Abstract
Background:
Imbalances between the oxidant-antioxidant status have been implicated in the pathogenesis of several diseases, including cancer. The aim of this study was to evaluate the extent of lipid peroxidation and antioxidants in the tissue samples of oral squamous cell carcinoma (OSCC) patients of different clinical stages in comparison with the healthy controls.
Materials and Methods:
A case-control study was designed with 20 new histopathologically proven oral carcinoma patients and an equal number of age, sex, and tobacco chewing habit matched healthy subjects. Their tissue samples were subjected to evaluation of lipid peroxidation product and antioxidant enzymes, namely, superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), and glutathione peroxidase (GPx) using spectrophotometric methods. The data are expressed as mean ± standard deviation. The statistical comparisons between the study groups were performed by independent Student's unpaired t-test and one-way analysis of variance. Post-hoc analysis was performed for within study group comparisons. Karl Pearson correlation was performed for the biochemical parameters within the group and between the groups. For statistically significant correlations, simple linear regression was performed using SPSS (α=0.05).
Results:
Significant reduction in lipid peroxidation (P < 0.001) SOD and CAT (P < 0.001) was observed in the tissue of OSCC patients as compared with the healthy controls. On the other hand, reduced GSH and GPx were significantly increased in tumor samples.
Conclusion:
Reduced lipid peroxidation and increased activity of reduced GSH and GPx provides the suitable environment for the local growth and invasion of the tumor and metastasis in the later stages. Among the antioxidant enzymes, GSH reductase appears to have a profound role in carcinogenesis and thus it can be considered as potential prognostic marker.
Keywords: Antioxidants, lipid peroxidation, oral cancer, oxidative stress
INTRODUCTION
Though oxygen is essential for the survival, it is also considered as a source of uncomplimentary reactive oxygen species (ROS) such as superoxide anion (O2−), hydrogen peroxide (H2 O2), and hydroxyl anion. The presence of an unpaired electron in their outer orbit makes them highly reactive. They are produced during several physiological processes like mitochondrial respiration.[1] Exposure to pathogens, environmental pollutants and inappropriate lifestyle like tobacco abuse are some of the external predisposing factors involved in their generation.[2] Under normal physiological conditions, cells are capable of counterbalancing the noxious effects of ROS with the antioxidant defense system which consists of free radical scavengers such as superoxide dismutase (SOD), glutathione reductase (GSH), glutathione peroxidase (GPx), and catalase (CAT). When production of free radicals exceeds the body's antioxidant defense system, it results in oxidative stress (OS). It is imposed on cells due to increase in oxidant generation, a decrease in antioxidant protection, and failure in the repair of oxidative damage.[3]
ROS can cause damage to nucleic acids (DNA), proteins, lipids, and carbohydrates that consequently affect the immune functions causing various diseases including cancer.[4,5] The OS has been implicated in the development and progress of various oral conditions such as lichen planus, recurrent aphthous ulcer, and periodontitis.[6,7] Recent studies have also demonstrated the association between OS and precancerous conditions such as oral submucous fibrosis and oral leukoplakia.[8,9,10]
Oral cancer, globally, is the sixth most common cancer and is a major problem in regions where various tobacco habits are common.[11] The incidence and prevalence of oral cancer in India is high constituting number 1 rank among men and third among women, with an exceedingly high rate of mortality.[12]
Adequate evidence has established the carcinogenic role of ROS in the initiation and progression of cancer. Though the final step in cancer development is mutations in DNA, peroxidation of lipids is considered to be the initial step. The end products of lipid peroxidation such as malondialdehyde are known to be a co-carcinogenic agent, owing to its high cytotoxicity and inhibitory action on protective enzymes.[13]
Despite the fact, that the oral cancer patients have developed OS in their circulation,[14] malignant tumor cells have shown an unrestrained growth. This indicates that malignant cells must have different mechanisms to deal with the free radical toxicity, when compared with the serum. Reduced lipid peroxidation have been reported in tumor cells of various cancers.[15] Subapriya et al.[16] have studied the levels of ROS and antioxidant enzymes assays in oral cancer tumor tissue, but conflicting results regarding thiobarbituric acid reactive substances (TBARS) and SOD have complicated the scenario.[17,18] Although earlier reports have documented the significance of GSH enzymes in oral squamous cell carcinoma (OSCC) patients,[19] no correlation has been demonstrated between GSH with other key parameters implicated in the development of OS. Oral cancer in majority of the cases are supposed to be preceded by potentially malignant disorders, which means that the continuum of disease exists starting from normal to premalignant and finally to a malignant disease. Therefore, considering the above-mentioned fact, we had initially observed levels of TBARS and antioxidant enzymes in tissue samples of leukoplakia and found reduced lipid peroxidation along with raised levels of GSH and GPx.[20]
We, therefore, undertook the present study to evaluate the extent of lipid peroxidation and the antioxidant SOD, GSH, GPx, and CAT in tumor samples of patients with various clinical stages of OSCC. The purpose of the study was to provide a clear picture on the TBARS and SOD levels in the tumor tissue. Furthermore, an attempt was made to explore the association of enzymes and TBARS in the progression of the disease.
