Abstract
Selective logging is one of the major drivers of tropical forest degradation, causing important shifts in species composition. Whether such changes modify interactions between species and the networks in which they are embedded remain fundamental questions to assess the ‘health’ and ecosystem functionality of logged forests. We focus on interactions between lianas and their tree hosts within primary and selectively logged forests in the biodiversity hotspot of Malaysian Borneo. We found that lianas were more abundant, had higher species richness, and different species compositions in logged than in primary forests. Logged forests showed heavier liana loads disparately affecting slow-growing tree species, which could exacerbate the loss of timber value and carbon storage already associated with logging. Moreover, simulation scenarios of host tree local species loss indicated that logging might decrease the robustness of liana–tree interaction networks if heavily infested trees (i.e. the most connected ones) were more likely to disappear. This effect is partially mitigated in the short term by the colonization of host trees by a greater diversity of liana species within logged forests, yet this might not compensate for the loss of preferred tree hosts in the long term. As a consequence, species interaction networks may show a lagged response to disturbance, which may trigger sudden collapses in species richness and ecosystem function in response to additional disturbances, representing a new type of ‘extinction debt’.
Keywords: ecosystem functioning, network structure, tree–liana networks, selective logging, Southeast Asia, wood density
1. Introduction
Selective logging is one of the major drivers of forest degradation, with 20% of all tropical forests logged between 2000 and 2005 [1], and more than 400 million hectares of tropical forest in permanent timber concessions [2]. Selective logging changes local microclimates [3], modifies resource availability [4], and alters forest structure [5], increasing the amount of edge-affected areas through felling of canopy trees and the creation of logging roads and skid trails [6]. In turn, selectively logged forests are more vulnerable to fires and drought events, especially under extreme weather events such as El Niño episodes [7], and are more accessible to hunters that exploit the network of logging roads [6], each of which can further degrade the remaining habitat and biological communities.
Despite these impacts, logged forests apparently maintain similar species richness to intact forests [5]. Yet, the identity of the species present does shift [8], with declines in forest-interior specialists balanced by increases in disturbance-tolerant taxa (e.g. lianas [9]) or edge-adapted ones (e.g. butterflies [10]). Such shifts notwithstanding, logged forests may retain over 75% of species found in a primary forest, including a suite of The International Union for Conservation of Nature and Natural Resources (IUCN) red-listed species [8].
Because selective logging changes the composition and abundance of species and modifies resource availability, it directly affects many pairwise interactions between species (e.g. pollination or seed dispersal [11]) and how these are organized within larger networks of interactions (network structure [12]), with important consequences for ecosystem stability within logged forests. For instance, the trophic organization of bird and ant communities shifts after logging, with species tending to feed from higher up the food chain, representing less frugivory and more insectivory for birds [13], and more predatory behaviour for ants [14] (see also [15]). In addition, the conversion of forest to agricultural plantations and pasturelands shifts the evenness of interaction frequencies and the rates of parasitism in host–parasitoid food webs, with cascading impacts on important ecosystem services, such as pollination [16]. Studies on plant–animal mutualistic interactions [17], on commensalistic interactions [18], and on antagonistic interactions [16] highlight that some of the most negative impacts of ecosystem degradation are only apparent when evaluating species interactions from a network perspective.
Disturbance of tropical forests generally increases the abundance and diversity of lianas which use trees as support to reach the canopy [9,19,20], although some recent African and Asian studies suggest otherwise [21,22]. The increased liana–host ratio has implications for tree recruitment, survival, and species composition, among others [23]. However, not all species of trees are equally sensitive to this effect [24]. For example, some exhibit traits that make them less prone to host lianas, such as structural defences including a flexible trunk or large leaves, or exhibit rapid growth rates, strongly related to low wood densities [25], which impede lianas from encircling the trees [26]. An alteration of tree community composition following selective logging as a consequence of different liana-induced mortality rates could have important consequences for the recovery of canopy cover, and important ecosystem services, including the rate of carbon sequestration and above-ground carbon storage (reviewed in [9]).
While previous research has focused on the organization of interactions between lianas and their natural hosts in primary forests (e.g. [27]), a basic unanswered question is how logging impacts the topology of liana–tree interaction networks, and the consequences of these changes for network robustness to further disturbance and species losses. Early work suggested that complexity (generally referring to network connectance, i.e. the density of realized links) was a key determinant of robustness [17], while more recent work points towards other network properties, such as nestedness [17,28] or modularity [29], as the actual drivers of network stability and robustness (e.g. to species extinctions [30]). The debate as to which parameter has the dominant role is still ongoing (e.g. nestedness in [31], connectance in [32]), while other studies criticize the simplifying assumptions of models used to test stability (e.g. lack of a dynamic redistribution of interaction frequencies following the extinction of certain species [33]). However, recent work suggests that such a debate might be misleading since most network metrics are strongly interrelated rather than independent properties [34], and their relationship varies with network size [29] and partner specialization (e.g. pollination syndromes [35]). More importantly, whether the level to which network properties such as nestedness patterns or ‘specialization asymmetry’ are caused by intrinsic properties of the species' interactions, rather than being a simple product of variable species abundances that can be explained by so-called ‘null models’ (e.g. [36]) is also controversial [37]. Hence, the use of appropriate null models (e.g. Patefield null models for nestedness [38]) is necessary to test whether or not observed changes in network topology can be attributed solely to different abundance patterns, or if they also reflect changes in interspecific relationships.
