Abstract
Why animal communication displays are so complex and how they have evolved are active foci of research with a long and rich history. Progress towards an evolutionary analysis of signal complexity, however, has been constrained by a lack of hypotheses to explain similarities and/or differences in signalling systems across taxa. To address this, we advocate incorporating a systems approach into studies of animal communication—an approach that includes comprehensive experimental designs and data collection in combination with the implementation of systems concepts and tools. A systems approach evaluates overall display architecture, including how components interact to alter function, and how function varies in different states of the system. We provide a brief overview of the current state of the field, including a focus on select studies that highlight the dynamic nature of animal signalling. We then introduce core concepts from systems biology (redundancy, degeneracy, pluripotentiality, and modularity) and discuss their relationships with system properties (e.g. robustness, flexibility, evolvability). We translate systems concepts into an animal communication framework and accentuate their utility through a case study. Finally, we demonstrate how consideration of the system-level organization of animal communication poses new practical research questions that will aid our understanding of how and why animal displays are so complex.
Keywords: degeneracy, evolvability, modularity, multimodal, redundancy, robustness
1. Introduction
Animals often use elaborate signalling displays to communicate with conspecifics and heterospecifics across a variety of contexts and for a variety of reasons [1–3]. Important contributions have helped to categorize and formalize hypotheses of complex signal form and function [4–8], yet our understanding of how and why animals incorporate multiple distinct components within and across sensory modalities (multicomponent and multimodal signalling, respectively) remains in its infancy [9,10]. A critical missing piece for the study of animal communication is an evolutionary framework that enables an analysis and comparison of entire signalling systems—an approach that encompasses multiple signalling traits, the complex interactions among traits, and the structure-to-function relationships throughout. Specifically, there is a dearth of quantitative approaches aimed at assessing and interpreting potential similarities and differences in the design and function of signalling systems. The lack of a unified evolutionary framework and shared terminology constrains our ability to uncover broad patterns and to generate and test evolutionary hypotheses. To that end, we advocate applying a systems approach to the study of animal communication—an approach that considers the organization and structure/function relationships of the signalling system, including how components of the system can interact within and across contexts and how these interactions may change across time [11].
Current studies in animal communication continue to focus, predominantly, on (multiple) signal function(s) within a single condition. In contrast, a systems approach champions the quantification and assessment of the structure-to-function relationships within and across conditions (e.g. behavioural context, receiver identity, or physiological state, time). Systems theory and terminology are based upon structure/function relationships, whereas current hypotheses of complex signalling are based upon signal function, irrespective of its relationship with structure. By adopting a framework that is more aligned with systems biology, animal communication research can borrow from, and build on, a tremendous knowledge base and toolset aimed at understanding how and why systems function the way they do. Importantly, it will also provide a shared terminology and methodologies that can facilitate cross-system, cross-species comparisons of system design and function. Re-directing the field's research focus to include structure/function relationships across conditions will require both adjustments to our empirical approach (e.g. experimental design and data collection) as well as the purposeful integration and application of systems concepts, terminology, and analytical tools (and the potential development of new ones).
We lay out our proposal for the integration of a systems approach to animal communication by highlighting the current state of the field. We underscore the challenge of fitting complex empirical data within existing categorical frameworks by highlighting specific studies that demonstrate intersignal interactions and the dynamic nature of animal signalling systems (§2). We follow this with an introduction of systems concepts and associated terminology. We translate these concepts into an animal communication framework, and briefly discuss their evolutionary implications (§3). We then provide suggestions for how we might use systems thinking in animal communication research, including proposing tools and techniques for visualizing and comparing complex signalling architectures and interactions among components within the system (§4). We elucidate the utility of such an approach with a detailed case study of barn swallows. We end by discussing how new hypotheses that arise from considering animal signals as signal systems can advance animal communication research (§5).
2. Current state of the field
(a). Modelling multiple signals and functions
The study of animal communication has largely moved past the early univariate models that analysed scenarios with one signaller, one receiver, and one signal serving one function [12,13]. It has importantly expanded its focus beyond selection for signal ‘content’, or information transfer, to a more inclusive view that acknowledges the importance of efficient signal transmission and the role of the receiver. Indeed, we now have good evidence for the existence of manipulative signals and signaller–receiver conflict [14,15]; and receivers are widely recognized as paramount in driving the evolution of signal form (reviewed in [4,16–20]). Empirical and conceptual progress in animal communication has even helped advance other areas of research focus, such as plant–insect interactions, as signalling theory has uncovered complex interactions between floral signals and their pollinator targets [21–23].
The first framework for classifying multimodal animal displays reflected a single function for a single signal [5]. Limitations of this approach, such as the difficulty of considering interactions between signal components and the possibility of individual signals having multiple functions, led to a suite of follow-up frameworks focused on intersignal interactions and potential sources of selection on signals [4,7,8,24]. The field has since been accruing multiple excellent case studies of complex signalling, including ground-dwelling spiders (reviewed in [25,26]), crustaceans (reviewed in [27]), anurans ([28], reviewed in [29]), insect pollinators (reviewed in [23,30]), birds [31,32], and primates [33,34], among others. Results from these studies and others have led to an appreciation that the function(s) of elements of communication displays are not fixed. Animal communication is multidimensional—it can encompass multiple strategies, multiple functions, multiple receivers, multiple components, and multiple sensory modalities [4,8,20]. We briefly elaborate on this with specific case studies.
(b). The dynamic nature of animal signalling
Animal displays can function differently across display compositions (system architecture) or timescales. Male Schizocosa crassipes, wolf spiders, for example, employ a multimodal (visual and vibratory) courtship display [35,36], the visual component of which includes dynamic waving of sexually dimorphic forelegs that possess conspicuous black brushes. Researchers have found that the function of the black brushes differs depending upon the presence versus absence of the multicomponent vibratory display. Specifically, females only respond to variation in brush size in the presence (versus absence) of the vibratory signal [37]. Thus, the relationship between the intensity of a signal component (visible brush size) and the behavioural response (likelihood to mate) is altered across display compositions (presence/absence of vibratory signal); and the vibratory signal interacts with a visual component (sensu [4]). Similar composition, environment, and receiver-dependent functions of complex signal components are found in other wolf spiders [38–42].
Functional interactions between signal components are also documented in the male túngara frogs, Engystomops pustulosus, which produce complex calls involving a whine and sometimes a chuck. Calls with both whines and chucks are more successful in attracting females and the temporal pattern of whines and chucks influences female responses [43–45]. The temporal coordination between the acoustic components and visual cues associated with calling (visible inflation of a vocal sac) also influences female responses [46]. Research on the squirrel treefrog, Hyla squirella, found similar cross-modal interactions [47]. Starnberger et al. [29] provide an excellent review of additional anuran signalling examples, including those in which temporal coordination among signal elements influences element function.