MATERIALS AND METHODS
Patients
Twenty newly diagnosed, biopsy-proven squamous cell carcinoma patients were recruited for the study (Group I). All the patients in this group had a history of tobacco usage. Twenty, age and sex matched healthy volunteers with tobacco chewing habit were included in the control group (Group II). The Institutional Ethical Committee approved the study and written informed consent was obtained from all the participants of the study. None of the subjects included in the study had any concomitant disease such as diabetes, hypertension, liver or kidney disorders or other systemic diseases. Oral cancer patients, who had previously undergone any treatment or reported carcinoma elsewhere in the body, were excluded from the study. All the subjects were interviewed before a thorough clinical examination. A questionnaire was used to collect data regarding demographic factors, type of habits, frequency and duration of habits. Histopathologically, Group I (OSCC) patients were divided into well, moderate or poorly differentiated carcinoma whereas clinically they were categorized into Stage II/III/IV on the basis of the tumor node and metastasis (TNM) staging system.
Tissue sample collection
Surgically resected tumor tissues were obtained. Later, the samples were homogenized in phosphate buffer saline, pH 7.4. The cytosols were separated by centrifugation at 20,000 (rpm) in a cooling centrifuge. Normal uninflamed tissues were taken from disease-free, healthy subjects who underwent vestibuloplasty.
Biochemical assays
Lipid peroxidation in tissue was analyzed by the method of Ohkawa et al.[21] The reaction of the thiobarbituric acid with broken-down products of lipid peroxidation will result in pink color chromogen, to be read colorimetrically at 532 nm.
Reduced GSH was estimated by the method of Ellman.[22] This method is based on the development of yellow color, read at 412 nm spectrophotometrically, when 5, 5'-dithiobis 2-nitrobenzoic acid reacts with the supernatant. SOD was assayed by the method of Kakkar et al.[23] It is based on the 50% inhibition of the formation of NADH-phenazine methosulfate nitroblue tetrazolium formazan at 520 nm. The activity of CAT was assayed by the method of Sinha,[24] based on the utilization of H2 O2 by the enzyme. The color developed was read at 620 nm. GPx activity was estimated by following the utilization of H2 O2 according to the method of Rotruck et al.[25]
Statistical analysis
All quantitative data were expressed as mean ± standard deviation (SD), whereas qualitative data in numbers and percentiles. Tabulation of the results was carried out for oral cancer and the control group. All the variables of the study were statistically analyzed for the mean values, SD and P value. The statistical comparison of biochemical parameters between case and control groups was performed by independent Student's t-test. Analysis of variance was used to compare parameters in various TNM stages. Pearson's correlation was used to evaluate whether any correlation exists between TBARS and antioxidant enzymes. A similar correlation analysis was carried out among antioxidant enzymes. For statistically significant correlations, linear regression was performed. The data were analyzed using SPSS version 13.0 package (SPSS Inc. Chicago, IL).
In all the above tests, P < 0.05 was considered statistically significant; P > 0.05 was taken to be statistically not significant; P < 0.01 was taken to be statistically highly significant and P < 0.001 as very highly significant.
RESULTS
Table 1 shows the clinicopathological characteristics of oral cancer patients and control subjects participated in the study. Patients with oral cancer had an average age of 56.35 ± 12.58 years and displayed male predominance. The habit of chewing tobacco with or without additives was a constant finding among all our patients, thus confirms the gravity of these risk factors.
Table 1.