Here, we assess the effect of selective logging on liana and tree species richness, community composition, biomass contribution, and on liana–tree interaction network topology within the global biodiversity hotspot of Borneo, Malaysia. Specifically, we test the hypotheses that: (i) species composition, but not species richness, of trees will change after logging, while both species composition and richness of lianas will change following logging, (ii) the liana and tree biomass will increase and decrease, respectively, following logging, (iii) lianas will preferentially colonize trees with slower growth rates, and hence greater wood densities, (iv) the number of links between lianas and trees (connectance) will increase after logging, following an increase in the abundance of lianas throughout disturbed forests, (v) partner specialization will decrease and network nestedness will increase after logging, following the increase in liana abundance and species richness, and the preferential colonization of trees lacking structural defences, and (vi) network robustness to host tree species loss will be greater in logged forests, due to its positive relationship with network nestedness. We further examine whether differences in network topology among the forest types are either a ‘neutral’ consequence of changes in species abundance or reflect changes in interaction patterns independent of such changes in abundance (or in addition to them).
2. Material and methods
(a). Tree and liana sampling
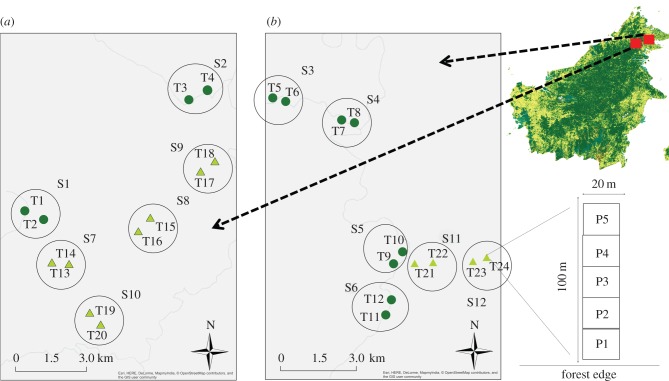
The study was carried out within the 1 million hectare Yayasan Sabah logging concession, Sabah, Malaysian Borneo (figure 1), where forests dominated by the Dipterocarpaceae family were selectively logged during the 1980s and 1990s, and again during the 2000s [39]. We selected six sites in primary forest and six sites in once-logged forest, with each site more than 2 km apart. Our protocol applies the frequently used space-for-time substitution (e.g. [8,40]) as an alternative to following land-use change impacts over decades [41]. Within each site, we established two 100 m transects separated by at least 500–800 m (12 sites × 2 transects/site = 24 transects, figure 1). Within each transect, we located five 20 × 20 m plots at increasing distances from the nearest forest edge (24 transects × 5 plots/transect = 120 plots, figure 1). Within each plot, we recorded tree diameter at breast height (DBH) and liana load for all trees above 10 cm DBH and all lianas above 1 cm DBH. For every individual liana measured, we recorded all trees used as support. Every liana and tree measured was identified to species or morphospecies by a botanist (electronic supplementary material, table S1).
Figure 1.
Study area location in Borneo (a) Maliau Basin and (b) Danum Valley, Malaysian Borneo. Inset shows location of sites (S), the two transects (T) sampled within each site as well as the five plots surveyed at increasing distances from the forest edge for each transect. Dark green circles: primary forest and light green triangles: logged forest. (Online version in colour.)
(b). Data analysis
(i). Species richness, composition, and biomass
To test our first hypothesis, we compared species richness patterns among forest types using individual-based rarefaction curves, at the plot level (n = 60 plots for primary and n = 60 plots for logged forests) with 95% confidence intervals (CIs), constructed in ESTIMATES v. 8.2 [42]. We then evaluated how logging and distance to the forest edge (as well as their interaction) affected liana and tree species richness per sample plot by fitting a generalized linear-mixed model (GLMM) with a Poisson error distribution and a log-link function. Transect was included as a random factor. Using non-metric multidimensional scaling, species composition of trees and lianas in the forest types was determined based on Bray–Curtis similarity [42,43]. A two-dimension ordination was employed. We based our interpretation on the first two dimensions for simplicity and because stress values were relatively low (less than 20%) after incorporating two dimensions (electronic supplementary material, figure S1). Actual differences between forest types were tested using a permutational multivariate analysis of variance with distance matrices. Our sampling protocol means that there are potential confounding effects of (i) spatial autocorrelation of sample transects and (ii) naturally occurring turnover of species across space (beta-diversity) rather than land-use change that explain changes in species richness and composition [44]. To exclude these possibilities, we performed Mantel tests for tree and liana species composition comparing compositional similarity as well as tree and liana species richness to geographical distance between transect pairs within logged forest, within primary forest, and across the whole dataset.
For our second hypothesis, we calculated liana and tree biomass (see electronic supplementary material for exact methodology) and computed average tree and liana biomass per plot as well as the per cent of the total biomass (tree and liana) contributed by lianas. The impact of logging on liana and tree abundance, average liana and tree biomass, average tree wood density, and per cent of total biomass contributed by lianas were then analysed via GLMMs with a Poisson error distribution and a log link for abundances and a gamma error distribution with log-link function for the rest.
To test our third hypothesis, we evaluated how host tree DBH, host tree wood density, and average canopy cover in the analyses affected liana load using a GLMM with a zero-inflated negative binomial distribution. As before, transect was included as a random factor. All analyses were performed using R v. 3.2.2. [45].
