Abstract
Animals use a number of different mechanisms to acquire crucial information. During social encounters, animals can pass information from one to another but, ideally, they would only use information that benefits survival and reproduction. Therefore, individuals need to be able to determine the value of the information they receive. One cue can come from the behaviour of other individuals that are already using the information. Using a previous extended dataset, we studied how individual decision-making is influenced by the behaviour of conspecifics in Drosophila melanogaster. We analysed how uninformed flies acquire and later use information about oviposition site choice they learn from informed flies. Our results suggest that uninformed flies adjust their future choices based on how coordinated the behaviours of the informed individuals they encounter are. Following social interaction, uninformed flies tended either to collectively follow the choice of the informed flies or to avoid it. Using social network analysis, we show that this selective information use seems to be based on the level of homogeneity of the social network. In particular, we found that the variance of individual centrality parameters among informed flies was lower in the case of a ‘follow’ outcome compared with the case of an ‘avoid’ outcome.
Keywords: social learning, social network, Drosophila, oviposition choice
1. Introduction
How do we know if the information we encounter in everyday life is beneficial or not? Deciding whether to use acquired information is a critical decision that could deeply affect an individual's behaviour, and ultimately its fitness. Theory and empirical evidence suggest that this decision is controlled by a wide array of factors affecting both informed and uninformed individuals [1,2]. Such factors range from environmental [3] to population [4] or individual characteristics [5–7]. For instance, Seppänen et al. [8] showed that female pied flycatchers (Ficedula hypoleuca) tended to not only copy the nest site choice of high-fitness individuals, but also to choose the opposite of low-fitness individuals. This discriminating decision-making process could accelerate the spread of adaptive behaviours by favouring those of individuals with higher fitness. Adult starlings (Sturnus vulgaris) exposed to unpredictable food availability copied the behaviour of informed demonstrators to make foraging decisions more than starlings in predictable environments did, and performed better when they could access social information than when they could not [9]. However, Japanese quails (Coturnix japonica) postnatally exposed to unpredictable food availability preferred to avoid the food source previously used by demonstrators [10]. These conflicting results highlight the need for a better understanding of the link between behavioural copying and environmental predictability.
At the group level, the social environment, and the social structure that follows, may greatly affect the way social information spreads among individuals [11,12]. Social network structures have been found to vary across and within species, and are sensitive to environmental factors [13]. Theoretically, the heterogeneity of individuals' roles in a network may directly impact how information is transmitted in a group [14]. Sueur et al. [15] showed that heterogeneous networks, characterized by high inter-individual variations in network measures, are less efficient to transmit information than homogeneous networks. Yet the process by which this heterogeneity may affect information transmission leads to contrasting results. For example, on the one hand, during collective decision-making, consensus among individuals is reached faster in homogeneous networks in which individuals are all well connected [16,17]. On the other hand, in strongly heterogeneous networks, a few central individuals, or nodes, hold most connections. When such individuals possess a piece of information, their network position allows them to boost the information-spreading process. In this case, centrality—which measures the influence of a node in a network—strongly affects an individual's power to transmit information [18]. So far, little experimental investigation into how the structure of the social network may impact individual use of social information and decision-making has taken place.
In a previous study, we showed that oviposition site preference could be socially transmitted in Drosophila melanogaster [19,20]. Uninformed flies are those that have no prior information about the quality of oviposition sites. After interacting with informed flies in an empty cage, uninformed flies tended to lay their eggs on the same medium that their informed conspecifics had previously learned to choose. We observed that the number of interactions (close contact) that occurred during the social interaction phase influenced how well the oviposition site choice of uninformed flies matched that of their informed counterparts [21]. Information about preferred oviposition sites is likely to be socially transmitted through smell or taste, because informed flies can carry traces of the medium they were conditioned to prefer. Using a larger dataset, we observed that, after social interaction, uninformed flies tended to show strong group behaviour, and that information use was variable among replicates. In 50% of the replicates, uninformed flies copied the oviposition choice of informed flies, whereas in 20% of the replicates, uninformed flies chose to lay all their eggs on the opposite medium (figure 1). Without prior social interaction with informed flies, uninformed flies did not show this strong group behaviour (figure 1). In all replicates, flies were treated the same way, which questions the source of this variation.
Figure 1.
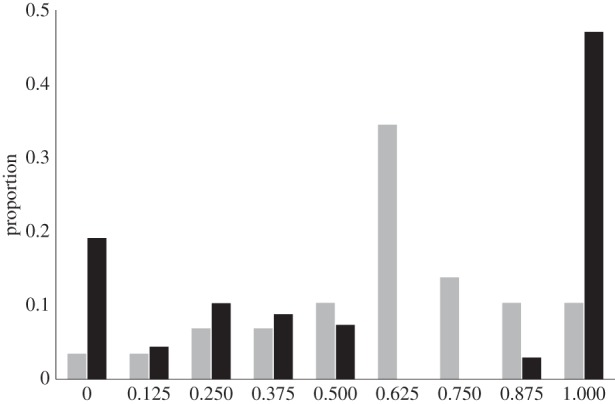
Black bars: distribution of the proportion of eggs that uninformed flies laid on the same medium that their informed counterparts had been conditioned to choose, after social interaction (n = 62). Grey bars: distribution of the proportion of eggs laid by uninformed flies on the banana-scented medium without any prior social interaction with informed flies (n = 29).