Individual receivers can vary in their perception and decision-making (reviewed in [19,48]) in an environment- or context-specific fashion, driven by past nutritional intake, hormone profiles, age, etc. Female round gobies, Neogobius melanostomus, for example, alter their response to uni- versus multimodal male stimuli across the breeding season [49], and the mate choice of female Rabidosa rabida, wolf spiders, is dependent upon both age and condition [50]. Even within a single display, female great bowerbirds are likely to perceive colours differently at the beginning versus a minute into a male display bout [20].
The social context of a display can similarly influence not only the functional response of receivers, but also characteristics of the signal architecture itself. In the lance-tailed manakin, Chiroxiphia lanceolata, pairs of males perform more coordinated, predictably choreographed acrobatic displays in the presence versus absence of females [51,52]. Similar variation in signal form is seen in male wolf spider courtship displays in the presence versus absence of a female [53], whereas in the Australian field cricket, Teleogryllus oceanicus, the expression of male chemical signals is influenced by past social experience [54].
These case studies demonstrate how research efforts focused on relating individual signals to individual functions or individual receivers, at single time points and in single contexts, may overlook important interactions or variation among display components that are crucial to system function. As evidenced by these examples, more inclusive approaches to animal signalling are gaining momentum. We suggest that the impact of such approaches and resulting data will be truly significant if we can integrate them into a framework that can provide an avenue for cross-taxa/cross-study synthesis and hypothesis testing.
3. System properties of animal signalling
In addition to the impacts of systems approaches on experimental design, data collection, and analyses, we propose that studies of animal communication can benefit by adopting systems concepts such as redundancy, degeneracy, pluripotentiality, and modularity [55]—key organizational principles of complex biological as well as other (e.g. engineering) systems. These systems design principles influence vital aspects of system properties, such as the robustness, flexibility and, most relevant to biologically complex systems, its evolvability. Robustness, evolvability, and modularity are terms currently found in animal communication literature, though some more commonly than others. The term redundancy, though commonly employed, is regularly used imprecisely; and ‘degeneracy’ and ‘pluripotentiality’ have yet to make a predictable appearance. We discuss how these key systems design principles might translate into animal signalling to provide a richer view of signal–receiver function (table 1).
Table 1.
Redundancy, degeneracy, and pluripotentiality in animal communication: their translations into scenarios of complex signalling and their implications for understanding evolutionary patterns of animal communication systems.
| concept | structure/function | communication scenario | system consequences | evolutionary implications |
|---|---|---|---|---|
| redundant | same structure/same independent function | repeated instances of a signal—repetition of a song or a display | increases robustness of a system | enables a system to maintain function in circumstances of loss (i.e. lack of transmission) of the element. Can relax selective pressure on duplicate structures and allow for functional or structural divergence |
| degenerate | different structures/overlapping function | two different signals or signal components serving similar functions in some signalling contexts | increases robustness and can increase the functionality of the system | enhances capacity to respond to selection. Elements can react independently to selection; can diverge over evolutionary time to incorporate new functions while maintaining, or before losing, original function(s) |
| pluripotent | one structure/multiple functions | the capacity of a particular signal or signal component to serve multiple functions in a display | increases efficiency and functional diversity of the system. Enables organization of coordinated responses to a signal | elements will likely be subject to multiple selective forces; any change in the signal will have multiple consequences across the system |
(a). Redundancy: structurally identical components have identical functions
Elements in a system that are structurally identical and perform the same function independently are redundant [56–58] (table 1). This systems terminology refines the traditional use of this term in animal communication, which considers redundancy as a set of elements performing the same function, regardless of structure [5]. Refining the use of redundancy—to distinguish between signal elements with the same structure or different structures—impacts our view of robustness or resistance to changing conditions, and the evolutionary potential of the communication system (table 1). Repeating identical signal elements, for example, cannot confer as much robustness across signal environments and is subject to greater pleiotropic constraints on signal production compared with employing distinct signals for the same function. Additionally, the tradition of categorizing signal elements as ‘redundant’ based solely on function has arguably stifled progress towards understanding the prevalence and importance of true system redundancy (similar structure and function), such as the rich possible causes and implications of repeating song components (e.g. redundant notes; figure 1). Interestingly, despite its exclusion from many conceptual frameworks of complex signalling, research exploring ‘consistency’ in signalling (i.e. redundancy) is gaining momentum [59–62], and this growing research focus may benefit tremendously from the recognition that repetition and consistency might relate to both system redundancy and modularity (discussed below).
Figure 1.
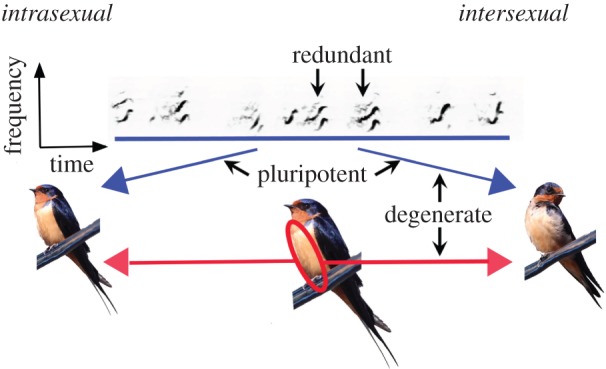
A heuristic example of the concepts and potential implementation of systems terminology based upon a recent study of animal communication [32]. Male barn swallows (centre), Hirundo rustica erythrogaster, communicate with conspecific males (left; intrasexual) and females (right; intersexual) using multimodal/multicomponent displays that encompass acoustic song (top; blue lines) and visual colour patches (red circle; red lines). Coloured lines with arrows indicate receivers (males and females) that respond to specific display components. Redundancy is seen in the repeated notes of the male's song. Degeneracy is seen in that two distinct display components (song and breast feather reflectance) overlap in function: territoriality (not highlighted) and female attraction [32]. Pluripotentiality is demonstrated by the dual function of song in both intra- and intersexual communication, and the similar dual function of breast feather colour (not highlighted) [32].
Redundancy provides a certain degree of robustness to a system [58]. If a redundant element is lost (e.g. a call is drowned out by environmental noise), repetition of the element can ensure that the signal still functions. In the king penguin, Aptenodytes patagonicus, for example, the repetition of similar syllables by adults is suggested to overcome the masking effects of the colony's background noise [63]. Such increased robustness, however, only occurs if the elements (syllables here) function independently; non-independent repeated elements do not fall as readily into the systems concept of redundancy and may not have the overall effect of increasing system robustness.
Redundancy changes the system's evolutionary potential [57,58]. This phenomenon is best illustrated by the classic example of gene duplication. Following duplication, selection on one gene copy might be relaxed, allowing mutations to accumulate that could, over time, result in the duplicated gene being exapted for a new function [56,64]. Similarly, redundant elements in displays have the potential to diverge, whereas at least one element maintains the original signal function [58]. A signal component could be co-opted, for example, from an initial function in mate attraction to a new function in competitor deterrence. Such an example would lead to pluripotentiality (same structure/multiple functions; table 1). Alternatively, though less likely, relaxed selection on a repeated signal component could enable change in component structure, resulting in system degeneracy (different structure/overlapping function) if the now structurally distinct elements maintain a similar function (table 1) [56,57].
