Abstract
Protamine sulfate is the only Food and Drug administration approved medication for reversal of intraoperative heparin-induced anticoagulation during cardiac and vascular surgeries. One of the rare side effects of protamine sulfate is an idiosyncratic reaction resulting in acute pulmonary hypertension (APH) and right ventricular (RV) failure occurring after protamine administration. These reactions are rare but catastrophic with high mortality. A 36-year-old female with severe congestive heart failure was undergoing cardiac transplant surgery. After successful implantation of the donor heart, the patient was weaned off cardiopulmonary bypass. Protamine was then administered to reverse the heparin anticoagulation. She immediately developed APH and RV failure immediately after protamine infusion. The patient required immediate administration of inotropic agents, nitric oxide (NO), and subsequently required a number of mechanical support devices including an RV assist device (RVAD) and ultimately full veno-arterial extracorporeal membrane oxygenation (VA-ECMO). Despite heroic efforts, the patient developed refractory multi-organ failure in the Intensive Care Unit and died after family requested discontinuation of resuscitative efforts. This case probably represents the first reported occurrence of fatal protamine-induced APH and ventricular failure in the setting of cardiac transplantation surgery. A number of interventions including inhaled NO, systemic vasopressors, RVAD, and ultimately VA-ECMO failed to reverse the situation, and the patient died of multi-organ failure.
Keywords: Protamine, pulmonary hypertension, right ventricular failure
Introduction
Protamine sulfate is used routinely all around the world to reverse heparin-induced anticoagulation at the end of major cardiac and vascular surgeries. Protamine is a small molecular weight cationic protein that covalently binds to the anionic heparin and inhibits its anticoagulant action. In general, protamine is a very safe medication, but very rarely is associated with potentially lethal side effects. In this case report, we discuss the development of acute pulmonary hypertension (APH) and right ventricular (RV) failure immediately after protamine administration during cardiac transplantation surgery. This idiosyncratic reaction to protamine is a poorly understood entity that needs to be immediately recognized and appropriately managed.
Case Report
A 36-year-old female was evaluated for heart failure. She had a past medical history of acute myeloid leukemia and had been treated with doxorubicin at the age of 13 months. She subsequently developed systolic heart failure with a left ventricular (LV) ejection fraction of 20–25%. Additional co-morbidities included chronic atrial fibrillation, nonsustained ventricular tachycardias with implantation of an automatic implantable cardiac defibrillator and chronic renal insufficiency. Her heart failure symptoms progressed over the course of 10 years leading to a consultation at our institution's heart failure clinic. A cardiac transplant was recommended given her refractory heart failure symptoms and young age. She then received an LV assist device as a bridge to cardiac transplant. A year later, she underwent cardiac transplant surgery. Implantation of the donor heart was uncomplicated, and she was successfully weaned off cardiac bypass. Protamine was then administered to reverse heparin anticoagulation and facilitate disconnection from the cardiac bypass circuit. Upon administration of protamine, the patient developed a sudden elevation of pulmonary artery pressures with profound systemic hypotension consistent with an acute protamine reaction [Figure 1]. The RV appeared acutely dilated and poorly functional on trans-esophageal echocardiography. Frantic attempts were made to reverse the situation with the use of nitric oxide (NO) (to reverse pulmonary vasoconstriction) and multiple vasoactive agents including milrinone, epinephrine, and vasopressin. However, she remained severely hypotensive in-spite of full pharmacological and mechanical support including an intra-aortic balloon pump. An RV assist device was then placed with some improvement in RV filling pressures and cardiac index. She was transferred to the Intensive Care Unit (ICU) where she developed continuous bloody output from her chest tube. She received multiple units of packed red blood cells, platelets, fresh frozen plasma, cryoprecipitate and Bebulin (factor IX) both during the surgery and in the ICU. She continued to deteriorate despite being on isoproterenol, milrinone, norepinephrine, vasopressin, and epinephrine. Due to progressive acidosis and worsening gas exchange, she was transitioned to full veno-arterial extracorporeal membrane oxygenation (VA-ECMO). Continuous renal replacement therapy was initiated for oliguria and severe acidosis. In the subsequent days, attempts to wean her from VA-ECMO failed on multiple occasions. Given her profound multi-organ failure including shock liver, renal failure, circulatory failure, and persistent cardiac failure, the family requested transition to comfort care measures. She died soon after discontinuation of supportive measures. Family declined an autopsy.
Figure 1.