Here we tested whether the observed bimodal pattern of behaviour may result from variation in information use, dependent on the structure of the social network during the social interaction phase. Using social network analysis, we tested how the network properties of both informed and uninformed flies impacted their oviposition site choice. Based on studies of network efficiency [22,23], we expected that homogeneous networks (in which the majority of individuals behave similarly) should lead uninformed individuals to adopt the behaviour of their informed counterparts more often than heterogeneous networks would.
2. Material and methods
(a). Information transmission experiment
We used interaction data from 62 social learning experiments, of which 49 were presented in Battesti et al. [9] and 13 new ones, to calculate and analyse social network measures, and to study their evolution over time. Each experiment was divided into three phases: (i) a conditioning phase, during which eight mated females (3–5 days old) were conditioned to prefer either a banana- or a strawberry-scented egg-laying medium (these were the informed females in the subsequent phases); (ii) an interaction phase during which we video-tracked the interactions among eight informed and four uninformed females, and later calculated network parameters; and (iii) a test phase during which the informed and uninformed females were tested separately for their oviposition site choice.
In the conditioning phase, we introduced a group of eight mated flies into a cage containing two egg-laying media: banana- and strawberry-scented. We added quinine beforehand to one of the media (selected randomly). Quinine is an alkaloid known to induce gustatory repulsion in fruit flies. The conditioning phase lasted 8 h.
In the interaction phase, we removed all eight informed flies from the cage. We placed them and four uninformed mated females—ones that had no experience with either scent or with quinine—in a semi-opaque white arena (diameter 100 mm; height 3 mm) covered with transparent Plexiglas. There were no egg-laying media in the arena. Flies were filmed for 4 h. The tracking system consisted of firewire cameras (Guppy Pro, Allied Vision Technologies) filming the interaction arena, which was backlit by a 150×150 mm IR backlight (R&D Vision). We used a vision software to analyse spatial data (open-source C-trax 0.3.7 [24]) that allowed us to automatically follow individuals and record their coordinates in the interaction arena, at a rate of 10 frames per second. Tracking corrections were made following the C-trax analysis using Fixerrors toolbox 0.2.11 in MATLAB software v. 7.11.0 to suppress swaps between individuals.
The test phase immediately followed the interaction phase. Separate groups of uninformed and informed flies (four in each group) were placed in separate arenas with two egg-laying sites: one with banana- and one with strawberry-scented media, both without quinine. We calculated the proportion of eggs both informed and uninformed individuals laid on each medium at the end of each experiment.
In order to focus on the main bimodal outcome observed in the real data, we omitted the few videos that showed random decision in oviposition site choice (figure 1). We thus defined two main categorical outcomes: (i) ‘follow’ means that uninformed flies laid most of their eggs on the same medium their informed counterparts had learned to choose (the proportion of eggs laid on the ‘right’ medium by uninformed flies was greater than 0.75; n = 29) and (ii) ‘avoid’ means that uninformed flies laid most of their eggs on the medium opposite to the one their informed counterparts had learned to choose (the proportion of eggs laid on the ‘right’ medium by uninformed flies was lower than 0.25; n = 20; figure 1).
(b). Data preparation
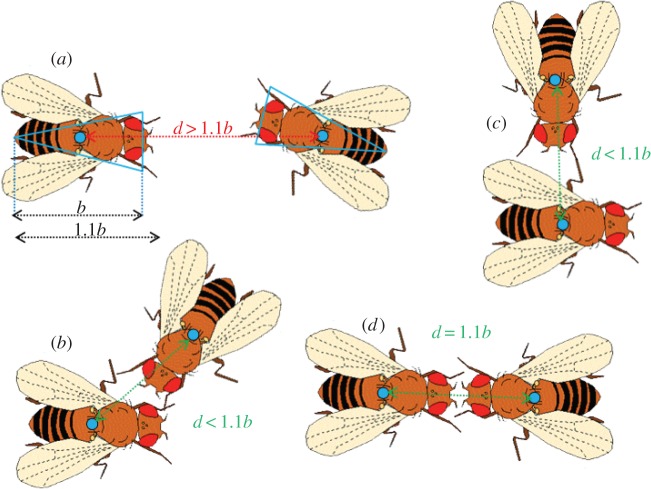
From fly coordinates, we built interaction matrices using an automated code specifically developed in R (available upon request). To this end, we defined an interaction between two individuals based on spatial and temporal constraints [21]: (i) proximity between two flies had to be smaller than 1.1 average fly body lengths and (ii) the duration of the contact had to be at least five time frames (0.5 s; figure 2). Moreover, to discriminate between the initiator and receiver of each interaction, we calculated the mean speed of the individuals over the four time frames that preceded each interaction. The initiator was defined as the faster of the two individuals involved in the interaction, under the assumption that the faster-moving individual was the one choosing to approach the other fly, and thus initiating the interaction.