(b). Degeneracy: structurally distinct components can have similar functions
Degenerate elements of a system differ structurally, but perform similar functions under certain conditions, although their functions may diverge across some environmental contexts or for some receivers (table 1 and figure 1) [56,57,64,65].
We have already mentioned the multimodal vibratory and visual sexual display of male S. crassipes, wolf spiders, [35,37,64,66] as an example of how total signal composition and interactions between signal elements can influence signal function; but this species also provides us with a good example of signal degeneracy. The integration of both vibratory and visual elements that can each subserve mate attraction reflects system degeneracy [37] and makes the display robust to changes in either light level or substrate properties. A similar example can be seen in the scent and colour of floral signals. Under low-light conditions, the presence of scent increases a nectar-foraging bumblebee's accuracy to a target, whereas target accuracy is reduced with unscented targets [67]. Indeed, owing to degeneracy, multimodal signalling specifically has been argued to be more robust than multicomponent signalling [68].
Degeneracy increases robustness to a greater degree than redundancy and can also extend the functional range of the system (table 1). Because components of degenerate systems can potentially react to selection independently, unlike redundant signals and because function is shared across components, degeneracy can more readily facilitate the evolution of novel signalling phenotypes. Appreciating the degeneracy of signalling systems is likely to be illuminating for understanding broad patterns of signal divergence between species, and even repeated loss of sexually dimorphic signals [64,69,70].
(c). Pluripotentiality: structurally similar components can have more than one function
When similar elements of a system can functionally diverge in diverse contexts, the system is said to exhibit pluripotentiality [57]. In animal signalling, many components may have distinct functions across contexts or with different receivers. For example, male snow buntings, Plectrophenax nivalis, display to other males with multiple visual plumage ornaments—breast feather and rectrices reflectance—one of which is also an attractive signal to females and is thus pluripotent [71]. An additional example can also be seen in the barn swallow, Hirundo rustica erythrogaster, in which both songs and breast feathers are signals to male competitors and to potential female mates [32] (figure 1). Whereas degeneracy increases system resilience in the face of environmental variation, pluripotentiality increases the functional diversity of a system across variation in environment or context (table 1).
Pluripotentiality can also introduce evolutionary constraints to the system, because signal components may be subject to a range of different selection pressures [72]; any evolved change could have multiple functional consequences. Accordingly, in the treefrog, Dendropsophus ebraccatus, shared production mechanisms across advertisement and aggressive calls are suggested to constrain signal structure owing to opposing selection pressures across social contexts [73]. Numerous studies support putative trade-offs between distinct signalling components within one context [74–78], but pluripotentiality could importantly lead to trade-offs within one display component across contexts [79].
(d). Modularity: a subset of components form tightly linked structural or functional clusters
Modularity refers to integrated groups of system elements that are distinct from other groups [55] (table 1). Integrated elements may be grouped, because either their structures or functions are linked. Structurally, display components might be grouped as a module owing to their tight covariance [32], or to their recurrence as a stereotyped unit in time or space [80]. Examples of structural modules in animal signalling might include particular notes, syllables, and phrases within a bird song, or different patches of colour in a fish; these elements likely share developmental and physiological bases and thus are interrelated, yet are independent of other structures or elements. Incorporating analyses and concepts of modularity has already enhanced our understanding of the elaborate and lineage-specific diverse displays of Parotia birds of paradise [81,82].
Functional modularity, with its focus on receiver responses to groups of signal components, is an important counterpart to structural modularity. Identifying functional modules requires observations of receiver responses to multivariate variation in display structure. Functional modules may help explain why responses to some signal elements vary depending on the composition of a display (e.g. §3), and may even reflect the underlying neural architecture and processing of receivers. This hypothesis has not been fully examined in a neural–behavioural setting.
Distinguishing between structural versus functional modularity and understanding their relationship (a focus of research often termed phenotypic integration) will be vital to our understanding of the evolutionary dynamics of signalling systems, as the extent to which structural modules also act as functional modules has important evolutionary implications. Shared mechanisms and underlying genetic correlations that give rise to structural modules might constrain the evolution of a communication system if these developmentally integrated elements subserve diverse functions with divergent selection pressures. And yet the genetic architecture of signal traits may also be the result of selection to produce effective, coordinated signal elements by aligning patterns of structural and functional modularity [55].
4. Implementing a systems approach in animal communication
To implement a systems approach to the study of animal communication, we must expand our scope of data collection and identify, adapt, and apply existing tools and techniques to the structure and dynamics of animal signalling. We must strive to implement an overarching approach that is broadly applicable to variation in signalling contexts, taxonomic groups, and experimental design.
(a). Visualizing and characterizing signalling systems
Heuristic visual representations will be vital to the successful integration of a systems approach in animal communication, just as Partan & Marler's [5] original multimodal classification scheme was compelling and appealing in part owing to its intuitive and elegant visual representations. Towards a more comprehensive visualization of complex signal function, Smith & Evans [83] recently proposed a geometric framework in which they suggested representing responses to multimodal signals as surface plots in a three-dimensional space (e.g. figure 2). To illustrate this advance, consider the multimodal sexual displays of the wolf spider, S. crassipes, discussed in §2b where the efficacy of the foreleg brushes for mating success was greater in the presence versus absence of the vibratory signal [37]. By plotting responses to the signal components geometrically, we acquire quantitative details of signal effects and interactions (figure 2). A geometrical visualization is inherently extensible as additional behavioural responses or signal elements can be included as additional dimensions, enabling one to consider the full functional range of the signalling system.
Figure 2.
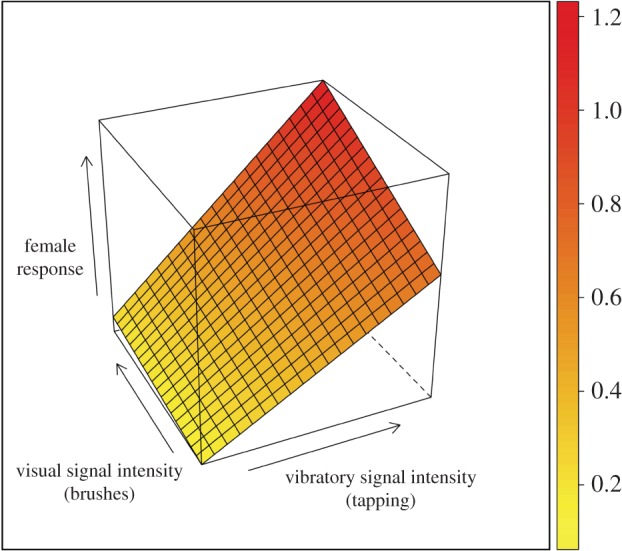
Building on the geometric framework of Smith & Evans [83], a heuristic graphical representation of a response surface highlighting interactions between vibratory and visual signal components of the courtship display of the wolf spider, Schizocosa crassipes [37]. Empirical data demonstrate that females respond to variation in brush size only in the presence, versus absence, of vibratory signalling [37]. This geometric visualization bears similarity to the multidimensional response surface methodology [e.g. 1], but rather than linking fitness to trait values to measure evolutionary responses to selection, here we plot behavioural responses as a function of trait (signal) values [84].
