with low levels of sperm competition (M. musculus). In bulls, as in M. musculus, sperm DNA integrity is not affected by incubation in capacitating medium [56], so all the cellular changes that occur during capacitation do not necessarily generate a negative impact on sperm DNA across species. In addition, no differences were observed between incubation in non-capacitating and capacitating media. That is, the increase in DNA damage that we observed in capacitating medium may not be related as much to the process of capacitation as to spermatozoa simply being incubated (i.e. motile).
Physical stress (i.e. freezing–thawing followed by a vortex treatment) did not result in any significant increase in sperm DNA damage in mice. Similarly, mechanical stress in other species as different as deer and dog does not lead to DNA damage [57,58], which suggests that mammalian sperm DNA can withstand some substantial amount of artificial physical stress and must also be very robust to any naturally occurring mechanical stress. By contrast, cryopreservation can lead to sperm DNA damage in some species such as ram, boar and alpaca [53,59,60], but not in others such as human, horse, bull and dog [58,61–63].
We found that different concentrations of DNase-I and H2O2 did not produce any significant sperm DNA fragmentation in three species of mice. These results are in line with those previously reported in a study on spermatozoa of the laboratory mouse [35]. In that study [35], spermatozoa from mice, along with those of humans and bulls were exposed to concentrations of DNase-I and H2O2 that were similar to the ones that we used. Human and bull spermatozoa were found to be very sensitive to both DNase-I and H2O2, whereas mouse DNA spermatozoa were only moderately affected by the highest concentrations of those stressors. We found no significant effects on mouse sperm DNA integrity even at the highest concentrations of DNase-I and H2O2. It is worth noting that in the study by Villani et al. [35], spermatozoa were frozen and thawed and then exposed to each treatment, whereas we treated fresh spermatozoa, which we then froze until thawing for SCSA analysis. Such methodological differences could account for the small differences between the two studies. In Villani et al.'s work [35], any membrane damage or decrease in compaction due to freezing and thawing may have rendered sperm DNA more accessible to chemical stressors. If so, we can argue that our methodological approach closer resembles the conditions that spermatozoa may encounter in the female reproductive tract, and that spermatozoa in these mouse species are very resistant to external chemical stressors.
In yet two other comparative studies—one examining spermatozoa of human, laboratory mouse and wallaby [29], the other comparing spermatozoa of human, boar, laboratory mouse, wombat, koala and grey kangaroo [64], and both studies using alternative methods to SCSA to assess sperm DNA fragmentation—the spermatozoa of mice were reported to be highly resistant to oxidative stress. In both of these studies, the sperm DNA of marsupial species were more sensitive to oxidative stress than the sperm DNA of eutherian species. This higher sensitivity to oxidative stress is likely to be due to a lack of disulfide cross-linking in marsupial sperm chromatin. The oxidation of thiols to disulfides during chromatin condensation in eutherian mammals can provide not only stability but also protection against genotoxic factors. Intracellular and seminal antioxidants may also protect sperm DNA from oxidative stress. For example, cryopreserved spermatozoa from red deer that were treated with H2O2 suffered an increase in sperm DNA damage, which was counterbalanced when antioxidants were also added [51,65]. Our study suggests that the DNA of mice is extremely well protected against oxidative stress and DNA-breaking molecules. Interestingly, rat spermatozoa treated with 2.5 mM H2O2 experienced a high increase in DNA damage compared with control treatments [66]. Further studies on other rodent species could elucidate why the sperm DNA of different rodent species may differ in their resistance to oxidative stress.
Finally, we investigated whether the spermatozoa from three Mus species in our study differed in their susceptibility to DNase-I and H2O2, and found no significant differences between these species. That is, high levels of sperm competition do not confer any further protection against external stressors in these mouse species.