Figure 2.
General principle used to spatially define an interaction between two flies. Each fly was identified by C-trax using polygons fitted to the flies' bodies (blue triangles in (a)), which were used to determine their body length b and geometric centre. In (a), the two flies' centres (and the distance between them d) are more than 1.1 body length apart; our system did not record this dyad as interacting at that point. In (b), the two flies are in contact (at least 0.5 s) with an angle between 0 and 180°, but close enough for the distance between their centres to be less than 1.1 body lengths. In (c), the flies are interacting (at least 0.5 s) at a 90° angle from each other. Contact is possible between the top fly's antennae or forelegs and the other fly's left legs, and will be recorded as such as long as they remain less than 1.1 body lengths apart. (d) In the most extreme case, flies are facing each other and are thus 1.1 body lengths apart but still interacting; the antennae and forelegs are still in contact, so information can be transmitted. Drawing of Drosophila: © Drosophila Interest Group, http://sigs.nih.gov/drosophila.
(c). Social network analysis
A network is composed of two main elements: actors and connections between actors. Actors are generally defined by attributes that can be used to describe their state (here ‘informed’ or ‘uninformed’). Connections can usually be ascribed to interactions that occur between actors. Because we could distinguish initiators and receivers, we were able to construct directed networks: each connection linked an initiator to its receiver. The weight of this link was proportional to the frequency of interaction between the two nodes. We built 16 consecutive interaction networks by dividing each 4 h video from the interaction phase into 15 min time intervals. Before running the analysis, we removed the first time interval from each experiment as flies were more active during the first interval of the transmission phase, probably because they were recently introduced into a new environment.
We focused on six different network measures that are commonly used to describe individual positions in a network (see figure 3 for network details). We examined the influence of each measure on the transmission process by comparing mean and variance in ‘follow’ versus ‘avoid’ replicates for each fly type (i.e. informed and uninformed). The mean and variance were calculated at each time interval (n = 15) for each video (n = 62). The network measures were as follows.
Figure 3.
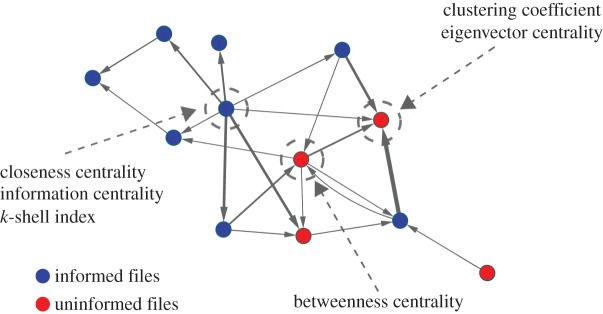
Example of an interaction network calculated at the 30th min of the transmission phase. Both informed (blue circles) and uninformed (red circles) flies are represented. The size of the black arrows is proportional to the number of contacts between flies, and their direction indicates which fly was the receiver of the interaction. The grey circles indicate the flies for which the corresponding network measures are maximized.
(i). Weighted closeness centrality
Calculated as the inverse of the geodesic distance, or the shortest path between two nodes [25], from one individual to all the others in the network, and weighting for the number of connections among actors (alpha parameter is equal to 1 [26]). It is generally used to identify nodes that, due to their position, rapidly increase the transmission process in the network.
(ii). Eigenvector centrality
Measures the influence of a node in the network [27]. It is directly related to the number of contacts a node has and to the relative weight of the nodes to which it is connected. The score of the focal node will be higher if the score of its neighbours is higher. This measure is used extensively in social network analysis, but it appears to be an unreliable predictor of how efficiently information spreads [28].
(iii). k-shell decomposition index
The decomposition procedure to obtain the k-shell index of each node is based on an iterative removal of nodes from the network, and their partition in respective k-shells. The first k-shell (ks = 1) of a network contains originally all nodes with a degree of 1 (k = 1). Then, these nodes are removed. If some remaining nodes have a degree of 1 in the new network, they are added to the first k-shell, and subsequently removed from the network. This iteration goes on until no more nodes can be removed. Then, the iteration for the second k-shell (ks = 2) starts. This shell contains all the remaining nodes of degree 2, which are then removed from the network. As long as at least some nodes have a degree lower than 3 in the new network, they are added to the second shell and removed from the network. And so on for higher levels, until all nodes of the network are assigned to a unique k-shell [28,29]. Thus, each k-shell consists of nodes characterized by k ≥ ks. It is a hierarchical measure of the importance of a node in spreading information within the network. It provides the absolute graph position of a node, ranging from peripheral (lowest values) to central (highest values).
(iv). Information centrality index
Calculated by combining all the paths present in a network and assigning a weight to them that is equal to the inverse of the length of the path [29,30]. It reflects the amount of information per individual contained in all possible paths that originate from and end with that individual. Information passes through these paths (via connections between individuals), so the information centrality index seems to be useful for studying information diffusion.