(ii). Structure of liana–tree interaction networks
To evaluate our fourth and fifth hypotheses, we explored the structure of weighted liana–tree interaction networks by using a bipartite graph and calculating different metrics for quantitative networks, which included interaction frequencies between particular species of lianas and trees using the bipartite 2.05 library in R v. 3.2.2 [45,46]. Analyses were carried out at the transect level given low sample sizes at the plot level and the fact that we found no differences in tree or liana species richness with distance to the edge (F = 0.78, p = 0.95 and F = 0.63, p = 0.97, respectively). Each network was characterized by a matrix in which rows represented host species (trees) and columns represented liana species with values in matrix cells representing the mean number of lianas of a given species per individual of a given host tree.
We estimated weighted connectance, measured as the proportion of realized interactions from all potential interactions in the network divided by the number of species in the network, and network nestedness, which increases when specialists tend to interact with a subset of the species that generalists interact with [17] using the weighted nestedness based on overlap and decreasing fill (NODF) metric which corrects for matrix fill and dimensions [47] and takes values of 0 for non-nested networks and 100 for perfectly nested ones. We also estimated complementary specialization, 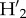 , a standardized index controlling for differences in observations and abundances, where generalized interactions yield a
, a standardized index controlling for differences in observations and abundances, where generalized interactions yield a 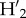 = 0 and
= 0 and 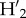 increases as interactions become reciprocally more specialized. Finally, we estimated specialization at the level of each guild, tree, and liana, which was estimated as the weighted mean of the specialization index for each species (
increases as interactions become reciprocally more specialized. Finally, we estimated specialization at the level of each guild, tree, and liana, which was estimated as the weighted mean of the specialization index for each species (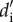 ), with the value for each species weighted by the total number of interactions [48].
), with the value for each species weighted by the total number of interactions [48].
We tested whether our estimated network metrics are caused by intrinsic properties of the species' interactions, rather than being a simple product of variable species abundances that can be explained by so-called ‘null models’ (e.g. [36]) by comparing the values we obtained from our logged and primary forest networks to those of 1 000 networks created based upon a Patefield null model [46] via GLMMs where site was always included as a random factor. We then evaluated how logging might affect each of the descriptive interaction network metrics. Every model included logging (logged versus primary) as a fixed factor and tree and liana species richness and abundances as covariates, to control for differences in sampling effort [49], and was fitted using a gamma error distribution with a log-link function.
(iii). Simulations of host tree and liana local species loss
Finally, to test our last hypothesis, we evaluated whether the different metrics of network topology affected the robustness of liana–tree interaction networks, defined as the area under the extinction curve, which has a maximum value of 1 (as in [18]) and measures the sensitivity to secondary species loss of lianas following the simulation of host tree species loss [50,51]. In our simulations, the cause for the disappearance or local extinction of any given liana species is neither the felling of trees, nor the loss of one of many tree–host species, rather each given liana species is only assumed to go locally extinct when all its host tree species disappear. We simulated three types of species loss scenarios for host trees: ‘random’, where a randomly chosen subset of tree species was eliminated each time; ‘rare-species’, where tree species were removed on the basis of their abundance, with the least abundant being removed first; and ‘connected-species’, where the most connected tree species were removed first.
We then fitted GLMMs including the following predictor variables: five measures of network topology (weighted NODF, weighted connectance, complementary specialization, and tree and liana specialization), logging, the extinction scenario fitted, and all interactions between the two qualitative and the five continuous variables (as common practice in other similar studies, e.g. [50]). We also included the combined number of tree and liana species and transect as a random factor. Prior to analyses, we evaluated the potential correlation between all independent variables, which was more than 0.5 (Spearman's ρ) only between weighted connectance and tree specialization, and therefore these two variables were never included within the same model. In all cases, we generated all possible subsets of the full model and selected the most parsimonious one based on their Akaike information criteria (AICc) value corrected for small sample sizes, the AICc score. All analyses were performed using R v. 3.2.2 [45].
3. Results and discussion
(a). Species richness, composition, and biomass
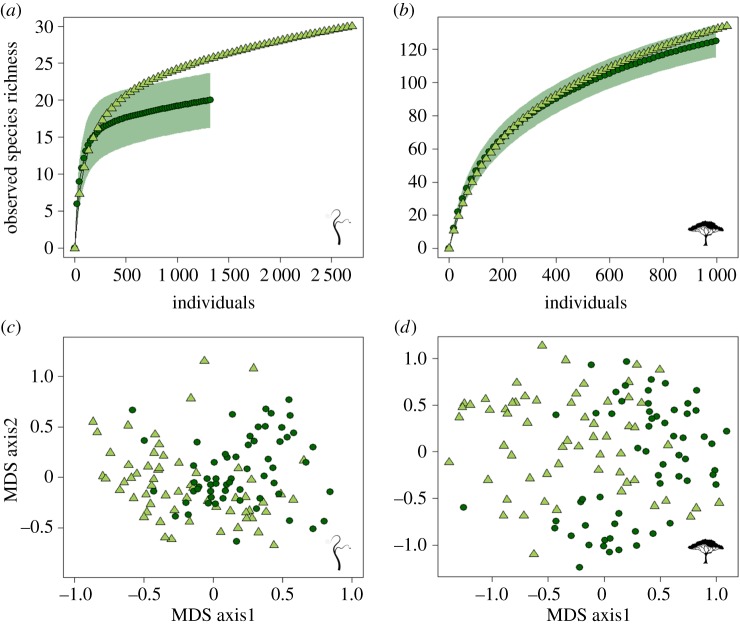
In our 120 sample plots, spanning primary and selectively logged forest, we found that logging leads to an increase in liana species richness (figure 2) and abundance (mean ± standard deviation (s.d.): 45.27 ± 23.10 and 22.28 ± 14.85 in logged and primary forests, respectively; z-value = 3.77, p < 0.001), as well as in liana load per tree (3.41 ± 2.83 in logged and 1.65 ± 1.51 in primary forests, z-value = 3.2, p = 0.001). In turn, we found changes in the composition of both liana (F = 9.05, p < 0.001) and tree (F = 5.77, p < 0.001) communities between forest types but not between plots within the same transect (F = 0.78, p = 0.95 and F = 0.63, p = 0.97, respectively, figure 2), with tendril (e.g. Bauhinia sp.) and hook climbing (e.g. several Strychnos sp.) lianas tending to increase after logging (electronic supplementary material, table S2).