In conclusion, our study suggests for the first time that the requirements to enhance sperm function in species with high levels of sperm competition may have a cost in terms of increasing the incidence of sperm DNA damage. In addition, the sperm DNA of rodent species, after release from the male tract, may be very resistant to the different stressors that spermatozoa can encounter in the female reproductive tract in their way to the fertilization site.
Supplementary Material
Acknowledgements
We are grateful to Annie Orth and François Bonhomme (Institut des Sciences de l'Evolution de Montpellier, CNRS-Université Montpellier 2, France) for facilitating access to animals, Juan José Luque-Larena and Leticia Arroyo for support with fieldwork, and Juan Antonio Rielo for managing the animal facilities and Esperanza Navarro for animal care at the Museo Nacional de Ciencias Naturales (CSIC). We thank María Varea-Sánchez, Alberto Vicens, Pilar Villar and Ester Sansegundo for their help collecting, processing, incubating and assessing spermatozoa in the laboratory. We also thank Diana Fisher and two anonymous referees for constructive criticism.
Ethics
The research protocol was approved by the Ethics Committee of the Spanish Research Council (CSIC). All procedures were carried out following Spanish Animal Protection Regulation RD53/2013, which conforms to European Union Regulation 2010/63.
Data Accessibility
Datasets have been uploaded to Dryad and are available for download: http://dx.doi.org/10.5061/dryad.cf2q1.
Authors' contributions
J.d.-T. designed the study, coordinated the study, collected and analysed data, and drafted the manuscript; O.G.-Á. collected SCSA data; A.J.S. coordinated the study and collected SCSA data; M.T. collected reproductive data; J.J.G. coordinated the collection of SCSA data; E.R.S.R. conceived the study, participated in the design of the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by a Ramón y Cajal fellowship from the Spanish Ministry of Economy and Competitiveness (RYC-2011-07943) and a Marie Curie Career Integration Grant (PCIG11-GA-2012-321888) to J.d.-T., a fellowship from Campus Científico y Tecnológico de la Energía y el Medioambiente—Universidad de Castilla-La Mancha (CYTEMA-UCLM) to O.G.-Á., a Juan de la Cierva fellowship from the Spanish Ministry of Economy and Competitiveness to M.T (JCI-2011-10381) and grants from the Spanish Ministry of Economy and Competitiveness (CGL2011-26341 to E.R.S.R., CGL2012-37423 to J.d.-T. and AGL2013-48421-R to A.J.S.).
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. San Diego, CA: Academic Press. [Google Scholar]
- 3.delBarco-Trillo J, Tourmente M, Roldan ERS. 2013. Metabolic rate limits the effect of sperm competition on mammalian spermatogenesis. PLoS ONE 8, e76510 ( 10.1371/journal.pone.0076510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.delBarco-Trillo J. 2011. Adjustment of sperm allocation under high risk of sperm competition across taxa: a meta-analysis. J. Evol. Biol. 24, 1706–1714. ( 10.1111/j.1420-9101.2011.02293.x) [DOI] [PubMed] [Google Scholar]