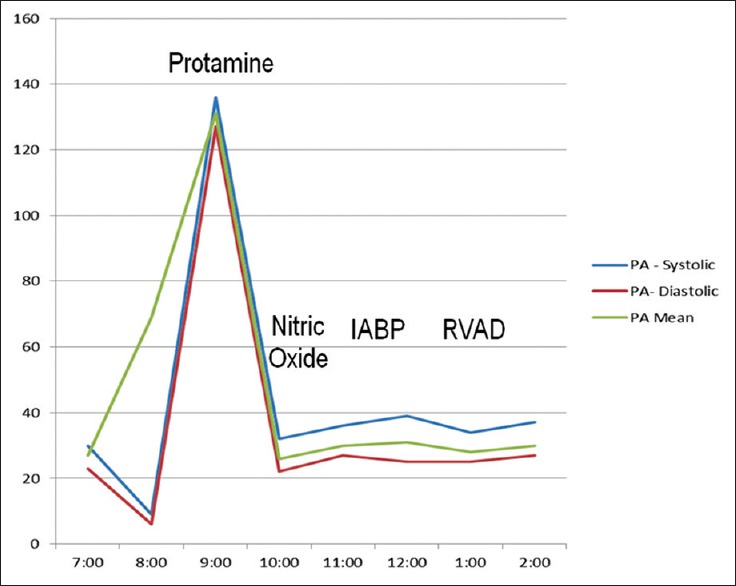
Pulmonary pressure tracing during cardiac transplantation surgery
Discussion
Protamine is a small (molecular weight ~4500 Daltons) highly cationic protein that was developed in the 1930's as a heparin reversal agent. It was originally produced from salmon sperm but is currently produced using recombinant techniques.[1] It covalently binds to anionic heparin forming a stable precipitate and neutralizes the anticoagulant effect of heparin. It is Food and Drug administration approved for reversal of heparin anticoagulation during vascular and cardiac surgical procedures and remains the primary agent used for this purpose even after more than 60 years of use[1,2] Acute reactions to protamine administration can be clinically classified into three distinct subtypes. In Type I reactions, there is significant hypotension following Protamine administration and is likely secondary to histamine release. Type II reactions are mainly allergic and can be further divided into true anaphylactic (Type IIA) and anaphylactoid type reactions (early IIB and delayed IIC). APH following Protamine is classified as a Type III reaction. APH is diagnosed clinically when a sudden rise in pulmonary artery pressure and associated RV failure and hypotension is noted shortly after protamine administration.[2,3,4] Systemic hypotension develops due to LV underfilling consequent to the RV dysfunction. Rarely, biventricular failure has also been documented.[5] It is an extremely rare complication, and the true incidence, and pathophysiological underpinnings are not entirely understood. APH is thought to be mediated by both immunological and nonimmunological factors. Risk factors for protamine reactions include preexisting pulmonary hypertension secondary to valvular heart disease, prior protamine exposure from insulin use, vasectomy and allergy to vertebrate fish.[6,7] Lowenstein et al.[3] documented five cases of APH without any fatal outcomes. Morel et al.[4] described a case series with 48 patients and calculated an incidence of 0.06%. The overall incidence of catastrophic reactions to protamine during cardiovascular surgery is reported to be around 0.13%.[6] Heparin reversal by protamine has been noted to cause leukocytosis,[8] increased levels of complement factor C5b-9, interleukin (IL)-6 and IL-8.[9] Thromboxane is another mediator implicated in animal models of APH.[10]
A number of agents have been used for the treatment of APH including NO and prostacyclin (PGI 2)[11,12] Both intravenous (IV) and inhaled forms of PGI 2 are available.[13] PGI 2 acts ultimately through the release of NO from pulmonary endothelial cells.[13] IV PGI 2 along with dopamine and norepinephrine has been successfully used in the management of APH occurring after open heart surgery.[14] Inhaled NO was used in our patient and quickly induces pulmonary vasodilatation by activating membrane guanylate cyclase to produce cyclic guanosine monophosphate. The typical starting dose for NO is 40 ppm. Side effects of NO include methemoglobinemia and buildup of oxygen free radicals such as nitrogen dioxide,[15] which can themselves lead to pulmonary toxicity. Both PGI 2 and NO can cause rebound pulmonary hypertension on withdrawal of therapy and can hamper in vitro platelet aggregation. The half-life of NO is much shorter than PGI 2 but is significantly more expensive.