Signalling phenotype networks provide another very promising avenue for visualizing and quantifying signalling dynamics, due in part to the potential for generating comparable indices that reflect key system design features for cross-system, cross-taxa comparisons. Network thinking is commonly employed in studies addressing the evolution of integrated systems at the gene, protein, and ecological community level [85], but has only recently been applied in animal communication research [32]. Transforming complex signal structure into signal phenotype networks facilitates a view of the whole architecture of the signalling system, and similar approaches have become important tools in related fields such as phenotypic integration [86,87]. We highlight a case study that used phenotype network analyses to compare barn swallow signalling across two social conditions to demonstrate how such an approach can be more fully integrated into systems thinking and terminology.
(b). Barn swallow case study
Wilkins and co-workers took an important step towards systems thinking in their study of multimodal signalling in the North American barn swallow, Hirundo rustica erythrogaster. In the field, the research team collected a comprehensive array of phenotypic data from 50 males including measurements of 28 presumed display components (two wing and 12 colour measurements; 14 measures of frequency, tempo, and repertoire per male) [32]. Field data were additionally collected to assess each male's success in intersexual communication (paternity analyses) and intrasexual communication (internest distance). Using a combination of principal component analyses and an information–theoretic approach, the research team was able to determine signal axes that best explained variation in paternity and internest distance. This innovative approach enabled them to assess both structure and function of display architecture within and across contexts (figure 3). Despite the limitation that some of their measured traits were not context-specific (i.e. not taken from a single individual at the time of displaying in a given context), the study nonetheless exemplifies an important advance towards an experimental systems approach to animal communication.
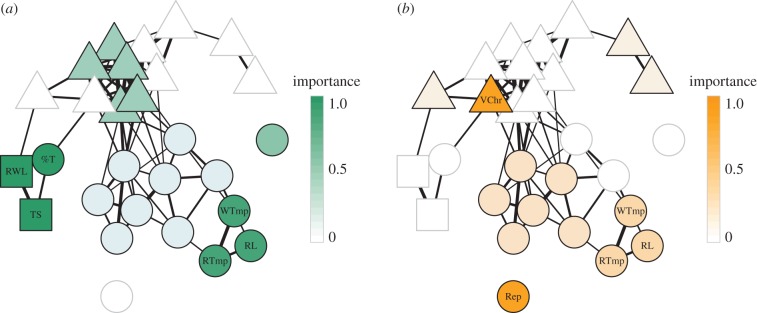
Figure 3.
Signalling phenotype networks adapted from Wilkins et al. [32], illustrating the traits predicting (a) within-nest paternity and (b) internest distance in the barn swallow, Hirundo rustica erythrogaster. Triangles, colour variables; squares, morphological traits; and circles, song components. Lines connecting shapes represent Spearman's correlations and shape colours are graded by importance of rotated principal components (for details, see [32]). The best predictor of paternity (a surrogate for mate choice) was a factor with high loadings for wing length (RWL), tail feather length (TS), and per cent complex syllables (%T). The best predictor of internest distance (a surrogate for competition) was a factor with high loadings for the darkness of undertail contour feathers (VChr) and syllable repertoire size (Rep). The factor loading highly for a triad of song traits—warble tempo (WTmp), trill tempo (RTmp), and trill length (RL)—was the only strong predictor of both intra- and intersexual selection measures.
We used this dataset to demonstrate how integrating systems terminology and thinking into animal communication research can provide much more than a roadmap for how experimental studies can incorporate holistic, multicontextual approaches; it can aid in data interpretation, synthesis, and in elucidating future research avenues. We note at the onset that the authors' use of the term ‘redundant’ is ‘degenerate’ in systems terminology. Redundancy would pertain to consistency or repeatability of song structure as it relates to function (same structure/same function), which was not assessed in this study. We follow systems terminology throughout.
Upon constructing a signalling phenotype network, Wilkins et al. [32] calculated network density, the proportion of correlation coefficients that were significant, based on a bootstrapping procedure to remove non-robust correlations. This value is hypothesized to reflect system degeneracy (strong correlations suggest shared information and possibly shared function), allowing the team to directly compare inter- versus intrasexual signalling system degeneracy. Although they did not take their data this far, their results lead to concrete testable hypotheses regarding system robustness (e.g. intrasexual displays should be more robust to change given the slightly higher network density/degeneracy). The ability to quantify a value that encapsulates a hypothesized proxy of degeneracy opens up the possibility of directly and quantitatively comparing degeneracy across contexts (as shown here) and across taxa (not shown)—it provides a path towards assessing similarities and differences across signalling systems and determining how these might influence the evolution and function of display architecture.
The research team also sorted their display traits into predetermined categories (morphology, colour, and song) and used assortativity coefficients to determine whether there were stronger correlations within the same trait types than across trait types. Indeed, they found that correlations within trait types were strong; morphological, acoustic, and colour traits form separate clusters [32] (figure 3). These structural modules might reflect shared production mechanisms, pleiotropic effects, or shared function; all of which are testable hypotheses. Another intriguing module groups a measurement of song complexity with wing and tail streamer length measurements (figure 3a; %T, RWL, TS); a grouping that might suggest a functional interaction between components, correlated outcomes of early-life conditions and/or, shared hormonal/genetic underpinnings.
Visually, the generated phenotype networks exemplify the limitations of our traditional view of multimodal signal function. Across both contexts, highly correlated sets of signal components are grouped into structural modules (e.g. RL, RTmp, WTmp). The strong covariance of these modular components might suggest degeneracy (e.g. similar information content potentially reflecting similar function, with distinct structures). This pattern is reflective of a commonly tested hypothesis of complex signalling—content backup [4,88,89]. Simultaneously, distinct modules (or single nodes) within the system are demonstrated to share in their function through their predictability of behavioural outcomes. For example, within-nest paternity is best predicted by two distinct modules (figure 3a—RL, RTmp, and WTmp and %T, TS, and RWL), whereas internest distance is similarly predicted by two distinct components (figure 3b; VChr and Rep) [32]. In these examples, despite the fact that the two modules/nodes share behavioural predictability, they do not covary and thus presumably do not share information content. This second scenario similarly suggests degeneracy (shared function with different structure) but without shared information (lack of covariance between modules/nodes). In contrast to the earlier pattern of content backup, this pattern can be directly related to another commonly tested hypothesis of complex signalling—multiple messages [4,88,89]. A systems approach importantly exposes the actuality that components within a single display can take on multiple functions within and across displays. It is also a useful example to caution against strict ‘binning’ of components or displays into discrete categories, even using the terminology we advocate here, as this can limit our understanding of animal signalling systems: ‘multiple messages’ and ‘content backup’ could simultaneously characterize parts of a complex signalling system.