Clinicopathological characteristics of oral cancer patients (Group I) and control group (Group II) participated in the study
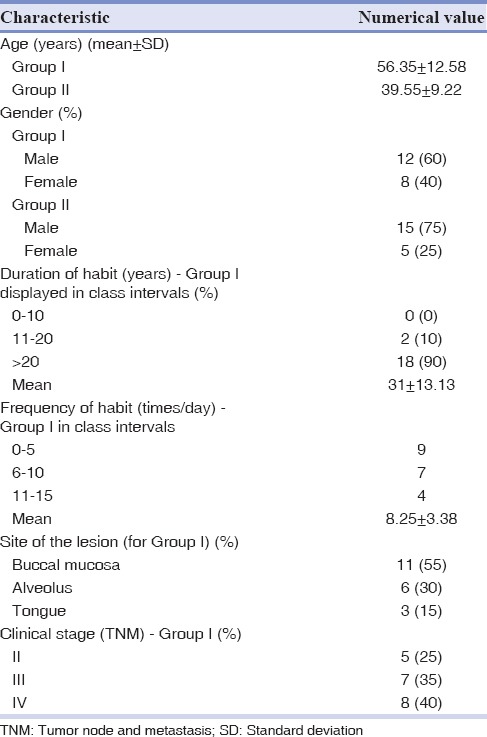
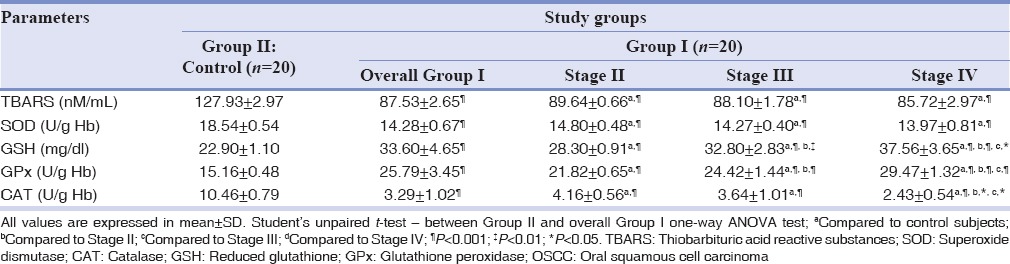
Table 2 shows a comparison of TBARS and various antioxidant enzyme profiles, between oral cancer patients and control subjects. The extent of lipid peroxidation was significantly (P < 0.001) decreased in oral cancer patients, as compared to control subjects. Among the antioxidant enzymes, SOD and CAT were significantly (P < 0.001) decreased, whereas GSH and Gpx were significantly increased, when compared with the controls. On comparing different tumor stages, TBARS did not show a significant (P < 0.05) decrease in transition from Stage II to Stage IV of oral cancer patients. Antioxidant enzymes, GSH and Gpx, showed significant (P < 0.001) decrease along the stages of the disease.
Table 2.
Comparison TBARS, SOD, GSH, GPx and CAT between the normal controls and OSCC groups and among clinical stages of OSCC group
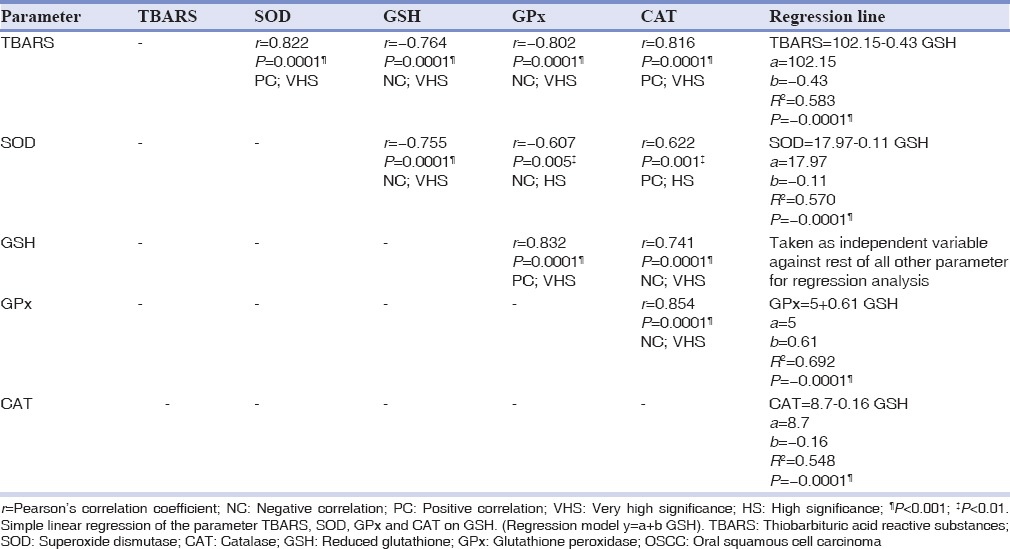
In an attempt, to study the relationship between TBARS and antioxidant enzymes, Pearson's correlation analysis was done. TBARS showed a significant (P < 0.001) positive correlation with SOD and CAT, and a significant (P < 0.001) negative correlation with GSH and GPx. It was also found that GSH had a significant negative correlation with SOD (P < 0.001) and CAT; and significant positive correlation with GPx (P < 0.001) [Table 3], thus laying stress on the crucial role played by GSH enzyme. Henceforth, exploration of GSH was performed with a linear regression model. With the presumption of GSH being an independent variable, a reliable prediction of the dependent variables such as GPx, SOD, CAT, and TBARS can be made. A significant proportion of variance (R2 = 0.69; 69%) in the Gpx is thought to be brought by GSH.