Figure 2.
Logging impacts on species richness and composition. (a,b) Observed species richness, constructed using incidence-based rarefaction curves for the two forest types, of (a) lianas and (b) trees. The x-axis is scaled to show the number of individuals; note that scales differ between figures. (c,d) Multi-dimensional scaling (MDS) plots showing the ordination of transects surveyed in relation to their community composition for (c) lianas and (d) trees. Dark green circles, primary forest; light green triangles, logged forest; green polygons, 95% CI for primary forest. (Online version in colour.)
We found no evidence that spatial autocorrelation or natural rates of species turnover across sample sites affected the species richness (electronic supplementary material, figures S2 and S3) or species composition (electronic supplementary material, figures S4 and S5) results. All comparisons for trees and lianas were not significant (p > 0.05), except for a single significant effect (p = 0.03) of distance on tree species composition across the entire dataset at large spatial scales. Thus, we are confident that our results reflect impacts of selective logging on tree and liana communities (see [8,40] for similar results for an array of taxa), rather than pre-logging heterogeneity between sites.
The great increase in liana abundance in logged forests (see also [9]) resulted in an increase in the above-ground biomass of lianas, whose contribution to total biomass was almost doubled in logged forest (4.41% ± 0.07) compared to primary forest (2.45% ± 0.04, z = 2.94, p= 0.003). Conversely, the decrease in tree wood density associated with logging (z-value = −4.04, p < 0.001) leads to considerable decreases in carbon stocking within logged forests which are probably further exacerbated by the heavier liana loads that increase tree mortality [52]. Yet despite their large increase in biomass, this carbon loss is not compensated by the contribution of greater densities of lianas. Further disturbances associated with logging (e.g. fire [53]) would probably lead to increased tree mortality, exacerbating the decrease in carbon sequestration and stocking.
(b). Structure of liana–tree interaction networks
While previous research focused on the organization of interactions between lianas and their hosts in primary forests (e.g. [27]), a fundamental question that remains unanswered is how logging impacts the topology of liana–tree interaction networks. In particular, little is known about the consequences of these changes for network robustness to further disturbance ([17,28,29], e.g. to species extinctions [30]).
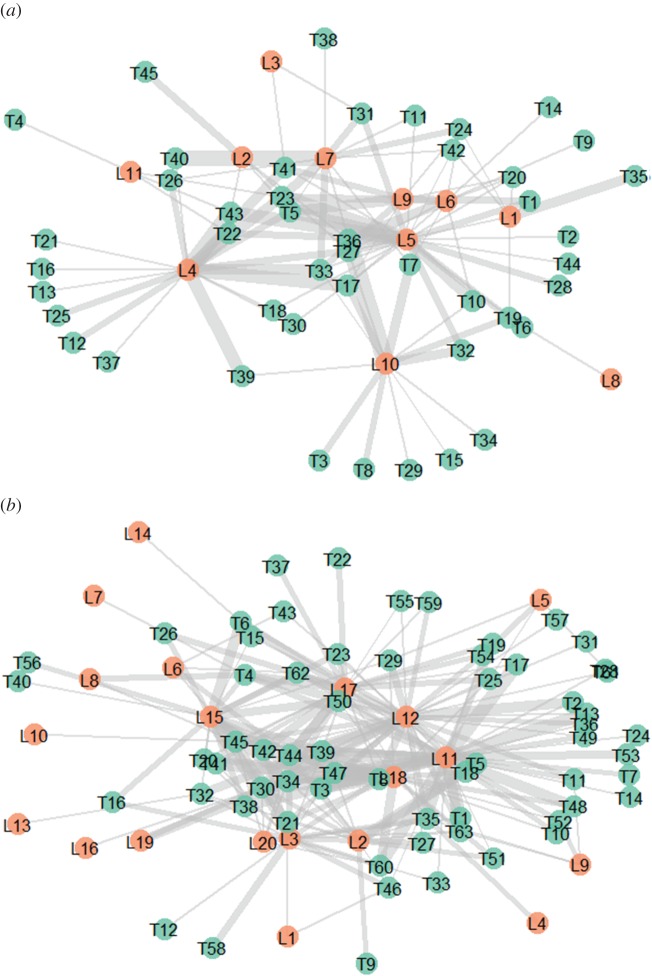
Only a small subset of all possible interactions between trees and lianas were realized; network connectance was thus low (significantly lower than expected from the null models, electronic supplementary material, table S3), particularly in primary forests. Indeed, lianas tend to preferentially colonize certain types of trees, such as those lacking morphological defences (e.g. flexible trunks [24]). In this study, they seem to colonize trees lacking functional defences exhibited by individuals with greater wood densities, a proxy for slower growth [25], which makes them available for colonization over longer periods of time (electronic supplementary material, figure S6). The increase in liana abundance and the differential colonization of slow-growing trees can be expected to decrease the survival and growth rates of these types of trees, exacerbating the effects of logging on biomass stocking, timber value, and ecosystem services described in the previous section [52,54].