(v). Weighted clustering coefficient
The probability that the individuals adjacent to the focal individual are connected to each other [31]. It assesses the degree to which nodes tend to cluster together. Strong clustering should have a negative effect on transmission processes in the overall network.
(vi). Weighted betweenness centrality
The number of shortest paths connecting nodes that pass through a focal node [26]. It reflects the importance of a node as a transmission channel in the network. High values of betweenness mean that a node is important as an intermediary in the transmission process.
(d). Statistical analysis
We used a Markov chain Monte Carlo approach to test whether the mean and the variance of the social network measures calculated for both informed and uninformed flies had an impact on subsequent oviposition site choice. In particular, we used a Bayesian bivariate mixed model [32] to estimate the correlation between the oviposition site choices (‘follow’ versus ‘avoid’) and both the mean and variance of each social network measure, while correcting for time interval as a fixed effect in the model [33]. We applied an idh variance structure, which allowed for different variances across the choice response and network measures, but assumed that the effect of the random factor for the first trait was not correlated with the one for the second trait [32]. Because we had multiple measures of both mean and variance for each video (one measure for each time interval), we added video identity as a random effect in the model. The Bayesian multiresponse model approach allowed us to estimate the strength and precision of the correlations by calculating the 95% credible intervals of the posterior distributions. We calculated the correlation between two traits as Cov(Px;Pbin)/sqrt(VPx × VPbin), where Cov(Px;Pbin) is the covariance between each trait (x) calculated for social network measures and oviposition site choice (bin), while VPx and VPbin are the corresponding variances [33]. A critical step in Bayesian statistical inference is to set priors before running the models. The term prior refers to a probability distribution that represents information about a particular parameter before the data are analysed. This information can vary according to different levels—from non-informative to highly informative. Because we had no knowledge of the relationship between the variables in the models, we used two sets of priors: a slightly informative prior (V = diag(2); n = 2) and a standard non-informative one (inverse Wishart prior; V = diag(2) × 1000; n = 2). We compared them using the deviance information criterion (DIC) obtained from the models [32]. We selected the prior that provided the lowest DICs for each model [34]. MCMCglmm models were run for 200 000 iterations after a burnin of 2000 (meaning that we omitted the first 2000 iterations to avoid autocorrelation problems) and a thinning interval of 200 (meaning that we only used one iteration from every 200 in the Markov chain to estimate the posterior distribution of the parameters).
3. Results
(a). Impact of social network measures on oviposition site choice in uninformed flies
For uninformed flies, the variance of three out of six network measures calculated for informed flies was smaller in the ‘follow’ replicates than in the ‘avoid’ ones. Most notably, uninformed flies showed a ‘follow’ reaction when: (i) informed flies had less variable network distances from the other individuals in the network (weighted closeness centrality, table 1a, column 1), meaning that hypothetical information starting from a single random informed actor in the network will spread with similar speed regardless of the identity of that actor; (ii) informed flies were similar in the number of interactions experienced (eigenvector centrality, table 1b, column 1), suggesting that uninformed flies chose the same medium as their informed peers when the network was more homogeneous; and (iii) all informed flies exchanged information to a similar extent (information centrality index, table 1d, column 1), suggesting each played a similar role in transmitting information. Neither the variance nor the mean of the k-shell decomposition index, weighted clustering coefficients and weighted betweenness centrality calculated for informed individuals influenced the uninformed flies' oviposition site choice (table 1c–f, column 1).
Table 1.
Behavioural correlations between oviposition site choices (‘follow’ versus ‘avoid’), calculated for both uninformed and informed flies, and mean and variance of each network measure. Positive values indicate that they ‘follow’ more frequently. Included are correlations, and the lower and upper 95% credible intervals (CI), derived from the variance and covariance matrices of the bivariate models. All Bayesian bivariate mixed models included video identification as a random effect, and time intervals as fixed effects. Bold values indicate significant correlations (the 95% CI does not overlap zero).