The potential for signalling systems to convey multiple messages can similarly be explored by identifying the number of distinct structural modules across systems. For example, six uncorrelated modules are found to play a role in predicting male paternity (proxy for attractiveness to females), whereas only four modules are predictive of internest distances (proxy for effectiveness of territorial defences). An increased number of uncorrelated modules might suggest that intersexual communication has the potential to convey more information than intrasexual communication. Indeed, females may require more information from potential mates than males do for guiding agonistic encounters. This hypothesis could be tested more generally by comparing degeneracy of inter- versus intrasexual signalling systems across divergent taxa.
Although they did not use the term, Wilkins and co-workers also calculated an estimate of pluripotentiality—or the degree to which identical display components function across disparate contexts. Thirty-two per cent of their quantified display components predicted both paternity and internest distance [32]. Interestingly, among these traits is a module with different patterns of predictability across contexts—in the module composing song tempo: faster, shorter warbles and rattles predict male paternity, whereas slower, longer rattles predict internest distance. If this truly reflects opposing selection pressures of module components across contexts, it provides an excellent example of how pluripotentiality might constrain evolution for optimal signalling.
We have used this case study to demonstrate how shifting our focus from a narrow range of trait interactions (e.g. two traits or signalling modalities) to overall system architecture provides new insights. We demonstrate how one could use a signalling phenotype network approach to calculate and interpret degeneracy, modularity, and pluripotentiality; and how adopting systems terminology and analytical approaches can generate new hypotheses and open the door to original research directions.
(c). Outstanding challenges
Unlike many other fields of study, one of the most formidable practical challenges for animal signalling systems is the conceptual and analytical incorporation of the dynamic and transient nature of communication displays (for review of spatiotemporal dimensions of visual signalling, see [90]). How can we quantify, analyse, and compare systems characterized by components that are continuously variable in their expression and/or perception? Temporal patterning, as a design feature (e.g. synchrony [91,92] versus asynchrony [93–95] of signal components), may hold significant explanatory power for the ubiquitous nature of multimodal animal signalling (e.g. sensory constraints, sensu [4]). We suspect that innovative analytical tools and techniques will best serve the purpose of quantifying and assessing temporal patterns of system design. We might borrow existing tools, for example, such as cyclic autocorrelation [96] to facilitate the measurement of relationships between signal components, or mutual information approaches to measure the extent to which one component/modality can be used to predict another. The relatively untapped aspect of temporal patterning in system design is likely to provide novel insights not only to animal communication, but also to systems biology more broadly.
5. Conclusion and future directions
The complex communicative displays that take place between many animals can be approached and studied as a system. This system can have multiple levels of analysis, from an individual signaller/receiver, to multiple signallers/receivers, to interacting species in a community [97]. Investigating how signal components function across contexts, including across signalling environments and variables receivers, will be essential for identifying systems design principles and properties; and clever experimental designs will remain vital to understanding signal interactions. We outline an innovative pathway for future research aimed at unifying and aligning studies of animal signalling systems with other scientific disciplines by adopting and adapting related concepts and tools. A system approach reorients readers from the current signal–function approach to an intuitive multidimensional/multifunctional approach that offers a more faithful evocation of animal communication.
Integrating systems thinking, experimental designs, terminology, and tools into animal communication research will provide a common language for cross-taxon comparisons of signal design. Thinking of animal signals as dynamic systems will (i) inspire testable evolutionary hypotheses addressing the patterns of system structure and function (e.g. degeneracy, modularity) and how systems respond to external factors (e.g. robustness, evolvability). It will (ii) lead to the development of innovative analytical tools and techniques integral for signalling system analyses and (iii) provide novel insights into cross-contextual selection pressure—e.g. intra- versus intersexual selection. A systems approach will also (iv) create avenues for comparing structure/function relationships within and across modalities to test the significance, or lack thereof, of modality-specific versus multimodal signalling. Finally, (v) a study of animal signals from a systems perspective will contribute to systems biology through its potential to assess and test systems design principles and properties in a comparative phylogenetic framework, enabling some of the first direct evolutionary tests of selection for systems design principles and properties.
Acknowledgements
This study was part of a working group on decision-making—Toward a unified evolutionary theory of decision-making in animals—sponsored by the National Evolutionary Synthesis Center (NESCent). We thank all members of the working group for stimulating discussions, with special thanks to Rebecca Safran and Tamra Mendelson for their leadership. Malcolm Rosenthal and Michael McCoy assisted in generating initial figures. We thank Jeffrey McKinnon, Tamra Mendelson, Rebecca Safran, Kyle Summers, William Wagner, Michael Reichert, Dan Papaj, and Matthew Wilkins for feedback on an earlier version of this manuscript. We also thank Dai Shizuka, Ximena Bernal, Gail Patricelli, Malcolm Rosenthal, and Arik Kershenbaum for additional stimulating discussions. Matt Wilkins provided figure 3, photos, and the spectrogram of barn swallows.
Competing interests
We declare we have no competing interests.
Funding
The NESCent working group received funding from the National Science Foundation (Grant #EF-0905606).