Table 3.
Correlation among TBARS and antioxidant enzymes in OSCC group
DISCUSSION
In our study, we observed significantly decreased (P < 0.001) tissue levels of TBARS in patients with oral cancer as compared to the control subjects [Table 2]. This is suggestive of decreased activity of lipid peroxidation at the tumor tissue level. Continuous cell proliferation is the essence of carcinogenesis. For such uninterrupted growth of tumor cells, lipid peroxidation has to be at a very low level. Inverse relationships have been observed between the levels of lipid peroxidation and the rate of cell proliferation.[3] Our result is in agreement with the previous researchers.[16] Diminished levels of lipid peroxidation have been linked with the two observations made regarding the tumor cell biology. First, the tumor cells have shown to have high concentrations of cholesterol and high cholesterol/phospholipid ratio. This reflects that, the most potent substrate for peroxidation, that is phospholipids, have depleted in large amounts, and thus lipid peroxidation too. At the same time, raised cholesterol had brought rigidity in the biomembrane, thus making it less assessable for attack by oxygen radical species.[3] Second, the altered levels of antioxidant enzymes will also prevent peroxidation of lipid. Previous studies have shown that serum exhibits increased levels of TBARS. Large volumes of TBARS in plasma had been attributed to the abundance of phospholipid as a substrate from erythrocyte membrane.[15] These facts establish that the tissue and serum are two different compartments with varied biological behavior. In this study, TBARS did not show a significant decreasing trend while comparing clinical stages (TNM) of oral cancer [Table 2]. Less number of subjects in each stage (Stage II/Stage III/Stage IV) might have influenced the result.
In the current study, it has been observed that antioxidant enzymes display different patterns of activity at the tissue level. Statistically, significant (P < 0.001) Decrease in SOD and CAT with increased GSH and GPx levels were recorded [Table 2].
As a matter of fact, SOD, CAT, and GPx are considered to be the first line of defense against the free radical attack. Increased lipid peroxidation in the serum will produce large quantities of free radicals including O2−. This gets easily diffused across the membrane and gets accumulated in large volumes even inside the cell. SOD gets thoroughly utilized in scavenging O2− via dismutation reaction.[26] This reaction produces H2 O2 as a byproduct, which is also a potent reactive radical. In an attempt to eliminate H2 O2, CAT, then gets consumed.[5] Apart from reaction's byproduct, H2 O2 is also generated in a considerable amount within the cell. With the increasing levels of H2 O2 and depleting CAT, GPx emerges as a savior for the cell. It has a higher affinity for H2 O2 than CAT or SOD and also has a wider range of action on various hydroperoxides produced during the process of lipid peroxidation.[27] In situations of increasing ROS within the cell, the neoplastic cell has shown to compensate the need for antioxidant enzyme by sequestering them from the serum. Previous reports have thus shown the reduced levels of antioxidant enzymes in the serum samples from oral cancer patients.[14,15] Along with GPx, GSH levels were also high in the current study. GSH has got high redox potential and thus it acts a potent antioxidant and a suitable cofactor for the enzymatic reaction. Because of these properties, GPx utilizes it as a cofactor in the process of neutralizing H2O2. GSH has also known to have a prominent role in detoxification of chemical carcinogens and protection of cell against cytotoxic oxygen free radicals. Known carcinogens from tobacco smoke or quid have found to be predominantly detoxified by glutathione-dependent enzymes. Prolonged direct contact of the quid with the oral mucosa leads to the seepage of the carcinogens and finally gets concentrated in high volumes in the local environment of the tissue. This leads to the increased activity of GSH in the tumor tissue.[28] Overexpression of the GPx and GSH has been reported in a wide range of malignant conditions.[29] In the present study, only GSH and GPx have shown a significant increase from Stage II to Stage IV oral cancer patients despite a small sample size in each category of Stage II/Stage III/Stage IV (5, 8 and 7 respectively) [Table 2]. These results also indicate the decisive role played by these enzymes. Thus, the host tumor cells have a low availability of the substrate for lipid peroxidation. This along with increased levels of GSH and GPx, facilitate the growth of the tumor.