| 1) uninformed fly choice correlation [lower—upper 95% CI] |
2) informed fly choice correlation [lower—upper 95% CI] |
||
|---|---|---|---|
| (a) weighted closeness centrality | |||
| informed | variance | −0.69 [−0.84; −0.41] | 0.09 [−0.33; 0.43] |
| mean | 0.10 [−0.19; 0.40] | 0.38 [0.05; 0.58] | |
| uninformed | variance | −0.36 [−0.61; 0.15] | −0.31 [−0.67; 0.12] |
| mean | −0.30 [−0.55; 0.11] | −0.29 [−0.67; 0.10] | |
| (b) weighted eigenvector centrality | |||
| informed | variance | −0.37 [−0.58; −0.09] | −0.32 [−0.53; 0.08] |
| mean | 0.03 [−0.25; 0.33] | −0.11 [−0.38; 0.26] | |
| uninformed | variance | −0.09 [−0.39; 0.26] | −0.12 [−0.35; 0.27] |
| mean | −0.13 [−0.39; 0.21] | −0.19 [−0.47; 0.12] | |
| (c) k-shell decomposition index | |||
| informed | variance | −0.41 [−0.66; 0.04] | −0.24 [−0.51; 0.11] |
| mean | 0.13 [−0.18; 0.42] | 0.09 [−011; 0.46] | |
| uninformed | variance | 0.01 [−0.38; 0.28] | 0.24 [−0.15; 0.55] |
| mean | 0.11 [−0.22; 0.38] | −0.03 [−0.31; 0.31] | |
| (d) information centrality index | |||
| informed | variance | −0.35 [−0.60; −0.10] | −0.20 [−0.47; 0.13] |
| mean | 0.16 [−0.22; 0.40] | 0.33 [0.06; 0.61] | |
| uninformed | variance | 0.02 [−0.38; 0.26] | 0.19 [−0.12; 0.51] |
| mean | −0.05 [−0.40; 0.23] | −0.37 [−0.58; 0.02] | |
| (e) weighted clustering coefficient | |||
| informed | variance | −0.23 [−0.55; 0.07] | −0.26 [−0.54; 0.08] |
| mean | −0.15 [−0.41; 0.18] | −0.39 [−0.66; −0.18] | |
| uninformed | variance | 0.20 [−0.14; 0.52] | 0.19 [−0.16; 0.50] |
| mean | −0.01 [−0.23; 0.35] | 0.20 [−0.13; 0.51] | |
| (f) weighted betweenness centrality | |||
| informed | variance | −0.67 [−0.83; 0.18] | −0.70 [−0.89; 0.15] |
| mean | −0.03 [−0.36; 0.30] | 0.29 [−0.16; 0.53] | |
| uninformed | variance | −0.91 [−0.98; −0.72] | −0.50 [−0.83; 0.31] |
| mean | −0.63 [−0.84; −0.27] | −0.30 [−0.54; 0.05] | |
Only one of the six network measures calculated for uninformed flies during the transmission phase was related to the oviposition site choice they made during the test phase. There was a negative correlation between the weighted betweenness centrality measure and uninformed flies choosing the same medium that their informed counterparts had been conditioned to choose (table 1f, column 1). In particular, uninformed flies were less likely to choose the same site as their informed counterparts when they interacted in networks where uninformed flies occupied important bridge positions (see the ‘mean’ component in table 1f). Also, when uninformed flies had similar betweenness centrality values, they were more likely to choose the same medium as their informed counterparts (see the ‘variance’ component in table 1f).
(b). Impact of social network measures on oviposition site choice in informed flies
Few of the social network measures calculated during the transmission phase affected oviposition site choice in informed flies during the test phase. Interestingly, only the means—not the variances—calculated for three out of six measures affected the oviposition site choice of informed flies. More precisely, informed flies were more likely to choose the opposite medium they were conditioned to choose when: (i) the network distance between them and the other individuals in the network was small (mean weighted closeness centrality table 1a, column 2), suggesting that informed flies with central positions during the transmission process changed their subsequent oviposition site choice; (ii) their degree of involvement in the transmission of information was lower (mean information centrality index, table 1d, column 2); and (iii) when they were more often part of clusters (mean clustering coefficient, table 1e, column 2).
Social network measures of uninformed individuals never influenced future oviposition site choice of informed individuals (table 1).
4. Discussion
In this study, we show that the oviposition site preference in Drosophila melanogaster can be strongly affected by social interactions between informed and uninformed flies, and that the behavioural outcome depends on the structure of the social network observed during the interaction phase.
First, uninformed flies tended to show group behaviour after interacting with informed ones. Without social interaction with informed flies, flies of uninformed groups tended to lay their eggs independently from each other, and we observed a random distribution of eggs on the two oviposition media. However, after social interaction with informed flies, for most group replicates, eggs were laid exclusively on a single oviposition medium (figure 1). The emergence of this collective behaviour is intriguing. Recent studies have shown that flies tend to show increased movement coordination and aggregation as a function of density and social encounters [35,36]. We previously found that the presence of informed flies within a group increases the number of interactions [21]. These interactions may potentially induce behavioural changes in uninformed flies, which become more gregarious even in the absence of informed peers.
In 59% of the selected replicates, the uninformed flies copied the oviposition choice of informed flies, whereas in 41% of the replicates uninformed flies chose the opposite. This ‘follow or avoid’ emergent behavioural group outcome appeared to rely on the organization of the social network during the interaction phase. In particular, we found that the variance of individual centrality parameters among informed flies was lower in the case of a ‘follow’ outcome compared with the case of an ‘avoid’ outcome. The impact of centrality variance but not mean centrality on the behavioural outcome of uninformed individuals suggests that the study of the entire group social structure, rather than individual network measures or network density, is important to understand information transfer. These results are in agreement with previous studies showing a link between network organization and information transmission [15]. In a comparative study, Pasquaretta et al. [37], using data from 80 primate species, demonstrated that heterogeneous groups had less efficient networks (efficiency being measured using the rate and accuracy of transmission, relative to the number of connections between agents [38]). How inter-replicate variation in social structures may arise remains to be investigated. In humans, heterogeneous networks have been found to arise when individuals are exposed to stress or high uncertainty. Argote et al. [13], using experimental manipulations on human choice, have demonstrated that groups of individuals exposed to stimulus ambiguity or a stressful environment showed increased heterogeneity, calculated from their communication network. Similarly, zebra finches exposed to developmental stress also show heterogeneous networks [39]. Whether inadvertent variation in stress levels occurred among our fly groups is unclear. Subtle variation in fly manipulation is well known to affect state and behaviour [40].