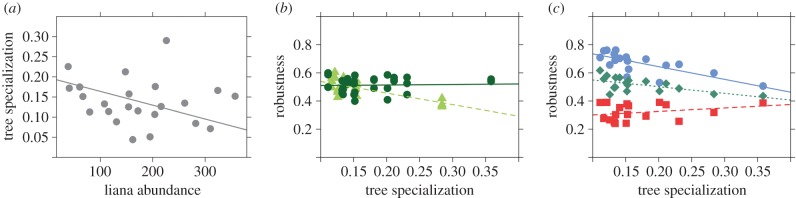
The number of links between trees and lianas (weighted connectance) increased following logging (figure 3), while the converse was true for complementary specialization (electronic supplementary material, table S4), whose decrease indicates that reciprocal specialization becomes less frequent (i.e. generalists tend to interact with generalists) in logged forests [55]. Furthermore, nestedness increased and tree specialization decreased with increasing liana abundance (figure 4a)—suggesting that, in logged forests where liana abundance increases greatly, host trees interact with larger numbers of liana species and become more generalist.
Figure 3.
Quantitative liana tree interaction networks for (a) primary and (b) logged forests. Orange circles represent liana species and green circles represent host tree species. Line width indicates the frequency of each interaction. Figures show data pooled across forest types. (Online version in colour.)
Figure 4.
Partial residual plots showing the effect of liana abundance on the specialization of the guild of trees (a), and how tree specialization relates to network robustness in logged and primary forests (b) as well as to the extinction of selected host trees under three extinction scenarios (c). Dark green circles and solid lines represent primary forest, light green triangles and dashed lines represent logged forests. Blue circles and solid lines represent the rare species extinction scenario, green diamonds and dotted lines represent the random species extinction scenario, and red squares and dashed lines represent the connected species extinction scenario. (Online version in colour.)
(c). Simulations of host tree and liana local species loss
Given that a fraction of host tree species could be locally eliminated by selective logging, a key question is how robust or resilient will liana–tree interaction networks be to such losses [50]. We evaluated this by simulating three different scenarios of host tree local species loss (rare, random, and connected) within our measured quantitative networks. The model with the lowest AICc score (table 1) shows that the decrease in tree specialization found in logged forests (0.11 ± 0.03 as compared to 0.17 ± 0.07 in primary forest) makes lianas less sensitive to host tree species loss in the short term (i.e. results in higher network robustness, figure 4b).
Table 1.
Effect of logging and topology on the robustness to secondary extinctions of tree–liana networks, analysed by means of GLMMs. Significance of p-values is provided by asterisks. Reference categories for the effects shown were ‘primary forest’ for forest type and ‘connected scenario’ for the extinction model.
| factor | level of factor | estimate | standard error (s.e.) | z-value |
|---|---|---|---|---|
| intercept | 0.36 | 0.09 | 4.19*** | |
| logging | logged forest | 0.12 | 0.09 | 1.23n.s. |
| extinction scenario | random | 0.32 | 0.03 | 9.25*** |
| rare | 0.55 | 0.03 | 15.88*** | |
| species richness | −0.004 | 0.001 | −2.96** | |
| tree specialization | 0.69 | 0.25 | 2.76** | |
| logging × tree specialization | logged forest | −0.90 | 0.29 | −3.15** |
| extinction scenario × tree specialization | rare | −0.72 | 0.21 | −3.40*** |
| random | −1.15 | 0.21 | −5.43*** |
**p < 0.01, ***p < 0.001, n.s. >0.05.
Over the longer term, however, higher liana loads can be expected to lead to an increased mortality of host trees. In this case, the most plausible extinction scenario will be the one in which the most connected tree species (i.e. those bearing larger liana loads) disappear preferentially (as opposed to the other two cases in which species are expected to disappear at random or based on their rarity [56]). This is also the scenario in which liana species show the greatest sensitivity to the local extinction of trees (i.e. the lowest network robustness, figure 4c, electronic supplementary material, figure S7). Hence, the increase in liana abundance in logged forests might have the paradoxical effect of reducing liana species richness over the long term—owing to the extinction of lianas that specialize on slow-growing trees, which are infested by larger liana loads and become locally extinct earlier.
Overall, network stability is primarily driven by the type of host tree species most likely to disappear and the functions they perform [50], which lead to cascading effects on the community of lianas. Analysing the effect of network topology on its robustness confirms this interpretation: robustness is mainly driven by the specialization at both the network and the guild level (as opposed to [50]). While these variables provide the best-performing model, an inspection of suboptimal models reveal other significant effects consistent with this interpretation (e.g. significant effects of nestedness and connectance). This should come as no surprise, given that most network metrics are strongly interrelated rather than independent descriptors of network properties [34].
(d). Interaction-driven extinction debt
Our results are analogous to a new type of ‘extinction debt’ [57], in which lagged responses of species interactions to disturbance may suddenly trigger accelerated species loss. This type of extinction debt is of particular importance because it is likely to cause cascading effects on tree community composition and its associated values for humans, including timber production and carbon storage.
Our findings demonstrate the strong impacts of selective logging on the provision of ecosystem services, in particular carbon storage. However, they also suggest that selectively logged forests maintain relatively large values of species richness for trees, and thus still have a high conservation value compared to other possible land uses common throughout the area (e.g. oil palm [40]). Furthermore, while the increase in liana abundance has detrimental effects for their host trees, lianas can benefit a host of wildlife: many lianas bear fleshy fruits that are consumed by an array of animals, including many frugivorous birds [58] and mammals [59]. Lianas provide dense microhabitats suitable for the foraging and nesting of many understorey bird species [58] and are used as a means of locomotion for species like the orangutan [60]. Therefore, the increase in lianas could help in retaining key elements of forest biodiversity and any management towards their complete removal in favour of host trees, perhaps to enhance the rate of timber and carbon recovery [61], should consider the potential side effects on other species in the network [58]. More studies on forest succession following logging, specifically targeting liana communities, are needed to understand the complexity of interactions between trees, lianas, and other dependent species and which, if any, management interventions should be conducted.