- 5.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. Camb. Philos. Soc. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 6.Gomendio M, Martin-Coello J, Crespo C, Magaña C, Roldan ERS. 2006. Sperm competition enhances functional capacity of mammalian spermatozoa. Proc. Natl Acad. Sci. USA 103, 15 113–15 117. ( 10.1073/pnas.0605795103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez Montoto L, Magaña C, Tourmente M, Martín-Coello J, Crespo C, Luque-Larena JJ, Gomendio M, Roldan ERS. 2011. Sperm competition, sperm numbers and sperm quality in muroid rodents. PLoS ONE 6, e18173 ( 10.1371/journal.pone.0018173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomendio M, Roldan ERS. 2008. Implications of diversity in sperm size and function for sperm competition and fertility. Int. J. Dev. Biol. 52, 439–447. ( 10.1387/ijdb.082595mg) [DOI] [PubMed] [Google Scholar]
- 9.Tourmente M, Gomendio M, Roldan ERS. 2011. Sperm competition and the evolution of sperm design in mammals. BMC Evol. Biol. 11, 12 ( 10.1186/1471-2148-11-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, Balshine S. 2009. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl Acad. Sci. USA 106, 1128–1132. ( 10.1073/pnas.0809990106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleven O, Fossøy F, Laskemoen T, Robertson RJ, Rudolfsen G, Lifjeld JT. 2009. Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution 63, 2466–2473. ( 10.1111/j.1558-5646.2009.00725.x) [DOI] [PubMed] [Google Scholar]
- 12.Lüpold S. 2013. Ejaculate quality and constraints in relation to sperm competition levels among eutherian mammals. Evolution 67, 3052–3060. ( 10.1111/evo.12132) [DOI] [PubMed] [Google Scholar]
- 13.Lewis S, Aitken R. 2005. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 322, 33–41. ( 10.1007/s00441-005-1097-5) [DOI] [PubMed] [Google Scholar]
- 14.Aitken RJ, Krausz C. 2001. Oxidative stress, DNA damage and the Y chromosome. Reproduction 122, 497–506. ( 10.1530/rep.0.1220497) [DOI] [PubMed] [Google Scholar]
- 15.Shen H-M, Chia S-E, Ong C-N. 1999. Evaluation of oxidative DNA damage in human sperm and its association with male infertility. J. Androl. 20, 718–723. ( 10.1002/j.1939-4640.1999.tb03376.x) [DOI] [PubMed] [Google Scholar]
- 16.Loft S, et al. 2003. Oxidative DNA damage in human sperm influences time to pregnancy. Hum. Reprod. 18, 1265–1272. ( 10.1093/humrep/deg202) [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-López MJ, Espeso G, Evenson DP, Roldan ERS, Gomendio M. 2010. Paternal levels of DNA damage in spermatozoa and maternal parity influence offspring mortality in an endangered ungulate. Proc. R. Soc. B 277, 2541–2546. ( 10.1098/rspb.2010.0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aitken RJ, De Iuliis GN. 2010. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 16, 3–13. ( 10.1093/molehr/gap059) [DOI] [PubMed] [Google Scholar]
- 19.Sakkas D, Alvarez JG. 2010. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 93, 1027–1036. ( 10.1016/j.fertnstert.2009.10.046) [DOI] [PubMed] [Google Scholar]
- 20.Tourmente M, Rowe M, González-Barroso M, Rial E, Gomendio M, Roldan ERS. 2013. Postcopulatory sexual selection increases ATP content in rodent spermatozoa. Evolution 67, 1838–1846. ( 10.1111/evo.12079) [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Cerezales S, Miranda A, Gutiérrez-Adán A. 2012. Comparison of four methods to evaluate sperm DNA integrity between mouse caput and cauda epididymidis. Asian J. Androl. 14, 335–337. ( 10.1038/aja.2011.119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Pastor F, Mata-Campuzano M, Álvarez-Rodríguez M, Álvarez M, Anel L, De Paz P. 2010. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Dom. Anim. 45, 67–78. ( 10.1111/j.1439-0531.2010.01622.x) [DOI] [PubMed] [Google Scholar]
- 23.Evenson DP, Larson KL, Jost LK. 2002. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J. Androl. 23, 25–43. ( 10.1002/j.1939-4640.2002.tb02599.x) [DOI] [PubMed] [Google Scholar]