Another effect called the illusion of majority could explain why the variance but not the mean has an effect on the behavioural choice ‘avoid or follow’. The majority illusion stipulates that individuals take their social cues from their local neighbours and the characteristics and positions in the network of these initial individuals can greatly influence the contagion of a behaviour [41]. The mathematical model developed by Lerman et al. [41] shows that the majority illusion is stronger in heterogeneous networks with active central nodes, potentially resulting in some behaviours spreading although they are outside the norm or maladaptive. In this case, homogeneous networks, whatever the quantity of interactions, should favour the transmission of correct and adaptive information.
Our results suggest that uninformed flies adjust their future choices based on how coordinated the behaviours of the informed individuals they encounter are. The homogeneous or heterogeneous organization of the informed flies' network may potentially create distinct environmental conditions for the uninformed flies, which may react accordingly. Environmental variability is often proposed as a major factor affecting social information use. In highly variable environments, individuals may face outdated social information and are expected to rely on their own, more reliable experience [42]. On the contrary, in environments where variability is low, individuals tend to discard personal information in favour of the more reliable and less costly social information [43]. In addition to weighted closeness and eigenvector centralities, we found that homogeneity in the information centrality index, a measure that describes an individual's potential to spread information, affects the oviposition site choice of uninformed flies (figure 3c). Homogeneous networks may constitute less variable environments where social information is evenly perceived from all informed flies, whereas heterogeneous networks may constitute highly variable environments where social information is received more from certain informed flies compared with others. This kind of model, as in all models that refer to complex contagion [44], requires a form of social mechanism that makes the signal more reliable as it is expressed by an increasing number of neighbours. In this study, uninformed flies had a very limited number of options (laying eggs either on banana- or on strawberry-flavoured medium). The existence of social environmental variability may thus induce the rejection of potentially out-dated information and the preference for the other, even if risky, oviposition medium. This process might be similar to the one observed in the study of uncertainty aversion in humans and primates, first introduced by Ellsberg [45]. Studies on rhesus macaques Macaca mulatta showed that animals preferred to choose a risky option rather than an ambiguous one [46].
Finally, we also observed that the more informed flies clustered in subgroups and were less central, the less willing they were to choose the medium they had been conditioned to prefer (weighted clustering coefficient). Interestingly, the means, but not the variances, of the social network parameters were associated with these oviposition preference changes. Because more informed flies (n = 8) than uninformed flies (n = 4) were present in the interaction arena, informed flies with high clustering values were more likely to form subgroups with other informed flies. In such cases, informed flies are likely to exchange many traces of the previously chosen oviposition medium. This accumulation of cues may decrease the attractiveness of this medium in the subsequent test, thus leading informed individuals to change their preferences. Besides, individuals with a high clustering coefficient are more likely to initiate behavioural contagion [47], which could explain the simultaneous change of most informed individuals.
Our experiment showed that, under similar social conditions, flies can exhibit contrasting group reactions. The bimodal pattern of reactions seems to correlate with subtle variation in social network structure, and especially with the level of homogeneity in the behaviour of informed flies. It is not yet clear how the level of homogeneity in the behaviour of informed flies influences the response of uninformed flies. Similarly, we cannot yet determine the factors that affect such variation in social network structures across replicates. Further studies of the source of this variation may help to understand the mechanisms that drive collective decision-making and the origin of behavioural diversification.
Acknowledgements
We thank S. Wardrop for useful corrections on the manuscript and V. Dufour for discussion on ambiguity theory in humans. We thank Prof. M. Brown and two anonymous reviewers for their constructive comments on previous versions of the manuscript.
Data accessibility
All data are deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.vb654.
Authors' contributions
M.B. and F.M. designed the experiments; M.B. conducted the experiments; C.P. and E.K. analysed the networks; C.P., M.B., E.K., C.A.H.B., C.S. and F.M. wrote the manuscript. All authors gave final approval for publication.
Competing interests
Authors declare no competing interests.
Funding
This project is funded by an ANR programme blanc (ANR 12 BSV7 0013 02) to F.M. and C.S. C.S. is funded by the University of Strasbourg Institute for Advanced Study (USIAS) and the Fyssen Foundation. C.P. is funded by an ANR programme blanc (ANR 12 BSV7 0013 02) to F.M. and C.S.