Supplementary Material
Acknowledgements
We thank three anonymous reviewers for comments on previous versions of the manuscript.
Data accessibility
Data are available from http://dx.doi.org/10.5061/dryad.6v75k.
Authors' contributions
A.M. and D.P.E. conceived and designed the study; A.M. coordinated the study; A.M., R.S., A.R., and D.N. collected field data; A.M. led data analysis, with participation from L.S. and D.P.E.; and A.M. wrote the first draft of the manuscript, with D.P.E., S.B., L.S., and W.F.L. contributing substantially to revisions. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
A.M. was funded by a Basque Government postdoctoral fellowship and an ETH postdoctoral fellowship. Field research support was provided by an ARC Laureate awarded to W.F.L.
References
- 1.Asner GP, Rudel TK, Aide TM, Defries R, Emerson R. 2009. A contemporary assessment of change in humid tropical forests. Conserv. Biol. 23, 1386–1395. ( 10.1111/j.1523-1739.2009.01333.x) [DOI] [PubMed] [Google Scholar]
- 2.Blaser J, Sarre A, Poore D, Johnson S. 2011. Status of tropical forest management 2011. ITTO technical series no. 38 (ed. Organisation I.T.T.). Yokohama, Japan.
- 3.Miller SD, Goulden ML, da Rocha HR. 2007. The effect of canopy gaps on subcanopy ventilation and scalar fluxes in a tropical forest. Agric. Forest Meteorol. 142, 25–34. ( 10.1016/j.agrformet.2006.10.008) [DOI] [Google Scholar]
- 4.Costa FRC, Magnusson WE. 2003. Effects of selective logging on the diversity and abundance of flowering and fruiting understory plants in a central Amazonian forest. Biotropica 35, 103–114. ( 10.2307/30043039) [DOI] [Google Scholar]
- 5.Putz FE, et al. 2012. Sustaining conservation values in selectively logged tropical forests: the attained and the attainable. Conserv. Lett. 5, 296–303. ( 10.1111/j.1755-263X.2012.00242.x) [DOI] [Google Scholar]
- 6.Laporte NT, Stabach JA, Grosch R, Lin TS, Goetz SJ. 2007. Expansion of industrial logging in Central Africa. Science 316, 1451 ( 10.1126/science.1141057) [DOI] [PubMed] [Google Scholar]
- 7.Nepstad DC, et al. 1999. Large-scale impoverishment of Amazonian forests by logging and fire. Nature 398, 505–508. ( 10.1038/19066) [DOI] [Google Scholar]
- 8.Edwards DP, Larsen TH, Docherty TDS, Ansell FA, Hsu WW, Derhe MA, Hamer KC, Wilcove DS. 2011. Degraded lands worth protecting: the biological importance of Southeast Asia's repeatedly logged forests. Proc. R. Soc. B 278, 82–90. ( 10.1098/rspb.2010.1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnitzer SA, Bongers F. 2011. Increasing liana abundance and biomass in tropical forests: emerging patterns and putative mechanisms. Ecol. Lett. 14, 397–406. ( 10.1111/j.1461-0248.2011.01590.x) [DOI] [PubMed] [Google Scholar]
- 10.Hamer KC, Hill JK, Benedick S, Mustaffa N, Sherratt TN, Maryati M, Chey VK. 2003. Ecology of butterflies in natural and selectively logged forests of northern Borneo: the importance of habitat heterogeneity. J. Appl. Ecol. 40, 150–162. ( 10.1046/j.1365-2664.2003.00783.x) [DOI] [Google Scholar]
- 11.Schleuning M, et al. 2011. Forest fragmentation and selective logging have inconsistent effects on multiple animal-mediated ecosystem processes in a tropical forest. PLoS ONE 6, e27785 ( 10.1371/journal.pone.0027785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris RJ. 2010. Anthropogenic impacts on tropical forest biodiversity: a network structure and ecosystem functioning perspective. Phil. Trans. R. Soc. B 365, 3709–3718. ( 10.1098/rstb.2010.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards DP, et al. 2013. Trophic flexibility and the persistence of understory birds in intensively logged rainforest. Conserv. Biol. 27, 1079–1086. ( 10.1111/cobi.12059) [DOI] [PubMed] [Google Scholar]
- 14.Woodcock P, Edwards DP, Newton RJ, Vun Khen C, Bottrell SH, Hamer KC. 2013. Impacts of intensive logging on the trophic organisation of ant communities in a biodiversity hotspot. PLoS ONE 8, e60756 ( 10.1371/journal.pone.0060756). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewers RM, et al. 2015. Logging cuts the functional importance of invertebrates in tropical rainforest. Nat. Commun. 6, 6836 ( 10.1038/ncomms7836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tylianakis J, Tscharntke T, Lewis O. 2007. Habitat modification alters the structure of tropical host–parasitoid food webs. Nature 445, 202–205. ( 10.1038/nature05429) [DOI] [PubMed] [Google Scholar]