One of the objectives of the current study is to explore the individual interactions among the various biochemical parameters under consideration, correlation, and regression statistical tools were used. TBARS have shown significant (P < 0.001) positive correlation with SOD and CAT, whereas negative correlation with GSH and GPx [Table 3]. On performing correlation analysis among the enzymes, GSH showed significantly (P < 0.001) positive correlation with GPx [Table 3]. This observation is widely documented in the literature, that both have an interplay in the glutathione redox cycle operating for the purpose of detoxifacating H2O2.[2] Another evidence for this observation comes from the significant (P < 0.001) regression analysis between GSH and GPx. Furthermore, considering the R2 value, the contribution of GSH in the variance seen in GPx was found to 69% [Table 3]. Hence, according to our results, GSH and GPx have emerged as the most influencing parameter.
Thus, the present study, supports the previous reports stating that tissue and serum show different behavior on exposure to OS. We, therefore, hypothesize that serum biology in comparison to tissue poses a considerable threat as it produces free radicals in a large amount. They get readily diffused inside the cell to cause various mutations, favoring carcinogenesis. The tissue, on the other hand, is producing the relatively lesser amount of free radical and at the same time, capable of neutralizing them with the available enzymes. Therefore, it can be assumed that along with the internal factors, external environment also influences the selective growth of the tumor cells.
Despite the small sample size, a new observation regarding the independent role of GSH and its highly significant association with GPx is made. Further studies on the molecular mechanisms of ROS-mediated carcinogenesis are warranted. This will be an attempt to unfold the role of GSH as a prognostic marker, thus evolving strategies for effective treatment for oral cancer.
CONCLUSION
Reduced lipid peroxidation supported by increased levels of two protective antioxidant enzymes namely GSH and Gpx can be an explanation of selective local growth nature shown by the malignant tumor tissue. This growth advantage will later facilitate invasion and metastasis. It can also be deduced from the current study that tumor tissue and plasma are two different compartments in respect to behavior toward OS. The ROS in blood is supposed to play a vital role in mutations in the cell; thus, normalization of oxidant-antioxidant status, might be used to improve the prognosis of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 2.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–62. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 3.Dianzani MU. Lipid peroxidation and cancer. Crit Rev Oncol Hematol. 1993;15:125–47. doi: 10.1016/1040-8428(93)90052-6. [DOI] [PubMed] [Google Scholar]
- 4.Abdi S, Ali A. Role of ROS modified human DNA in the pathogenesis and etiology of cancer. Cancer Lett. 1999;142:1–9. doi: 10.1016/s0304-3835(99)00112-3. [DOI] [PubMed] [Google Scholar]
- 5.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Arikan S, Durusoy C, Akalin N, Haberal A, Seckin D. Oxidant/antioxidant status in recurrent aphthous stomatitis. Oral Dis. 2009;15:512–5. doi: 10.1111/j.1601-0825.2009.01580.x. [DOI] [PubMed] [Google Scholar]
- 7.Ergun S, Trosala SC, Warnakulasuriya S, Özel S, Önal AE, Ofluoglu D, et al. Evaluation of oxidative stress and antioxidant profile in patients with oral lichen planus. J Oral Pathol Med. 2011;40:286–93. doi: 10.1111/j.1600-0714.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- 8.Khanna R, Thapa PB, Khanna HD, Khanna S, Khanna AK, Shukla HS. Lipid peroxidation and antioxidant enzyme status in oral carcinoma patients. Kathmandu Univ Med J (KUMJ) 2005;3:334–9. [PubMed] [Google Scholar]