References
- 1.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: J. Murray. [Google Scholar]
- 2.Bradbury JW, Vehrencamp. SL: 1998. Principles of animal communication. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 3.Andersson M. 1994. Sexual selection, p. 441 Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Hebets EA, Papaj DR. 2005. Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. ( 10.1007/s00265-004-0865-7) [DOI] [Google Scholar]
- 5.Partan S, Marler P. 1999. Behavior—communication goes multimodal. Science 283, 1272–1273. ( 10.1126/science.283.5406.1272) [DOI] [PubMed] [Google Scholar]
- 6.Partan SR, Marler P. 2005. Issues in the classification of multimodal communication signals. Am. Nat. 166, 231–245. ( 10.1086/431246) [DOI] [PubMed] [Google Scholar]
- 7.Candolin U. 2003. The use of multiple cues in mate choice. Biol. Rev. 78, 575–595. ( 10.1017/S1464793103006158) [DOI] [PubMed] [Google Scholar]
- 8.Bro-Jorgensen J. 2010. Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol. Evol. 25, 292–300. ( 10.1016/j.tree.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 9.Hebets EA. 2011. Current status and future directions of research in complex signaling. Curr. Zool. 57, i–v. [Google Scholar]
- 10.Higham JP, Hebets EA. 2013. An introduction to multimodal communication. Behav. Ecol. Sociobiol. 67, 1381–1388. ( 10.1007/s00265-013-1590-x) [DOI] [Google Scholar]
- 11.Kitano H. 2002. Systems biology: a brief overview. Science 295, 1662–1664. ( 10.1126/science.1069492) [DOI] [PubMed] [Google Scholar]
- 12.Shannon CE. 1948. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. ( 10.1002/j.1538-7305.1948.tb01338.x) [DOI] [Google Scholar]
- 13.Maynard-Smith J, Harper D, Maynard-Smith J, Harper D. Animal signals. 2003. i–ix, pp. 1–166; Animal Signals. New York, NY: Oxford University Press. [Google Scholar]
- 14.Hauber ME, Zuk M. 2010. Social influences on communication signals: from honesty to exploitation. In Social behaviour: genes, ecology and evolution (eds Szekely T, Moore AJ, Komdeur J), pp. 185–199. New York, NY: Cambridge University Press. [Google Scholar]
- 15.Christy JH, Rittschof D. 2011. Deception in visual and chemical communication in crustaceans. In Chemical Communication in Crustaceans, pp. 313–333. New York, NY: Springer. [Google Scholar]
- 16.Rowe C. 1999. Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921–931. ( 10.1006/anbe.1999.1242) [DOI] [PubMed] [Google Scholar]
- 17.Rowe C. 2013. Receiver psychology: a receiver's perspective. Anim. Behav. 85, 517–523. ( 10.1016/j.anbehav.2013.01.004) [DOI] [Google Scholar]
- 18.Miller CT, Bee MA. 2012. Receiver psychology turns 20: is it time for a broader approach? Anim. Behav. 83, 331–343. ( 10.1016/j.anbehav.2011.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz NE, Blumstein DT. 2012. Multisensory perception in uncertain environments. Behav. Ecol. 23, 457–462. ( 10.1093/beheco/arr220) [DOI] [Google Scholar]
- 20.Endler JA, Gaburro J, Kelley LA. 2014. Visual effects in great bowerbird sexual displays and their implications for signal design. Proc. R. Soc. B 281, 20140235 ( 10.1098/rspb.2014.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raguso RA, Weiss MR. 2015. Concerted changes in floral colour and scent, and the importance of spatio-temporal variation in floral volatiles. J. Indian Inst. Sci. 95, 69–92. [Google Scholar]
- 22.Leonard AS, Dornhaus A, Papaj DR. 2011. Forget-me-not: complex floral displays, inter-signal interactions, and pollinator cognition. Curr. Zool. [Google Scholar]
- 23.Leonard AS, Masek P. 2014. Multisensory integration of colors and scents: insights from bees and flowers. J. Comp. Physiol. 200, 463–474. ( 10.1007/s00359-014-0904-4) [DOI] [PubMed] [Google Scholar]
- 24.Guilford T, Dawkins MS. 1991. Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14. ( 10.1016/S0003-3472(05)80600-1) [DOI] [Google Scholar]
- 25.Hebets EA, Vink CJ, Sullivan-Beckers L, Rosenthal M. 2013. The dominance of seismic signaling and selection for signal complexity in Schizocosa multimodal courtship displays. Behav. Ecol. Sociobiol. 67, 1483–1498. ( 10.1007/s00265-013-1519-4) [DOI] [Google Scholar]
- 26.Elias DO, Maddison WP, Peckmezian C, Girard MB, Mason AC. 2013. Orchestrating the score: complex multimodal courtship in the Habronattus coecatus group of Habronattus jumping spiders (Araneae: Salticidae). Biol. J. Linn. Soc. 105, 522–547. ( 10.1111/j.1095-8312.2011.01817.x) [DOI] [Google Scholar]
- 27.Hebets EA, Rundus A. Chemical communication in a multimodal context. 2011. In Chemical Communication in Crustaceans, pp. 335–354. New York, NY: Springer. [Google Scholar]
- 28.Halfwerk W, Page RA, Taylor RC, Wilson PS, Ryan MJ. 2014. Crossmodal comparisons of signal components allow for relative-distance assessment. Curr. Biol. 24, 1751–1755. ( 10.1016/j.cub.2014.05.068) [DOI] [PubMed] [Google Scholar]
- 29.Starnberger I, Preininger D, Hoedl W. 2014. From uni- to multimodality: towards an integrative view on anuran communication. J. Comp. Physiol. 200, 777–787. ( 10.1007/s00359-014-0923-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulahci IG, Dornhaus A, Papaj DR. 2008. Multimodal signals enhance decision making in foraging bumble-bees. Proc. R. Soc. B 275, 797–802. ( 10.1098/rspb.2007.1176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauglund K, Hagen SB, Lampe HM. 2006. Responses of domestic chicks (Gallus gallus domesticus) to multimodal aposematic signals. Behav. Ecol. 17, 392–398. ( 10.1093/beheco/arj038) [DOI] [Google Scholar]
- 32.Wilkins MR, Shizuka D, Joseph MB, Hubbard JK, Safran RJ. 2015. Multimodal signalling in the North American barn swallow: a phenotype network approach. Proc. R. Soc. B 282, 20151574 ( 10.1098/rspb.2015.1574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones CB, Van Cantfort TE. 2007. Multimodal communication by male mantled howler monkeys (Alouatta palliata) in sexual contexts: a descriptive analysis. Folia Primatol. 78, 166–185. ( 10.1159/000099138) [DOI] [PubMed] [Google Scholar]
- 34.Leavens DA. 2007. Animal cognition: multimodal tactics of orangutan communication. Curr. Biol. 17, R762–R764. ( 10.1016/j.cub.2007.07.010) [DOI] [PubMed] [Google Scholar]
- 35.Miller GL, Stratton GE, Miller PR, Hebets E. 1998. Geographical variation in male courtship behaviour and sexual isolation in wolf spiders of the genus Schizocosa. Anim. Behav. 56, 937–951. ( 10.1006/anbe.1998.0851) [DOI] [PubMed] [Google Scholar]
- 36.Stratton GE. 1997. A new species of Schizocosa from the southeastern USA (Araneae, Lycosidae). J. Arachnol. 25, 84–92. [Google Scholar]