- 17.Bascompte J, Jordano P, Melián CJ, Olesen JM. 2003. The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA 100, 9383–9387. ( 10.1073/pnas.1633576100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piazzon M, Larrinaga AR, Santamaría L. 2011. Are nested networks more robust to disturbance? A test using epiphyte-tree, comensalistic networks. PLoS ONE 6, e19637 ( 10.1371/journal.pone.0019637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurance WF, Pérez-Salicrup D, Delamônica P, Fearnside PM, D'Angelo S, Jerozolinski A, Pohl L, Lovejoy TE. 2001. Rain forest fragmentation and the structure of Amazonian liana communities. Ecology 82, 105–116. ( 10.1890/0012-9658(2001)082%5B0105:rffats%5D2.0.co;2) [DOI] [Google Scholar]
- 20.Ledo A, Schnitzer SA. 2014. Disturbance and clonal reproduction determine liana distribution and maintain liana diversity in a tropical forest. Ecology 95, 2169–2178. ( 10.1890/13-1775.1) [DOI] [PubMed] [Google Scholar]
- 21.Addo-Fordjour P, Rahmad ZB, Shahrul AMS. 2012. Effects of human disturbance on liana community diversity and structure in a tropical rainforest, Malaysia: implication for conservation. J. Plant Ecol. 5, 391–399. ( 10.1093/jpe/rts012) [DOI] [Google Scholar]
- 22.Addo-Fordjour P, Rahmad ZB, Amui J, Pinto C, Dwomoh M. 2013. Patterns of liana community diversity and structure in a tropical rainforest reserve, Ghana: effects of human disturbance. Afr. J. Ecol. 51, 217–227. ( 10.1111/aje.12025) [DOI] [Google Scholar]
- 23.Schnitzer SA, Dalling JW, Carson WP. 2000. The impact of lianas on tree regeneration in tropical forest canopy gaps: evidence for an alternative pathway of gap-phase regeneration. J. Ecol. 88, 655–666. ( 10.1046/j.1365-2745.2000.00489.x) [DOI] [Google Scholar]
- 24.Schnitzer SA, Bongers F. 2002. The ecology of lianas and their role in forests. Trends Ecol. Evol. 175, 223–230. ( 10.1016/S0169-5347(02)02491-6) [DOI] [Google Scholar]
- 25.Philipson CD, et al. 2014. A trait-based trade-off between growth and mortality: evidence from 15 tropical tree species using size-specific relative growth rates. Ecol. Evol. 4, 3675–3688. ( 10.1002/ece3.1186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putz FE. 1984. How trees avoid and shed lianas. Biotropica 16, 19–23. ( 10.2307/2387889) [DOI] [Google Scholar]
- 27.Sfair JC, Rochelle ALC, Rezende AA, van Melis J, Weiser VDL, Martins FR. 2010. Nested liana–tree network in three distinct neotropical vegetation formations. Perspect. Plant Ecol. 12, 277–281. ( 10.1016/j.ppees.2010.09.001) [DOI] [Google Scholar]
- 28.Bascompte J, Jordano P. 2007. Plant–animal mutualistic networks: the architecture of biodiversity. Annu. Rev. Ecol. Evol. Sci. 38, 567–593. ( 10.1146/annurev.ecolsys.38.091206.095818) [DOI] [Google Scholar]
- 29.Olesen JM, Bascompte J, Dupont YL, Jordano P. 2007. The modularity of pollination networks. Proc. Natl Acad. Sci. USA 104, 19 891–19 896. ( 10.1073/pnas.0706375104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortuna MA, Bascompte J. 2006. Habitat loss and the structure of plant–animal mutualistic networks. Ecol. Lett. 9, 278–283. ( 10.1111/j.1461-0248.2005.00868.x) [DOI] [PubMed] [Google Scholar]
- 31.Bastolla U, Fortuna MA, Pascual-Garcia A, Ferrera A, Luque B, Bascompte J. 2009. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 458, U1018–U1091. ( 10.1038/nature07950) [DOI] [PubMed] [Google Scholar]
- 32.James A, Pitchford JW, Plank MJ. 2012. Disentangling nestedness from models of ecological complexity. Nature 487, 227–230. ( 10.1038/nature11214) [DOI] [PubMed] [Google Scholar]
- 33.Benadi G, Blüthgen N, Hovestadt T, Poethke H-J. 2013. When can plant–pollinator interactions promote plant diversity? Am. Nat. 182, 131–146. ( 10.1086/670942) [DOI] [PubMed] [Google Scholar]
- 34.Fortuna MA, Stouffer DB, Olesen JM, Jordano P, Mouillot D, Krasnov BR, Poulin R, Bascompte J. 2010. Nestedness versus modularity in ecological networks: two sides of the same coin? J. Anim. Ecol. 79, 811–817. ( 10.1111/j.1365-2656.2010.01688.x) [DOI] [PubMed] [Google Scholar]
- 35.Danieli-Silva A, de Souza JMT, Donatti AJ, Campos RP, Vicente-Silva J, Freitas L, Varassin IG. 2012. Do pollination syndromes cause modularity and predict interactions in a pollination network in tropical high-altitude grasslands? Oikos 121, 35–43. ( 10.1111/j.1600-0706.2011.19089.x) [DOI] [Google Scholar]
- 36.Vázquez DP, Aizen MA. 2003. Null model analyses of specialization in plant–pollinator interactions. Ecology 84, 2493–2501. ( 10.1890/02-0587) [DOI] [Google Scholar]
- 37.Santamaría L, Rodríguez-Gironés MA. 2007. Linkage rules for plant–pollinator networks: trait complementarity or exploitation barriers? PLoS Biol. 5, e31 ( 10.1371/journal.pbio.0050031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patefield WM. 1981. An efficient method of generating random RxC tables with given row and column totals. Appl. Stat. 30, 91–97. ( 10.2307/2346669) [DOI] [Google Scholar]