References
- 1.Laland KN. 2004. Social learning strategies. Learn. Behav. 32, 4–14. ( 10.3758/BF03196002) [DOI] [PubMed] [Google Scholar]
- 2.Bonnie KE, Earley RL. 2007. Expanding the scope for social information use. Anim. Behav. 74, 171–181. ( 10.1016/j.anbehav.2006.12.009) [DOI] [Google Scholar]
- 3.Kameda T, Nakanishi D. 2002. Cost–benefit analysis of social/cultural learning in a nonstationary uncertain environment: an evolutionary simulation and an experiment with human subjects. Evol. Hum. Behav. 23, 373–393. ( 10.1016/S1090-5138(02)00101-0) [DOI] [Google Scholar]
- 4.Rendell L, Fogarty L, Laland KN. 2010. Rogers’ paradox recast and resolved: population structure and the evolution of social learning strategies. Evolution 64, 534–548. ( 10.1111/j.1558-5646.2009.00817.x) [DOI] [PubMed] [Google Scholar]
- 5.Range F, Huber L. 2007. Attention in common marmosets: implications for social-learning experiments. Anim. Behav. 73, 1033–1041. ( 10.1016/j.anbehav.2006.07.015) [DOI] [Google Scholar]
- 6.Grüter C, Segers FH, Ratnieks FL. 2013. Social learning strategies in honeybee foragers: do the costs of using private information affect the use of social information? Anim. Behav. 85, 1443–1449. ( 10.1016/j.anbehav.2013.03.041) [DOI] [Google Scholar]
- 7.Trompf L, Brown C. 2014. Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulata. Anim. Behav. 88, 99–106. ( 10.1016/j.anbehav.2013.11.022) [DOI] [Google Scholar]
- 8.Seppänen J-T, Forsman JT, Mönkkönen M, Krams I, Salmi T. 2010. New behavioural trait adopted or rejected by observing heterospecific tutor fitness. Proc. R. Soc. B 278, 1736–1741. ( 10.1098/rspb.2010.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafacz M, Templeton JJ. 2003. Environmental unpredictability and the value of social information for foraging starlings. Ethology 109, 951–960. ( 10.1046/j.0179-1613.2003.00935.x) [DOI] [Google Scholar]
- 10.Boogert NJ, Zimmer C, Spencer KA. 2013. Pre- and post-natal stress have opposing effects on social information use. Biol. Lett. 9, 20121088 ( 10.1098/rsbl.2012.1088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atton N, Galef B, Hoppitt W, Webster M, Laland K. 2014. Familiarity affects social network structure and discovery of prey patch locations in foraging stickleback shoals. Proc. R. Soc. B 281, 20140579 ( 10.1098/rspb.2014.0579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aplin L, Farine D, Morand-Ferron J, Sheldon B. 2012. Social networks predict patch discovery in a wild population of songbirds. Proc. R. Soc. B 281, 20121591 ( 10.1098/rspb.2012.1591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argote L, Turner ME, Fichman M. 1989. To centralize or not to centralize: The effects of uncertainty and threat on group structure and performance. Organ Behav. Hum. Decis. Process 43, 58–74. ( 10.1016/0749-5978(89)90058-7) [DOI] [Google Scholar]
- 14.Rogers Everett M. 1995. Diffusion of innovations. New York, NY: Free Press. [Google Scholar]
- 15.Sueur C, Deneubourg J-L, Petit O. 2012. From social network (centralized vs. decentralized) to collective decision-making (unshared vs. shared consensus). PLoS ONE 7, e32566 ( 10.1371/journal.pone.0032566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H-T, Chen MZ, Zhou T. 2009. Improve consensus via decentralized predictive mechanisms. Europhys. Lett. 86, 40011 ( 10.1209/0295-5075/86/40011) [DOI] [Google Scholar]
- 17.Sueur C, King AJ, Pelé M, Petit O. 2013. Fast and accurate decisions as a result of scale-free network properties in two primate species. In Proc. of the European Conf. on Complex Systems 2013, pp. 579–584. Berlin, Germany: Springer. [Google Scholar]
- 18.Canright GS, Engø-Monsen K. 2006. Spreading on networks: a topographic view. Complexus 3, 131–146. ( 10.1159/000094195) [DOI] [Google Scholar]
- 19.Battesti M, Moreno C, Joly D, Mery F. 2012. Spread of social information and dynamics of social transmission within Drosophila groups. Curr. Biol. 22, 309–313. ( 10.1016/j.cub.2011.12.050) [DOI] [PubMed] [Google Scholar]
- 20.Giurfa M. 2012. Social learning in insects: a higher-order capacity? Front. Behav. Neurosci. 6, 57 ( 10.3389/fnbeh.2012.00057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battesti M, Pasquaretta C, Moreno C, Teseo S, Joly D, Klensch E, Petit O, Sueur C, Mery F. 2015. Ecology of information: social transmission dynamics within groups of non-social insects. Proc. R. Soc. B 282, 20142480 ( 10.1098/rspb.2014.2480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sueur C. 2011. Social network, information flow and decision-making efficiency: a comparison of humans and animals. In Social networking and community behavior modeling: qualitative and quantitative measures (ed. M Safar), pp. 164–177. Hershey, PA: IGI Global. [Google Scholar]