- 37.Stafstrom JA, Hebets EA. 2013. Female mate choice for multimodal courtship and the importance of the signaling background for selection on male ornamentation. Curr. Zool. 59, 200–209. [Google Scholar]
- 38.Hebets EA. 2005. Attention-altering signal interactions in the multimodal courtship display of the wolf spider Schizocosa uetzi. Behav. Ecol. 16, 75–82. ( 10.1093/beheco/arh133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilgers DJ, Hebets EA. 2011. Complex courtship displays facilitate male reproductive success and plasticity in signaling across variable environments. Curr. Zool. 57, 175–186. ( 10.1093/czoolo/57.2.175) [DOI] [Google Scholar]
- 40.Rundus AS, Sullivan-Beckers L, Wilgers DJ, Hebets EA. 2011. Females are choosier in the dark: environment-dependent reliance on courtship components and its impact on fitness. Evolution 65, 268–282. ( 10.1111/j.1558-5646.2010.01125.x) [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal M, Hebets E. 2012. Resource heterogeneity interacts with courtship rate to influence mating success in the wolf spier S. floridana. Anim. Behav. 84, 1341–1346. ( 10.1016/j.anbehav.2012.08.028) [DOI] [Google Scholar]
- 42.Rosenthal MF, Hebets EA. 2015. Temporal patterns of nutrition dependence in secondary sexual traits and their varying impacts on male mating success. Anim. Behav. 103, 75–82. ( 10.1016/j.anbehav.2015.02.001) [DOI] [Google Scholar]
- 43.Rand AS, Ryan MJ. 1981. The adaptive significance of a complex vocal repertoire in a neotropical frog. J. Comp. Ethol. 57, 209–214. ( 10.1111/j.1439-0310.1981.tb01923.x) [DOI] [Google Scholar]
- 44.Ryan MJ. 1980. Female mate choice in a neotropical frog. Science 209, 523–525. ( 10.1126/science.209.4455.523) [DOI] [PubMed] [Google Scholar]
- 45.Wilczynski W, Rand AS, Ryan MJ. 1999. Female preferences for temporal order of call components in the tungara frog: a Bayesian analysis. Anim. Behav. 58, 841–851. ( 10.1006/anbe.1999.1208) [DOI] [PubMed] [Google Scholar]
- 46.Taylor RC, Ryan MJ. 2013. Interactions of multisensory components perceptually rescue Tungara frog mating signals. Science 341, 273–274. ( 10.1126/science.1237113) [DOI] [PubMed] [Google Scholar]
- 47.Taylor RC, Klein BA, Ryan MJ. 2011. Inter-signal interaction and uncertain information in anuran multimodal signals. Curr. Zool. 57, 153–161. ( 10.1093/czoolo/57.2.153) [DOI] [Google Scholar]
- 48.Ronald KL, Fernandez-Juricic E, Lucas JR. 2012. Taking the sensory approach: how individual differences in sensory perception can influence mate choice. Anim. Behav. 84, 1283–1294. ( 10.1016/j.anbehav.2012.09.015) [DOI] [Google Scholar]
- 49.Kasurak AV, Zielinski BS, Higgs DM. 2012. Reproductive status influences multisensory integration responses in female round gobies, Neogobius melanostomus. Anim. Behav. 83, 1179–1185. ( 10.1016/j.anbehav.2012.02.008) [DOI] [Google Scholar]
- 50.Wilgers DJ, Hebets EA. 2012. Age-related female mating decisions are condition dependent in wolf spiders. Behav. Ecol. Sociobiol. 66, 29–38. ( 10.1007/s00265-011-1248-5) [DOI] [Google Scholar]
- 51.Vanderbilt CC, Kelley JP, Duval EH. 2015. Variation in the performance of cross-contextual displays suggests selection on dual-male phenotypes in a lekking bird. Anim. Behav. 107, 213–219. ( 10.1016/j.anbehav.2015.06.023) [DOI] [Google Scholar]
- 52.DuVal EH. 2007. Social organization and variation in cooperative alliances among male lance-tailed manakins. Anim. Behav. 73, 391–401. ( 10.1016/j.anbehav.2006.05.017) [DOI] [Google Scholar]
- 53.Rosenthal MF. 2015. Mating in a variable world: the implications of environmental variation for male and female mating behavior. Lincoln, NE: University of Nebraska-Lincoln. [Google Scholar]
- 54.Thomas ML, Gray B, Simmons LW. 2011. Male crickets alter the relative expression of cuticular hydrocarbons when exposed to different acoustic environments. Anim. Behav. 82, 49–53. ( 10.1016/j.anbehav.2011.03.023) [DOI] [Google Scholar]
- 55.Chen C-C, Crilly N. 2014. Modularity, redundancy and degeneracy: cross-domain perspectives on key design principles. In 8th Annual IEEE Int. Syst. Conf., SysCon 2014—Proc., pp. 546–553.
- 56.Maleszka R, Mason PH, Barron AB. 2014. Epigenomics and the concept of degeneracy in biological systems. Brief Funct. Genome 13, 191–202. ( 10.1093/bfgp/elt050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mason PH. 2015. Degeneracy: demystifying and destigmatizing a core concept in systems biology. Complexity 20, 12–21. ( 10.1002/cplx.21534) [DOI] [Google Scholar]
- 58.Friston K, Price CJ. 2003. Degeneracy and redundancy in cognitive anatomy. Trends Cogn. Sci. 7, 151–152. ( 10.1016/S1364-6613(03)00054-8) [DOI] [PubMed] [Google Scholar]
- 59.Botero CA, de Kort SR. 2013. Learned signals and consistency of delivery: a case against receiver manipulation in animal communication. In Animal communication theory: information and influence, pp. 281–296. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 60.Botero CA, Rossman RJ, Caro LM, Stenzler LM, Lovette IJ, de Kort SR, Vehrencamp SL. 2009. Syllable type consistency is related to age, social status and reproductive success in the tropical mockingbird. Anim. Behav. 77, 701–706. ( 10.1016/j.anbehav.2008.11.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Kort SR, Eldermire ERB, Valderrama S, Botero CA, Vehrencamp SL. 2009. Trill consistency is an age-related assessment signal in banded wrens. Proc. R. Soc. B 276, 2315–2321. ( 10.1098/rspb.2009.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rivera-Gutierrez HF, Pinxten R, Eens M. 2011. Songs differing in consistency elicit differential aggressive response in territorial birds. Biol. Lett. 7, 339–342. ( 10.1098/rsbl.2010.0962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aubin T, Jouventin P. 2002. Localisation of an acoustic signal in a noisy environment: the display call of the king penguin Aptenodytes patagonicus. J. Exp. Biol. 205, 3793–3798. [DOI] [PubMed] [Google Scholar]
- 64.Whitacre J, Bender A. 2010. Degeneracy: a design principle for achieving robustness and evolvability. J. Theor. Biol. 263, 143–153. ( 10.1016/j.jtbi.2009.11.008) [DOI] [PubMed] [Google Scholar]
- 65.Edelman GM, Gally JA. 2001. Degeneracy and complexity in biological systems. Proc. Natl Acad. Sci. USA 98, 13 763–13 768. ( 10.1073/pnas.231499798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stratton GE. 2005. Evolution of ornamentation and courtship behavior in Schizocosa: Insights from a phylogeny based on morphology (Araneae, Lycosidae). J. Arachnol. 33, 347–376. ( 10.1636/04-80.1) [DOI] [Google Scholar]