- 24.Visconti PE, Galantino-Homer H, Moore GD, Bailey JL, Ning X, Fornes M, Kopf GS. 1998. The molecular basis of sperm capacitation. J. Androl. 19, 242–248. ( 10.1002/j.1939-4640.1998.tb01994.x) [DOI] [PubMed] [Google Scholar]
- 25.Florman HM, Ducibella T. 2006. Fertilization in mammals. In Knobil and Neill's physiology of reproduction (ed. Neill JD.), pp. 55–112, 2nd edn San Diego, CA: Elsevier. [Google Scholar]
- 26.Aoki VW, Carrell DT. 2003. Human protamines and the developing spermatid: their structure, function, expression and relationship with male infertility. Asian J. Androl. 5, 315–324. [PubMed] [Google Scholar]
- 27.Kosower NS, Katayose H, Yanagimachi R. 1992. Thiol-disulfide status and acridine orange fluorescence of mammalian sperm nuclei. J. Androl. 13, 342–348. ( 10.1002/j.1939-4640.1992.tb00335.x) [DOI] [PubMed] [Google Scholar]
- 28.Cho C, Jung-Ha H, Willis WD, Goulding EH, Stein P, Xu Z, Schultz RM, Hecht NB, Eddy EM. 2003. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol. Reprod. 69, 211–217. ( 10.1095/biolreprod.102.015115) [DOI] [PubMed] [Google Scholar]
- 29.Bennetts LE, Aitken RJ. 2005. A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol. Reprod. Dev. 71, 77–87. ( 10.1002/mrd.20285) [DOI] [PubMed] [Google Scholar]
- 30.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi P, Bianchi U. 1999. Origin of DNA damage in ejaculated human spermatozoa. Rev. Reprod. 4, 31–37. ( 10.1530/ror.0.0040031) [DOI] [PubMed] [Google Scholar]
- 31.Rueff J, Brás A, Cristóvão L, Mexia J, Sáda Costa M, Pires V. 1993. DNA strand breaks and chromosomal aberrations induced by H2O2 and 60Co γ-radiation. Mutat. Res. 289, 197–204. ( 10.1016/0027-5107(93)90070-V) [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Macias V, Martinez-Pastor F, Alvarez M, Garde JJ, Anel E, Anel L, de Paz P. 2006. Assessment of chromatin status (SCSA (R)) in epididymal and ejaculated sperm in Iberian red deer, ram and domestic dog. Theriogenology 66, 1921–1930. ( 10.1016/j.theriogenology.2006.05.011) [DOI] [PubMed] [Google Scholar]
- 33.Shi QX, Roldan ERS. 1995. Bicarbonate/CO2 is not required for zona pellucida- or progesterone-induced acrosomal exocytosis of mouse spermatozoa but is essential for capacitation. Biol. Reprod. 52, 540–546. ( 10.1095/biolreprod52.3.540) [DOI] [PubMed] [Google Scholar]
- 34.Tourmente M, Villar-Moya P, Varea-Sánchez M, Luque-Larena JJ, Rial E, Roldan ERS. 2015. Performance of rodent spermatozoa over time Is enhanced by increased ATP concentrations: the role of sperm competition. Biol. Reprod. 93, 64 ( 10.1095/biolreprod.114.127621) [DOI] [PubMed] [Google Scholar]
- 35.Villani P, Eleuteri P, Grollino MG, Rescia M, Altavista P, Spanò M, Pacchierotti F, Cordelli E. 2010. Sperm DNA fragmentation induced by DNAse I and hydrogen peroxide: an in vitro comparative study among different mammalian species. Reproduction 140, 445–452. ( 10.1530/REP-10-0176) [DOI] [PubMed] [Google Scholar]
- 36.Evenson DP, Wixon R. 2006. Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology 65, 979–991. ( 10.1016/j.theriogenology.2005.09.011) [DOI] [PubMed] [Google Scholar]
- 37.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 38.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726. ( 10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 39.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2013. caper: comparative analysis of phylogenetics and evolution in R. R package version 0.5.2. See http://CRAN.R-project.org/package=caper.