Conclusion
In summary, we present a case of fatal protamine-induced APH and RV failure occurring during cardiac transplantation surgery. This case highlights a rare but potentially fatal side effect of protamine administration that clinicians need to be aware of. Treatment is primarily directed at providing RV and LV support using a range of pharmacological and mechanical measures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Horrow JC. Protamine: A review of its toxicity. Anesth Analg. 1985;64:348–61. [PubMed] [Google Scholar]
- 2.Park KW. Protamine and protamine reactions. Int Anesthesiol Clin. 2004;42:135–45. doi: 10.1097/00004311-200404230-00011. [DOI] [PubMed] [Google Scholar]
- 3.Lowenstein E, Johnston WE, Lappas DG, D’Ambra MN, Schneider RC, Daggett WM, et al. Catastrophic pulmonary vasoconstriction associated with protamine reversal of heparin. Anesthesiology. 1983;59:470–3. doi: 10.1097/00000542-198311000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Morel DR, Zapol WM, Thomas SJ, Kitain EM, Robinson DR, Moss J, et al. C5a and thromboxane generation associated with pulmonary vaso- and broncho-constriction during protamine reversal of heparin. Anesthesiology. 1987;66:597–604. doi: 10.1097/00000542-198705000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Jerath A, Srinivas C, Vegas A, Brister S. The successful management of severe protamine-induced pulmonary hypertension using inhaled prostacyclin. Anesth Analg. 2010;110:365–9. doi: 10.1213/ANE.0b013e3181c6bbf0. [DOI] [PubMed] [Google Scholar]
- 6.Levy JH, Zaidan JR, Faraj B. Prospective evaluation of risk of protamine reactions in patients with NPH insulin-dependent diabetes. Anesth Analg. 1986;65:739–42. [PubMed] [Google Scholar]
- 7.Kimmel SE, Sekeres MA, Berlin JA, Ellison N, DiSesa VJ, Strom BL. Risk factors for clinically important adverse events after protamine administration following cardiopulmonary bypass. J Am Coll Cardiol. 1998;32:1916–22. doi: 10.1016/s0735-1097(98)00484-7. [DOI] [PubMed] [Google Scholar]
- 8.Seghaye MC, Duchateau J, Grabitz RG, Faymonville ML, Messmer BJ, Buro-Rathsmann K, et al. Complement activation during cardiopulmonary bypass in infants and children. Relation to postoperative multiple system organ failure. J Thorac Cardiovasc Surg. 1993;106:978–87. [PubMed] [Google Scholar]
- 9.Ashraf SS, Tian Y, Zacharrias S, Cowan D, Martin P, Watterson K. Effects of cardiopulmonary bypass on neonatal and paediatric inflammatory profiles. Eur J Cardiothorac Surg. 1997;12:862–8. doi: 10.1016/s1010-7940(97)00261-3. [DOI] [PubMed] [Google Scholar]
- 10.Conzen PF, Habazetti H, Gutmann R, Hobbhahn J, Goetz AE, Peter K, et al. Thromboxane mediation of pulmonary hemodynamic responses after neutralization of heparin by protamine in pigs. Anesth Analg. 1989;68:25–31. [PubMed] [Google Scholar]
- 11.Ralley FE. The use of nitric oxide for managing catastrophic pulmonary vasoconstriction arising from protamine administration. Anesth Analg. 1999;88:505–7. doi: 10.1097/00000539-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Abe K, Sakakibara T, Miyamoto Y, Ohnishi K. Effect of prostaglandin E1 on pulmonary hypertension after protamine injection during cardiac surgery. Eur J Clin Pharmacol. 1998;54:21–5. doi: 10.1007/s002280050414. [DOI] [PubMed] [Google Scholar]
- 13.Wetzel RC. Aerosolized prostacyclin. In search of the ideal pulmonary vasodilator. Anesthesiology. 1995;82:1315–7. doi: 10.1097/00000542-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Ocal A, Kiris I, Erdinç M, Peker O, Yavuz T, Ibrisim E. Efficiency of prostacyclin in the treatment of protamine-mediated right ventricular failure and acute pulmonary hypertension. Tohoku J Exp Med. 2005;207:51–8. doi: 10.1620/tjem.207.51. [DOI] [PubMed] [Google Scholar]
- 15.Moller JC, Schaible TF, Reiss I, Artlich A, Gortner L. Treatment of severe non-neonatal ARDS in children with surfactant and nitric oxide in a “pre-ECMO”-situation. Int J Artif Organs. 1995;18:598–602. [PubMed] [Google Scholar]