- 67.Kaczorowski RL, Leonard AS, Dornhaus A, Papaj DR. 2012. Floral signal complexity as a possible adaptation to environmental variability: a test using nectar-foraging bumblebees, Bombus impatiens. Anim. Behav. 83, 905–913. ( 10.1016/j.anbehav.2012.01.007) [DOI] [Google Scholar]
- 68.Ay N, Flack J, Krakauer DC. 2007. Robustness and complexity co-constructed in multimodal signalling networks. Phil. Trans. R. Soc. B 362, 441–447. ( 10.1098/rstb.2006.1971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE. 2014. Female song is widespread and ancestral in songbirds. Nat. Commun. 5 ( 10.1038/ncomms4379) [DOI] [PubMed] [Google Scholar]
- 70.Wiens JJ. 2001. Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol. Evol. 16, 517–523. ( 10.1016/S0169-5347(01)02217-0) [DOI] [Google Scholar]
- 71.Guindre-Parker S, Gilchrist HG, Baldo S, Doucet SM, Love OP. 2013. Multiple achromatic plumage ornaments signal to multiple receivers. Behav. Ecol. 24, 672–682. ( 10.1093/beheco/ars215) [DOI] [Google Scholar]
- 72.Hunt J, Breuker CJ, Sadowski JA, Moore AJ. 2009. Male–male competition, female mate choice and their interaction: determining total sexual selection. J. Evol. Biol. 22, 13–26. ( 10.1111/j.1420-9101.2008.01633.x) [DOI] [PubMed] [Google Scholar]
- 73.Reichert MS. 2013. Patterns of variability are consistent across signal types in the treefrog Dendropsophus ebraccatus. Biol. J. Linn. Soc. 109, 131–145. ( 10.1111/bij.12028) [DOI] [Google Scholar]
- 74.Podos J. 1997. A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51, 537–551. ( 10.2307/2411126) [DOI] [PubMed] [Google Scholar]
- 75.Podos J. 2001. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature 409, 185–188. ( 10.1038/35051570) [DOI] [PubMed] [Google Scholar]
- 76.Wagner WE, Beckers OM, Tolle AE, Basolo AL. 2012. Tradeoffs limit the evolution of male traits that are attractive to females. Proc. R. Soc. B 279, 2899–2906. ( 10.1098/rspb.2012.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ward JL, Love EK, Velez A, Buerkle NP, O'Bryan LR, Bee MA. 2013. Multitasking males and multiplicative females: dynamic signalling and receiver preferences in Cope's grey treefrog. Anim. Behav. 86, 231–243. ( 10.1016/j.anbehav.2013.05.016) [DOI] [Google Scholar]
- 78.Reichert MS, Gerhardt HC. 2012. Trade-offs and upper limits to signal performance during close-range vocal competition in gray tree frogs hyla versicolor. Am. Nat. 180, 425–437. ( 10.1086/667575) [DOI] [PubMed] [Google Scholar]
- 79.Arnold SJ. 1992. Constraints on phenotypic evolution. Am. Nat. 140, S85–S107. ( 10.1086/285398) [DOI] [PubMed] [Google Scholar]
- 80.Dalziell AH, Peters RA, Cockburn A, Dorland AD, Maisey AC, Magrath RD. 2013. Dance choreography is coordinated with song repertoire in a complex avian display. Curr. Biol. 23, 1132–1135. ( 10.1016/j.cub.2013.05.018) [DOI] [PubMed] [Google Scholar]
- 81.Scholes EI. 2008. Evolution of the courtship phenotype in the bird of Paradise genus Parotia (Aves: Paradisaeidae): homology, phylogeny, and modularity. Biol. J. Linn. Soc. 94, 491–504. ( 10.1111/j.1095-8312.2008.01012.x) [DOI] [Google Scholar]
- 82.Scholes EI. 2008. Structure and composition of the courtship phenotype in the bird of paradise Parotia lawessi (Aves: Paradisaeidae). Zoology 111, 260–278. ( 10.1016/j.zool.2007.07.012) [DOI] [PubMed] [Google Scholar]
- 83.Smith CL, Evans CS. 2013. A new heuristic for capturing the complexity of multimodal signals. Behav. Ecol. Sociobiol. 67, 1389–1398. ( 10.1007/s00265-013-1490-0) [DOI] [Google Scholar]
- 84.Blows MW, Brooks R, Kraft PG. 2003. Exploring complex fitness surfaces: multiple ornamentation and polymorphism in male groups. Evolution 57, 1622–1630. ( 10.1111/j.0014-3820.2003.tb00369.x) [DOI] [PubMed] [Google Scholar]
- 85.Proulx SR, Promislow DEL, Phillips PC. 2005. Network thinking in ecology and evolution. Trends Ecol. Evol. 20, 345–353. ( 10.1016/j.tree.2005.04.004) [DOI] [PubMed] [Google Scholar]
- 86.Magwene PM. 2001. New tools for studying integration and modularity. Evolution 55, 1734–1745. ( 10.1111/j.0014-3820.2001.tb00823.x) [DOI] [PubMed] [Google Scholar]
- 87.Mikolajewski DJ, Rusen L, Mauersberger R, Johansson F, Rolff J. 2015. Relaxed predation results in reduced phenotypic integration in a suite of dragonflies. J. Evol. Biol. 28, 1354–1363. ( 10.1111/jeb.12658) [DOI] [PubMed] [Google Scholar]
- 88.Johnstone RA. 1996. Multiple displays in animal communication: ‘backup signals’ and ‘multiple messages’. Phil. Trans. R. Soc. Lond. B 351, 329–338. ( 10.1098/rstb.1996.0026) [DOI] [Google Scholar]
- 89.Møller AP, Pomiankowski A. 1993. Why have birds got multiple sexual ornaments. Behav. Ecol. Sociobiol. 32, 167–176. ( 10.1007/BF00173774) [DOI] [Google Scholar]
- 90.Rosenthal GG. 2007. Spatiotemporal dimensions of visual signals in animal communication. Annu. Rev. Ecol. Evol. Syst. 38, 155–178. ( 10.1146/annurev.ecolsys.38.091206.095745) [DOI] [Google Scholar]
- 91.Narins PM, Grabul DS, Soma KK, Gaucher P, Hodl W. 2005. Cross-modal integration in a dart-poison frog. Proc. Natl Acad. Sci. USA 102, 2425–2429. ( 10.1073/pnas.0406407102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Narins PM, Hodl W, Grabul DS. 2003. Bimodal signal requisite for agonistic behavior in a dart-poison frog, Epipedobates femoralis. Proc. Natl Acad. Sci. USA 100, 577–580. ( 10.1073/pnas.0237165100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grafe TU, Preininger D, Sztatecsny M, Kasah R, Dehling JM, Proksch S, Hodl W. 2012. Multimodal communication in a noisy environment: a case study of the bornean rock frog Staurois parvus. PLoS ONE 7, e37965 ( 10.1371/journal.pone.0037965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grafe TU, Wanger TC. 2007. Multimodal signaling in male and female foot-flagging frogs Staurois guttatus (Ranidae): an alerting function of calling. Ethology 113, 772–781. ( 10.1111/j.1439-0310.2007.01378.x) [DOI] [Google Scholar]
- 95.Preininger D, Boeckle M, Hodl W. 2009. Communication in noisy environments II: visual signaling behavior of male foot-flagging frogs Staurois latopalmatus. Herpetologica 65, 166–173. ( 10.1655/08-037R.1) [DOI] [Google Scholar]
- 96.Dale MRT, Fortin MJ. 2009. Spatial autocorrelation and statistical tests: some solutions. J. Agric. Biol. Environ. Stat. 14, 188–206. ( 10.1198/jabes.2009.0012) [DOI] [Google Scholar]
- 97.Leal M, Losos JB. 2015. A naturalist's insight into evolution of signal redundance. Am. Nat. 186, i–iv. ( 10.1086/682704) [DOI] [PubMed] [Google Scholar]