- 40.Ramm SA, Parker GA, Stockley P. 2005. Sperm competition and the evolution of male reproductive anatomy in rodents. Proc. R. Soc. B 272, 949–955. ( 10.1098/rspb.2004.3048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firman RC, Simmons LW. 2008. The frequency of multiple paternity predicts variation in testes size among island populations of house mice. J. Evol. Biol. 21, 1524–1533. ( 10.1111/j.1420-9101.2008.01612.x) [DOI] [PubMed] [Google Scholar]
- 42.Soulsbury CD. 2010. Genetic patterns of paternity and testes size in mammals. PLoS ONE 5, e9581 ( 10.1371/journal.pone.0009581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenagy GJ, Trombulak SC. 1986. Size and function of mammalian testes in relation to body size. J. Mammal. 67, 1–22. ( 10.2307/1380997) [DOI] [Google Scholar]
- 44.Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS. 1998. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 59, 1037–1046. ( 10.1095/biolreprod59.5.1037) [DOI] [PubMed] [Google Scholar]
- 45.González-Marín C, Gosálvez J, Roy R. 2012. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int. J. Mol. Sci. 13, 14 026–14 052. ( 10.3390/ijms131114026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tourmente M, Roldan ERS. 2015. Mass-specific metabolic rate influences sperm performance through energy production in mammals. PLoS ONE 10, e0138185 ( 10.1371/journal.pone.0138185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tourmente M, Villar-Moya P, Rial E, Roldan ER. 2015. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 290, 20 613–20 626. ( 10.1074/jbc.M115.664813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.delBarco-Trillo J, Mateo R, Roldan ERS. 2015. Differences in the fatty-acid composition of rodent spermatozoa are associated to levels of sperm competition. Biol. Open 4, 466–473. ( 10.1242/bio.201411288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.delBarco-Trillo J, Roldan ERS. 2014. Effects of metabolic rate and sperm competition on the fatty-acid composition of mammalian sperm. J. Evol. Biol. 27, 55–62. ( 10.1111/jeb.12275) [DOI] [PubMed] [Google Scholar]
- 50.Lüke L, Campbell P, Varea Sánchez M, Nachman MW, Roldan ERS. 2014. Sexual selection on protamine and transition nuclear protein expression in mouse species. Proc. R. Soc. B 281, 20133359 ( 10.1098/rspb.2013.3359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domínguez-Rebolledo AE, Martínez-Pastor F, Bisbal AF, Ros-Santaella JL, García-Álvarez O, Maroto-Morales A, Soler AJ, Garde JJ, Fernández-Santos MR. 2011. Response of thawed epididymal red deer spermatozoa to increasing concentrations of hydrogen peroxide, and importance of individual male variability. Reprod. Dom. Anim. 46, 393–403. ( 10.1111/j.1439-0531.2010.01677.x) [DOI] [PubMed] [Google Scholar]
- 52.Portas T, Johnston SD, Hermes R, Arroyo F, López-Fernadez C, Bryant B, Hildebrandt TB, Göritz F, Gosalvez J. 2009. Frozen-thawed rhinoceros sperm exhibit DNA damage shortly after thawing when assessed by the sperm chromatin dispersion assay. Theriogenology 72, 711–720. ( 10.1016/j.theriogenology.2009.05.008) [DOI] [PubMed] [Google Scholar]
- 53.Peris SI, Bilodeau JF, Dufour M, Bailey JL. 2007. Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol. Reprod. Dev. 74, 878–892. ( 10.1002/mrd.20686) [DOI] [PubMed] [Google Scholar]