- 23.Flack A, Biro D, Guilford T, Freeman R. 2015. Modelling group navigation: transitive social structures improve navigational performance. J. R. Soc. Interface 12, 20150213 ( 10.1098/rsif.2015.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. 2009. High-throughput ethomics in large groups of Drosophila. Nat Meth 6, 451–457. ( 10.1038/nmeth.1328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider J, Dickinson MH, Levine JD. 2012. Social structures depend on innate determinants and chemosensory processing in Drosophila. Proc. Natl Acad. Sci. USA 109, 17 174–17 179. ( 10.1073/pnas.1121252109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opsahl T, Agneessens F, Skvoretz J. 2010. Node centrality in weighted networks: generalizing degree and shortest paths. Soc. Networks 32, 245–251. ( 10.1016/j.socnet.2010.03.006) [DOI] [Google Scholar]
- 27.Bonacich P. 2007. Some unique properties of eigenvector centrality. Soc. Networks 29, 555–564. ( 10.1016/j.socnet.2007.04.002) [DOI] [Google Scholar]
- 28.Pei S, Makse HA. 2013. Spreading dynamics in complex networks. J. Stat. Mech. Theory Exp. 2013, P12002 ( 10.1088/1742-5468/2013/12/P12002) [DOI] [Google Scholar]
- 29.Seidman SB. 1983. Network structure and minimum degree. Soc. Networks 5, 269–287. ( 10.1016/0378-8733(83)90028-X) [DOI] [Google Scholar]
- 30.Stephenson K, Zelen M. 1989. Rethinking centrality: methods and examples. Soc. Networks 11, 1–37. ( 10.1016/0378-8733(89)90016-6) [DOI] [Google Scholar]
- 31.Opsahl T, Panzarasa P. 2009. Clustering in weighted networks. Soc. Networks 31, 155–163. ( 10.1016/j.socnet.2009.02.002) [DOI] [Google Scholar]
- 32.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Software 33, 1–22. ( 10.18637/jss.v033.i02) [DOI] [Google Scholar]
- 33.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 34.Teplitsky C, Mouawad N, Balbontin J, De Lope F, Møller A. 2011. Quantitative genetics of migration syndromes: a study of two barn swallow populations. J. Evol. Biol. 24, 2025–2039. ( 10.1111/j.1420-9101.2011.02342.x) [DOI] [PubMed] [Google Scholar]
- 35.Ramdya P, Lichocki P, Cruchet S, Frisch L, Tse W, Floreano D, Benton R. 2015. Mechanosensory interactions drive collective behaviour in Drosophila. Nature 519, 233–236. ( 10.1038/nature14024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lihoreau M, Clarke I, Buhl J, Sumpter D, Simpson S. 2016. Collective selection of food patches in Drosophila. J. Exp. Biol. ( 10.1242/jeb.127431) [DOI] [PubMed] [Google Scholar]
- 37.Pasquaretta C, et al. 2014. Social networks in primates: smart and tolerant species have more efficient networks. Sci. Rep. 4, 7600 ( 10.1038/srep07600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clune J, Mouret J-B, Lipson H. 2013. The evolutionary origins of modularity. Proc. R. Soc. B 280, 20122863 ( 10.1098/rspb.2012.2863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boogert NJ, Farine DR, Spencer KA. 2014. Developmental stress predicts social network position. Biol. Lett. 10, 20140561 ( 10.1098/rsbl.2014.0561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trannoy S, Chowdhury B, Kravitz EA. 2015. Handling alters aggression and ‘loser’ effect formation in Drosophila melanogaster. Learn. Mem. 22, 64–68. ( 10.1101/lm.036418.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lerman K, Yan X, Wu X-Z. 2015. The majority illusion in social networks. See http://arxiv:150603022.
- 42.Giraldeau LA, Valone TJ, Templeton JJ. 2002. Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. B 357, 1559–1566. ( 10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Bergen Y, Coolen I, Laland KN. 2004. Nine-spined sticklebacks exploit the most reliable source when public and private information conflict. Proc. R. Soc. Lond. B 271, 957–962. ( 10.1098/rspb.2004.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centola D, Macy M. 2007. Complex contagions and the weakness of long ties. Am. J. Sociol. 113, 702–734. ( 10.1086/521848) [DOI] [Google Scholar]
- 45.Ellsberg D. 1961. Risk, ambiguity, and the savage axioms. Q. J. Econ. 75, 643–669. ( 10.2307/1884324) [DOI] [Google Scholar]
- 46.Hayden BY, Heilbronner SR, Platt ML. 2010. Ambiguity aversion in rhesus macaques. Front. Neurosci. 4, 1–7. ( 10.3389/fnins.2010.00166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenthal SB, Twomey CR, Hartnett AT, Wu HS, Couzin ID. 2015. Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc. Natl Acad. Sci. USA 112, 4690–4695. ( 10.1073/pnas.1420068112) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.vb654.