- 39.Fisher B, Edwards DP, Larsen TH, Ansell FA, Hsu WW, Roberts CS, Wilcove DS. 2011. Cost-effective conservation: calculating biodiversity and logging trade-offs in Southeast Asia. Conserv. Lett. 4, 443–450. ( 10.1111/j.1755-263X.2011.00198.x) [DOI] [Google Scholar]
- 40.Edwards DP, et al. 2014. Selective-logging and oil palm: multitaxon impacts, biodiversity indicators, and trade-offs for conservation planning. Ecol. Appl. 24, 2029–2049. ( 10.1890/14-0010.1) [DOI] [PubMed] [Google Scholar]
- 41.Pickett STA. 1989. Space-for-time substitution as an alternative to long-term studies. In Long-term studies in ecology: approaches and alternatives (ed. Likens PGE.), pp. 110–135. New York, NY: Springer. [Google Scholar]
- 42.Colwell RK. 2006. EstimateS: statistical estimation of species richness and shared species from samples, v. 8.2. Storrs, CT: University of Connecticut; (http://viceroy.eeb.uconn.edu/EstimateS) [Google Scholar]
- 43.Magurran AE. 2004. Measuring biological diversity. Oxford, UK: Blackwell Science Ltd. [Google Scholar]
- 44.Ramage BS, et al. 2013. Pseudoreplication in tropical forests and the resulting effects on biodiversity conservation. Conserv. Biol. 27, 364–372. ( 10.1111/cobi.12004) [DOI] [PubMed] [Google Scholar]
- 45.Team RDC. 2005. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 46.Dormann CF, Frund J, Bluthgen N, Gruber B. 2009. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol. J. 2, 7–24. ( 10.2174/1874213000902010007) [DOI] [Google Scholar]
- 47.Almeida-Neto M, Ulrich W. 2011. A straightforward computational approach for measuring nestedness using quantitative matrices. Environ. Model Softw. 26, 173–178. ( 10.1016/j.envsoft.2010.08.003) [DOI] [Google Scholar]
- 48.Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N. 2007. Specialization, Constraints, and conflicting interests in mutualistic networks. Curr. Biol. 17, 341–346. ( 10.1016/j.cub.2006.12.039) [DOI] [PubMed] [Google Scholar]
- 49.Blüthgen N, Fründ J, Vázquez DP, Menzel F. 2008. What do interaction network metrics tell us about specialization and biological traits. Ecology 89, 3387–3399. ( 10.1890/07-2121.1) [DOI] [PubMed] [Google Scholar]
- 50.Dunne JA, Williams RJ, Martinez ND. 2002. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol. Lett. 5, 558–567. ( 10.1046/j.1461-0248.2002.00354.x) [DOI] [Google Scholar]
- 51.Vieira MC, Almeida-Neto M. 2015. A simple stochastic model for complex coextinctions in mutualistic networks: robustness decreases with connectance. Ecol. Lett. 18, 144–152. ( 10.1111/ele.12394) [DOI] [PubMed] [Google Scholar]
- 52.Ingwell LL, Joseph Wright S, Becklund KK, Hubbell SP, Schnitzer SA. 2010. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island, Panama. J. Ecol. 98, 879–887. ( 10.1111/j.1365-2745.2010.01676.x) [DOI] [Google Scholar]
- 53.Siegert F, Ruecker G, Hinrichs A, Hoffmann AA. 2001. Increased damage from fires in logged forests during droughts caused by El Nino. Nature 414, 437–440. ( 10.1038/35106547) [DOI] [PubMed] [Google Scholar]
- 54.Gerwing JJ. 2002. Degradation of forests through logging and fire in the eastern Brazilian Amazon. Forest Ecol. Manag. 157, 131–141. ( 10.1016/S0378-1127(00)00644-7) [DOI] [Google Scholar]
- 55.Fayle TM, Edwards DP, Foster WA, Yusah KM, Turner EC. 2015. An ant-plant by-product mutualism is robust to selective logging of rain forest and conversion to oil palm plantation. Oecologia 178, 441–450. ( 10.1007/s00442-014-3208-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyons KG, Schwartz MW. 2001. Rare species loss alters ecosystem function—invasion resistance. Ecol. Lett. 4, 358–365. ( 10.1046/j.1461-0248.2001.00235.x) [DOI] [Google Scholar]
- 57.Valiente-Banuet A, et al. 2014. Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307. ( 10.1111/1365-2435.12356) [DOI] [Google Scholar]
- 58.Ansell FA, Edwards DP, Hamer KC. 2011. Rehabilitation of logged rain forests: avifaunal composition, habitat structure, and implications for biodiversity-friendly REDD+. Biotropica 43, 504–511. ( 10.1111/j.1744-7429.2010.00725.x) [DOI] [Google Scholar]
- 59.Putz FE, Mooney HA. 1991. The biology of vines. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 60.Thorpe SKS, Holder R, Crompton RH. 2009. Orangutans employ unique strategies to control branch flexibility. Proc. Natl Acad. Sci. USA 106, 12 646–12 651. ( 10.1073/pnas.0811537106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards DP, Fisher B, Boyd E. 2010. Protecting degraded rainforests: enhancement of forest carbon stocks under REDD+. Conserv. Lett. 3, 313–316. ( 10.1111/j.1755-263X.2010.00143.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from http://dx.doi.org/10.5061/dryad.6v75k.