- 9.Avinash Tejasvi ML, Bangi BB, Geetha P, Anulekha Avinash CK, Chittaranjan B, Bhayya H, et al. Estimation of serum superoxide dismutase and serum malondialdehyde in oral submucous fibrosis: A clinical and biochemical study. J Cancer Res Ther. 2014;10:722–5. doi: 10.4103/0973-1482.139160. [DOI] [PubMed] [Google Scholar]
- 10.Shetty SR, Babu SG, Kumari S, Rao V, Vijay R, Karikal A. Malondialdehyde levels in oral sub mucous fibrosis: A clinicopathological and biochemical study. N Am J Med Sci. 2012;4:125–8. doi: 10.4103/1947-2714.93887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González-Ramírez I, García-Cuellar C, Sánchez-Pérez Y, Granados-García M. DNA methylation in oral squamous cell carcinoma: Molecular mechanisms and clinical implications. Oral Dis. 2011;17:771–8. doi: 10.1111/j.1601-0825.2011.01833.x. [DOI] [PubMed] [Google Scholar]
- 12.Byakodi R, Byakodi S, Hiremath S, Byakodi J, Adaki S, Marathe K, et al. Oral cancer in India: An epidemiologic and clinical review. J Community Health. 2012;37:316–9. doi: 10.1007/s10900-011-9447-6. [DOI] [PubMed] [Google Scholar]
- 13.Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, et al. The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect. 1998;106(Suppl 1):289–95. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava KC, Austin RD, Shrivastava D, Sethupathy S, Rajesh S. A Case control study to evaluate oxidative stress in plasma samples of oral malignancy. Contemp Clin Dent. 2012;3:271–6. doi: 10.4103/0976-237X.103617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manoharan S, Kolanjiappan K, Suresh K, Panjamurthy K. Lipid peroxidation and antioxidants status in patients with oral squamous cell carcinoma. Indian J Med Res. 2005;122:529–34. [PubMed] [Google Scholar]
- 16.Subapriya R, Kumaraguruparan R, Ramachandran CR, Nagini S. Oxidant-antioxidant status in patients with oral squamous cell carcinomas at different intraoral sites. Clin Biochem. 2002;35:489–93. doi: 10.1016/s0009-9120(02)00340-5. [DOI] [PubMed] [Google Scholar]
- 17.Gokul S, Patil VS, Jailkhani R, Hallikeri K, Kattappagari KK. Oxidant-antioxidant status in blood and tumor tissue of oral squamous cell carcinoma patients. Oral Dis. 2010;16:29–33. doi: 10.1111/j.1601-0825.2009.01598.x. [DOI] [PubMed] [Google Scholar]
- 18.Patel BP, Rawal UM, Shah PM, Prajapati JA, Rawal RM, Dave TK, et al. Study of tobacco habits and alterations in enzymatic antioxidant system in oral cancer. Oncology. 2005;68:511–9. doi: 10.1159/000086995. [DOI] [PubMed] [Google Scholar]
- 19.Rawal RM, Patel DD, Patel BP, Patel MM, Wadhwa MK, Patel PS, et al. Assessment of glutathione-S-transferase and glutathione reductase in patients with squamous-cell carcinoma of buccal mucosa. Int J Cancer. 1999;83:727–31. doi: 10.1002/(sici)1097-0215(19991210)83:6<727::aid-ijc5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava KC, Austin RD, Shrivastava D, Pranavadhyani G. Oxidant-antioxidant status in tissue samples of oral leukoplakia. Dent Res J (Isfahan) 2014;11:180–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 24.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 25.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 26.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 27.Fiaschi AI, Cozzolino A, Ruggiero G, Giorgi G. Glutathione, ascorbic acid and antioxidant enzymes in the tumor tissue and blood of patients with oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2005;9:361–7. [PubMed] [Google Scholar]
- 28.Coles B, Ketterer B. The role of glutathione and glutathione transferases in chemical carcinogenesis. Crit Rev Biochem Mol Biol. 1990;25:47–70. doi: 10.3109/10409239009090605. [DOI] [PubMed] [Google Scholar]
- 29.Moghadasian MH, Freeman HJ, Godin DV. Endogenous antioxidant status in neoplastic and adjacent tissues in 1,2-dimethylhydrazine-induced colon cancer in rats: Effects of olsalazine. Carcinogenesis. 1996;17:983–7. doi: 10.1093/carcin/17.5.983. [DOI] [PubMed] [Google Scholar]