- 54.Pérez-Llano B, López-Fernández C, García-Casado P, Arroyo F, Gosalbez A, Sala R, Gosálvez J. 2010. Dynamics of sperm DNA fragmentation in the swine: ejaculate and temperature effects. Anim. Reprod. Sci. 119, 235–243. ( 10.1016/j.anireprosci.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 55.Zhang XD, Chen MY, Gao Y, Han W, Liu DY, Huang GN. 2011. The effects of different sperm preparation methods and incubation time on sperm DNA fragmentation. Hum. Fertil. 14, 187–191. ( 10.3109/14647273.2011.604817) [DOI] [PubMed] [Google Scholar]
- 56.Reckova Z, Machatkova M, Rybar R, Horakova J, Hulinska P, Machal L. 2008. Evaluation of chromatin integrity of motile bovine spermatozoa capacitated in vitro. Zygote 16, 195–202. ( 10.1017/s0967199408004772) [DOI] [PubMed] [Google Scholar]
- 57.Domínguez-Rebolledo ÁE, Fernández-Santos MR, García-Álvarez O, Maroto-Morales A, Garde JJ, Martínez-Pastor F. 2009. Washing increases the susceptibility to exogenous oxidative stress in red deer spermatozoa. Theriogenology 72, 1073–1084. ( 10.1016/j.theriogenology.2009.06.027) [DOI] [PubMed] [Google Scholar]
- 58.Koderle M, Aurich C, Schafer-Somi S. 2009. The influence of cryopreservation and seminal plasma on the chromatin structure of dog spermatozoa. Theriogenology 72, 1215–1220. ( 10.1016/j.theriogenology.2009.07.015) [DOI] [PubMed] [Google Scholar]
- 59.Fraser L, Strzezek J, Kordan W. 2011. Effect of freezing on sperm nuclear DNA. Reprod. Dom. Anim. 46, 14–17. ( 10.1111/j.1439-0531.2011.01815.x) [DOI] [PubMed] [Google Scholar]
- 60.Acosta AS, Vargas SE, Cuya MV, González JR, Gutiérrez RS. 2013. Effect of the addition of two superoxide dismutase analogues (Tempo and Tempol) to alpaca semen extender for cryopreservation. Theriogenology 79, 842–846. ( 10.1016/j.theriogenology.2012.12.012) [DOI] [PubMed] [Google Scholar]
- 61.López-Fernández C, Crespo F, Arroyo F, Fernández JL, Arana P, Johnston SD, Gosálvez J. 2007. Dynamics of sperm DNA fragmentation in domestic animals. II. The stallion. Theriogenology 68, 1240–1250. ( 10.1016/j.theriogenology.2007.08.029) [DOI] [PubMed] [Google Scholar]
- 62.Martin G, Sabido O, Durand P, Levy R. 2004. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 71, 28–37. ( 10.1095/biolreprod.103.024281) [DOI] [PubMed] [Google Scholar]
- 63.Duru NK, Morshedi MS, Schuffner A, Oehninger S. 2001. Cryopreservation-thawing of fractionated human spermatozoa is associated with membrane phosphatidylserine externalization and not DNA fragmentation. J. Androl. 22, 646–651. ( 10.1002/j.1939-4640.2001.tb02225.x) [DOI] [PubMed] [Google Scholar]
- 64.Enciso M, Johnston SD, Gosálvez J. 2011. Differential resistance of mammalian sperm chromatin to oxidative stress as assessed by a two-tailed comet assay. Reprod. Fertil. Dev. 23, 633–637. ( 10.1071/RD10269) [DOI] [PubMed] [Google Scholar]
- 65.Domínguez-Rebolledo AE, Fernández-Santos MR, Bisbal A, Ros-Santaella JL, Ramón M, Carmona M, Martínez-Pastor F, Garde JJ. 2010. Improving the effect of incubation and oxidative stress on thawed spermatozoa from red deer by using different antioxidant treatments. Reprod. Fertil. Dev. 22, 856–870. ( 10.1071/RD09197) [DOI] [PubMed] [Google Scholar]
- 66.Zubkova EV, Wade M, Robaire B. 2005. Changes in spermatozoal chromatin packaging and susceptibility to oxidative challenge during aging. Fertil. Steril. 84(Suppl 2), 1191–1198. ( 10.1016/j.fertnstert.2005.04.044) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets have been uploaded to Dryad and are available for download: http://dx.doi.org/10.5061/dryad.cf2q1.


